Abstract
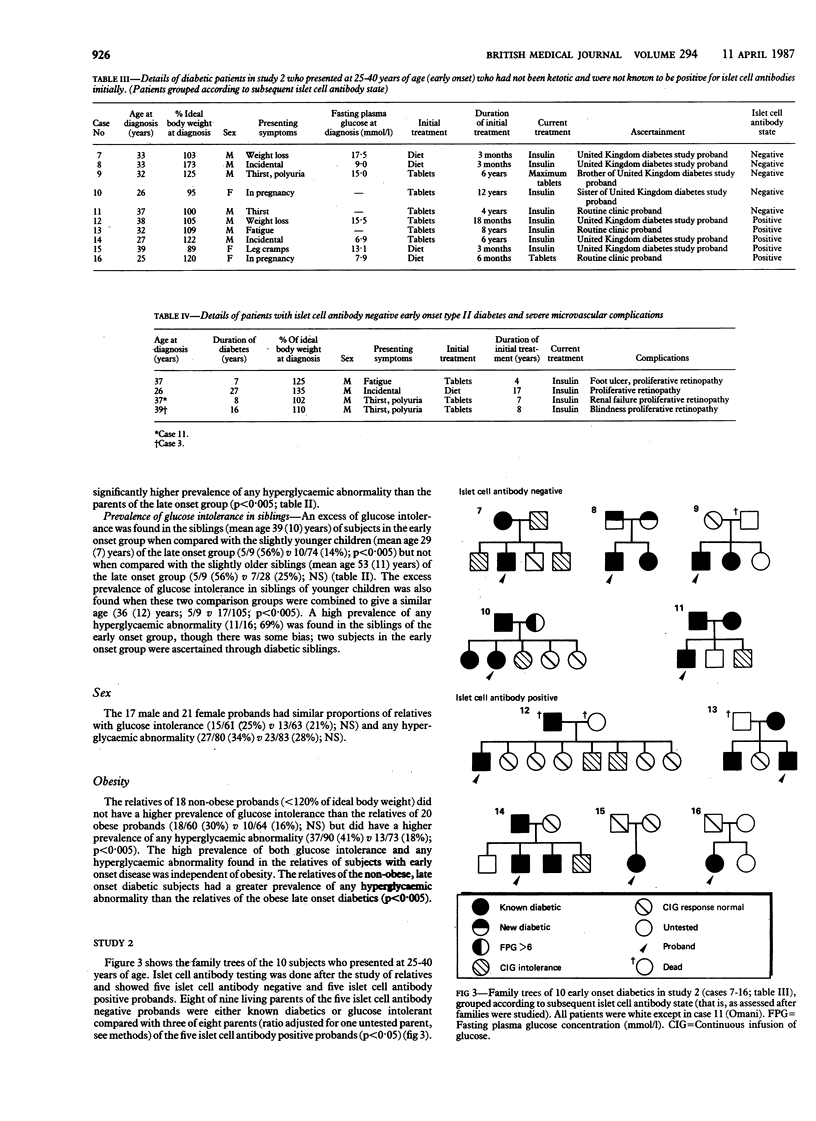
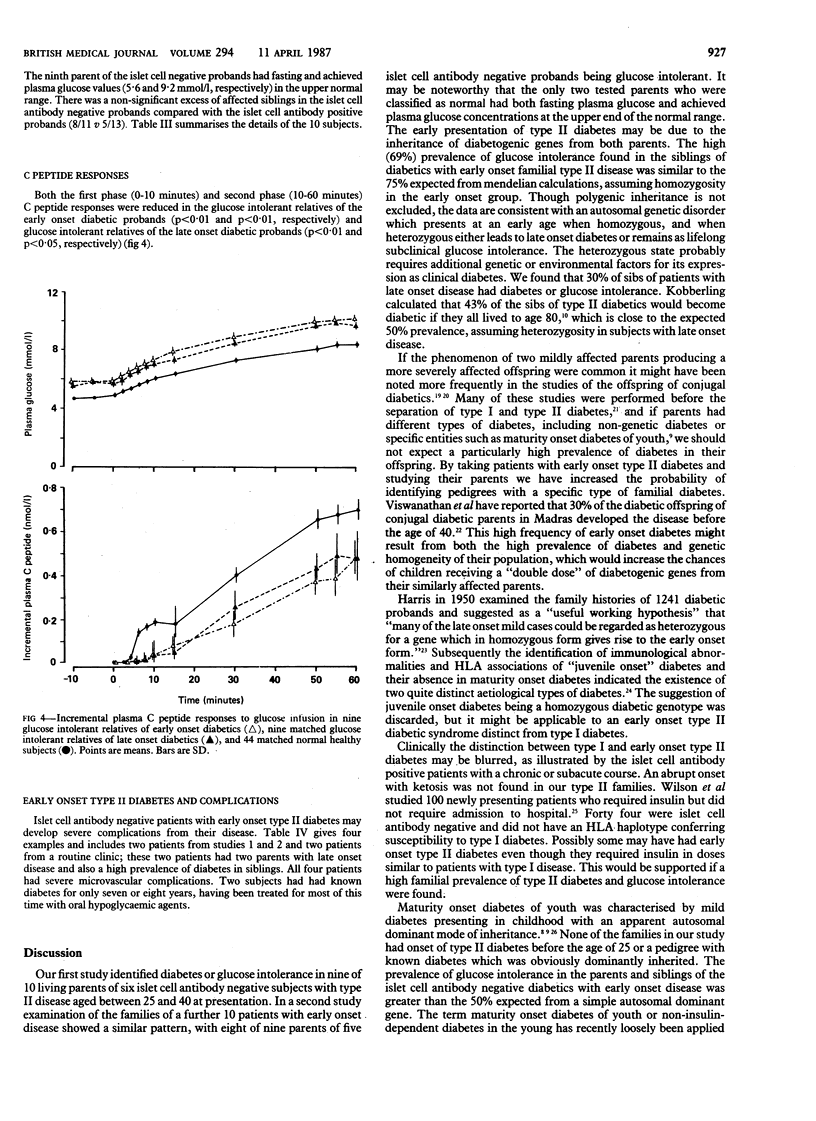
The inheritance of non-insulin-dependent (type II) diabetes was studied by a continuous infusion of glucose test in all available first degree relatives of 48 diabetic probands of various ages and with differing severity of disease. In an initial study of 38 type II diabetic subjects and their first degree relatives six islet cell antibody negative patients with early onset disease (aged 25-40 at diagnosis) were found to have a particularly high familial prevalence of diabetes or glucose intolerance. Nine of 10 parents available for study either had type II diabetes or were glucose intolerant. A high prevalence of diabetes or glucose intolerance was also found in their siblings (11/16;69%). In a second study of the families of a further 10 young diabetic probands (presenting age 25-40) whose islet cell antibody state was unknown a similar high prevalence of diabetes or glucose intolerance was found among parents of the five islet cell antibody negative probands (8/9; 89%) but not among parents of the five islet cell antibody positive probands (3/8;38%). Islet cell antibody negative diabetics with early onset type II disease may have inherited a diabetogenic gene or genes from both parents. They commonly need insulin to maintain adequate glycaemic control and may develop severe diabetic complications. Early onset type II diabetes may represent a syndrome in which characteristic pedigrees, clinical severity, and absence of islet autoimmunity make it distinct from either type I diabetes, maturity onset diabetes of the young, or late onset type II diabetes.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albano J. D., Ekins R. P., Maritz G., Turner R. C. A sensitive, precise radioimmunoassay of serum insulin relying on charcoal separation of bound and free hormone moieties. Acta Endocrinol (Copenh) 1972 Jul;70(3):487–509. doi: 10.1530/acta.0.0700487. [DOI] [PubMed] [Google Scholar]
- Barnett A. H., Eff C., Leslie R. D., Pyke D. A. Diabetes in identical twins. A study of 200 pairs. Diabetologia. 1981 Feb;20(2):87–93. doi: 10.1007/BF00262007. [DOI] [PubMed] [Google Scholar]
