Abstract
An infectious nonpathogenic molecular clone (19-2-6A) of equine infectious anemia virus (EIAV) was modified by substitution of a 3.3-kbp fragment amplified by PCR techniques from a pathogenic variant (EIAVPV) of the cell culture-adapted strain of EIAV (EIAVPR). This substitution consisted of coding sequences for 77 amino acids at the carboxyl terminus of the integrase, the S1 (encoding the second exon of tat), S2, and S3 (encoding the second exon of rev) open reading frames, the complete env gene (including the first exon of rev), and the 3′ long terminal repeat (LTR). Modified 19-2-6A molecular clones were designated EIAVPV3.3, and infection of a single pony (678) with viruses derived from a mixture of five of these molecular clones induced clinical signs of acute equine infectious anemia (EIA) at 23 days postinfection (dpi). As a consequence of this initial study, a single molecular clone, EIAVPV3.3#3 (redesignated EIAVUK), was selected for further study and inoculated into two ponies (613 and 614) and two horses (700 and 764). Pony 614 and the two horses developed febrile responses by 12 dpi, which was accompanied by a 48 to 64% reduction in platelet number, whereas pony 613 did not develop fever (40.6°C) until 76 dpi. EIAV could be isolated from the plasma of these animals by 5 to 7 dpi, and all became seropositive for antibodies to this virus by 21 dpi. Analysis of the complete nucleotide sequence demonstrated that the 3.3-kbp 3′ fragment of EIAVUK differed from the consensus sequence of EIAVPV by just a single amino acid residue in the second exon of the rev gene. Complete homology with the EIAVPV consensus sequence was observed in the hypervariable region of the LTR. However, EIAVUK was found to contain an unusual 68-bp nucleotide insertion/duplication in a normally conserved region of the LTR sequence. These results demonstrate that substitution of a 3.3-kbp fragment from the EIAVPV strain into the infectious nonpathogenic molecular clone 19-2-6A leads to the production of progeny virus particles with the ability to induce clinical signs of EIA. Therefore, EIAVUK, which is the first pathogenic, cell culture-adapted molecular clone of EIAV to be described, should be of value in identifying viral determinants of pathogenicity.
Infection of horses and ponies (Equus caballus) with the equine lentivirus equine infectious anemia virus (EIAV) results in a persistent, life-long infection in which evidence of extensive active viral replication can be detected in blood samples as much as 20 years after the initial diagnosis (30). However, the course and severity of the disease following infection vary considerably, with some animals suffering debilitating cycles of illness and occasionally even death, while others show no obvious clinical signs. In experimental infections where disease is apparent, the first clinical episode usually occurs within 42 days after administration of the virus (reviewed in references 6, 10, 40, and 54). By convention, this first fever episode is referred to as the acute stage of equine infectious anemia (EIA) and is characterized by pyrexia, thrombocytopenia, anorexia, depression, and high plasma viremia levels (reviewed in references 6, 10, 40, and 54). Anemia is not usually detected at this stage (reviewed in references 6, 10, 40, and 54). Resolution of this first febrile episode is normally observed after 1 to 5 days and occurs concomitantly with a dramatic drop in the amount of plasma-associated virus (reviewed in references 6, 10, 40, and 54). Following the acute stage, some animals may remain clinically normal (20), while others go on to experience multiple bouts of illness in which severe anemia may accompany pyrexia, thrombocytopenia, edema, and dramatic weight loss (reviewed in references 6, 10, 40, and 54).
The mechanisms that control the broad range of clinical responses are unknown but almost certainly involve both host and viral factors, including the probable existence of EIAV strains with widely different pathogenic potentials. In the laboratory, it is possible to attenuate virulent wild-type isolates of EIAV by adaptation to growth in fibroblastic cell types (3, 19). These wild-type isolates of EIAV are normally tropic for cells of the monocyte/macrophage lineage (24–27, 39, 53, 59), and so virulence determinants must either be lost or altered when selection pressure in the form of growth in nonnatural target cells is applied.
In this laboratory, the avirulent, cell-adapted version of the macrophage tropic Wyoming isolate (36) is designated the prototype (EIAVPR) strain of EIAV. Infection of horses and ponies with this virus produces no discernible signs of disease as monitored by rectal temperature, platelet numbers, or behavioral changes, within a normal observation period of 100 to 150 days (21, 22). However, pathogenic properties were restored when the EIAVPR strain was subjected to three serial passages in ponies (44), followed by selection and biological cloning of a neutralization escape mutant (50). This antigenic variant is designated EIAVPV. Infection of horses and ponies with this strain routinely induces symptoms of acute EIA within 42 days (11, 20, 22, 60), although variability between individual animals in the severity of disease and the number of febrile episodes is observed. Interestingly, EIAVPV retained the in vitro cell tropism characteristics of the EIAVPR strain, indicating that virulence and ability to grow in fibroblastic cell cultures are not mutually exclusive (50). The existence of related strains of EIAV with clearly defined virulence characteristics provides the basis of an excellent system to study viral factors associated with pathogenicity. However, to exploit this system fully, the development of pathogenic infectious molecular clones of EIAV with properties similar to those of the EIAVPV strain is required. This would allow the identification of pathogenic determinants within the EIAV genome by the production of recombinants between pathogenic and the nonpathogenic molecular clones that have been described previously (45).
A number of infectious molecular clones have been produced from fetal equine kidney (FEK) cells apparently infected with the EIAVPV strain (45). However, viruses derived from these molecular clones were found to be nonpathogenic when inoculated into ponies (45). Furthermore, a comparison of the nucleotide sequence of the surface unit (SU) glycoprotein demonstrated a closer homology to the EIAVPR strain (49) than to a consensus sequence for the EIAVPV strain (32, 34). This conclusion was reinforced by neutralization studies using two of the infectious molecular clones (44-1 and 19-2), where greater reactivity was observed with serum samples taken from ponies infected with the EIAVPR strain than the EIAVPV strain (7, 9).
We have confirmed that the infectious clone 19-2 is not pathogenic in ponies and that pathogenicity is not restored (33) by the introduction of a second copy of the putative transcription regulatory element, CAAT, into the long terminal repeat (LTR). The rationale for this modification was that while a double CAAT-box motif is present in the LTR of the virulent EIAVWY strain, only a single copy is found in the nonpathogenic EIAVPR strain (29, 35, 46, 49). Furthermore, pathogenicity was not restored to 19-2 by substitution of a EIAVPV strain-like 3′ LTR or by a double substitution involving the EIAVPV 3′ LTR and modification of the variable regions of the SU glycoprotein gene to make them identical to those of the consensus sequence for the EIAVPV strain (34).
In an attempt to produce an infectious molecular clone of EIAV with the ability to induce disease in horses and ponies, we modified the version of molecular clone 19-2 containing a double CAAT motif (19-2-6A) in the 3′LTR by substituting a 3.3-kbp fragment obtained by PCR amplification of EIAVPV-infected FEK cells. This fragment was replaced using a unique NcoI site situated in the pol gene of EIAV. Therefore, it encompassed sequences encoding 77 amino acids from the carboxyl terminus of the integrase protein, the S1 (encoding the second exon of tat [14, 42, 56]), S2 (encoding a 7-kDa polypeptide of unknown function [52]), and S3 (encoding the second exon of rev [17, 42, 48, 56]) open reading frames (ORFs), the complete env gene (including the first exon of rev [42, 48, 56]), and the 3′LTR. These modified molecular clones were tested for the ability to induce disease in horses and ponies.
MATERIALS AND METHODS
Virus strains and cell culture.
The pathogenic EIAVPV strain (44, 50), derived from the avirulent cell-adapted EIAVPR strain (36), was grown in primary FEK cell cultures as described previously (22).
Preparation of DNA from EIAVPV-infected cell cultures.
