Abstract
Microbiota sharing between people and their companion animals is a concern for development of antimicrobial resistance. To assess the risks associated with feeding raw products to cats, with an emphasis on previously understudied freeze-dried products, a collection of 112 conventional and raw products was purchased and investigated using a combination of cultivation and high-throughput sequencing techniques. Here we show that bacterial cultures were exclusively isolated from raw foods. A total of 19 genera were cultured including Salmonella, Clostridium, Escherichia, Klebsiella, Enterobacter, and Cronobacter. Carbapenem-resistant Pseudomonas aeruginosa and Pseudomonas fulva, and Stenotrophomonas lactitubi were isolated from frozen raw products, and 6 Bacillus strains harbored carbapenemase gene bla2. Multidrug efflux pumps were highly abundant in frozen raw isolates. Clostridium sensu stricto I genus detection predicted a raw, freeze-dried product with 95% sensitivity and 78% specificity. Genera Pseudomonas, Paraclostridium and Peptostreptococcus were associated with frozen raw food products while the Bacillus genus was associated with conventional processing. Parasite genes were exclusively detected in raw foods. The presence of pathogenic species and high load of resistance genes in raw commercial food products, particularly those sold on shelves at room temperature, suggests a considerable health risk to cats and the families who care for them.
Subject terms: Policy and public health in microbiology, Microbial ecology, Infectious-disease diagnostics
Predictive microbial ecology sheds light on human health risks of freeze-dried and frozen raw foods for cats.
Introduction
For more than 30 years, there have been attempts to return companion animal nutrition to its ancestral roots with bone and raw food diets (BARF)1. For clarity, we will refer to BARF diets as raw diets in this article. While there is potential evidence for the benefits of raw diets, further studies have underscored the risks of these diets and their One Health implications2–4. As such, it is important to understand the true risk of raw diets for all household members, meaning owners and pets. Despite news articles and even regulatory actions indicating that raw diets may be contaminated with zoonotic bacteria, viruses, and parasites when compared to conventionally processed foods, American and European pet owners continue to pursue this dietary endeavor in pursuit of better animal health5. As the dietary focus gains popularity, online communities have begun describing the benefits of this lifestyle for pets. A review article from 2013 by Freeman et al. identified how online proponents deliver anecdotal evidence of better digestion, immune health, and overall healthier appearance6. The article also emphasized that pet owners are easily influenced by the desire to do better for their companion animals. On the nutritional side, Buff et al. analyzed the metabolic needs for both domestic dogs and cats compared to their non-domestic relatives7. Their data support that raw diets adequately meet the nutritional needs of companion animals and offer more bioavailable nutrition compared to conventional foods. Subsequently, consumer conformity has led to the rapid spread of the tenets of raw diets throughout online spaces, leading to larger groups of adherents8. Approximately two-thirds of survey respondents indicated they would gather pet health information from the internet or other online resources as opposed to veterinarians, including food and diet recommendations5. The lack of proper information gathering also belies increased risk for both animal and owner from using raw diets.
Several studies have approached consumers who feed their pets non-conventional diets to determine their level of knowledge about the foods that they are handling. In particular, two separate studies by Anturaniemi et al. and Thomas and Feng aimed to evaluate the knowledge of owners who feed their animals raw diets9,10. Consumer use and handling of food represent critical control points for food safety because they have the opportunity to reduce hazards to an acceptable level11. The aforementioned studies indicate that American consumers lack adequate education on the proper handling of foods, especially those intended to be served raw9,10. As such, they place themselves and their pets at a higher risk for infection. A 2019 case study by O’Halloran et al. identified a cluster of thirteen cats in the United Kingdom who suffered from a Mycobacterium bovis infection (a member of the Mycobacterium tuberculosis complex)12. The investigation revealed that the six index cases in the five geographically isolated clusters were each indoor-only cats that ate frozen raw food products. Further, these index cases were associated with seven other contact cases who were all indicated as M. bovis positive, suggesting the highly communicable nature of this infection. In addition to M. bovis, a number of other pathogenic or antimicrobial resistant species have been attributed to raw diets. For instance, Baede et al. reported finding extended-spectrum β-lactamase (ESBL) producing enteric species from frozen raw pet food3 and Nemser et al. investigated the presence of Listeria, Salmonella, and toxigenic Escherichia species in several pet foods13. The latter finding 10% of the raw foods (all frozen products) tested positive for one of the listed pathogens, as opposed to less than 0.05% in conventional dry and semimoist foods. Ahmed et al. brought to light the heightened risks of parasitic infections that come with raw diets2. Species groups such as Echinococcus cestodes and Sarcocystis protozoa rely on the predator-prey interactions for the persistence of their lifecycles14. Additionally, Toxoplasma gondii commonly infects cats in rural regions14. However, Ahmed et al. noted that pet animals will encounter these species more frequently in raw feed than in conventional feed, thus increasing their risk of colonization and subsequent human infection2.
In addition to the presence of viable pathogenic microbes in raw meat, either from the original animal(s) or from cross-contamination during processing, another concern is the spreading of antimicrobial resistance genes (ARGs). A study by Nilsson in 2015 found evidence of transferrable ESBL genes in E. coli isolated from frozen raw poultry diets for dogs in Europe, with 100% of products tested containing viable bacteria15. Raw foods also harbor ARGs in viable microbes not considered foodborne (e.g., Klebsiella pneumoniae)16. As reviewed by Verraes et al., interspecies ARG transfer by conjugation has been experimentally demonstrated in a number of food matrices17. The ARGs could be transferred among the bacterial species present in the products and incorporated into the gut microbiota of household members via horizontal gene transfer. The addition of bacteriophages, lactic acid bacteria, and live “probiotics” such as Enterococcus sp. during processing may exacerbate the risk by adding additional potential vehicles for horizontal ARG transfer15,17.
While there is mounting evidence that the increased risk of foodborne diseases or presence of antimicrobial-resistant (AMR) pathogens in animals fed frozen raw diets poses health concerns to both humans and animals, new types of formulations (e.g., freeze-dried raw treats) have come on the market that may be perceived as safer because they are sold as shelf-stable products. Our aims for this study were to expand the scope of known species and AMR mechanisms present in non-conventional cat foods to understand the risks involved with the various raw diets within a One Health perspective. In particular, we sought to identify microbial taxa of interest for guiding future regulatory actions involving different product formulations.
Results
To complete this study, a total of 112 commercial cat food products were selected for testing (Table 1; Supplementary Data 1). To reflect what an average USA-based pet owner would purchase, all food products were purchased from easily accessible major online vendors and grocery store chains. Products were then categorized by processing method (raw or conventional) and texture (canned, freeze-dried raw, frozen raw, kibble or refrigerated). Overall, 27 conventional foods were purchased and tested, and 85 raw (including raw and partially cooked) products were tested. The food type was also noted as “refrigerated” (n = 3), “frozen raw” (n = 25), “canned” (n = 22), “freeze-dried raw” (n = 49), or “kibble” (n = 13).
Table 1.
Summary of foods purchased and studied
| Food type | Number of products studied | Total | |
|---|---|---|---|
| Raw | Conventional | ||
| Refrigerated | 3 | 0 | 3 |
| Frozen raw | 25 | 0 | 25 |
| Canned | 0 | 22 | 22 |
| Freeze-dried raw | 49 | 0 | 49 |
| Kibble | 8 | 5 | 13 |
| Total | 85 | 27 | 112 |
Commercial foods were stratified by “raw or partially cooked” and “conventional”.
Protein source verification
We started our study by carrying out mitochondrial 16S rRNA gene amplification in order to verify the labeled protein source of our selected 112 products. Two products, FR08 and C18, did not show results of amplification and one product FD17 was removed from the study (see methods). Using ASV (Amplicon Sequence Variant) relative abundance, we first assigned primary, secondary and tertiary protein sources for the 109 other products when possible before grading the results as “matched” (protein sources detected in accordance with labeled ingredients), “neutral” (protein sources detected partially in accordance with labeled ingredients without major discrepancies), “discrepant” (major discrepancies between protein sources detected and labeled) for each of them. The full results are given in Supplementary Data 2 and summarized in Table 2. In brief, 59% of the food products were marked as “matched” and 28% as “neutral”. Matches were particularly high in raw products (66%) while conventional products were more distributed (~33% each grade). More precisely, freeze-dried raw (75%) and kibble (62%) were the food textures with the highest concordance, while freeze-dried raw products were the least discrepant (4%). We found that pork was the major (>75% ASV abundance) protein source detected for three canned samples (C12–14) with the ingredient “Meat By-Products” despite those products being labeled as chicken or fish. Overall, chicken and turkey were the most common unlabeled ingredients.
Table 2.
Summary of protein source comparison between product label and mitochondrial barcoding
| Canned | Frozen | Freeze-dried | Kibble | All raw | All conventional | Total | |
|---|---|---|---|---|---|---|---|
| Matched | 6 | 12 | 36 | 8 | 55 | 9 | 64 |
| Neutral | 7 | 10 | 10 | 2 | 22 | 8 | 30 |
| Discrepant | 8 | 2 | 2 | 3 | 6 | 9 | 15 |
Numbers shown are the counts of products tested.
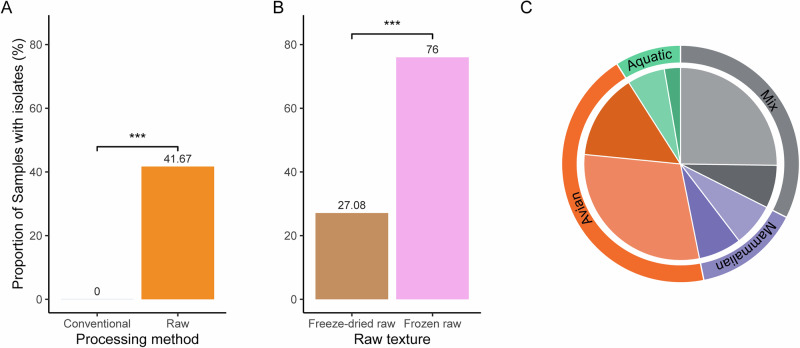
Despite adequately confirming protein source compositions in our samples, we chose for this study an unbiased approach and decided to use labeled ingredients and not detected protein sources (Supplementary Data 1). Based on those labeled protein sources, we then assigned each sample a protein source category: mammalian (e.g., beef, pork, rabbit, deer, and lamb; n = 17), avian (e.g., turkey, chicken, and duck; n = 49), aquatic (all fish; n = 10) and mix (any product with protein source from multiple categories; n = 36).
16S rRNA gene metabarcoding
Diversity analyses
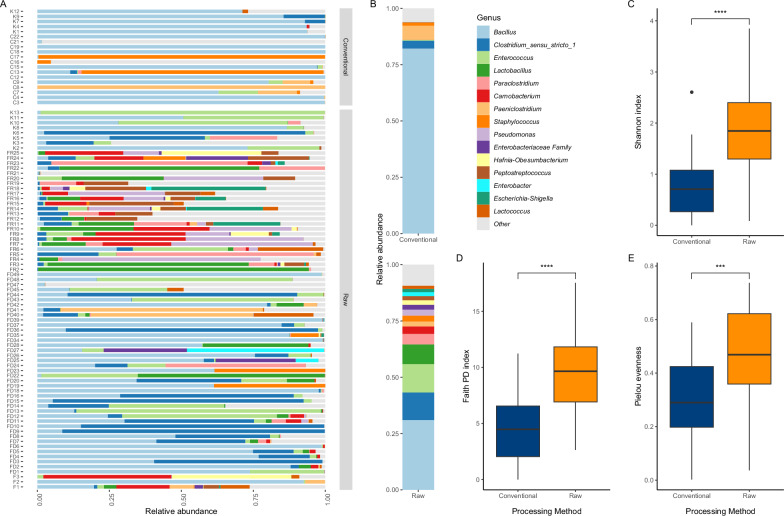
To characterize the microbial composition of the different sources of food, we performed 16S rRNA gene sequencing on all the samples (Fig. 1). Taxonomic analysis of the resultant ASV revealed the presence of 68 bacterial genera in all food products (Fig. 1A, B; Supplementary Fig. S2). Bacillus was by far the most abundant genus for both processing methods (Fig. 1B). The detected genera included Pseudomonas, Escherichia-Shigella, Enterococcus, Staphylococcus, and, in smaller abundance, Acinetobacter, Serratia and Aeromonas (Supplementary Fig. S2).
