Abstract
Advancing age increases cardiovascular disease risk, in part, because of impaired glycocalyx thickness and endothelial dysfunction. Glycocalyx-targeted therapies, such as Endocalyx Pro, could improve both glycocalyx thickness and endothelial function in older adults; however, this has yet to be tested. We hypothesized that Endocalyx Pro supplementation would increase glycocalyx thickness and endothelial function in older adults. Twenty-three older adults aged 66 ± 7 yr (52% female) were enrolled in a randomized, double-blind, placebo-controlled, parallel-arms study to investigate the effect of 12-wk Endocalyx Pro supplementation (3,712 mg/day) on glycocalyx thickness and endothelial function. Glycocalyx thickness was assessed using the GlycoCheck, and endothelial function was determined via brachial artery flow-mediated dilation (FMD). Between-group comparisons revealed Endocalyx Pro did not increase glycocalyx thickness in microvessels 4–25 µm (P = 0.33), 4–7 µm (P = 0.07), or 10–25 µm (P = 0.47) in diameter when compared with placebo. In addition, Endocalyx Pro did not significantly improve FMD [mean ratio (95%) confidence interval [CI]) for between-group comparisons, 1.16 (0.77–1.74); P = 0.48]. However, Endocalyx Pro improved FMD normalized to shear rate (SR) area under the curve [mean ratio (95% CI) for between-group comparisons, 2.41 (1.14,4.13); P = 0.001]. Moreover, Endocalyx Pro increased capillary glycocalyx thickness more than placebo in individuals not taking antihypertensive medication [mean difference (95% CI) for between-group comparison, −0.08 (−0.15, −0.01); P = 0.02]. Our pilot study suggests that Endocalyx Pro supplementation is feasible in older adults but has no measurable effect on overall glycocalyx thickness and FMD. However, Endocalyx Pro may have select effects on capillary glycocalyx thickness and FMD normalized to shear rate among older adults, but further investigation is warranted.
Keywords: endocalyx, endothelium, glycocalyx
NEW & NOTEWORTHY
Endothelial glycocalyx thickness and vascular endothelial function decline with advancing age. Endocalyx Pro is a glycocalyx-targeted therapy that may improve endothelial glycocalyx thickness and vascular endothelial function in older adults. This study demonstrated that 12-wk Endocalyx Pro supplementation did not improve overall endothelial glycocalyx thickness or flow-mediated dilation in older adults; however, Endocalyx Pro did increase capillary glycocalyx thickness in individuals not taking antihypertensive medication and improve flow-mediated dilation normalized to the shear stimulus.
INTRODUCTION
Cardiovascular disease (CVD) is the leading cause of death worldwide, and advancing age is a primary risk factor (1). A key mechanism contributing to the progression of CVD with aging is vascular endothelial dysfunction (2). One potential cause of age-related vascular endothelial dysfunction in humans is the degradation of the endothelial glycocalyx (3). The endothelial glycocalyx is a dynamic, gel-like layer that lines the luminal surface of endothelial cells and is essential for maintaining vascular endothelial homeostasis (4). Of importance, endothelial glycocalyx thickness declines with advancing age (5), and lower endothelial glycocalyx thickness is associated with worse vascular endothelial function in adults (6). Furthermore, lower endothelial glycocalyx thickness is associated with a higher risk of major adverse CVD events in middle-aged and older adults (7). Therefore, restoring endothelial glycocalyx thickness could be a novel approach to improving vascular endothelial function and attenuating CVD in older adults.
Endocalyx Pro is a patented glycocalyx-targeted therapy intended to synthesize, repair, and provide antioxidant support to the endothelial glycocalyx and may increase endothelial glycocalyx thickness in humans (8). In a preclinical study, old mice given Endocalyx Pro for 10 wk had a greater increase in endothelial glycocalyx thickness and endothelium-dependent vasodilation compared with control mice (9). In addition, old mice given Endocalyx Pro had a significant reduction in systolic blood pressure and aortic stiffness, indicating that restoring endothelial glycocalyx thickness could lead to lower blood pressure and aortic stiffness (9). However, there are currently no published clinical trials testing the effect of Endocalyx Pro on endothelial glycocalyx thickness and vascular function in humans. Thus, the purpose of our study was to determine the effects of Endocalyx Pro on endothelial glycocalyx thickness, vascular endothelial function, and aortic stiffness in older adults. Our primary hypothesis was that 12 wk of Endocalyx Pro supplementation would increase endothelial glycocalyx thickness and vascular endothelial function in older adults. Our secondary hypothesis was that 12 wk of Endocalyx Pro supplementation would reduce aortic stiffness in older adults.
METHODS
Study Population
Older men and postmenopausal women were recruited from Iowa City and the surrounding communities to complete a randomized, double-blind, placebo-controlled, parallel-arms intervention examining the effect of 12 wk of Endocalyx Pro supplementation on endothelial glycocalyx thickness and vascular endothelial function. Exclusion criteria included body mass index ≥40 kg/m2, resting systolic blood pressure of ≥140 mmHg and/or diastolic blood pressure ≥100 mmHg, clinically abnormal thyroid function, current tobacco use or history of tobacco use within the past 3 mo, current use or previous use of hormone therapy within the past 6 mo, self-reported history of stroke, dementia, diabetes mellitus, pulmonary/renal/neurological/hepatic disease, previous CVD events (myocardial infarction, stent, bypass surgery, and heart failure), current CVD or evidence of CVD during a resting 12-lead electrocardiogram, and allergy to artichokes, grapes, olives, or melons (Endocalyx Pro ingredients).
