Abstract
Human immunodeficiency virus type 1 Vpu is a multifunctional phosphoprotein composed of the N-terminal transmembrane (VpuTM) and C-terminal cytoplasmic domains. Each of these domains regulates a distinct function of the protein; the transmembrane domain is critical in virus release, and phosphorylation of the cytoplasmic domain is necessary for CD4 proteolysis. We carried our experiments to identify amino acids in the VpuTM domain that are important in the process of virus-like particle (VLP) release from HeLa cells. VLPs are released from the plasma membrane of HeLa cells at constitutive levels, and Vpu expression enhanced the release of VLPs by a factor of 10 to 15. Deletion of two to five amino acids from both N- and C-terminal ends or the middle of the VpuTM domain generated mutant Vpu proteins that have lost the ability to enhance VLP release. These deletion mutants have not lost the ability to associate with the wild-type or mutant Vpu proteins and formed complexes with equal efficiency. They were also transported normally to the Golgi complex. Furthermore, a Vpu protein having the CD4 transmembrane and Vpu cytoplasmic domains was completely inactive, and Vpu proteins harboring hybrid Vpu-CD4 TM domains were also defective in the ability to enhance the release of VLPs. When tested for functional complementation in cotransfected cells, two inactive proteins were not able to reconstitute Vpu activity that enhances the release of Gag particles. Coexpression of functional CD4/Vpu hybrids or wild-type Vpu with inactive mutant CD4/Vpu proteins revealed that mutations in the VpuTM domain could dominantly interfere with Vpu activity in Gag release. Taken together, these results demonstrated that the structural integrity of the VpuTM domain is critical for Vpu activity in the release of VLPs from the plasma membrane of mammalian cells.
The vpu reading frame is unique to human immunodeficiency virus type 1 (HIV-1) with the exception of simian immunodeficiency virus SIVcpz, which could potentially encode a Vpu-like protein (11, 56). HIV-1 Vpu is organized in two distinct protein domains: the N-terminal 27-amino-acid (aa) hydrophobic domain and the C-terminal 54-aa hydrophilic domain. Each of the two domains regulates one of the cellular processes that appear to be critical in the HIV-1 life cycle (30). The N-terminal transmembrane domain of Vpu (VpuTM domain) directs the protein to the secretory pathway by way of inserting it in the endoplasmic reticulum (ER) membrane, and the cytoplasmic domain is phosphorylated by the ubiquitous casein kinase II at two conserved serine residues (Ser52 and Ser56) (50, 52, 57). The phosphorylation of Vpu is absolutely essential for sequence-specific degradation of CD4 in the ER (39, 50, 59, 61). The Vpu protein recognizes sequences in the cytoplasmic and/or transmembrane domains of CD4 to bind and form complexes with CD4. Such complex formation appears to signal the selective ER degradation of CD4 or proteins having the Vpu response elements (6, 8, 10, 30, 35, 39, 43, 45, 59, 62, 64, 68). Even though the role of CD4 down-regulation in the virus life cycle is not clearly understood, HIV-1 has been shown to down-regulate CD4 by multiple mechanisms (1, 3, 5, 9, 13, 14, 21, 31, 60, 65, 66).
The other function of Vpu is to enhance the release of HIV-1 particles, which occurs at the cell surface. HIV-1 mutants defective in Vpu expression are poorly released from infected cells, and the mutant virus particles are localized in intracellular vacuoles or as tethers on the infected cell surface (2, 19, 24, 33, 51, 53, 58, 69). Immunolocalization studies revealed that Vpu was present predominantly in the perinuclear region (ER-Golgi) of infected cells (33), and this localization pattern would be consistent with its activity in CD4 proteolysis. Experiments with brefeldin-A, a microphenolic fungal metabolite that inhibits the transport of proteins from the ER to the Golgi, suggested a requirement for Vpu to move beyond the ER (a post-Golgi compartment) to be effective in the release of virus particles (50). The movement of Vpu in the intracellular compartments of the secretory pathway is difficult to monitor, as this protein lacks diagnostic markers (e.g., glycosylation sites) for easy detection for transport patterns inside the cell. This problem is compounded by the lack of an extracellular domain in the Vpu protein as well. To circumvent these problems, we appended the CD4 extracellular domain to Vpu and analyzed the biological activities of the modified Vpu protein. We reported that such modifications of HIV-1 Vpu did not alter the Vpu activity that induced the ER degradation of CD4 or proteins bearing the Vpu-responsive element (43). Importantly, we could detect CD/Vpu hybrids on the cell surface of HeLa cells, and the hybrid proteins underwent endoglycosidase H (endo-H)-resistant modifications indicative of their movement through the Golgi complex (43). These analyses have revealed that the Vpu protein does not possess sequence information to sequester CD4 in the internal compartments of the cell and therefore is free to move to the cell surface.
Göttlinger et al. (24) reported that Vpu expression not only enhanced the release of infectious virions but also released noninfectious particles having uncleaved Gag precursor protein in them, suggesting that cleavage of Gag precursor is not a prerequisite for Vpu to engage in the release process. Moreover, these studies revealed that heterologous viruses (HIV-2, SIV, and Moloney murine leukemia virus) having different Gag molecules were released as efficiently as were HIV-1 particles. Some of these viruses encode Gag precursor proteins that lack the myristoylation signal. The Gag proteins of type C retroviruses move to the plasma membrane to catalyze the assembly of virus particles that are released as immature virions. The maturation of immature virions occurs sequentially by proteolytic cleavage of the Gag precursor by the virus-encoded protease. To assemble an infectious HIV virion, the full complement of viral proteins is required (22, 29, 67). The results of Göttlinger et al. (24) have thus demonstrated that the expression of Vpu has biologic consequences in the early phase of the budding process. Furthermore, the enhanced release of virus particles by Vpu occurs independent of the expression of HIV envelope glycoproteins (gp160) or CD4, the HIV receptor (23, 70). The glycoproteins of HIV-1 follow a well-defined secretory pathway for delivery to the plasma membrane and subsequent incorporation into budding virions (15, 16, 29). The transport of Gag to the assembly site is poorly defined, but biochemical studies have revealed that HIV-1 Gag precursor is necessary and sufficient to assemble virus-like particles that are released into the medium (22, 26, 29, 67). Mutational analysis of the Gag protein has demonstrated that both the myristoylation signal and basic amino acids at the N terminus of Gag are required for proper intracellular targeting, and some of the mutations have conferred assembly defects in HIV-1-infected cells (18, 25, 32, 42, 55, 63, 71–73).
Schubert et al. (52) have reported that the VpuTM domain contains sequence information for the enhanced release of virus particles and the Vpu cytoplasmic domain could modulate the activity of the VpuTM domain in the virus release process. Experiments with a scrambled VpuTM domain provided some insight into the importance of amino acid organization within the VpuTM domain, and viruses having randomized VpuTM domains were severely defective in promoting virus release at enhanced rates (52). Further studies provided evidence that HIV-1 Vpu is capable of forming cation-selective ion channels presumably at the plasma membrane (17, 49). Like Vpu-mediated CD4 proteolysis, the mechanisms by which the HIV-1 Vpu protein enhances the release of virus particles are not clearly understood.
To begin to address some of the mechanistic details of Vpu action in virus release, we focused in on the VpuTM domain. Mutational analysis has revealed that the structural integrity of the VpuTM domain is an important feature for efficient regulation of virus-like particle release from the plasma membrane of mammalian cells.
MATERIALS AND METHODS
Expression vectors.
The recombinant vaccinia virus vTF7-3 (20) was used to drive the expression of Gag, Vpu, and CD4/Vpu proteins in HeLa cells. The gag gene was derived from a dual-tropic HIV-1 isolate 89.6 as described previously (12). vTF7-3 synthesizes T7 RNA polymerase in the cytoplasm of infected cells to activate the genes under control of the T7 promoter in expression vectors as described previously (36, 43, 44).
Mutagenesis.
Table 1 lists the primers used to introduce mutations in the vpu gene by the method of Ho et al. (27).
TABLE 1.
Primers
| No. | Sequence | Name |
|---|---|---|
| 1 | 5′CATAACTTACGGTAAATGGC3′ | A |
| 2 | 5′TCTCTGTAGGTAGTTTGTCC3′ | D |
| 3 | 5′CAGCCAATGCAACCTGTAGCAATAGTAGCA3′ | VpuNΔ2-B |
| 4 | 5′TGCTACTATTGCTACAGGTTGCATTGGCTG3′ | VpuNΔ2-C |
| 5 | 5′CAGCCAATGCAACCTGCAATAGTAGCATTA3′ | VpuNΔ3-B |
| 6 | 5′TAATGCTACTATTGCAGGTTGCATTGGCTG3′ | VpuNΔ3-C |
| 7 | 5′GTGCAGCCAATGCAACCTATAGTAGCATTAGTAGTA3′ | VpuNΔ4-B |
| 8 | 5′TACTACTAATGCTACTATAGGTTGCATTGGCTGCAC3′ | VpuNΔ4-C |
| 9 | 5′GTGCAGCCAATGCAACCTGTAGCATTAGTAGTAGCA3′ | VpuNΔ5-B |
| 10 | 5′TGCTACTACTAATGCTACAGGTTGCATTGGCTGCAC3′ | VpuNΔ5-C |
| 11 | 5′GTGTGGTCCATAGTAGAATATAGGAAAATA3′ | VpuCΔ2-B |
| 12 | 5′TATTTTCCTATATTCTACTATGGACCACAC3′ | VpuCΔ2-C |
| 13 | 5′TGTTGTGTGGTCCATAGAATATAGGAAAATATTA3′ | VpuCΔ3-B |
| 14 | 5′TAATATTTTCCTATATTCTATGGACCACACAAC3′ | VpuCΔ3-C |
| 15 | 5′GCAATAGTTGTGTGGTCCGAATATAGGAAAATATTA3′ | VpuCΔ4-B |
| 16 | 5′TAATATTTTCCTATATTCGGACCACACAACTATTGC3′ | VpuCΔ4-C |
| 17 | 5′ATAGCAATAGTTGTGTGGGAATATAGGAAAATATTA3′ | VpuCΔ5-B |
| 18 | 5′TAATATTTTCCTATATTCCCACACAACTATTGCTAT3′ | VpuCΔ5-C |
| 19 | 5′ATAATAGCAATAGTTGTGTGGTCCATAGTAATCATAG3′ | Vpu-CNVC-B |
| 20 | 5′CACAACTATTGCTATTATGAGGCCGGCGACGCCCCCCAG3′ | Vpu-CNVC-C |
| 21 | 5′CTGCTTTTCATTGGGCTAGGCATCTTCTTCTGTGTC3′ | Vpu-VNCC-B |
| 22 | 5′TAGCCCAATGAAAAGCAGTATTGCTACTACTAATGCTAC3′ | Vpu-VNCC-C |
| 23 | 5′ATAGTAGCAAGAGTAGCATTAG3′ | VpuI8R-B |
| 24 | 5′TAATGCTACTCTTGCTACTAT3′ | VpuI8R-C |
| 25 | 5′GTAGTAGCAAGAATAATAGCA3′ | VpuI15R-B |
| 26 | 5′TGCTATTATTCTTGCTACTAC3′ | VpuI15R-C |
| 27 | 5′TTAGTAGTAGCAAGAATAAGAGCAATAGTT3′ | VpuI15, 17R-B |
| 28 | 5′ACCTATTGCTCTTATTCTTGCTACTACTAA3′ | VpuI15, 17R-C |
Primers A and D represent sequences (5′ and 3′ ends of the vpu gene) within the plasmid pCDNA1. Mutation primers denoted by suffixes B and C were combined with primers A and D, respectively, in pairwise combinations to generate AB and CD gene fragments that were used in the fusion reaction for the generation of desired mutations in the vpu gene. Briefly, the primers (250 nM each) were annealed to template DNA (100 ng) encoding Vpu to generate two DNA fragments (AB or CD) in PCRs using Pfu polymerase (Strategene, San Diego, Calif.). Gel-purified fragments (AB and CD) were combined in the fusion reaction to obtain full-length Vpu clones after digestion with EcoRI and XbaI. All PCRs were performed in 25 to 30 cycles, using a Thermal Cycler. The fusion products were digested with EcoRI and XbaI and cloned into pCDNA1.
