Abstract
Somapacitan, a once-weekly GH preparation used to treat GH deficiency, has effects on glucose tolerance similar to those of daily GH (somatropin) treatment. However, its effects on glycemic control in patients with type 1 diabetes mellitus (T1DM) remain unclear. A 50-year-old man with hypopituitarism and T1DM switched from somatropin to somapacitan at his request. Soon thereafter, he experienced frequent hypoglycemia, which led to an emergency department visit. Continuous glucose monitoring revealed weekly glucose fluctuations, which correlated with GH levels. Serum free fatty acids showed a parallel trend. These findings suggested that insulin dose adjustments throughout the week were necessary. Despite increasing the somapacitan dose, insulin titration was still required to stabilize glycemic variability. After treatment modifications, the number of hypoglycemic episodes significantly decreased, and his quality of life improved. This case highlights the need for close glucose monitoring and individualized insulin management when transitioning from daily to long-acting GH therapy in insulin-dependent patients. This report provides insights into the metabolic effects of GH fluctuations in T1DM and the potential impact of somapacitan on insulin sensitivity. Further studies are warranted to establish optimal GH dosing strategies for patients with diabetes undergoing GH replacement therapy.
Keywords: growth hormone deficiency, type 1 diabetes mellitus, somapacitan, hypoglycemia
Introduction
Somapacitan is a once-weekly, long-acting GH derivative recently approved for patients with GH deficiency (GHD). Somapacitan does not differ from somatropin in its impact on glucose tolerance in healthy individuals [1]. However, its effect on patients with diabetes remains largely unknown. Although the coexistence of type 1 diabetes mellitus (T1DM) and GHD is rare, it is associated with a high risk of hypoglycemia [2]. This is partially because patients with T1DM have inherently low IGF-I levels due to hepatic GH resistance caused by reduced portal insulin levels [3]. In patients who also have GHD, this combined effect may further lower serum IGF-I levels and increase susceptibility to glycemic fluctuations when GH therapy is adjusted. GH replacement therapy can mitigate this risk by improving IGF-I levels and reducing episodes of hypoglycemia [4]. However, there are no reports on the actual use of long-acting GH preparations, including somapacitan, in patients with T1DM. We present the first case of weekly GH preparations for GHD complicated by T1DM. The glycemic trends we found suggest points healthcare providers should be aware of when treating similar cases.
Case Presentation
A 50-year-old man was diagnosed with T1DM 36 years before presentation. Five years previously, he was diagnosed with panhypopituitarism because of a Rathke cleft cyst, for which he underwent transsphenoidal surgery 1 year later. His medical history also included epilepsy because of severe heatstroke and osteoporosis. His medications comprised desmopressin, eldecalcitol, denosumab, and levetiracetam, along with insulin therapy and hormone replacement therapy for anterior pituitary dysfunction, which are described in detail later. In addition to hydrocortisone (15 mg/day), levothyroxine (100 mcg/day), and gonadotropin treatment (4000 units/day), GH replacement therapy using somatropin was initiated 3 years ago and was titrated to 0.7 mg/day. Because weekly GH preparations and somapacitan were approved for adult GHD, somatropin was switched to somapacitan at his request, starting from the recommended initiation dose (1.5 mg/week). Following this switch, he experienced an increase in episodes of hypoglycemia. Two weeks prior, he visited the emergency department for severe hypoglycemia and was subsequently hospitalized for evaluation and adjustment of his treatment plan to prevent further episodes.