Study Procedures
The primary investigator and research team, as well as the study participants, were blinded to which treatment participants received. The randomization of participants was generated by an unblinded research coordinator using permuted blocks of two and four with a random number generator. On randomization and after baseline measurements, participants were provided Endocalyx Pro (Microvascular Health Solutions, Alpine, UT) or a matching placebo (Nu-Mag, 10 mg; rice flour, 830 mg; and magnesium stearate veggie powder, 10 mg). Participants were instructed to take three instant-release capsules of Endocalyx Pro twice per day (3,712 mg/day), preferably with breakfast and dinner, or placebo for 12 wk. The dose of Endocalyx Pro was selected based on the study duration and similar Endocalyx Pro trials registered on ClinicalTrials.gov that are in progress in different populations (heart failure, diabetes, diabetic nephropathy, psoriasis, and COVID-19). Participants returned to the laboratory for follow-up measurements following 12 wk of supplementation. Adherence was measured at the end of the trial with a pill count. The total number of pills ingested was calculated as the number of pills provided – the number of pills returned. Percent adherence was then calculated as pills ingested/number of pills provided × 100.
On the day of vascular testing, participants were instructed to arrive following an overnight fast, a minimum of 8 h, and abstain from caffeine intake the morning of the study (10). In addition, participants were instructed to abstain from exercise and alcohol consumption for at least 24 h before testing (10). Furthermore, participants taking vasoactive medications (e.g., antihypertensives) were asked to withhold their medication the morning of vascular testing. All procedures were approved by the University of Iowa Institutional Review Board and conducted in accordance with the Declaration of Helsinki. All participants provided written informed consent before participating in the research study. ClinicalTrials.gov ID (NCT06071728).
Clinical Characteristics
Race, ethnicity, menopause status, smoking status, and health history were evaluated by a questionnaire. Body mass index was computed after measurement of weight and height in kg/m2. Blood samples were measured at the University of Iowa Diagnostics Laboratories and included thyroid-stimulating hormone, fasting lipid panel, and fasting glucose.
Endothelial Glycocalyx Thickness
Glycocalyx thickness was determined in the placebo (n = 10) and Endocalyx Pro (n = 12) groups by noninvasively imaging the sublingual microcirculation using a sidestream dark field imaging camera (KK Technology) and automated acquisition software (GlycoCheck, Microvascular Health Solutions, Alpine, UT) as previously described (11). Briefly, five trials were performed, with each trial lasting 2–3 min and consisting of at least 10 2-s video recordings of 40 frames in length. The values from the five trials were averaged to obtain a single value per measurement. In each recording, microvessels 4–25 µm in lumen diameter were identified using a contrast between red blood cells (RBCs) and the background and were divided into 10-µm long microvessel segments. Video recordings were repeated until 3,000 microvessel segments had been identified. The perfused boundary region (PBR) reflects the depth at which RBCs penetrate the glycocalyx, with a larger PBR indicative of decreased glycocalyx thickness (12). To calculate PBR, the GlycoCheck software identifies the radial movement of RBCs in each valid segment to determine the median RBC width (RBCW) as well as the outer edge of the RBC perfused lumen (Dperf). PBR was reported in vessels 4–25 µm in diameter and calculated using the following equation: PBR = (Dperf – RBCW)/2. Microvessels ranging from 4 to 7 µm in diameter were considered capillaries, and microvessels ≥10 µm in diameter were considered feed vessels (11).
Endothelial Function
Endothelial function was determined from brachial artery flow-mediated dilation (FMD) and assessed by high-resolution ultrasonography (GE Logiq 7), as previously described (13). Following baseline diameter measurements, a cuff placed on the upper forearm was inflated to suprasystolic pressure (250 mmHg) for 5 min. After 5 min, the cuff was rapidly deflated, and measurements continued for an additional 2 min. Baseline and deflation images were analyzed using offline software with automatic edge detection (Vascular Analysis Tools 5.5, Medical Imaging Applications, LLC, Coralville, IA) to determine changes in brachial artery diameter and velocity in response to reactive hyperemia. FMDmm was reported as the mm difference in peak diameter from baseline diameter and FMD% was calculated as the percent difference in peak diameter from baseline diameter using the following equation:
Brachial artery shear rate (SR) was determined in the placebo (n = 10) and Endocalyx Pro (n = 10) groups and calculated as SR = 8 × mean blood velocity (Vmean)/diameter. The SR area under the curve (SRAUC) was quantified as the area from the time of cuff deflation to the time of peak diameter (Dpeak) and calculated using the trapezoidal rule as previously described (14). Normalization of FMD% to SR was expressed as the peak FMD:SRAUC ratio (15). Reactive hyperemia was determined in the placebo (n = 10) and Endocalyx Pro (n = 11) groups as the difference in blood flow area under the curve (BFAUC) between baseline and 120-s postocclusion and calculated using the trapezoidal rule equation as was used for SRAUC.
Blood Pressure and Aortic Stiffness
Supine brachial blood pressure was obtained in triplicate using an inflatable cuff with a built-in microphone and determined via auscultation, where the first and fifth Korotkoff sounds denoted systolic and diastolic blood pressures, respectively (NIHem workstation, Cardiovascular Engineering, Inc., Norwood, MA). Aortic stiffness was assessed by the reference-standard carotid-femoral pulse wave velocity (cfPWV; NIHem workstation, Cardiovascular Engineering, Inc., Norwood, MA) as previously described (16). Briefly, a tonometer was used to collect pressure waveforms from the brachial, radial, carotid, and femoral arteries. The carotid and femoral artery waveforms were gated to the R-wave of the ECG to determine the foot-to-foot time delay. Carotid-femoral transit distance was quantified as the distance between the suprasternal notch and the femoral waveform pulse site minus the distance from the suprasternal notch to the carotid waveform pulse site. cfPWV was calculated using the following equation: cfPWV = carotid-femoral transit distance/carotid-femoral foot-to-foot time delay.