EIAVPV-infected cell cultures were trypsinized and washed in Hanks buffered saline solution to remove contaminating proteins from the maintenance medium. The cells were resuspended and lysed in 0.6% sodium dodecyl sulfate (SDS)–50 mM Tris hydrochloride (pH 7.5)–10 mM EDTA. This lysate was digested with RNase (100 μg/ml) at 50°C for 30 min and with proteinase K (0.1 mg/ml) at 50°C for 15 h and then subjected to phenol-chloroform-isoamyl alcohol extractions (25:24:1) to remove protein, followed by two chloroform-isoamyl alcohol (24:1) extractions and ethanol precipitation.
PCR amplification and molecular cloning of the 3.3-kbp 3′-terminal fragments from the EIAVPV strain.
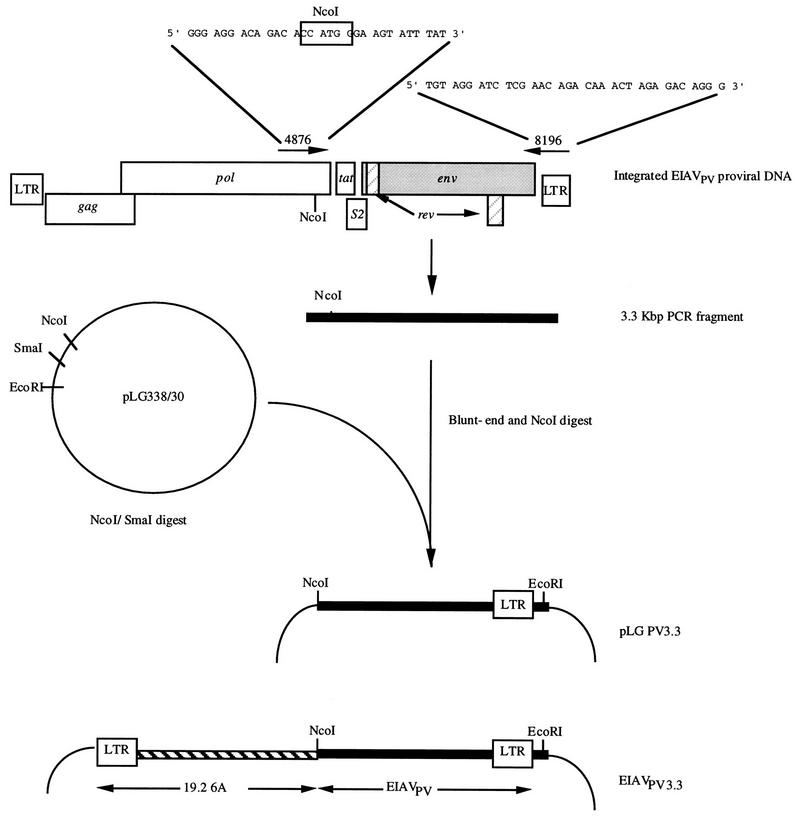
The 3.3-kbp 3′-terminal fragment of the EIAVPV strain was amplified from FEK-infected cell DNA by using primers 4876 and 8196 (Fig. 1). To increase fidelity, this amplification was conducted with Klentaq 1 (Ab Peptides, Inc., St. Louis, Mo.) and Pfu (Strategene, La Jolla, Calif.) thermostable polymerases as described by Barnes (2). Briefly, each 100-μl reaction mixture contained 20 mM Tris-HCl (pH 9.1), 1.6 mM (NH4)2SO4, 3.5 mM MgCl2, 15 μg of bovine serum albumin, 200 μM each deoxynucleoside triphosphate, 20 pmol of each primer, 25 U of Klentaq 1, and 0.156 U of Pfu polymerase. Following the addition of 0.1 μg of EIAVPV-infected FEK DNA, each reaction was subjected to 30 cycles of melting at 94°C for 20 s, annealing at 66°C for 30 s, and extension at 68°C for 8 min. An aliquot of each reaction mixture was analyzed for the presence of a 3.3-kbp product on an 0.8% agarose-Tris borate gel (51), while additional aliquots (approximately 500 ng) were blunt ended by using a PCR polishing kit (Strategene) according to the manufacturer’s instructions. Blunt-ended PCR products were extracted once with phenol-chloroform-isoamyl alcohol (25:24:1) and twice with chloroform-isoamyl alcohol (24:1) before being ethanol precipitated. This precipitated material was resuspended in sterile distilled H2O, digested with NcoI, and separated in a 1% low-melting-point agarose gel in TAE (51) buffer. The 3.3-kbp fragment was purified from agarose gel by digestion with Agar ACE (Promega, Madison, Wis.) according to the manufacturer’s recommendations. This purified fragment was ligated into NcoI-SmaI-digested pLG338/30 plasmid DNA (Fig. 1), and the products of this ligation were used to transform Escherichia coli Sure cells (Strategene). Plasmid pLG338/30 is a low-copy-number vector designed to stabilize the EIAV env gene during molecular cloning in E. coli (12). The resultant molecular clones were designated pLG338/PV3.3.
FIG. 1.
Amplification and molecular cloning of the 3.3-kbp 3′-terminal fragment of EIAVPV. A 3.3-kbp fragment from EIAVPV-infected cell cultures was amplified by PCR, blunt ended, and digested with NcoI prior to ligation with NcoI/SmaI-digested pLG338/30 vector DNA. The resultant pLG338/PV3.3 molecular clones were digested with NcoI and EcoRI, and the 3.3-kbp EIAVPV fragment was inserted into the infectious molecular clone 19-2-6A. This produced chimeric proviral molecular clones designated EIAVPV3.3.
Construction of EIAVPV3.3 chimeric molecular clones.
A modified 19-2 infectious molecular clone of EIAV (45) containing a double CAAT-box motif in the 3′ LTR, designated 19-2-6A (33), was digested with NcoI and EcoRI, and the fragments (9.7 and 3.7 kbp) were separated on a low-melting-point agarose gel. The 9.7-kbp fragments, containing the plasmid vector, 5′ LTR, gag, and pol sequences, was recovered from the agar by digestion with Agar ACE. Five pLG338/PV3.3 molecular clones were selected after partial sequencing of the env gene, their plasmid DNAs were digested with NcoI and EcoRI, and the 3.3-kbp EIAVPV fragment was recovered by Agar ACE digestion from a 1% low-melting-point agarose gel. These EIAVPV 3.3-kbp NcoI-EcoRI fragments were ligated to the 9.7-kbp purified fragment from 19-2-6A to generate chimeric proviral molecular clones of EIAV designated EIAVPV3.3 (Fig. 1).
Transfection of cell cultures.
Plasmid DNA preparations from five EIAVPV3.3 molecular clones of EIAV were purified with minor modifications by a polyethylene glycol precipitation procedure described previously (28). These modifications included a phenol-chloroform-isoamyl alcohol (25:24:1) extraction of the supernatant obtained after centrifugation of the bacterial cell lysate and digestion of RNase A-treated nucleic acids with proteinase K (250 μg/ml) in the presence of 0.25% SDS for 30 min at 37°C. Purified EIAVPV3.3 plasmid DNAs (4 μg) were transfected into FEK cell cultures (at 2 × 105 cells per 35-mm-diameter well) by using a polycationic lipid transfection reagent (Lipofectamine; Gibco Life Technologies, Gaithersburg, Md.) according to the manufacturer’s instructions. After a 21- to 24-day incubation period (which included passage and amplification of the cell numbers), tissue culture fluids from transfected cells cultures were screened for released reverse transcriptase activity (8), aliquoted, and stored at −70°C until further use. The titers of the chimeric virus stocks were determined by performing serial log10 dilutions in modified Eagle’s medium containing 3% fetal bovine serum, 25 mM HEPES, 10 mM NaHCO3, 100 IU of penicillin/ml, and 100 μg of streptomycin/ml and inoculating each dilution onto fresh FEK cell cultures. These FEK cell cultures were monitored for 21 days, after which the culture fluids were assayed for reverse transcriptase activity (8) and the titration endpoint was determined by the Karber method (15).
