Abstract
Purpose
Sepsis-associated endothelial injury drives thrombomodulin (TM) shedding and severe coagulopathy. We hypothesized that plasma TM levels predict sepsis severity and prognosis. This study investigated the prognostic association of plasma TM as a sepsis biomarker and validated it in a murine model.
Patients and Methods
In a prospective cohort (Gongli Hospital, Shanghai; July 2024–January 2025), 68 sepsis patients (45 survivors, 23 nonsurvivors) and 50 controls underwent plasma TM, CD64 index, procalcitonin (PCT), C-reactive protein (CRP), Sequential Organ Failure Assessment (SOFA) score, and Acute Physiology and Chronic Health Evaluation II (APACHE II) score assessments. Survival analysis, multivariable regression, and receiver operating characteristic (ROC) curve modeling were performed. Mechanistic validation utilized Cecal Ligation and Puncture (CLP) mice with plasma/aortic TM quantification.
Results
Elevated plasma TM and CD64 index correlated with poor prognosis (p<0.05). The combined TM/CD64 Index ROC model achieved superior predictive performance (AUC=0.9671, p<0.05) compared with TM alone (AUC=0.9333) or CD64 alone (AUC=0.8628). The multiple linear regression model indicated a positive correlation between TM levels in sepsis patients and SOFA and APACHE II scores. In vivo, experiments indicate plasma TM increased while aortic endothelial TM expression decreased.
Conclusion
This study demonstrates that the combined plasma TM and CD64 index assessment enhances early prediction of adverse sepsis outcomes, with strong correlations to clinical severity scores. The paradoxical TM dynamics (plasma elevation vs endothelial depletion) suggest endothelial injury as a key mechanism. Future research should focus on multicenter validation, and mechanistic studies are warranted to optimize clinical translation.
Keywords: sepsis, thrombomodulin, TM, CD64 index, biomarker
Introduction
Sepsis, a life-threatening condition characterized by multiorgan dysfunction resulting from an uncontrolled host response to infection, is a common systemic inflammatory response syndrome in clinical practice.1 It is associated with high morbidity, mortality, and substantial treatment costs.2,3 Coagulation abnormalities are frequently observed in sepsis, often progressing to disseminated intravascular coagulation (DIC).4 Septic coagulopathy (SIC) is an acquired condition that manifests as DIC, characterized by hypercoagulability, intravascular fibrin deposition, and hematologic dysfunction arising from various causes, potentially leading to multiple organ dysfunction syndrome and death.5,6 SIC occurs in 30–50% of sepsis patients, accounting for nearly half of all DIC cases, and is linked to a twofold increase in mortality among sepsis patients with SIC complications.7–9
The pathophysiology of sepsis is complex, with endothelial cell injury playing a central role in its progression.10 Under normal conditions, endothelial cells act as a selective barrier within the vasculature, regulating the movement of fluids, proteins, and inflammatory mediators. However, during sepsis, inflammatory responses, immune dysregulation, and oxidative stress damage endothelial cells, impairing microcirculatory blood flow. This leads to reduced vascular density, disrupted oxygen homeostasis, compromised endothelial integrity, and a hypercoagulable state, all of which contribute to sepsis-induced coagulopathy.10–13 Recent research highlights the role of thrombomodulin (TM), a type I transmembrane protein expressed on vascular endothelial cells, in controlling inflammation, coagulation, and maintaining endothelial barrier function.14 When endothelial injury occurs, TM is cleaved and released into the bloodstream, with plasma TM levels serving as a marker of endothelial damage.15 Specifically, soluble TM has been shown to interact with leukocytes through lectin-like domains.16
Current sepsis diagnosis lacks a single high-specificity biomarker. Conventional markers like CRP and PCT exhibit critical limitations: CRP elevation delays ≥12–24 hours post-infection, while PCT increases in non-infectious contexts (eg, trauma/surgery), compromising early diagnostic accuracy.17–19 In contrast, neutrophil CD64 (FcγRI)—minimally expressed basally but rapidly upregulated by bacterial components and cytokines (eg, LPS/G-CSF/IFN-γ)—demonstrates superior diagnostic utility, enabling early infection detection and severity stratification.20,21 Notably, thrombomodulin (TM) reflects endothelial injury and modulates immune dysregulation, serving as a nexus between coagulopathy and hyperinflammation (immunothrombosis). While TM and CD64 cover distinct pathophysiological axes (endothelial dysfunction vs neutrophil activation), their synergistic prognostic potential remains unexplored. Our study bridges this gap by correlating plasma TM dynamics with CD64 index to elucidate endothelial-immune crosstalk in sepsis progression, with parallel animal models validating TM-endothelial injury relationships to inform therapeutic strategies.
Materials and Methods
Patients
A total of 68 sepsis patients admitted to the Emergency Department of Shanghai Gongli Hospital between July 2024 and January 2025, along with 50 individuals undergoing routine medical checkups, were included in this study. The inclusion criteria for the healthy control group were over 18 years old, no history of infectious diseases within the past 3 months, no pregnancy, no history of chronic diseases, and no history of tumors. The cohort consisted of 33 males and 35 females, with a mean age of 62.10 (±10.33) years. All sepsis patients received treatments by standard diagnostic and therapeutic guidelines. Based on their 28-day survival status, sepsis patients were classified into a Survived group (n=45) and a Deceased group (n=23), while healthy individuals formed the Control group (n=50). The study was approved by the Ethics Committee of Gongli Hospital, Pudong New District, Shanghai, China (GLYYls2024-034), and written informed consent was obtained from all participants.
Case Selection Criteria
Inclusion criteria: Compliance with Sepsis 3.0 diagnostic criteria; hospital admission within 24 hours of symptom onset; age between 18 and 85 years; provision of informed consent for study participation.
Exclusion criteria: Patients were excluded if they had pre-existing major organ insufficiency or severe underlying diseases, malignant tumors, allergic conditions, immunodeficiency disorders, or autoimmune diseases. Other exclusion factors included prior exposure to radiotherapy, chemotherapy, or immunosuppressant therapy within the past six months, incomplete clinical test results within 24 hours of admission to the EICU, and pregnancy or lactation.
Detection Indicators and Methods
Upon admission, demographic data, clinical characteristics, and laboratory findings were recorded. Blood samples were collected on the day of diagnosis (D1) and subsequently on Days 3 and 7 post-admissions. A total of 5 mL of blood was drawn into EDTA anticoagulant tubes for analysis. Thrombomodulin (TM) expression levels were measured using enzyme-linked immunosorbent assay (ELISA), while C-reactive protein (CRP) and procalcitonin (PCT) concentrations were quantified using magnetic bead immunoassay. CD64 expression was assessed via flow cytometry. Additionally, the Sequential Organ Failure Assessment (SOFA) score and the Acute Physiology and Chronic Health Evaluation (APACHE) score were calculated.
Experimental Animals and Experimental Design
Male C57BL/6J mice (4–8 weeks, n=20) from Speyford (Suzhou) Biotechnology Co., Ltd (China). All animal experiments were rigorously conducted, adhering strictly to the protocols approved by the Animal Care and Use Committee of Shanghai Gongli Hospital (approval number: GLYYls2024-036). It was randomized into sham (abdominal exposure only, n=10) or cecal ligation and puncture (CLP, n=10) groups. Mortality was assessed every 3–4h. Postoperatively, mice received 0.9% normal saline (0.2 mL per dose, twice daily) for 7 days before euthanasia. Aortic tissues were dissected immediately post-euthanasia, and immunofluorescence characterized thrombomodulin alterations on the endothelium. Aortic tissues were also homogenized for protein (Western blot), RNA (quantified via quantitative polymerase chain reaction, qPCR) extraction.
