Abstract
This case report discusses a 32-year-old woman with Friedreich ataxia (FA) and suboptimally managed diabetes mellitus (DM), focusing on a treatment strategy aimed at improving mitochondrial function for better glycemic control and symptom management. Her regimen included insulin, a dipeptidyl peptidase-4 (DPP-4) inhibitor, neurotropic vitamins, and mitochondriotropic agents and antioxidants, specifically L-carnitine, coenzyme Q10 (CoQ10), and vitamin E. Imeglimin, a mitochondriotropic antihyperglycemic agent, was also part of her regimen. While glycemic stability initially fluctuated, it reached stability over 3 to 4 months. During the 3-year follow-up, her fasting C-peptide levels decreased from 1.15 ng/mL (SI: 0.38 nmol/L) to 0.5 ng/mL (SI: 0.17 nmol/L) (reference range, 0.78-1.89 ng/mL [SI: 0.26-0.62 nmol/L]), yet her glycemic stability improved significantly, and her International Cooperative Ataxia Rating Scale (ICARS) score improved from 85 to 71 points. These findings highlight the potential of mitochondriotropic agents in the management of FA and related DM, possibly improving insulin sensitivity and neurodegeneration and underscores the need for further studies on the efficacy of specific agents in improving metabolic and neurological outcomes.
Keywords: Friedreich ataxia, diabetes mellitus, mitochondrial dysfunction, glycemic control, antioxidants, mitochondriotropic agents
Introduction
Friedreich ataxia (FA), caused by pathogenic variants in the FXN gene (9q13–21.1), impairs frataxin (FXN) production, disrupting mitochondrial iron-sulfur clusters and oxidative phosphorylation [1]. This leads to adenosine triphosphate (ATP) deficiency, iron overload, and oxidative stress, with a global prevalence of ∼1:40 000 [2]. An autosomal recessive disorder, FA typically manifests in childhood (8-15 years) with progressive ataxia, dysarthria, cardiomyopathy, diabetes mellitus (DM), and sensory loss [3]. Life expectancy averages 39 years, with cardiomyopathy being the primary mortality cause, though supportive care can extend survival [4]. Despite its identification over 150 years ago, FA has no cure, and treatment focuses on managing symptoms, including DM and heart failure.
FA-related DM (5%-40% prevalence) usually develops 10 to 15 years after FA onset [5, 6]. Mitochondrial dysfunction in FA causes both insulin deficiency (β-cell loss) and insulin resistance, akin to maternally inherited diabetes (MIDD) [7, 8]. The insulin resistance is mediated by impaired glucose uptake in skeletal muscle from defective oxidative phosphorylation, ectopic lipid accumulation in liver and muscle due to disrupted fat metabolism, chronic inflammation and oxidative stress impairing insulin signaling, autonomic dysregulation of hepatic glucose production, and secondary metabolic deterioration from ataxia-related physical inactivity and muscle atrophy [9, 10]. Given the mitochondrial basis of DM in both FA and MIDD, the use of mitochondriotropic agents that preserve pancreatic β-cell mass and enhance mitochondrial function may improve metabolic status. Our previous studies on MIDD demonstrated improved glycemic control with these agents [11, 12]. Mitochondrial deoxyribonucleic acid (DNA) mutations also exacerbate cardiovascular disease [13, 14], due to transfer RNA leucine (tRNA-Leu) deficiency impairing protein synthesis, highlighting the need for therapies targeting mitochondria to mitigate cardiovascular risk. This case report discusses the clinical course of FA-related DM treated with mitochondriotropic agents and insulin.
