Abstract
Glaucoma is a heterogeneous group of diseases which is one of the leading causes of irreversible blindness worldwide. Although the eye-brain axis has been proposed, its functional connectivity remains poorly defined. This study aimed to explore the mechanisms and causal relationship between glaucoma and brain cortical structure, focusing on the eye-brain axis. A Mendelian randomization (MR) study was conducted using inverse variance weighting as the primary estimator, alongside MR-PRESSO, MR-Egger, and weighted median methods to assess sensitivity, heterogeneity, and pleiotropy. Pathway analysis, transcriptomic analysis, and weighted gene co-expression network analysis (WGCNA) were applied to investigate brain-eye interactions in Alzheimer’s disease (AD) and primary open-angle glaucoma (POAG), revealing shared pathogenic mechanisms. Significant associations between glaucoma and brain cortex regions, including the superior temporal sulcus, anterior cingulate, cuneus, entorhinal, inferior temporal, and insula, were identified. About 18 overlapping genes between AD and POAG were found, including MYH14, EFNA1, FZD1, and CACNG3. Using WGCNA, 11 overlapping genes were identified as most related to both AD and POAG, including TSC2, MAGED4, LSS, and DNM1. These results contributed to understanding the association between glaucoma and the brain, indicating the eye-brain axis and may provide clues for early screening of high-risk populations.
Keywords: Alzheimer’s disease, brain cortical structure, glaucoma, IOP, Mendelian randomization, visual field defects
1. Introduction
Glaucoma is a heterogeneous group of diseases characterized by cupping of the optic nerve head and visual field damage, potentially leading to irreversible blindness.[1–3] A study using UN World Population Prospects data estimated that by 2040, 3.54% of affected people will be 40 to 80 years old and 111.8 million will be affected overall.[4] Glaucoma shares similar pathogenic mechanisms with several neurodegenerative and psychiatric diseases, such as Alzheimer’s disease (AD) and depression.[5] This suggested a potential correlation between glaucoma and structural changes in the brain.
The concept of the eye-brain axis has recently emerged, emphasizing the necessity of an intact functional connection between the retina and brain for proper visual function.[6] Functional magnetic resonance imaging provides a noninvasive method to evaluate neuronal activity, revealing neuronal degeneration in the lateral geniculate nucleus (LGN), primary visual cortex (V1), and other visual areas in the context of visual defects, optic disc damage, and retinal nerve fiber layer (RNFL) thinning.[6–8] Additionally, recent research has established a correlation between glaucoma and white matter abnormalities.[9] Previous studies have shown that amyloid-β (Aβ) was involved in the loss of RGCs, as well as the degeneration of the LGN and V1 in glaucoma models. The deposition of Aβ was broadly discovered in the chronic glaucoma model.[10] Therefore, a comprehensive understanding of the eye-brain axis could lead to more advanced treatments for patients (Fig. 1).
Figure 1.
The conception atlas of eye-brain axis. Description of the concept construction of eye-brain axis. IOP = intraocular pressure, RNFL = retinal nerve fiber layer.
We conducted Mendelian randomization (MR) analysis to explore the causal relationship between glaucoma and brain function, specifically focusing on the human brain’s cortical surface area (SA) and cortical thickness (TH). We further discussed the similarities and differences between AD and glaucoma, uncovering the correlation between the 2 diseases. Our research addressed a critical gap, offering new insights into the connection between glaucoma and brain cortex structure, which could enhance early diagnosis and detection of both conditions.
2. Methods
2.1. Study overview
We used a 2-sample MR study to investigate the causal relationship of glaucoma including glaucoma, intraocular pressure (IOP), vertical optic cup-disc ratio (CDR), visual field defects, and RNFL thickness on brain functional cortex. An overview of the study is presented in Figure 2. Glaucoma, IOP, CDR, visual field defects, and RNFL thickness were the exposures, while SA and TH of specific brain regions were the outcomes in the MR analysis. The overall design of this MR study was based on the below assumption: all the included genetic variants were closely related to the interest exposures (glaucoma and its related vision indicators); the genetic variants were not correlated with potential confounders; and the affected effect on the outcome (brain regional cortex) was the only the result of our exposures.
Figure 2.
Overview of Mendelian randomization study design. A flow diagram of our MR study design to study the causal relationship of glaucoma on the SA and TH of total and 34 regional brain cortexes. The MR analysis was primarily estimated using inverse variance weighted (IVW). CDR = cup-disc ratio, MR‑Egger and weighted median. GWAS = genome-wide association study, IOP = intraocular pressure, IVW = inverse variance weighted, MR = Mendelian randomization, RNFL = retinal nerve fiber layer, SA = surface area, SNPs = single nucleotide polymorphisms, TH = thickness.
2.2. Data source of glaucoma, IOP, CDR, visual field defects, RNFL thickness
The genome-wide association study (GWAS) data associated with glaucoma were extracted from UK Biobank (UKB) participants (n = 3,51,696).[11] The GWAS data associated with left or right eye of IOP (Goldmann-correlated) were extracted from UKB participants. The meta-analysis data of CDR gene loci included 18 cohorts of the European descent (n = 32,067, enrolled cohorts were listed in Table S1, Supplemental Digital Content, https://links.lww.com/MD/P928).[12] The visual field defects data was extracted from the results of FINGEN (n = 3,77,277, https://www.finngen.fi/fi).[13] The FinnGen study combined the genome information with the digital healthcare data of participants whose were over 18 years lived in Finland. The meta-analysis data of RNFL was derived from the optical coherence tomography images of 31,434 UKB participants.[14]
2.3. Data source of brain cortex SA and cortex TH
The meta-analysis data of brain cortex SA and TH was obtained from ENIGMA Consortium.[15] The study identified cortical SA and TH measures in 51,665 individuals from 60 cohorts all over the world primarily consisting of ~94% European descent (enrolled cohorts were listed in Table S2, Supplemental Digital Content, https://links.lww.com/MD/P928). The brain cortex was divided into 34 regions according to Desikan–Killiany atlas.[15] The boundaries of region were defined by gyral anatomy labeled from the depths of the sulci.[16] The data used here included total SA and average TH, as well as regional SA and TH averaged across both hemispheres.[15] We performed MR analysis for glaucoma, IOP (including the right and the left IOP), CDR, visual field defects, retinal nerve fiber layer (RNFL) thickness on the total SA and TH, and the respective regional SA and TH for 34 regions with or without global weighted. The MR analysis totally generated 828 outcomes.
2.4. Genetic variants selection criteria
To identify the causal relationship between glaucoma and the brain cortical function, we used 6 sets of genetic instruments indicating different aspects of glaucoma pathophysiology, including index single nucleotide polymorphisms (SNPs) representing glaucoma (listed in Table S3, Supplemental Digital Content, https://links.lww.com/MD/P928), index SNPs representing right IOP (RIOP, listed in Table S4, Supplemental Digital Content, https://links.lww.com/MD/P928), index SNPs representing left IOP (LIOP, listed in Table S5, Supplemental Digital Content, https://links.lww.com/MD/P928), index SNPs representing CDR (listed in Table S6, Supplemental Digital Content, https://links.lww.com/MD/P928), index SNPs representing visual field defects (listed in Table S7, Supplemental Digital Content, https://links.lww.com/MD/P928), and index SNPs representing RNFL thickness (listed in Table S8, Supplemental Digital Content, https://links.lww.com/MD/P928). Since the diagnosis of glaucoma can’t be accomplished by using a single index, we included a series of index in our MR to fix the bias.