Immunofluorescence
Immediately after euthanasia, aortas were harvested and fixed in 4% paraformaldehyde, followed by paraffin embedding and sectioning. Sections were baked at 60°C for 60 min, then sequentially immersed in xylene (3 times, 5 min each), ethanol (100%, 100%, 70%; 3 min each), and distilled water (5 min). Citrate antigen retrieval solution (pH 6.0; ZSGB-BIO, #ZL1-9065) was prepared. Antigen retrieval was performed using microwave irradiation: 8 min at 80% power, 8 min standing, then 7 min at 50% power. Sections were cooled at room temperature for 20 min and rinsed with PBS buffer (Beyotime, #C0221A; 3 times, 5 min each). Blocking was performed at room temperature for 1 h using blocking buffer (Beyotime, #P0102). Primary antibodies - rabbit polyclonal anti-thrombomodulin (TM) antibody (HUABIO, #ET7107-92; 1:500) and mouse monoclonal anti-CD31 antibody (Abcam, #Ab182981; 1:200) - were applied to sufficiently cover the tissue and incubated overnight at 4°C. Following incubation, sections were washed with PBS (3 times, 5 min each). Tissues were then incubated sequentially with anti-rabbit IgG (H+L) Alexa Fluor®488 conjugate (Cell Signaling Technology, #4412) and anti-mouse IgG (H+L) Alexa Fluor®594 conjugate (Cell Signaling Technology, #8890) for 2h at room temperature. DAPI (Beyotime, C1005) was used for nuclear staining. After washed with PBS, the tissues were visualized using Pannoramic MIDI scanning microscopy (3DHISTECH).
Western Blot
TM protein expression was assessed using 10% SDS-PAGE and transferred to active polyvinylidene difluoride (PVDF) membranes. After the transfer, the membranes were blocked with 5% nonfat powdered milk for 1h at room temperature and then probed with antibodies TM (ThermoFisher #MA5-34718, 1:1000) and GAPDH (HUABIO #ET1601-4, 1:5000) at 4°C overnight. The membranes were probed with GAPDH to control for protein loading. Then incubated for 2h at room temperature with HRP Conjugated Goat anti-Rabbit IgG polyclonal Antibody (HUABIO #HA1001, 1:10,000).
Quantitative RT-PCR
TM mRNA was quantified via qPCR with GAPDH normalization (Table 1).
Table 1.
Primer Sequences
| Primer | Sequences |
|---|---|
| Mouse GAPDH | Forward: CATCACTGCCACCCAGAAGACTG |
| Reverse: ATGCCAGTGAGCTTCCCGTTCAG | |
| Mouse TM | Forward: GGAGAATGGTGGCTGTGAGTAC |
| Reverse: GCACGATTGAACCACAGGTCTTG | |
| Human GAPDH | Forward: GTCTCCTCTGACTTCAACAGCG |
| Reverse: ACCACCCTGTTGCTGTAGCCAA | |
| Human TM | Forward: AACGACCTCTGCGAGCACTTCT |
| Reverse: CCAGTATGCAGTCATCCACGTC |
Abbreviation: TM, Thrombomodulin.
Elisa
TM in plasma was measured with commercially available ELISA kits (KeshunBio, #KS11551) according to the manufacturer’s instructions.
Statistical Methods
This study’s data were analyzed using SPSS 29.0, MedCalc 23.1, R, and GraphPad Prism 10. Normally distributed variables (mean ± SD) were compared via t-tests; non-normal variables (median [IQR]) using Mann–Whitney U-tests. Correlations between biomarkers (TM, CD64 index, PCT, CRP) and prognosis were assessed using Pearson/Spearman tests (p <0.05). Receiver operating characteristic (ROC) curves evaluated diagnostic performance including under the curve (AUC), sensitivity, specificity) and Kaplan-Meier curves analyzed survival. Stepwise multiple linear regression (entry p <0.05, removal p >0.1) modeled APACHE II/SOFA scores against biomarkers.
Results
Study Population Characteristics
A total of 68 sepsis patients were included in the study, with the following distribution of infection sites: pulmonary (n=46), intra-abdominal (n=12), urinary tract (n=5), skin and soft tissue (n=2), and infections of unknown origin (n=4). The control group consisted of 50 individuals with documented pre-admission comorbidities. Table 2 presents the baseline characteristics of the study participants.
Table 2.
Baseline of Clinical Features of Cases
| Variable | Groups | p-valueb | |
|---|---|---|---|
| Control N = 50 (42%)a | Sepsis N = 68 (58%)a | ||
| Age (years) | 56.26 (8.80) | 62.10 (10.33) | 0.002 |
| Gender | 0.11 | ||
| Female | 34 (68.00%) | 35 (51.47%) | |
| Male | 16 (32.00%) | 33 (48.53%) | |
| TM (ng/mL) | 1.08 [0.87, 1.38] | 55.77 [40.47, 77.45] | <0.001 |
| CRP (mg/l) | 4.35 [3.71, 4.70] | 35.60 [17.90, 69.98] | <0.001 |
| PCT (ng/mL) | 0.03 [0.02, 0.04] | 2.68 [1.15, 16.50] | <0.001 |
| CD64 Index | 1.26 [0.66, 2.11] | 8.65 [6.33, 13.52] | <0.001 |
Notes: aMean (SD); n (%); Median [IQR]. bTwo Sample t-test; Pearson’s Chi-squared test; Wilcoxon rank sum test.
Abbreviations: TM, Thrombomodulin; CRP, C-reactive protein; PCT, procalcitonin.
Dynamic Biomarker Profiles and Survival Correlation
Biomarkers were evaluated at three time points (D1, D3, and D7) in the study population. Significant differences (p < 0.05) were observed between the sepsis group and healthy controls in plasma TM, CRP, PCT, CD64 index, APACHE II score, and SOFA score. Among sepsis patients, plasma TM, CD64 index, APACHE II score, and SOFA score differed significantly (p < 0.05) between survivors and non-survivors. CRP levels showed significant variation on D3 and D7 (p < 0.05), while PCT levels were significantly different only on D7 (p < 0.05) (Table 3 and Table 4).
Table 3.
Analysis of the Clinical Parameters in Each Group on the First Day (D1) of Sepsis Diagnosis
| Variable | Sepsis | p-valueb | |
|---|---|---|---|
| Survived N = 45 (66%)a |
Deceased N = 23 (34%)a |
||
| Age (years) | 62.00 [56.00, 68.00] | 68.00 [57.50, 72.50] | 0.14 |
| Gender | 0.49 | ||
| Female | 25 (55.56%) | 10 (43.48%) | |
| Male | 20 (44.44%) | 13 (56.52%) | |
| Length of Stay | 16.00 [12.00, 24.00] | 9.00 [7.00, 14.00] | <0.001* |
| TM (ng/mL) | 43.74 [36.30, 55.32] | 91.62 [70.29, 119.16] | <0.001* |
| CRP (mg/l) | 33.00 [15.70, 53.50] | 42.90 [21.40, 87.35] | 0.25 |
| PCT (ng/mL) | 4.00 [1.56, 25.30] | 1.90 [1.05, 5.91] | 0.14 |
| CD64 Index | 7.45 [5.55, 9.14] | 16.68 [12.06, 18.45] | <0.001* |
| SOFA Score | 7.00 [6.00, 8.00] | 13.00 [11.50, 14.00] | <0.001* |
| APACHEII Score | 16.00 [12.00, 19.00] | 30.00 [26.50, 32.00] | <0.001* |
| Initial Infection | 0.083 | ||
| Pulmonary infection | 34 (75.56%) | 11 (47.83%) | |
| Abdominal infection | 7 (15.56%) | 5 (21.74%) | |
| Urinary infection | 2 (4.44%) | 3 (13.04%) | |
| Skin/tissue infection | 1 (2.22%) | 1 (4.35%) | |
| *Other site | 1 (2.22%) | 3 (13.04%) | |
Notes: *Other site: blood stream infection from unknown origin. aMean (SD); n (%); Median [IQR]. bTwo Sample t-test; Pearson’s Chi-squared test; Wilcoxon rank sum test.
Abbreviations: TM, Thrombomodulin; CRP, C-reactive protein; PCT, procalcitonin; SOFA, Sequential Organ Failure Assessment; APACHE, Acute Physiology and Chronic Health Evaluation score.
Table 4.