Experimental subjects.
The ponies (613, 614, and 678) used in these studies were of mixed breed origin, while the horses (700 and 764) were thoroughbreds. Prior to the experiment, serum samples from all animals were tested by the agar gel immunodiffusion test (IDEXX Laboratories Inc., Westbrook, Mass.) to ensure that they were seronegative for EIAV. The animals were housed in screened box stalls to exclude hematophagous insects, and all animal handling protocols were approved by the University of Kentucky Institutional Animal Care and Use Committee. The horses and ponies were treated with Ivermectin for internal parasites and vaccinated against tetanus toxoid (Merck, Sharp and Dohme, Rahway, N.J.). Animals were inoculated intravenously, using clarified (3,000 × g for 30 min at 4°C) cell culture fluids from EIAVPV3.3-transfected cell cultures at 4 × 106 or 1 × 105 50% tissue culture infective doses (TCID50) and monitored on a daily basis for rectal temperature and overall clinical status. Blood samples were collected at regular intervals by jugular venipuncture into sterile glass evacuated tubes (Becton Dickinson, Rutherford, N.J.) for serum or with EDTA for plasma (Sherwood Medical, St. Louis, Mo.). Serum and plasma were stored at −20 and −70°C, respectively. In addition, fresh blood samples collected in the presence of EDTA were analyzed to determine platelet numbers, using the Unopette system (Becton Dickinson). Platelet numbers were determined at least weekly and during each febrile episode (>39.4°C). Virus isolation from plasma samples on FEK cells cultures was described previously (22, 60), and determination of viral titers in these samples was conducted by inoculation of serial log10 dilutions onto FEK cells (22, 60). All cultures were maintained for at least 21 days, after which they were assayed for reverse transcriptase activity (8). Serum samples were tested for antibodies to the p26 antigen of EIAV by equine infectious anemia-competitive enzyme-linked immunosorbent assay (EIA-CELISA; IDEXX Laboratories).
Nucleotide sequencing of EIAVUK (EIAVPV3.3#3).
Plasmid DNA was extracted and purified with a Midiprep kit (Qiagen, Chatsworth, Calif.). This purified DNA was sequenced automatically with a Taq Dye Deoxy Terminator Cycle Sequencer kit (Perkin-Elmer, Applied Biosystems Foster City, Calif.), using overlapping EIAV primers. DNA sequences were resolved with an ABI Prism 373 DNA sequencer (Applied Biosystems). The sequences were analyzed by using the Genetics Computer Group (Madison, Wis.) package analysis software (18). Oligonucleotide primers for these sequencing reactions were derived from published sequences of the EIAV genome (32, 45, 49, 55 [GenBank accession no.) U01866]).
Nucleotide sequence accession number.
The sequence of EIAVUK was submitted to GenBank and has been assigned accession no. AFO16316.
RESULTS
Amplification of the 3.3-kbp 3′-terminal fragment from EIAVPV-infected cell cultures and production of EIAVPV3.3 chimeric proviral DNA molecular clones.
A modified version of the PCR (2) was used with the oligonucleotide primer pair 4876-8196 (Fig. 1) against a template DNA prepared from EIAVPV-infected FEK cells cultures to produce 3′-terminal fragments of the proviral genome. This primer pair was designed to produce a 3,340-bp fragment encompassing coding sequences for 77 amino acids at the carboxyl terminus of integrase, the S1, S2, and S3 ORFs, the complete env gene, and the 3′ LTR. Analysis of the PCR products by agarose gel electrophoresis, followed by ethidium bromide staining, demonstrated the presence of a single band with the predicted size of approximately 3.3 kbp. These PCR products were incubated with Pfu polymerase to remove possible 3′ single nucleotide overhangs that may have been added during PCR and then digested with NcoI. A unique NcoI site is situated at position 4889 within the genomes of all strains of EIAV which have been examined in this laboratory, and this was included in the oligonucleotide primer 4876 (Fig. 1) to simplify subsequent molecular cloning steps. The 3.3-kbp fragments containing a cleaved NcoI site at the 5′ terminus and a blunt 3′ terminus were ligated into the low-copy-number plasmid vector pLG338/30 that had been digested with NcoI and SmaI (Fig. 1). Five of the resultant molecular clones, designated pLG338/PV3.3, were selected at random and partially characterized by determining the nucleotide sequence of a 459-bp segment encompassing four of the eight variable regions (32) within the coding domain for the SU glycoprotein. At both nucleotide and amino acid levels, two of the pLG338/PV3.3 molecular clones showed complete homology with the consensus sequence for the EIAVPV strain (32, 34), while two differed by just a single amino acid (99.95% homology) and the remaining clone differed by three amino acids (98.04% homology). These results suggested that the pLG338/PV3.3 molecular clones were a family of closely related sequences, representative of those found in viral stocks of the EIAVPV strain (31, 32, 34). Therefore, the 3.3-kbp EIAVPV sequences were excised from the plasmid by digestion with NcoI/EcoRI and substituted into the infectious molecular clone 19-2-6A (Fig. 1) to create chimeric proviral molecular clones designated EIAVPV3.3. These chimeric molecular clones were transfected into FEK cell cultures, either individually or as a mixture. In the case of the mixture, each of the five plasmid DNA preparations was added in equal proportions (0.8 μg) to produce a total of 4.0 μg of DNA per transfection. Following an incubation period of 3 weeks, reverse transcriptase activity was detected in cell culture fluids from both the mixed and individual molecular clone transfections. The amount of virus produced by the transfected cell cultures was determined by titration of cell culture fluids in FEK cells. The mixture and each of the individual EIAVPV3.3 molecular clones produced infectious progeny virus titers of 106.0 TCID50/ml, which is equivalent to the yields obtained following transfection with the parental 19-2-6A molecular clone.
Clinical responses following infection with viruses derived from a mixture of EIAVPV3.3 molecular clones.
Infection of ponies with viruses from molecular clone 19-2-6A produced none of the clinical responses (6, 10, 40, 54), such as fever or thrombocytopenia, which are normally associated with acute EIA (Fig. 2A). Therefore, a preliminary experiment was conducted to investigate the effects in vivo of replacing the 3.3-kbp 3′-terminal fragment from 19-2-6A with equivalent sequences derived from the EIAVPV strain. For this preliminary experiment, a single pony (678) was inoculated intravenously with 4 × 106.0 TCID50 of the virus population derived from the mixed transfection of five EIAVPV3.3 molecular clones. A mixture rather than a single source was used because it was considered potentially more economical in terms of animal resources for the initial screening of chimeric molecular clones.
FIG. 2.
Clinical responses in animals infected with EIAV derived from infectious molecular clones. Body temperature and platelet count are shown for animals infected with viruses from molecular clone 19-2-6A (A), viruses from a mixture of EIAVPV3.3 molecular clones (B), and viruses from molecular clone EIAVUK (C to F).
Pony 678 experienced a febrile episode (39.6°C) 23 days postinfection (dpi) with a concomitant decline in platelet levels from 220,000/μl before infection to 105,000μl at 28 dpi (Fig. 2B). This pony developed antibodies to the major core protein (p26) of EIAV as measured by the commercially available EIA-CELISA by 18 dpi (Table 1), and EIAV could be isolated on FEK cell cultures from plasma samples as early as 2 dpi (Table 1). In addition, the plasma viremia titer during the febrile episode (23 dpi) was 104.0 TCID50/ml, which is similar to the levels found in many ponies infected with the EIAVPV (11, 20, 22, 60).
TABLE 1.