Fig. 1. Microbial diversity of raw and conventional food samples based on 16S rRNA gene amplicon sequencing data.
A Relative abundance of the 15 most abundant bacterial genera between conventional and raw food samples at the genus level. B Relative abundance as in (A), summarized by food processing method. All other genera were grouped under the Other category. C–E Comparison of alpha diversity indexes in conventional (n = 27) and raw (n = 84) food samples. C Shannon Diversity index (p = 0.00000077, Wilcoxon rank sum test; effect size large, 0.510). D Faith’s Phylogenetic Diversity index (p = 0.00000316, Wilcoxon rank sum test; effect size moderate, 0.481). E Pielou Evenness index (p = 0.000216, Wilcoxon rank sum test; effect size moderate, 0.384). (****p < 0.0001; ***p < 0.001). All boxplots display 1st quartile (Q1) to 3rd quartile (Q3) with median (Q2) as the black horizontal line.
We first characterized alpha aiversity metrics to compare the richness of bacterial species between raw food samples and conventional food samples. Raw food samples exhibited higher Shannon diversity (Fig. 1 C), higher Faith’s Phylogenetic Diversity (Fig. 1D), and higher Pielou’s evenness diversity (Fig. 1E) than conventional food samples. These results support the hypothesis that raw food products have a phylogenetically diverse variety of microbes. In addition, beta diversity analyses on rarefied ASV data revealed that microbial community structures are statistically dissimilar between raw food samples and conventional food samples (weighted UniFrac: p = 0.0001; R2 = 0.06924, unweighted UniFrac: p = 0.0001; R2 = 0.06272 and Bray–Curtis dissimilarity: p = 0.0001; R2 = 0.04746; pairwise PERMANOVA tests, 9999 permutations, controlling for sequencing run effect).
Differential abundance analysis/indicator species analysis applied to raw vs conventional
We next sought the particular genera that were most likely to vary by food processing method. Differential abundance analysis (DAA) of our ASV table aggregated at the genus level highlighted several genera for which relative abundances were significantly different between raw and conventional products (Supplementary Fig. S3). Three Bacilli genera (Bacillus, Paenibacillus and Aneurinibacillus) were found with higher relative abundances in conventional products, while a total of 17 genera were higher for raw food products. These genera included Clostridium sensu stricto 1 (CSS1), Enterococcus, Esherichia-Shigella, Serratia and Pseudomonas, which were found in significantly higher abundance in raw products (p < 0.05, internal Corncob R package test result).
To investigate further, we applied an indicator species analysis (ISA) (Fig. 2A). This analysis showed a strong association between the detection of genera CSS1, Enterococcus, and Pseudomonas, as well as other potentially pathogenic genera such as Paraclostridium and Peptostreptococcus and the presence of raw ingredients in the given food product (high specificity). Of these, only CSS1 and Enterococcus had also a high sensitivity, i.e., a >90% probability to be detected in a raw food product. The genus Bacillus was the only highly sensitive (100% of the time) taxonomic group significantly associated with conventional food products. Despite that, the specificity of Bacillus was quite low, with only a 70% chance that a product will be conventional if Bacillus is detected. This result is in accordance with other results showing Bacillus as the most detected genus in both pet food processing methods (Fig. 1A, B). Every other genus significantly associated with conventional products was also a very good predictor (high specificity).
Fig. 2. Statistical analysis of key microbial genera linked to types of food using 16S rRNA gene amplicon sequencing data.
Indicator Species Analysis (ISA) sensitivity and specificity for genera found significantly linked to A processing methods (n = 111) and B raw food textures (n = 73). Bars were colored to reflect the processing method or the food texture for which a genus was found significantly associated with, p values are reported in parenthesis.
Investigating raw product food texture associations (freeze-dried raw vs frozen raw)
Given that our results indicate that raw pet food products are more likely to contain harmful microbial taxonomic groups, we next investigated the differences within raw pet foods divided by food texture. We performed ISA to compare frozen raw and freeze-dried raw products (Fig. 2B). We found that 5 genera were significantly associated with freeze-dried raw products, while 23 were for frozen raw products. Of the genera enriched in freeze-dried raw products, Bacillus genera had high sensitivity (100%) and high specificity (96%). Genus CSS1, previously strongly associated with raw products, seems to be an even better signal of freeze-dried raw products (95% sensitivity, 78% specificity). Of the genera enriched in frozen raw products, Pseudomonas, Lactobacillus, and Carnobacterium were predicted with a high sensitivity (>75%). All three also had high specificity (>80%).
Overall, considering the pathogenic genera strongly associated with raw products by the ISA of processing methods (Fig. 2A), CSS1 is a marker of freeze-dried raw products, while Pseudomonas, Paraclostridium and Peptostreptococcus are associated with frozen raw food products. Finally, one of the most important genus predictors was not found significantly associated with either raw texture and it is therefore likely that Enterococcus was widely distributed across raw products and not specific to either frozen raw or freeze-dried raw products.
Investigating CSS1
Given the importance of the genus CSS1 as a marker of freeze-dried products, we investigated all 43 CSS1 ASVs to determine the species-level proportions. We found that 23 ASVs (53%) were Clostridium perfringens, but accounted for ~85% of the total CSS1 relative abundance (Supplementary Fig. S4). On the other end, 20 ASVs were classified as other Clostridium genera but accounted for less than 15% of the total relative abundance of CSS1 genus (Supplementary Fig. S4). These findings included C. botulinum DNA detected in 6 frozen raw (FR2-7) and one freeze-dried product (FD11).
Bacterial culture and subtyping
Gram-negative enrichments
Given that we established that there are traces of microbial pathogen genomes strongly associated with raw food products, we aimed to evaluate the direct risks of poor handling of such products. Indeed, given the extensive usage of raw food diets, we sought to verify if the food products contained viable pathogens. We first aimed at enriching Gram-negative bacteria from all food products using a protocol from the Food and Drug Administration’s Bacteriological Analytical Manual (see method for details). In total, 92 good-quality bacterial isolates were obtained from the foods using general Gram-negative enrichment methods (Supplementary Data 3). Raw foods were found statistically more likely to harbor viable and cultivable Gram-negative bacteria than conventional food (Fig. 3A). Within raw foods, we found that cultivable Gram-negative bacteria were more prevalent in the frozen raw foods compared to the freeze-dried raw foods (Fig. 3B). Finally, we found that the mammalian protein source category was the only category for which the proportion of samples yielding or not yielding bacterial growth were comparable (Fig. 3C; Supplementary Fig. S5).
Fig. 3. Gram-negative bacterial isolate summary for non-selective culture.
Bar plots representing the proportion (%) of samples for which at least one isolate was cultured. A by processing methods (n = 111, p = 0.0001359) B by raw food textures (n = 73, p = 0.0001779) (Pearson’s Chi-squared test with Yates’ continuity correction. P value significance: ***p < 0.001). C Distribution of food samples by protein source categories (aquatic, avian, mammalian, mix). Darker colors represent samples for which at least one bacterium was isolated using FDA BAM procedures, while lighter colors represent no bacteria isolated (the mix category gathers non-unique protein sources, full list of categories in Supplementary Fig. S5).
As highlighted by Fig. 3A, all samples that yielded bacterial isolates were from raw processing methods. A major foodborne pathogen, Salmonella enterica, was detected in 16% (4/25) of frozen raw samples, all single-protein diets (mammalian, aquatic and avian*2). Other genera of human health concern identified included Cronobacter, Enterobacter, Enterococcus, Escherichia and Klebsiella. Escherichia coli isolates were, like Salmonella enterica, exclusively found in frozen raw products (n(isolates)=5;n(samples)=4). Enterobacter and Klebsiella isolates were grown from numerous products from both freeze-dried raw (n(isolates)=3;n(samples)=2 and n(isolates)=1;n(samples)=1) and frozen raw (n(isolates)=5;n(samples)=5 and n(isolates)=4;n(samples)=2) textures. The only Cronobacter sakazakii isolate was from a raw kibble product. Finally, Enterococcus isolates were cultured mostly from freeze-dried raw (n(isolates)=5;n(samples)=5) but were also isolated from one kibble. Other genera identified in our pool of isolates included Citrobacter, Hafnia, and Morganella (Supplementary Data 3).
Anaerobic cultures
While analyzing 16S rRNA gene amplicon data, we highlighted the very high relative abundance in many samples of DNA from genus CSS1, mostly comprised of the species Clostridium perfringens. Thus, we attempted to isolate anaerobic microbes to grow C. perfringens. We chose all 11 samples from our cohort with more than 35% of their total amplicon reads belonging to genus CSS1 (Supplementary Fig. S6). While we failed to grow C. perfringens retrospectively using the moderately anaerobic conditions (on frozen homogenates), we successfully grew, isolated, and sequenced 6 Bacillus species, 3 Enterococcus species, 2 Macrococccoides caseolyticum, 1 Clostridium tertium, and 1 Weizmannia agrestimuris. Untargeted sequencing yielded two samples (FD9 and FD15) with more than half the microbial DNA composition belonging to C. perfringens (642,631 and 641,547 reads per million).
Carbapenem cultures
We isolated carbapenem-resistant bacteria using carbapenem-selective enrichment on the food products for which carbapenemase genes were detected using the AMR panel direct sequencing (See Section ARGs). Those enrichments yielded cultures from 3 frozen raw products. Ultimately, four good-quality genomes were sequenced, and the species identified were Pseudomonas aeruginosa, Pseudomonas lundensis, Pseudomonas fulva, and Stenotrophomonas lactitubi. Of the four P. aeruginosa clones sequenced, two had carbapenem-associated point mutations (oprD_V359L or cmrA_A68V). The predicted Stenotrophomonas mechanism was blaL1, resulting in an imipenem MIC > 8. Multidrug efflux pumps were predicted in 3 isolates. Strain FR19-erta1a (P. aeruginosa) had an imipenem MIC of 4, with ARGs mexA, mexE and mexX, FR24-erta1 (P. fulva) had only mexE predicted (imipenem MIC of 1), while FR24-erta2 (S. lactitubi) had multidrug efflux genes emrA, emrB and emrC predicted on one contig.