Protein analysis.
Transfections of HeLa cells and immunoprecipitation of detergent lysates were carried out as described previously (39, 43). To analyze both extracellular and intracellular Gag proteins, we transfected HeLa cells with the Gag expression plasmid in the presence or absence of Vpu plasmids. At 16 h posttransfection, the cells were labeled with [35S]methionine for 10 min and chased in the presence of medium containing excess unlabeled amino acids. At the indicated times during the chase, media (1 ml of each) were withdrawn from the dishes and concentrated in Centricon-30 columns. The concentrates were solubilized in a radioimmunoprecipitation assay (RIPA) for immunoprecipitation assays. Both extracellular (medium) and intracellular (detergent cell lysates) were immunoprecipitated with anti-HIV sera. The Vpu and CD4/Vpu hybrid proteins were immunoprecipitated with anti-Vpu and anti-CD4 antibodies, respectively. After immunoprecipitations, proteins were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). CD4/Vpu-Vpu protein complexes were identified in digitonin lysates as described previously (39). Proteins were quantified with a PhosphorImager (Molecular Dynamics, Sunnyvale, Calif.).
RESULTS
The VpuTM domain plays an essential role in the enhanced release of HIV-1 Gag.
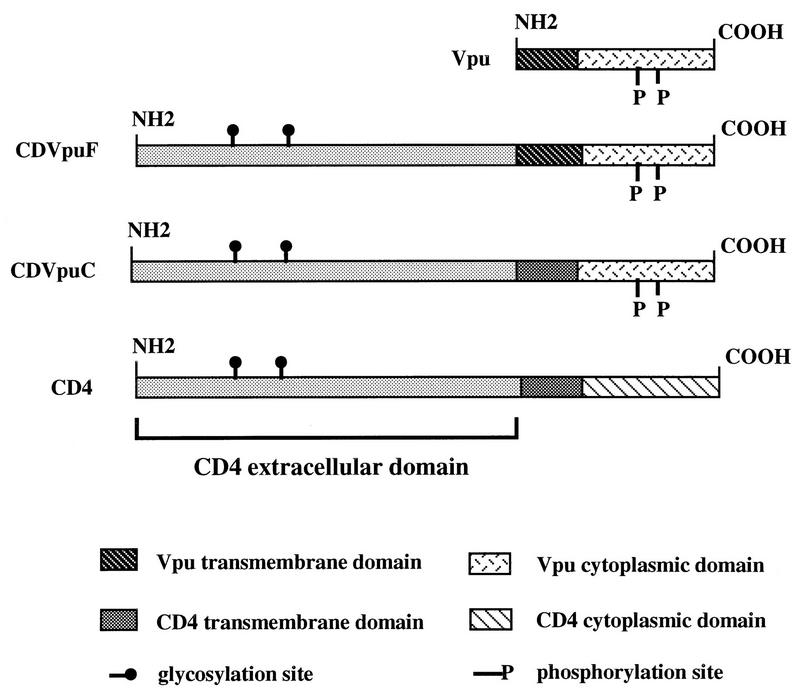
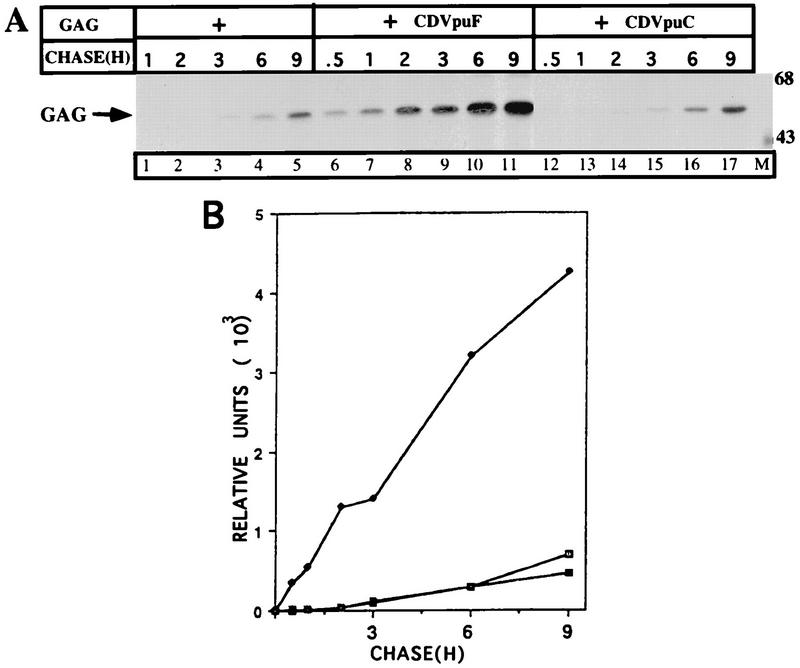
CDVpuF is a hybrid protein consisting of the CD4 extracellular domain the entire Vpu protein sequence at its C terminus, and CDVpuC is a derivative of CDVpuF that harbors the CD4 transmembrane domain in place of the Vpu counterpart (Fig. 1). Like Vpu, the CD4/Vpu hybrid proteins are phosphorylated when expressed in HeLa cells, and this phosphoryl modification has been shown to be essential to activate a pathway that lead to the proteolysis of CD4 in the ER (39, 43). To examine the functional activity of the VpuTM domain in CD4/Vpu hybrid proteins, we transfected cells with plasmids encoding Gag alone and in the presence of CD4/Vpu proteins. Figure 2 shows the kinetics of Gag release in the absence or presence of CD4/Vpu hybrid proteins. When expressed alone, the Gag protein was released from the cells, and accumulated in the extracellular medium with increasing chase times (lanes 1 to 5). Cotransfection of CDVpuF with Gag dramatically enhanced the release of Gag particles from the cell and after a chase of 1 h, the level of Gag in the medium reached the same level as that of Gag particles released from singly transfected cells. During the chase period, the Gag protein continued to be shed from the cell and accumulated for the entire period of 9 h (lanes 6 to 11). Cotransfection of CDVpuC with Gag did not enhance the release of Gag particles over the level observed in the absence of any CD/Vpu addition (lanes 12 to 17) CDVpuF and CDVpuC exhibit two profoundly distinct activities in cells expressing HIV-1 Gag. The only major difference between these proteins is the composition of their TM domains. CDVpuF has the VpuTM domain, whereas the CD4TM domain anchors the CDVpuC protein in the lipid bilayer (Fig. 1) (43). Thus, these experiments have demonstrated that the Vpu protein in the context of a hybrid configuration (CDVpuF) is active in enhancing the release of Gag particles from the plasma membrane of HeLa cells.
FIG. 1.
Construction and schematics of CD4/Vpu fusion proteins. CDVpuF has the CD4 extracellular domain and full-length Vpu at its C terminus. CDVpuC contains CD4 extracellular and TM domains and the cytoplasmic domain of Vpu.
FIG. 2.
The VpuTM domain plays an essential role in the enhanced release of HIV-1 Gag. (A) Plasmids (3 μg) encoding Gag were transfected with 3 μg of pCDNA (lanes 1 to 5) or cotransfected with those (3 μg of each) expressing CDVpuF (lanes 6 to 11) or CDVpuC (lanes 12 to 17). At 16 h posttransfection, cells were pulse-labeled for 15 min with [35S]methionine (200 μCi/ml) and chased at the indicated times in the medium containing unlabeled amino acids and 2.5% serum. At the end of chase, media were collected, concentrated in Centricon-30 columns as described previously (39), and immunoprecipitated with anti-HIV serum before being analyzed by SDS-PAGE (10% gel). Sizes are indicated in kilodaltons. (B) Extracellular levels of Gag (□), Gag plus CDVpuF (•), and Gag plus CDVpuC (⊡) were quantified in a PhosphorImager.
Construction and expression of CD4/Vpu proteins.
