Abstract
Background
Pseudotyped lentiviral vectors (LVs) were used in cancer therapy as gene delivery systems for inhibiting tumor angiogenesis by targeting cells overexpressing “vascular endothelial growth factor receptor-2 (VEGFR2)”. Herein, we report that switching from chimeric sindbis virus glycoprotein (SVG) harboring VEGFR2-specific nanobody (VEGFR2-Nb) to Two-Molecules Targeting Approach (TMTA: independent co-display of binding and fusogenic moieties) highly enhanced the transduction efficiency (TE) of the targeted LVs.
Methods
Several LVs co-displaying either the VEGFR2-Nb or the natural ligand (VEGF121) as targeting moiety, along with a de-targeted mutant form of SVG (as a binding deficient and fusion competent) fusogenic moiety were produced. LVs were constructed via various backbones and linkers (platelet-derived growth factor receptor “PDGFR” and CD28 as transmembrane domains, and HL and Fc as spacer domains).
Results
Expression and incorporation of the VEGFR2-Nb and SVG onto lentiviral particles were confirmed by flowcytometry and Western blotting while their co-display was demonstrated by virus-capture ELISA and virus-cell binding assays. LVs co-enveloped with fusogen and either VEGFR2-Nb or VEGF121 showed higher TEs in VEGFR2-expressing cells (72% and 91%, respectively) over LVs pseudotyped with chimeric fusogen containing the same nanobody (30%). In silico analyses indicated a direct correlation for the TE and the distance between the nanobody and the lipid bilayer.
Conclusion
Compared to the chimeric strategy, the two-molecule targeting approach of LVs, due to its flexible and modular nature provides higher TE and thus great potentials for targeted gene delivery.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12985-025-02923-3.
Introduction
Gene therapy against a disseminated disease such as cancer is likely to achieve optimal results when delivered systemically to target metastatic lesions [1, 2]. Nevertheless, the concept of “systemic gene therapy” faces significant challenges related to specificity, efficacy, and safety, which underscores the need for “targeted delivery” in gene therapies [3, 4]. To address these issues, the engineering of targeted delivery vectors, such as Lentiviral vectors (LVs), to recognize cancer-specific or associated markers has been proposed as a promising solution [5]. Tumor-associated endothelial cells (TAECs) play a crucial role in tumor angiogenesis [6, 7] and are accessible to vectors administered systemically [8, 9]. Furthermore, the capacity of a single endothelial cell to support up to 100 tumor cells [8, 10], provides an excellent opportunity for delivery of transgenes that can exert bystander effects [11]. Given that vascular endothelial growth factor Receptor 2 (VEGFR2), a key mediator of angiogenesis [12–14], is upregulated on TAECs across various tumor types [15], makes TAECs an intriguing candidate for targeted gene therapy via VEGFR2.
A prevalent method for achieving transductional targeting of LVs at the cell surface involves pseudotyping these vectors with heterologous viral glycoproteins (GPs) that are engineered to include a specific targeting moiety [16, 17]. However, a significant limitation of this strategy is the potential impact of the targeting moiety’s insertion on the fusion activity of the GP, which may reduce the infectivity of the viruses [18]. One strategy to bypass this obstacle takes advantage of sindbis virus GP (SVG). The SVG is composed of E1 and E2 GPs where E1 mediates fusion at low pH, independent of binding activity of E2 [19]. In previous work, we developed a binding-deficient SVG and created chimeric SVGs by inserting a VEGFR2-specific nanobody (3VGR19) or VEGF121, a natural ligand of VEGFR2, between residues 71–74 of the sindbis virus E2 GP [20]. LVs pseudotyped with these chimeric glycoproteins (GPs) demonstrated a transduction efficiency of approximately 30% in VEGFR2-expressing, 293/KDR cells; however, there remains a persistent need for improved transduction rates. Indeed, the insertion of a notably large targeting moiety into the sindbis GP presents significant challenges in preserving the structural integrity of both the targeting moiety and the envelope GPs in the constructed chimeras [21, 22]. In this context, it is shown that insertion of avidin or streptavidin into E2 of the sindbis GP disrupted the production of infectious pseudotyped LVs likely due to the destabilization of trimers of E1/E2 heterodimers [23]. To address this concern, several groups have exploited adaptor-based approaches via bispecific antibodies [24] or insertion of monomeric biotin-binding domain [23], SpyTag [25], and PDZ1 peptide [26] into E2 molecule of detargeted sindbis GPs. While these strategies may alleviate the necessity for developing functional chimeric GPs for each targeting ligand, concerns regarding the potential instability of the vector-adaptor complex in vivo, particularly with bispecific antibodies, and the difficulties associated with the separate production of vector and adaptor molecules, including regulatory challenges, pose significant barriers to clinical implementation [1].