Functional genomics on bacterial isolate genomes
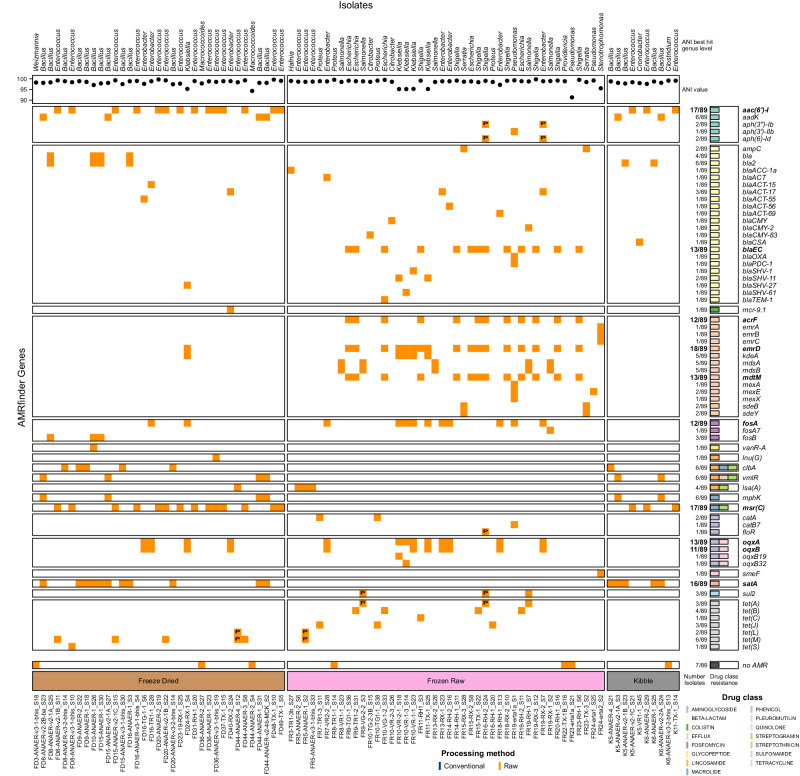
In total, whole-genome sequencing of pure culture yielded 89 non-clonal bacterial isolates that passed quality thresholds (48 from gram-negative enrichment, four from carbapenem enrichment and 37 from anaerobic conditions). With the idea to highlight the broad range of ARGs and virulence genes in our entire pool of isolates, we performed functional genomic analyses on all 89 isolates. All isolates except seven were predicted with at least one ARG (Fig. 4). In total, 69 unique ARGs were detected in isolates, predicting resistance to 15 different antimicrobial classes. Ten of those genes were predicted in at least ten isolates, including three that encoded efflux pumps. Eight predicted genes are known to be part of resistance mechanisms against multiple drug classes. In particular, two Bacillus genus-specific genes with predicted resistance to three antimicrobial classes, clbA and vmlR, were found in our anaerobic isolates from freeze-dried raw foods and kibbles. The most common genes found were emrD, msr(C) and aac(6’)-I, respectively granting resistance to the Efflux, Macrolide/Streptogramin and Aminoglycoside classes. emrD was detected mostly in frozen raw foods and in three different genera (Escherichia, Klebsiella and Shigella). On the contrary, msr(C) and aac(6’)-I were not found in any of the frozen raw foods isolates and were all predicted on genomes from the genus Enterococcus. In fact, msr(C) is known to be a chromosomal gene of E. faecium, and all nine products yielding live cultures of this species were freeze-dried treats or raw-coated kibbles. Here, we detected msr(C) in multiple E. lactis isolates, a closely related species to E. faecium, also used as a probiotic. Efflux class-resistant isolates were mostly found in frozen raw foods, with the exception of one freeze-dried raw Klebsiella isolate. One Enterobacter hormaechei isolate from raw product FD40 carried mcr-9.1, a mobile resistance gene against colistin. No Glycopeptide, Lincosamide (except lsa(A)), Macrolide or Streptothricin ARGs were detected in the Frozen raw isolates. Also, only one gene conferring resistance to the Glycopeptide antimicrobial class was found in our isolates. This gene, vanR-A, was predicted in a Bacillus strain (closest predicted species B. cereus, ANI = 96.5%) from a freeze-dried raw product. The same isolate was also isolated in another isolation condition (Supplementary Data 3). Similarly, only one gene conferring resistance to sulfonamide was predicted in our isolate genomes. This gene, sul2, was predicted in three isolates from three different frozen raw samples. Interestingly, for two of these isolates, the gene was predicted on putative plasmid contig with high confidence and on the genome itself with low confidence for the third isolate (Fig. 4; Supplementary Data 4). A total of three Fosfomycin resistance genes were found, but mostly one was widespread (fosA). Last but not least, despite not being able to grow isolates with the carbapenem genes detect in the AMR panel analyses on carbapenem selective media (see carbapenem culture section) we have successfully grown 6 Bacillus strains from 6 different samples with the gene bla2.
Fig. 4. Presence-absence heatmap representing ARGs detected in isolates using WGS data.
The colors on the heatmap are indicative of the sample processing method when an ARG was detected or blank otherwise. Presence of “P” indicates that the corresponding ARG was detected on a putative plasmid. The numbers beside the boxes on the right describe the number of isolates in which an ARG was found and the drug class(es) this ARG confers resistance to. Bold text highlights ARGs found in 10 or more isolates. The upper legend indicates ANI best hit with the corresponding ANI value for each isolate at the genus level.
Stress response genes were abundant in isolates with 54 different genes representing four categories of stress resistance: Acid (n = 2), Biocide (n = 8), Heat (n = 10) and Metal (n = 34). In addition to being the most diverse with 34 different genes predicted, resistance to metals was the highest category predicted on isolates with 276 total predictions, including 71 genes encoding copper and silver resistance, 66 genes encoding arsenic resistance and 60 genes to help resist copper only. Resistance against heat (35 predictions), acid tolerance (24 predictions) and biocide (20 predictions) were observed. Furthermore, 19 isolates (of the genera Citrobacter, Enterococcus, Escherichia coli, and Klebsiella) across all raw food textures were predicted with one contig harboring at least a heat stress gene. For 10/19 isolates, those predictions (15 heat stress genes) were found on putative plasmid contigs (confidence in prediction >0.745). The other 9/19 isolates had those heat stress genes predicted on genomic contigs, but with very low confidence (<0.3). Particularly, one Escherichia coli isolate of concern, FR9-TG1-1 was predicted with 5 different heat stress-related genes on the same putative plasmid contig. Also, that contig did not yield any results when comparing it to the plsdb database18. Virulence genes (n(total ARGs)=245; n(unique ARGs)=42) were also detected, including cytolysins, hemolytic enterotoxins, and adhesins.
Clustering of bacterial isolate genomes with strains in NCBI pathogen detection
Bacterial genomes from this study were uploaded to the US National Center for Biotechnology Information (NCBI) Pathogen Detection Database19 as they were generated. The associated Pathogen Browser (https://www.ncbi.nlm.nih.gov/pathogens) matched 24 SNP clusters from this study as of August 3, 2024. Of these, 16 contained only isolates from this study, and the other eight connected strains were submitted by other submitters. The connected SNP clusters were all for Salmonella enterica, Enterococcus faecium, or Bacillus cereus. The isolated S. enterica serovars were Typhimurium (two different strains, one shown in Fig. 5), Kentucky (two different strains), and Montevideo. The Typhimurium isolates from Frozen raw product FR9 were within one single nucleotide polymorphism (SNP) of a presumed human clinical sample from the USA, submitted by the PulseNet program (strain PNUSAS214272). Eleven other presumed human clinical strains were located in the overall SNP cluster. The next closest strain (eight SNPs) came from a chicken carcass in Indiana, USA, tested by the US Department of Agriculture in 2020 (FSIS12032032), the same labeled protein source as the FR9 food product (produced in 2021). All other non-clinical strains in this cluster came from chicken sources and included antimicrobial resistance determinants for tetracyclines, sulfonamides, and multidrug efflux. The other S. enterica clusters had a similar source composition, with primarily either chicken or beef interspersed with clinical strains. One included several recent clinical strains, from 2023, and harbored the blaCMY-2 gene encoding resistance to cefoxitin (Fig. 5). The E. faecium cluster consisted of one clinical strain from India and one bovine isolate from the USA.
Fig. 5. Evidence of zoonotic infection from raw cat food isolates using NCBI Pathogen Detection.
Snapshot of an NCBI SNP cluster issued from Pathogen Detection containing a Salmonella enterica serovar Typhimurium strain from FR19, a frozen raw cat food diet. The genome from this study (sample FR19) is highlighted in red. The scale bar indicates SNP distance, and the dates indicated are the collection dates submitted. Isolates labeled clinical are presumed to be from human infections.
Direct amplification of ARGs
ARGs screening by deep amplicon sequencing
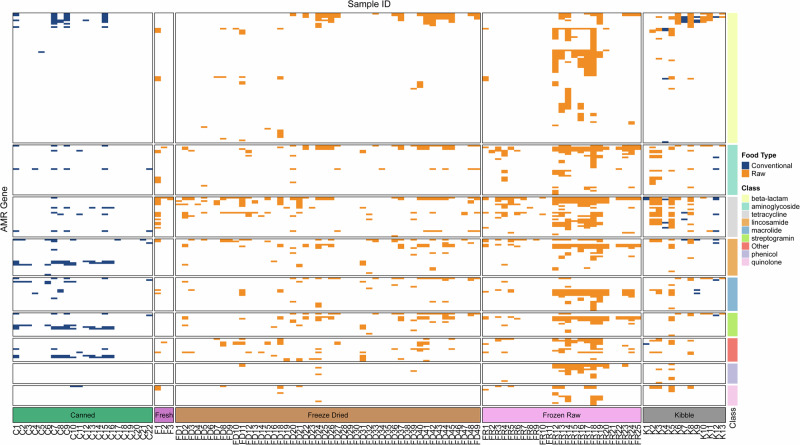
In addition to highlighting the drivers of our different microbial communities and isolating the potential species with One Health concern, we aimed at inventorying the presence of ARGs in our samples using deep amplicon sequencing directly from food homogenates. This approach found that 91 food samples had at least one ARG detected (Fig. 6). We investigated the effect of processing methods and found that raw products have significantly higher ARGs detected than conventional food products (Fig. 7A). Following that result, we tested if the food texture was an important factor of detection of ARGs and found that Frozen raw products are expected to have larger ARG repertoire than freeze-dried products (Fig. 7B). With the idea that raw products may harbor ARGs from the protein source they are based on, we first carried out a one-way ANOVA to test for the effect of protein categories on ARG detection counts in each sample. We found a small, significant effect of protein categories through the ANOVA (p = 0.021, effect size = 0.114). To examine the effects of protein category ARG detection in raw foods, we conducted pairwise Wilcoxon rank sum tests between our 4 protein source categories (Fig. 7C). Despite visual clues showing that mammalian-based feline raw foods would tend to harbor more ARGs, we found only one significant difference between mammalian and avian-based raw food products (p = 0.007, p.adj = 0.041).
Fig. 6. Presence-absence heatmap representing ARGs detected directly in cat foods by deep amplicon sequencing.
Presence-absence heatmap representing ARGs detected directly in cat foods by deep amplicon sequencing. Only the main eight antimicrobial classes are displayed, as well as an “Other” category grouping all other classes included on the panel. The colors on the heatmap are indicative of the sample processing method when a gene was detected or blank otherwise. Categories at the bottom represent food textures. Categories on the right represent antimicrobial classes, sorted by the number of genes detected.
Fig. 7. Distribution of ARG counts per food sample using deep amplicon sequencing.
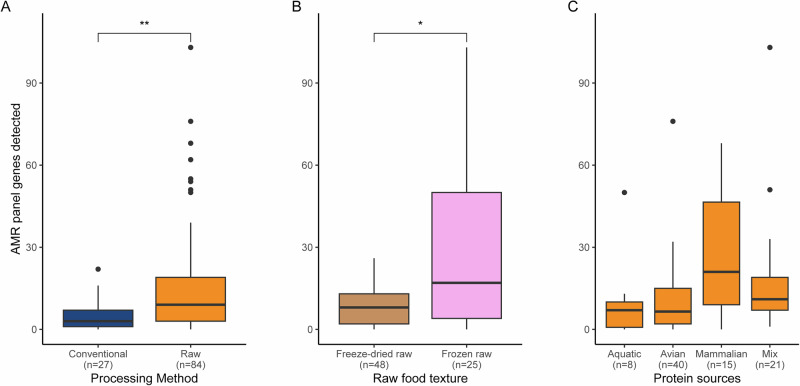
Boxplots representing the distribution of ARG counts per sample A by processing method (n = 111, p = 0.00234), B Raw food textures (n = 73, p = 0.0111) and C Protein sources categories of raw samples (n = 84). Statistical test results testing for a difference between categories are displayed (Wilcoxon rank sum tests, **p < 0.01; *p < 0.05; ns = non-significant p > 0.05). All boxplots display the 1st quartile (Q1) to the 3rd quartile (Q3) with the median (Q2) as the black horizontal line.
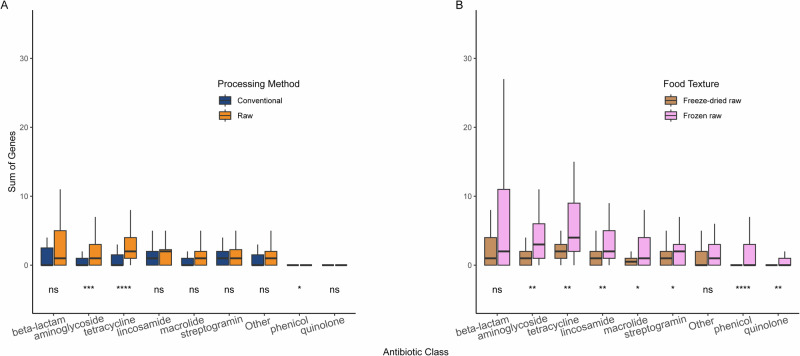
Overall, between 15% and 53% of the total number of genes present in the panel for each antimicrobial class was detected, with the tetracycline antibiotic class being the highest (Supplementary Fig. S7). Using this panel, we aimed at measuring the effect of cat food processing method on the presence of ARG counts per class (Fig. 8A). As expected from previous results (Fig. 7A), raw foods were found with a higher count of ARGs than conventional foods for three antimicrobial classes, including aminoglycoside and tetracycline, with very high confidence. All other antimicrobial classes were statistically similar in regard to the processing method. Following results displayed in Fig. 7B, we aimed at looking at differences between frozen raw and freeze-dried raw products. To do so, we carried out ARG counts comparison for every antimicrobial class in regard to the two raw food textures of interest (Fig. 8B). In total, seven out of the eight major antimicrobial classes were found significantly higher in frozen raw products than in freeze-dried raw products, with the only exception being the beta-lactam. Of note, the artificial “Other” class values were also found not to be significantly different between the two raw food textures. These results using direct amplification of antimicrobial resistance genes suggest that foods containing raw ingredients are bigger reservoirs of ARGs than purely conventional foods. This is especially true for the antibiotic classes aminoglycoside and tetracycline, and the frozen raw food products.
