Ventricular tachycardia (VT) significantly impacts morbidity and mortality, particularly in patients with cardiomyopathies. Catheter ablation (CA) is a vital treatment, yet outcomes vary notably between ischemic cardiomyopathy (ICMP) and non-ischemic cardiomyopathy (NICMP). The recent study by Khosravi et al. provides valuable insights into these differences, highlighting long-term outcomes after VT ablation [1].
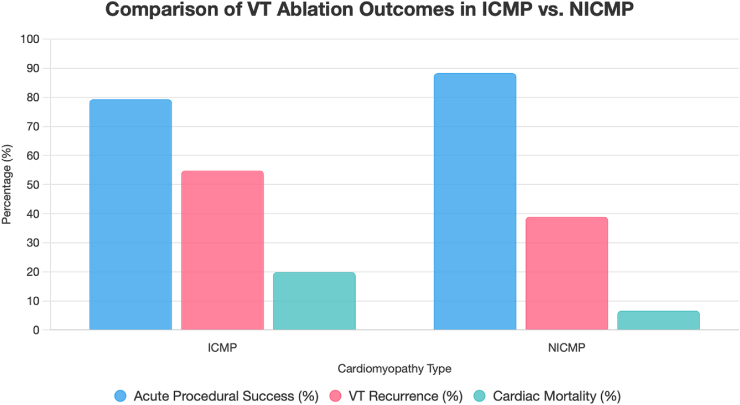
Khosravi and colleagues retrospectively analyzed 212 patients, finding higher procedural success rates in NICMP compared to ICMP (88.3 % vs. 79.3 %). Over a median follow-up of 36 months, NICMP patients experienced lower VT recurrence (38.9 % vs. 54.8 %, P = 0.26), though the difference was not statistically significant. They also had reduced cardiac mortality (6.6 % vs 19.9 %) and improved survival rates (7 % vs. 22 %) [1]. These outcomes are summarized in Fig. 1.
Fig. 1.
Bar chart comparing acute procedural success, VT recurrence, and cardiac mortality in ICMP and NICMP patients undergoing VT ablation, based on Khosravi et al. [1]. Note: VT recurrence difference was not statistically significant (P = 0.26).
Historically, ICMP has been viewed as more amenable to VT ablation due to well-defined scar pathways identifiable by endocardial mapping [2]. NICMP presents a more heterogeneous substrate, often requiring extensive intramural or epicardial mapping techniques [3]. Contrary to conventional expectations, this study showed superior outcomes in NICMP patients, potentially due to patient selection bias, as ICMP patients were older, had lower left ventricular ejection fraction (LVEF), and had more comorbidities.
The complexity of NICMP substrates necessitates combined endo-epicardial strategies. Recent research emphasizes the importance of epicardial mapping for comprehensive substrate characterization and improved success in NICMP ablation [3]. Additionally, detailed unipolar voltage mapping has emerged as a critical tool in predicting the recovery of left ventricular ejection fraction (LVEF) in recent-onset NICMP patients and correlates closely with VT substrate complexity and ablation outcomes [4,5].
Further, myocardial strain analysis by cardiac magnetic resonance (CMR) feature tracking has demonstrated strong correlation with electro-anatomical substrate abnormalities, providing complementary information for identifying arrhythmogenic substrates and refining ablation strategies [6].
Khosravi et al. identified lower LVEF, history of AF, and the need for hemodynamic support as predictors of higher VT recurrence. These findings resonate with prior work highlighting relationships between procedural success, substrate characteristics, and clinical outcomes [7,8].
Our earlier research has also underscored the complexity of para-Hisian ventricular arrhythmias, demonstrating unique electrocardiographic characteristics and ablation outcomes, thereby stressing the necessity for nuanced ablation strategies based on precise anatomical and electroanatomical mapping [9]. Additionally, research characterizing the phenotype of mixed cardiomyopathy in patients with implantable cardioverter-defibrillators (ICD) has provided essential insights into the clinical profile and arrhythmic risk, guiding tailored ablation strategies [8].
The limitations cited by Khosravi, including retrospective design and limited use of cardiac magnetic resonance imaging (CMR), underscore ongoing clinical challenges. Advanced imaging like late gadolinium-enhanced CMR, despite ICD-related limitations, remains crucial for defining scar characteristics, optimizing ablation strategies, and improving outcomes [6].