Diagnostic Assessment
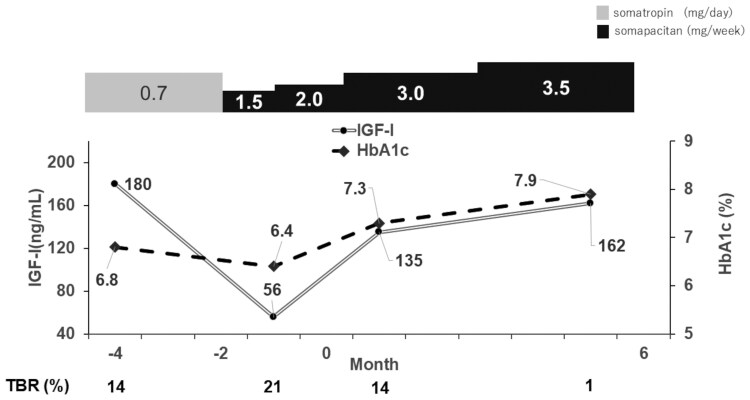
At the admission, he was 170 cm tall and weighed 62.0 kg, with a body mass index of 21.4 kg/m2. His blood pressure was 98/76 mmHg, and his pulse rate was 65 beats/min. A physical examination revealed no abnormalities. General laboratory tests showed that the blood count and biochemical findings were unremarkable, and hemoglobin A1c (HbA1c) levels were 6.8% [normal reference range (ref): 4.9-6.0%]. Fasting plasma glucose levels were 95 mg/dL (5.3 mmol/L) (ref: 73-109 mg/dL; 4.0-6.1 mmol/L), serum C-peptide immunoreactivity levels were <0.01 ng/mL (<0.003 nmol/L) (ref: 0.69-2.45 ng/mL; 0.23-0.82 nmol/L), and the 24-hour urinary C-peptide immunoreactivity levels were <0.1 μg/day (<0.03 nmol/day) (ref: 22.8~155.2 μg/day; 7.5~51.3 nmol/day), indicating that endogenous insulin secretion was completely depleted. His pituitary associated hormone levels were as follows: thyrotropin, 0.01 mIU/L (ref: 0.610-4.230 mIU/L); free T3, 1.28 ng/dL (16.5 pmol/L) (ref: 0.90-1.70 ng/dL; 11.6-21.9 pmol/L); free T4, 1.9 pg/mL (2.92 pmol/L) (ref: 2.3-4.0 pg/mL; 3.53-6.14 pmol/L); cortisol: 0.9 µg/dL (24.8 nmol/L) (ref: 7.1-19.6 μg/dL;196-540 nmol/L); adrenocorticotropin, 2.9 pg/mL (0.638 pmol/L) (ref: 7.7-63.1 pg/mL;7-13.9 nmol/L); total testosterone, 8.56 ng/mL (29.7 nmol/L) (ref: 1.31-8.71 ng/mL; 4.54-30.2 nmol/L); FSH, <0.3 mIU/mL (ref: 1.5-12.4 mIU/mL); and LH, <0.3 mIU/mL (1.7-8.6 mIU/mL). His serum IGF-I levels were 86 ng/mL, with a calculated SD of −2.1, adjusted for sex and age [5]. While treated with somatropin at 0.7 mg/day, his IGF-I levels were 180 ng/mL (+0.7 SD) and this decreased significantly after switching to the recommended starting dose of somapacitan (Fig. 1). On day 2 after somapacitan administration, the average sensory glucose (SG) value increased from 143 to 275 mg/dL compared to the day before administration (day 0), despite an increase in the total daily dose (TDD) from 26.5 units/day to 32.5 units/day (Fig. 2). The increase in the TDD and SG values showed a weekly cycle, peaking on day 3 of administration and gradually declining thereafter. In addition, serum free fatty acid (FFA) levels, thought to be one of the causes of GH-induced insulin resistance when elevated, were measured on days 0, 1, and 7. Of note, serum FFA levels increased on day 1 compared with day 0 and subsequently declined, paralleling the changes in the TDD and SG values (Fig. 3). Furthermore, weekly TDD fluctuations and SG value changes were recorded over 4 weeks. The day of somapacitan administration during hospitalization was designated as day 0, and the data are summarized accordingly (Fig. 4). This parallel fluctuation in SG and TDD values following somapacitan administration was observed as a recurring weekly pattern. The clinical course of glycemic control, fluctuations, and management during the switching of GH preparations is shown in Fig. 1. During GH replacement with somatropin at 0.7 mg/day, the IGF-I levels were 180 ng/mL (+0.7 SD), with a hypoglycemia frequency of 14% within 1 month and an HbA1c level of 6.8% over a 2-month period. However, after switching to somapacitan at 1.5 mg/week, the IGF-I levels rapidly dropped to 56 ng/mL (−3.4 SD), while the frequency of hypoglycemia increased to 21% within 1 month.
Figure 1.
Comparison of weekly somapacitan and daily somatropin treatments in terms of IGF-I levels and TBR. Changes in IGF-I levels over time (months) are shown for patients treated with somatropin (0.7 mg/day) and somapacitan (1.5-3.5 mg/week). The percentage of TBR is also presented for each group. After switching from somatropin to somapacitan, IGF-I levels temporarily decreased and TBR increased, but both parameters improved following an increase in the somapacitan dose. Month 0 corresponds to the time of hospital admission.
Abbreviation: TBR, time below range.
Figure 2.