Fig. 8. Distribution of ARG counts per antimicrobial class using deep amplicon sequencing data.
Boxplots representing the distribution of ARG counts per antimicrobial class. Results were grouped and colored by A Processing method (n = 111) and B Raw food textures (n = 73). Statistical test results testing for a difference between both categories are displayed for each class (Wilcoxon rank sum test, ****p < 0.0001; ***p < 0.001; **p < 0.01; *p < 0.05; ns = non-significant p > 0.05). All boxplots display 1st quartile (Q1) to 3rd quartile (Q3) with median (Q2) as the black horizontal line.
Eight carbapenemase genes were detected directly from food homogenates. Six canned (conventional) foods, two conventional kibble products, and 13 raw products had bla2 detected. This gene is typically found in the chromosome of Bacillus sp. The other seven genes (cphA, blab, blaBIC, blaMUS, blaOXA.24, blaOXA.134, and blaOXA.51-like) were exclusively detected in raw foods. The ambler class B cphA was the next most common gene detected (n = 7, across all raw textures including kibble).
Eukaryotic pathogen 18S rRNA
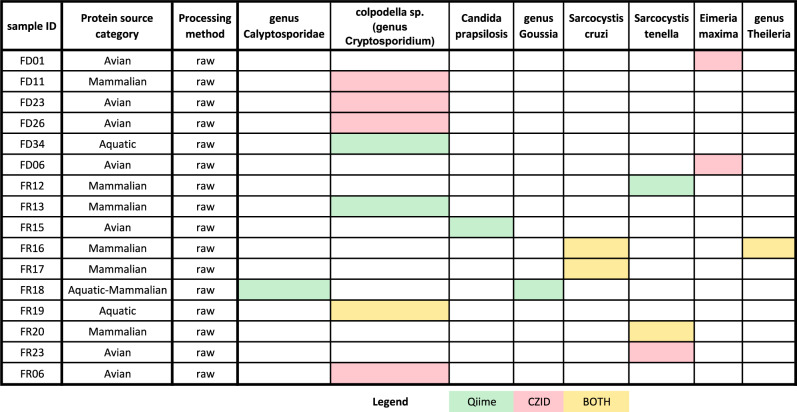
Through an 18S rRNA gene metabarcoding approach, we aimed at detecting eukaryotic pathogens within food products. In order to broaden our chances of detecting parasites, we applied two bioinformatic pipelines to the reads dataset: an ASV approach through Qiime220 and a pathogen-oriented workflow using CZID21. Despite most of the data being taxonomically assigned to plants or fungi, we successfully detected the presence of parasite DNA in 16 samples. All of these samples were raw food products, with six being freeze-dried raw and ten frozen raw. Colpodella sp. (genus Cryptosporidium) was the most detected in both pipelines (see Fig. 9). Two species of Sarcocystis, Sarcocystis cruzi and Sarcocystis tenella, were detected by both approaches in multiple Frozen raw food products. Additionally, fish and aquatic invertebrate pathogen genera Calyptosporidae and Goussia were detected by the Qiime approach in one Frozen raw sample labeled with an aquatic protein source. On the same note, but using the CZID approach, the intracellular protozoan parasite species Eimeria maxima, causing avian coccidiosis, was found in two freeze-dried samples labeled avian. Also detected by a single approach (Qiime), Candida parapsilosis was found in an avian frozen raw product. Notably, both pipelines agreed on only four samples (FR16, FR17, FR19, FR20). All five are frozen raw and from the same manufacturer. Only FR16, a frozen raw mammalian protein source-based product, was found with two different potential parasites. One of the parasites is Sarcocystis cruzii, and the other is from genus Theileria. Sarcosystis cruzi was also detected in FR17. Another species of Sarcocystis, S. tenella, was found by both approaches in FR20, while FR19 was found with Colpodella sp./genus Cryptosporidium. No helminth parasites were found in our dataset while looking for phyla Platyhelminthes, Nematoda, Cestoda or Trematoda. As well, no pathogenic fungi other than Candida parapsilosis were detected by 18S rRNA gene amplicon sequencing. By untargeted RNAseq, the C. parapsilosis in this sample (FR15) was confirmed, and also detected in sample FR10. Additionally, Aspergillus flavus was found at fairly high levels in FR10 (458 reads per million) by RNAseq.
Fig. 9. Presence-absence heatmap of major parasite taxa based on 18S rRNA gene amplicon sequencing data.
Heatmap of parasite taxa detected in our 18S rRNA gene ASV dataset. Detections were carried out using two pipelines. We report here only the samples with hits and parasites found at least once in a food product. Hits are reported in green if detected locally with Qiime2 using reference databases Silva138Ref and PR2v5, in red if detected online using CZID or yellow if detected using both pipelines.
Influenza A screening
Due to reported outbreaks of highly pathogenic Influenza A linked to raw cat food in Poland22 and Korea23 while this study was ongoing, we undertook RNA purification from all products to assess the potential risk of viral RNA. The overall RNA yield ranged from 0-70 ng/µL and was significantly higher for raw products (p = 0.0008). No Influenza A RNA, however, was detected in any sample. We performed untargeted RNAseq on the five samples with the highest RNA yield (>50 ng/µL) and integrity to assess if the genomes of any other human viruses could be detected. Only traces of livestock viruses were seen, including Rotavirus F, Bovine alphaherpesvirus 5, and Avian leukosis virus. This sequencing also contained results that corroborated the amplicon-based microbial findings from those food products.
Discussion
By expanding the ‘Bacteriological Analytical Manual Salmonella spp. procedures24 to characterize all growth, we sought to determine the prevalence of other related Gram-negative organisms in addition to the primary objective of culturing Salmonella. Although using selective media likely biased the results towards certain Gram-negative species, we decided not to discard the non-Salmonella isolates in order to examine the potential context for gene transfer. The high abundance of Gram-positives of public health significance appreciated through molecular barcoding, particularly CSS1 and Carnobacterium, was an unexpected finding. We attempted anaerobic culture retrospectively on a subset of samples, but conditions may not have been ideal due to the products being exposed to oxygen and frozen in BPW prior to those attempts. Future studies and potential regulatory investigations should include cultures specifically for these organisms from the initial processing.
Our focus here was primarily on AMR organisms and AMR genes. The presence of ARGs in a bacterial community allows for the movement of these genes into sensitive recipients. Thus, while not all a concern for foodborne transmission per se, the co-infection of live Klebsiella, E. coli, P. aeruginosa, S. enterica, S. maltophilia, E. cloacae, and/or E. faecium in a feline food product poses real concern for horizontal gene transfer of ARGs between these species. The close genetic proximity of some of these strains with clinical cases in the NCBI Pathogen Browser19 also highlights that such transfers would not be limited to infecting cats. Two of our Salmonella isolates (serovars Typhimurium and Montevideo) were in fact nearly identical to presumed human case isolates, implying a very close common source and a Public Health concern.
The application of some bacterial and bacteriophage species in the food industry as bioprotective agents has been proven effective against pathogens such as Listeria monocytogenes, Candida albicans or Pseudomonas aeruginosa, as recently been reviewed by Evangelista et al.25. One notable case is the genus Carnobacterium, associated with raw products with very high specificity (99.6%) by our ISA analysis and significantly detected with higher abundance by one of the DAA methods used (corncob, p-value = 1.236549e-07). The presence of this genus could be due either to intentional addition as a bioprotectant or due to spoilage. While not as well documented, we can also expect genera Lactococcus and Vagococcus to be part of a Lactic Acid Bacteria (LAB) solution sprayed on Raw food products to reduce pathogen loads due to their antimicrobial properties26. Considering the potential risk to immunocompromised persons, adding these to the label would be prudent. A number of products did have “probiotics” on the label, including, in some cases, Enterococcus faecium. This organism, although an opportunistic pathogen, is permitted as a direct-fed microorganism for animals in the USA if it is a non-toxigenic strain27. The toxigenic potential of this species was only recently recognized28, and the plasmid-borne genes encoding enterococcus toxins could easily be transferred between strains. Given our previous findings of multidrug-resistant E. faecium colonization in dogs29, constantly feeding even a benign strain to companion animals could serve as a vehicle for both resistance and toxin genes to transfer into the same strains.
The last genera significantly associated with raw products were not found as theoretically protective. In fact, most of them contain species that are associated with diseases and/or conditions, even in humans. Genus Paraclostridium, for example, contains species Paraclostridium bifermentans, which has recently been characterized30 as a potential emerging human pathogen while genus Peptostreptococcus has been associated with multiple infections31 despite being a normal inhabitant of human microbiomes. Finally, three genera of the family Enterobacteriaceae, often used to determine the quality of food products, were found significantly linked to raw food products: Enterobacteriaceae family, Escherichia-Shigella and Hafnia-Obesumbacterium.
The food resistomes not only threaten to seed other bacteria present with those mechanisms, but also to introduce these genes into the gut microbiota of the animals eating them. For example, K. pneumoniae, found in a number of our Raw products, is known as a vector for interspecies AMR gene transfer via outer membrane vesicles32. These vesicles are resistant to bacteriophage treatments commonly used for knocking down bacterial loads in the raw pet food industry. A 2008 study demonstrated the ability of AMR K. pneumoniae species to transfer their AMR genes to sensitive E. coli species colonizing in the same digestive tract of mice33. Another study34 found the presence of transposons Tn4451/Tn4453 that carry ARGs in several commensal bacterial hosts, such as Clostridioides, indicating the potential transferability of ARGs from food animals to host via foodborne route. At present, the US Food and Drug Administration (FDA) only routinely inspects pet foods for STEC, Salmonella, and L. monocytogenes35,36. Another species, Cronobacter sakazakii, is also now under greater scrutiny37 due to contamination events in powdered infant formula, and our results suggest that freeze-dried raw pet treats could be another important reservoir for this species. Reportable foods under the regulation of the US FDA are defined as “an article of food/feed for which there is a reasonable probability that the use of, or exposure to, such article of food will cause serious adverse health consequences or death to humans or animals”36. Because K. pneumoniae, non-STEC E. coli, and P. aeruginosa are not amongst the diseases to qualify as reportable, they would largely go undetected in commercial food products. This likely explains the lack of NCBI Pathogen Detection database matches in regard to the strains of those species sequenced for this study. However, the unregulated species could introduce ARGs into the larger microbiome of the food and gut, not only of the animals eating the food but also the humans in the household.
The detection of ESBL genes in the food products aligns with the current literature that has identified ESBL-producing species in the feces of animals fed raw diets15,38,39. This may suggest that ARGs are persistent in most animal-source foods regardless of the antimicrobial exposure history of the food animal. A study from 2015 on the microbiomes of an isolated Amerindian population showed evidence of AMR genes protecting against new-age synthetic antimicrobials such as quinolones and sulfonamides40. The presence of ARGs in a population isolated from Western medicine suggests a persistence or high rate of horizontal transfer, even without the selective pressures of antimicrobial usage. This data may also corroborate an increased rate of gene shedding into the environment, putting humans, animals, and plants all at an increased risk of contact with a resistant species41. Encountering ARGs in both cooked and uncooked foods underscores the importance of the intersection of animal, human, and environmental health outcomes, and further studies are needed to explore the health implications of ARG consumption.
We detected a higher ARG load in food samples with mammalian protein source, which could reflect efforts to regulate antimicrobials in the US poultry industry. The lack of significance for other comparisons might be due to the limited raw sample size of the category aquatic and the fact that the mix includes products with mammalian ingredients (Supplementary Fig. S5). We chose a targeted method for ARG detection, rather than untargeted metagenomic sequencing, to maximize sensitivity for genes in low abundance. The breadth of resistance genes identified directly from food was limited to those included in the panel42 utilized here, which lacks any genes discovered since 2019, and does not include any of the mobilized colistin resistance (mcr) genes. Due to this limitation, we supplemented this analysis with whole-genome sequencing of all cultured isolates, which could be screened with the latest database of known ARGs in addition to virulence and stress response genes. One mcr gene was identified with this approach, mcr-9.143, which has the potential to confer colistin resistance when expressed with an active promoter. Resistance mechanisms for glycopeptides, considered “last resort” antimicrobials to combat gram-positive bacteria, were found, as well as for the broad-spectrum fosfomycin. A perspective paper in 201744 raised the question about the use of Fosfomycin from UTI infections to other applications, but, to date, it remains mostly used for human-based infections, especially UTIs. Nonetheless, it has been used in Central and South American countries in chicken and swine farms, and increasing resistance has been shown in Asia, as the drug is used against ESBL infections45. Additionally, finding putative mobile heat stress response genes was also concerning given that some of these products are claimed to be “partially” or “gently” cooked.