Another sophisticated strategy in transductional targeting takes advantage of the inherent ability of lentiviral virions to incorporate host cell plasma membrane proteins during the budding process. In this approach, the recombinant targeting moiety is co-expressed independently alongside a separate detargeted fusogenic GP on the surface of virus-producing cells, and therefore, both components are co-displayed on the resulting lentiviral particles [18]. This approach allows for the production of targeted lentiviral vectors as a unified product, which is advantageous for large-scale manufacturing and regulatory compliance. Furthermore, this strategy mitigates the difficulties associated with chimeric targeting methods, particularly in maintaining the correct functional conformation of the envelope glycoprotein and/or targeting component, thereby facilitating the creation of novel targeted LVs. Several studies adopted this approach and targeted LVs by co-displaying detargeted mutant forms of SVG that are binding-deficient yet retain fusogenic capabilities, in conjunction with various targeting moieties such as antibodies [27, 28], natural ligands [29], and receptors [30]. This approach has proven to be flexible and readily optimized through modifications to transmembrane or spacer domains [31, 32]. The targeting ligand plays a crucial role in the functionality of targeted LVs, as its specific and high-affinity binding to tumor cell-specific or associated markers represents the initial and vital step in the transduction of the intended cell types. Nanobodies, characterized by their small size, stability, and high-affinity antigen-binding properties, present a promising candidate as a targeting ligand [33]. As mentioned above, we previously showed that LVs pseudotyped with sindbis virus E2 GPs harboring VEGFR2-specific nanobody (3VGR19) or VEGF121 as a targeting ligand reached to a transduction efficiency of around 30% in VEGFR2-expressing, 293/KDR cells. In the current study, we investigated whether switching the targeting strategy from chimeric to two-molecule (i.e.: co-displaying targeting/binding and fusogenic moieties) will improve the transduction efficiency of targeted LVs. To this end, we examined various backbones/frameworks to display 3VGR19 nanobody [34] alongside the de-targeted mutant form of SVG. Additionally, to assess whether this strategy is independent of the targeting moiety, we used VEGF121 as a control for the targeting modality.
Materials and methods
Plasmids
Plasmids pLOX-CWgfp (LV transfer vector encoding GFP as the reporter gene; Addgene plasmid # 12241 [35]), psPAX2 (packaging plasmid; Addgene plasmid # 12260), and pMD2.G (encoding vesicular stomatitis virus glycoprotein G (VSV-G); Addgene plasmid # 12259) were gifts from Didier Trono (Department of Genetics and Microbiology, Faculty of Medicine, CMU, Geneva, Switzerland). Plasmids p2.2-L [20] and pDis-Nb [36] were previously described. p2.2-L encodes a binding deficient and fusion competent mutant form of SVG, in which HA- and His-tag linkers are inserted into E2 GP. pDis-Nb is a derivative of pDisplay (Invitrogen, USA) which expresses a membrane-anchored form of 3VGR19 nanobody. Plasmid pcDNA3.1-PS11-scFvFc-CD28-gp41 (706–713) [37], herein designated as pDF, was kindly provided by Dr. Wayne Marasco (Dana-Farber Cancer Institute, Boston, Massachusetts). This plasmid has been used to display antibody fragments on the surface of LVs and human cells. The pDis-Nb-HL vector was generated by insertion of a synthetic sequence encoding a helical linker (RGSGA(EAAAK)7ALGS) (Biomatik, Canada) [38], into the SalI recognition site of pDis-Nb. To construct pDF-Nb, the sequence corresponding to 3VGR19 nanobody was PCR amplified from pHEN6C-3VGR19 [34] and the resulting fragment was cloned at SfiI and NotI restriction sites, in frame with Fc domain of pDF to replace the ScFv. The pDF-Nb-ΔFc plasmid was constructed by cloning 3VGR19 nanobody into SfiI and XbaI restriction sites of pDF. The same strategies were used to construct different plasmids encoding membrane-bound VEGF121 (a natural isoform of VEGF, consisting of 121 amino acids) and the constructs were designated as pDis-VE, pDis-VE-HL, pDF-VE, and pDF-VE-ΔFc, respectively.