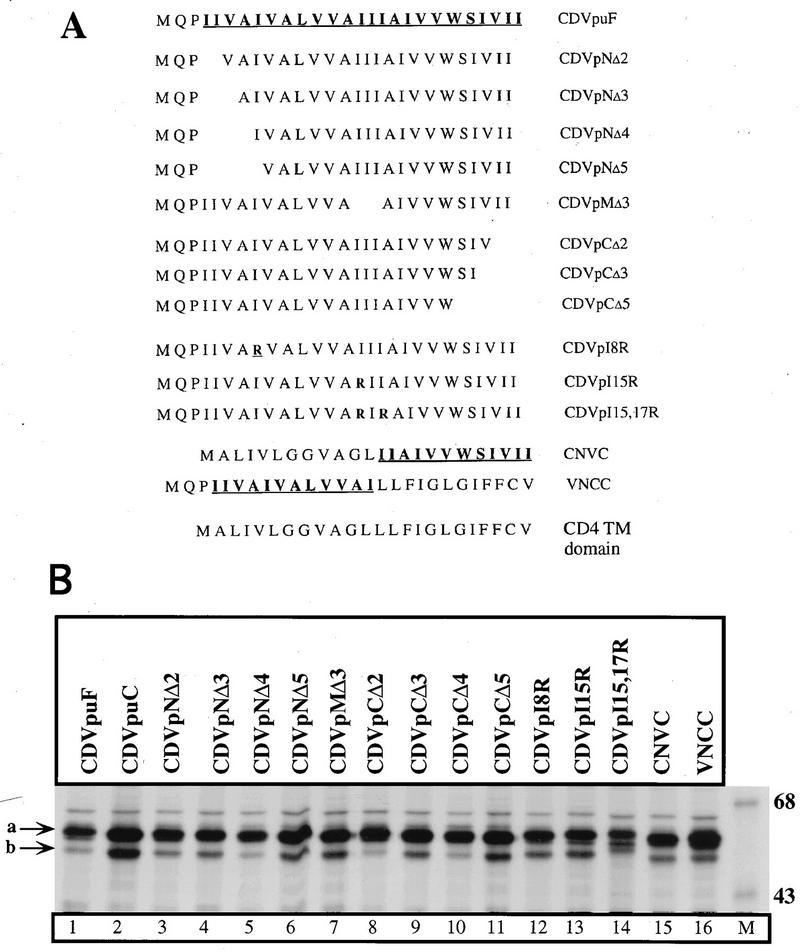
To determine the role of amino acids which are critical in Vpu-mediated Gag release process, we carried out mutational analysis of the Vpu and CD4/Vpu proteins. The genes encoding Vpu and CDVpuF were subject to in vitro mutagenesis as described previously (39, 43). Figure 3A shows the primary sequences of wild-type (wt) and mutant VpuTM domains in CD4/Vpu hybrid proteins. We first assessed the expression of parental and mutant CD4/Vpu proteins in HeLa cells by immunoprecipitation assays. Plasmids encoding CDVpuF and each of the mutant CD4/Vpu proteins were introduced into HeLa cells. Transfected cells were labeled with [35S]methionine for 1 h, and the cells were lysed in RIPA for immunoprecipitation with an anti-Vpu antibody. Figure 3B shows the expression of CD4/Vpu proteins. Each of the expression plasmids produced one major protein species (indicated by arrow a) that corresponds to the fully glycosylated CD4/Vpu protein. A minor species (indicated by arrow b) could represent the unglycosylated CD4/Vpu protein that failed to be translocated under these expression conditions. The wt and mutant CD4/Vpu proteins were synthesized with the relative molecular mass of 58 kDa (lanes 1 to 16). This molecular mass is consistent with each of the CD4/Vpu hybrid proteins having the entire CD4 extracellular domain and portions of the Vpu protein (76 to 81 aa). Importantly, the parental and mutant derivatives of CD4/Vpu showed comparable expression patterns, suggesting that mutation of the VpuTM domain did not affect the biogenesis of CD4/Vpu proteins in HeLa cells.
FIG. 3.
Construction and expression of CD4/Vpu proteins in HeLa cells. (A) Primary sequences of wt and mutant Vpu transmembrane domains in CD4/Vpu hybrid proteins. (B) Expression of CD4/Vpu proteins (lanes 1 to 16). Plasmids (3 μg of each) encoding CD4 VpuF and each of the mutant CD4/Vpu proteins were introduced into vTF7-3-infected HeLa cells. Transfected cells were labeled with [35S]methionine for 1 h and lysed by RIPA. Detergent lysates were immunoprecipitated with anti-Vpu antibody and resolved by SDS-PAGE (10% gel). Arrow a indicates fully glycosylated proteins, and arrow b represents partially glycosylated or unglycosylated CD4/Vpu proteins. Sizes are indicated in kilodaltons.
Mutations in the VpuTM domain inactivate Vpu activity in the enhanced release of Gag particles.
We examined the ability of some of the VpuTM mutants to enhance the release of Gag particles from transfected cells. Accordingly, plasmids encoding CDVpMΔ3, CDVpCΔ2, and CDVpNΔ2 were transfected with the Gag expression plasmid to assay for Gag release. Each of these mutants has a deletion of two (CDVpCΔ2 and CDVpNΔ2) or three (CDVpuMΔ3) amino acids in the VpuTM domain. As shown in Fig. 2, CDVpuF enhanced the release of Gag particles which accumulate in the extracellular medium during a chase of up to 9 h (Fig. 4). The expression of CDVpCΔ2, CDVpNΔ2, and CDVpMΔ3 with Gag did not provide any Vpu activity that was capable of enhancing the release of Gag particles from the cell surface (Fig. 4A and C). Two of the mutants (CDVpMΔ3 and CDVpNΔ2) appeared to have retained some activity in the Gag release process (Fig. 4A, lanes 5 to 8 and 13 to 16), but this activity was only slightly above the constitutive Gag release activity in cells expressing Gag alone (Fig. 2). The mutant protein, CDVpCΔ2, was highly defective in the release of Gag from the transfected cell (Fig. 4A, lanes 9 to 12). The introduction of single-point mutations within the VpuTM domain had moderate to profound effects (depending on the position of amino acids in the VpuTM domain) on the ability of HIV-1 Vpu to enhance the release of Gag particles from the cell surface (data not shown). These studies demonstrated that minor changes in the VpuTM domain have profound consequences in terms of the ability of the VpuTM domain to engage in the Gag release process.
FIG. 4.
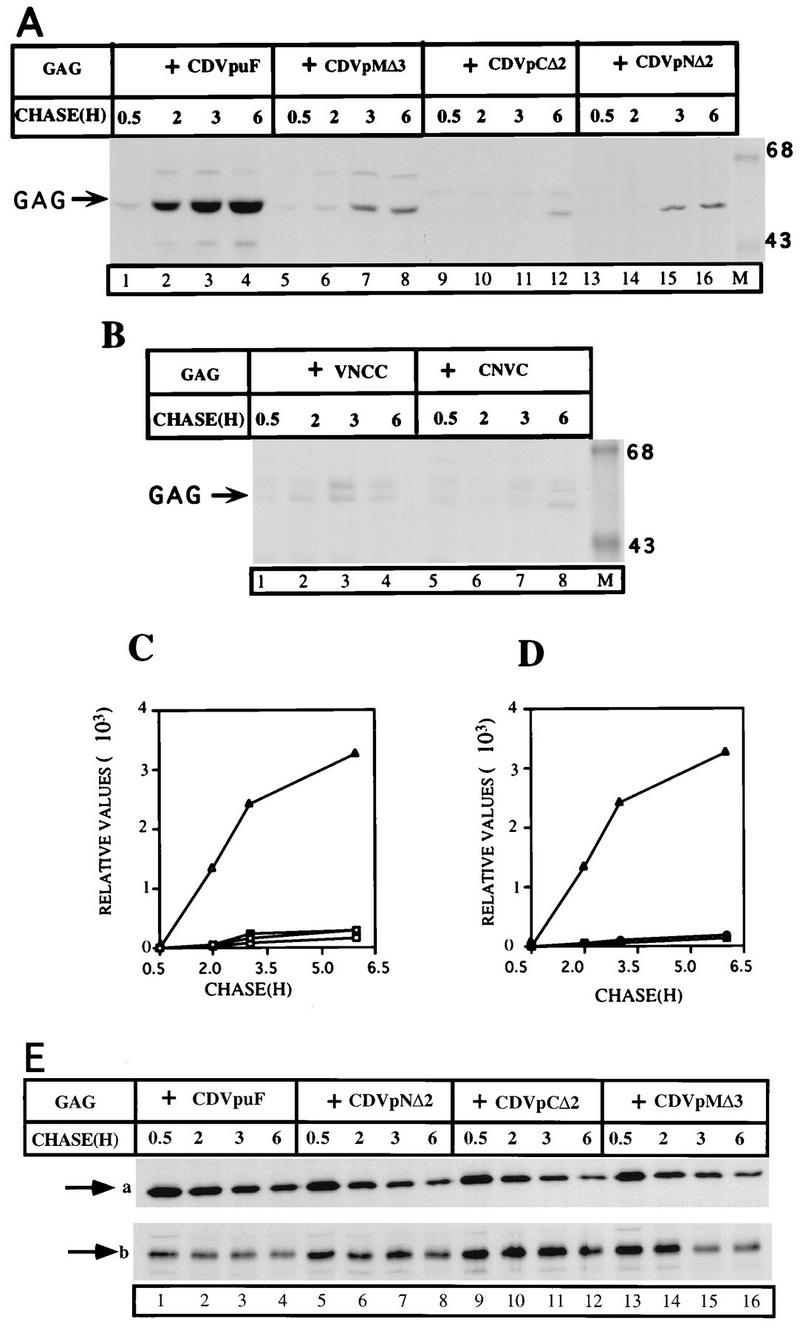
Mutations in the VpuTM domains of CD4/Vpu hybrid proteins inactivate the Vpu activity that enhances the release of Gag particles. (A and B) Plasmids (3 μg) encoding Gag were cotransfected with those (3 μg of each) expressing the parental CDVpuF protein or CD4/VpuF mutant hybrid proteins. The experimental protocol is the same as described for Fig. 2. (C and D) Gag proteins (C, Gag + CDVpuF [▵], Gag + CDVpMΔ3 [○], Gag + CDVpNΔ2 [○], and Gag + CDVpCΔ2 [□]; D, Gag + CDVpuF [▵], Gag + VNCC [○], and Gag + NCVC [○] were quantified by a PhosphorImager. (E) Intracellular levels of Gag and CD4/Vpu hybrid proteins. The transfected cells in A were lysed by RIPA, immunoprecipitated with anti-HIV (for Gag) or anti-CD4 (for CD/Vpu proteins) serum, and analyzed by SDS-PAGE (10% gel). Arrow a indicates the intracellular Gag proteins; arrow b corresponds to the CD4/Vpu proteins.