We used stringent clumping criteria (GWAS-correlated P-value of 5 × 10−8, a linkage disequilibrium [LD] r2 of <0.001, and <10,000 kb from the index variant) to select independent SNPs.[17] The exception of GWAS-correlated P-value for visual defects was 5 × 10−6, as applying a more stringent threshold of P < 5 × 10−8 resulted in too few available instrumental variables. This threshold was chosen to ensure sufficient statistical power while capturing a broader range of genetic variants potentially relevant to visual defects. And we harmonized the SNPs of exposures to exclude palindromic and incompatible SNPs. The concrete selection process is listed below as Figure 2.
2.5. Statistics analysis
All analyses were performed using the TwoSampleMR package (version 0.5.6) in R software (version 4.2.2).[18] For a global-level test, a significant 2-sided P-value was set as .05. For region-level analyses, given the 816 MR estimates, a Bonferroni-corrected P-value was set as 0.05/816 (6.127 × 10−5), and meanwhile P < .05 was regarded as nominally significant.
We used random-effect inverse variance weighted (IVW),[19] MR-Egger,[20] and weighted median[21] to conduct MR analysis which guaranteed the accuracy and credibility of these results. The primary analysis was conducted using IVW, which relies on inverse variance weighting. This approach incorporated SNP-specific estimates derived from Wald ratios, under the assumption that no directional pleiotropic effects were present for any SNP. MR-Egger and weighted median were used as sensitivity analyses to assess the robustness of IVW. MR-Egger intercept test was utilized to assess the horizontal pleiotropy. No significant evidence of horizontal pleiotropy was detected as indicated by the MR-Egger intercept. Additionally, a leave-one-out analysis was conducted by systematically excluding 1 SNP at a time and applying the IVW method to the remaining SNPs to assess the influence of individual variants on the estimates. If the results of IVW, MR-Egger and weighted median were not consistent, a tighten instrument P-value threshold was used and then the MR analysis was re-performed (Table S9, Supplemental Digital Content, https://links.lww.com/MD/P928).[22]
We also used the MR-PRESSO outlier test to remove the outlier results and calculated P-values to assess pleiotropy for each SNP, while the MR-PRESSO global test evaluated overall horizontal pleiotropy.[23,24] In addition, the Cochran’Q test was used to exam the heterogeneity. The funnel plot was used to test directional pleiotropy. After retrieving significant results, we also checked whether the SNPs were associated with potential risk factors on the website the PhenoScanner including hypertension, hyperlipemia, body mass index, drinking, obesity, smoking, and neuropsychiatric diseases, and removed the related SNPs, after which we conducted the MR analysis again to confirm the accuracy of results.[25]
2.6. Integration of transcriptomic analysis in the GEO database
Data for POAG and AD were extracted from Gene Expression Omnibus (GEO) microarray expression profiling dataset (http://www.ncbi.nlm.nih.gov/geo/). We chose dataset including GSE27276 and GSE260873. Dataset GSE27276 involved genome-wide expression in human trabecular meshwork tissue between 13 controls and 15 POAG cases. Dataset GSE260873 utilized in this study involved RNA sequencing data isolated from the gray matter in the superior frontal gyrus from 12 patients with AD and 12 individuals without AD. The data in GSE260873 and GSE27276 have been normalized when uploaded in GEO datasets. Specifically, for GSE260873, we applied a log2 transformation to the normalized expression values (log2(normalized expression + 1)). For GSE27276, the expression levels have already been normalized and log2-transformed, making the data ready for downstream analysis without the need for further preprocessing. For batch correction, we applied the surrogate variable analysis to both datasets. The differential expressed genes (DEGs) respectively related to POAG and AD were screened by the “limma” package in R software. The genes with P-value < .05 and |log2(FC)| > 0.5 were considered to be DEGs. Then, the “ggplot2” packages in R software are used to draw heatmap and volcano plot respectively to visualize the differential genes. The venn plot of overlapping protein between POAG and suicide/AD were pictured by using “ggvenn” package. Gene Ontology (GO) terms and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways enrichment analysis were performed to find the functional enrichment of the above overlapping mRNA.
2.7. WGCNA analysis
Weighted correlation network analysis (WGCNA) was used in this study to discovering clusters (modules) of highly correlated genes and summarize such clusters using the module eigengene.[26] For GSE27276 and GSE260873, we used WGCNA analysis and find the most related modules and key genes. We chose R2 = 0.9 and the soft-threshold β = 18. Subsequently, the adjacency matrix was transformed into a topological overlap matrix (TOM). Modules were identified with hierarchical clustering (minModuleSize = 50). The eigengene was calculated, and the modules were hierarchically clustered. The module eigengene (ME) was used to distinguish the vital modules associated with POAG or AD. ME shows the first principal component in the module, and describes the expression pattern of the module. GO terms and KEGG pathways enrichment analysis were performed to find the functional enrichment of the gene module above.
2.8. Ethical review
Since all data used in this study were obtained from publicly available databases, ethical approval and patient consent were not required. The data for this study was sourced from the the UKB, FinnGen, ENIGMA and GEO public database and can be extracted respectively from https://www.nealelab.is/uk-biobank, https://www.finngen.fi/fi, https://enigma.ini.usc.edu/ and http://www.ncbi.nlm.nih.gov/geo/.
3. Results
3.1. Causal effects of glaucoma on brain cortex
A total of 143 index SNPs were selected to genetically predict glaucoma, while 92 and 93 index SNPs were chosen to indicate left and right IOP, respectively. Additionally, 25 index SNPs were selected for RNFL thickness, 28 for CDR, and 3 for visual defects. Notably, 34 SNPs overlapped between left and right IOP, which was acceptable as they were representative index SNPs for IOP. Consequently, we conducted MR analysis between 6 representative aspects of glaucoma and 34 regional cortical SA and TH measurements, both with and without global weighting (Fig. 3). Furthermore, we analyzed the causal relationship between the glaucoma and brain cortex SA and TH (Fig. 3). The IVW results were the primary components of our analysis. No significant glaucoma-associated SNPs were found to influence the global cortical level. However, we identified several nominally significant SNPs that may affect specific functional regions of the gyrus in relation to glaucoma (Fig. 3). Glaucoma index SNPs had no causal effect on the regional brain cortex SA with or without global weighted. Data indicated that glaucoma function had a potential influence on the SA and TH region including gyrus of bankssts, caudal anterior cingulate, cuneus, entorhinal, fusiform, inferior parietal, inferior temporal, insula, lateral orbitofrontal, lingual, medial orbitofrontal, middle temporal, paracentral, pars opercularis, pars orbitalis, pericalcarine, postcentral, superior temporal, supramarginal, temporal pole, transverse temporal. Detailed information is provided in Table 1 and the supplementary tables. We further created scatter plots, funnel plots, and leave-out plots for all genetically predicted SNPs for 6 aspects of glaucoma and the potentially affected SA and TH with or without global weighted (Figs. S1–S18, Supplemental Digital Content, https://links.lww.com/MD/P927). For all nominally significant SNPs, we used the Phenoscanner website to exclude those influenced by risk factors (as listed in Table S10, Supplemental Digital Content, https://links.lww.com/MD/P928). The MR-Egger intercept test P-values were all >.05 for IVW significant SNPs, indicating no horizontal pleiotropy.