Cells and transfections
293T cells were obtained from National cell bank of Iran (Pasteur Institute of Iran, Iran). 293/KDR cells were purchased from Sibtech (USA). Cells were grown in DMEM supplemented with 10% FBS, 1% v/v Glutamax (Life Technologies, USA), and Pen/Strep (100 units/mL of penicillin and 100 µg/mL of streptomycin). Transfections were performed by Turbofect transfection reagent (Thermoscientific, Lithuania) according to the manufacturer’s instructions. Briefly, one night before transfection, 5 × 105 or 1 × 106 293T cells were seeded in 6-well plates or 6-cm cell culture dishes, respectively. Subsequently, cells were transfected with a total of 4 µg of plasmids and 6 µl of Turbofect transfection reagent for 6-well plates or 6 µg of plasmids and 12 µl of Turbofect transfection reagent for 6-cm cell culture dishes.
LV production and titration
To produce LVs co-displaying the targeting motif (3VGR19 nanobody or VEGF121) and SVG, 293T cells were seeded in 6-cm culture dishes one night before transfection. The following day, cells were transfected with 2.6 µg pLOX-CWgfp, 1.7 µg psPAX2, 0.85 µg of p2.2-L, and 0.85 µg of either of the plasmids: pDis-Nb, pDis-Nb-HL, pDF-Nb, pDF-Nb-ΔFc, pDis-VE, pDis-VE-HL, pDF-VE or pDF-VE-ΔFc, using Turbofect transfection reagent. The resultant viruses were designated as Dis-Nb/2.2-L, Dis-Nb-HL/2.2-L, DF-Nb/2.2-L, DF-Nb-ΔFc/2.2-L, Dis-VE/2.2-L, Dis-VE-HL/2.2-L, DF-VE/2.2-L, and DF-VE-ΔFc/2.2-L, respectively. As controls, 293T cells were transfected with 3 µg pLOX-CWgfp, 2 µg psPAX2, and 1 µg of either pMD2.G (positive non-targeting controls, designated as VSV-G viruses) or p2.2-L (negative controls, designated as 2.2-L viruses). Viral supernatants were collected 48 and 72 h post-transfection. The supernatants were centrifuged at 3000 g for 15 min at 4 °C to remove cell debris. To concentrate viral vectors, centrifugation was performed at 48,000 g for 3 h at 4 °C and viral pellets were resuspended in cold PBS. Physical titration of viral vectors was performed by p24 (capsid) ELISA (Pasto Lentivirus HIV p24, Pasteur Institute of Iran, Iran) according to the manufacturer’s instructions.
SDS-PAGE and western blot analyses
Around 1 × 106 virus-producing cells were harvested and centrifuged at 300 g for 5 min. Cell pellets were resuspended in appropriate volumes of SDS loading buffer. In parallel, equal amounts of concentrated viruses (normalized by p24 content) were mixed with SDS loading buffer. Cell and virus suspensions were then placed in boiling water for 5 min. The denatured suspensions were separated by 12% SDS-PAGE and finally electrotransferred to a PVDF membrane. To detect the expression of Dis-Nb, Dis-Nb-HL proteins (expressed from pDis-Nb and pDis-Nb-HL, respectively) and 2.2-L (expressed from p2.2-L), the membranes were incubated with primary mouse anti-HA tag antibody (1:1000, Cell Signaling, USA) and secondary rabbit anti-mouse HRP conjugated antibody (1:2000, Cell Signaling, USA). Protein bands were finally visualized using Amersham ECL Western Blotting Detection Reagents (GE Healthcare Life Sciences, UK) and X-ray films. Intensities of bands were quantified by Fiji distribution of ImageJ software [39].