CDVpuF and CDVpuC showed opposing properties with respect to the Gag release phenotype (Fig. 2). CDVpuC bearing the CD4TM domain is completely inactive, whereas CDVpuF bearing the VpuTM domain is highly active, in the release of Gag particles. To test which half of the VpuTM domain would provide functional activity in the Gag release process, we made two CD4/Vpu proteins, CNVC and VNCC, which have hybrid VpuTM domains (Fig. 3). Expression of Gag with either VNCC or CNVC did not exhibit Vpu activity that enhances the release of Gag particles (Fig. 4B and D). These analyses provided additional evidence that VpuTM half domains (12 aa each of the N- and C-terminal ends of the VpuTM domain) in the contexts of corresponding CD4TM domains are not enough to reconstitute Gag release enhancing activity in HeLa cells.
The intracellular levels of Gag are largely unchanged in the presence of wt or mutant CD4/Vpu proteins.
We have shown convincingly that CDVpuF enhances the release of Gag at an accelerated rate from the cell surface of HeLa cells, but some of the VpuTM domain mutants were defective in this process (Fig. 4A to C). Since the amount of extracellular Gag is strictly dependent on intracellular Gag levels, we used a pulse-chase protocol to assess the synthesis and stability of Gag proteins made in cotransfected cells (Fig. 4E). The Gag protein was made during the 15-min pulse (indicated by arrow a), and the levels of intracellular Gag decreased with time during the chase period in cells expressing Gag and CDVpuF (lanes 1 to 4). This pattern of Gag expression was unchanged in cells expressing defective CD4/Vpu hybrid proteins, which failed to enhance the release of Gag (lanes 5 to 16). The possibility that some of the CD4/Vpu mutants could be unstable in the cell and thus would not have the opportunity to engage in the Gag release process was addressed. As shown in Fig. 4E, the expression levels of CD4/Vpu proteins are comparable in both active and inactive states (arrow b). We observed slight decreases in the intracellular levels of the CD4/Vpu proteins after 3 h of chase, but this would be unlikely to be the reason for defects in the ability of CD4/Vpu mutants in the Gag release process. The extent of the decrease in the level was also noticed for CDVpuF, which is the parental wt protein (lanes 1 to 4). These experiments clearly demonstrated that the defective phenotypes of the CD4/Vpu mutant proteins were due to their inherent biological properties only under the condition in which equivalent amounts of intracellular Gag and CD/Vpu proteins were maintained.
Vpu proteins traffic normally through the secretory pathway with and without Gag.
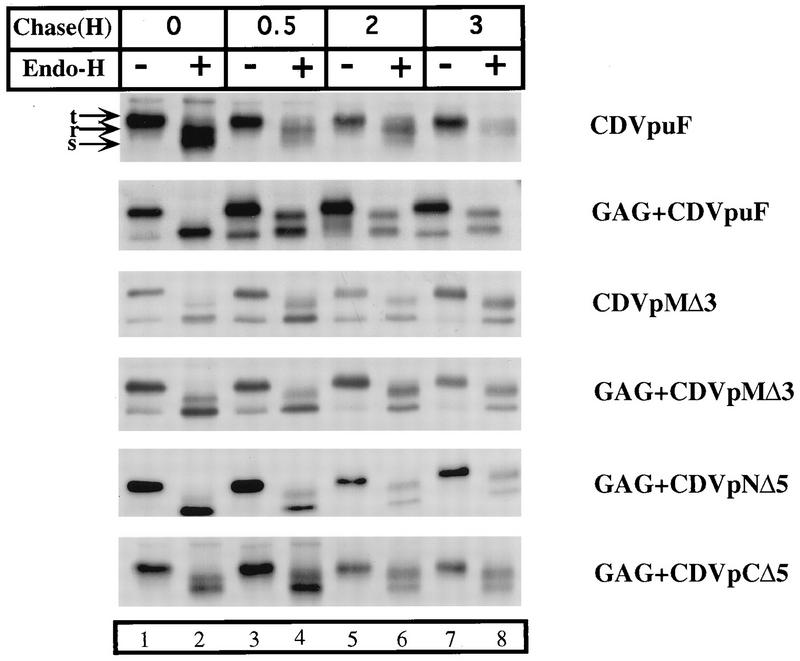
We have shown that the CD4/Vpu hybrid proteins CDVpuF and CDVpuC are delivered to the plasma membrane with kinetics similar to that of CD4 (43). However, these proteins have opposing biological properties in relation to the Gag release process. The VpuTM mutants tested in this study are all defective in enhancing the release of Gag particles. We reasoned that the normal trafficking of Vpu sequences to the cell surface could be essential for biological activity in the Gag release process. To test this possibility, we transfected cells with plasmids encoding the parental and each of the mutant derivatives of the CD4/Vpu proteins in the absence or presence of Gag. CDVpuF was transported to the Golgi complex undergoing characteristic endo-H-resistant modifications, and this pattern did not change in cells expressing Gag (Fig. 5). This is indicative of CDVpuF being properly delivered to the plasma membrane via the Golgi complex irrespective of its expression status (with or without Gag; top two panels). Importantly, the CD4/Vpu proteins that are defective in Gag release showed transport kinetics very similar to that of CDVpuF (bottom four panels). Thus, the Gag release defect exhibited by each of the Vpu mutants tested in this study was not due to any defects in intracellular trafficking profiles of the Vpu sequences in the secretory pathway.
FIG. 5.
CD4/Vpu proteins traffic normally through the secretory pathway with and without Gag. HeLa cells were transfected with plasmids (3 μg) expressing the parental hybrid protein, CDVpuF, and each mutant derivative of the CD4/Vpu protein in the absence or presence of Gag. Transfected cells expressing the CD4/Vpu proteins were pulse-labeled with [35S]methionine for 30 min and chased at the indicated times in the presence of unlabeled amino acids in medium containing 2.5% serum. Detergent lysates were made and immunoprecipitated with anti-CD4 serum. The immunoprecipitated proteins were divided into two portions; to one was added 5 U of endo-H (+), and the other was left untreated (−). Samples were incubated overnight at 37°C and analyzed by SDS-PAGE (10% gel). t, total proteins; r, endo-H-resistant proteins; s, endo-H-sensitive proteins.
Mutations in the TM domains of Vpu and CD4/Vpu have similar phenotypic consequences in the release of Gag.
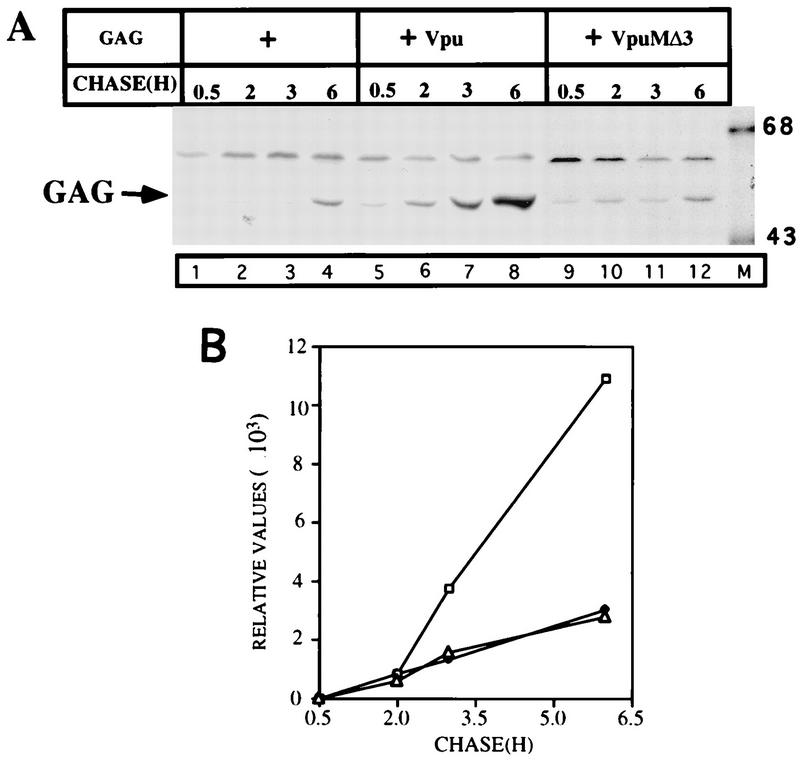
The majority of the experiments presented in this report were carried out in the CD4/Vpu hybrid context. This approach has been very fruitful in terms of correlating protein trafficking profiles with biological activities of Vpu sequences in Gag release. Even though we demonstrated that CDVpuF enhanced the extracellular release of Gag (Fig. 2), the question remained as to whether the biological properties of CDVpuF could be comparable to those of wt Vpu. To address this question, we introduced mutations in the TM domains of CD4VpuF and Vpu at identical positions (deletion of three isoleucines) and tested loss-of-function phenotypes of these mutants in the Gag release assay (Fig. 6). We showed above that CDVpMΔ3 having a deletion of three amino acids in the TM domain was highly defective in Gag release (Fig. 4A, lanes 5 to 8). A Vpu protein with the same mutations in its TM domain failed to enhance the release of Gag particles above the constitutive level (Fig. 6, lanes 9 to 12). Taken together, the defects exhibited by both CDVpMΔ3 and VpuMΔ3 strengthen the view that the molecular bases of Vpu action in both contexts (CD4/Vpu and Vpu) appear to be identical in nature. We have shown that CDVpMΔ3 is normally transported to the Golgi complex, and the transport kinetics of VpuMΔ3 would presumably mirror that of CDVpMΔ3. It is therefore unlikely that a defective intracellular transport phenotype would be the primary cause of VpuMΔ3 being not able to engage in the Gag release process.
FIG. 6.
Mutations in the TM domains of the Vpu and CD4/Vpu proteins confer similar phenotypes in the Gag release process. (A) Plasmid (3 μg) encoding Gag was transfected with 3 μg of either pCDNA or Vpu protein. The protocol used was as described in the legend to Fig. 2. (B) PhosphorImager quantification of Gag (○), Gag plus VpuMΔ3 (▵), and Gag plus wt Vpu (□) particles released into the medium.
Vpu forms complexes with both active and inactive CD4/Vpu hybrid proteins.