Another limitation of our approach was that the carbapenem-selective enrichment was only performed retrospectively on a small subset of food products with carbapenem ARGs detected by this panel. Additionally, the method we used may not have recovered Acinetobacter that was likely present in the foods containing blaOXA51-like, blaOXA2.4, and blaOXA134. Carbapenem-resistant bacteria pose a substantial threat to mortality worldwide, and the finding of ARGs that have been associated with carbapenem-resistant Acinetobacter baumannii (CRAB) is possibly the finding of highest public health significance from this study. The World Health Organization’s 2023 analysis of antibacterial agents in development and their 2024 bacterial priority pathogens report categorically position carbapenem-resistant strains of Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacteriaceae -- all of which are Gram-negative bacterial species -- as occupying the highest level of urgency, denoted as “critical” priority46,47. In the USA, carbapenem-resistant Enterobacteriaceae (CRE) are primarily identified among patients with healthcare exposure, but there is potential for CRE to spread outside of healthcare settings, given that Enterobacteriaceae are a common cause of community-associated infections48,49. The detection of several carbapenemase genes, such as bla2 (also in isolated strains) and blaOXA-51, in both raw and conventionally processed foods cannot be ignored either. This suggests that the prevalence of carbapenem-resistant bacterial species in companion animals may be higher than previously estimated. Past research has identified the presence of carbapenemase-producing Enterobacteriaceae in food-producing animals50. Notably, these animals are a principal protein source in the cat foods examined in this investigation. Our research implies that carbapenemase-encoding genes from zoonotic or commensal bacteria could be transferred to companion animals through the foods ingested by these animals. Given the mounting evidence of shared gut microbiome carriage between people and their pets51,52, this would also pose a risk to humans in the household.
Metabarcoding approaches for Eukaryotic pathogens are far less well developed compared to bacteria. We opted for an 18S rRNA gene-based approach53 here in order to get a snapshot of potential eukaryotic pathogen presence. No culture was attempted for parasites or fungi, limiting interpretation of those results to the presence of the genome only. The results presented, therefore, do not imply that parasites were viable. The majority of sequences identified belonged to plant components of the foods, e.g., corn, squash, rice, etc. The presence of Sarcocystis sp. DNA was of interest, identified in raw products marketed for both cats and dogs. This genus comprises intracellular, apicomplexan protozoans. Dogs are the definitive host for both of the Sarcocystis species identified (S. cruzi and S. tenella), and cattle or sheep are the intermediate hosts. It could be that the carcasses used for these food products were downgraded towards pet food due to associated lesions observed during meat inspection. Although we did not evaluate infectivity, it would be prudent to keep dogs fed these products away from livestock so as not to perpetuate the life cycle. The finding of Aspergillus flavus, known for the production of mycotoxins, is also a potential indicator of suboptimal or possibly spoiled grain ingredients. Finally, Candida parapsilosis is a human pathogen of particular concern for immunocompromised individuals54,55.
The ongoing outbreak of Highly Pathogenic Avian Influenza A(H5N1) Clade 2.3.4.4b in US dairy cattle and cats56 raises the possible concern of foodborne viral transmission. Infection in mammalian wildlife could be from contact with mucous membranes during bird predation. The feline infections in the US reported by Burrough et al.56 however, were linked to consumption of raw milk. Findings from recent outbreaks in Korea23 and Poland22 indicate that Influenza A is also transmissible to cats from commercial foods. We did not detect Influenza A in any products tested here, which were all purchased prior to the Clade 2.3.4.4b outbreak. Our finding of substantially higher total RNA yield in raw products supports a focus on these products for future surveillance efforts.
The principle of One Health considers the intersection of animal, plant, and environmental health as facets of any individual’s health in the ecosystem57. The close interactions of humans and their companion animals is an important and often overlooked component of this principle. For example, there is now ample evidence (reviewed by Singla et al.58) that SARS-CoV-2 can be transmitted to animals by humans and vice versa. Just as easily, there is concern over parasitic species for which humans are accidental hosts and animals are definitive hosts. Ahmed et al. cite five specific parasites and eight different bacterial genera which present a zoonotic hazard2. Great concern lies within the risk of introducing these species into populations that would not normally encounter them. Specifically, urban developments would not typically encounter domestic animals infected with most parasites. However, the feeding of raw diets to companion animals opens a portal of entry for urban populations2.
Close-quarters living poses a significant risk of transmission between companion animals and their families, as well as other animals. A study conducted in the UK among pet owners who chose raw diets for their pets revealed low perceived susceptibility to food-borne illness associated with the diet and limited knowledge on safe raw pet food handling59. Eighty-nine percent of participants were not aware that raw pet food poses a risk of food-borne illness to themselves and their family. In addition, improper raw pet food handling, including rinsing raw meat and no segregation between utensil and kitchen preparation areas, was reported in 27% and 52% of participants, respectively. Subsequently, another UK study associated raw diets for dogs with shedding of multidrug-resistant E. coli60. When pet food products are contaminated with pathogenic microbiomes, unsafe raw food handling, including sharing utensils and the absence of cleaning kitchen surfaces after food preparation open the route of infection for pet owners, their family, and other pets in the household38.
Households with immunocompromised members and young children should take particular care in selecting pet foods. One of the most striking and unexpected findings of our study was our observation that not a single freeze-dried product we purchased was fully cooked prior to freeze-drying. This determination was made after extensive efforts to contact the manufacturers. Out of 49 freeze-dried products purchased, all containing uncooked meat in the form of treats or coated kibble, only a small minority indicated some place on the packaging that it was raw. Freeze-drying slows microbial growth and preserves viability61,62. This is consistent with reports of dried pig ear treats for dogs having very high Salmonella prevalence63 and causing large outbreaks64. Given that these products are sold on store shelves at ambient temperature, a consumer could easily purchase them without being aware of the risks or even realizing that they are purchasing an uncooked product containing viable microbes. Easily legible label warnings on packaging would potentially reduce morbidity and mortality in both humans and animals.
In this study, we identified raw food products as potential vehicles for the lateral gene transfer of AMR mechanisms into pathogens of significant consequence to One Health. Also, we highlighted freeze-dried products as a concern to the health of companion animals and owners from viable pathogens. Finally, we have highlighted general concerns regarding the lack of information and clear feeding safety guidance on the raw products. Overall, this study expands the evidence base for investigation of illness in relation to cat foods.
Methods
Food selection
A total of 112 commercial food products were selected and purchased for testing between 2021 and 2023 (Table 1). Food products were purchased from three major online vendors based in the USA and three chain grocery stores with locations in NY, USA. Direct-to-consumer commercial products were also purchased from the manufacturer’s websites after identifying them by web search for “raw cat food”. A total of 31 different manufacturers were included (identities intentionally withheld from publication). Products from the same manufacturer were different formulations, with an emphasis on diversity of animal protein sources and textures. The foods were classified based on their labeling on the food package and website materials for the processing method: “raw” (not fully cooked) or “conventional” (fully cooked). Texture was classified as either canned, freeze-dried raw, frozen raw, kibble, or fresh (refrigerated paté). “Partially” or “gently” cooked products were classified as raw, and shelf-stable kibble products with freeze-dried raw coating were classified as raw kibble. For instances when the packaging was not clear, the manufacturer was anonymously contacted to confirm. One sample (FD17) was removed from the study due to apparent cross-contamination of the homogenate with another sample.
DNA extraction
One ml of food homogenate (as described below for bacterial culture), or broth culture from a single colony, was centrifuged at 8000 × g for 5 min to pellet the bacterial cells for DNA extraction (Supplementary Fig. S1). DNA was extracted using the MagMAX Microbiome Ultra Nucleic Acid Isolation kit and associated consumables from Thermo Fisher Scientific USA (A42354). In brief, samples were suspended in lysis buffer and lysed using a prepared silicon bead tube at 4 m/s for 10 minutes. After centrifugation, the supernatant was transferred to a deep-well KingFisher sample plate, and Proteinase K was added to each sample. The plate was loaded into the KingFisher Apex instrument (Thermo Fisher Scientific USA) and run with the manufacturer’s MagMAX_Microbiome_Soil_Flex program. Extracted DNA was quantified with fluorometry (Qubit 2.0; Thermo Fisher Scientific USA).
Protein source detection using 16S mitochondrial rRNA gene amplicon sequencing
For protein source detection, amplification of the host 16S mitochondrial rRNA gene was performed using the following untagged primers: UnivMamF: 5’- GACGAGAAGACCCTATGGAGC-3’, UnivMamR: 5’- TCCGAGGTCGCCCCAACC-365. Amplification was performed using Platinum II Hot-Start Green PCR Master Mix (Invitrogen USA) with 1 µM final concentration of each primer in 50 µL reactions using the following cycling conditions: 94 °C for 2 minutes; 35 cycles of denature 94 °C 15 seconds, anneal 55 °C for 15 seconds, extend 68 °C for 30 seconds; final extension 72 °C for 10 minutes. Those samples with a band of 98–120 bp (visualized on a 2% agarose gel) were moved forward for sequencing. Purification was performed with Ampure XP beads (Beckman Coulter USA) with a 1.85X bead to sample ratio, followed by an abbreviated DNA Prep library preparation (Illumina USA), replacing the final cleanup with the Ampure XP cleanup as before. Cleaned, barcoded amplicons were adjusted to 4 nM, pooled, and sequenced with 2 × 100 bp Illumina MiSeq v3 chemistry. Controls included remnant diagnostic tissue from a rabbit and negative setup controls at the extraction and PCR stages.
Analysis of sequences was performed using Qiime220. First, sequences were denoised into amplicon sequence variants (ASVs) using the ‘dada2 denoised-paired’ plugin with trimming options set to 6 and truncating options to 70. Taxonomy predictions were assigned to the ASVs with plugin ‘feature-classifier classify-sklearn’ using a classifier based on complete mitochondrial genomes downloaded from NCBI. A reference classifier was adapted to our primer set using plugin ‘feature-classifier extract-reads’ options ‘--p-f-primer GACGAGAAGACCCTATGGAGC --p-r-primer TCCGAGGTCGCCCCAACC --p-min-length 70 --p-max-length 150’. Then those trimmed sequences were trained into a classifier using plugin ‘feature-classifier fit-classifier-naïve-bayes’. The final ASV matrix was exported using plugin ‘tools export’ (ASV table in biom format) followed by ‘biom convert’ from biom-format66 v2.1.0 to create a tsv file. Taxonomy was downloaded from the online view (qiime2 view) of the qzv taxonomy file made with ‘metadata tabulate’ plugin. ASV tsv file and taxonomy tsv file downloaded were combined and investigated manually to detect protein sources. A major discrepancy was defined as an expected category (Mammalian, Avian, or Aquatic) entirely missing or an unexpected category identified. Figures and panels were made with R (script isolate_presence_vs_metadata.R) using ggplot267 and statistical tests with R base and ggpubr68. Final figures were made using svglite69, patchwork70 and Inkscape (https://inkscape.org).
Sample preparation and bacterial culture
Using the US Food and Drug Administration’s Bacteriological Analytical Manual (BAM), food products were processed based on the Salmonella protocols (Supplementary Fig. S1)24. Products were weighed into 25 g aliquots, added to 225 ml of 11% (m/v) in buffered peptone water (BD#218105), and blended for 2 minutes. Aside from aliquots saved for direct DNA extraction (or other follow up studies) and frozen neat at −80 °C, the homogenate was transferred to a sterile screw-cap jar and incubated 1 hour at room temperature with jar loosely closed. The sample jars were tightly closed, shaken, and then incubated overnight at 35 °C with a loose lid.