Glycemic variability over a week in the patient treated with somapacitan 2.0 mg. SG values over a week are shown for the patient receiving somapacitan 2.0 mg. On day 2 of somapacitan administration, the average SG value increased markedly to 275 mg/dL compared to 143 mg/dL on day 0, despite an increase in total daily dose from 26.5 units/day to 32.5 units/day.
Abbreviation: SG, sensor glucose.
Figure 3.
Weekly cycle of insulin dose, glycemic control, and FFA levels during somapacitan treatment. TDD and average SG values showed a weekly cyclical pattern, peaking on day 3 after somapacitan administration and gradually declining thereafter. Serum FFA levels—considered one of the contributors to GH-induced insulin resistance—were measured on days 0, 1, and 7. FFA levels increased on day 1 compared to day 0 and subsequently declined by day 7, paralleling the trends observed in TDD and SG values.
Abbreviations: FFA, free fatty acid; SG, sensor glucose; TDD, total daily dose.
Figure 4.
Weekly trends in insulin dose and glycemic control over 4 weeks of somapacitan treatment. Weekly fluctuations in total daily dose and changes in average sensor glucose values were recorded over 4 weeks. The day of somapacitan administration during hospitalization was designated as day 0 each week, and the data were aligned and summarized based on this timeline.
Treatment
To stabilize these intraweekly glycemic fluctuations, carbohydrate counting was performed, and the carbohydrate-insulin ratio was increased to accommodate the peak observed on days 2 to 5 following somapacitan administration. Since the dose of somapacitan was considered low based on his serum IGF-I levels and seemed to contribute to his frequent hypoglycemic attacks, we increased the dose from 2 to 3 mg/week and then observed the SG values. After increasing somapacitan and adjusting insulin, hypoglycemia was rare. However, the TDD still needed to be increased to ensure that the SG value trend was not altered.
Outcome and Follow-up
Two months after discharge, his continuous glucose monitoring records demonstrated a decline in the time below range from 14% before admission to 1%. No further instances of severe hypoglycemia were observed. His serum IGF-I levels increased to 135 ng/mL (−0.5 SD) 1 month after discharge. Given the suboptimal outcomes observed in the Adult Hypopituitarism Questionnaire, particularly regarding restrictions in social activities and overall physical well-being [6], along with the insufficient increase in IGF-I levels, an escalation of the somapacitan dosage from 3.0 to 3.5 mg/week was deemed necessary. Five months after discharge, his IGF-I levels increased to 162 (+0.3 SD), and his quality of life improved, specifically regarding restrictions on social activities and overall physical well-being. Although his HbA1c levels increased to approximately 8%, an increased insulin dosage helped reduce it to approximately 7.0% within 1 year.
Discussion
This is the first case report of the use of weekly GH preparations for adult GHD complicated by T1DM. Patients with GHD with the coexistence of T1DM are at high risk of hypoglycemia, and our case revealed the importance of the interaction between the GH/IGF-I axis and insulin signaling in glycemic homeostasis. Patients with GHD have reduced muscle glycogen synthesis and glucose storage capacity [7]. While GH promotes lipolysis and elevates FFAs, contributing to insulin resistance, IGF-I counters this by enhancing glucose uptake and glycogen synthesis, thereby improving insulin sensitivity [8]. Thus, alterations in this axis, whether due to GHD itself or adjustments in GH therapy, can profoundly affect glucose metabolism. Although other pituitary hormone deficiencies could contribute to hypoglycemia, stable replacement without signs of adrenal insufficiency suggests GH was the main factor; nonetheless, impaired cortisol response to hypoglycemia may have had an negative impact in the present case. Although severe hypoglycemia is well documented in pediatric GHD and mitigated by somatropin therapy, it is rare in adults, possibly due to larger fat reserves [4, 9]. Patients with T1DM inherently have low IGF-I levels owing to hepatic GH resistance caused by reduced portal insulin levels [3]. In individuals who have both T1DM and GHD, this combined effect may further lower IGF-I levels and heighten vulnerability to glycemic instability, especially when GH therapy is modified. Given this potential for increased glycemic volatility in such patients, it is important to consider how different GH replacement regimens might affect glucose metabolism. Although no studies have directly compared daily vs weekly GH replacement in GHD patients with diabetes, trials in nondiabetic GHD have shown comparable effects on glucose metabolism, including HbA1c [1]. Our case highlights that, in patients with coexisting GHD and T1DM, switching GH formulations may necessitate careful monitoring and individualized dose adjustments due to potential impacts on glycemic variability. Markedly