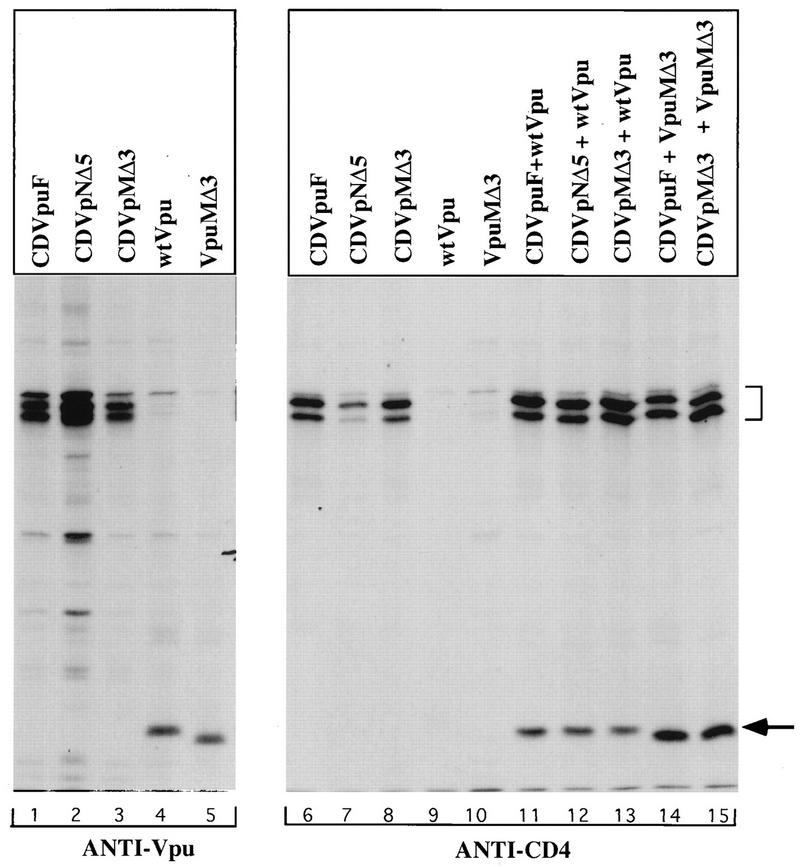
We have previously shown that the Vpu protein forms complexes with CDVpuF in HeLa cells and that the ability to form complexes does not depend on the phosphorylation state of either the Vpu or CD4/Vpu hybrid proteins (39). We hypothesized that the VpuTM domain could possess critical sequence elements that regulate the assembly process to reconstitute Vpu activity on the biological membrane. To test this possibility, we performed coimmunoprecipitation experiments as described previously (39). We used two antibodies (anti-Vpu and anti-CD4) in this assay. As expected, the Vpu antibody precipitated the Vpu and CD4/Vpu hybrid proteins (Fig. 7, lanes 1 to 5), and only the CD4/Vpu hybrid proteins were recovered from anti-CD4 precipitates (lanes 6 to 8). The failure to precipitate Vpu proteins strongly indicated that the antibody is not cross-reactive, and this property is useful in coprecipitation assays (lanes 9 and 10). Expression of wt Vpu with both active and inactive CD/Vpu mutants revealed that Vpu existed in a complex with either CDVpuF (lane 11) or two of the CD4/Vpu mutant proteins (lanes 12 and 13). Comparable levels of wt Vpu were recovered in all immunoprecipitates, strongly indicating that the inactivating mutations in the VpuTM domain have not interfered with Vpu assembly on the membrane. We did a reciprocal experiment in which we tested the mutant Vpu protein, VpuMΔ3, for the ability to form complexes with CDVpuF. VpuMΔ3 formed complexes not only with CDVpuF but also with CDVpMΔ3, a hybrid protein that carries the exact deletion as in VpuMΔ3 (lanes 14 and 15). These studies clearly demonstrate that deletions within the VpuTM domain have not disrupted the ability of mutant proteins to assemble as oligomeric complexes in the cell.
FIG. 7.
VpuTM domain mutants have not lost the ability to oligomerize and form hetero-oligomeric protein complexes: Plasmid (3 μg) encoding CD4VpuF, CDVpNΔ5, or CDVpMΔ3 was transfected into vTF7-3-infected HeLa cells alone and in combination with 3 μg of plasmid expressing wt Vpu and VpuMΔ3. Both singly transfected (lanes 1 to 10) and cotransfected (lanes 11 to 15) cells were lysed in digitonin buffer before immunoprecipitations with appropriate antibodies (anti-Vpu and anti-CD4). The proteins were resolved on an SDS–12% polyacrylamide gel. The arrow indicates wt Vpu and VpuMΔ3, and the bracket denotes CD4/Vpu hybrids (CD4VpuF, CDVpNΔ5, and CDVpMΔ3).
VpuTM domain mutations failed to functionally complement Vpu activity that enhances the release of Gag particles.
As shown in Fig. 7, CDVpNΔ5 was able to assemble in an oligomeric complex with wt Vpu, suggesting that this mutant protein had not lost the ability to oligomerize in the cell. We observed the same phenotype for CDVpCΔ5 (data not shown). To test for functional complementation in Gag release, we set up an experiment in which two inactive VpuTM domain mutants would be expressed at equimolar amounts in the same cell. Figure 8 illustrates such an analysis. The proteins CDVpNΔ5 and CDVpCΔ5 were defective in enhancing the release of Gag particles when they were expressed alone, and in fact extracellular Gag levels were less than the level produced constitutively in cells expressing Gag (Fig. 8A, lanes 5 to 12). This experiment suggests that mutations within the VpuTM domain failed to reconstitute Vpu activity in the cell. Importantly, when these mutants were transfected together into the same cell, we did not observe any Vpu activity that enhances Gag release, indicating that the mutants proteins, CDVpNΔ5 and CDVpCΔ5, do not have the ability to correct each other’s defects in the Gag release process.
FIG. 8.
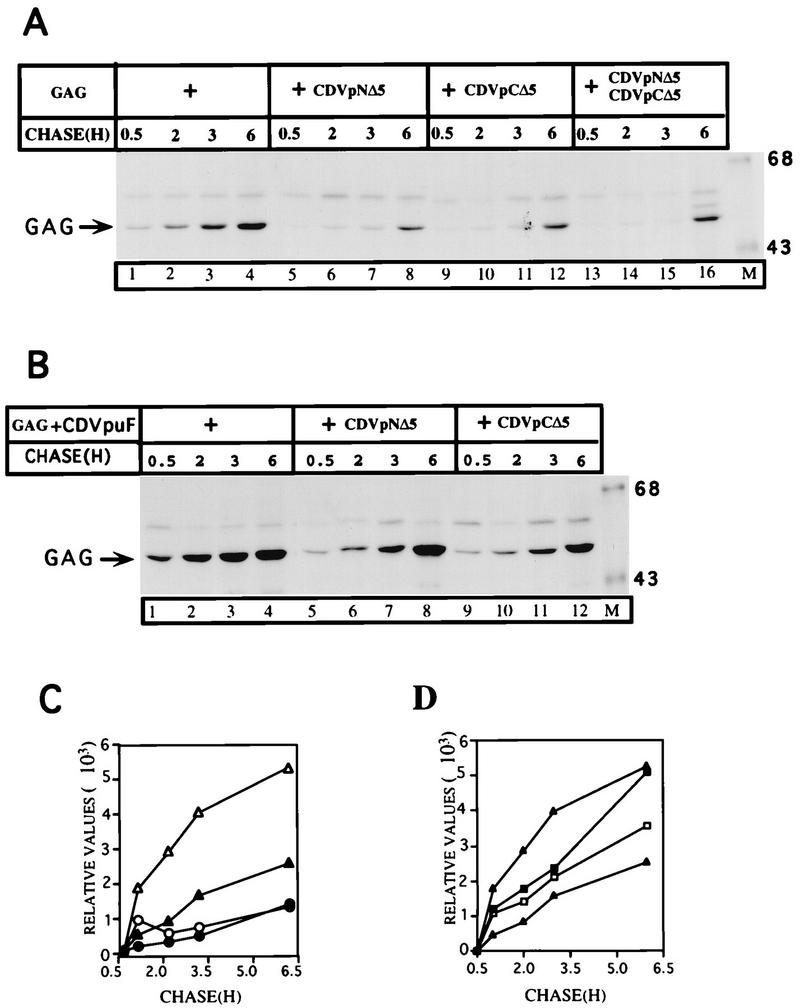
VpuTM domain deletion mutants failed to functionally complement and to reconstitute Vpu activity in the enhanced release of Gag particles. (A) HeLa cells were transfected with plasmids (3 μg of each) expressing Gag alone and coexpressed with those coding for the mutant hybrid proteins, CDVpNΔ5 and CDVpCΔ5 (lanes 5 to 16). To control for equivalent amounts of plasmid DNA in all transfections, the empty vector pcDNA1 was added to cells at appropriate amounts (for single Gag transfections, 3 μg of the Gag plasmid with 6 μg of pcDNA1; for cotransfections, 3 μg of pcDNA with 3 μg of each of the desired plasmids; for triple transfections, no empty vector and 3 μg of each of all three desired plasmids). The extracellular Gag was analyzed by SDS-PAGE (10% gel). Sizes are indicated in kilodaltons. (B) The VpuTM domain mutants partially interfere with Vpu activity that enhances the release of Gag particles. The protocol used was the same as for panel A. (C and D) Extracellular Gag proteins (Gag + CDVpuF [▵]; Gag [▴]; Gag + CDVpNΔ5 [○]; Gag + CDVpNΔ5 + CDVpCΔ5 [•], Gag + CDVpuF + CDVpCΔ5 [▪]; Gag + CDVpuF + CDVpNΔ5 [□] were quantified in a PhosphorImager.
Mutations in the VpuTM domain partially interfere with Vpu activity that enhances the release of Gag particles.
