Abstract
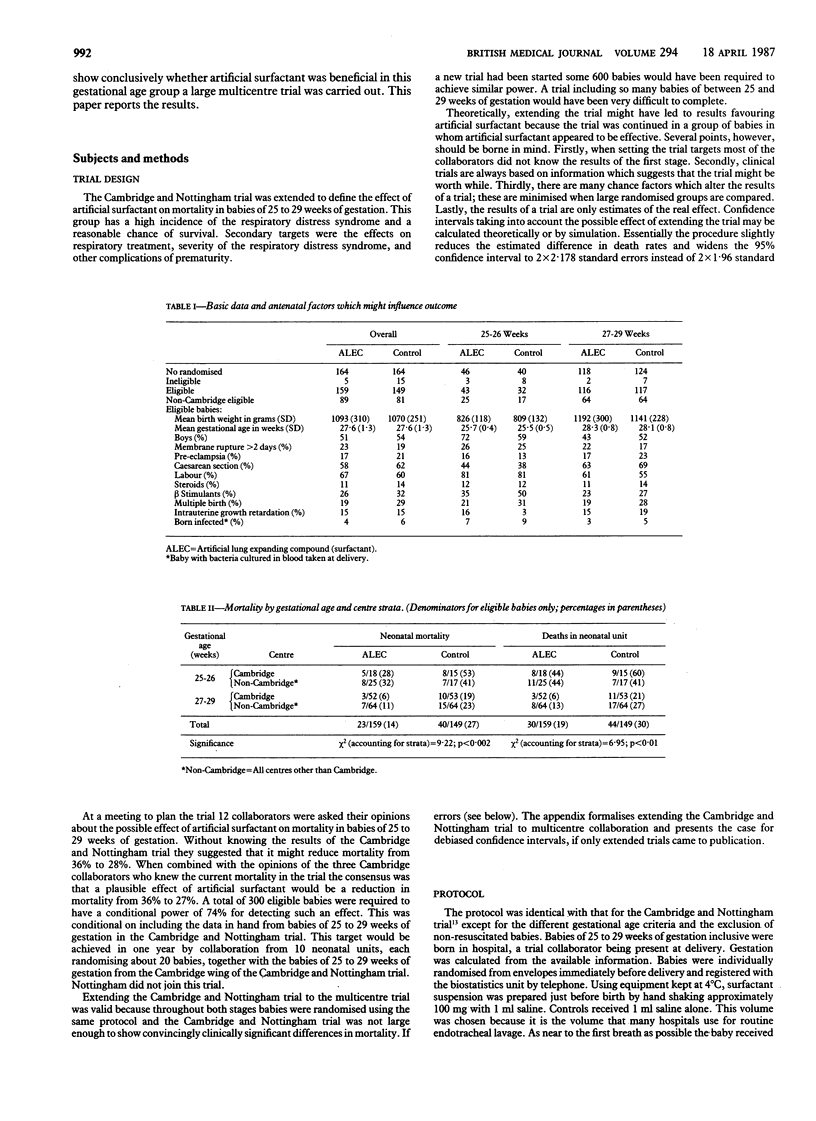
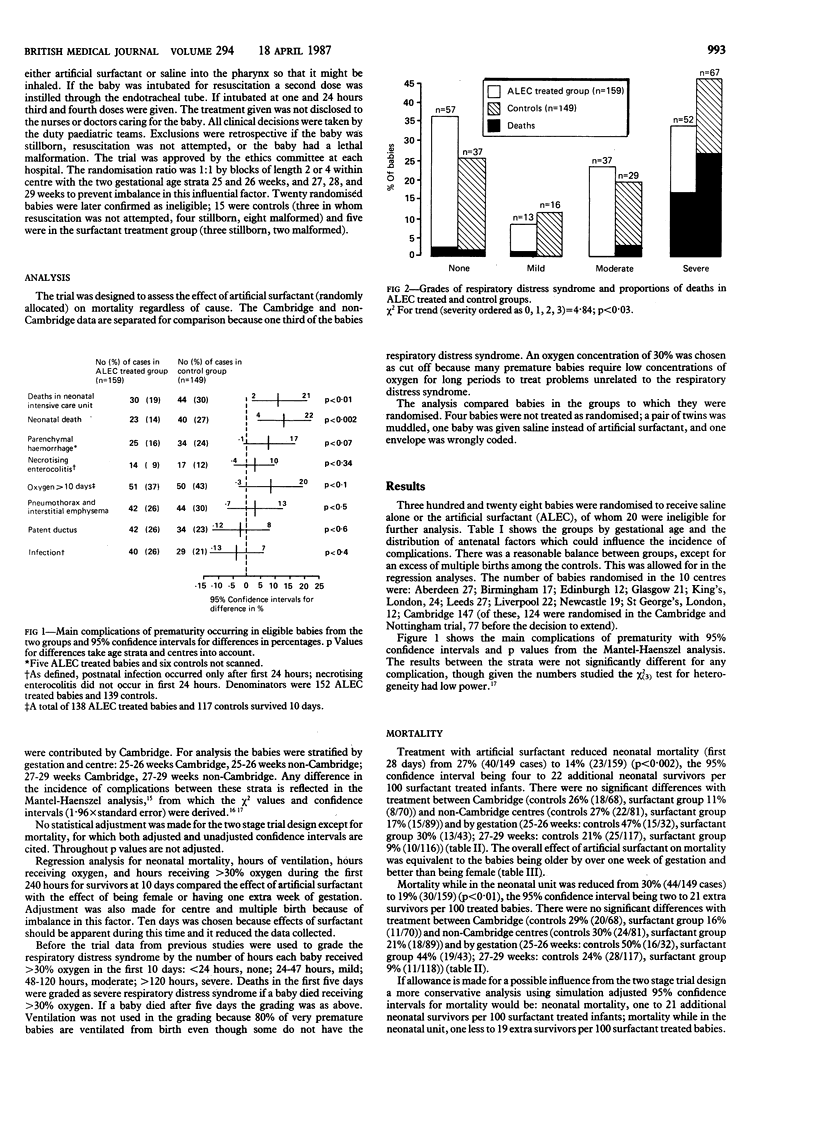
A protein free artificial surfactant (artificial lung expanding compound; ALEC) composed of dipalmitoylphosphatidylcholine and phosphatidylglycerol was assessed for its effect on the main complications of prematurity in a prospective two stage randomised trial of 328 unselected babies delivered at between 25 and 29 weeks of gestation. Babies were randomised to receive approximately 100 mg artificial surfactant suspension or 1 ml saline. This was given at birth into the pharynx with up to three more endotracheal doses if the baby was intubated during the first day. Treatment with artificial surfactant reduced the neonatal mortality from 27% to 14%, the incidence of parenchymal brain haemorrhages from 24% to 16%, and the severity of the respiratory distress syndrome. In the first 10 days babies treated with artificial surfactant who survived averaged 19 hours less in greater than 30% oxygen, 20 hours less ventilation, and 17 hours less supplemental oxygen. Artificial surfactant had no effect on the incidence of pneumothoraces, pulmonary interstitial emphysema, patent ductus arteriosus, or postnatal infections and no serious side effects. Artificial surfactant (ALEC) given to very premature babies at birth significantly reduces their mortality and the respiratory support needed and should prove a valuable addition to treatment.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bangham A. D. Lung surfactant: how it does and does not work. Lung. 1987;165(1):17–25. doi: 10.1007/BF02714417. [DOI] [PubMed] [Google Scholar]
- Enhorning G., Shennan A., Possmayer F., Dunn M., Chen C. P., Milligan J. Prevention of neonatal respiratory distress syndrome by tracheal instillation of surfactant: a randomized clinical trial. Pediatrics. 1985 Aug;76(2):145–153. [PubMed] [Google Scholar]
- Fujiwara T., Maeta H., Chida S., Morita T., Watabe Y., Abe T. Artificial surfactant therapy in hyaline-membrane disease. Lancet. 1980 Jan 12;1(8159):55–59. doi: 10.1016/s0140-6736(80)90489-4. [DOI] [PubMed] [Google Scholar]
- Gore S. M., Oldham J. A. Randomized trials of high-versus-low-dose steroids in renal transplantation. Does the evidence favor a consensus? Transplantation. 1986 Mar;41(3):319–327. doi: 10.1097/00007890-198603000-00008. [DOI] [PubMed] [Google Scholar]