- Barnett A. H., Spiliopoulos A. J., Pyke D. A., Stubbs W. A., Burrin J., Alberti K. G. Metabolic studies in unaffected co-twins of non-insulin-dependent diabetics. Br Med J (Clin Res Ed) 1981 May 23;282(6277):1656–1658. doi: 10.1136/bmj.282.6277.1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaty T. H., Neel J. V., Fajans S. S. Identifying risk factors for diabetes in first degree relatives of non-insulin dependent diabetic patients. Am J Epidemiol. 1982 Mar;115(3):380–397. doi: 10.1093/oxfordjournals.aje.a113316. [DOI] [PubMed] [Google Scholar]
- Bottazzo G. F., Florin-Christensen A., Doniach D. Islet-cell antibodies in diabetes mellitus with autoimmune polyendocrine deficiencies. Lancet. 1974 Nov 30;2(7892):1279–1283. doi: 10.1016/s0140-6736(74)90140-8. [DOI] [PubMed] [Google Scholar]
- Chen M., Bergman R. N., Pacini G., Porte D., Jr Pathogenesis of age-related glucose intolerance in man: insulin resistance and decreased beta-cell function. J Clin Endocrinol Metab. 1985 Jan;60(1):13–20. doi: 10.1210/jcem-60-1-13. [DOI] [PubMed] [Google Scholar]
- Davis T. M., Turner R. C. CIGMA assessment of insulin resistance and pancreatic beta cell function in the elderly. Age Ageing. 1985 Jul;14(4):220–224. doi: 10.1093/ageing/14.4.220. [DOI] [PubMed] [Google Scholar]
- Efendić S., Luft R., Wajngot A. Aspects of the pathogenesis of type 2 diabetes. Endocr Rev. 1984 Summer;5(3):395–410. doi: 10.1210/edrv-5-3-395. [DOI] [PubMed] [Google Scholar]
- Fajans S. S., Cloutier M. C., Crowther R. L. The Banting Memorial Lecture 1978. Clinical and etiologic heterogeneity of idiopathic diabetes mellitus. Diabetes. 1978 Nov;27(11):1112–1125. doi: 10.2337/diab.27.11.1112. [DOI] [PubMed] [Google Scholar]
- Holman R. R., Turner R. C. Diabetes: The quest for basal normoglycaemia. Lancet. 1977 Feb 26;1(8009):469–474. doi: 10.1016/s0140-6736(77)91954-7. [DOI] [PubMed] [Google Scholar]
- Hosker J. P., Matthews D. R., Rudenski A. S., Burnett M. A., Darling P., Bown E. G., Turner R. C. Continuous infusion of glucose with model assessment: measurement of insulin resistance and beta-cell function in man. Diabetologia. 1985 Jul;28(7):401–411. doi: 10.1007/BF00280882. [DOI] [PubMed] [Google Scholar]
- Kahn C. B., Soeldner J. S., Gleason R. E., Rojas L., Camerini-Davalos R. A., Marble A. Clinical and chemical diabetes in offspring of diabetic couples. N Engl J Med. 1969 Aug 14;281(7):343–347. doi: 10.1056/NEJM196908142810703. [DOI] [PubMed] [Google Scholar]
- Keen H., Track N. S. Age of onset and inheritance of diabetes: the importance of examining relatives. Diabetologia. 1968 Dec;4(6):317–321. doi: 10.1007/BF01211765. [DOI] [PubMed] [Google Scholar]