low IGF-I levels after switching to somapacitan likely contributed to glycemic fluctuations, which improved with dose escalation. However, even after IGF-I normalized, weekly adjustments in insulin dose and carbohydrate ratio were still required, suggesting that the pharmacokinetics of somapacitan influence glycemic variability. Therefore, individualized titration and vigilance for hypoglycemia are essential when transitioning from daily to weekly GH, especially in patients with coexisting GHD and T1DM. This case highlights the need for individualized GH dosing strategies when transitioning from daily to weekly therapy in patients with insulin dependence. A previously reported maintenance dose of somapacitan was 8.2 times that of somatropin [10], suggesting that the switching dose may need to be carefully considered in this context. GH replacement therapy has been shown to increase the daily insulin requirements in patients with insulin-dependent diabetes, likely due to FFA-induced insulin resistance [11]. In the present case, we observed what appeared to be parallel changes in serum FFA levels and impaired insulin sensitivity after somapacitan administration. In our case, weekly fluctuations in insulin sensitivity appeared to parallel serum FFA levels, aligning with the pharmacokinetics of somapacitan, which peaks 1 to 2 days postadministration and then declines [12]. These observations support our hypothesis that FFA dynamics may underline the glycemic variability seen with weekly GH therapy. Increasing the somapacitan dose reduced the frequency of hypoglycemia. It also led to elevated blood glucose levels, necessitating appropriate adjustment of the TDD. Therefore, when treating patients with GHD and insulin-dependent diabetes mellitus, including T1DM, with somapacitan, various measures should be taken to stabilize the blood glucose levels. It was recommended that the patient transition back to somatropin or consider an insulin pump for more stable glycemic control. However, the patient expressed a strong preference for not wearing a continuous infusion device and preferred the convenience of fewer injections and the prolonged duration of action offered by once-weekly somapacitan. Therefore, we decided to continue the current treatment regimen. In conclusion, we report the first case of coexistent T1DM and GHD in which the GH preparation was switched from a daily somatropin dose to a weekly somapacitan dose. Severe hypoglycemia occurred during switching. We further showed that somapacitan induced intraweekly blood glucose fluctuations, which might be parallel to serum FFA levels. Daily intraweekly insulin dose variations were required even after somapacitan titration. Further studies are required to determine whether this phenomenon is common in patients with both insulin-dependent diabetes mellitus and GHD.
Learning Points
When switching from daily to long-acting GH preparations in patients with GHD and T1DM, initiating GH at a dose that leads to a decrease in IGF-I levels may increase the risk of hypoglycemia.
Increasing the somapacitan dose reduced the frequency of hypoglycemia; however, the patient required insulin dose adjustments throughout the week.
This case suggests that weekly GH preparations may aid in elucidating the relationship between blood GH levels, FFA levels, and insulin sensitivity.
Acknowledgments
We sincerely thank Dr. K. Yoshino, Ms. M. Saito, and Ms. T. Soki for their valuable assistance in preparing this case report.
Contributor Information
Yuichi Higuchi, Division of Diabetes and Endocrinology, Department of Internal Medicine, Kobe University Hospital, Kobe 650-0017, Japan.
Hironori Bando, Division of Diabetes and Endocrinology, Department of Internal Medicine, Kobe University Graduate School of Medicine, Kobe 650-0017, Japan.
Masaaki Yamamoto, Division of Diabetes and Endocrinology, Department of Internal Medicine, Kobe University Graduate School of Medicine, Kobe 650-0017, Japan.
Yushi Hirota, Division of Diabetes and Endocrinology, Department of Internal Medicine, Kobe University Graduate School of Medicine, Kobe 650-0017, Japan.
Wataru Ogawa, Division of Translational Research, Section of Metabolic Disease, Kobe University Graduate School of Medicine, Kobe 650-0017, Japan.
Hidenori Fukuoka, Division of Diabetes and Endocrinology, Department of Internal Medicine, Kobe University Hospital, Kobe 650-0017, Japan.
Contributors
All authors made individual contributions to authorship. Yui.H. was involved in diagnosis and management of the patient and in the preparation of the manuscript. Yus.H. contributed to the management and follow-up of the patient and reviewed the manuscript. H.B., M.Y., and W.O. were involved in the management of the patient and reviewed the manuscript. H.F. was involved in the diagnosis, management, and follow-up of the patient; edited the manuscript; and was responsible for its submission. All authors reviewed and approved the final draft.
Funding
This work was supported by the Foundation for Growth Science (H.F.).