Figure 3.
Heatmap for the IVW estimates of glaucoma and brain cortical structure. IVW estimates from glaucoma, right intraocular pressure, left intraocular pressure, CDR, visual field defects and retinal nerve fiber layer on brain cortical structure as defined using magnetic resonance imaging-measured brain cortical surficial area and thickness. The color of each block represents the IVW-derived P-values in which P-values of <.05 were shown in red and P-values of >.05 were shown in white or blue. P-value < .05 is set as nominally significant, whereas <6.127 × 10−5 is set as significant. CDR = cup-disc ratio, IVW = inverse variance weighted, LIOP = left intraocular pressure, RIOP = right intraocular pressure, RNFL = retinal nerve fiber layer, SA = surface area, TH = thickness.
Table 1.
Nominally significant Mendelian randomization estimates from glaucoma, RIOP, LIOP, CD ratio, visual defects, RNFL on genetically predicted cortical structure.
| Exposure | Regions | Outcome | IVW-derived P-value | SE | β (95% confidence intervals) | Cochran’s Q-derived P-value (IVW) | MR-Egger intercept-derived P-value |
|---|---|---|---|---|---|---|---|
| RIOP | SA with global weighted | Bankssts | .01688415 | 3.153644 | −7.534751 (−13.71589324 to −1.35360876) | .1610958 | .789299 |
| RNFL | SA with global weighted | Cuneus | .03577377 | 0.9637074 | 0.03577377 (1.853092734–1.924640274) | .3953804 | .8227772 |
| Visual field defect | SA with global weighted | Insula | .04248628 | 5.820073 | 11.80733 (0.39999–23.21467) | .885334 | .7933541 |
| RNFL | SA with global weighted | Lingual | .01522818 | 2.28831 | 5.553515 (1.068–10.0386) | .07561273 | .3415702 |
| CDR | SA with global weighted | Medial orbitofrontal | .01158722 | 6.282351 | −15.85962 (−28.1730 to −3.5462) | .4161612 | .06997356 |
| LIOP | SA with global weighted | Middle temporal | .03853515 | 7.749717 | −16.035069 (−31.2245 to −0.8456) | .004543756 | .5970609 |
| RIOP | SA with global weighted | Middle temporal | .01902528 | 7.288442 | −17.09165 (−37.3770 to −2.8064) | .04548642 | .7422792 |
| RIOP | SA with global weighted | Pars orbitalis | .03452194 | 1.61511 | 3.414216 (0.2486–6.5798) | .8465002 | .8388516 |
| RNFL | SA with global weighted | Pericalcarine | .03788488 | 1.423034 | 2.954358 (0.1652–5.7435) | .1622568 | .6868197 |
| CDR | SA with global weighted | Supramarginal | .03867818 | 14.80571 | 30.6122 (1.5930–59.6314) | .5295423 | .9152001 |
| LIOP | TH with global weighted | Inferior temporal | .01585135 | 0.003035155 | 0.007321765 (1.372E−3–0.0133) | .482825 | .6329547 |
| RIOP | TH with global weighted | Inferior temporal | .03885252 | 0.003152263 | 0.006511773 (3.33E−04–1.27E−02) | .3137848 | .161031 |
| LIOP | TH with global weighted | Insula | .02825338 | 0.003590151 | 0.007875894 (8.391E−04–1.49E−02) | .04422644 | .4502293 |
| RIOP | TH with global weighted | Insula | .032344755 | 0.003395193 | 0.007266134 (6.12E−04–1.39E−02) | .1659844 | .3000331 |
| CDR | TH with global weighted | Lateral orbitofrontal | .01427768 | 0.005478756 | 0.01342405 (2.69E−03–2.42E−02) | .2847521 | .2742373 |
| CDR | TH with global weighted | Paracentral | .04550759 | 0.004907963 | 0.009815593 (1.96E−04–1.94E−02) | .3908524 | .2844665 |
| LIOP | TH with global weighted | Pars opercularis | .03217115 | 0.002545815 | −0.005453843 (−1.04E−02 to −4.64E−04) | .279893 | .4653224 |
| CDR | TH with global weighted | Postcentral | .01336995 | 0.004271543 | −0.01056677 (−1.89E−02 to −2.19E−3) | .3777455 | .5570059 |
| LIOP | TH with global weighted | Superior temporal | .03562407 | 0.003011846 | −0.006328468 (−1.22E−02 to −4.25E−04) | .2280699 | .9167432 |
| RIOP | TH with global weighted | Superior temporal | .01696313 | 0.003398077 | −0.008112929 (−1.48E−02 to −1.45E−03) | .01128277 | .2270669 |
| Visual field defect | TH with global weighted | Superior temporal | .01183891 | 0.003803505 | −0.00957308 (−1.70E−02 to −2.12E−03) | .3329308 | .4519864 |
| Visual field defect | TH with global weighted | Temporal pole | .01089616 | 0.009326843 | −0.023746252 (−4.20E−02 to −5.47E−03) | .6769357 | .8193353 |
| LIOP | TH with global weighted | Transverse temporal | .00420875 | 0.004599944 | −0.01316539 (−2.22E−02 to −4.15E−03) | .4847958 | .1118366 |
| RIOP | TH with global weighted | Transverse temporal | .00905521 | 0.004880191 | −0.01273712 (−2.23E−02 to −3.17−03) | .2405437 | .3972771 |
| RIOP | SA without global weighted | Bankssts | .003989793 | 3.966734 | −11.4200986 (−19.2 to −3.65) | .1100141 | .3803361 |
| RNFL | TH without global weighted | Caudal anterior cingulate | .0382417 | 0.001339957 | −0.002776732 (−0.00540304772 to −0.00015041628) | .552215 | .2748972 |
| RNFL | SA without global weighted | Cuneus | .00629431 | 1.209682 | 3.304901 (0.93392428–5.67587772) | .4015765 | .516554 |
| CDR | TH without global weighted | Entorhinal | .03263177 | 0.01494659 | 0.03193465 (0.00263933360000001–0.0612299664) | .6248359 | .4863026 |
| Glaucoma | TH without global weighted | Fusiform | .01402422 | 0.001588878 | −0.003903305 (−0.00701750588 to −0.00078910412) | .3124801 | .1755189 |
| Glaucoma | TH without global weighted | Inferior parietal | .03089072 | 0.001408483 | −0.003040179 (−0.00580080568 to −0.00027955232) | .9090512 | .9706877 |
| Glaucoma | TH without global weighted | Inferior temporal | .03629676 | 0.001669538 | −0.003495323 (−0.00676761748 to −0.00022302852) | .8403206 | .5607643 |
| CDR | TH without global weighted | Lateral orbitofrontal | .005211349 | 0.006671737 | 0.01863864 (0.00556203548–0.03171524452) | .7788618 | .2786813 |
| RNFL | TH without global weighted | Lingual | .002939873 | 2.774716 | 8.251884 (2.81344064–13.69032736) | .10954548 | .7468527 |
| RIOP | SA without global weighted | Middle temporal | .01372171 | 12.0184 | −29.61896 (−53.175024 to −6.062896) | .009174386 | .1796473 |
| CDR | TH without global weighted | Paracentral | .140958 | 0.007239849 | 0.01559431 (0.00140420596–0.02978441404) | .3719876 | .7373556 |
| RNFL | SA without global weighted | Pericalcarine | .009817014 | 1.64111 | 4.237689 (1.0211134–7.4542646) | .1488886 | .7752245 |
| RIOP | SA without global weighted | Temporal pole | .04377713 | 1.710582 | −3.4489069 (−6.80164762 to −0.09616618) | .001727006 | .5758234 |
| Visual field defect | TH without global weighted | Temporal pole | .009320226 | 0.01030391 | −0.02679099 (−0.0469866536 to −0.0065953264) | .5633278 | 5.59E-01 |
| LIOP | TH without global weighted | Transverse temporal | .01610277 | 0.006087239 | −0.01464941 (−0.02658039844 to −0.00271842156) | .07879453 | 5.19E-01 |
| RIOP | TH without global weighted | Transverse temporal | .03021788 | 0.005888416 | −0.01276151 (−0.02430280536 to −0.00122021464) | .1714643 | .6457767 |
CDR = cup-disc ratio, IVW = inverse variance weighted, LIOP = left intraocular pressure, MR = Mendelian randomization, RIOP = right intraocular pressure, RNFL = retinal nerve fiber layer, SA = surface area, TH = thickness.