Mutant oligomeric proteins could acquire the ability to dominantly interfere with the wt by forming mixed oligomers. The results of the experiments in Fig. 7 indicated that CDVpNΔ5 was able to assemble and form hetero-oligomeric complexes with Vpu. We wanted to test whether such assembly properties could result in the attenuation of Vpu activity. To examine this possibility, we transfected HeLa cells with plasmids encoding CDVpuF and Gag with or without either of the mutant CD4/Vpu hybrid proteins. In another set of transfections, the plasmid encoding CDVpNΔ5 or CDVpCΔ5 was introduced into cotransfected cells. As expected, CDVpuF enhanced the release of Gag particles at an accelerated rate (Fig. 8B, lanes 1 to 4). However, the expression of CDVpNΔ5 or CDVpCΔ5 with CDVpuF and Gag had differential effects in terms of the ability of CDVpuF to participate in Gag release. In cells expressing CDVpuF and Gag, the kinetics of Gag release was rather rapid, and Gag particles began to accumulate in the extracellular medium after 30 min of chase. The Gag particles continued to be shed from the cell surface up to 6 h of chase (Fig. 8B, lanes 1 to 4). When CDVpuF and Gag were expressed with CDVpNΔ5 (lanes 5 to 8) or CDVpCΔ5 (lanes 9 to 12), the initial kinetics of Gag particle shedding was less pronounced (lanes 5 to 7 and 9 to 11), but after 6 h of chase, significant amounts of Gag accumulated in the medium (lanes 8 and 12). Interestingly, the expression of CDVpuF and Gag with CDVpCΔ5 appears to dampen the release of Gag to a greater extent than that exhibited by CDVpNΔ5. In experiments in which wtVpu was used instead of CDVpuF, we obtained similar phenotypes for both CDVpNΔ5 and CDVpCΔ5 (data not shown). Taken together, these results demonstrated that the activity of Vpu could be attenuated when expressed with Vpu mutants bearing deletions in their TM domains. This partial attenuation of Vpu activity appears to be due to the formation of hetero-oligomeric complexes between wt and mutant proteins.
DISCUSSION
In this study, we have elucidated the role of the VpuTM domain in the Gag release process. Mutational analysis revealed that the removal of hydrophobic amino acids from both ends (N and C terminal) or the middle of the domain generated proteins that are highly defective to enhance the release of virus-like Gag particles. The assembly and budding of HIV particles occur at the plasma membrane. We have provided evidence that mutations in the VpuTM domain did not have any discernible effects on Vpu movement through the secretory pathway. Mutant Vpu proteins were transported to the Golgi complex but were unable to engage in reactions that enhance the release of Gag particles. Furthermore, experiments with CD4/Vpu proteins bearing hybrid TM domains have also revealed that intracellular movement and Vpu activity are the two separable properties of the Vpu protein. Properly delivered mutant Vpu proteins in the secretory pathway have failed to exhibit Vpu activity that has the ability to enhance Gag release from the cell. Vpu has been shown to be assembled as an oligomeric protein, and Vpu oligomerization could be critical for virus release processes. Using an in vivo assembly assay, we provided evidence that mutant Vpu proteins were able to form specific complexes with wt or mutant Vpu proteins, suggesting that Vpu oligomerization per se might not be the sole determinant that regulates Vpu activity in the Gag release process. We suggest that the structural integrity of the VpuTM domain is a paramount factor that reconstitutes Vpu activity that enhances the release of virus-like Gag particles from the plasma membrane.
The Vpu protein is synthesized from the bicistronic mRNA as a transmembrane phosphoprotein of 16 kDa and the vpu open reading frame is conserved in the majority of primary HIV-1 isolates (11, 54, 56). The N-terminal hydrophobic domain of Vpu appears to serve two functions in the biogenesis of Vpu in the mammalian cells: to translocate and to anchor the protein to the appropriate cell membranes. Type 1 transmembrane proteins (e.g., CD4 and gp160) have physically separable domains for each of the two functions. The N-terminal signal sequence serves only to translocate proteins to the ER before being cleaved off in the ER lumen by signal peptidase, and the C-terminal hydrophobic region serves as a stop-transfer sequence to anchor the protein in the ER membrane. In CD4/Vpu, the normally N-terminal end of Vpu has been transposed to the C terminus, which serves only the anchor function in the CD4/Vpu context (43). We have demonstrated that the Vpu protein in CD4/Vpu hybrids is biologically active in inducing the degradation of Vpu-sensitive proteins in the ER, and this activity of Vpu is strictly dependent on phosphorylation of the Vpu cytoplasmic domain at Ser52 and Ser56 in both Vpu and CD4/Vpu (39, 43, 50). In the present study, we have shown that the Vpu protein at the C terminus of CDVpuF and the parental wt Vpu are equally active in enhancing the release of virus-like Gag particles from the plasma membrane. It is interesting that Nef, a peripheral membrane protein of HIV-1, was shown to be active in CD4 down-regulation and T-cell activation even when it was expressed as the CD8/Nef or CD4/Nef hybrid transmembrane proteins (1, 3).
The vpu reading frame is unique to the HIV-1 genome (11, 56). The biological activities of HIV-1 Vpu are rather diverse and point to critical functions in the virus life cycle. Recent studies of a subset of HIV-2 isolates have demonstrated that the glycoproteins of HIV-2 appear to possess Vpu-like activity that enhances the release of HIV-2 virus particles (4, 7, 46). This activity of the HIV-2 viral glycoprotein maps to its transmembrane segment (4, 46). HIV glycoproteins are very complex having multiple oligosaccharide sites and also assemble as oligomeric proteins in the ER (16). In this study, we converted a simple transmembrane protein (Vpu) into a rather complex glycoprotein which undergoes glycosylation in the secretory pathway. Such modifications have not altered the biological activities of the Vpu protein in both CD4 proteolysis and Gag release (43) (Fig. 2). The Vpu-like activity of the HIV-2 envelope glycoprotein has the ability to enhance only the release of virus particles without having any apparent activity that induces the degradation of CD4 in the ER (4, 7, 46). However, the HIV-1 Vpu protein possesses both activities in a single polypeptide but in two separate modular protein domains. Preservation of the membrane topology of Vpu in the HIV-1 genome could have some biological relevance that cannot be adequately addressed by using minimal experimental systems such as ours. HIV-1 Vpu is coordinately synthesized with gp160 and appears to regulate gp160 trafficking in the infected cell (54, 66). It is likely that the requirements for gp160 processing in the maturation processes of HIV-1 and HIV-2 are quite distinct and that both viruses devised strategies appropriate for a particular virus life cycle. The Vpu activity that enhances the release of virus particles is rather promiscuous in that this activity does not discriminate viruses on the basis of their Gag polyproteins (4, 24). This property of Vpu lends credence to the notion that the assembly processes of a majority of retroviruses are dependent on a common but poorly understood intracellular pathway in mammalian cells.
HIV-1 Vpu is structurally related to the influenza virus M2 protein, which is a bona fide prototype ion channel protein (34, 41). The ion channel activity of M2 protein maps to its TM domain and has roles in the early and late stages of the influenza virus life cycle (28, 41). M2 is a tetrameric protein, and the ion channel activity of M2 depends on its ability to assemble as a tetramer in the cell (48). Furthermore, recent experiments suggest that M2 expression induces a secretion block at the Golgi stage of the secretory pathway, and M2 ion channel activity appears to be critical in this process (47). Interestingly, the HIV-1 Vpu protein has also been shown to induce ER accumulation of a subset of proteins that traverse the secretory pathway, and the phosphorylation of Vpu appears to regulate this Vpu activity (39, 61). Both in vitro and in vivo data suggest that the HIV-1 Vpu protein forms high-order structures, and the nature of these protein structures is not known (37, 39). If HIV-1 Vpu forms ion channels, it could presumably exist as an oligomeric protein on the membrane. Recent reports have demonstrated that the Vpu protein indeed has the ability to form cation-selective ion channels in biological membranes (17, 49). This ion channel activity of Vpu appears to be responsible for inducing the enhanced release of virus particles from the cell surface (49). Some of our VpuTM domain mutants could be defective in the putative channel activity that participates in reactions that lead to the release of virus-like Gag particles at enhanced rates from the plasma membrane. However, none of the VpuTM domain mutants were defective in the ability to form hetero-oligomeric complexes and therefore had not lost the ability to assemble as oligomeric proteins on the membrane (Fig. 7), but all of them were defective in enhancing the release of Gag particles into the extracellular medium. Thus, mutations in the VpuTM domain could have interfered with the ability of the Vpu protein to form presumptive ion channels that are capable of extruding Gag (virus) particles from the cell surface. Ion channels are compared to enzymes, which catalyze biochemical reactions with high specificity and speed (38). The VpuTM domain mutations have perhaps disrupted some of the structural elements that constitute an active ion channel activity on the membrane.
We have provided evidence that the VpuTM domain plays a critical role in the Gag release process. By extension, the VpuTM domain mutants would also be defective in the release of virus particles when the corresponding mutations are incorporated into the HIV-1 genome. The question still remains as to the role of the Vpu cytoplasmic domain in the release of Gag particles. Schubert et al. (52) reported that a mutant virus with deletions in the Vpu cytoplasmic domain was highly attenuated in its ability to enhance the release of virus particles, and this study was a clear indication that the Vpu cytoplasmic domain could provide regulatory functions in Vpu-mediated virus release. Mutations in the two phosphoacceptor sites of the Vpu cytoplasmic domain reduced the efficiency with which virus or Gag particles are released, suggesting a direct role for the domain in the virus assembly and release processes (40, 50). The Vpu cytoplasmic domain contains α-helical regions that are important for protein-protein interactions and mutations in the α-helical regions have prevented Vpu from binding to CD4 (59). The Gag protein is the primary machine that drives the process of virus assembly and budding in infected cells (22, 29, 67). The mechanisms by which Gag catalyzes the assembly of virus-like particles or the pathway of Gag delivery in the cell are not clearly understood. It is possible that the Vpu cytoplasmic domain can provide signals for Gag association and cotransport with Vpu to the plasma membrane from which virus particles are released at an enhanced rate through the action of the VpuTM domain. In addition to N-myristoylation, basic amino acids in the N-terminal region of Gag and in turn of MA-p17 appear to regulate membrane binding and Gag transport in the intracellular compartments of mammalian cells (18, 25, 32, 42, 55, 65, 71–73). We have shown that the Vpu protein is transported to the plasma membrane via membrane vesicles that bud from distinct compartments (ER and Golgi) of the secretory pathway (43). Vpu expression could thus potentially enhance membrane trafficking of the Gag proteins that would ultimately result in high concentrations of Gag at the sites of virus assembly. Future studies would clarify the molecular mechanisms of Vpu function in virus assembly and release.