Serological and virological responses following infection with EIAVPV3.3 strains
| Animal no. | Virus strain | Time (dpi) of viremiaa | Viremia titerb during 1st fe- brile episode | Time (dpi) of serocon- versionc |
|---|---|---|---|---|
| 678 | EIAVPV3.3 mixd | 2–21 | 104.0 | 18 |
| 613 | EIAVUK | 5–14, 16, 21, 28, 35 | 103.5 | 19 |
| 614 | EIAVUK | 5–16, 22 | 104.5 | 19 |
| 700 | EIAVUK | 7–18 | 104.0 | 21 |
| 764 | EIAVUK | 7–18 | 105.0 | 21 |
Virus isolation in FEK cells was carried out on plasma samples collected until 21 dpi for animal 678, up to 40 dpi for animals 613 and 614, and until 18 dpi for animals 700 and 764.
Expressed as TCID50/ml of plasma sample.
Seroconversion is defined as the time taken following infection to produce a positive reaction in the EIA-CELISA.
Mixture of five EIAVPV3.3 infectious molecular clones.
Clinical responses following infection of horses and ponies with viruses derived from a single EIAVPV3.3 molecular clone.
The results obtained with pony 678 suggested that a pathogenic phenotype typical of acute EIA in terms of involving pyrexia and thrombocytopenia (reviewed references 6;0, 10;0, 40;0, and 54;0) could be conferred on virions from the 19-2-6A molecular clone by replacement of the 3.3-kbp 3′-terminal fragment with equivalent sequences derived from the EIAVPV strain. However, it was important to determine if the clinical responses in pony 678 could be duplicated in other animals by using viruses derived from a single EIAVPV3.3 molecular clone. As a starting point for these experiments, the chimeric molecular clone EIAVPV3.3#3 (redesignated EIAVUK) was selected because it had complete homology to the consensus sequence of the EIAVPV strain in the 459-bp region of the EIAV SU glycoprotein that was used in preliminary characterization of the 3.3-kb EIAVPV fragments.
Two ponies (613 and 614) and two horses (700 and 764) were inoculated intravenously with viruses derived from molecular clone EIAVUK at doses of 4 × 106.0 and 1 × 105.0 TCID50, respectively. The rectal temperature of pony 614 reached 39.3°C at 10 dpi, declined to normal levels (37 to 38°C) at 11 dpi, and then rose again to 39.9°C at 12 dpi (Fig. 2D). Platelet numbers in this animal declined 56% from 215,000/μl before infection to 95,000/μl at 13 dpi (Fig. 2D). Febrile episodes (≥39.4°C) were also observed in the two horses at 12 dpi, with maximal declines in platelet numbers of 48% for horse 700 and 64% for horse 764 during the first 28 dpi (Fig. 2E and F). In contrast, pony 613 did not exhibit a febrile response (40.6°C) until 76 dpi and showed only a slight decrease (27%) in platelet levels during the first 28 dpi. However, during the febrile episode, platelet levels in pony 613 did decline to 105,000/μl, which is a decrease of 49% from the preinfection level (Fig. 2C). Ponies 613 and 614 and horse 700 experienced only a single bout of disease during the postinfection observation periods of 98 and 84 dpi, respectively. However, horse 764 had a second febrile episode (39.2°C) at 28 dpi, with a decrease in platelets from 120,000/μl at 25 dpi to 85,000/μl at 28 dpi. In addition, at 55 dpi this horse became severely depressed and anorexic, with a body temperature of 40.8°C. Platelet levels in 764 decreased to 60,000/μl, and the animal was euthanized at 56 dpi. At necropsy, no gross macroscopic lesions were detected, although histochemical examination revealed Kupffer cell hyperplasia in sections of the liver. These cells contained hemosiderin aggregates, which is a pathological feature associated with EIA (6, 10, 40, 54). Bacteriologic examination of tissues (lung, liver, small intestine, and colon) from 764 produced no evidence of pathogenic bacteria.
Serological and virological responses following infection with EIAVUK.
Serological responses to the infection with viruses from EIAVUK plasmid were examined by using a commercially available EIA-CELISA. In this test, both ponies (613 and 614) became seropositive to p26 at 19 dpi, with the horses (700 and 764) becoming positive at 21 dpi (Table 1). All animals remained seropositive throughout the duration of the experiment.
Plasma samples from all animals (613, 614, 700, and 764) were assayed at various time points postinfection for the presence of EIAV by inoculation onto susceptible FEK cell cultures, followed by measurement of reverse transcriptase activity in cell culture fluids. Using this technique, we detected virus in pony 613 plasma samples from 5 to 14 dpi, with additional positive samples at 16, 21, 28, and 35 dpi (Table 1). Similarly, virus was detected in samples from pony 614 at each time point from 5 to 16 dpi and then again at 22 dpi (Table 1). In the case of the horses, virus was detected at each time point from 7 to 18 dpi.
This analysis was extended to determine the amounts of virus present during the first febrile episode. Titrations of plasma samples revealed peak viremia of 104.5 TCID50/ml for pony 614, 103.5 TCID50/ml for pony 613, 104.0 TCID50/ml for horse 700, and 105.0 TCID50/ml for horse 764 (Table 1). These titers are very similar to those observed during febrile episodes in ponies infected with the EIAVPV strain (11, 20, 22, 60).
Complete nucleotide sequence of EIAVUK.
The complete nucleotide sequence of molecular clone EIAVUK was determined as a prerequisite to the identification of viral determinants of pathogenicity. Comparison of these sequences revealed complete homology with those of molecular clone 19-2-6A from nucleotide position 1 in the 5′ LTR to the NcoI site at position 4889 (data not shown). Although this result might be expected, it demonstrates that no mutations had been introduced into the sequence upstream of the NcoI site during molecular cloning steps. The nucleotide sequences downstream of the NcoI site were compared with the consensus sequence for the EIAVPV strain (32, 34), a cloned representative of EIAVPR and molecular clone 19-2 (Fig. 3). The nucleotide sequence of ORF S1, which encodes the second exon of tat (14, 42, 56), was identical in all viruses examined (data not shown). In the case of ORF S2, 19-2 was found to differ from the other viruses at amino acid positions 4 and 19 (Fig. 3A; Table 2). In the second exon of rev (ORF S3), the avirulent 19-2 and EIAVPR strains contained valine and tyrosine at amino acid positions 14 and 91, whereas the same positions in EIAVPV and EIAVUK were occupied by isoleucine and histidine (Fig. 3B; Table 2). Furthermore, 19-2 was found to contain a glycine rather than a serine residue at position 8 and EIAVUK was found to contain an arginine rather than a tryptophan at position 103 (Fig. 3B; Table 2). In the sequences encoding polypeptides downstream of the NcoI, there were just three differences at the nucleotide level between EIAVUK and the consensus sequence for the EIAVPV strain. Of these differences, only one, resulting in the arginine residue at position 103 in ORF S3, was nonsynonymous. In sequences encoding the SU glycoprotein, EIAVUK differed from 19-2 and EIAVPR by seven and nine amino acids, respectively. Furthermore, five of these differences resulted in the creation of potential N-linked glycosylation sites (Table 2). The transmembrane (TM) glycoprotein contained just two amino acid differences in which isoleucine at position 589 and cysteine at 701 in EIAVUK were represented by threonine and tyrosine in 19-2 and EIAVPR (Fig. 3C; Table 2). It is interesting that the differences between EIAVUK and 19-2 amounted to only 1.1% of the total amino acid content. However, a similar low level of variation has been observed previously between pathogenic and nonpathogenic (or weakly pathogenic) molecular clones of simian immunodeficiency virus (SIV) and visna virus (1, 38, 58). With one exception, extensive homology between EIAVUK and the EIAVPV consensus sequence was observed in the 3′ LTR, which contains the cis-acting transcriptional control elements (Fig. 4). The major difference between the two sequences was the presence of an unusual 68-bp duplication/insertion located in a normally conserved U3 region, 85 nucleotides from the 5′ end of the 3′ LTR (Fig. 5). With the exception of this duplication, the sequences downstream of the NcoI site in molecular clone EIAVUK are typical of the predominant forms found in the quasispecies comprising the EIAVPV strain.