Analysis of Indices in Each Group on the Third (D3) and Seventh (D7) days of Sepsis Diagnosis
| Variable | Sepsis | p-valueb | ||
|---|---|---|---|---|
| Survived N = 45 (70%)a | Deceased N = 19 (30%)a | |||
| Day 3 | TM (ng/mL) | 49.62 [36.54, 64.86] | 83.82 [68.76, 110.67] | <0.001 |
| CRP (mg/l) | 40.00 [18.10, 62.20] | 65.60 [26.83, 109.99] | 0.020 | |
| PCT (ng/mL) | 3.87 [0.49, 12.60] | 11.85 [6.06, 20.71] | 0.011 | |
| CD64 Index | 5.05 [3.32, 7.88] | 12.83 [12.31, 15.78] | <0.001 | |
| SOFA Score | 7.00 [6.00, 8.00] | 14.00 [13.00, 15.00] | <0.001 | |
| APACHEII Score | 31.00 [18.00, 33.00] | 36.00 [35.00, 44.50] | <0.001 | |
| Day 7 | TM (ng/mL) | 37.50 [21.42, 62.88] | 93.66 [85.29, 115.44] | <0.001 |
| CRP (mg/l) | 29.60 [13.40, 50.90] | 143.82 [92.04, 197.46] | <0.001 | |
| PCT (ng/mL) | 2.27 [0.77, 9.45] | 17.84 [12.66, 27.34] | <0.001 | |
| CD64 Index | 7.22 [4.76, 10.86] | 13.79 [12.74, 15.43] | <0.001 | |
| SOFA Score | 7.00 [6.00, 8.00] | 14.00 [13.50, 15.50] | <0.001 | |
| APACHEII Score | 32.00 [31.00, 35.00] | 40.00 [37.00, 44.50] | <0.001 | |
Notes: aMedian [IQR]. bWilcoxon rank sum test; Wilcoxon rank sum exact test.
Abbreviations: TM, Thrombomodulin; CRP, C-reactive protein and PCT, procalcitonin; SOFA, Sequential Organ Failure Assessment; APACHE, Acute Physiology and Chronic Health Evaluation score.
Plasma TM levels were categorized into three groups: A (<60 ng/mL), B (60–80 ng/mL), and C (>80 ng/mL). Similarly, CD64 index values were classified into Group A (≤10) and Group B (>10). The results revealed an inverse relationship between plasma TM and CD64 index levels and the 28-day survival rate of sepsis patients (p < 0.05) (Figure 1).
Figure 1.
Analysis of survival curves in patients with sepsis (A and B) Kaplan-Meier survival curve analysis of TM and CD64 indices affecting sepsis prognosis (upper) and risk table (lower). (C and D) Cumulative risk function curves of TM, CD64 index affecting prognosis in sepsis (upper) and risk table (lower). TM values were divided into group A (<60ng/mL), group B (60–80ng/mL), group C (>80ng/mL).CD64 index values were divided into group A (≤10), group B (>10).
Biomarker Interdependence and Severity Association
Pearson correlation coefficients were calculated to assess the linear relationships among TM, CD64 index, PCT, and CRP. The results showed a positive correlation between TM and CD64 index on D1 and D3 (r=0.315, p=0.009; r=0.404, p<0.001). Additionally, TM was positively correlated with CRP on D3 and D7 (r=0.435, p < 0.001; r=0.493, p<0.001) and with PCT on D7 (r=0.392, p=0.003). CD64 index also showed a positive correlation with CRP on D7 (r=0.432, p<0.001). Scatter plots confirmed a linear correlation between TM and CD64 index on D1 and D3, as well as between TM and both PCT and CRP on D7 (Tables 5,6 and Figures 2,3).
Table 5.
Linear Correlation Analysis Between TM and CD64 Index, CRP, and PCT in the Sepsis Group
| Pearson | TM | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| D1 | D3 | D7 | ||||||||
| r | *P | n | r | *P | n | r | *P | n | ||
| CD64 Index | D1 | 0.315 | 0.009 | 68 | ||||||
| D3 | 0.404 | <0.001 | 64 | |||||||
| D7 | 0.164 | 0.437 | 56 | |||||||
| CRP | D1 | 0.175 | 0.154 | 68 | ||||||
| D3 | 0.435 | <0.001 | 64 | |||||||
| D7 | 0.493 | <0.001 | 56 | |||||||
| PCT | D1 | −0.091 | 0.459 | 68 | ||||||
| D3 | 0.013 | 0.917 | 64 | |||||||
| D7 | 0.392 | 0.003 | 56 | |||||||
Notes: r: respectively; *P: Correlation is significant at the 0.05 level (2-tailed).
Abbreviations: TM, Thrombomodulin; CRP, C-reactive protein; PCT, procalcitonin.
Table 6.
Linear Correlation Analysis Between CD64 Index and CRP, PCT in the Sepsis Group
| Pearson | CD64 Index | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| D1 | D3 | D7 | ||||||||
| r | *P | n | r | *P | n | r | *P | n | ||
| PCT | D1 | −0.085 | 0.492 | 68 | ||||||
| D3 | 0.037 | 0.769 | 64 | |||||||
| D7 | 0.116 | 0.393 | 56 | |||||||
| CRP | D1 | 0.038 | 0.758 | 68 | ||||||
| D3 | 0.071 | 0.575 | 64 | |||||||
| D7 | 0.432 | <0.001 | 56 | |||||||
Notes: r: respectively; *P: Correlation is significant at the 0.05 level (2-tailed).
Abbreviations: TM, Thrombomodulin; CRP, C-reactive protein; PCT, procalcitonin.
Figure 2.
Bivariate scatter plot between TM and CRP, PCT, CD64 Index in different time. (A–C) Bivariate scatter plot between TM and CRP, PCT, CD64 Index at first day (D1) diagnosed the sepsis. (D–F) Bivariate scatter plot between TM and CRP, PCT, CD64 Index at third day (D3) diagnosed the sepsis. (G–I) Bivariate scatter plot between TM and CRP, PCT, CD64 Index at seventh day (D7) diagnosed the sepsis.
Abbreviations: TM, Thrombomodulin; CRP, C-reactive protein; PCT, procalcitonin.
Figure 3.
Bivariate scatter plot between CD64 Index and CRP, PCT in different time. (A and D) Bivariate scatter plot between CD64 Index and CRP, PCT at first day (D1) diagnosed the sepsis. (B and E) Bivariate scatter plot between CD64 Index and CRP, PCT at third day (D3) diagnosed the sepsis. (C and F) Bivariate scatter plot between CD64 Index and CRP, PCT at seventh day (D7) diagnosed the sepsis.
Abbreviations: CRP, C-reactive protein; PCT, procalcitonin.
Spearman correlation coefficients were used to evaluate the association between disease severity and TM and CD64 index levels. A negative correlation was observed between TM and survival status on D1 (r=−0.710, p<0.001), as well as between CD64 index and survival status on D1 (r=−0.595, p<0.001) (Table 7).
Table 7.
Linear Correlation Analysis Between TM and CD64 Index Under Different Survival Conditions
| Spearman | Survival Conditions | ||
|---|---|---|---|
| r | *P | n | |
| TM (D1) | −0.710 | <0.001 | 68 |
| CD64 Index (D1) | −0.595 | <0.001 | 68 |
Notes: r: respectively; *P: Correlation is significant at the 0.05 level (2-tailed).
Abbreviation: TM, Thrombomodulin.
Prognostic Value of Plasma TM and CD64 Index in Sepsis Severity
ROC Analysis for Diagnostic Prediction
We performed ROC curve analysis to evaluate plasma TM and CD64 index as predictors of 28-day mortality across serial time points (D1/D3/D7), computing area under the curve (AUC) with 95% confidence intervals. Prognostic utility of the combined biomarker panel (TM+CD64) was benchmarked against conventional severity scores (SOFA and APACHE II) using DeLong’s test for statistical comparison. Sensitivity (Sen), specificity (Spe), Youden’s index, positive likelihood ratio (PLR), and negative likelihood ratio (NLR) were calculated to determine the predictive performance of plasma TM combined with the CD64 index in assessing sepsis severity and prognosis.