ACKNOWLEDGMENTS
We thank the AIDS Research and Reagent Program, Division of AIDS, NIAID, NIH, for HIV serum and Vpu antibody (K. Strebel and K. Maldarelli) and CD4 (T4-4) antibody (R. Sweet). We thank Jim Lang for photographic assistance.
This work was supported by the Lerner Research Institute, Cleveland Clinic Foundation, through a seed support program (to M.A.J.) in the Department of Molecular Biology.
REFERENCES
- 1.Aiken C, Konner J, Landau N R, Lenburg M E, Trono D. Nef induces CD4 endocytosis: requirement for a critical di leucine motif in the membrane-proximal CD4 cytoplasmic domain. Cell. 1994;76:853–864. doi: 10.1016/0092-8674(94)90360-3. [DOI] [PubMed] [Google Scholar]
- 2.Balleit J W, Kolson D L, Eiger G, Kim F M, McGann K A, Srinivasan A, Collman R. Distinct effects in primary macrophages and lymphocytes of the human immunodeficiency virus type 1 accessory genes vpr, vpu, and nef: mutational analysis of a primary isolate. Virology. 1994;200:623–623. doi: 10.1006/viro.1994.1225. [DOI] [PubMed] [Google Scholar]
- 3.Baur A S, Sawai E T, Dazin P, Fantl W J, Chen-Mayer C, Peterlin B M. HIV-1 Nef leads to inhibition or activation of T cells depending on its intracellular localization. Immunity. 1994;1:373–384. doi: 10.1016/1074-7613(94)90068-x. [DOI] [PubMed] [Google Scholar]
- 4.Bour S, Strebel K. The human immunodeficiency virus (HIV) type 2 envelope glycoprotein is a functional complement of to HIV-1 Vpu that enhances particle release of heterologous retroviruses. J Virol. 1996;70:8285–8300. doi: 10.1128/jvi.70.12.8285-8300.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bour S, Geleziunas R, Wainberg M A. The human immunodeficiency virus type 1 (HIV-1) CD4 receptor and its central role in promotion of HIV-1 infection. Microbiol Rev. 1995;59:63–93. doi: 10.1128/mr.59.1.63-93.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bour S, Schubert U, Strebel K. The human immunodeficiency virus type 1 Vpu protein specifically binds to the cytoplasmic domain of CD4: implications for the mechanism of degradation. J Virol. 1995;69:1510–1520. doi: 10.1128/jvi.69.3.1510-1520.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bour S, Schubert U, Peden K, Strebel K. The envelope glycoprotein of human immunodeficiency virus type 2 enhances viral particle release: a Vpu-like factor? J Virol. 1996;70:820–829. doi: 10.1128/jvi.70.2.820-829.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buonocore L, Turi T G, Crise B, Rose J K. Stimulation of heterologous protein degradation by the Vpu protein of HIV-1 requires the transmembrane and cytoplasmic domains of CD4. Virology. 1994;204:482–486. doi: 10.1006/viro.1994.1560. [DOI] [PubMed] [Google Scholar]
- 9.Chen B K, Gandhi R T, Baltimore D. CD4 down-modulation during infection of human T cells with human immunodeficiency virus type 1 involves independent activities of vpu, env, and nef. J Virol. 1996;70:6044–6053. doi: 10.1128/jvi.70.9.6044-6053.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen M-T, Malderelli F, Karczewski M K, Willey R L, Strebel K. Human immunodeficiency virus type 1 Vpu protein induces degradation of CD4 in vitro: the cytoplasmic domain of CD4 contributes to Vpu sensitivity. J Virol. 1993;67:3877–3884. doi: 10.1128/jvi.67.7.3877-3884.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen E A, Terwilliger E F, Sodroski J G, Haseltine W A. Identification of a protein encoded by the vpu gene of HIV-1. Nature (London) 1988;334:532–534. doi: 10.1038/334532a0. [DOI] [PubMed] [Google Scholar]
- 12.Collman R, Balleit J W, Gregory S A, Friedman H, Kolson D L, Nathanson N, Srinivasan A. An infectious molecular clone of an unusual macrophage-tropic and highly cytopathic strain of human immunodeficiency virus type 1. J Virol. 1992;66:7517–7521. doi: 10.1128/jvi.66.12.7517-7521.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crise B, Buonocore L, Rose J K. CD4 is retained in the endoplasmic reticulum by the human immunodeficiency virus envelope glycoprotein precursor. J Virol. 1990;64:5585–5593. doi: 10.1128/jvi.64.11.5585-5593.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cullen B R. The role of Nef in the replication cycle of the human and simian immunodeficiency viruses. Virology. 1994;205:1–6. doi: 10.1006/viro.1994.1613. [DOI] [PubMed] [Google Scholar]
- 15.Doms R W, Lamb R A, Rose J K, Helenius A. Folding and assembly of viral membrane proteins. Virology. 1993;193:545–562. doi: 10.1006/viro.1993.1164. [DOI] [PubMed] [Google Scholar]
- 16.Earl P L, Moss B, Doms R W. Folding, interaction with GRP78-BiP, assembly, and transport of the human immunodeficiency virus type 1 envelope protein. J Virol. 1991;65:2047–2055. doi: 10.1128/jvi.65.4.2047-2055.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ewart G D, Sutherland T, Gage P W, Cox G B. The Vpu protein of human immunodeficiency virus type forms cation-selective ion channels. J Virol. 1996;70:7108–7115. doi: 10.1128/jvi.70.10.7108-7115.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Facke M, Janetzko A, Shoeman R L, Kräusslich H G. A large deletion in the matrix domain of the human immunodeficiency virus gag gene redirects virus particle assembly from the plasma membrane to the endoplasmic reticulum. J Virol. 1993;67:4972–4980. doi: 10.1128/jvi.67.8.4972-4980.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Friborg J, Ladha Z, Göttlinger H, Haseltine W A, Cohen E A. Functional analysis of the phosphorylation sites on the human immunodeficiency virus type 1 Vpu protein. J Acquired Immune Defic Syndr Hum Retrovirol. 1995;8:10–22. [PubMed] [Google Scholar]
- 20.Fuerst T R, Earl P L, Moss B. Use of a hybrid virus-T7 RNA polymerase system for expression of target genes. Mol Cell Biol. 1987;7:2538–2544. doi: 10.1128/mcb.7.7.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garcia R J, Miller A D. Serine phosphorylation-independent down-regulation of cell surface CD4 by nef. Nature (London) 1991;350:508–511. doi: 10.1038/350508a0. [DOI] [PubMed] [Google Scholar]
- 22.Gelderblom H R. Assembly and morphology of HIV: potential effect of structure on viral function. AIDS. 1991;5:617–637. [PubMed] [Google Scholar]
- 23.Geraghty R J, Panganiban A T. Human immunodeficiency virus type 1 Vpu has a CD4− and an envelope glycoprotein-independent function. J Virol. 1993;67:4190–4194. doi: 10.1128/jvi.67.7.4190-4194.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Göttlinger H G, Dorfman T, Cohen E A, Haseltine W A. Vpu protein of human immunodeficiency virus type 1 enhances the release of capsids produced by gag gene constructs of widely divergent retroviruses. Proc Natl Acad Sci USA. 1993;90:7381–7385. doi: 10.1073/pnas.90.15.7381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Göttlinger H G, Dorfman T, Sodroski J G, Haseltine W A. Role of capsid precursor processing and myristoylation in morphogenesis and infectivity of human immunodeficiency virus type 1. Proc Natl Acad Sci USA. 1989;86:5781–5785. doi: 10.1073/pnas.86.15.5781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haffar O, Garrigues J, Travis B, Moran P, Zarling J, Hu S L. Human immunodeficiency virus-like, nonreplicating, gag-env particles assemble in a recombinant vaccinia virus expression system. J Virol. 1990;64:2653–2659. doi: 10.1128/jvi.64.6.2653-2659.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ho S N, Hunt H D, Horton R M, Pullen J K, Pease L R. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989;77:51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- 28.Holsinger L J, Nichani D, Pinto L H, Lamb R A. Influenza A virus M2 ion channel protein: a structure-function analysis. J Virol. 1994;68:1551–1563. doi: 10.1128/jvi.68.3.1551-1563.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hunter E. Macromolecular interactions in the assembly of HIV and other retroviruses. Semin Virol. 1994;5:71–83. [Google Scholar]
- 30.Jabbar M A. The human immunodeficiency virus type 1 Vpu protein: roles in virus release and CD4 down-modulation. Curr Top Microbiol Immunol. 1995;193:107–120. doi: 10.1007/978-3-642-78929-8_6. [DOI] [PubMed] [Google Scholar]
- 31.Jabbar M A, Nayak D P. Intracellular interaction of human immunodeficiency virus type 1 (ARV-2) envelope glycoprotein gp160 with CD4 blocks the movement and maturation of CD4 to the plasma membrane. J Virol. 1990;64:6297–6304. doi: 10.1128/jvi.64.12.6297-6304.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaplan A H, Swanstrom R. Human immunodeficiency virus type 1 Gag proteins are processed in two cellular compartments. Proc Natl Acad Sci USA. 1991;88:4528–4532. doi: 10.1073/pnas.88.10.4528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klimkait T, Strebel K, Hoggan M D, Martin M A, Orenstein J M. The human immunodeficiency virus type 1-specific protein Vpu is required for efficient virus maturation and release. J Virol. 1990;64:621–629. doi: 10.1128/jvi.64.2.621-629.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lamb R A, Pinto L H. Do Vpu and Vpr of human immunodeficiency virus type 1 and of influenza B virus have ion channel activities in the virus life cycles? Virology. 1997;229:1–11. doi: 10.1006/viro.1997.8451. [DOI] [PubMed] [Google Scholar]
- 35.Lenburg M E, Landau N R. Vpu-induced degradation of CD4: requirement for specific amino acid residues in the cytoplasmic domain of CD4. J Virol. 1993;67:7238–7245. doi: 10.1128/jvi.67.12.7238-7245.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mahalingam S, Khan S A, Jabbar M A, Monken C, Collman R, Srinivasan A. Identification of residues in the N-terminal acidic domain of HIV-1 Vpr essential for virion incorporation. Virology. 1995;207:297–302. doi: 10.1006/viro.1995.1081. [DOI] [PubMed] [Google Scholar]
- 37.Maldarelli F, Chen M-Y, Willey R L, Strebel K. Human immunodeficiency virus type 1 Vpu protein is an oligomeric type 1 integral membrane protein. J Virol. 1993;67:5056–5061. doi: 10.1128/jvi.67.8.5056-5061.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marban E, Tomaselli G E. Ion channels as enzymes: analogy or homology. Trends Neurosci. 1997;20:144–147. doi: 10.1016/s0166-2236(96)01008-9. [DOI] [PubMed] [Google Scholar]
- 39.Paul M, Jabbar M A. Phosphorylation of both phosphoacceptor sites in the HIV-1 Vpu cytoplasmic domain is essential for Vpu-mediated ER degradation of CD4. Virology. 1997;232:207–216. doi: 10.1006/viro.1997.8541. [DOI] [PubMed] [Google Scholar]
- 40.Paul, M., and M. A. Jabbar. Unpublished observations.