Statistical Analysis
Variables were assessed for normality using Shapiro–Wilk tests. Normally distributed data are presented as means ± standard deviation, nonnormally distributed data as median (interquartile range), and categorical data as count (percentage of participants). Unpaired Student’s t tests were used to test differences in normally distributed variables between groups at baseline, and unpaired Wilcoxon rank-sum tests were used to test differences in nonnormally distributed variables between groups at baseline. To determine the effect of Endocalyx Pro supplementation on our primary and secondary outcomes, a linear mixed-effects model was used to examine the significance of condition, time, and their interaction for normally distributed variables, and a Gamma mixed-effects model was used for nonnormally distributed variables. In each model, a random intercept was built for each participant to account for inherent variability in the participant’s baseline values. Mean differences and their corresponding (95%) confidence interval (CI) and P values are reported for normally distributed outcomes, whereas mean ratios are reported for nonnormally distributed variables along with their corresponding 95% confidence intervals and P values. A complete case analysis was used when analyzing primary and secondary outcomes. Significance was set at an α level of 0.05. All analyses were performed in R, version 4.3.2 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
In total, 24 participants, free of overt CVD and metabolic disease, were screened and consented. Of those, 1 (4%) participant was excluded because of elevated blood pressure, and the remaining 23 (96%) participants were randomized (Fig. 1). All 23 (100%) participants that were randomized completed the intervention (Fig. 1). Baseline clinical characteristics are presented in Table 1. On average, participants were 66 ± 7 yr of age, and 52% were female. Most participants were non-Hispanic (100%), white individuals (96%) with clinically optimal fasting glycemic control and blood lipid profile. In addition, on average, participants had clinically elevated blood pressure (17), and 6 (26%) participants were receiving antihypertensive therapy.
Figure 1.

Study randomization scheme.
Table 1.
Baseline clinical characteristics
| Variable | Placebo (n = 11) | Endocalyx Pro (n = 12) | P Value |
|---|---|---|---|
| Age, yr | 67 (61,71) | 64 (62,67) | 0.99 |
| Sex, no (%) | |||
| Female | 6 (54) | 6 (50) | 0.99 |
| Male | 5 (46) | 6 (50) | |
| Race, no (%) | |||
| White | 11 (100) | 11 (92) | 0.99 |
| Non-white | 0 (0) | 1 (8) | |
| Ethnicity, no (%) | |||
| Non-Hispanic | 11 (100) | 12 (100) | 0.99 |
| Antihypertensives, no (%) | |||
| ACEi or ARB | 2 (18) | 2 (17) | |
| Calcium channel blocker | 1(9) | 1 (8) | 0.99 |
| Diuretic | 2 (18) | 1 (8) | |
| No medication | 8 (73) | 9 (75) | |
| Weight, kg | 69.6 ± 11.5 | 77.3 ± 20.2 | 0.28 |
| Body mass index, kg/m2 | 24.2 ± 3.0 | 25.9 ± 4.6 | 0.30 |
| Glucose, mg/dL | 89 ± 8 | 92 ± 10 | 0.50 |
| Total cholesterol, mg/dL | 210 ± 32 | 182 ± 40 | 0.08 |
| Triglycerides, mg/dL | 102 (75,112) | 67 (52,86) | 0.12 |
| LDL-C, mg/dL | 123 ± 29 | 112 ± 28 | 0.35 |
| HDL-C, mg/dL | 67 ± 19 | 56 ± 17 | 0.16 |
| Systolic blood pressure, mmHg | 123 ± 12 | 125 ± 17 | 0.83 |
| Diastolic blood pressure, mmHg | 69 ± 8 | 70 ± 10 | 0.97 |
| FMD, % | 3.73 (2.77,5.23) | 3.43 (2.95,6.22) | 0.57 |
| PBR 4–25, µm | 1.98 ± 0.14 | 2.10 ± 0.14 | 0.07 |
| cfPWV, m/s | 10.3 (7.2,12.3) | 8.2 (8.1,10.7) | 0.83 |
Variables are presented as means ± SD, median (interquartile range), or number (percentage). Unpaired t tests were used to test differences in normally distributed variables and unpaired Wilcoxon rank-sum tests were used to test differences in nonnormally distributed variables. ACEi/ARB, angiotensin-converting enzyme inhibitor or angiotensin receptor blocker; cfPWV, carotid-femoral pulse wave velocity (aortic stiffness); FMD, flow-mediated dilation; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; PBR, perfused boundary region.
Endothelial Glycocalyx Thickness
Following Endocalyx Pro supplementation at 3,712 mg/day for 12 wk, we observed no significant difference in overall endothelial glycocalyx thickness, expressed as PBR, between groups in vessels 4–25 μm in diameter (Table 2, Supplemental Table S1). In addition, there was no significant difference between groups in capillary endothelial glycocalyx thickness (PBR 4–7 µm) and feed vessel endothelial glycocalyx thickness (PBR 10–25 µm) after supplementation (Table 2, Supplemental Table S1). Supplemental Table S2 presents the change in endothelial glycocalyx thickness divided into 1-µm microvessel subgroups in the whole cohort. Between-group comparisons revealed that only microvessels 7 µm in diameter had a greater increase in endothelial glycocalyx thickness with Endocalyx Pro compared with placebo (Supplemental Table S2). We performed a post hoc sensitivity analysis excluding individuals taking antihypertensive medication to assess whether Endocalyx Pro had differential effects in participants not prescribed antihypertensive medication (n = 16). We observed no difference in PBR 4–25 µm (P = 0.38) and PBR 10–25 µm (P = 0.92) between groups after supplementation. Conversely, 12 wk of Endocalyx Pro supplementation reduced PBR 4–7 µm greater than placebo in individuals not taking antihypertensive medication [mean difference and 95% CI for between-group comparison, −0.08 (−0.15, −0.01); P = 0.02].
Table 2.