FIG. 3.

Comparison of the deduced amino acid sequences encoded by EIAVUK with the 19-2 sequence, the EIAVPV consensus sequence, and the sequence of a cloned representative of the EIAVPR strain. Comparisons shown are for ORF S2 (A), ORF S3, which encodes the second exon of rev (B), and the envelope glycoprotein (C). Only the amino acid residues that differ from the total consensus sequence are indicated. A dash indicates that no consensus sequence was found. Potential N-linked glycosylation sites are underlined. Potential N-linked glycosylation sites that differ between (EIAVUK and EIAVPV) and EIAVPR and 19-2) are marked by asterisks.
TABLE 2.
Differences in amino acid content between EIAVUK, 19-2, EIAVPV (consensus), and EIAVPR
| Coding sequence | Amino acid position | Amino acida
|
|||
|---|---|---|---|---|---|
| EIAVUK | 19-2 | EIAVPV | EIAVPR | ||
| ORF S2 | 4 | L | V | L | L |
| 19 | G | E | G | G | |
| ORF S3 | 8 | S | G | S | S |
| 14 | I | V | I | V | |
| 91 | H | Y | H | Y | |
| 103 | R | W | W | W | |
| Env SU | 193 | P | S | P | S |
| 200 | M | M | M | T | |
| 203 | Tb | A | Tb | A | |
| 282 | Nb | D | N b | D | |
| 308 | Nb | D | Nb | D | |
| 315 | Sb | R | Sb | R | |
| 318 | K | N | K | N | |
| 337 | Nb | T | Nb | T | |
| 426 | I | I | I | V | |
| TM | 589 | I | T | I | T |
| 701 | C | Y | C | Y | |
Indicated in single-letter code.
Change creates a potential N-linked glycosylation site.
FIG. 4.
Comparison of the U3-LTR hypervariable regions of the EIAVWY strain with the EIAVPV consensus sequence EIAVUK,the 19-2-6A sequence, and a consensus sequence for the EIAVPR strain. Potential cis-acting sequences (PEA-2, CAAT, ets, AP-1, and TATA) are underlined. The presence of a possible new cis-acting element is indicated by ?. Gaps (indicated by dashes) were introduced to align the sequence. In EIAVPR, R indicates either G or A, as at this location 54% of the population is represented by G and 46% is represented by A (29).
FIG. 5.
Comparison of nucleotide sequences between EIAVUK and EIAVPV consensus sequences showing the location of the insertion/duplication in the 3′ LTR. The first repeat of the 68-nucleotide region in U3 is indicated by a white box; the second repeat of the same sequence in EIAVUK is marked by a shaded box.
DISCUSSION
Previously it was established that viruses produced by transfection of the 19-2 and 19-2-6A proviral molecular clones of EIAV in equine cell cultures can establish a persistent infection in an equine host but are unable to induce clinical responses (33, 45). However, in this report, it is demonstrated that a virulent phenotype is conferred on viruses produced from the 19-2-6A molecular clone by replacement of the 3′-terminal 3,340-bp fragment with corresponding sequences derived from the pathogenic biological clone EIAVPV. The in vivo pathogenic properties of these chimeric molecular clones were evaluated by using viruses derived by transfection of FEK cell cultures at dose levels equivalent to those used for the 19-2, 19-2-6A, and EIAVPR strains (11, 22, 33, 34, 60). In the case of the infection with the single clone, EIAVUK, disease was observed by 12 dpi in three of the four recipients, which is well within the time frame expected for the onset of clinical signs following infection with similar amounts of the EIAVPV strain (21). The remaining recipient (613) did not experience a febrile episode until 76 dpi; however, the earliest detectable plasma viremia, the duration of the first viremia, and the approximate time of seroconversion were similar to those for the other EIAVUK-infected animals. A delayed onset of disease (which in this laboratory is defined as clinical signs occurring after 42 dpi) despite apparently normal kinetics of initial viral replication (as defined by initial detection of plasma viremia, duration of initial viremia, and time of seroconversion) has been previously observed in 3 of 23 EIAVPV-infected ponies (21). This finding suggests that although all ponies are susceptible to infection by EIAVPV, some are partially resistant to disease. In addition to a delayed onset of clinical signs, there is also variation between animals in the severity of disease following infection with EIAV. In a recent study investigating maturation of immune responses in four EIAVPV-infected ponies, two animals experienced only a single bout of disease with a modest 38% decline in platelet count, whereas the remaining two had multiple febrile episodes and a maximum 86% drop in platelets from preinfection levels (20). Likewise, in results reported here, ponies 614 and 700 experienced only a single febrile response, whereas horse 764 had three febrile episodes in 56 days. It appears that the spectrum of clinical responses obtained with viruses from molecular clone EIAVUK is very similar to that observed for the EIAVPV strain.
The initial reason for selecting the 3.3-kb fragment for generation of chimeric molecular clones was based on nucleotide sequence comparisons between pathogenic and nonpathogenic strain of EIAV. These comparisons demonstrated that most of the differences which could account for the phenotypic variation between strains are located in the 3′-terminal half of the viral genome. For example, compared to the virulent EIAVWY strain, the avirulent proviral molecular clone, CL-22, has nucleotide differences of 0.26% in the gag gene and 0.67% in the pol gene but differences of 3.09% in ORF S1, 3.83% in ORF S3, 5.57% in the SU glycoprotein coding sequences, 3.04% in the TM glycoprotein coding sequence, and 5.3% in the 3′ LTR (46). Preliminary studies had also indicated that the majority of differences between the avirulent cell culture adapted Wyoming strain (EIAVPR) and the EIAVPV strain were also located in the 3′ half of the viral genome. It seemed logical to assume that the regions of the viral genome having the greatest variability between pathogenic and nonpathogenic strains of EIAV should contain some, if not all, of the pathogenic determinants. This prediction has been confirmed by the results of this study.
The nucleotide sequence from position 1 in the 5′ LTR to position 4889 in EIAVUK was identical to that of the parental 19-2-6A molecular clone. This finding demonstrated that no mutations which could alter the biological properties of the chimeric molecular clones had been introduced into the 5′ half of the viral genome during the molecular cloning steps. A highly significant finding was that the coding sequences downstream of position 4889 in EIAVUK differed by just three nucleotides from the EIAVPV consensus sequence. These differences resulted in just a single amino acid change in which tryptophan at position 103 in ORF S3 in the EIAVPV consensus sequence is replaced by arginine in EIAVUK. An extensive analysis of the EIAVPV population both in vitro and in vivo has been undertaken. Originally this analysis was restricted to the variable regions of the SU glycoprotein and the LTR (34). However, it was recently extended to include amplification and nucleotide sequencing of 3′ 3,901-bp fragments obtained both from the EIAVPV stock and from plasma samples taken during febrile episodes (32). This demonstrated that the predominant members of the population present in the biologically cloned (50), tissue culture-derived EIAVPV stock maintain their dominance during initial replication in ponies and during the first febrile episode (32, 34). Therefore, replication in vivo does not result in major shifts in the population and selection of minority species. Consequently, the predominant members of the population in the EIAVPV stock, which may be defined as those closest to the consensus sequence (32), are probably the ones responsible for inducing disease. The nucleotide sequence analysis presented here demonstrates that EIAVUK contains amino acid sequences typical of those comprising the majority of the EIAVPV population. Complete homology was also observed between EIAVUK and the EIAVPV consensus sequence in all of the 3′ LTR cis-acting elements that have been previously associated with the control of viral transcription (4, 5, 13). This extensive homology at the nucleotide level probably accounts for the very similar biological properties between viruses derived from the molecular clone EIAVUK and the EIAVPV strain. One major difference was, however, detected between the EIAVPV consensus sequence and the molecular clone EIAVUK. This was the presence of a 68-bp insertion/duplication which was located in a normally conserved area of the U3 region of the 3′ LTR, not previously associated with cis-acting transcriptional control elements (4, 5, 13, 39). The role of this insertion/duplication in pathogenesis will be examined in future studies, although the available evidence indicates that LTRs containing it, both in the initial viral stock and during the first febrile episode, are a very minor species in the EIAVPV population (7, 34). Furthermore, comparisons between the titer in vivo and in vitro of the EIAVPV stock suggest that induction of disease is not restricted to a minority of the EIAVPV quasispecies (21). Therefore, it is questionable if this 68-bp insertion/duplication in the LTR functions as a major pathogenic determinant in the EIAVPV population. However, this does not preclude it from having a role within the specific context of molecular clone EIAVUK. It should be noted that substitutions of 3′ LTR sequences in 19-2 molecular clones with ones identical to those in the EIAVPV strain, but without the insertion/duplication, have failed to produce virulent progeny viruses (34).