- 41.Pinto L H, Holsinger L J, Lamb R A. Influenza virus M2 protein has ion channel activity. Cell. 1992;69:517–528. doi: 10.1016/0092-8674(92)90452-i. [DOI] [PubMed] [Google Scholar]
- 42.Platt E J, Haffar O K. Characterization of human immunodeficiency virus type 1 Pr55gag membrane association in a cell-free system: requirement for a C-terminal domain. Proc Natl Acad Sci USA. 1994;91:4594–4598. doi: 10.1073/pnas.91.10.4594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Raja N U, Jabbar M A. The human immunodeficiency virus type 1 Vpu protein tethered to the CD4 extracellular domain is localized to the plasma membrane and is biologically active in the secretory pathway of mammalian cells: implications for the mechanisms of Vpu function. Virology. 1996;220:141–151. doi: 10.1006/viro.1996.0294. [DOI] [PubMed] [Google Scholar]
- 44.Raja N U, Vincent M J, Jabbar M A. Analysis of endoproteolytic cleavage, and intracellular transport of human immunodeficiency virus type 1 envelope glycoproteins using mutant CD4 molecules bearing the transmembrane endoplasmic reticulum retention signal. J Gen Virol. 1993;74:2085–2097. doi: 10.1099/0022-1317-74-10-2085. [DOI] [PubMed] [Google Scholar]
- 45.Raja N U, Vincent M J, Jabbar M A. Vpu-mediated proteolysis of gp160/CD chimeric envelope glycoproteins in the endoplasmic reticulum: requirement for both the anchor and cytoplasmic domains of CD4. Virology. 1994;204:357–366. doi: 10.1006/viro.1994.1540. [DOI] [PubMed] [Google Scholar]
- 46.Ritter G D, Jr, Yamshchikov G, Cohen S J, Mulligan M J. Human immunodeficiency virus type 2 enhancement of particle budding: role of the cytoplasmic domain. J Virol. 1996;70:2669–2673. doi: 10.1128/jvi.70.4.2669-2673.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sakaguchi T, Leser G P, Lamb R A. The ion channel activity of the influenza virus M2 protein affects transport through the Golgi apparatus. J Cell Biol. 1996;133:733–747. doi: 10.1083/jcb.133.4.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sakaguchi T, Tu Q, Pinto L H, Lamb R A. The active oligomeric state of the minimalistic influenza virus M2 ion channel is a tetramer. Proc Natl Acad Sci USA. 1997;94:5000–5005. doi: 10.1073/pnas.94.10.5000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schubert U, Ferrer-Montal A V, Oblatt-Montal M, Henklein P, Strebel K, Montal M. Identification of an ion channel activity of the Vpu transmembrane domain and its involvement in the regulation of virus release from HIV-infected cells. FEBS Lett. 1996;378:12–18. doi: 10.1016/s0014-5793(96)01146-5. [DOI] [PubMed] [Google Scholar]
- 50.Schubert U, Strebel K. Differential activities of the human immunodeficiency virus type 1-encoded Vpu protein are regulated by phosphorylation and occur in different cellular compartments. J Virol. 1994;68:2260–2271. doi: 10.1128/jvi.68.4.2260-2271.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schubert U, Clouse K, Strebel K. Augmentation of virus secretion by the human immunodeficiency virus type 1 Vpu protein is cell type independent and occurs in cultured human primary macrophages and lymphocytes. J Virol. 1995;69:7699–7711. doi: 10.1128/jvi.69.12.7699-7711.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schubert U, Bour S, Ferrer-Montiel A V, Mantiel M, Maldarelli F, Strebel K. The two biological activities of human immunodeficiency virus type 1 Vpu protein involves two separable structural domains. J Virol. 1996;70:809–819. doi: 10.1128/jvi.70.2.809-819.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schwartz, M. D., R. J., Geraghty, and A. T. Panganiban. HIV particle release mediated by Vpu is distinct from that mediated by p6. Virology 224:302–309. [DOI] [PubMed]
- 54.Schwartz S, Felber B K, Fenyo E M, Pavlakis G N. Env and Vpu proteins of human immunodeficiency virus type 1 are produced from multiple bicistronic mRNAs. J Virol. 1990;64:5448–5456. doi: 10.1128/jvi.64.11.5448-5456.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Spearman P, Wang J-J, Heyden N V, Ratner L. Identification of human immunodeficiency virus type 1 Gag protein domains essential to membrane binding and particle assembly. J Virol. 1994;68:3232–3242. doi: 10.1128/jvi.68.5.3232-3242.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Strebel K, Klimkait T, Martin M A. A novel gene of HIV-1, vpu, and its 16-kilodalton product. Science. 1988;241:1221–1223. doi: 10.1126/science.3261888. [DOI] [PubMed] [Google Scholar]
- 57.Strebel K, Klimkait T, Maldarelli F, Martin M A. Molecular and biochemical analyses of human immunodeficiency virus type 1 Vpu protein. J Virol. 1989;63:3784–3791. doi: 10.1128/jvi.63.9.3784-3791.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Terwilliger E F, Cohen E A, Lu Y, Sodroski J G, Haseltine W A. Functional role of human immunodeficiency virus type 1 vpu. Proc Natl Acad Sci USA. 1989;86:5163–5167. doi: 10.1073/pnas.86.13.5163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tiganos E, Yao X-Y, Friforg J, Daniel N, Cohen E A. Putative α-helical structures in the human immunodeficiency virus type 1 Vpu protein and CD4 are involved in binding and degradation of the CD4 molecule. J Virol. 1997;71:4452–4460. doi: 10.1128/jvi.71.6.4452-4460.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Trono D. HIV accessory proteins: leading role for the supporting cast. Cell. 1995;82:189–192. doi: 10.1016/0092-8674(95)90306-2. [DOI] [PubMed] [Google Scholar]
- 61.Vincent M J, Jabbar M A. The human immunodeficiency virus type 1 Vpu protein: a potential regulator of proteolysis and protein trafficking in the secretory pathway of mammalian cells. Virology. 1995;213:639–649. doi: 10.1006/viro.1995.0035. [DOI] [PubMed] [Google Scholar]
- 62.Vincent M J, Raja N U, Jabbar M A. The human immunodeficiency virus Vpu protein induces degradation of chimeric envelope glycoproteins bearing the cytoplasmic and anchor domains of CD4: role of cytoplasmic domain in Vpu-induced degradation in the endoplasmic reticulum. J Virol. 1993;67:5538–5549. doi: 10.1128/jvi.67.9.5538-5549.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang C-T, Barklis E. Assembly, processing, and infectivity of human immunodeficiency virus type 1 Gag mutants. J Virol. 1993;67:4264–4273. doi: 10.1128/jvi.67.7.4264-4273.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Willey R L, Buckler-White A, Strebel K. Sequences present in the cytoplasmic domain of CD4 are necessary and sufficient to confer sensitivity to the human immunodeficiency virus type I Vpu protein. J Virol. 1994;68:1207–1212. doi: 10.1128/jvi.68.2.1207-1212.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Willey R L, Maldarelli F, Martin M A, Strebel K. Human immunodeficiency virus type 1 Vpu protein induces rapid degradation of CD4. J Virol. 1992;66:7193–7200. doi: 10.1128/jvi.66.12.7193-7200.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Willey R L, Maldarelli F, Martin M A, Strebel K. Human immunodeficiency virus type I Vpu protein regulates the formation of intracellular gp160-CD4 complexes. J Virol. 1992;66:226–234. doi: 10.1128/jvi.66.1.226-234.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wills J W, Craven R C. Form, function, and use of retroviral Gag proteins. AIDS. 1991;5:639–654. doi: 10.1097/00002030-199106000-00002. [DOI] [PubMed] [Google Scholar]
- 68.Yao X-J, Friborg J, Checroune F, Gratton S, Boisvert F, Sekaly R P, Cohen E A. Degradation of CD4 by human immunodeficiency virus type 1 Vpu protein: a predicted alpha-helix structure in the proximal cytoplasmic region of CD4 contributes to Vpu sensitivity. Virology. 1995;209:615–623. doi: 10.1006/viro.1995.1293. [DOI] [PubMed] [Google Scholar]
- 69.Yao X-J, Garzon S, Boisvert F, Haseltine W A, Cohen E A. The effect of vpu on HIV-1-induced syncytia formation. J Acquired Immune Defic Syndr. 1993;6:135–141. [PubMed] [Google Scholar]
- 70.Yao X-J, Gottlinger H, Haseltine W A, Cohen E A. Envelope glycoprotein and CD4 independence of vpu-facilitated human immunodeficiency virus type 1 capsid export. J Virol. 1992;66:5119–5126. doi: 10.1128/jvi.66.8.5119-5126.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yu G, Shen F S, Sturch S, Aquino A, Glazer R I, Felsted R I. Regulation of HIV-1 gag protein subcellular targeting by protein kinase C. J Biol Chem. 1995;270:4792–4796. doi: 10.1074/jbc.270.9.4792. [DOI] [PubMed] [Google Scholar]
- 72.Yuan X, Yu X, Lee T-H, Essex M. Mutations in the N-terminal region of human immunodeficiency virus type 1 matrix protein block intracellular transport of the Gag precursor. J Virol. 1993;67:6387–6394. doi: 10.1128/jvi.67.11.6387-6394.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhou W, Parent L J, Wills J W, Resh M D. Identification of a membrane-binding domain within the amino-terminal region of human immunodeficiency virus type 1 protein which interacts with acidic phospholipids. J Virol. 1994;68:2556–2569. doi: 10.1128/jvi.68.4.2556-2569.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]