Perfused boundary region at baseline and after 12 wk of placebo or Endocalyx Pro
| Variable | Placebo (n = 10) |
Endocalyx Pro (n = 12) |
Between-Group Comparison |
|||
|---|---|---|---|---|---|---|
| Baseline | 12-wk | Baseline | 12-wk | B (95% CI) | Between-Group P Value | |
| PBR 4–25, µm | 1.98 ± 0.14 | 2.02 ± 0.17 | 2.10 ± 0.14 | 2.05 ± 0.15 | −0.08 (−0.25,0.09)a | 0.33 |
| PBR 4–7, µm | 0.91 ± 0.06 | 0.93 ± 0.04 | 0.94 ± 0.06 | 0.91 ± 0.04 | −0.05 (−0.11,0.00)a | 0.07 |
| PBR 10–25, µm | 2.48 ± 0.25 | 2.47 ± 0.33 | 2.51 ± 0.26 | 2.51 ± 0.27 | 0.13 (−0.27,0.53)a | 0.47 |
A linear mixed-effects model was used for variables denoted means ± standard deviation. CI, confidence interval; PBR, perfused boundary region.
Normally distributed variables are shown as the mean difference.
Endothelial Function
As presented in Fig. 2, Table 3, and Supplemental Table S1, 12 wk of Endocalyx Pro supplementation did not significantly increase FMD% compared with placebo, with the mean ratio for between-group comparisons of 1.16 (95% confidence interval, 0.77,1.74; P = 0.48). In addition, Fig. 2, Table 3, and Supplemental Table S1 reveal that 12 wk of supplementation with Endocalyx Pro did not increase FMDmm compared with placebo, with a mean ratio for the between-group comparison of 1.16 (95% confidence interval, 0.79,1.70; P = 0.46). A post hoc sensitivity analysis of individuals not taking antihypertensive medication (n = 17) revealed no difference in FMD% (P = 0.96) or FMDmm (P = 0.98) between groups after 12 wk of supplementation.
Figure 2.
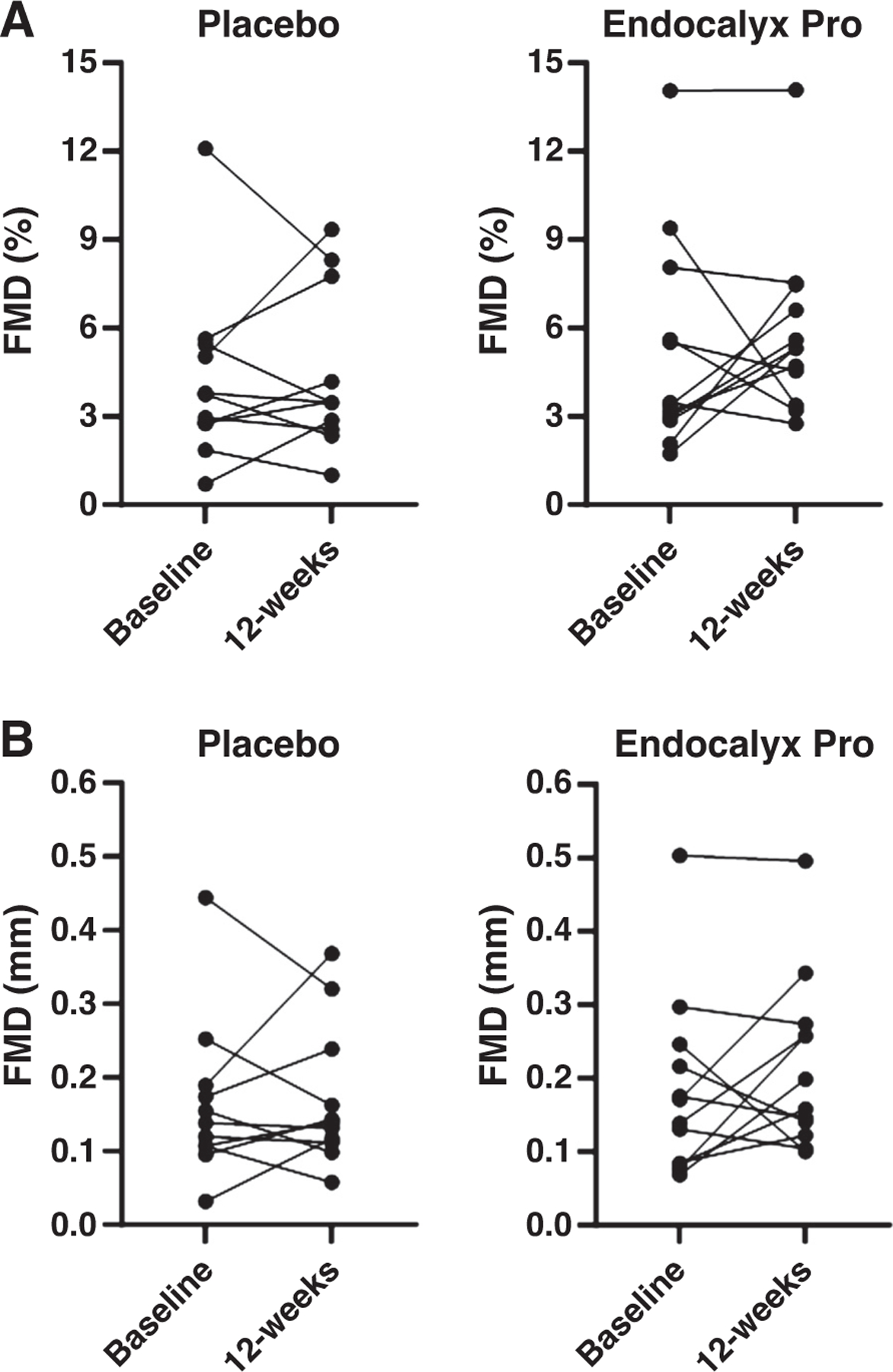
Change in FMD% (A) and FMDmm (B) after 12 wk of placebo (n = 11) or Endocalyx Pro (n = 12). A Gamma mixed-effects model was used to examine the significance of condition, time, and their interaction. FMD, flow-mediated dilation.
Table 3.