The results showed that on Day 1, plasma TM, CD64 index, and their combination demonstrated strong prognostic capabilities, with the combined approach achieving the highest area under the curve (AUC=0.9671, p<0.05). This exceeded the predictive performance of plasma TM alone (AUC=0.9333, p<0.05) and CD64 index alone (AUC=0.8628, p<0.05). Pairwise comparison of mortality prediction models using DeLong’s test demonstrated significant disparities in discriminatory capacity. The combined biomarker panel (TM + CD64 index) exhibited significantly enhanced discriminatory power compared to APACHE II (ΔAUC = −0.128; 95% CI: −0.235 to −0.020; p=0.020), while demonstrating statistical equivalence to SOFA scores (ΔAUC = 0.019; 95% CI: −0.028–0.066; p=0.422) (Tables 8,9 and Figure 4).
Table 8.
Analysis of the Diagnostic Efficacy of Different Indicators at Three Time Points Using ROC
| Indexes | AUC [95% CI] |
P | Youden’s Index | Associated Criterion (ng/mL) |
Sensitivity (%) | Specificity (%) | PLR | NLP | |
|---|---|---|---|---|---|---|---|---|---|
| Day 1 | TM | 0.93 [0.87,0.99] |
<0.0001 | 0.801 | >63.87 | 95.65 | 84.44 | 6.15 | 0.05 |
| CD64 Index | 0.86 [0.75,0.96] |
<0.0001 | 0.759 | >11.8 | 82.61 | 93.33 | 12.39 | 0.19 | |
| TM+CD64 Index | 0.96 [0.92–1.00] |
<0.0001 | 0.869 | —— | 91.30 | 95.56 | 20.54 | 0.09 | |
| SOFA Score | 0.98 [0.95,1.00] |
<0.0001 | 0.957 | >9.5 | 100 | 95.74 | 23.26 | 0.00 | |
| APACHE-II Score | 0.83 [0.74,0.93] |
<0.0001 | 0.734 | >21.5 | 95.70 | 77.80 | 4.31 | 0.06 | |
| Day 3 | TM | 0.80.6 [0.78,0.95] |
<0.0001 | 0.606 | >61.59 | 89.47 | 71.11 | 3.10 | 0.15 |
| CD64 Index | 0.94 [0.89,0.99] |
<0.0001 | 0.784 | >11.48 | 89.47 | 88.89 | 8.05 | 0.12 | |
| TM+CD64 Index | 0.96 [0.92,1.00] |
<0.0001 | 0.933 | —— | 100 | 93.33 | 15.00 | 0.00 | |
| SOFA Score | 0.97 [0.93–1.00] |
<0.0001 | 0.964 | >11.5 | 90.00 | 97.81 | 40.91 | 0.10 | |
| APACHE-II Score | 0.89 [0.81–0.96] |
<0.0001 | 0.636 | >32.5 | 94.73 | 68.93 | 3.05 | 0.08 | |
| Day 7 | TM | 0.90 [0.81,0.98] |
<0.0001 | 0.846 | >84.63 | 84.62 | 88.89 | 7.62 | 0.17 |
| CD64 Index | 0.90 [0.81,0.98] |
<0.0001 | 0.822 | >11.75 | 100 | 82.22 | 5.63 | 0.00 | |
| TM+CD64 Index | 0.94 [0.88,1.00] |
<0.0001 | 0.911 | —— | 100 | 91.11 | 11.25 | 0.00 | |
| SOFA Score | 0.99 [0.97,1.00] |
<0.0001 | 0.928 | >10 | 95.13 | 97.84 | 43.18 | 0.05 | |
| APACHE-II Score | 0.93 [0.86,1.00] |
<0.0001 | 0.539 | >34.5 | 85.72 | 68.91 | 2.73 | 0.21 |
Abbreviations: TM, Thrombomodulin; SOFA, Sequential Organ Failure Assessment; APACHE, Acute Physiology and Chronic Health Evaluation (APACHE) score.
Table 9.
Pairwise Comparison of AUC Values for Prediction Models
| Compared ROC Pairs | z Statistic | p-valuea | AUC Difference | Standard Errorb | 95% Confidence Interval |
|---|---|---|---|---|---|
| SOFA vs APACHE Score | 2.995 | 0.003* | 0.147 | 0.049 | [0.051,0.243] |
| SOFA vs.Combined (TM+CD64 Index) | 0.803 | 0.422 | 0.019 | 0.024 | [−0.028,0.066] |
| APACHE Score vs Combined (TM+CD64 Index) | −2.327 | 0.020* | −0.128 | 0.055 | [0.020,0.235] |
Notes: aNull hypothesis: true AUC difference = 0. bDeLong test. *Indicates statistical significance.
Abbreviations: TM, Thrombomodulin; SOFA, Sequential Organ Failure Assessment; APACHE, Acute Physiology and Chronic Health Evaluation score.
Figure 4.
The summary receiver operating characteristic (ROC) curves of the combination Thrombomodulin (TM), CD64 Index, SOFA Score, and APACHE II Score diagnostic effectiveness analysis for sepsis at 3 different time points. (A) The first day, the patient was diagnosed the sepsis (Day 1). (B) The third day, the patient was diagnosed the sepsis (Day 3). (C) The seventh day, the patient was diagnosed the sepsis (Day 7).
Regression Analysis with Severity Scores
Let YSOFA represent the SOFA score, YAPACHE the APACHE II score, X1 = plasma TM, X2 = CRP, X3 = PCT, and X4 = CD64 Index. Time points D1, D3, and D7 are denoted as −1, −3, and −7, respectively, for the variables.
SOFA Score Association
The overall F-test for the regression model demonstrated significance only at D1 (p < 0.05), indicating a meaningful association (Table 10). Further t-tests for plasma TM, CD64 index, CRP, and PCT showed that only at D1, plasma TM exhibited a significant effect (p < 0.05), suggesting that its regression coefficient was nonzero and had a strong impact on the SOFA score (Table 11). The model’s goodness of fit was validated through adjusted R² and variance analysis (Table 12), while residual histograms indicated that standardized residuals followed an approximately normal distribution (Figure 5). In summary, a linear regression analysis was conducted using plasma TM (X1), CRP (X2), PCT (X3), and CD64 index (X4) as independent variables, with the SOFA score as the dependent variable. The model was significant (F = 7.291, p<0.05), with TM showing a notable positive effect (Beta = 0.536, p < 0.05). The regression equation was established as:
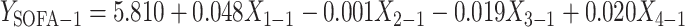 |
Table 10.
ANOVA for SOFA Score as the Dependent Variable: Test of Overall Model Significance
| Factor | Sum of Squares | Degrees of Freedom | Mean Square | F | Significance | |
|---|---|---|---|---|---|---|
| D1 SOFA | Regression | 183.28 | 4 | 45.82 | 7.291 | 0.000a* |
| Residual | 395.941 | 63 | 6.285 | |||
| Total | 579.221 | 67 | ||||
| D3 SOFA | Regression | 48.056 | 4 | 12.014 | 2.43 | 0.064b |
| Residual | 192.853 | 39 | 4.945 | |||
| Total | 240.909 | 43 | ||||
| D7 SOFA | Regression | 8.417 | 4 | 2.104 | 1.32 | 0.289c |
| Residual | 41.454 | 26 | 1.594 | |||
| Total | 49.871 | 30 |
Notes: aPredicted Variable: (Constant), D1 TM, D1 CRP, D1 PCT, D1 CD64 Index. bPredicted Variable: (Constant), D3 TM, D3 CRP, D3 PCT, D3 CD64 Index. cPredicted Variable: (Constant), D7 TM, D7 CRP, D7 PCT, D7 CD64 Index. *Indicates statistical significance.
Abbreviation: SOFA, Sequential Organ Failure Assessment.
Table 11.