Cell surface expression analyses of membrane-bound forms of nanobody (mNbs) by flow cytometry
Flow cytometry was used to detect the surface expression of mNbs. To this end, 293T cells were transfected with corresponding constructs and 48 h post-transfection, cells were dissociated by PBS containing 10 mM EDTA and 1 × 106 cells were stained for each group. For pDisplay-based construct (pDis-Nb or pDis-Nb-HL), mouse anti-c-myc-tag mAb IgG2a (1 µg/ml, Genscript, USA) and Anti-Mouse IgG2a PerCP-eFlour 710 (0.25 µg/test, eBioscience, USA) were used as primary and secondary antibodies, respectively. To detect pDF-Nb, cells were stained with FITC conjugated-anti-Fc antibody (Razibiotech, Iran). Mock-transfected cells were also stained as negative controls.
All flow cytometric analyses were performed by Cyflow SL (Partec, Germany) and the percentage of positive populations was determined by Super-Enhanced Dmax Subtraction (SED) algorithm of FlowJo software (FLOWJO LLC, USA).
Virus capture ELISA
Virus capture ELISA was performed as previously described [16]. Briefly, a 96-well plate was coated with 2.5 µg/ml of extracellular domain of human VEGFR2 (G&P biosciences, USA) or 2.5 µg/ml bovine serum albumin (BSA) (Sigma, USA) using carbonate/bicarbonate buffer (pH 9.5). Wells were blocked with PBS-BSA 3%. Equal amounts of concentrated lentiviral particles (determined by p24 content) were added to each well and incubated for 1 h at 37 °C. Wells were then washed four times with PBS-FBS 2%. Captured viruses were lysed and the virus lysates were transferred to a p24 pre-coated ELISA plate (Pasto Lentivirus HIV p24, Pasteur Institute of Iran, Iran). Subsequently, p24 contents were determined according to the manufacturer’s protocol.
Virus-cell binding assay
0.5 × 106 293/KDR cells were incubated with equal amounts of concentrated LVs (normalized by p24 content) for 1 h at 4˚C. The virus-bound cells were detected by staining with His-probe mouse monoclonal antibody (1:50, Santa Cruz, USA) followed by Anti-mouse IgG, F(ab´)2 Fragment (PE Conjugate) (1:500, Cell Signaling, USA). As negative controls, 293/KDR cells or 293/KDR cells incubated with 2.2-L LVs were stained with the above-mentioned antibodies. Finally, cells were analyzed by Cyflow SL flow cytometer (Partec, Germany).
Targeted transduction
293T or 293/KDR cells were seeded in 24-well cell culture plates. The next day (at 70% confluency), cell medium was removed and cells were incubated with equal amounts of each un-concentrated viral group (normalized by p24 content) for 8 h. Subsequently, viruses were replaced with fresh medium and expression of GFP was assessed 96 h later by flow cytometry using Cyflow SL (Partec, Germany).
In Silico analyses
The molecular model for each transmembrane, linker, and main nanobody region was constructed and eventually assembled into a full-length molecule. In this regard, the secondary structure was predicted for each of designed molecule using PSIpred [40] and SPIDER2 [41] webservers. The transmembrane (TM) domain residues were predicted using TMHMM 2.0 webserver [42]. Molecular models in atomic scale were predicted by homology modeling, threading, and ab-initio methods using RaptorX [43], I-TASSER [44], QUARK [45], Swiss-model [46], and MODELLER [47] webservers. The best partial structure templates for each molecule, which matched more with the predicted secondary structures and transmembrane region prediction, were assembled and applied for threading one more time. The final molecular models were quality checked, energy minimized, and tested for stability by molecular dynamics (MD) simulations using NAMD version 2.12 software [48] and analyzed using VMD software [48]. A membrane patch of 50 Å dimensions, including all POPC (1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine) molecules was constructed using VMD. Four molecular models were implemented in separate membrane patches, solvated by explicit TIP3 water molecules, and ionized to the physiological concentration of NaCl. MD simulation runs were carried out for 500 to 1200 nanoseconds using NAMD version 2.12 to determine the behavior and the distance of the nanobody to the membrane in each model. All movies were generated by VMD software. Surface area accessibility (SASA) of the nanobody segment and the complementarity-determining regions (CDRs) were determined for each full-length molecule. Root mean square deviation (RMSD) and root mean square fluctuation (RMSF) were calculated and analyzed for the full-length molecules and nanobody segments after simulation runs.