3.2. Transcriptomic analyses in GEO database
To further testify the causal relationship of glaucoma on brain cortex, we compared DEGs for the data in GEO database for primary open-angle glaucoma (POAG) and AD. This study aimed to reveal the similarities and differences of these 2 diseases and find the close relationship between POAG and AD from the disease level. In particular, the data of patients with AD were extracted from inferior frontal which was found in the previous study to be related with glaucoma. In GSE27276 dataset for POAG, we found 444 upregulated genes and 439 downregulated genes (Fig. 4A, Table S11, Supplemental Digital Content, https://links.lww.com/MD/P928). In GSE260873 dataset for AD, we found 509 up-regulated genes and 352 down-regulated genes (Fig. 4B, Table S12, Supplemental Digital Content, https://links.lww.com/MD/P928). We identified 18 overlapping mRNA between POAG and AD including MYH14, EFNA1, FZD1, CACNG3, LTBP3, DIAPH2, GADD45B, ELF3, CRLF1, KCNJ2, SLC24A3, GP1BB, GRP, SLC25A10, ATP6AP2, SCARF2, LLGL2, and CST3 (Fig. 4C). Then we conducted KEGG enrichment analysis and GO enrichment analysis to find the common influenced pathway. We found these DEGs involved in tight junction and proximal tubule bicarbonate reclamation (Fig. 4D). From the GO enrichment analysis, the crucial common involved in the common pathological process can be identified (Fig. 4E).
Figure 4.
Integration of transactional analysis of AD and POAG. (A) Volcano plot for DEGs in POAG. (B) Volcano plot for DEGs in AD. (C) Venn diagram summarizing the differential and overlapping DEGs for POAG and AD. (D) KEGG enrichment analysis for overlapping DEGs. (E) GO analysis for overlapping DEGs. AD = Alzheimer’s disease, DEGs = differentially expressed genes, KEGG = Kyoto Encyclopedia of Genes and Genomes, GO = Gene Ontology, POAG = primary open-angle glaucoma.
3.3. Module hub gene analysis by WGCNA
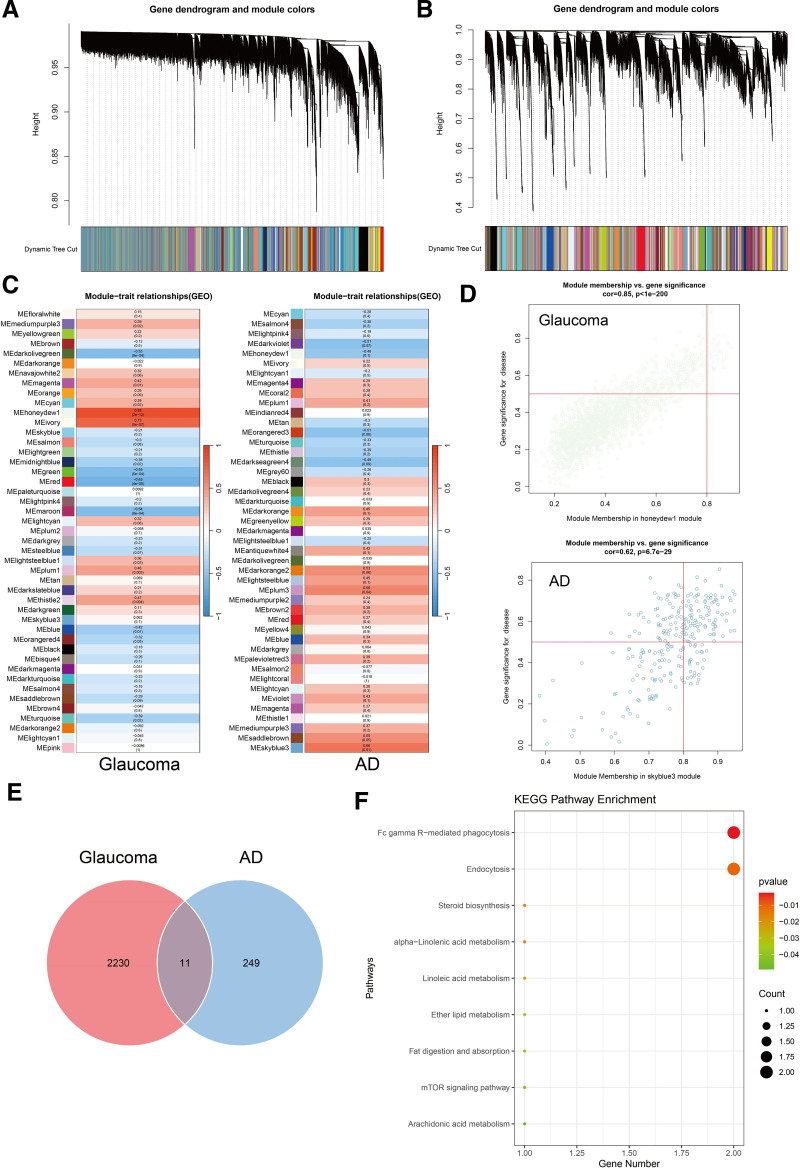
We used WGCNA analysis to establish gene modules which contained a group of high expression genes (Fig. 5A, B). For the GSE27276 dataset, 45 gene modules were identified (Fig. 5C). Among these, module MEhoneydew1 was found to be the most correlated with glaucoma development (Fig. 5D, Table S13, Supplemental Digital Content, https://links.lww.com/MD/P928). Also, for GSE260873, 45 gene modules were identified (Fig. 5C), among which MEskyblue3 was considered to be the most intimate module for AD development (Fig. 5D, Table S13, Supplemental Digital Content, https://links.lww.com/MD/P928). By combining the 2 modules, we identified 11 overlapping genes including TSC2, MAGED4, LSS, MIDN, EPS15, DNM1, GAK, LGALS3BP, PLA2G6, TUBG2, and MAEA (Fig. 5E). Later, we performed KEGG analysis at the RNA level (Fig. 5F). We identified several signal pathways involved in both AD and glaucoma pathology. They included Fc gamma R-mediated phagocytosis, endocytosis, lipid metabolism (steroid biosynthesis, alpha-linolenic acid metabolism, linoleic acid metabolism, ether lipid metabolism, fat digestion and absorption and arachidonic acid metabolism) and mammalian target of rapamycin (mTOR) signaling pathway (Table S14, Supplemental Digital Content, https://links.lww.com/MD/P928).