Significance Test of Regression Coefficients: SOFA Score as the Dependent Variable
| Model | Unstandardized Coefficients | Standardized Coefficients | t | Significance | ||
|---|---|---|---|---|---|---|
| B | Standard Residual | Beta | ||||
| D1 SOFA | (Constant) | 5.810 | 0.845 | 6.877 | 0.000 | |
| D1 TM | 0.048 | 0.010 | 0.536 | 5.006 | 0.000* | |
| D1 CRP | −0.001 | 0.010 | −0.011 | −0.095 | 0.925 | |
| D1 PCT | −0.019 | 0.018 | −0.120 | −1.083 | 0.283 | |
| D1 CD64 Index | 0.020 | 0.030 | 0.072 | 0.675 | 0.502 | |
Note:*Indicates statistical significance.
Abbreviation: SOFA, Sequential Organ Failure Assessment.
Table 12.
Combined Evaluation of the Regression Model: SOFA Score as the Dependent Variable
| Factor | R | R2 | Adjusted R² | Standard Error of Estimate | Change Statistics | ||||
|---|---|---|---|---|---|---|---|---|---|
| R² Change | F Change | Degrees of Freedom 1 | Degrees of Freedom 2 | Significance (F Change) | |||||
| D1 SOFA | 0.563a | 0.316 | 0.273 | 2.507 | 0.316 | 7.291 | 4.000 | 63.000a | 0.000 |
Notes: aPredicted Variable: (Constant), D1 TM, D1 CRP, D1 PCT, D1 CD64 Index.
Figure 5.
Histogram of Standardized Residuals from Regression Model for SOFA Score in Day 1. Standardized residuals show near-ideal distribution (n = 68). Mean = 4.13×10−16 (computationally equivalent to 0), standard deviation = 0.97 (theoretically expected ≈1), supporting homoscedasticity and normality assumptions.
APACHE II Score Association
A multiple linear regression analysis was performed with the APACHE II score as the dependent variable. The F-test was used to evaluate the overall significance of the regression model. The results showed that the F-test value was significant (p < 0.05) on D1 and D3, indicating that the model was statistically meaningful (Table 13). A subsequent t-test was conducted on plasma TM, CD64 index, CRP, and PCT. The analysis revealed that on D1 and D3, plasma TM values were significant, indicating that its regression coefficient was not zero and that it had a notable impact on the APACHE II score (Table 14). The model’s goodness of fit was verified using adjusted R² and variance analysis (Table 15). Additionally, an examination of the residual histogram demonstrated that the standardized residuals followed an approximately normal distribution (Figure 6). In summary, a linear regression analysis was conducted using X1 (plasma TM), X2 (CRP), X3 (PCT), and X4 (CD64 index) as independent variables, with the APACHE II score as the dependent variable. The regression model was significant for D1 (F=3.194, p<0.05) and D3 (F=3.982, p<0.05). Specifically, D1 plasma TM (Beta=0.401, p<0.05) and D3 plasma TM (Beta=0.352, p<0.05) exhibited a significant positive effect on the APACHE II score.
Table 13.
ANOVA for APACHE II Score as the dependent Vairable: Test of Overall Model Significance
| Factor | Sum of Squares | Degrees of Freedom | Mean Square | F | Significance | |
|---|---|---|---|---|---|---|
| D1 APACHEII | Regression | 1121.439 | 4 | 280.36 | 3.194 | 0.019a* |
| Residual | 5529.429 | 63 | 87.769 | |||
| Total | 6650.868 | 67 | ||||
| D3 APACHEII | Regression | 1276.187 | 4 | 319.047 | 3.982 | 0.008b* |
| Residual | 3124.79 | 39 | 80.123 | |||
| Total | 4400.977 | 43 | ||||
| D7 APACHEII | Regression | 110.003 | 4 | 27.501 | 1.368 | 0.272c |
| Residual | 522.706 | 26 | 20.104 | |||
| Total | 632.71 | 30 |
Notes: aPredicted Variable: (Constant), D1 TM, D1 CRP, D1 PCT, D1 CD64 Index. bPredicted Variable: (Constant), D3 TM, D3 CRP, D3 PCT, D3 CD64 Index. cPredicted Variable: (Constant), D7 TM, D7 CRP, D7 PCT, D7 CD64 Index. *Indicates statistical significance.
Abbreviation: APACHE, Acute Physiology and Chronic Health Evaluation score.
Table 14.
Significance Test of Regression Coefficients: APACHE II Score as the Dependent Variable
| Model | Unstandardized Coefficients | Standardized Coefficients | t | Significance | ||
|---|---|---|---|---|---|---|
| B | Standard Residual | Beta | ||||
| D1 APACHE II | (Constant) | 14.134 | 3.157 | 4.477 | 0.000 | |
| D1 TM | 0.121 | 0.036 | 0.401 | 3.393 | 0.001* | |
| D1 CRP | 0.011 | 0.036 | 0.036 | 0.291 | 0.772 | |
| D1 PCT | 0.039 | 0.066 | 0.071 | 0.584 | 0.561 | |
| D1 CD64 | −0.028 | 0.111 | −0.029 | −0.248 | 0.805 | |
| D3 APACHE II | (Constant) | 18.782 | 3.608 | 5.206 | 0.000 | |
| D3 TM | 0.121 | 0.051 | 0.352 | 2.361 | 0.023* | |
| D3 CRP | 0.067 | 0.045 | 0.225 | 1.491 | 0.144 | |
| D3 PCT | 0.131 | 0.083 | 0.236 | 1.583 | 0.121 | |
| D3 CD64 | −0.392 | 0.314 | −0.183 | −1.250 | 0.219 | |
Note: *Indicates statistical significance.
Abbreviation: APACHE, Acute Physiology and Chronic Health Evaluation score.
Table 15.
Combined Evaluation of the Regression Model: APACHE II Score as the Dependent Variable
| Factor | R | R2 | Adjusted R² | Standard Error of Estimate | Change Statistics | ||||
|---|---|---|---|---|---|---|---|---|---|
| R² Change | F Change | Degrees of Freedom 1 | Degrees of Freedom 2 | Significance (F Change) | |||||
| D1 APACHEII | 0.411a | 0.169 | 0.116 | 9.369 | 0.169 | 3.194 | 4.000 | 63.000 a | 0.019 |
| D3 APACHEII | 0.538b | 0.290 | 0.217 | 8.951 | 0.290 | 3.982 | 4.000 | 39.000 b | 0.008 |
Notes: aPredicted Variable: (Constant), D1 TM, D1 CRP, D1 PCT, D1 CD64 Index. bPredicted Variable: (Constant), D3 TM, D3 CRP, D3 PCT, D3 CD64 Index.
Figure 6.
Distribution of Standardized Residuals for APACHE-II Score Models. (A) APACHE II regression standardized residual in Day 1. Standardized residuals show near-ideal distribution (n = 68). Mean = 4.65×10−16 (computationally equivalent to 0), standard deviation = 0.97 (theoretically expected ≈1). (B) APACHE II regression standardized residual in Day3. Standardized residuals show near-ideal distribution (n = 44). Mean = −2.12×10−16 (computationally equivalent to 0), standard deviation = 0.95 (theoretically expected ≈1). Both distributions demonstrate successful standardization (mean ≈ 0, SD ≈ 1), validating regression assumptions for sequential APACHE-II measurements.
Model formula at time D1:
 |
Model formula at time D3:
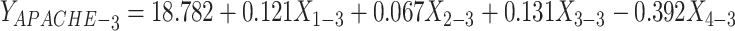 |
Murine Model Validation of Endothelial Injury
The plasma TM expression levels in each group of mice at various time points were assessed using ELISA. Results indicated that the levels in the CLP group were significantly elevated compared to the Sham group, with plasma TM expression progressively increasing in correlation with disease severity, and the differences were statistically significant (p<0.05), as illustrated in Figure 7.
Figure 7.
Expression of plasma Thrombomodulin (TM) in mice in different groups at different time points. **means p < 0.01, ***means p < 0.001, and ****means p<0.0001 versus in different groups.