Case Presentation
This case involves a 32-year-old wheelchair-bound woman, born to consanguineous parents, who presented to us 3 years ago with suboptimally managed DM and symptoms including tiredness, imbalance, bilateral lower limb edema, and polyuria. She struggled with standing, walking, and performing routine self-care activities. She was diagnosed with sensory ataxic neuropathy at the age of 15 and later diagnosed with DM at the age of 27, following evaluation for polyuria and fatigue with documented fasting blood sugar at 295 mg/dL (SI: 16.4 mmol/L) (reference range, 70-99 mg/dL [SI: 3.9-5.6 mmol/L]), postprandial blood sugar (PPBS) 350 mg/dL (SI: 19.4 mmol/L) (reference range, <140 mg/dL [SI: <7.8 mmol/L]), and glycated hemoglobin (HbA1c) at 10.9% (SI: 96 mmol/mol) (reference range, 4.0-5.6% [SI: 20-38 mmol/mol]). She had no history of diabetic ketoacidosis or DM-related hospitalizations. She was on metformin, glimepiride, vildagliptin, and B-complex vitamins for 5 years before presenting to us.
Diagnostic Assessment
Clinical examination revealed hypotonia, hyporeflexia, impaired posterior column sensation, absent joint position and vibratory sensation, an extensor plantar response, scoliosis, and peripheral signs of cerebellar dysfunction, without nystagmus or definitive dysarthria. There was no evidence of optic atrophy. Previous records indicated a positive Romberg sign and an ataxic gait at the onset of her symptoms.
Laboratory tests showed elevated fasting blood sugar at 336 mg/dL (SI: 18.6 mmol/L), PPBS at 371 mg/dL (SI: 20.6 mmol/L), and HbA1c at 13.3% (SI: 122 mmol/mol). Her fasting C-peptide level was 1.15 ng/mL (SI: 0.38 nmol/L) (reference range, 0.78-1.89 ng/mL [SI: 0.26-0.62 nmol/L]), postprandial C-peptide was 1.9 ng/mL (SI: 0.63 nmol/L) (reference range, 3.0-9.0 ng/mL [SI: 0.99-2.98 nmol/L]), fasting insulin level was 4 µU/mL (SI: 27.8 pmol/L) (reference range, 2-20 μU/mL [SI: 13.9-138.9 pmol/L]), and islet autoantibodies (glutamic acid decarboxylase 65 [GAD65], insulinoma-associated protein-2 [IA-2] and zinc transporter 8 [ZnT8] antibodies) were negative. Comprehensive metabolic assessment demonstrated severe insulin resistance (homeostatic model assessment 2 for insulin resistance [HOMA2-IR] of 4.5 [normal range, 1.0-1.5] and homeostatic model assessment 2 for beta cell function [HOMA2-%B] of 5 [normal range, 60-120]), suggestive of relative insulin deficiency.
Nerve conduction study indicated normal latency, amplitude, and motor nerve conduction velocity, while sensory nerve responses could not be obtained from all tested nerves, suggestive of sensory polyneuropathy. The nerve biopsy revealed features suggestive of hereditary motor sensory neuropathy with an axonal form, exhibiting mild reparative processes. Symmetric fiber loss in both large and small fibers, without onion bulb formation, occasional thin myelinated fibers, and randomly distributed focal sprouts were observed. Computed tomography scan showed mild brain atrophy. Cardiovascular examination with echocardiography revealed a small patent ductus arteriosus with a left-to-right shunt, an ejection fraction of 59%, left ventricular diastolic dysfunction, and trivial pulmonary regurgitation. The electrocardiogram showed mild conduction defects. Following clinical suspicion of FA, genetic testing was ordered.
Treatment
The patient's treatment course was implemented in 3 distinct phases. Initial management involved discontinuation of metformin and glimepiride, followed by initiation of insulin therapy (total daily dose: 26 units, approximately 0.5 U/kg/day) and sitagliptin 100 mg daily. This regimen produced modest glucose reduction but was marked by significant glycemic fluctuations, with coefficient of variation (CV) reaching 43% and mean amplitude of glycemic excursions (MAGE) of 120 mg/dL (SI: 6.7 mmol/L) during the first 6 weeks.