Figure 5.
WGCNA analysis for GSE27276, and GSE260873. (A) Dynamic tree plot for glaucoma. (B) Dynamic tree plot for AD. (C) Specific and concrete gene module division and correlation index respectively for glaucoma and AD. (D) Significance and correlation index for genes in the most related gene modules in POAG and AD. (E) Venn diagram summarizing the differential and overlapping genes between the most related gene module for POAG and AD. (F) KEGG analysis for overlapping genes in the most related gene modules. POAG = primary open-angle glaucoma, AD = Alzheimer’s disease, KEGG = Kyoto Encyclopedia of Genes and Genomes, WGCNA = weighted gene co-expression network analysis.
4. Discussion
To date, this was the first large-scale MR analysis focusing on the relationship between glaucoma function and the brain functional structure. Our findings indicated that glaucoma, IOP, RNFL thickness, CDR, and visual field defects impacted brain function, further supporting the existence of the eye-brain axis. This discovery explained the reason why glaucoma patients often exhibited a higher prevalence of brain structure changes and brain diseases. These results had significant implications for diagnosing and preventing brain diseases and offered valuable insights for clinical practice.
RGCs degeneration is one of the main characters for glaucoma. Previous study has demonstrated that multiple forms of death were involved in the RGCs degeneration including apoptosis, ferroptosis, PANoptosis, and necrosis.[27–32] We mainly demonstrated that several brain cortical regions including visual and nonvisual ways where SA and TH were affected by glaucoma. On the one hand, the mechanism of transneuronal degeneration can be a contributing factor to defects in the visual pathway. Anterograde transsynaptic degeneration is the process in which retinal degeneration causes subsequent degeneration of the posterior visual pathway. The axons of retinal neurons extend to form the optic nerve and converge at the optic chiasm, then project to the visual cortex through the LGN.[33] Thus, transneuronal degeneration may be one of the causes of cortical changes in the brain. Glaucoma involves transneuronal degeneration of the posterior structures along the central visual pathway, as the neurotrophic factor like brain-derived neurotrophic factor (BDNF) and ciliary neurotrophic factor (CTNF), and metabolites like choline plays a significant part in it.[34] The volume of primary visual cortex and visual pathway including LGN, V1, lingual gyrus and calcarine fissure decreases indicating the atrophy of these structures. Meanwhile, the degeneration of brain cortex along the visual pathway was discovered in POAG including hippocampus, thalamus and midbrain as the magnetic resonance imaging indicating decreased volume.[35] On the other hand, nonvisual cognitive pathways were also influenced by POAG like cingulate cortex, caudate nucleus, corpus callosum and claustrum.[35] This might explain the emotion change in glaucoma patients.[36] Intrinsically photosensitive retinal ganglion cells detect light via the G-protein-coupled receptor melanopsin, depolarize, generate electrical spikes, and influence physiology, behavior, perception, and mood through widespread brain projections to cortex.[37]This will be the physiological basis for brain cortex changes.
Among these, most of the temporal cortical regions, including the superior, middle, transverse, and inferior temporal gyri, fusiform gyrus, and temporal pole, were found to be affected by glaucoma-related SNPs. Observation of Tau protein in brain can be the preclinical characteristics of AD which widespread deposits in these area.[38] Our results proved the influence of glaucoma on the temporal part on the genetic level which potentially explains the phenomena and outcomes of high incident rates of AD in the population of glaucoma patients. The impact of glaucoma risk SNPs on the insula further elucidated the cognitive dysfunction and memory loss observed in glaucoma patients. Specifically, the pars opercularis (Brodmann area 44), which was involved in language function, was affected. The significant SNPs identified could potentially serve as preclinical markers for brain diseases such as AD. The above results indicated that the nominally significant findings from the MR analysis align with the transneuronal degeneration caused by glaucoma and brain cortical atrophy.