In addition to the possible involvement of this duplication in the 3′ LTR, some predictions concerning the location of other potential pathogenic determinants can be inferred from the comparative nucleotide sequence analysis between EIAVUK, 19-2, and EIAVPV. For example, the sequences of integrase from nucleotide position 4889 and ORF S1 are identical between EIAVUK, the EIAVPV consensus sequence, 19-2, and a cloned representative of the EIAVPR strain. Therefore, within the context of 19-2- and EIAVPV3.3-based clones, it is unlikely that the integrase or Tat protein play a direct role in determining virulence. This is in contrast to a study based on the production of chimeras between SIV and human immunodeficiency virus type 1, where tat was implicated in pathogenicity (23).
In ORF S2, there are two amino acid substitutions at positions 4 and 19 between 19-2 and EIAVUK (Fig. 3A). At present, it is difficult to assign a role to these amino acid residues in pathogenicity. The fact that the nucleotide sequence of S2 in the avirulent EIAVPR strain is identical to that of EIAVUK and EIAVPV suggests that S2 is not associated with determinants of virulence. However, it should be noted that although the function of S2 is unknown, the majority of sequences examined (including those of EIAVPR, EIAVPV, and EIAVUK) contain a single nucleoporin motif (Fig. 3A) at the amino terminus (reviewed in reference 41). The leucine-to-valine change found in 19-2 could potentially disrupt this motif and perhaps further reduce the ability of viruses derived from this molecular clone to induce disease in vivo. Obviously further studies, including experiments to examine the functional significance of the nucleoporin motif, will be required to determine the role of S2 in pathogenesis.
A more consistent demarcation between virulent and avirulent viruses is seen in ORF S3, where isoleucine at position 14 and histidine at position 91 in EIAVUK (and EIAVPV) are represented by valine and tyrosine in 19-2 and EIAVPR (Fig. 3B; Table 2). However, less uniform substitutions were also observed in ORF S3. In 19-2, glycine is located at position 8, whereas the other strains contain a serine residue. Similarly, EIAVUK contained an arginine residue at position 103 instead of tryptophan found in EIAVPV, EIAVPR, and 19-2 (Fig. 3B; Table 2). Although the lack of complete correlation between these substitutions and pathogenicity suggests that they are not associated with virulence determinants, it is still possible that they are important in the context of an individual molecular clone. Several other amino acid differences between EIAVUK, 19-2, and EIAVPR are also apparent in the envelope gene, with most being found in the SU glycoprotein (Fig. 3C; Table 2).
In addition to variation in amino acid residues, there are clearly identifiable differences between EIAVWY, EIAVPR, and EIAVPV in the potential cis-acting transcriptional control elements located in the LTR. These differences appear to have evolved in response to viral growth conditions, which in turn suggests that they could have a role in cell tropism and/or pathogenesis. The hypervariable region in the LTR of the macrophage-tropic virulent EIAVWY strain contains two CAAT-box motifs, three potential ets protein binding domains, and a single AP-1 binding site (Fig. 4). Recently, we have undertaken an extensive analysis of the population of LTR sequences present in the EIAVPR strain (29) and found that the majority do not contain a 22-bp deletion as reported previously (35, 49). Instead, this analysis demonstrates that apparently during adaptation of the EIAVWY strain to fibroblastic cell cultures, one ets and one CAAT-box motif were replaced with two PEA-2 sites and an additional AP-1 binding motif (Fig. 4). Similarly, generation of the EIAVPV from the EIAVPR strain resulted in further changes, such as an alteration in the position of the CAAT box, the loss of PEA-2 and AP-1 binding sites and the creation of a possible new motif occupying the same relative position as the 5′ ets site in the EIAVWY strain (Fig. 4). This new motif has some resemblance to the ets binding site except that the central core sequence is TTTC rather than TTCC (Fig. 4). However, as mentioned above, replacement of a 3′ LTR with the EIAVPV consensus sequence in molecular clone 19-2-6A (designated EIAVPV100) did not result in the production of virulent progeny viruses (34). In fact, 19-2-6A was itself created with a 3′ LTR resembling that of the EIAVWY strain in terms of having two CAAT-box motifs and three ets protein binding domains (Fig. 4). Therefore, if as suggested by the differences in cis-acting control elements between EIAVWY, EIAVPR, and EIAVPV the LTR does play a part in pathogenesis, it must act in concert with sequences elsewhere in the viral genome.
Originally it was considered that these additional sequences might be located in the coding region of the SU glycoprotein, as this glycoprotein has been implicated in the pathogenesis of some lentivirus diseases (23, 43, 47, 57). Furthermore, in EIAV most of the differences in amino acid content between isolates are located in this area of the viral genome, and in many cases these differences result in changes in the number of N-linked glycosylation sites (32, 34). However, viruses produced from a chimeric molecular clone containing a consensus EIAVPV SU glycoprotein gene sequence in addition to a EIAVPV 3′ LTR (designated EIAVPV101) were also nonpathogenic when tested in one animal (34). If confirmed, this result would demonstrate that areas of the viral genome other than the hypervariable region of the LTR and/or the SU glycoprotein are involved in pathogenicity. These other areas could act either in isolation, with each other, or in combination with LTR and/or SU glycoprotein sequences. Perhaps one of the most intriguing conclusions from the comparative nucleotide sequence analysis and from the previously reported studies (34) is that these other areas must be limited to a very small number of nucleotides. In fact, based on differences between EIAVUK and 19-2, confirmation of the result with viruses from EIAVPV101 would restrict these areas to two nucleotides in ORF S2 (at positions 4 and 19), potentially four nucleotides in ORF S3 (at positions 8, 14, 91, and 103), and two nucleotides in the TM glycoprotein (at positions 589 in the extracellular domain and 701 in the cytoplasmic domain). Interestingly, residues in TM in combination with SU sequences and an intact nef gene have recently been demonstrated to be essential for the optimal replication of SIV in brain cells derived from the micro-vessel endothelium and for SIV-mediated neurovirulence in macaques (16, 37). Experiments are in progress to test the involvement of differences observed between EIAVUK and 19-2-6A in pathogenesis. It is anticipated that production of additional chimeric molecular clones, coupled with the relatively simple genome organization of EIAV, will eventually enable identification of viral sequences associated with pathogenicity in this and possibly other lentiviruses. A potentially more difficult challenge may be to determine how these viral sequences interact with their host, thereby discovering why variation in relatively few nucleotides between strains of EIAV can produce such dramatic differences in clinical outcome.
ACKNOWLEDGMENTS
We thank Beth Frost for excellent technical assistance in DNA sequencing and Gary Thomas and Brian Meade for maintaining the highest standards of animal care.
This work was supported by National Institutes of Health grant R01 CA49296, by funds from the Lucille P. Markey Charitable Trust and the Kentucky Agricultural Experimental Station, and in part by resource grant 2 P41 RR06009 from the Pittsburgh Supercomputing Center through NIH National Center for Research Resources. C.L. was a recipient of postdoctoral grants from the Fondation pour la Recherche Medicale and the Alain Philippe Foundation.