Disclosures
Yus.H. has received lecture fees from Eli Lilly Japan K.K., Sanofi, Terumo Corp., Sumitomo Pharma Co. Ltd., Novo Nordisk Pharma Ltd., and Abbott Japan LLC; research expenses (including for contracted research, joint research, and clinical trials) and grant from Sumitomo Pharma Co. Ltd., Kyowa Kirin Co. Ltd., Medtronic Japan Co. Ltd., and Nippon Boehringer Ingelheim Co. Ltd.; and scholarship donations from Abbott Japan LLC. W.O. has received lecture fees from Sumitomo Pharma Co., Ltd., Boehringer Ingelheim Japan, Inc., Novo Nordisk Pharma Ltd., Eli Lilly Japan K.K.; research expenses (including for contracted research, joint research, and clinical trials) and grant from Boehringer Ingelheim Japan, Inc., Eli Lilly Japan K.K., Sumitomo Pharma Co., Ltd., Novo Nordisk Pharma Ltd.; and scholarship donations from Sumitomo Pharma Co., Ltd. H.F. has received lecture fees from Novo Nordisk Pharma Ltd. Yui.H., M.Y., and H.B. have nothing to disclose.
Informed Patient Consent for Publication
Signed informed consent obtained directly from patient.
Data Availability Statement
Data sharing is not applicable to this article, as no datasets were generated or analyzed in the current study.
References
- 1. Takahashi Y, Biller BMK, Fukuoka H, et al. Weekly somapacitan had no adverse effects on glucose metabolism in adults with growth hormone deficiency. Pituitary. 2023;26(1):57‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Christ ER, Simpson HL, Breen L, Sönksen PH, Russell-Jones DL, Kohner EM. The effect of growth hormone (GH) replacement therapy in adult patients with type 1 diabetes mellitus and GH deficiency. Clin Endocrinol (Oxf). 2003;58(3):309‐315. [DOI] [PubMed] [Google Scholar]
- 3. Nambam B, Schatz D. Growth hormone and insulin-like growth factor-I axis in type 1 diabetes. Growth Horm IGF Res. 2018;38:49‐52. [DOI] [PubMed] [Google Scholar]
- 4. Smyczyńska J, Pawelak N, Hilczer M, Lewiński A. Delayed diagnosis of congenital combined pituitary hormone deficiency including severe growth hormone deficiency in children with persistent neonatal hypoglycemia—case reports and review. Int J Mol Sci. 2022;23(19):11069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Isojima T, Shimatsu A, Yokoya S, et al. Standardized centile curves and reference intervals of serum insulin-like growth factor-I (IGF-I) levels in a normal Japanese population using the LMS method. Endocr J. 2012;59(9):771‐780. [DOI] [PubMed] [Google Scholar]
- 6. Ishii H, Shimatsu A, Okimura Y, et al. Development and validation of a new questionnaire assessing quality of life in adults with hypopituitarism: adult hypopituitarism questionnaire (AHQ). PLoS One. 2012;7(9):e44304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hew FL, Koschmann M, Christopher M, et al. Insulin resistance in growth hormone-deficient adults: defects in glucose utilization and glycogen synthase activity. J Clin Endocrinol Metab. 1996;81(2):555‐564. [DOI] [PubMed] [Google Scholar]
- 8. Aghili ZS, Khoshnevisan G, Mostoli R, Alibaglouei M, Zarkesh-Esfahani SH. Growth hormone signaling and clinical implications: from molecular to therapeutic perspectives. Mol Biol Rep. 2025;52(1):202. [DOI] [PubMed] [Google Scholar]
- 9. Pia A, Piovesan A, Tassone F, et al. A rare case of adulthood-onset growth hormone deficiency presenting as sporadic, symptomatic hypoglycemia. J Endocrinol Invest. 2004;27(11):1060‐1064. [DOI] [PubMed] [Google Scholar]
- 10. Rasmussen MH, Nedjatian N, Svaerke C, et al. Dose–exposure–IGF-I response of once-weekly somapacitan in adults with GH deficiency. Eur J Endocrinol. 2022;187(1):27‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sharma R, Kopchick JJ, Puri V, Sharma VM. Effect of growth hormone on insulin signaling. Mol Cell Endocrinol. 2020;518:111038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rasmussen MH, Brændholt Olsen MW, Alifrangis L, Klim S, Suntum M. A reversible albumin-binding growth hormone derivative is well tolerated and possesses a potential once-weekly treatment profile. J Clin Endocrinol Metab. 2014;99(10):E1819‐E1829. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article, as no datasets were generated or analyzed in the current study.