Following genetic confirmation of FA characterized by the pathogenic (GAA)n repeat expansion in the FXN gene (ClinVar accession number SCV006277911), the treatment protocol was expanded to include targeted mitochondrial support. This second phase incorporated L-carnitine 500 mg once a day, coenzyme Q10 (CoQ10) 100 mg once a day, and vitamin E 400 IU daily. Neurotropic vitamin supplementation (B1 10 mg, B2 10 mg, B3 45 mg, B5 50 mg, B6 3 mg, and B12 15 mcg daily) was also given.
The third therapeutic phase began approximately 17 months after mitochondrial agent initiation when imeglimin became available in India. This novel antihyperglycemic agent was added at 500 mg twice daily to further target mitochondrial dysfunction while providing additional glycemic control.
Outcome and Follow-Up
Metabolic outcomes improved progressively throughout all treatment phases. In the initial 6 weeks on insulin and a dipeptidyl peptidase-4 (DPP-4) inhibitor, glycemic control was unstable, with marked fluctuations: mean glucose 194 mg/dL, SD 83.5 mg/dL (SI: 10.8 mmol/L, SD 4.6 mmol/L), CV 43%, and MAGE 120 mg/dL (SI: 6.7 mmol/L). Glucose excursions ranged from 94 mg/dL (SI: 5.2 mmol/L) to 314 mg/dL (SI: 17.4 mmol/L).
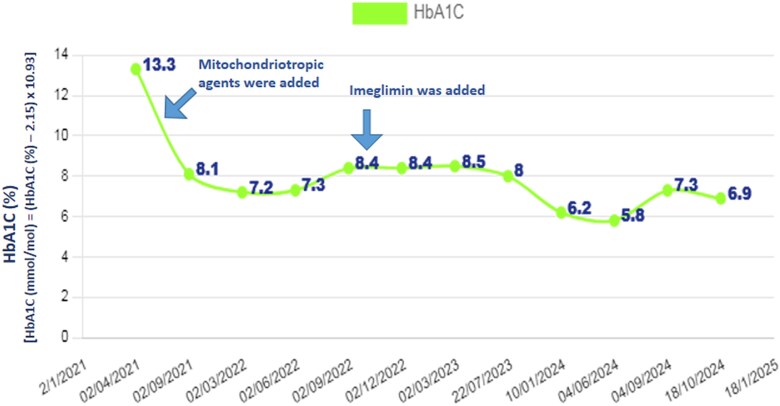
After the addition of mitochondriotropic agents, glycemic variability declined significantly over 17 months. Mean glucose dropped to 116 mg/dL (SI: 6.4 mmol/L), SD decreased to 44.2, CV reduced to 38.1%, and MAGE fell to 65 mg/dL (SI: 3.6 mmol/L). Extreme hyperglycemic episodes diminished, with a peak PPBS of 214 mg/dL (SI: 11.9 mmol/L). HbA1c also improved from 13.3% (SI: 122 mmol/mol) at baseline to 8.4% (SI: 68 mmol/mol) after 17 months, reflecting better long-term glycemic control and stability.
With the introduction of imeglimin over the subsequent 19 months, glycemic control was further optimized. Glucose variability was maintained within target (CV 34.9%; target < 36%), MAGE was 70 mg/dL (SI: 3.9 mmol/L), and HbA1c reached 6.9% (SI: 52 mmol/mol) (Figs. 1 and 2). Follow-up assessments showed a fasting C-peptide of 0.5 ng/mL (SI: 0.17 nmol/L), fasting insulin of 2.9 μU/mL (SI: 20.1 pmol/L), a robust postprandial C-peptide response (3.8 ng/mL [SI: 1.26 nmol/L]), and 24-hour urinary C-peptide excretion of 15.44 μg/day (SI: 5.11 nmol/day), indicating near-normal insulin sensitivity (HOMA2-IR 1.2) and improved glucose-responsive insulin secretion (HOMA2-%B 60). These metabolic improvements occurred without increasing insulin dosage.
Figure 1.