Endothelial function at baseline and after 12 wk of placebo or Endocalyx Pro
| Variable | Placebo (n = 11) |
Endocalyx Pro (n = 12) |
Between-Group Comparison |
|||
|---|---|---|---|---|---|---|
| Baseline | 12-wk | Baseline | 12-wk | B (95% CI) | Between-Group P Value | |
| FMD, % | 3.7 (2.8,5.2) | 3.5 (2.7,6.0) | 3.4 (2.9,6.2) | 5.3 (4.3,6.8) | 1.16 (0.77,1.74)b | 0.48 |
| FMD, mm | 0.14 (0.11,0.18) | 0.13 (0.11,0.20) | 0.16 (0.09,0.22) | 0.18 (0.14,0.26) | 1.16 (0.79,1.70)b | 0.46 |
| Baseline diameter, mm | 4.05 ± 0.72 | 4.10 ± 0.70 | 3.64 ± 0.73 | 3.68 ± 0.75 | −0.02 (−0.20,0.16)a | 0.81 |
| Time to peak, s | 35.3 ± 27.7 | 42.1 ± 22.6 | 55.2 ± 25.8 | 56.3 ± 20.7 | −3.8 (−35,24)a | 0.70 |
| SRAUC, 1/s | 17,184 ± 12,071 | 20,751 ± 8,910 | 42,339 ± 20,449 | 37,484 ± 12,967 | −8,062 (−23,819, 7,695)a | 0.30 |
| Peak FMD:SRAUC, a.u. | 0.29 (0.16,0.63) | 0.21 (0.13,0.41)* | 0.10 (0.07,0.16) | 0.18 (0.12,0.20) | 2.41 (1.41,4.13)b | 0.001* |
| BFAUC, a.u. | 13,132 (9,480, 14,505) | 14,407 (11,493, 16,213) | 15,595 (12,465, 18,273) | 18,493 (12,179, 20,202) | 0.95 (0.79,1.14)b | 0.55 |
A linear mixed-effects model was used for variables denoted means ± SD and a Gamma mixed-effects model was used for variables denoted with median (interquartile range). SRAUC and peak FMD:SRAUC were calculated in n = 10 participants in both the Endocalyx Pro groups. BFAUC was calculated in n = 10 participants in the placebo group and n = 11 participants in the Endocalyx Pro group. BF, blood flow; CI, confidence interval; FMD, flow-mediated dilation; SR, shear rate.
P < 0.05.
Normally distributed variables are shown as the mean difference.
Nonnormally distributed variables are shown as the mean ratio.
As shown in Table 3 and Supplemental Table S1, between-group comparisons reveal that Endocalyx Pro significantly increased brachial artery peak FMD:SRAUC compared with placebo (mean ratio, 2.41; 95% confidence interval, 1.14,4.13; P = 0.001). However, there was no change in baseline diameter, time to peak dilation, or SRAUC after supplementation (Table 3, Supplemental Table S1). In addition, brachial artery BFAUC, an index of microvascular function, was unchanged with Endocalyx Pro and placebo (Table 3, Supplemental Table S1).
Blood Pressure and Aortic Stiffness
Table 4 presents the change in hemodynamic outcome variables according to group. Following 12 wk of supplementation, we observed no between-group differences in systolic or diastolic blood pressure (Table 4, Supplemental Table S1). Furthermore, we observed no significant change in cfPWV between groups after supplementation (Table 4, Supplemental Table S1).
Table 4.
Blood pressure and aortic stiffness at baseline and after 12 wk of placebo or Endocalyx Pro
| Variable | Placebo (n = 11) |
Endocalyx Pro (n = 12) |
Between-Group Comparison |
|||
|---|---|---|---|---|---|---|
| Baseline | 12-wk | Baseline | 12-wk | B (95% CI) | Between-Group P Value | |
| Systolic BP, mmHg | 123 ± 12 | 126 ± 15 | 125 ± 17 | 120 ± 15 | −7.4 (−18,2.7)a | 0.14 |
| Diastolic BP, mmHg | 69 ± 8 | 69 ± 10 | 70 ± 10 | 67 ± 10 | −2.0 (−8.2,4.2)a | 0.50 |
| cfPWV, m/s | 10.3 (7.2,12.3) | 9.2 (8.3,10.7) | 8.2 (8.1,10.7) | 8.3 (7.5,10.0) | 0.96 (0.87,1.07)b | 0.49 |
A linear mixed-effects model was used for variables denoted means ± SD and a Gamma mixed-effects model was used for variables denoted with median (interquartile range). BP, blood pressure; cfPWV, carotid-femoral pulse wave velocity (aortic stiffness); CI, confidence interval. *P < 0.05.
Normally distributed variables are shown as the mean difference.
Nonnormally distributed variables are shown as the mean ratio.
Adverse Events and Adherence
There was no evidence of differences in adverse events between the Endocalyx Pro and placebo groups (P = 0.58). Overall, nine participants (75%) reported no side effects from Endocalyx Pro, and 10 participants (91%) reported no side effects from the placebo. In the Endocalyx Pro group, there were two reports of gastrointestinal distress (17%) and one report of extremities feeling slightly warmer (8%). Median study supplement adherence was 98.1% (94.2,99.7) in the Endocalyx Pro group and 97.7% (92.7,99.3) in the placebo group (P = 0.68).
DISCUSSION
In this randomized, double-blind, placebo-controlled, parallel-arms study involving older men and postmenopausal women, 12 wk of Endocalyx Pro supplementation did not significantly increase overall endothelial glycocalyx thickness, expressed as microvascular PBR, or vascular endothelial function, determined by brachial artery FMD. However, Endocalyx Pro did increase capillary endothelial glycocalyx thickness in individuals not taking antihypertensive medication and improve FMD normalized to shear. These data suggest that Endocalyx Pro may exert a select beneficial effect on the endothelial glycocalyx and vascular endothelium; however, larger clinical trials are needed to determine whether Endocalyx Pro improves overall endothelial glycocalyx thickness and endothelial function in older adults.