Results
Construction of membrane-bound forms of 3VGR19 nanobody (mNbs) and membrane-bound forms of VEGF121 (mVEs) encoding plasmids
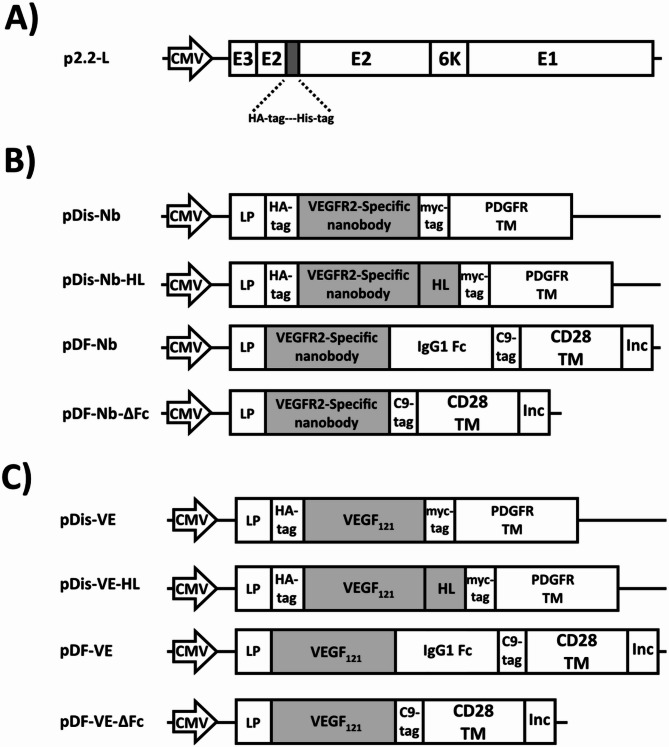
P2.2-L and pDF are presented in Fig. 1a and supplementary Fig. 1, respectively. Schematic images of plasmids encoding mNbs (pDis-Nb, pDis-Nb-HL, pDF-Nb, and pDF-Nb-ΔFc) are illustrated in Fig. 1b and images related to plasmids encoding mVEs (pDis-VE, pDis-VE-HL, pDF-VE, and pDF-VE-ΔFc) are shown in Fig. 1c. pDis-Nb-HL was generated by insertion of a helical linker between the nanobody and PDGFR transmembrane domain of pDis-Nb. pDF-Nb was generated by insertion of the nanobody into pDF upstream of the human IgG1 Fc domain. To remove the Fc domain, pDF-Nb-ΔFc was constructed by cloning 3VGR19 nanobody between human IgG1 signal peptide and CD28 transmembrane domain of pDF. The same procedures were taken for construction of mVE encoding counterparts. All constructs were verified by restriction enzyme digestion and sequencing analyses (data not shown).
Fig. 1.