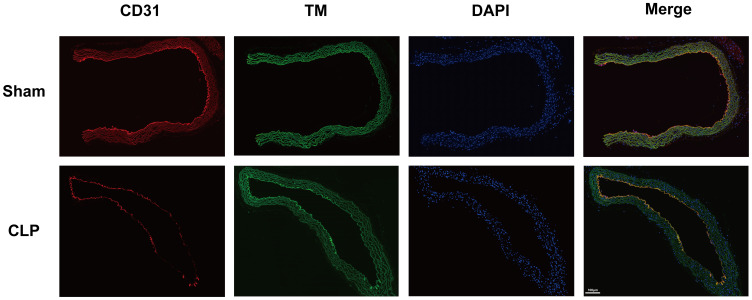
Immunofluorescence analysis revealed aortic endothelial injury with concomitant reduction in surface TM and CD31 expression in CLP-induced septic mice versus sham controls (Figure 8). Consistent with these findings, Western blot and qPCR confirmed significantly decreased TM protein and mRNA levels in CLP-group aortic endothelium (p<0.05) (Figure 9).
Figure 8.
Immunofluorescence analysis of aortic endothelial integrity in septic mice. Immunostaining of mouse aortic sections showing CD31 (endothelial marker, red), thrombomodulin (TM, green), and DAPI (nuclear stain, blue) in Sham-operated (top) versus CLP-induced septic mice (bottom). Merged images demonstrate disrupted endothelial architecture and attenuated TM expression in the CLP group compared to intact endothelium in Sham controls, indicating sepsis-induced vascular injury. Scale bar: 100 μm. CLP: cecal ligation and puncture.
Figure 9.
Expression of Thrombomodulin (TM) in aortic endothelial. (A) Expression efficiency of TM by quantitative real-time polymerase chain reaction (qRT-PCR). (B) Expression efficiency of TM by Western Blot. (C) Quantitated by densitometric analysis (n=6). ***means p < 0.001, and ****means p<0.0001 versus in different groups.
Discussion
Sepsis is a life-threatening condition characterized by a complex interplay of immune dysregulation, coagulation disturbances, and endothelial dysfunction.22–24 TM, an essential anticoagulant and anti-inflammatory regulator expressed on the endothelial cell surface, has gained attention for its potential role in sepsis pathophysiology.25 This study systematically investigated the dynamic changes in TM and CD64 index among sepsis patients, highlighting their significance in early diagnosis and prognosis. The results demonstrated a significant elevation in plasma TM levels in sepsis patients, which positively correlated with disease severity, as reflected in the SOFA and APACHE II scores, and with 28-day mortality. Moreover, the combined evaluation of TM and CD64 index provided greater prognostic accuracy (AUC=0.9671) than either marker alone, suggesting a synergistic predictive effect. Animal experiments further confirmed a significant reduction in TM protein and mRNA expression in the aortic endothelium of septic mice, reinforcing clinical findings at a mechanistic level. These results align with existing literature, highlighting the scientific and clinical significance of TM as a potential biomarker for sepsis assessment and prognosis.
Pathophysiological significance of TM as a marker of endothelial injury. TM is a key anticoagulant and anti-inflammatory regulatory protein expressed on endothelial cell surfaces.26 The release of TM into the bloodstream reflects endothelial barrier dysfunction and microcirculatory disturbances.27,28 This study identified a significant elevation in plasma TM levels in sepsis patients at the time of admission, with a strong positive correlation with SOFA score (β=0.536, p<0.05) and APACHE II score (β=0.401, p<0.05). These findings align with the concept of TM as a biomarker of endothelial injury, as previously described by Hemant.29 Notably, in vivo experiments demonstrated a marked reduction in TM expression on the aortic endothelial surface in CLP-induced septic mice, suggesting that decreased endothelial TM contributes directly to increased plasma TM levels. This observation is closely associated with endothelial cell apoptosis, inflammatory mediator release, and oxidative stress, key mechanisms implicated in sepsis pathophysiology.13,30–32Furthermore, elevated plasma TM levels in mice were detected by ELISA assay, supporting the detrimental impact of inflammation on endothelial integrity and reinforcing clinical findings. Endothelial injury and coagulation disturbances are central to sepsis progression.33 Therefore, changes in TM levels represent a valuable indicator for early diagnosis and disease monitoring.
This study examined fluctuations in plasma TM levels in sepsis patients and their relationship with clinical severity scores (APACHE II, SOFA score) and inflammatory markers (CD64 index, CRP, PCT). Elevated plasma TM levels not only signify endothelial damage, leading to the release of TM from endothelial surfaces into circulation but may also be linked to sepsis-induced coagulation abnormalities and microcirculatory dysfunction.34 By dynamically monitoring plasma TM levels alongside other biomarkers, this study aims to establish new reference parameters for early sepsis diagnosis and prognosis. TM’s distinct role in coagulation regulation highlights its potential clinical significance in sepsis, providing a theoretical basis for more precise diagnostic and therapeutic strategies. These findings contribute to a deeper understanding of TM’s involvement in sepsis and offer a new perspective for improving clinical management, particularly in early detection and intervention.
The paradoxical dynamics of TM—elevated plasma levels coupled with reduced endothelial expression—observed in both patients and CLP mice suggest a feedforward loop between endothelial injury and systemic inflammation. Proteolytic shedding of TM from damaged endothelium depletes local anticoagulant capacity and releases soluble TM fragments into circulation. Recent studies indicate that TM may bind to leukocyte surface receptors (such as Mac-1 integrin),35 priming neutrophils for CD64 upregulation and pro-inflammatory cytokine release.36
The synergistic diagnostic value of TM and CD64 index. The CD64 index, a marker of neutrophil activation, reflects heightened immune activity in the early stages of sepsis.37,38 This study identified a significant correlation between TM and the CD64 index on the first day of sepsis diagnosis (D1) (r=0.315, p=0.009). The combined use of these markers significantly improved prognostic prediction, achieving a sensitivity of 91.30% and a specificity of 95.56%, surpassing conventional inflammatory markers such as CRP and PCT. These findings suggest that TM and the CD64 index represent distinct pathological aspects of sepsis, with TM indicating endothelial damage and the CD64 index reflecting immune activation. This aligns with murine data showing aortic TM depletion coinciding with plasma TM rise, implying that endothelial TM loss is not merely a passive biomarker but an active contributor to immune dysregulation.39,40 Aligned with the emphasis on host dysregulation in the “Sepsis 3.0” definition by Singer et al1 the combined assessment of TM and CD64 index provides a more comprehensive evaluation of disease progression and facilitates early stratified treatment.9,41 Tracking the dynamic changes in these markers enables a more precise representation of the immune response and disease pathology in sepsis, offering timely guidance for clinical intervention.
Notably, the correlation between TM and CRP or PCT becomes apparent only in the later stages of sepsis (D7) (r=0.493 and 0.392, p<0.001), while the association between the CD64 index and CRP is also delayed until D7 (r=0.432, p<0.001). This temporal difference may reflect distinct pathological phases of sepsis: early elevation of TM suggests that endothelial damage and coagulation abnormalities precede systemic inflammation, whereas the delayed association with CRP and PCT indicates the later amplification of the inflammatory response.42,43 Endothelial dysfunction plays a central role in sepsis pathogenesis and is a major contributor to multi-organ failure.44,45 This further highlights TM’s unique value as an early warning biomarker. The increase in TM levels provides new opportunities for the early diagnosis of sepsis by capturing endothelial injury before the onset of widespread inflammation and immune activation, thereby offering a more sensitive early warning signal for clinical application.
Limitations and future directions of the study. The predictive model incorporating TM and the CD64 index, with an AUC exceeding 0.96, presents a promising approach for early sepsis risk stratification. Compared to conventional scoring systems such as SOFA and APACHE II, biomarker-based testing offers advantages in simplicity, ease of use, and real-time monitoring, making it particularly valuable for rapid decision-making in emergency settings. Integrating the TM/CD64 index into sepsis diagnostic protocols could improve resource allocation and enhance treatment efficacy for critically ill patients. The simultaneous assessment of TM and CD64 index not only increases the sensitivity and specificity of early sepsis detection but also facilitates timely clinical intervention, potentially reducing sepsis-related mortality and complications.