Long-term changes in blood sugar levels during therapy in Friedreich ataxia (FA). Serial measurements of fasting blood sugar (FBS) and postprandial blood sugar (PPBS) illustrating glycemic trends throughout the treatment course in patient with FA.
Figure 2.
Long-term changes in glycated hemoglobin (HbA1c) levels during therapy in FA Longitudinal changes in HbA1c levels depicting the progression of glycemic control during therapy in patient with FA.
Neurological function, measured by the International Cooperative Ataxia Rating Scale (ICARS), also showed improvement over 3 years: scores improved from 85 to 71 points. Enhanced kinetic function was evident, as illustrated by more accurate Archimedes spiral drawing (Fig. 3A and 3B). The simultaneous improvement in glycemic variability and neurological scores underscores a shared mitochondrial pathophysiology in both metabolic and neurological manifestations.
Figure 3.
Drawings of the Archimedes spiral to assess kinetic function in FA. (A). Baseline drawing of Archimedes spiral, illustrating impaired kinetic function prior to therapy initiation. (B). Follow-up drawing of the Archimedes spiral, showing improved accuracy and enhanced kinetic function after 3 years of treatment.
Discussion
Mitochondrial dysfunction in FA necessitates therapeutic strategies targeting mitochondrial protection. Mitochondriotropic agents improve metabolic control by enhancing mitochondrial function, reducing oxidative damage, and slowing apoptosis, as demonstrated in MIDD [11, 12]. However, certain antihyperglycemic agents (eg, metformin, thiazolidinediones) may worsen mitochondrial impairment by inhibiting complex I. While sulfonylureas were historically used, their risks (hypoglycemia, β-cell stress) favor safer alternatives like glucagon-like peptide-1 (GLP-1) analogs or DPP-4 inhibitors, which lack direct mitochondrial toxicity.
In FA, mitochondrial dysfunction disrupts pancreatic β- and α-cell ATP production, impairing insulin secretion and causing dysregulated glucagon release [15]. This leads to paradoxical hyperglucagonemia during hyperglycemia and inadequate glucagon response to hypoglycemia, exacerbating glycemic variability. Our patient's initial presentation demonstrated this dual pathophysiology, with both insulin resistance and deficiency. Mitochondriotropic therapy produced 3 key metabolic improvements, such as enhanced peripheral insulin sensitivity, preserved and potentially enhanced glucose-stimulated insulin secretion, and HbA1c reduction without escalating insulin therapy. These therapeutic effects likely represent both the reversal of chronic glucotoxicity and direct mitochondrial protection, with the dissociation between fasting and postprandial C-peptide responses indicating reduced basal β-cell workload due to normalized fasting glucose levels, and restored glucose-responsive insulin secretion capacity. Future studies involving glucagon dynamics and insulin clamp techniques could further characterize these dynamic endocrine effects.
Imeglimin, a novel antihyperglycemic agent, uniquely targets mitochondrial dysfunction by improving respiratory chain activity, reducing oxidative stress, and boosting ATP/NAD+ synthesis [16]. Its dual action, enhancing insulin secretion and sensitivity, makes it particularly relevant for FA-related DM, where mitochondrial failure drives both β-cell loss and insulin resistance.
There is evidence supporting the role of antioxidants in FA therapy, as oxidative stress has been identified as a consequence of reduced FXN levels [17]. Jauslin et al demonstrated that CoQ10 and its analogues could prevent cell death in fibroblasts from FA patients [18]. In a clinical trial published in 2008, CoQ10 was combined with vitamin E which demonstrated significant improvements in the ICARS scores after 2 years of treatment [19]. These results suggest that the combination of CoQ10 and vitamin E can help improve motor coordination and reduce the progression of FA-related symptoms, likely by enhancing mitochondrial function and reducing oxidative stress. L-carnitine plays a vital role in transporting long-chain fatty acids into mitochondria for ATP generation via β-oxidation [20]. In recent years, there has been increasing interest in the therapeutic potential of L-carnitine and acetyl L-carnitine for conditions associated with nervous system injury and degeneration [21]. Schols et al reported a significant improvement in mitochondrial ATP production in patients with FA who received L-carnitine [22].