Given the close connection between glaucoma and brain function, our study focused on the relationship and similarities between AD and POAG.[39,40] Previous studies have identified several shared genetic loci and protein-coding genes, such as MTCH2, NDUFS3, SPI1, MYBPC3, and PTPMT1, suggesting common genetic markers between the 2 conditions.[41] In our study, we identified 18 overlapping DEGs between AD and POAG. Among them, GADD45B, FZD1, EFNA1, LTBP3, and CST3 have been reported to potentially play a role in the development of both AD and glaucoma. GADD45B is directly involved in stress-induced DNA repair, cell cycle arrest, cell survival, and apoptosis. It is widely expressed in the nervous system, and its dysregulation may lead to neuronal damage.[42] FZD1 is a receptor in the Wnt signaling pathway, which plays a crucial role in neuronal development, synaptic plasticity, and neurodegeneration. Dysregulation of FZD1 has been linked to neurodegenerative processes in AD.[43] Given its involvement in retinal development, our study suggested that FZD1 dysregulation may contribute to RGCs damage in glaucoma.[44] EFNA1, which is essential for axon guidance and neuronal communication, also plays a key role in synaptic plasticity.[45] The activation of EFNA1 may therefore promote neuronal regeneration, offering potential therapeutic insights for neurodegenerative diseases.[46] LTBP3 regulates the activation of TGF-β, a cytokine involved in tissue repair and fibrosis. In the context of glaucoma, LTBP3 contributes to extracellular matrix remodeling,[47] while mutations in LTBP1 have been identified in clinical families with AD. CST3 encodes cystatin C, a potent inhibitor of cysteine proteases. Elevated levels of CST3 have been associated with modulation of Aβ metabolism in AD.[47] Cysteine, as part of this process, may also be involved in aqueous humor outflow. Consequently, elevated CST3 levels could potentially contribute to glaucoma by increasing intraocular pressure.[48] In addition, these DEGs were found to be enriched in pathways such as tight junctions. The results suggested that alterations in tight junctions may represent a common pathological feature of both diseases. Under stress, downregulation of VE-cadherin, β-catenin, VEGFR-1, VEGFR-2, vimentin, Cdc42, and ACK1 was observed in the retina.[49] In AD mouse model, tight junctions in the intestine can be disrupted by Aβ deposition.[50] For example, Aβ and Tau protein deposits in the retina and central visual systems of experimental high IOP model.[51]
To identify the most co-expressed genes in AD and glaucoma, we performed WGCNA analysis. We identified 2 modules highly related to both diseases, showing a positive association with disease progression. By integrating AD and glaucoma data, we discovered 11 overlapping genes. KEGG pathway analysis revealed common functions in lipid metabolism, the mTOR pathway, endocytosis, and phagocytosis. Lipid metabolism plays a complex role in the neurodegenerative changes observed in glaucoma. For example, the leukotriene and arachidonic acid metabolic pathways promote retinal neuroinflammation,[52] which directly leads to RGCs damage. In contrast, the metabolism of lipoxins has been shown to exert a protective effect in glaucoma models. In AD, abnormal lipid levels accelerate Aβ deposition and promote tangled Tau protein.[53] Thus, disruption of lipid metabolism homeostasis may serve as a common mechanism of degeneration in both glaucoma and AD, potentially acting as a bridge in the eye-brain axis. The mTOR signaling pathway plays a crucial role in coordinating various neuronal functions and maintaining neuronal homeostasis in the brain and retina.[54,55] Abnormalities in the mTOR pathway can disrupt autophagic processes, leading to neurotoxic cell death. In AD, glaucoma and other neurodegenerative disorders, misfolded and ubiquitylated proteins accumulate in autophagic vacuoles, clogging the system, and causing the buildup of toxic proteins in dystrophic neurites. Neuronal cells, unlike other cell types, cannot divide to eliminate excess macromolecules or organelles, making them highly dependent on autophagy.[56,57] Any disruption in autolysosome clearance can impair the entire endocytic machinery, which may worsen metabolic and immune-related damage, thereby exacerbating AD and RGCs loss in glaucoma.[55] As noted, damage to endocytosis and phagocytosis can also lead to RGCs loss and neuronal degeneration, contributing to the development of neurodegenerative diseases. What’s more, the transcriptomic analysis presented above reveals that the common pathways identified in both glaucoma and AD may contribute to RGCs loss, and therefore may result in the transneuronal degeneration, and brain cortical atrophy.
All in all, this MR study and transcriptomic analysis revealed common pathways and a close relationship between glaucoma and AD. Existing clinical studies also suggested that glaucoma patients were at a higher risk of developing dementia.[58] Additionally, clinical research indicated that, compared to cataract patients, individuals with normal-tension glaucoma were more likely to experience cognitive impairments.[58,59] These findings aligned with our results and further emphasized the importance of identifying shared mechanisms in neurodegenerative diseases, as well as the need to search for potential preclinical biomarkers.
A limitation of our study was that the databases predominantly consist of European populations, which may not fully represent the genetic and environmental diversity of other populations. This may lead to biased estimates and limit the generalizability of the findings to other ethnic and racial groups. Future studies should aim to include more diverse cohorts to enhance the applicability and robustness of the results across different populations. What’s more, new methods accounting for potential participation biases can be considered in the future.[60] Another potential limitation of our study was the sample overlap between the exposure (glaucoma-related indicators, including glaucoma, IOP, CDR, and RNFL) and outcome (cerebral cortical structure) datasets, both derived from the UKB. Based on Burgess’s simulation,[61] the bias introduced by sample overlap is minimal when the instrument strength is high, with bias tending toward the null. In our study, the total overlap between the CDR cohort and outcome was fewer than 1716 participants, constituting <5% of the sample. Moreover, according to study of Minelli et al,[62] 2-sample MR remains valid even with overlap when both exposure and outcome come from large biobanks like the UKB. Given the large sample size and strong instrument strength in our study, we expected the bias from sample overlap to be limited. As such, no additional sensitivity analysis was deemed necessary. To further ensure the robustness of our findings, we plan to conduct sensitivity analyses, such as MRlap, in future studies.[63] Additionally, previous study employed a similar MR approach using the same outcome as in our research, with exposure data from the UKB, further supporting the validity of this approach.[64] The wide confidence intervals observed for some β coefficients, such as for visual defects and SA of the insula (β = 11.807, 95% CI [0.399, 23.214]), indicate instability in the results. This could be due to factors such as sample size, data variability, or measurement inconsistencies. These uncertainties highlighted the need for caution in interpreting the findings and suggested that further studies with larger sample sizes and more precise measurements are necessary to validate these associations. Additionally, adjusting for P-values revealed no significant estimates in the current data and analysis. Furthermore, we relied on public databases to explore the disease pathology between glaucoma and brain diseases. Due to data source constraints and the cross-sectional nature of the study, we were only able to compare AD with glaucoma. As a result, we could not determine the temporal sequence or causal relationship between the 2 conditions. For instance, AD-related cognitive or behavioral symptoms can be examined in commonly used glaucoma animal models, such as the acute glaucoma model,[27] glutamate excitotoxicity model,[28] and chronic magnetic bead-induced model.[65] Based on the insightful feedback provided, we have also emphasized the importance of prospective studies, which will be critical in clarifying the sequence of pathological events and uncovering the molecular basis of glaucoma-related brain alterations.
5. Conclusions
This study revealed the relationship between the functional brain cortex and glaucoma, IOP, RNFL, CDR, and visual field defects. We found that glaucoma-related functions might drive broad structural brain changes. Brain magnetic resonance imaging of different functional regions may offer clues for early screening of high-risk populations for psychiatric and neurodegenerative disorders in glaucoma patients. Additionally, we uncovered similarities and differences between POAG and AD, identifying significant and notable potential preclinical markers. Neurologists should consider both retinal and neurodegenerative changes. This study highlighted the potential eye-brain axis and offers valuable clinical insights.
Acknowledgments
We gratefully thank for the data supplied by GEO database, UK Biobank, FINGEN, and ENIGMA Consortium.
Author contributions
Conceptualization: Xingyi Chen, Xiaobo Xia.
Investigation: Chaoran Shi.
Methodology: Meihui He, Xingyi Chen.
Supervision: Xiaobo Xia.
Writing – review & editing: Chaoran Shi.
Writing – original draft: Xingyi Chen.