Jars were removed from the incubator and gently shaken again with the lid tightened. 100 µL of the homogenate was transferred to 10 mL of Rappaport-Vassiliadis (RV) medium (Becton Dickinson USA #218581), and 1 mL of the homogenate transferred to 10 mL of tetrathionate (TT) broth (Becton Dickinson USA #210430). Liquid cultures were incubated overnight at 42 °C. Liquid cultures were removed from the incubator, and each liquid culture was streaked onto both xylose lysine desoxycholate (XLD) agar (Becton Dickinson USA #221284) and Hektoen enteric (HE) agar (Becton Dickinson USA #221366). Plates were incubated overnight at 35 °C. Plates were examined the next day for growth, and all unique and distinct colonies were selected for isolation. Selected colonies were inoculated into fresh Luria-Bertani (LB) broth (Becton Dickinson USA #241420) and incubated overnight at 35 °C. After incubation, broth cultures were transferred to a 15% glycerol-LB solution and saved at −80 °C. Isolates were also grown for 24 hours on 5% sheep’s blood trypticase soy agar plates from BBL (Becton Dickinson USA #221261) and identified by matrix-assisted laser desorption/ionization (MALDI) mass spectrometry (Bruker Corporation USA) for the Gram-negative enrichments, or by Whole-genome sequencing (WGS) for the carbapenem and anaerobic isolates as described below.
Carbapenem-selective enrichment was attempted retrospectively on the food products harboring carbapenem resistance genes (based on applying annotations from the AMRFinder database71 to the Illumina Ampliseq AMR panel output). Frozen (−80 °C) food homogenates in BPW were thawed, and 100 µl was inoculated separately into 2.5 ml MacConkey broth tubes with a 10 µg ertapenem or meropenem disc for 17 hours at 37 °C. A loop was dipped into the broth culture and streaked on carbapenem-resistant Enterobacteriaceae (CRE) chromogenic agar (HardyCHROM, Hardy Diagnostics USA). Positive colonies were re-streaked for isolation on TSA + 5% sheep blood and cultured overnight at 35 °C. Antimicrobial susceptibility testing was performed on the Sensititre Companion Animal Gram Negative COMPGN1F Vet AST Plate (Thermo Fisher USA).
Anaerobic culture was attempted retrospectively on samples with more than 3.5% of their total reads in 16S rRNA gene amplicon data belonging to genus Clostridium sensu stricto 1. Homogenates were streaked on MacConkey agar (Becton Dickinson USA #212123) and brain heart infusion agar (BHIS, Becton Dickinson USA #211059) with 5 g/L yeast extract (Becton Dickinson USA #212750)) and incubated in an anaerobe container system (GasPak, Becton Dickinson USA #260678) at 42 °C for up to 48 hours. Individual colonies were inoculated into 3 mL brain heart infusion broth (supplemented with 5 g/L yeast extract as above) in sealed tubes and incubated at 37 °C overnight in the anaerobe container with three of the manufacturer’s carbon-based packets.
WGS and bioinformatics analyses
DNA extractions of bacterial isolates were processed for WGS (Supplementary Fig. S1). Genomic libraries were prepared and barcoded using the DNA Prep Kit (Illumina USA), then sequenced on the Illumina MiSeq platform using the MiSeq Reagent Kit v3 (Illumina USA) with 2 × 250 bp chemistry. Sequencing quality for each isolate was checked using an adapted standalone version of the GalaxyTrakr MicroRunQC pipeline72, which is based on de novo assembly of trimmed reads with SKESA v2.4.073 followed by computation of coverage, N50 and reads QC30 as well as multilocus sequence typing (MLST) using the PubMLST database schemes, to which we added ANI prediction with fastANI74 v1.31 against a database from NCBI RefSeq sequences. WGS data were submitted to the NCBI Pathogen Detection database (https://www.ncbi.nlm.nih.gov/pathogens) under BioProject Accession PRJNA832331 for comparison with other genomes with Pathogen Detection19 and are globally accessible. AMR gene detection for bacterial isolates was performed using AMRFinderPlus v3.12.819,71. The tool Deeplasmid75 vDecember_22_2022 was used to determine if contigs matching known AMR mechanisms were likely to belong to a plasmid or chromosomal sequence. Contigs of interest that were predicted to be plasmidic were further analyzed with plsdb API18. Finally, we carried out all-vs-all ANI analysis using fastANI74 on the good-quality isolates and used NCBI Pathogen Detection to screen and remove duplicate clones (based on SNP distance) (Supplementary Data 3). Analyses were carried out in R76 (v4.3.1) (script AMR_isolates.R) using dplyr77, stringr78, tidyr79, tibble80 and gtools81. The heatmap was made using ComplexHeatmap82 and svglite69 R packages.
Bacterial 16S and parasite 18S rRNA gene microbiome profiles
Extracted food homogenate DNA was sequenced to target amplification of the V3 and V4 regions of the 16S rRNA gene83 (Supplementary Fig. S1) with Illumina adapter tags (underlined) added to the amplification primers as follows: 16S rRNA gene amplicon PCR Forward Primer = 5’ TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGCCTACGGGNGGCWGCAG 16S rRNA gene amplicon PCR Reverse Primer = 5’ GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGGACTACHVGGGTATCTAATCC. DNA fragments were amplified using the Platinum Hot Start PCR Master Mix (2X) kit (Invitrogen USA) with all primers at a final concentration of 0.2 μM. The PCR cycling condition was an initial denaturation at 95 °C for 5 minutes; 25 cycles of 95 °C for 30 seconds, 55 °C for 30 seconds, and 72 °C for 30 seconds; and a final extension at 72 °C for 5 minutes84. Amplicons were cleaned with Ampure XP beads (Beckman Coulter USA), barcoded with Nextera UD Indexes (Illumina USA), then purified again. These libraries were normalized to 2 nM and sequenced using Illumina 2×300 bp chemistry. Half the samples were pooled and sequenced using a NextSeq Instrument, the other half using a MiSeq instrument (both manufactured by Illumina USA).
The V4 region of the 18S rRNA gene from DNA extracted from homogenates was also amplified to explore the relative risk of parasite exposures between different diets. PCR was carried out using Platinum II Hot Start (Invitrogen USA #14001013) with the following primers53: EK-565F (GCAGTTAAAAAGCTCGTAGT), 18S-EUK-1134-R-UNonMet (TTTAAGTTTCAGCCTTGCG). The cycling conditions were: 94 °C for 3 min, 35 cycles of 94 °C for 1 min, 60 °C for 1 min, 72 °C for 1 min, and final extension of 10 min at 72 °C. Size selection (~550 bp) and PCR product cleanup were done with Ampure XP Beads (Beckman Coulter USA #A63881) at 1.8x concentration. Libraries were prepared with the Illumina DNA Prep Kit (Illumina USA #20060059) and sequenced with Illumina 2 × 300 bp chemistry (MiSEQ v3).
Reads produced with 16S rRNA gene sequencing were processed using QIIME220 version ‘qiime2-amplicon-2023.9-py38’ in a conda environment (detailed command lines in doCommands_16SrRNA_QiimePipelineCommandLines.sh file). First, for each run, ASV tables were created by denoising the reads trimmed of primers via cutadapt trim-paired command. Denoising happened with dada2 plugin with option ‘--p-min-fold-parent-over-abundance’ set to 4 and, options ‘--p-trunc-len-f’ and ‘--p-trunc-len-r’ set based on their own sequencing qualities, respectfully 0 and 235 for the MiSeq and 0 and 0 for the NextSeq (0 means no size selection). The option ‘p-trim-left’ was also used in both cases set to 0 in order to merge the denoising results of both runs into one ASV table for downstream analyses. ASVs were then filtered using the ‘filter-features-conditionally’ plugin to retain ASVs with at least 0.25% relative abundance in one sample as suggested by Reitmeier et al.85. A phylogenetic tree using the ‘sepp’ plugin was done to place our filtered ASVs into a reference phylogenetic tree based on SILVA128 generated and provided by QIIME to later compute phylogenetic distance-based diversity metrics. Taxonomy was assigned to the ASVs using the ‘classify-sklearn’ plugin using a reference database adapted from SILVA138 (available from QIIME) using our primers via the plugin ‘extract-reads’. Other plugins like ‘tabulate’, ‘import’ or ‘export’ were used to navigate between the analyses, display plugin results along twith he overall data processing.
Diversity analyses were carried out in R76 (v4.3.1 – script 16SrRNA_diversity_analyses.R) by importing QIIME2 outputs (relative abundance and sampling depth filtered ASV table, phylogenetic placement tree and taxonomy classifications) into a phyloseq86 object using the package Qiime2R. ASVs were cleaned from Mitochondrial and Chloroplast sequences, and abundances were adjusted for batch effect using ComBat-seq87 from the sva R package88 v. 3.21. ASVs were then grouped at the genus level and plotted with packages microViz89 (‘tax_select’, ’tax_agg’, ‘ps_get’, ’comp_barplot’) and ggplot267. Alpha diversity metrics were computed using ‘ps_calc_diversity’ and ‘ps_calc_richness’ from microViz89 (Shannon and Pielou), ‘phyloseq_phylo_div’ from metagMisc90 (FaithPD). Boxplots were made using ggplot267 and statistical significance computed using ‘wilcox_test’ from rstatix91 and displayed using ggpubr68. The final figure was crafted using the patchwork70 package. Beta diversity was computed on a rarefied matrix (using ‘phyloseq_mult_raref_avg’ from metagMisc) using ‘dist_calc’ and then permanova was conducted using ‘dist_permanova’ (both from microViz89 R package). DAA on non-rarefied data was investigated using ‘differentialTest’ from R package corncob92. ISA was performed at the genus level on the rarefied ASV table using ‘multipatt’ from indicspecies93. Barplots of Sensitivity and Specificity were produced with ggplot267 with data crafted with dplyr77, tibble80, and tidyr79 R packages. The final figure was made with the patchwork70 R package (script ISA-DA.R). Clostridium Sensu Stricto 1 ASVs were manually checked in the sepp tree made with Qiime (see above), and manual taxonomy annotation was chosen. Pie charts were made with ggplot267 (script CSS1.R).
Analysis of 18S rRNA gene amplicons was performed using Qiime220 version ‘qiime2-amplicon-2023.9-py38’ in a conda environment. First, sequences were denoised using plugin ‘dada2 denoised-paired’ with trimming options set to 0, truncating options adapted to the quality of each sequencing run individually and option --p-min-fold-parent-over-abundance set to 4. Then, denoised sequences (ASV) from each sequencing run were merged (‘feature-table merge’ plugin) to create only one ASV dataset for further analyses (116 samples and 25,969 ASVs). Next, a fixed filter of 10 was applied using the plugin ‘feature-table filter-features’ option –p-min-frequency (106 samples and 13,277 ASVs). Finally, two taxonomy predictions were assigned to the ASVs with plugin ‘feature-classifier classify-sklearn’ using classifiers from reference databases PR2 and Silva138Ref. Reference classifiers derived from the reference database sequences that were trimmed in accordance with our primer sets to fit our data better using plugin ‘feature-classifier extract-reads’ options ‘--p-f-primer GCAGTTAAAAAGCTCGTAGT --p-r-primer TTTAAGTTTCAGCCTTGCG --p-min-length 300 --p-max-length 650. Then those trimmed sequences were trained into a classifier using plugin ‘feature-classifier fit-classifier-naïve-bayes’. The final ASV matrix was exported using plugin ‘tools export’ (ASV table in biom format), followed by ‘biom convert’ from biom-format66 v2.1.0 to create a tsv file. Taxonomy was downloaded from the online view (qiime2 view) of the qzv taxonomy file made with the’metadata tabulate’ plugin. The ASV tsv file and taxonomy tsv file downloaded were used manually to detect parasites of interest.