Gene therapy offers a promising approach for definitive treatment of FA by correcting faulty genes using techniques like adeno-associated viral (AAV) vectors and CRISPR-Cas9 gene editing [23]. These methods have shown success in preclinical models by delivering functional FXN to affected tissues, particularly the heart and nervous system. Several AAV-based therapies, such as AAVrh.10hFXN are currently in clinical trials, showing early promise in improving FXN expression and disease markers [24]. However, these therapies are still in the trial phase, with challenges like immune responses and safe delivery to be addressed before approval for widespread patient use. Until such gene therapies become widely available, agents that enhance mitochondrial function may play a crucial role in delaying disease progression and stabilizing symptoms in patients with FA.
Natural history studies indicate FA typically progresses at 0.77 points annually on the Scale for Assessment and Rating of Ataxia (SARA) (SE 0.06) [25], with ICARS scores showing strong correlation to SARA measurements across disease stages [26]. For our patient, this would predict a 2.31-point worsening (ICARS 87.31) over 3 years. Remarkably, we observed a 14-point ICARS improvement (final score 71), representing a 16-point positive deviation from expected progression.
This case represents the first reported instance of concurrent long-term glycemic stabilization and ataxia improvement in FA, achieved through combined mitochondriotropic therapy. While previous studies have demonstrated neuroprotective benefits of these agents, our findings uniquely highlight their dual metabolic and neurological efficacy, particularly through the synergistic use of these agents. The observed outcomes—a 14-point ICARS improvement (in contrast to expected disease progression) and sustained glycemic control with reduced variability—suggest these agents may collectively, though partially, address FA's mitochondrial pathophysiology. The temporal association of clinical improvements with therapeutic interventions, despite progressive β-cell failure, supports the hypothesis that targeting multiple modes of mitochondrial dysfunction may be more effective than isolated therapies, highlighting the synergy of these agents. However, the individual contributions of each component remain unclear due to their complementary mechanisms and warrant future comparative studies to delineate agent-specific effects. This case reinforces mitochondriotropic therapy as a promising interim strategy for FA management, bridging the gap until gene therapies become clinically viable.
Learning Points
Mitochondriotropic agents stabilize glycemic control and improve ataxic symptoms in FA.
The stable glycemic control achieved is likely due to improved insulin sensitivity & preserved β-cell function.
Mitochondriotropic agents reduced ICARS for ataxic symptoms by 14 points in 3 years.
Ours is the first report to demonstrate improvement in both glycemia and ataxia in FA.
Acknowledgments
We would like to thank the Madras Diabetes Research Foundation (MDRF) in Chennai for their help with the genetic studies. We also acknowledge Dr. Anusree E, Pharm D for her assistance with manuscript preparation and typing. The authors further acknowledge the use of DeepSeek, an AI-based tool, which was employed to improve readability and language. The authors reviewed and edited the content as needed and take full responsibility for the final version of the manuscript.
Abbreviations
- AAV
adeno-associated virus
- CoQ10
coenzyme Q10
- CV
coefficient of variation
- DM
diabetes mellitus
- DPP-4
dipeptidyl peptidase-4
- FA
Friedreich ataxia
- FXN
frataxin
- HbA1c
glycated hemoglobin
- HOMA2-%B
homeostatic model assessment 2 of beta cell function
- HOMA2-IR
homeostatic model assessment 2 of insulin resistance
- ICARS
International Cooperative Ataxia Rating Scale
- MAGE
mean amplitude of glycemic excursions
- MIDD
maternally inherited diabetes
- PPBS
postprandial blood sugar
Contributor Information
Pichakacheri Sureshkumar, Dr. Suresh’s Diabcare India Diabetes Center, Calicut, Kerala 673017, India; Department of Life Science, Calicut University, Kerala 673635, India; Faculty of Medicine, Kerala University of Health Sciences, Thrissur, Kerala 680596, India.