Supplementary Material
Abbreviations:
- AD
- Alzheimer’s disease
- Aβ
- amyloid-β
- CDR
- cup-disc ratio
- DEGs
- differential expressed genes
- GEO
- Gene Expression Omnibus
- GO
- Gene Ontology
- GWAS
- genome-wide association study
- IOP
- intraocular pressure
- IVW
- inverse variance weighted
- KEGG
- Kyoto Encyclopedia of Genes and Genomes
- LGN
- lateral geniculate nucleus
- MR
- Mendelian randomization
- POAG
- primary open-angle glaucoma
- RNA
- ribonucleic acid
- RNFL
- retinal nerve fiber layer
- SA
- surface area
- SNPs
- single nucleotide polymorphisms
- TH
- thickness
- UKB
- UK Biobank
- V1
- primary visual cortex
- WGCNA
- weighted gene co-expression network analysis
This study was financially supported by National Key Research and Development Program of China (grant number 2024YFA1108704), National Natural Science Foundation of China (grant number 82171058), Research and Development Program of Hunan Fu Rong Laboratory (grant number 2024PT5107). This study was supported by National Clinical Key Specialty of Ophthalmology.
The datasets generated during and/or analyzed during the current study are publicly available.
The authors have no conflicts of interest to disclose.
The data for this study was sourced from the the UKB, FinnGen, ENIGMA and GEO public database and can be extracted respectively from https://www.nealelab.is/uk-biobank, https://www.finngen.fi/fi, https://enigma.ini.usc.edu/ and http://www.ncbi.nlm.nih.gov/geo/.
How to cite this article: Chen X, Shi C, He M, Xia X. Novel insights into the relationship between glaucoma and brain diseases from the genetic to diseases levels: A cross-sectional study. Medicine 2025;104:38(e44416).
This manuscript was previously posted to SSRN: http://dx.doi.org/10.2139/ssrn.4549226.
Supplemental Digital Content is available for this article.
Contributor Information
Xingyi Chen, Email: xingyichen@csu.edu.cn.
Chaoran Shi, Email: 2204170217@csu.edu.cn.
Meihui He, Email: hemeihui222@163.com.
References
- [1].GBD 2019 Blindness and Vision Impairment Collaborators; Vision Loss Expert Group of the Global Burden of Disease Study. Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: the right to sight: an analysis for the global burden of disease study. Lancet Glob Health. 2021;9:e144–60.33275949 [Google Scholar]
- [2].Dandona L, Dandona R. What is the global burden of visual impairment? BMC Med. 2006;4:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Casson RJ, Chidlow G, Wood JP, Crowston JG, Goldberg I. Definition of glaucoma: clinical and experimental concepts. Clin Exp Ophthalmol. 2012;40:341–9. [DOI] [PubMed] [Google Scholar]
- [4].Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng C-Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121:2081–90. [DOI] [PubMed] [Google Scholar]
- [5].Jeong WC, Min JY, Kang TG, Bae H. Association between pseudoexfoliation and Alzheimer’s disease-related brain atrophy. PLoS One. 2023;18:e0286727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Duncan RO, Sample PA, Weinreb RN, Bowd C, Zangwill LM. Retinotopic organization of primary visual cortex in glaucoma: Comparing fMRI measurements of cortical function with visual field loss. Prog Retin Eye Res. 2007;26:38–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Carvalho J, Invernizzi A, Martins J, Renken RJ, Cornelissen FW. Local neuroplasticity in adult glaucomatous visual cortex. Sci Rep. 2022;12:21981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Tellouck L, Durieux M, Coupé P, et al. Optic radiations microstructural changes in glaucoma and association with severity: a study using 3tesla-magnetic resonance diffusion tensor imaging. Invest Ophthalmol Vis Sci. 2016;57:6539–47. [DOI] [PubMed] [Google Scholar]
- [9].Frezzotti P, Giorgio A, Motolese I, et al. Structural and functional brain changes beyond visual system in patients with advanced glaucoma. PLoS One. 2014;9:e105931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Guo L, Salt TE, Luong V, et al. Targeting amyloid-beta in glaucoma treatment. Proc Natl Acad Sci U S A. 2007;104:13444–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Craig JE, Han X, Qassim A, et al. Multitrait analysis of glaucoma identifies new risk loci and enables polygenic prediction of disease susceptibility and progression. Nat Genet. 2020;52:160–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Springelkamp H, Iglesias AI, Mishra A, et al. New insights into the genetics of primary open-angle glaucoma based on meta-analyses of intraocular pressure and optic disc characteristics. Hum Mol Genet. 2017;26:438–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kurki MI, Karjalainen J, Palta P, et al. FinnGen provides genetic insights from a well-phenotyped isolated population. Nature. 2023;613:508–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Currant H, Hysi P, Fitzgerald TW, et al. Genetic variation affects morphological retinal phenotypes extracted from UK Biobank optical coherence tomography images. PLoS Genet. 2021;17:e1009497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Grasby KL, Jahanshad N, Painter JN, et al. The genetic architecture of the human cerebral cortex. Science. 2020;367:eaay6690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Desikan RS, Ségonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–80. [DOI] [PubMed] [Google Scholar]
- [17].Qin Y, Havulinna AS, Liu Y, et al. Combined effects of host genetics and diet on human gut microbiota and incident disease in a single population cohort. Nat Genet. 2022;54:134–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hemani G, Zheng J, Elsworth B, et al. The MR-Base platform supports systematic causal inference across the human phenome. Elife. 2018;7:e34408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Burgess S, Butterworth A, Thompson SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol. 2013;37:658–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015;44:512–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. 2016;40:304–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Chen X, Kong J, Diao X, et al. Depression and prostate cancer risk: a Mendelian randomization study. Cancer Med. 2020;9:9160–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Long Y, Tang L, Zhou Y, Zhao S, Zhu H. Causal relationship between gut microbiota and cancers: a two-sample Mendelian randomisation study. BMC Med. 2023;21:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. 2018;50:693–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Staley JR, Blackshaw J, Kamat MA, et al. PhenoScanner: a database of human genotype-phenotype associations. Bioinformatics. 2016;32:3207–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Langfelder P, Horvath S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinf. 2008;9:559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Yao F, Peng J, Zhang E, et al. Pathologically high intraocular pressure disturbs normal iron homeostasis and leads to retinal ganglion cell ferroptosis in glaucoma. Cell Death Differ. 2023;30:69–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Liu M, Li H, Yang R, Ji D, Xia X. GSK872 and necrostatin-1 protect retinal ganglion cells against necroptosis through inhibition of RIP1/RIP3/MLKL pathway in glutamate-induced retinal excitotoxic model of glaucoma. J Neuroinflammation. 2022;19:262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Zeng Z, You M, Fan C, Rong R, Li H, Xia X. Pathologically high intraocular pressure induces mitochondrial dysfunction through Drp1 and leads to retinal ganglion cell PANoptosis in glaucoma. Redox Biol. 2023;62:102687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].McKinnon SJ. Glaucoma, apoptosis, and neuroprotection. Curr Opin Ophthalmol. 1997;8:28–37. [DOI] [PubMed] [Google Scholar]