While these findings carry notable implications, essential limitations must be acknowledged: the single-center design and modest sample size (*n*=68) may constrain generalizability; infection-source variations in TM levels remain unaddressed; the 28-day follow-up precludes long-term prognostic assessment; and multivariate models inadequately explain clinical heterogeneity. Consequently, future research should employ larger multicenter cohorts to validate TM’s applicability, conduct infection-source subgroup analyses, extend observation periods, and refine predictive modeling. These concerted efforts will clarify TM’s pathophysiological role in sepsis and advance targeted therapies.
The mechanistic link between TM shedding and CD64 activation has direct therapeutic implications. Targeting endothelial stabilization (eg, via recombinant TM supplementation)46,47 may not only restore anticoagulant function but also mitigate neutrophil hyperactivation, as suggested by preclinical studies. Our data provide a rationale for combined biomarker monitoring (TM + CD64) to identify patients who would benefit from such dual-pathway interventions. Future studies should explore whether TM/CD64-guided therapies improve outcomes in sepsis-associated endothelial-immune axis disruption.
Conclusion
Plasma TM, an indicator of endothelial damage, significantly improves the prognostic accuracy of sepsis when combined with the CD64 index. The early rise in plasma TM levels and its strong correlation with disease severity scores establish this biomarker combination as a valuable tool in clinical practice. Future studies should aim to validate its underlying mechanisms and expand its clinical application to support precise sepsis management. Through rigorous clinical validation and mechanistic investigations, plasma TM has the potential to serve as a novel biomarker for early sepsis diagnosis, risk assessment, and therapeutic decision-making, contributing to more effective and personalized treatment strategies.
Plasma thrombomodulin (TM), an indicator of endothelial damage, significantly enhances the prognostic accuracy of sepsis when integrated with the CD64 index. The early elevation of plasma TM levels and its strong correlation with disease severity establish this biomarker combination as a valuable clinical tool. While a modest sample size constrains this study, future validation through multicenter large-cohort studies remains imperative. Subsequent research will prioritize elucidating mechanistic interactions between soluble TM and CD64—using in vitro models such as endothelial-leukocyte co-cultures—while expanding clinical applications to enable precision sepsis management. Therapeutically, recombinant TM trials represent a critical translational pathway. Through these concerted efforts, the TM-CD64 biomarker combination holds significant promise for advancing early diagnosis, risk stratification, and tailored therapies, ultimately driving personalized sepsis care.
Acknowledgments
The first three authors contributed equally to this study. This study was supported by Characteristic Discipline Project (PWYts2021-17); Shanghai Pudong New Area Health Commission Discipline Construction Project (Key Discipline Group)(PWZxq2022-12); The Training Plan of discipline Leader of Shanghai Pudong new area Health Commission (PWRd2022-04); Pudong New Area Science and Technology Development Fund for Public Welfare Research Projects in Public Institutions, Shanghai(PKJ2024-Y28).
Ethical Approve
This study complies with the Declaration of Helsinki and was approved by the Ethics Committee of Gongli Hospital, Pudong New District, Shanghai, China (Approval No. GLYYls2024-034; No. GLYYls2024-036).
Disclosure
The authors declare that they have no competing interests.
References
- 1.Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315(8):801. doi: 10.1001/jama.2016.0287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou J, Qian C, Zhao M, et al. Epidemiology and outcome of severe sepsis and septic shock in intensive care units in Mainland China. PLoS One. 2014;9(9):e107181. doi: 10.1371/journal.pone.0107181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Markwart R, Saito H, Harder T, et al. Epidemiology and burden of sepsis acquired in hospitals and intensive care units: a systematic review and meta-analysis. Intensive Care Med. 2020;46(8):1536–1551. doi: 10.1007/s00134-020-06106-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Unar A, Bertolino L, Patauner F, Gallo R, Durante-Mangoni E. Decoding sepsis-induced disseminated intravascular coagulation: a comprehensive review of existing and emerging therapies. JCM. 2023;12(19):6128. doi: 10.3390/jcm12196128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iba T, Levi M, Thachil J, Helms J, Scarlatescu E, Levy JH. Communication from the scientific and standardization committee of the international society on thrombosis and haemostasis on sepsis-induced coagulopathy in the management of sepsis. J Thromb Haemost. 2023;21(1):145–153. doi: 10.1016/j.jtha.2022.10.022 [DOI] [PubMed] [Google Scholar]
- 6.Okamoto K, Tamura T, Sawatsubashi Y. Sepsis and disseminated intravascular coagulation. J Intensive Care. 2016;4(1):23. doi: 10.1186/s40560-016-0149-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iba T, Levy JH. Sepsis-induced coagulopathy and disseminated intravascular coagulation. Anesthesiology. 2020;132(5):1238–1245. doi: 10.1097/ALN.0000000000003122 [DOI] [PubMed] [Google Scholar]
- 8.Taylor FB, Keith W, Wada H, Levi M. Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb Haemost. 2017;86(11):1327–1330. doi: 10.1055/s-0037-1616068 [DOI] [PubMed] [Google Scholar]
- 9.Czempik PF, Wiórek A. Management strategies in septic coagulopathy: a review of the current literature. Healthcare. 2023;11(2):227. doi: 10.3390/healthcare11020227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Joffre J, Hellman J. Oxidative stress and endothelial dysfunction in sepsis and acute inflammation. Antioxidants & redox signaling. doi: 10.1089/ars.2021.0027 [DOI] [PubMed] [Google Scholar]
- 11.Whitney JE, Zhang B, Koterba N, et al. Systemic endothelial activation is associated with early acute respiratory distress syndrome in children with extrapulmonary sepsis. Crit Care Med. 2020;48(3):344–352. doi: 10.1097/CCM.0000000000004091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Umbro I, Gentile G, Tinti F, Muiesan P, Mitterhofer AP. Recent advances in pathophysiology and biomarkers of sepsis-induced acute kidney injury. J Infect. 2016;72(2):131–142. doi: 10.1016/j.jinf.2015.11.008 [DOI] [PubMed] [Google Scholar]
- 13.Iba T, Helms J, Neal MD, Levy JH. Mechanisms and management of the coagulopathy of trauma and sepsis: trauma-induced coagulopathy, sepsis-induced coagulopathy, and disseminated intravascular coagulation. J Thromb Haemost. 2023. doi: 10.1016/j.jtha.2023.05.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suzuki K. Thrombomodulin: a key regulator of intravascular blood coagulation, fibrinolysis, and inflammation, and a treatment for disseminated intravascular coagulation. Proc Jpn Acad Ser B Phys Biol Sci. 2025;101(2):75–97. doi: 10.2183/pjab.101.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wenzel J, Assmann JC, Schwaninger M. Thrombomodulin – a new target for treating stroke at the crossroad of coagulation and inflammation. CMC. 2014;21(18):2025–2034. doi: 10.2174/0929867321666131228204839 [DOI] [PubMed] [Google Scholar]
- 16.Lin WL, Chang CF, Shi CS, Shi GY, Wu HL. Recombinant lectin-like domain of thrombomodulin suppresses vascular inflammation by reducing leukocyte recruitment via interacting with lewis y on endothelial cells. ATVB. 2013;33(10):2366–2373. doi: 10.1161/ATVBAHA.113.301221 [DOI] [PubMed] [Google Scholar]