Sidharth S Kumar, Dr. Suresh’s Diabcare India Diabetes Center, Calicut, Kerala 673017, India.
Johny Cheriyan, Department of Internal Medicine, Korambayil Hospital and Diagnostic Center, Manjeri, Kerala 676122, India.
Asif Masood, Department of General Medicine, Malabar Hospital, Manjeri, Kerala 676121, India.
Contributors
All authors made individual contributions to this work. P.S., S.S.K., J.C., and A.M. were involved in the clinical diagnosis, management, and longitudinal follow-up of the patient. P.S. and S.S.K. conceptualized the case report and drafted the manuscript. J.C. contributed to the neurological assessment and interpretation of ataxia-related outcomes. A.M. and P.S. provided expertise in diabetes management and metabolic monitoring. All authors critically reviewed, edited, and approved the final manuscript.
Funding
No public or commercial funding.
Disclosures
None declared.
Informed Patient Consent for Publication
Signed informed consent obtained directly from the patient.
Data Availability Statement
Original data generated and analyzed during this study are included in this published article.
References
- 1. Ristow M, Pfister MF, Yee AJ, et al. Frataxin activates mitochondrial energy conversion and oxidative phosphorylation. Proc Natl Acad Sci U S A. 2000;97(22):12239‐12243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Williams CT, De Jesus O. Friedreich ataxia. StatPearls. StatPearls Publishing; 2023. Updated June 27, 2023. Accessed June 10, 2024. https://www.ncbi.nlm.nih.gov/books/NBK563199/
- 3. Dürr A, Cossee M, Agid Y, et al. Clinical and genetic abnormalities in patients with Friedreich's ataxia. N Engl J Med. 1996;335(16):1169‐1175. [DOI] [PubMed] [Google Scholar]
- 4. Tamaroff J, DeDio A, Wade K, et al. Friedreich's ataxia related diabetes: epidemiology and management practices. Diabetes Res Clin Pract. 2022;186:109828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Melo M, Fagulha A, Barros L, Guimaraes J, Carrilho F, Carvalheiro M. Friedreich ataxia and diabetes mellitus-family study. Acta Med Port. 2005;18(6):479‐483. [PubMed] [Google Scholar]
- 6. Greeley NR, Regner S, Willi S, Lynch DR. Cross-sectional analysis of glucose metabolism in Friedreich ataxia. J Neurol Sci. 2014;342(1-2):29‐35. [DOI] [PubMed] [Google Scholar]
- 7. McCormick A, Farmer J, Perlman S, et al. Impact of diabetes in the Friedreich ataxia clinical outcome measures study. Ann Clin Transl Neurol. 2017;4(9):622‐631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Norose T, Ito Y, Ohike N. Two autopsy cases of mitochondrial disease (MELAS and MERRF) with special reference to the histological and immunohistochemical findings of the pancreatic islets. Pathol Int. 2020;70(11):915‐917. [DOI] [PubMed] [Google Scholar]
- 9. Befroy DE, Petersen KF, Dufour S, et al. Impaired mitochondrial substrate oxidation in muscle of insulin-resistant offspring of type 2 diabetic patients. Diabetes. 2007;56(5):1376‐1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cnop M, Mulder H, Igoillo-Esteve M. Diabetes in Friedreich ataxia. J Neurochem. 2013;126(Suppl 1):94‐102. [DOI] [PubMed] [Google Scholar]
- 11. Sureshkumar P, Radha V, Mohan V, Kumar SS, Babu AS. Maternally inherited diabetes and deafness (MIDD)—a series of case reports. Int J Diabetes Dev Ctries. 2023;43(4):583‐586. [Google Scholar]
- 12. Sureshkumar P, Radha V, Unnikrishnan R, Mohan V. Clinical profile of monogenic diabetes: a case series from a single south Indian diabetes clinic. Int J Diabetes Dev Ctries. 2024;13:1‐7. [Google Scholar]