Deep amplicon sequencing for AMR gene (ARG) detection
All food homogenates were directly tested for the presence of ARGs using an 815-primer ARG detection panel. To amplify ARGs from the food homogenates, the AmpliSeq for Illumina AMR Panel was used with the AmpliSeq Library PLUS kit (Illumina USA). All samples were first diluted to 4 ng/µl and amplified with each of the two provided primer pools separately for 16 cycles with an annealing time of 4 minutes. Amplicons were then pooled, digested, and barcoded with Unique Dual indexes following the manufacturer’s instructions. The final amplification cleanup was performed with AMPure XP beads (Beckman Coulter USA). Libraries were normalized to 2 nM and sequenced using Illumina 2 × 150 bp chemistry on the MiSeq platform (three different runs). Alignment to the reference gene sequences provided with the kit was performed using the DNA Amplicon application in the Basespace platform (https://basespace.illumina.com/apps/), which outputs coverage.csv files (one per sample). For each sample, the maximum coverage found in a negative control from the same run was subtracted, and any resultant value below zero was set to zero. Then the coverage values were compiled into a matrix and transformed into presence/absence to remove amplification bias and only keep gene detection information. Antibiotic class annotation of the genes included in this panel was conducted primarily using AMRFinderPlus19,71 (v2024-07-22.1), as well as the Comprehensive Antibiotic Resistance Database94 (CARD) when necessary (file available in the GitLab repository). For genes annotated to confer resistance to multiple antibiotic classes, coverage metrics were duplicated during data analysis to reflect multi-drug resistance when necessary (analyses focusing on antibiotic class). Analyses were carried out in R76 (v4.3.1) with the following packages: dplyr77, stringr78, tidyr79, forcats95, gtools81 and tibble80 (script AMR_panel.R). AMR results were displayed with R packages ggplot267, patchwork70 and ComplexHeatmap82. Statistical tests were carried out with ggpubr68 and rstatix91. Figures were exported from R in a vectorial image using svglite69.
Influenza A screening and RNAseq
RNA purification from food homogenates was performed using the MagMAX mirVana Total RNA Isolation Kit (Thermo Fisher USA). Real-time PCR was performed using the CDC Universal Influenza A96 assay using FastVirus master mix on the QuantStudio 3 platform (both Thermo Fisher USA). Untargeted RNAseq on a subset of samples was performed by the Cornell Transcriptional Regulation and Expression Facility. Library preparation was performed using the NEB directional library preparation kit. Sequencing was performed with PE 2 × 150 bp read length on the NovaSeq 6000 instrument (Illumina USA) with 100 M reads per sample. Taxonomic analysis of RNAseq data was performed using the CZID21 Illumina mNGS Pipeline v8.3, NCBI Index Date: 2021-01-22.
Statistics and reproducibility
The Shapiro-Wilk test for normality was used to assess the normality of the distribution of the investigated parameters. Neither parameter (cultured bacteria count and AMR gene count) was distributed normally. Differences in medians were tested by the Wilcoxon Rank Sum Test. Pearson’s Chi-squared tests with Yates’ continuity correction were used to verify associations between growing isolates and groups of samples. One-way ANOVA tests were performed separately to evaluate the effect of protein source categories on the number of cultured bacteria species and ARG count. All analyses were performed using R Statistical Software76 (v4.3.1), and the R packages cited throughout the methods were managed with renv97 (versions in renv.lock file).
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
This project was funded by the Cornell Feline Health Center Natural Nutrition Award. The authors are grateful to Stephen Parry from the Cornell Statistical Consulting Unit for guidance on the statistics methods used in this study, Lars Westblade for guidance on carbapenem selection and manuscript review, and Mani Lejeune for guidance on parasite biology. The authors also thank Ralitsa Todorova and Joel J. Brown for constructive feedback and statistical advice. The RNAseq was performed at the BRC Genomics Facility (RRID: SCR_022532) at the Cornell Institute of Biotechnology. Sequencing equipment used for this study was provided by the US Food and Drug Administration’s Veterinary Laboratory Investigation and Response Network under Research Collaboration Agreement FY16-RCA-CVM-01-CU.
Author contributions
L.B.G. obtained funding. L.B.G. and G.R. designed the study with help from A.C.M. (cultures), Y.T.Y. (AMR panel), K.S. (cultures), and B.J.S. (sample selection). A.C.M. performed sample homogenization. J.M.U. and K.S. performed protein source lab work. Y.T.Y., G.R. and J.M.U. analyzed and interpreted the protein source results. A.C.M., K.S., L.B.G., B.W. performed cultures, glycerol stocks and whole-genome sequencing of isolates. R.F.G. performed MALDI-TOF isolate identifications. G.R. performed bioinformatics analyses on isolate genomic data and interpretation. A.C.M., Y.T.Y., L.B.G. performed ampliseq lab work. G.R., Y.T.Y., A.C.M., L.B.G. analyzed and interpreted ampliseq data. Y.G. performed parasite lab work. G.R., Y.G., L.B.G. analyzed and interpreted parasite data. Y.J., Z.C., K.S., L.B.G. performed Influenza lab work and interpreted the data. A.C.M., Y.T.Y., Y.G. drafted portions of the manuscript. G.R., L.B.G. wrote the final manuscript. G.R., L.B.G., A.C.M. uploaded data to NCBI. All authors read and approved the final manuscript.
Peer review
Peer review information
Communications Biology thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editors: Dr Sabina La Rosa and Dr Ophelia Bu.
Data availability
All sequences generated as part of this study are available on NCBI in the umbrella Project PRJNA1088075. The cultured bacterial isolate genomes are under this umbrella in BioProject PRJNA832331. Amplicon and RNAseq data are in BioProject PRJNA1129155. Specific format files compiling raw data required for analyses and summary tables can be found on the GitLab: https://gitlab.com/goodmanlab/cat-food page. All other source data can be obtained from Supplementary data 1–5:
File name: Supplementary Data 1
Description: Metadata for all samples tested
File name: Supplementary Data 2
Description: Protein source analysis results
File name: Supplementary Data 3
Description: Bacterial isolate genome quality metrics
File name: Supplementary Data 4
Description: AMR and mobility annotations for bacterial isolate genomes
File name: Supplementary Data 5
Description: Direct deep AMR amplicon sequencing results from food samples.
Code availability
All command lines used to generate QIIME data from raw sequences and R scripts written to analyze them are available on GitLab: https://gitlab.com/goodmanlab/cat-food.
Competing interests
The authors declare the following competing interests: L.B.G. held an expert witness contract with the US Food and Drug Administration at the time the experiments were performed. The authors declare no other competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Aaron C. Malkowski, Y. Tina Yu.
Contributor Information
Guillaume Reboul, Email: gcr58@cornell.edu.
Laura B. Goodman, Email: laura.goodman@cornell.edu
Supplementary information
The online version contains supplementary material available at 10.1038/s42003-025-08756-8.
References
- 1.Billinghurst, I. Give your dog a bone. (Dogwise Publishing, 1993).
- 2.Ahmed, F. et al. Raw meat based diet (RMBD) for household pets as potential door opener to parasitic load of domestic and urban environment. Revival of understated zoonotic hazards? A review. One Health13, 100327 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baede, V. O. et al. Raw pet food as a risk factor for shedding of extended-spectrum beta-lactamase-producing Enterobacteriaceae in household cats. PLoS One12, e0187239 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Szakacs, A.-R., Ștefănuț, L.-C., Matei, S., Borz, B.-I. & Macri, A.-M. Assessment of digestibility and fecal score of raw meatbased diet (B.A.R.F.) in dog feeding. (2021).
- 5.Hoummady, S. et al. Comparison of canine owner profile according to food choice: an online preliminary survey in France. BMC Vet. Res.18, 163 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Freeman, L. M., Chandler, M. L., Hamper, B. A. & Weeth, L. P. Current knowledge about the risks and benefits of raw meat-based diets for dogs and cats. J. Am. Vet. Med. Assoc.243, 1549–1558 (2013). [DOI] [PubMed] [Google Scholar]
- 7.Buff, P. R., Carter, R. A., Bauer, J. E. & Kersey, J. H. Natural pet food: a review of natural diets and their impact on canine and feline physiology. J. Anim. Sci.92, 3781–3791 (2014). [DOI] [PubMed] [Google Scholar]
- 8.Park, J. & Feinberg, R. E-formity: consumer conformity behaviour in virtual communities: an International Journal. J. Res. Interact. Mark.4, 197–213 (2010). [Google Scholar]
- 9.Anturaniemi, J., Barrouin-Melo, S. M., Zaldivar-López, S., Sinkko, H. & Hielm-Björkman, A. Owners’ perception of acquiring infections through raw pet food: a comprehensive internet-based survey. Vet. Rec.185, 658 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thomas, M. & Feng, Y. Risk of foodborne illness from pet food: assessing pet owners’ knowledge, behavior, and risk perception. J. Food Prot.83, 1998–2007 (2020). [DOI] [PubMed] [Google Scholar]
- 11.F. D. A. HACCP Principles & Application Guidelines. FDA (2023).
- 12.O’Halloran, C. et al. Tuberculosis due to Mycobacterium bovis in pet cats associated with feeding a commercial raw food diet. J. Feline Med. Surg.21, 667–681 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nemser, S. M. et al. Investigation of Listeria, Salmonella, and toxigenic Escherichia coli in various pet foods. Foodborne Pathog. Dis.11, 706–709 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leventhal, R. & Cheadle, R. F. Medical Parasitology: A Self-Instructional Text. (F. A. Davis Company, 2019).
- 15.Nilsson, O. Hygiene quality and presence of ESBL-producing Escherichia coli in raw food diets for dogs. Infect. Ecol. Epidemiol.5, 28758 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hartantyo, S. H. P. et al. Foodborne Klebsiella pneumoniae: Virulence Potential, Antibiotic Resistance, and Risks to Food Safety. J. Food Prot.83, 1096–1103 (2020). [DOI] [PubMed] [Google Scholar]
- 17.Verraes, C. et al. Antimicrobial resistance in the food chain: a review. Int. J. Environ. Res. Public Health10, 2643–2669 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmartz, G. P. et al. PLSDB: advancing a comprehensive database of bacterial plasmids. Nucleic Acids Res.50, D273–D278 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Library of Medicine (US). The NCBI Pathogen Detection Project. National Center for Biotechnology Information https://www.ncbi.nlm.nih.gov/pathogens/ (2016).
- 20.Bolyen, E. et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol.37, 852–857 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kalantar, K. L. et al. IDseq-An open source cloud-based pipeline and analysis service for metagenomic pathogen detection and monitoring. Gigascience9, giaa111 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rabalski, L. et al. Emergence and potential transmission route of avian influenza A (H5N1) virus in domestic cats in Poland, June 2023. Eurosurveillance28, 2300390 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Korea Times. Highly pathogenic avian influenza virus confirmed in cat food. koreatimes (2023).
- 24.FDA. Bacteriological Analytical Manual (BAM) Chapter 5: Salmonella. (2019).
- 25.Evangelista, A. G. et al. Carnobacterium as a bioprotective and potential probiotic culture to improve food quality, food safety, and human health - a scoping review. Crit. Rev. Food Sci. Nutr.63, 6946–6959 (2023). [DOI] [PubMed] [Google Scholar]
- 26.Hernández-Aquino, S., Miranda-Romero, L. A., Fujikawa, H., Maldonado-Simán, E. D. J. & Alarcón-Zuñiga, B. Antibacterial activity of lactic acid bacteria to improve shelf life of raw meat. Biocontrol Sci.24, 185–192 (2019). [DOI] [PubMed] [Google Scholar]
- 27.AAFCO. Chapter 6: Official Feed Terrms, Common or Usual Ingredient Names and Ingredient Definitions. In AAFCO Official Publication vol. (Association of American Feed Control Officials, 2023).
- 28.Xiong, X. et al. Emerging enterococcus pore-forming toxins with MHC/HLA-I as receptors. Cell185, 1157–1171.e22 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Menard, J. et al. Effect of antimicrobial administration on fecal microbiota of critically ill dogs: dynamics of antimicrobial resistance over time. Anim. Microbiome4, 36 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cai, X. et al. Populational genomic insights of Paraclostridium bifermentans as an emerging human pathogen. Front Microbiol14, 1293206 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Public Health Agency of Canada. Pathogen safety data sheets: infectious substances – peptostreptococcus spp. https://www.canada.ca/en/public-health/services/laboratory-biosafety-biosecurity/pathogen-safety-data-sheets-risk-assessment/peptostreptococcus.html (2011).
- 32.Wyres, K. L. & Holt, K. E. Klebsiella pneumoniae as a key trafficker of drug resistance genes from environmental to clinically important bacteria. Curr. Opin. Microbiol.45, 131–139 (2018). [DOI] [PubMed] [Google Scholar]
- 33.Schjørring, S., Struve, C. & Krogfelt, K. A. Transfer of antimicrobial resistance plasmids from Klebsiella pneumoniae to Escherichia coli in the mouse intestine. J. Antimicrob. Chemother.62, 1086 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cao, H. et al. Sharing of antimicrobial resistance genes between humans and food animals. mSystems7, e00775-22 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.FDA. Microbiological surveillance sampling. FDAhttps://www.fda.gov/food/sampling-protect-food-supply/microbiological-surveillance-sampling (2024).