The endothelial glycocalyx is an extracellular matrix structure consisting of core glycoproteins and their attached glycosaminoglycan side chains. Collectively, distinct glycosaminoglycan side chains contribute to endothelial glycocalyx structure and function (4). Therefore, supplements that aid glycosaminoglycan synthesis and prevent glycosaminoglycan degradation could improve endothelial glycocalyx thickness with aging. Endocalyx Pro is a glycocalyx-targeted supplement consisting of glucosamine (glycocalyx precursor), hyaluronan and fucoidan (glycocalyx mimetics), and a mixture of antioxidants (8). Together, this patented formula was created to synthesize, repair, and provide antioxidant support to the endothelial glycocalyx (8). To our knowledge, our study is the first randomized, placebo-controlled clinical trial to examine the effect of Endocalyx Pro supplementation on endothelial glycocalyx thickness in older adults. In our study, 12-wk Endocalyx Pro supplementation (53 mg/kg/day) did not increase overall endothelial glycocalyx thickness (PBR 4–25), endothelial glycocalyx thickness in capillaries (PBR 4–7), and feed vessels (PBR 10–25). However, a sensitivity analysis performed in individuals not taking antihypertensive medication revealed a greater increase in capillary endothelial glycocalyx thickness (PBR 4–7) with Endocalyx Pro supplementation. In contrast to our study, Machin et al. (9) reported that supplementing old mice with Endocalyx Pro (37 mg/kg/day) for 10 wk increased overall endothelial glycocalyx thickness in the mesenteric microvasculature greater than old mice fed a standard rodent diet. Of note, the mean weight of participants in our study was 70.6 kg; therefore, participants in the Endocalyx Pro group received 53 mg/kg/day on average. Moreover, our participants had lower PBR 4–25 µm at baseline than older adults in a previous study (17), suggesting that Endocalyx Pro may not change overall endothelial glycocalyx thickness in populations with lower PBR 4–25 µm (thicker endothelial glycocalyx) at baseline. Taken together, our results indicate that Endocalyx Pro may have promise as a supplement to increase capillary endothelial glycocalyx thickness in older adults not taking antihypertensive medication; however, larger adequately powered studies are needed.
Endocalyx Pro might contribute to increased endothelial glycocalyx thickness via several mechanisms, including the restoration of endothelial glycocalyx components and attenuated oxidative stress. Hyaluronan accounts for 5–20% of all glycosaminoglycans within the endothelial glycocalyx (4). In addition, hyaluronan synthase 2 (Has2) gene expression, responsible for synthesizing high molecular-weight hyaluronan, declines with age in mice (5), and Has2 knockout mice have reduced endothelial glycocalyx thickness compared with age-matched controls (9). Of note, supplementing old mice with hyaluronan increases endothelial glycocalyx thickness to a similar degree as Endocalyx Pro (9). Alternatively, Endocalyx Pro may increase endothelial glycocalyx thickness by enhancing heparan sulfate production through the actions of fucoidan and glucosamine. Heparan sulfate is the primary glycosaminoglycan within the endothelial glycocalyx, comprising 50–90% of all glycosaminoglycans (4). Fucoidan is a heparan sulfate mimetic that prevents heparan sulfate degradation and restores endothelial glycocalyx thickness in cultured cells treated with proinflammatory serum from patients with COVID-19 (18). In addition, glucosamine provides essential building blocks for heparan sulfate synthesis and may have influenced endothelial glycocalyx thickness in our study and the preclinical study by Machin et al. (9, 19, 20). Last, antioxidants incorporated into Endocalyx Pro may have attenuated endothelial cell oxidative stress, thereby preventing endothelial glycocalyx degradation and augmenting endothelial glycocalyx thickness, but we did not measure oxidative stress to confirm this supposition (21). Taken together, in addition to further clinical trials, more studies will be needed to expand on our findings and investigate the mechanisms by which Endocalyx Pro may enhance capillary endothelial glycocalyx thickness.
In addition to targeting endothelial glycocalyx thickness, Endocalyx Pro may also be a promising therapy to improve endothelial function with advancing age. A widely used technique to determine endothelial function in humans is brachial artery FMD (14). In our study, 12 wk of Endocalyx Pro supplementation did not increase FMD or FMDmm greater than the placebo group. In addition, Endocalyx Pro did not increase the time to peak dilation, SRAUC, or BFAUC but did increase peak FMD:SRAUC greater than the placebo group. Considering SRAUC is a primary determinant of the peak FMD response (15, 22), our data suggest that a lower shear stress stimulus was needed to elicit peak FMD after Endocalyx Pro supplementation but not placebo. Of note, the Endocalyx Pro group had a significantly higher SRAUC than the placebo group (P = 0.003) at baseline, indicating that the Endocalyx Pro group required more shear stress to achieve a similar peak dilation as the placebo group. In addition, the placebo group had a 43% reduction in peak FMD:SRAUC after 12 wk, which presumably influenced our significant between-group finding regarding peak FMD:SRAUC. Our findings concerning vascular endothelial function build on a study by Machin et al. (9) demonstrating that 10-wk Endocalyx Pro supplementation improved nitric oxide-mediated vasodilation in ex vivo evaluations of carotid artery endothelial function in old mice. Taken together, our data suggest that 12 wk of Endocalyx Pro supplementation may improve shear stress-mediated brachial artery vasodilation in older adults. However, further investigation is required to corroborate our findings regarding the effect of Endocalyx Pro on endothelial function in older adults, given that FMD was not improved with Endocalyx Pro and that there was a reduction in peak FMD:SRAUC in the placebo group after 12 wk. Alternatively, it is possible that Endocalyx Pro could enhance vascular endothelial function independent of improvements in brachial artery mechanotransduction.