Future research must focus on prospective multicenter studies, advanced imaging integration, and exploring novel technologies specifically for NICMP substrates. Robust randomized clinical trials will further clarify optimal procedural strategies, enhancing prognostic accuracy and patient outcomes.
In summary, Khosravi et al. provide significant insights into VT ablation for cardiomyopathies, highlighting efficacy and safety, especially in NICMP. Their findings advocate a shift toward personalized substrate-based ablation strategies, critical for future management.
Declaration of competing interest
Dr Pathak reports having served on the advisory board of Medtronic, Abbott, Boston Scientific and Biotronik. Dr Pathak reports that Canberra Heart Rhythm Foundation/Australian National University has received on his behalf lecture and/or consulting fees from Medtronic, Abbott, Boston Scientific and Biotronik. Dr Sanders reports having served on the advisory board of Medtronic, Abbott, Boston Scientific, CathRx and PaceMate and that the University of Adelaide has received on his behalf lecture and/or consulting fees from Medtronic, Abbott, and Boston Scientific. Dr Sanders reports that the University of Adelaide has received on his behalf research funding from Medtronic, Abbott Medical, Boston Scientific, BD, and Microport.
References
- 1.Khosravi R., Kamali F., Simiyari S., et al. Long-term outcomes of ventricular tachycardia ablation in ischemic and non-ischemic cardiomyopathy patients: data from a single-center ablation registry. Indian Pacing Electrophysiol J. 2025;25(4):220–226. doi: 10.1016/j.ipej.2025.07.002. [DOI] [PubMed] [Google Scholar]
- 2.Stevenson W.G., Soejima K. Catheter ablation for ventricular tachycardia. Circulation. 2007;115(21):2750–2760. doi: 10.1161/CIRCULATIONAHA.106.655720. [DOI] [PubMed] [Google Scholar]
- 3.Vaseghi M., Hu T.Y., Tung R., et al. Outcomes of catheter ablation of ventricular tachycardia based on etiology in nonischemic heart disease: an international VT ablation center collaborative study. JACC Clin Electrophysiol. 2018;4(9):1141–1150. doi: 10.1016/j.jacep.2018.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chaumont C., Peyster E.G., Siontis K.C., et al. Unipolar voltage mapping to predict recovery of left ventricular ejection fraction in patients with recent-onset nonischemic cardiomyopathy. Circulation. 2025;151(6):368–378. doi: 10.1161/CIRCULATIONAHA.124.070501. [DOI] [PubMed] [Google Scholar]
- 5.Raja D.C., Shroff J., Nair A., et al. Correlation of extent of left ventricular endocardial unipolar low-voltage zones with ventricular tachycardia in non-ischaemic cardiomyopathy. Heart Rhythm. 2024;21(10):1970–1977. doi: 10.1016/j.hrthm.2024.04.065. [DOI] [PubMed] [Google Scholar]
- 6.Raja D.C., Samarawickrema I., Srinivasan J.R., et al. Correlation of myocardial strain by CMR-feature tracking with substrate abnormalities detected by electro-anatomical mapping in patients with nonischemic cardiomyopathy. J Intervent Card Electrophysiol. 2023;66(9):2113–2123. doi: 10.1007/s10840-022-01236-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ammar A., Sharief M., Abouelmagd K., et al. Outcomes of catheter ablation of ventricular tachycardia in non-ischemic idiopathic dilated cardiomyopathy: a systematic review and meta-analysis. Front Cardiovasc Med. 2022;9 doi: 10.3389/fcvm.2022.1007392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raja D.C., Samarawickrema I., Menon S.K., et al. Characteristics of the phenotype of mixed cardiomyopathy in patients with implantable cardioverter-defibrillators. J Intervent Card Electrophysiol. 2023;67(1):129–137. doi: 10.1007/s10840-022-01220-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nair A., Shroff J.P., Tuan L.Q., et al. Electrocardiographic characteristics and ablation outcomes associated with para-Hisian ventricular arrhythmias. JACC Asia. 2025;5(2):299–312. doi: 10.1016/j.jacasi.2024.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]