Schematic representation of the constructs (not drawn to scale) used for co-enveloping LVs in this study. (a) p2.2-L is a mutant form of SVG (binding deficient and fusion competent) containing HA-tag and His-tag. (b) Plasmids encoding membrane-bound forms of 3VGR19 nanobody (mNbs). (c) Plasmids encoding membrane-bound forms of VEGF121 (mVEs): CMV: Human cytomegalovirus immediate-early promoter/enhancer, LP: leader peptide, HA-tag (YPYDVPDYA), myc-tag (EQKLISEEDL), PDGFR TM: Platelet-derived growth factor receptor transmembrane domain, C9-tag (TETSQVAPA), CD28 TM: CD28 transmembrane domain, Inc: HIV gp41 derived incorporation motif (NRVRQGYS), His-tag: (HHHHHH), E1 performs the fusogenic activity. E2 is responsible for recognizing and attaching to the cell receptors. E3 and 6 K are leader sequences of E2 and E1, respectively
The mNbs were displayed on the surface of 293T cells
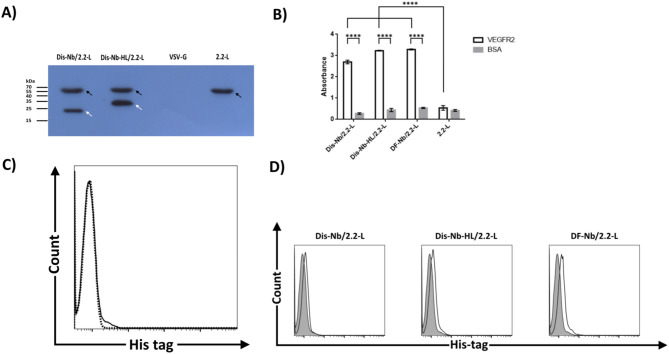
Efficient surface expression of proteins on producer cells is an essential prerequisite for incorporation of such proteins onto lentiviral particles [49]. Before investigating the surface presentation of mNbs on 293T cells, the expression of Dis-Nb and Dis-Nb-HL in virus-producing cells were assessed by western blotting using an anti-HA tag antibody. As depicted in Fig. 2a, protein bands with approximate sizes of 22 and 26 kDa (indicated by white arrows) corresponding to the expressed mNbs were detected in Dis-Nb/2.2-L and Dis-Nb-HL/2.2-L virus-producing cells, respectively. None of these protein bands were detected in 2.2-L or VSV-G virus-producing cells (used as negative control for nanobody expression). Notably, the band intensity of Dis-Nb-HL was higher than that of the Dis-Nb (approximately a 1.5-fold increased intensity). Furthermore, in Dis-Nb/2.2-L, Dis-Nb-HL/2.2-L, and 2.2-L virus-producing cells, a band with the approximate size of 67 kDa (indicated by black arrows) corresponding to E2 GP was observed. Accordingly, the presence of mNbs on the surface of 293T cells was also assessed by flow cytometry. As depicted in Fig. 2b, about 97%, 98%, and 71% of cells were transfected with pDis-Nb, pDis-Nb-HL, and pDF-Nb, respectively, indicating surface expression of the corresponding mNbs. Of note, cells expressing Dis-Nb-HL demonstrated 50% increase in mean fluorescent intensity compared to Dis-Nb expressing cells (1.21 and 0.78, respectively).
Fig. 2.
Surface expression of nanobody. (a) Western blotting of lentivirus-producing cells using anti-HA tag antibody. 293T cells were transfected with transfer (pLOX-CWgfp), packaging (psPAX2), and envelope plasmids (pDis-Nb/p2.2-L, pDis-Nb-HL/p2.2-L, pMD2.G or p2.2-L). 72 h post-transfection equal number of cells were subjected to SDS-PAGE and western blotting. White arrows indicate bands with approximate sizes of 22 and 26 kDa which correspond to Dis-Nb and Dis-Nb-HL, respectively. Black arrows show bands with an approximate size of 67 kDa which are related to sindbis E2 GP. No band was observed in cells transfected with pMD2.G (encoding VSV-G). (b) Analysis of nanobody expression of the cell surface via flow cytometry. 293T cells were transfected with pDis-Nb, pDis-Nb-HL, or pDF-Nb. 48 h post-transfection cells were stained with anti-myc tag antibody for pDisplay derived mNbs (pDis-Nb, pDis-Nb-HL) and anti-human Fc antibody for pDF-Nb. The percentage of positive cells was determined by SED algorithm of FlowJo software. Shaded histograms represent stained mock-transfected cells (negative controls) and open histograms represent cells transfected with mNb encoding plasmids
SVG (fusogen) and functional targeting molecules were co-incorporated onto the same LV virions