- [31].Wan H, Ban X, He Y, et al. Voltage-dependent anion channel 1 oligomerization regulates PANoptosis in retinal ischemia-reperfusion injury. Neural Regen Res. 2026;21:1652–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Zhao WJ, Fan C-L, Hu X-M, et al. Regulated cell death of retinal ganglion cells in glaucoma: molecular insights and therapeutic potentials. Cell Mol Neurobiol. 2023;43:3161–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].You M, Rong R, Zeng Z, Xia X, Ji D. Transneuronal degeneration in the brain during glaucoma. Front Aging Neurosci. 2021;13:643685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Faiq MA, Wollstein G, Schuman JS, Chan KC. Cholinergic nervous system and glaucoma: from basic science to clinical applications. Prog Retin Eye Res. 2019;72:100767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Chen WW, Wang N, Cai S, et al. Structural brain abnormalities in patients with primary open-angle glaucoma: a study with 3T MR imaging. Invest Ophthalmol Vis Sci. 2013;54:545–54. [DOI] [PubMed] [Google Scholar]
- [36].Sharma AA, Goodman AM, Allendorfer JB, et al. Regional brain atrophy and aberrant cortical folding relate to anxiety and depression in patients with traumatic brain injury and psychogenic nonepileptic seizures. Epilepsia. 2022;63:222–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Do MTH. Melanopsin and the intrinsically photosensitive retinal ganglion cells: biophysics to behavior. Neuron. 2019;104:205–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Schultz SA, Gordon BA, Mishra S, et al. Widespread distribution of tauopathy in preclinical Alzheimer’s disease. Neurobiol Aging. 2018;72:177–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Zhao W, Lv X, Wu G, et al. Glaucoma is not associated with Alzheimer’s disease or dementia: a meta-analysis of cohort studies. Front Med (Lausanne). 2021;8:688551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Xu XH, Zou JY, Geng W, Wang AY. Association between glaucoma and the risk of Alzheimer’s disease: a systematic review of observational studies. Acta Ophthalmol. 2019;97:665–71. [DOI] [PubMed] [Google Scholar]
- [41].Zheng C, Liu S, Zhang X, et al. Shared genetic architecture between the two neurodegenerative diseases: Alzheimer’s disease and glaucoma. Front Aging Neurosci. 2022;14:880576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Shen XY, Shi S-H, Li H, et al. The role of Gadd45b in neurologic and neuropsychiatric disorders: an overview. Front Mol Neurosci. 2022;15:1021207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Palomer E, Martín-Flores N, Jolly S, et al. Epigenetic repression of Wnt receptors in AD: a role for Sirtuin2-induced H4K16ac deacetylation of Frizzled1 and Frizzled7 promoters. Mol Psychiatry. 2022;27:3024–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Van Raay TJ, Vetter ML. Wnt/frizzled signaling during vertebrate retinal development. Dev Neurosci. 2004;26:352–8. [DOI] [PubMed] [Google Scholar]
- [45].Kozulin P, Natoli R, Madigan MC, O’Brien KM, Provis JM. Gradients of Eph-A6 expression in primate retina suggest roles in both vascular and axon guidance. Mol Vis. 2009;15:2649–62. [PMC free article] [PubMed] [Google Scholar]
- [46].Jing X, Miwa H, Sawada T, et al. Ephrin-A1-mediated dopaminergic neurogenesis and angiogenesis in a rat model of Parkinson’s disease. PLoS One. 2012;7:e32019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].De Maria A, Zientek KD, David LL, et al. Compositional analysis of extracellular aggregates in the eyes of patients with exfoliation syndrome and exfoliation glaucoma. Invest Ophthalmol Vis Sci. 2021;62:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Swaminathan SS, Oh D-J, Kang MH, Shepard AR, Pang I-H, Rhee DJ. TGF-β2-mediated ocular hypertension is attenuated in SPARC-null mice. Invest Ophthalmol Vis Sci. 2014;55:4084–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Brockhaus K, Melkonyan H, Prokosch-Willing V, Liu H, Thanos S. Alterations in tight- and adherens-junction proteins related to glaucoma mimicked in the organotypically cultivated mouse retina under elevated pressure. Invest Ophthalmol Vis Sci. 2020;61:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].He J, Liu Y, Li J, et al. Intestinal changes in permeability, tight junction and mucin synthesis in a mouse model of Alzheimer’s disease. Int J Mol Med. 2023;52:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Yan Z, Liao H, Chen H, et al. Elevated intraocular pressure induces amyloid-β deposition and tauopathy in the lateral geniculate nucleus in a monkey model of glaucoma. Invest Ophthalmol Vis Sci. 2017;58:5434–43. [DOI] [PubMed] [Google Scholar]
- [52].Phillis JW, Horrocks LA, Farooqui AA. Cyclooxygenases, lipoxygenases, and epoxygenases in CNS: their role and involvement in neurological disorders. Brain Res Rev. 2006;52:201–43. [DOI] [PubMed] [Google Scholar]
- [53].Tong B, Ba Y, Li Z, et al. Targeting dysregulated lipid metabolism for the treatment of Alzheimer’s disease and Parkinson’s disease: current advancements and future prospects. Neurobiol Dis. 2024;196:106505. [DOI] [PubMed] [Google Scholar]
- [54].Lipton JO, Sahin M. The neurology of mTOR. Neuron. 2014;84:275–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Wang Y, Fung NSK, Lam WC, Lo ACY. mTOR signalling pathway: a potential therapeutic target for ocular neurodegenerative diseases. Antioxidants (Basel). 2022;11:1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Komatsu M, Waguri S, Chiba T, et al. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature. 2006;441:880–4. [DOI] [PubMed] [Google Scholar]
- [57].Tanik SA, Schultheiss CE, Volpicelli-Daley LA, Brunden KR, Lee VM. Lewy body-like α-synuclein aggregates resist degradation and impair macroautophagy. J Biol Chem. 2013;288:15194–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Wang X, Chen W, Zhao W, Miao M. Risk of glaucoma to subsequent dementia or cognitive impairment: a systematic review and meta-analysis. Aging Clin Exp Res. 2024;36:172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Daveckaite A, Grusauskiene E, Petrikonis K, Vaitkus A, Siaudvytyte L, Januleviciene I. Cognitive functions and normal tension glaucoma. Indian J Ophthalmol. 2017;65:974–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Casanova F, O'Loughlin J, Karageorgiou V, et al. Effects of physical activity and sedentary time on depression, anxiety and well-being: a bidirectional Mendelian randomisation study. BMC Med. 2023;21:501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Burgess S, Davies NM, Thompson SG. Bias due to participant overlap in two-sample Mendelian randomization. Genet Epidemiol. 2016;40:597–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Minelli C, Del Greco M F, van der Plaat DA, Bowden J, Sheehan NA, Thompson J. The use of two-sample methods for Mendelian randomization analyses on single large datasets. Int J Epidemiol. 2021;50:1651–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Mounier N, Kutalik Z. Bias correction for inverse variance weighting Mendelian randomization. Genet Epidemiol. 2023;47:314–31. [DOI] [PubMed] [Google Scholar]
- [64].Chen X, Kong J, Pan J, et al. Kidney damage causally affects the brain cortical structure: a mendelian randomization study. EBioMedicine. 2021;72:103592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Tribble JR, Otmani A, Kokkali E, Lardner E, Morgan JE, Williams PA. Retinal ganglion cell degeneration in a rat magnetic bead model of ocular hypertensive glaucoma. Transl Vis Sci Technol. 2021;10:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.