REFERENCES
- 1.Banapour B, Marthas M, Ramos R, Lohman B, Unger R, Gardner M, Pedersen N, Luciw P. Identification of viral determinants of macrophage tropism for simian immunodeficiency virus SIVmac. J Virol. 1991;65:5798–5805. doi: 10.1128/jvi.65.11.5798-5805.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnes W M. PCR amplification of up to 35-kb DNA with high fidelity and high yield from λ bacteriophage templates. Proc Natl Acad Sci USA. 1994;91:2216–2220. doi: 10.1073/pnas.91.6.2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carpenter S, Chesebro B. Change in host cell tropism associated with in vitro replication of equine infectious anemia virus. J Virol. 1989;63:2492–2496. doi: 10.1128/jvi.63.6.2492-2496.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carvalho M, Derse D. Physical and functional characterization of transcriptional control elements in the equine infectious anemia virus promoter. J Virol. 1993;67:2064–2074. doi: 10.1128/jvi.67.4.2064-2074.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carvalho M, Kirkland M, Derse D. Protein interactions with DNA elements in variant equine infectious anemia virus enhancers and their impact on transcriptional activity. J Virol. 1993;67:6586–6595. doi: 10.1128/jvi.67.11.6586-6595.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheevers W P, McGuire T C. Equine infectious anemia virus: immunopathogenesis and persistence. Rev Infect Dis. 1985;7:83–88. doi: 10.1093/clinids/7.1.83. [DOI] [PubMed] [Google Scholar]
- 7.Cook, R. F., and C. J. Issel. Unpublished observations.
- 8.Cook R F, Cook S J, Issel C J. A non-radioactive micro-assay for released reverse transcriptase activity of a lentivirus. BioTechniques. 1992;13:380–386. [PubMed] [Google Scholar]
- 9.Cook R F, Berger S L, Rushlow K E, McManus J M, Cook S J, Harrold S, Raabe M L, Montelaro R C, Issel C J. Enhanced sensitivity to neutralizing antibodies in a variant of equine infectious anemia virus is linked to amino acid substitutions in the surface unit envelope glycoprotein. J Virol. 1995;69:1493–1499. doi: 10.1128/jvi.69.3.1493-1499.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cook R F, Issel C J, Montelaro R C. Equine infectious anemia. In: Studdert M J, editor. Virus infections of equines. Amsterdam, The Netherlands: Elsevier Science B.V.; 1996. pp. 297–323. [Google Scholar]
- 11.Costa L R, Issel C J, Montelaro R C, Cook S J, Cook R F, Rushlow K E, Grund C, Wang S Z-S. Responses of ponies to challenge with equine infectious anemia virus following exposure to recombinant gp90 or viral p26 immunogens. In: Nakajima H, Plowright W, editors. Equine infectious diseases. VII. Newmarket, England: R&W Publications; 1994. pp. 85–94. [Google Scholar]
- 12.Cunningham T P, Montelaro R C, Rushlow K E. Lentivirus envelope sequences and proviral genomes are stabilized in Escherichia coli when cloned into low copy-number plasmid vectors. Gene. 1993;124:93–98. doi: 10.1016/0378-1119(93)90766-v. [DOI] [PubMed] [Google Scholar]
- 13.Derse D, Dorn P L, Levy L, Stephens R M, Rice N R, Casey J W. Characterization of equine infectious anemia virus long terminal repeat. J Virol. 1987;61:743–747. doi: 10.1128/jvi.61.3.743-747.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dorn P, DaSilva L, Martarano L, Derse D. Equine infectious anemia virus Tat: insights into the structure, function, and evolution of lentivirus transactivation proteins. J Virol. 1990;64:1616–1624. doi: 10.1128/jvi.64.4.1616-1624.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dougherty R M. In: Techniques in experimental virology. Harris R J C, editor. London, England: Academic Press; 1964. pp. 169–220. [Google Scholar]
- 16.Flaherty M T, Hauer D A, Mankowski J L, Zink M C, Clements J E. Molecular and biological characterization of a neurovirulent molecular clone of simian immunodeficiency virus. J Virol. 1997;71:5790–5798. doi: 10.1128/jvi.71.8.5790-5798.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fridell R A, Partin K M, Carpenter S, Cullen B R. Identification of the activation domain of equine infectious anemia virus rev. J Virol. 1993;67:7317–7323. doi: 10.1128/jvi.67.12.7317-7323.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Genetics Computer Group. Program manual for the Wisconsin Package. Madison, Wis: Genetics Computer Group; 1994. [Google Scholar]
- 19.Gutekunst D E, Becvar C S. Responses in horses infected with equine infectious anemia virus adapted to tissue culture. Am J Vet Res. 1979;40:974–977. [PubMed] [Google Scholar]
- 20.Hammond S A, Cook S J, Lichtenstein D L, Issel C J, Montelaro R C. Maturation of the cellular and humoral immune responses to persistent infection in horses by equine infectious anemia is a complex and lengthy process. J Virol. 1997;71:3840–3852. doi: 10.1128/jvi.71.5.3840-3852.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Issel, C. J. Unpublished observations.
- 22.Issel C J, Horohov D W, Lea D F, Adams W V, Jr, Hagius S D, McManus J M, Allison A C, Montelaro R C. Efficacy of inactivated whole-virus and subunit vaccines in preventing infection and disease caused by equine infectious anemia virus. J Virol. 1992;66:3398–3408. doi: 10.1128/jvi.66.6.3398-3408.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karlsson G B, Halloran M, Li J, Park I-W, Gomila R, Reimann K A, Axthelm M K, Iliff S A, Letvin N L, Sodroski J. Characterization of molecularly cloned simian-human immunodeficiency viruses causing rapid CD4+ lymphocyte depletion in rhesus monkeys. J Virol. 1997;71:4218–4225. doi: 10.1128/jvi.71.6.4218-4225.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kobayashi K. Studies on the cultivation of equine infectious anemia virus in vitro. I. Serial cultivation of the virus in the culture of various horse tissues. Virus. 1961;11:177–189. [Google Scholar]
- 25.Kobayashi K, Kono Y. Propagation and titration of equine infectious anemia virus in horse leukocyte culture. Natl Inst Anim Health Q. 1967;7:8–20. [PubMed] [Google Scholar]
- 26.Kono Y, Yokomizo Y. Attempts to cultivate the equine infectious anemia virus in various cell types. Natl Inst Anim Health Q. 1968;8:182–186. [PubMed] [Google Scholar]
- 27.Kono Y, Yoshino T, Fukunaga Y. Growth characteristics of equine infectious anemia virus in horse leukocyte cultures. Arch Virol. 1970;30:252–256. doi: 10.1007/BF01250196. [DOI] [PubMed] [Google Scholar]
- 28.Kraft R, Tardiff J, Krauter K S, Leinwand L A. Using miniprep plasmid DNA for sequencing double stranded template with Sequenase. BioTechniques. 1988;6:544–546. [PubMed] [Google Scholar]
- 29.Langemeier, J. L., and C. J. Issel. Unpublished observations.
- 30.Langemeier J L, Cook S J, Cook R F, Rushlow K E, Montelaro R C, Issel C J. Detection of equine infectious anemia viral RNA in plasma samples from recently infected and long-term inapparent carrier animals by PCR. J Clin Microbiol. 1996;34:1481–1487. doi: 10.1128/jcm.34.6.1481-1487.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leroux, C., and R. C. Montelaro. Unpublished observations.