Recently, it was demonstrated that elevated blood pressure and aortic stiffness are associated with reduced endothelial glycocalyx thickness in middle-aged and older adults with hypertension (23). Therefore, it is plausible that restoring endothelial glycocalyx thickness could lower blood pressure and aortic stiffness in older adults. We did not observe a reduction in either systolic or diastolic blood pressure after Endocalyx Pro. Furthermore, there was no change in aortic stiffness after 12-wk supplementation. Previously, Machin et al. (9) observed a significant reduction in systolic blood pressure after 10-wk Endocalyx Pro supplementation in old mice. In the same study, there was a significant reduction in aortic stiffness, medial aortic cross-sectional area, and medial aortic collagen content, as well as an increase in medial aortic elastin content (9). However, aortic biomechanics and hemodynamics differ considerably between mice and humans with aging (24, 25), which may explain our disparate results. Collectively, the potential antihypertensive effect of Endocalyx Pro warrants further investigation, given that blood pressure was not a primary endpoint in our study and most of our participants did not have clinical hypertension. Thus, future trials testing whether Endocalyx Pro lowers blood pressure in individuals with hypertension are needed.
There were several limitations to our study. First, PBR is an indirect measure of microvascular endothelial glycocalyx thickness estimated from the lateral movement of RBCs in sublingual microvessels. Although PBR has been validated in humans (9), it is unknown the extent to which sublingual endothelial glycocalyx thickness determined from PBR reflects whole body endothelial glycocalyx thickness. In addition, we did not conduct acute pharmacokinetic assessments to evaluate the blood concentrations of Endocalyx Pro components following oral administration in humans. As such, we cannot confirm the absorption of Endocalyx Pro. Next, Endocalyx Pro contains glucosamine, fucoidan, hyaluronan, and antioxidants, making it difficult to parse out the specific effects of each component on endothelial glycocalyx thickness and vascular endothelial function with aging. Further trials are needed to separately assess the effects of each Endocalyx Pro component on endothelial glycocalyx thickness and vascular endothelial function in older adults. Finally, although we were able to detect discrete changes in endothelial glycocalyx thickness and FMD normalized to shear stress, it is unclear if endothelial glycocalyx restoration was responsible for the observed change in shear stress-mediated brachial artery vasodilation. Future studies could include endothelial glycocalyx thickness as a mediating variable in adequately powered clinical trials examining the effect of Endocalyx Pro on FMD in humans.
In conclusion, our pilot study suggests that Endocalyx Pro supplementation is feasible in older adults, although it did not enhance FMD and overall endothelial glycocalyx thickness. However, our findings regarding the effect of Endocalyx Pro on capillary endothelial glycocalyx thickness and FMD normalized to shear stress are worthy of additional investigation. Moreover, larger clinical trials are needed to expand on our pilot findings in older adults. Finally, additional trials are needed to test whether Endocalyx Pro improves endothelial glycocalyx thickness and vascular function in older adults with additional CVD risk factors such as hypertension, hyperlipidemia, and hyperglycemia or overt CVD.
Supplementary Material
Supplemental Table S2: https://doi.org/10.6084/m9.figshare.25632336.
Supplemental Table S1: https://doi.org/10.6084/m9.figshare.25632333.
ACKNOWLEDGMENTS
The authors thank the nurses and staff of the Clinical Research Unit at the University of Iowa Hospitals and Clinics for their help in completing the study.
GRANTS
C.J.G. is supported by the American Heart Association predoctoral Fellowship Grant 23PRE1012593. G.L.P. is supported by the Russell B. Day and Florence D. Day Endowed Chair in Liberal Arts and Sciences at the University of Iowa.
Footnotes
DISCLAIMERS
Microvascular Health Solutions provided Endocalyx Pro and the matching placebo free of charge but were not involved in data collection, analysis, or interpretation of the results.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
DATA AVAILABILITY
The data that support this study are available at https://doi.org/10.6084/m9.figshare.25632369.
REFERENCES
- 1.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises. Circulation 107: 139–146, 2003. doi: 10.1161/01.CIR.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- 2.Donato AJ, Machin DR, Lesniewski LA. Mechanisms of dysfunction in the aging vasculature and role in age-related disease. Circ Res 123: 825–848, 2018. doi: 10.1161/CIRCRESAHA.118.312563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Machin DR, Phuong TT, Donato AJ. The role of the endothelial glycocalyx in advancing age and cardiovascular disease. Curr Opin Pharmacol 45: 66–71, 2019. doi: 10.1016/j.coph.2019.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Foote CA, Soares RN, Ramirez-Perez FI, Ghiarone T, Aroor A, Manrique-Acevedo C, Padilla J, Martinez-Lemus L. Endothelial glycocalyx. Compr Physiol 12: 3781–3811, 2022. doi: 10.1002/cphy.c210029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Machin DR, Bloom SI, Campbell RA, Phuong TTT, Gates PE, Lesniewski LA, Rondina MT, Donato AJ. Advanced age results in a diminished endothelial glycocalyx. Am J Physiol Heart Circ Physiol 315: H531–H539, 2018. doi: 10.1152/ajpheart.00104.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smilowitz NR, Luttrell-Williams E, Golpanian M, Engel A, Buyon JP, Katz SD, Berger JS. Microvascular endothelial glycocalyx thickness is associated with brachial artery flow-mediated dilation. Vasc Med 26: 563–565, 2021. doi: 10.1177/1358863X211026765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ikonomidis I, Thymis J, Simitsis P, Koliou GA, Katsanos S, Triantafyllou C, Kousathana F, Pavlidis G, Kountouri A, Polyzogopoulou E, Katogiannis K, Vlastos D, Kostelli G, Triantafyllidi H, Parissis J, Papadavid E, Lekakis J, Filippatos G, Lambadiari V. Impaired endothelial glycocalyx predicts adverse outcome in subjects without overt cardiovascular disease: a 6-year follow-up study. J Cardiovasc Transl Res 15: 890–902, 2022. doi: 10.1007/s12265-021-10180-2. [DOI] [PubMed] [Google Scholar]