- Köbberling J. Studies on the genetic heterogeneity of diabetes mellitus. Diabetologia. 1971 Feb;7(1):46–49. doi: 10.1007/BF02346253. [DOI] [PubMed] [Google Scholar]
- O'Rahilly S. P., Nugent Z., Rudenski A. S., Hosker J. P., Burnett M. A., Darling P., Turner R. C. Beta-cell dysfunction, rather than insulin insensitivity, is the primary defect in familial type 2 diabetes. Lancet. 1986 Aug 16;2(8503):360–364. doi: 10.1016/s0140-6736(86)90052-8. [DOI] [PubMed] [Google Scholar]
- Rotter J. I., Rimoin D. L. The genetics of the glucose intolerance disorders. Am J Med. 1981 Jan;70(1):116–126. doi: 10.1016/0002-9343(81)90418-6. [DOI] [PubMed] [Google Scholar]
- Rushforth N. B., Bennett P. H., Steinberg A. G., Burch T. A., Miller M. Diabetes in the Pima Indians. Evidence of bimodality in glucose tolerance distributions. Diabetes. 1971 Nov;20(11):756–765. doi: 10.2337/diab.20.11.756. [DOI] [PubMed] [Google Scholar]
- Tattersal R. B., Fajans S. S. Prevalence of diabetes and glucose intolerance in 199 offspring of thirty-seven conjugal diabetic parents. Diabetes. 1975 May;24(5):452–462. doi: 10.2337/diab.24.5.452. [DOI] [PubMed] [Google Scholar]
- Tattersall R. B., Fajans S. S. A difference between the inheritance of classical juvenile-onset and maturity-onset type diabetes of young people. Diabetes. 1975 Jan;24(1):44–53. doi: 10.2337/diab.24.1.44. [DOI] [PubMed] [Google Scholar]
- Tattersall R. B. Mild familial diabetes with dominant inheritance. Q J Med. 1974 Apr;43(170):339–357. [PubMed] [Google Scholar]
- Thompson G. S. Genetic factors in diabetes mellitus studied by the oral glucose tolerance test. J Med Genet. 1965 Dec;2(4):221–226. doi: 10.1136/jmg.2.4.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanathan M., Mohan V., Snehalatha C., Ramachandran A. High prevalence of type 2 (non-insulin-dependent) diabetes among the offspring of conjugal type 2 diabetic parents in India. Diabetologia. 1985 Dec;28(12):907–910. doi: 10.1007/BF00703134. [DOI] [PubMed] [Google Scholar]
- Wilson R. M., Van der Minne P., Deverill I., Heller S. R., Gelsthrope K., Reeves W. G., Tattersall R. B. Insulin dependence: problems with the classification of 100 consecutive patients. Diabet Med. 1985 May;2(3):167–172. doi: 10.1111/j.1464-5491.1985.tb00627.x. [DOI] [PubMed] [Google Scholar]
- Zimmet P., Whitehouse S. Bimodality of fasting and two-hour glucose tolerance distributions in a Micronesian population. Diabetes. 1978 Aug;27(8):793–800. doi: 10.2337/diab.27.8.793. [DOI] [PubMed] [Google Scholar]