- 32.Leroux C, Issel C J, Montelaro R C. Novel and dynamic evolution of equine infectious anemia virus genomic quasispecies associated with sequential disease cycles in an experimentally infected pony. J Virol. 1997;71:9627–9639. doi: 10.1128/jvi.71.12.9627-9639.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lichtenstein D L, Rushlow K E, Cook R F, Raabe M L, Swardson C J, Kociba G J, Issel C J, Montelaro R C. Replication in vitro and in vivo of an equine infectious anemia virus mutant deficient in dUTPase activity. J Virol. 1995;69:2881–2888. doi: 10.1128/jvi.69.5.2881-2888.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lichtenstein D L, Issel C J, Montelaro R C. Genomic quasispecies associated with the initiation of infection and disease in ponies experimentally infected with equine infectious anemia virus. J Virol. 1996;70:3346–3354. doi: 10.1128/jvi.70.6.3346-3354.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Madden C R, Shih D S. Analysis of the long terminal repeat from a cytopathic strain of equine infectious anemia virus. Virology. 1996;225:395–399. doi: 10.1006/viro.1996.0614. [DOI] [PubMed] [Google Scholar]
- 36.Malmquist W A, Barnett D, Becvar C S. Production of equine infectious anemia antigen in a persistently infected cell line. Arch Virol. 1973;42:361–370. doi: 10.1007/BF01250717. [DOI] [PubMed] [Google Scholar]
- 37.Mankowski J L, Flaherty M T, Spelman J P, Hauer D A, Didier P J, Amedee A M, Murphey-Corb M, Kirstein L M, Mumoz A, Clements J E, Zink M C. Pathogenesis of simian immunodeficiency virus encephalitis: viral determinants of neurovirulence. J Virol. 1997;71:6055–6060. doi: 10.1128/jvi.71.8.6055-6060.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marthas M L, Ramos R A, Lohman B L, van Rompay K K, Unger R E, Miller C J, Banapour B, Pedersen N C, Luciw P L. Viral determinants of simian immunodeficiency virus (SIV) virulence in rhesus macaques assessed by using attenuated and pathogenic molecular clones of SIVmac. J Virol. 1993;67:6047–6055. doi: 10.1128/jvi.67.10.6047-6055.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maury W. Monocyte maturation controls expression of equine infectious anemia virus. J Virol. 1994;68:6270–6279. doi: 10.1128/jvi.68.10.6270-6279.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Montelaro R C, Ball J M, Rushlow K E. Equine retroviruses. In: Levy J A, editor. The Retroviridae. Vol. 2. New York, N.Y: Plenum Press; 1993. pp. 257–360. [Google Scholar]
- 41.Nigg E A. Nucleocytoplasmic transport: signals, mechanisms and regulation. Nature. 1997;2386:779–787. doi: 10.1038/386779a0. [DOI] [PubMed] [Google Scholar]
- 42.Noiman S, Yaniv A, Sherman L, Tronick S R, Gazit A. Pattern of transcription of the genome of equine infectious anemia virus. J Virol. 1990;64:1839–1843. doi: 10.1128/jvi.64.4.1839-1843.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Novembre F J, Johnson P R, Lewis M G, Anderson D C, Klumpp S, McClure H M, Hirsch V A. Multiple viral determinants contribute to pathogenicity of the acutely lethal simian immunodeficiency virus SIVsmmPBj variant. J Virol. 1993;67:2466–2474. doi: 10.1128/jvi.67.5.2466-2474.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Orrego A, Issel C J, Montelaro R C, Adams W V., Jr Virulence and in vitro growth of a cell-adapted strain of equine infectious anemia virus after serial passage in ponies. Am J Vet Res. 1982;43:1556–1560. [PubMed] [Google Scholar]
- 45.Payne S L, Rausch J, Rushlow K, Montelaro R C, Issel C, Flaherty M, Perry S, Sellon D, Fuller F. Characterization of infectious molecular clones of equine infectious anemia virus. J Gen Virol. 1994;75:425–429. doi: 10.1099/0022-1317-75-2-425. [DOI] [PubMed] [Google Scholar]
- 46.Perry S T, Flaherty M T, Kelley M J, Clabough D L, Tronick S R, Coggins L, Whetter L, Lengel C R, Fuller F. The surface envelope protein gene region of equine infectious anemia virus is not an important determinant of tropism in vitro. J Virol. 1992;66:4085–4097. doi: 10.1128/jvi.66.7.4085-4097.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Power C, McArthur J C, Johnson R T, Griffin D E, Glass J D, Perryman S, Chesebro B. Demented and nondemented patients with AIDS differ in brain-derived human immunodeficiency virus type 1 envelope sequences. J Virol. 1994;68:4643–4649. doi: 10.1128/jvi.68.7.4643-4649.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rosin-Arbesfeld R, Rivlin M, Noiman S, Mashiah P, Yaniv A, Miki T, Tronick S R, Gazit A. Structural and functional characterization of rev-like transcripts of equine infectious anemia virus. J Virol. 1993;67:5640–5646. doi: 10.1128/jvi.67.9.5640-5646.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rushlow K, Olsen K, Stiegler G, Payne S L, Montelaro R C, Issel C J. Lentivirus genomic organization: the complete nucleotide sequence of the env gene region of equine infectious anemia virus. Virology. 1986;155:309–321. doi: 10.1016/0042-6822(86)90195-9. [DOI] [PubMed] [Google Scholar]
- 50.Rwambo P M, Issel C J, Hussain K A, Montelaro R C. In vitro isolation of a neutralization escape mutant of equine infectious anemia virus (EIAV) Arch Virol. 1990;111:275–280. doi: 10.1007/BF01311062. [DOI] [PubMed] [Google Scholar]
- 51.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 52.Schiltz R L, Shih D S, Rasty S, Montelaro R C, Rushlow K E. Equine infectious anemia virus gene expression: characterization of the RNA splicing pattern and the protein products encoded by open reading frames S1 and S2. J Virol. 1992;66:3455–3465. doi: 10.1128/jvi.66.6.3455-3465.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sellon D C, Perry S T, Coggins L, Fuller F J. Wild-type equine infectious anemia virus replicates in vivo predominantly in tissue macrophages, not in peripheral blood monocytes. J Virol. 1992;66:5906–5913. doi: 10.1128/jvi.66.10.5906-5913.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sellon D C, Fuller F J, McGuire T C. The immunopathogenesis of equine infectious anemia virus. Virus Res. 1994;32:111–138. doi: 10.1016/0168-1702(94)90038-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stephens R M, Casey J W, Rice N R. Equine infectious anemia virus gag and pol genes: relatedness to Visna and AIDS virus. Science. 1986;231:589–594. doi: 10.1126/science.3003905. [DOI] [PubMed] [Google Scholar]
- 56.Stephens R M, Derse D, Rice N R. Cloning and characterization of cDNA encoding equine infectious anemia virus tat and putative rev proteins. J Virol. 1990;64:3716–3725. doi: 10.1128/jvi.64.8.3716-3725.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Toggas S M, Masliah E, Rockenstein E M, Rall G F, Abraham C R, Mucke L. Central nervous system damage produced by expression of the HIV-1 coat protein gp120 in transgenic mice. Nature. 1994;367:188–193. doi: 10.1038/367188a0. [DOI] [PubMed] [Google Scholar]
- 58.Torsteinsdottir S, Agnarsdottir G, Matthiasdottir S, Rafnar B, Andresdottir V, Andresson O S, Staskus K, Petursson G, Palsson P A, Georgsson G. In vivo and in vitro infection with two different molecular clones of visna virus. Virology. 1997;229:370–380. doi: 10.1006/viro.1996.8428. [DOI] [PubMed] [Google Scholar]
- 59.Ushimi C, Henson J B, Gorham J R. Study of the one-step growth curve of equine infectious anemia virus by immunofluorescence. Infect Immun. 1972;5:890–895. doi: 10.1128/iai.5.6.890-895.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang S Z-S, Rushlow K E, Issel C J, Cook R F, Cook S J, Raabe M L, Chong Y-H, Costa L, Montelaro R C. Enhancement of EIAV replication and disease by immunization with a baculovirus-expressed recombinant envelope surface glycoprotein. Virology. 1994;199:247–251. doi: 10.1006/viro.1994.1120. [DOI] [PubMed] [Google Scholar]