- 36.FDA. The reportable food registry: a five year overview of targeting inspection resources and identifying patterns of adulteration. https://www.fda.gov/food/reportable-food-registry-industry/reportable-food-registry-annual-report (2016).
- 37.FDA. Summary of FDA’s strategy to help prevent cronobacter sakazakii illnesses associated with consumption of powdered infant formula. FDA (2023).
- 38.Davies, R. H., Lawes, J. R. & Wales, A. D. Raw diets for dogs and cats: a review, with particular reference to microbiological hazards. J. Small Anim. Pr.60, 329–339 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Bree, F. P. J. et al. Zoonotic bacteria and parasites found in raw meat-based diets for cats and dogs. Vet. Rec.182, 50 (2018). [DOI] [PubMed] [Google Scholar]
- 40.Clemente, J. C. et al. The microbiome of uncontacted Amerindians. Sci. Adv.1, e1500183 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhuang, M. et al. Distribution of antibiotic resistance genes in the environment. Environ. Pollut.285, 117402 (2021). [DOI] [PubMed] [Google Scholar]
- 42.Urbaniak, C. et al. Detection of antimicrobial resistance genes associated with the International Space Station environmental surfaces. Sci. Rep.8, 814 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carroll, L. M. et al. Identification of novel mobilized colistin resistance gene mcr-9 in a multidrug-resistant, colistin-susceptible salmonella enterica serotype typhimurium isolate. mBio10, e00853-19 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Silver, L. L. Fosfomycin: Mechanism and Resistance. Cold Spring Harb. Perspect. Med.7, a025262 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zurfluh, K., Treier, A., Schmitt, K. & Stephan, R. Mobile fosfomycin resistance genes in Enterobacteriaceae—an increasing threat. Microbiologyopen9, e1135 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.WHO. 2023 antibacterial agents in clinical and preclinical development: an overview and analysis. https://www.who.int/publications/i/item/9789240094000 (2024).
- 47.WHO. WHO bacterial priority pathogens list, 2024: bacterial pathogens of public health importance to guide research, development and strategies to prevent and control antimicrobial resistance. https://www.who.int/publications/i/item/9789240093461 (2024). [DOI] [PMC free article] [PubMed]
- 48.Kelly, A. M., Mathema, B. & Larson, E. L. Carbapenem-resistant Enterobacteriaceae in the community: a scoping review. Int. J. Antimicrob. Agents50, 127 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sabour, S. et al. Detection and characterization of targeted carbapenem-resistant health care-associated threats: findings from the antibiotic resistance laboratory network, 2017 to 2019. Antimicrob. Agents Chemother. 65, e0110521 (2021). [DOI] [PMC free article] [PubMed]
- 50.Guerra, B., Fischer, J. & Helmuth, R. An emerging public health problem: acquired carbapenemase-producing microorganisms are present in food-producing animals, their environment, companion animals and wild birds. Vet. Microbiol.171, 290–297 (2014). [DOI] [PubMed] [Google Scholar]
- 51.Elankumaran, P. et al. Genomic and temporal trends in canine ExPEC reflect those of human ExPEC. Microbiol. Spectr.10, e0129122 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Harrison, L. et al. Use of large-scale genomics to identify the role of animals and foods as potential sources of extraintestinal pathogenic escherichia coli that cause human illness. Foods11, 1975 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Simon, M. et al. Complex communities of small protists and unexpected occurrence of typical marine lineages in shallow freshwater systems. Environ. Microbiol.17, 3610–3627 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Franconi, I., Rizzato, C., Tavanti, A., Falcone, M. & Lupetti, A. Paradigm shift: candida parapsilosis sensu stricto as the most prevalent candida species isolated from bloodstream infections with increasing azole-non-susceptibility rates: trends from 2015–2022 Survey. J. Fungi9, 1012 (2023). [DOI] [PMC free article] [PubMed]
- 55.Trofa, D., Gácser, A. & Nosanchuk, J. D. Candida parapsilosis, an emerging fungal pathogen. Clin. Microbiol Rev.21, 606–625 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Burrough, E. R. et al. Early release - highly pathogenic avian influenza A(H5N1) Clade 2.3.4.4b Virus Infection in Domestic Dairy Cattle and Cats, United States, 2024 - Volume 30, Number 7—July 2024 - emerging infectious diseases journal - CDC 10.3201/eid3007.240508 (2024). [DOI] [PMC free article] [PubMed]
- 57.Mwangi, W., de Figueiredo, P. & Criscitiello, M. F. One health: addressing global challenges at the nexus of human, animal, and environmental health. PLoS Pathog.12, e1005731 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Singla, R. et al. Human animal interface of SARS-CoV-2 (COVID-19) transmission: a critical appraisal of scientific evidence. Vet. Res. Commun.44, 119–130 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bulochova, V. & Evans, E. W. Exploring food safety perceptions and self-reported practices of pet owners providing raw meat-based diets to pets. J. Food Prot.84, 912–919 (2021). [DOI] [PubMed] [Google Scholar]
- 60.Morgan, G., Pinchbeck, G., Haldenby, S., Schmidt, V. & Williams, N. Raw meat diets are a major risk factor for carriage of third-generation cephalosporin-resistant and multidrug-resistant E. coli by dogs in the UK. Front. Microbiol.15, 1460143 (2024). [DOI] [PMC free article] [PubMed]
- 61.Miyamoto-Shinohara, Y., Sukenobe, J., Imaizumi, T. & Nakahara, T. Survival of freeze-dried bacteria. J. Gen. Appl. Microbiol.54, 9–24 (2008). [DOI] [PubMed] [Google Scholar]
- 62.Beuchat, L. R. et al. Low-water activity foods: increased concern as vehicles of foodborne pathogens. J. Food Prot.76, 150–172 (2013). [DOI] [PubMed] [Google Scholar]
- 63.Adley, C., Dillon, C., Morris, C. P., Delappe, N. & Cormican, M. Prevalence of Salmonella in pig ear pet treats. Food Res. Int.44, 193–197 (2011). [Google Scholar]
- 64.Nichols, M. et al. Outbreak of multidrug-resistant Salmonella infections in people linked to pig ear pet treats, United States, 2015-2019: results of a multistate investigation. Lancet Reg. Health Am.34, 100769 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tillmar, A. O., Dell’Amico, B., Welander, J. & Holmlund, G. A universal method for species identification of mammals utilizing next generation sequencing for the analysis of DNA mixtures. PLoS One8, e83761 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McDonald, D. et al. The biological observation matrix (BIOM) format or: how I learned to stop worrying and love the ome-ome. GigaScience1, 2047–217X-1–7 (2012). [DOI] [PMC free article] [PubMed]
- 67.Wickham, H. et al. ggplot2: create elegant data visualisations using the grammar of graphics. https://ggplot2.tidyverse.org/ (2024).
- 68.Kassambara, A. ggpubr: ‘ggplot2’ based publication ready plots. https://rpkgs.datanovia.com/ggpubr/ (2023).
- 69.Wickham, H. et al. svglite: An ‘SVG’ Graphics Device. (2023).
- 70.Pedersen, T. L. patchwork: the composer of plots. https://github.com/thomasp85/patchwork (2024).
- 71.Feldgarden, M. et al. AMRFinderPlus and the Reference Gene Catalog facilitate examination of the genomic links among antimicrobial resistance, stress response, and virulence. Sci. Rep.11, 12728 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Timme, R. E. et al. Optimizing open data to support one health: best practices to ensure interoperability of genomic data from bacterial pathogens. One Health Outlook2, 20 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Souvorov, A., Agarwala, R. & Lipman, D. J. SKESA: strategic k-mer extension for scrupulous assemblies. Genome Biol.19, 153 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jain, C., Rodriguez-R, L. M., Phillippy, A. M., Konstantinidis, K. T. & Aluru, S. High throughput ANI analysis of 90K prokaryotic genomes reveals clear species boundaries. Nat. Commun.9, 5114 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Andreopoulos, W. B. et al. Deeplasmid: deep learning accurately separates plasmids from bacterial chromosomes. Nucleic Acids Res.50, e17 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.R. Core Team. R: a language and environment for statistical computing. https://www.R-project.org. R Foundation for Statistical Computing (2017).
- 77.Wickham, H. et al. dplyr: a grammar of data manipulation. https://dplyr.tidyverse.org/ (2023).
- 78.Wickham, H. stringr: simple, consistent wrappers for common string operations. https://stringr.tidyverse.org/ (2023).
- 79.Wickham, H. et al. tidyr: tidy messy data. https://tidyr.tidyverse.org/ (2024).
- 80.Müller, K., Wickham, H., Francois, R., Bryan, J. & RStudio. tibble: simple data frames. https://tibble.tidyverse.org/ (2023).
- 81.Warnes, G. R. et al. gtools: Various R Programming Tools. https://github.com/r-gregmisc/gtools (2023).
- 82.Gu, Z., Eils, R. & Schlesner, M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics32, 2847–2849 (2016). [DOI] [PubMed] [Google Scholar]
- 83.Klindworth, A. et al. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res.41, e1 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Caporaso, J. G. et al. Earth microbiome protocol 16s illumina amplicon protocol. https://protocols.io/view/emp-16s-illumina-amplicon-protocol-cpisvkee (2023).
- 85.Reitmeier, S. et al. Handling of spurious sequences affects the outcome of high-throughput 16S rRNA gene amplicon profiling. ISME Commun.1, 31 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.phyloseq R package: http://bioconductor.org/packages/phyloseq/.
- 87.Zhang, Y., Parmigiani, G. & Johnson, W. E. ComBat-seq: batch effect adjustment for RNA-seq count data. NAR Genom. Bioinform.2, lqaa078 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.LLeek, J. T., Storey, J. D. & Johnson, W. E. sva: Surrogate Variable Analysis. R package 1032 version 3.56.0 (2025).
- 89.Barnett, D. J. M., Arts, I. C. W. & Penders, J. microViz: an R package for microbiome data visualization and statistics. J. Open Source Softw.6, 3201 (2021). [Google Scholar]
- 90.Mikryukov, V. metagMisc R package available at https://github.com/vmikk/metagMisc (2024).
- 91.Kassambara, A. rstatix: pipe-friendly framework for basic statistical tests. https://rpkgs.datanovia.com/rstatix/ (2023).
- 92.Martin, B.D., Witten, D. & Willis, A.D. Modeling microbial abundances and dysbiosis with beta-binomial regression. Ann. Appl. Stat.14, 94–115 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cáceres, M. D. & Legendre, P. Associations between species and groups of sites: indices and statistical inference. Ecology90, 3566–3574 (2009). [DOI] [PubMed] [Google Scholar]
- 94.Alcock, B. P. et al. CARD 2023: expanded curation, support for machine learning, and resistome prediction at the Comprehensive Antibiotic Resistance Database. Nucleic Acids Res.51, D690–D699 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wickham, H. forcats: tools for working with categorical variables (Factors). R package version 1.0.0. https://github.com/tidyverse/forcats, https://forcats.tidyverse.org/.
- 96.Shu, B. et al. Design and performance of the CDC real-time reverse transcriptase PCR swine flu panel for detection of 2009 A (H1N1) pandemic influenza virus. J. Clin. Microbiol.49, 2614–2619 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ushey, K. & Wickham, H. renv: project environments. R package version 1.0.7, https://github.com/rstudio/renv, https://rstudio.github.io/renv/.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
All sequences generated as part of this study are available on NCBI in the umbrella Project PRJNA1088075. The cultured bacterial isolate genomes are under this umbrella in BioProject PRJNA832331. Amplicon and RNAseq data are in BioProject PRJNA1129155. Specific format files compiling raw data required for analyses and summary tables can be found on the GitLab: https://gitlab.com/goodmanlab/cat-food page. All other source data can be obtained from Supplementary data 1–5:
File name: Supplementary Data 1
Description: Metadata for all samples tested
File name: Supplementary Data 2
Description: Protein source analysis results
File name: Supplementary Data 3
Description: Bacterial isolate genome quality metrics
File name: Supplementary Data 4
Description: AMR and mobility annotations for bacterial isolate genomes
File name: Supplementary Data 5
Description: Direct deep AMR amplicon sequencing results from food samples.
All command lines used to generate QIIME data from raw sequences and R scripts written to analyze them are available on GitLab: https://gitlab.com/goodmanlab/cat-food.