- 13. Sazonova MA, Sinyov VV, Ryzhkova AI, et al. Role of mitochondrial genome mutations in pathogenesis of carotid atherosclerosis. Oxid Med Cell Longev. 2017;2017(1):6934394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Heidari MM, Mirfakhradini FS, Tayefi F, Ghorbani S, Khatami M, Hadadzadeh M. Novel point mutations in mitochondrial Mt-Co2 gene may be risk factors for coronary artery disease. Appl Biochem Biotechnol. 2020;191(3):1326‐1339. [DOI] [PubMed] [Google Scholar]
- 15. Grubelnik V, Markovič R, Lipovšek S, et al. Modelling of dysregulated glucagon secretion in type 2 diabetes by considering mitochondrial alterations in pancreatic α-cells. R Soc Open Sci. 2020;7(1):191171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hallakou-Bozec S, Vial G, Kergoat M, et al. Mechanism of action of imeglimin: a novel therapeutic agent for type 2 diabetes. Diabetes Obes Metab. 2021;23(3):664‐673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Edzeamey FJ, Ramchunder Z, Pourzand C, Virmouni SA. Emerging antioxidant therapies in Friedreich's ataxia. Front Pharmacol. 2024;15:1359618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jauslin ML, Meier T, Smith RA, Murphy MP. Mitochondria-targeted antioxidants protect Friedreich ataxia fibroblasts from endogenous oxidative stress more effectively than untargeted antioxidants. FASEB J. 2003;17(13):1‐10. [DOI] [PubMed] [Google Scholar]
- 19. Cooper JM, Korlipara LV, Hart PE, Bradley JL, Schapira AH. Coenzyme Q10 and vitamin E deficiency in Friedreich's ataxia: predictor of efficacy of vitamin E and coenzyme Q10 therapy. Eur J Neurol. 2008;15(12):1371‐1379. [DOI] [PubMed] [Google Scholar]
- 20. Ribas GS, Vargas CR, Wajner M. L-carnitine supplementation as a potential antioxidant therapy for inherited neurometabolic disorders. Gene. 2014;533(2):469‐476. [DOI] [PubMed] [Google Scholar]
- 21. Zhang R, Zhang H, Zhang Z, et al. Neuroprotective effects of pre-treatment with l-carnitine and acetyl-L-carnitine on ischemic injury in vivo and in vitro. Int J Mol Sci. 2012;13(2):2078‐2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schöls L, Zange J, Abele M, et al. L-carnitine and creatine in Friedreich's ataxia: a randomized, placebo-controlled crossover trial. J Neural Transm (Vienna). 2005;112(6):789‐796. [DOI] [PubMed] [Google Scholar]
- 23. Ouellet DL, Cherif K, Rousseau J, Tremblay JP. Deletion of the GAA repeats from the human frataxin gene using the CRISPR-Cas9 system in YG8R-derived cells and mouse models of Friedreich ataxia. Gene Ther. 2017;24(5):265‐274. [DOI] [PubMed] [Google Scholar]
- 24. Munoz-Zuluaga C, Gertz M, Yost-Bido M, et al. Identification of safe and effective intravenous dose of AAVrh.10hFXN to treat the cardiac manifestations of Friedreich's ataxia. Hum Gene Ther. 2023;34(13-14):605‐615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Reetz K, Dogan I, Hilgers RD, et al. Progression characteristics of the European Friedreich's Ataxia Consortium for Translational Studies (EFACTS): a 2 year cohort study. Lancet Neurol. 2016;15(13):1346‐1354. [DOI] [PubMed] [Google Scholar]
- 26. Rummey C, Harding IH, Delatycki MB, et al. Harmonizing results of ataxia rating scales: mFARS, SARA, and ICARS. Ann Clin Transl Neurol. 2022;9(12):2041‐2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Original data generated and analyzed during this study are included in this published article.