- Halliday H. L., McClure G., Reid M. M., Lappin T. R., Meban C., Thomas P. S. Controlled trial of artificial surfactant to prevent respiratory distress syndrome. Lancet. 1984 Mar 3;1(8375):476–478. doi: 10.1016/s0140-6736(84)92849-6. [DOI] [PubMed] [Google Scholar]
- Kwong M. S., Egan E. A., Notter R. H., Shapiro D. L. Double-blind clinical trial of calf lung surfactant extract for the prevention of hyaline membrane disease in extremely premature infants. Pediatrics. 1985 Oct;76(4):585–592. [PubMed] [Google Scholar]
- Merritt T. A., Hallman M., Bloom B. T., Berry C., Benirschke K., Sahn D., Key T., Edwards D., Jarvenpaa A. L., Pohjavuori M. Prophylactic treatment of very premature infants with human surfactant. N Engl J Med. 1986 Sep 25;315(13):785–790. doi: 10.1056/NEJM198609253151301. [DOI] [PubMed] [Google Scholar]
- Morley C. J., Bangham A. D., Miller N., Davis J. A. Dry artificial lung surfactant and its effect on very premature babies. Lancet. 1981 Jan 10;1(8211):64–68. doi: 10.1016/s0140-6736(81)90002-7. [DOI] [PubMed] [Google Scholar]
- Morley C. J., Banhham A. D., Johnson P., Thorburn G. D., Jenkin G. Physical and physiological properties of dry lung surfactant. Nature. 1978 Jan 12;271(5641):162–163. doi: 10.1038/271162a0. [DOI] [PubMed] [Google Scholar]
- Morley C., Robertson B., Lachmann B., Nilsson R., Bangham A., Grossmann G., Miller N. Artificial surfactant and natural surfactant. Comparative study of the effects on premature rabbit lungs. Arch Dis Child. 1980 Oct;55(10):758–765. doi: 10.1136/adc.55.10.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro D. L., Notter R. H., Morin F. C., 3rd, Deluga K. S., Golub L. M., Sinkin R. A., Weiss K. I., Cox C. Double-blind, randomized trial of a calf lung surfactant extract administered at birth to very premature infants for prevention of respiratory distress syndrome. Pediatrics. 1985 Oct;76(4):593–599. [PubMed] [Google Scholar]
- Simpson H. C., Simpson R. W., Lousley S., Carter R. D., Geekie M., Hockaday T. D., Mann J. I. A high carbohydrate leguminous fibre diet improves all aspects of diabetic control. Lancet. 1981 Jan 3;1(8210):1–5. doi: 10.1016/s0140-6736(81)90112-4. [DOI] [PubMed] [Google Scholar]
- Wilkinson A., Jenkins P. A., Jeffrey J. A. Two controlled trials of dry artificial surfactant: early effects and later outcome in babies with surfactant deficiency. Lancet. 1985 Aug 10;2(8450):287–291. doi: 10.1016/s0140-6736(85)90346-0. [DOI] [PubMed] [Google Scholar]