- 17.Castelli GP, Pognani C, Meisner M, Stuani A, Bellomi D, Sgarbi L. Procalcitonin and C-reactive protein during systemic inflammatory response syndrome, sepsis and organ dysfunction. Crit Care. 2004;8(4):R234–242. doi: 10.1186/cc2877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sridharan P, Chamberlain RS, Sridharan P, Chamberlain RS. The efficacy of procalcitonin as a biomarker in the management of sepsis: slaying dragons or tilting at windmills? Surg Infect. 2013;14(6):489–511. doi: 10.1089/sur.2012.028 [DOI] [PubMed] [Google Scholar]
- 19.Anand D, Das S, Bhargava S, et al. Procalcitonin as a rapid diagnostic biomarker to differentiate between culture-negative bacterial sepsis and systemic inflammatory response syndrome: a prospective, observational, cohort study. J Crit Care. 2015;30(1):218.e7–12. doi: 10.1016/j.jcrc.2014.08.017 [DOI] [PubMed] [Google Scholar]
- 20.Shang YX, Zheng Z, Wang M, et al. Diagnostic performance of neutrophil CD64 index, procalcitonin, and C-reactive protein for early sepsis in hematological patients. World J Clin Cases. 2022;10(7):2127–2137. doi: 10.12998/wjcc.v10.i7.2127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patnaik R, Azim A, Agarwal V. Neutrophil CD64 a diagnostic and prognostic marker of sepsis in adult critically ill patients: a brief review. Indian J Crit Care Med. 2020;24(12):1242–1250. doi: 10.5005/jp-journals-10071-23558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Delabranche X, Helms J, Meziani F. Immunohaemostasis: a new view on haemostasis during sepsis. Ann Intensive Care. 2017;7(1):117. doi: 10.1186/s13613-017-0339-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Font MD, Thyagarajan B, Khanna AK. Sepsis and septic shock – basics of diagnosis, pathophysiology and clinical decision making. Med Clin North Am. 2020;104(4):573–585. doi: 10.1016/j.mcna.2020.02.011 [DOI] [PubMed] [Google Scholar]
- 24.Song JW, Zullo J, Lipphardt M, et al. Endothelial glycocalyx—the battleground for complications of sepsis and kidney injury. Nephrol Dial Transplant. 2018;33(2):203–211. doi: 10.1093/ndt/gfx076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levi M, Van Der Poll T. Thrombomodulin in sepsis. Minerva Anestesiol. 2013;79(3):294–298. [PubMed] [Google Scholar]
- 26.Watanabe-Kusunoki K, Nakazawa D, Ishizu A, Atsumi T. Thrombomodulin as a physiological modulator of intravascular injury. Front Immunol. 2020;11:575890. doi: 10.3389/fimmu.2020.575890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang J, Xue M, Chen Y, et al. Identification of soluble thrombomodulin and tissue plasminogen activator-inhibitor complex as biomarkers for prognosis and early evaluation of septic shock and sepsis-induced disseminated intravascular coagulation. Annals Palliative Med. 2021;10(10):101700184–101710184. doi: 10.21037/apm-21-2222 [DOI] [PubMed] [Google Scholar]
- 28.Nguyen VT, Nguyen-Phan HN, Hoang BB. Serum thrombomodulin level can predict mortality in patients with sepsis? Med Arch. 2023;77(6):433–439. doi: 10.5455/medarh.2023.77.433-439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Giri H, Panicker SR, Cai X, Biswas I, Weiler H, Rezaie AR. Thrombomodulin is essential for maintaining quiescence in vascular endothelial cells. Proc Natl Acad Sci U S A. 2021;118(11):e2022248118. doi: 10.1073/pnas.2022248118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iba T, Helms J, Connors JM, Levy JH. The pathophysiology, diagnosis, and management of sepsis-associated disseminated intravascular coagulation. J Intensive Care. 2023;11:24. doi: 10.1186/s40560-023-00672-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu S, Ilyas I, Little PJ, et al. Endothelial dysfunction in atherosclerotic cardiovascular diseases and beyond: from mechanism to pharmacotherapies. Pharmacol Rev. 2021;73(3):924–967. doi: 10.1124/pharmrev.120.000096 [DOI] [PubMed] [Google Scholar]
- 32.Lee WL, Slutsky AS. Sepsis and endothelial permeability. N Engl J Med. 2010;363(7):689–691. doi: 10.1056/NEJMcibr1007320 [DOI] [PubMed] [Google Scholar]
- 33.Ostrowski SR, Gaïni S, Pedersen C, Johansson PI. Sympathoadrenal activation and endothelial damage in patients with varying degrees of acute infectious disease: an observational study. J Crit Care. 2015;30(1):90–96. doi: 10.1016/j.jcrc.2014.10.006 [DOI] [PubMed] [Google Scholar]
- 34.Ikezoe T. Thrombomodulin/activated protein C system in septic disseminated intravascular coagulation. J Intensive Care. 2015;3(1):1. doi: 10.1186/s40560-014-0050-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nguyen VT, Nguyen-Phan HN, Ton TN, Hoang BB. Value of serum thrombomodulin as a marker and predictor in patients with sepsis-associated acute kidney injury. Int J Gen Med. 2023;16:2933–2941. doi: 10.2147/IJGM.S417410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goswami DG, Garcia LF, Dodoo C, et al. Evaluating the timeliness and specificity of CD69, CD64, and CD25 as biomarkers of sepsis in mice. Shock. 2021;55(4):507–518. doi: 10.1097/SHK.0000000000001650 [DOI] [PubMed] [Google Scholar]
- 37.Meghraoui-Kheddar A, Chousterman B, Guillou N, et al. Two new neutrophil subsets define a discriminating sepsis signature. Am J Respir Crit Care Med. 2022;205(1):46–59. doi: 10.1164/rccm.202104-1027OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang L, Chen Y, Hu R, Chen H, Peng X, Yuan H. A single-center study of reference intervals for TAT, PIC, TM and t-PAIC in healthy older Chinese adults. Thromb J. 2024;22:82. doi: 10.1186/s12959-024-00651-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wallen TE, Singer KE, Elson NC, et al. Defining endotheliopathy in murine polytrauma models. Shock. 2022;57(6):291–298. doi: 10.1097/SHK.0000000000001940 [DOI] [PubMed] [Google Scholar]
- 40.Imaura M, Katagiri F, Nagase S, et al. Optimal plasma concentration of thrombomodulin alfa to treat sepsis-induced disseminated intravascular coagulation. Shock. 2023;60(2):221–226. doi: 10.1097/SHK.0000000000002172 [DOI] [PubMed] [Google Scholar]
- 41.Wiedermann CJ. Anticoagulant therapy for septic coagulopathy and disseminated intravascular coagulation: where do KyberSept and SCARLET leave us? Acute Med Surg. 2020;7(1):e477. doi: 10.1002/ams2.477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haasper C, Kalmbach M, Dikos GD, et al. Prognostic value of procalcitonin (PCT) and/or interleukin-6 (IL-6) plasma levels after multiple trauma for the development of multi organ dysfunction syndrome (MODS) or sepsis. THC. 2010;18(2):89–100. doi: 10.3233/THC-2010-0571 [DOI] [PubMed] [Google Scholar]
- 43.Peschanski N, Chenevier-Gobeaux C, Mzabi L, et al. Prognostic value of PCT in septic emergency patients. Ann Intensive Care. 2016;6(1):47. doi: 10.1186/s13613-016-0146-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Levi M, T IBA. Organ dysfunction in sepsis-associated intravascular coagulation. Juntendo Iji Zasshi. 2024;70(1):26–28. doi: 10.14789/jmj.JMJ23-0042-P [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kazune S, Piebalga A, Strike E, Vanags I. Impaired vascular reactivity in sepsis – a systematic review with meta-analysis. Arch Med Sci Atheroscler Dis. 2019;4:e151–e161. doi: 10.5114/amsad.2019.86754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Uzawa A, Mori M, Masuda H, Ohtani R, Uchida T, Kuwabara S. Recombinant thrombomodulin ameliorates experimental autoimmune encephalomyelitis by suppressing high mobility group box 1 and inflammatory cytokines. Clin Exp Immunol. 2018;193(1):47–54. doi: 10.1111/cei.13123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tamura K, Saito H, Asakura H, et al. Recombinant human soluble thrombomodulin (thrombomodulin alfa) to treat disseminated intravascular coagulation in solid tumors: results of a one-arm prospective trial. Int J Clin Oncol. 2015;20(4):821–828. doi: 10.1007/s10147-014-0768-1 [DOI] [PubMed] [Google Scholar]