- 8.Long R, Vink H. Synergistic Glycocalyx Treatment Composition and Methods. US Patent US9943572B2. April 17, 2018. [Google Scholar]
- 9.Machin DR, Trott DW, Gogulamudi VR, Islam MT, Bloom SI, Vink H, Lesniewski LA, Donato AJ. Glycocalyx-targeted therapy ameliorates age-related arterial dysfunction. GeroScience, 45: 2351–2365, 2023. doi: 10.1007/s11357-023-00745-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eickhoff MK, Winther SA, Hansen TW, Diaz LJ, Persson F, Rossing P, Frimodt-Møller M. Assessment of the sublingual microcirculation with the GlycoCheck system: reproducibility and examination conditions. PLoS One 15: e0243737, 2020. doi: 10.1371/journal.pone.0243737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rovas A, Sackarnd J, Rossaint J, Kampmeier S, Pavenstädt H, Vink H, Kümpers P. Identification of novel sublingual parameters to analyze and diagnose microvascular dysfunction in sepsis: the NOSTRADAMUS study. Crit Care 25: 112, 2021. doi: 10.1186/s13054-021-03520-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee DH, Dane MJC, Van Den Berg BM, Boels MGS, Van Teeffelen JW, De Mutsert R, Heijer M, Den Rosendaal FR, Van Der Vlag J, Van Zonneveld AJ, Vink H, Rabelink TJ, NEO study group. Deeper penetration of erythrocytes into the endothelial glycocalyx is associated with impaired microvascular perfusion. PLoS One 9: e96477, 2014. doi: 10.1371/journal.pone.0096477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gimblet CJ, Ernst JW, Bos KD, Stroud AK, Donato AJ, Jalal DI, Pierce GL. Effect of acute heparin administration on glycocalyx thickness and endothelial function in healthy younger adults. J Appl Physiol (1985) 136: 330–336, 2024. doi: 10.1152/japplphysiol.00767.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thijssen DHJ, Black MA, Pyke KE, Padilla J, Atkinson G, Harris RA, Parker B, Widlansky ME, Tschakovsky ME, Green DJ. Assessment of flow-mediated dilation in humans: a methodological and physiological guideline. Am J Physiol Heart Circ Physiol 300: H2–H12, 2011. doi: 10.1152/ajpheart.00471.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Padilla J, Johnson BD, Newcomer SC, Wilhite DP, Mickleborough TD, Fly AD, Mather KJ, Wallace JP. Normalization of flow-mediated dilation to shear stress area under the curve eliminates the impact of variable hyperemic stimulus. Cardiovasc Ultrasound 6: 44, 2008. doi: 10.1186/1476-7120-6-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gimblet CJ, Armstrong MK, Nuckols VR, DuBose LE, Holwerda SW, Luehrs RE, Lane AD, Voss MW, Pierce GL. Sex-specific associations of reservoir-excess pressure parameters with age and subclinical vascular remodeling. J Hypertens 41: 624–631, 2023. doi: 10.1097/HJH.0000000000003378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA, Williamson JD, Wright JT. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. Hypertension 71: 1269–1324, 2018. [Erratum in Hypertension 7: e136-e139, 2018, and in Hypertension 72: e33, 2018]. doi: 10.1161/HYP.0000000000000066. [DOI] [PubMed] [Google Scholar]
- 18.Yuan L, Cheng S, Sol WMPJ, Velden AIM, van der Velden AIM, Vink H, Rabelink TJ. van den Berg BM. Heparan sulfate mimetic fucoidan restores the endothelial glycocalyx and protects against dysfunction induced by serum of COVID-19 patients in the intensive care unit. ERJ Open Res 8: 00652–2021, 2022. doi: 10.1183/23120541.00652-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCarty MF. Glucosamine may retard atherogenesis by promoting endothelial production of heparan sulfate proteoglycans. Med Hypotheses 48: 245–251, 1997. doi: 10.1016/S0306-9877(97)90328-5. [DOI] [PubMed] [Google Scholar]
- 20.Pretorius D, Richter RP, Anand T, Cardenas JC, Richter JR. Alterations in heparan sulfate proteoglycan synthesis and sulfation and the impact on vascular endothelial function. Matrix Biol Plus 16: 100121, 2022. doi: 10.1016/j.mbplus.2022.100121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Villalba N, Baby S, Yuan SY. The Endothelial glycocalyx as a double-edged sword in microvascular homeostasis and pathogenesis. Front Cell Dev Biol 9: 711003, 2021. doi: 10.3389/fcell.2021.711003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pyke KE, Tschakovsky ME. The relationship between shear stress and flow-mediated dilatation: implications for the assessment of endothelial function. J Physiol 568: 357–369, 2005. doi: 10.1113/jphysiol.2005.089755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ikonomidis I, Voumvourakis A, Makavos G, Triantafyllidi H, Pavlidis G, Katogiannis K, Benas D, Vlastos D, Trivilou P, Varoudi M, Parissis J, Iliodromitis E, Lekakis J. Association of impaired endothelial glycocalyx with arterial stiffness, coronary microcirculatory dysfunction, and abnormal myocardial deformation in untreated hypertensives. J Clin Hypertens (Greenwich) 20: 672–679, 2018. doi: 10.1111/jch.13236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hopper SE, Cuomo F, Ferruzzi J, Burris NS, Roccabianca S, Humphrey JD, Figueroa CA. Comparative study of human and murine aortic biomechanics and hemodynamics in vascular aging. Front Physiol 12: 746796, 2021. doi: 10.3389/fphys.2021.746796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pierce GL, Coutinho TA, DuBose LE, Donato AJ. Is it good to have a stiff aorta with aging? Causes and consequences. Physiology (Bethesda) 37: 154–173, 2022. doi: 10.1152/physiol.00035.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table S2: https://doi.org/10.6084/m9.figshare.25632336.
Supplemental Table S1: https://doi.org/10.6084/m9.figshare.25632333.
Data Availability Statement
The data that support this study are available at https://doi.org/10.6084/m9.figshare.25632369.