In addition to cell surface expression, it is important to have both functional targeting and fusogen molecules on the same virions. To investigate the incorporation of both pDisplay-derived mNbs (Dis-Nb and Dis-Nb-HL) and 2.2-L onto lentiviral particles, concentrated viruses were subjected to western blotting with anti-HA tag antibody. As has been shown in Fig. 3a, western blot analysis demonstrated the presence of SVG (approximately 67 kDa; black arrows) along with Dis-Nb or Dis-Nb-HL (22 and 26 kDa, respectively; white arrows) in LV particles. Notably, in these virions, Dis-Nb-HL showed higher band intensity than Dis-Nb (approximately a 1.5-fold increase). To evaluate whether incorporated mNbs retained their ability to recognize their corresponding antigens, two-step capture ELISA was employed. As presented in Fig. 3b, Dis-Nb/2.2-L, Dis-Nb-HL/2.2-L, and DF-Nb/2.2-L LVs bound to VEGFR2 with high selectivity in comparison to BSA-coated wells (P value < 0.0001), while 2.2-LVs showed only background binding to VEGFR2, similar to that of BSA. Finally, to examine the co-incorporation of mNbs and SVG onto the same virions, a virus-cell binding assay was performed. 293/KDR cells were incubated with LVs at 4˚C for 1 h. Following the washing steps, virus-cell complexes were stained with an anti-His tag antibody that recognizes the His-tag present in SVG. In this condition, only mNb and SVG co-incorporating virions could bind to 293/KDR cells and be detected by antibody against SVG. The flow cytometric results of cells incubated with 2.2-L LVs revealed that they did not bind to 293/KDR cells and no histogram distribution shift was observed in comparison to mock treated cells (cells without any virus incubation) (Fig. 3c). On the other hand, as presented in Fig. 3d, overlaying the virus-cell binding data of Dis-Nb/2.2-L, Dis-Nb-HL/2.2-L, and DF-Nb/2.2-L viruses against 2.2-L LVs demonstrated clear fluorescence distribution shifts, indicating functional mNbs are incorporated onto LVs’ surfaces and both mNbs and SVG are co-displayed on the same virion. The histogram shift was also observed for Dis-VE/2.2-L, Dis-VE-HL/2.2-L, and DF-VE/2.2-L but with greater intensities in comparison to mNb-harboring viruses (supplementary Fig. 2).
Fig. 3.
Co-incorporation of SVG (fusogen) and functional mNbs onto the same LV virion. (a) Western blotting of LVs using anti-HA tag antibody. 293T cells were transfected with transfer, packaging, and envelope (pDis-Nb/p2.2-L, pDis-Nb-HL/p2.2-L, pMD2.G or p2.2-L) plasmids. 48 and 72 h post-transfection, viral supernatants were harvested and LVs were concentrated by centrifugation. Equal amounts of viruses (determined by p24) were subjected to SDS-PAGE and western blotting. White arrows indicate bands with approximate sizes of 22 and 26 kDa which correspond to Dis-Nb and Dis-Nb-HL, respectively. Black arrows show bands with an approximate size of 67 kDa which are related to sindbis E2 GP. No band was observed in cells transfected with pMD2.G (encoding VSV-G). This result showed the presence of SVG, along with Dis-Nb or Dis-Nb-HL in corresponding LVs. (b) Virus capture ELISA. Viral supernatants were added to ELISA wells containing either BSA or the extracellular domain of human VEGFR2. Following incubation, wells were washed and bound viruses were measured by p24 ELISA kit. Bars and error bars represent means and standard deviations, respectively. All three mNb bearing LV groups demonstrated significant increase in attachment to VEGFR2-coated wells compared to BSA-coated wells (P value < 0.0001), while 2.2-L viruses showed background levels of attachment in both types of wells. This result indicated that functional nanobodies are displayed on the surface of LVs. (c) and (d) Virus cell binding assay. 293/KDR cells were incubated with LVs at 4˚C for 1 h. Virus-cell complexes were then stained with anti-His tag antibody (sindbis virus E2 GP used in this study contains His-tag). As negative control, 293/KDR cells were also stained with the anti-His tag antibody (c) Dotted-line histogram represents 293/KDR cells and solid-line histogram represents cells incubated with 2.2-L LVs. No shift was observed when the two histograms were overlaid. (d) Shaded histograms represent cells incubated with 2.2-L LVs. Open histograms represent cells incubated with Dis-Nb/2.2-L, Dis-Nb-HL/2.2-L, and DF-Nb/2.2-L viruses. The observed shifts in fluorescence distribution indicate the co-incorporation of SVG and functional mNbs onto the same lentiviral particles
Two-molecule targeting system results in selective transduction of VEGFR2 expressing cells and is readily optimizable through modification of targeting module
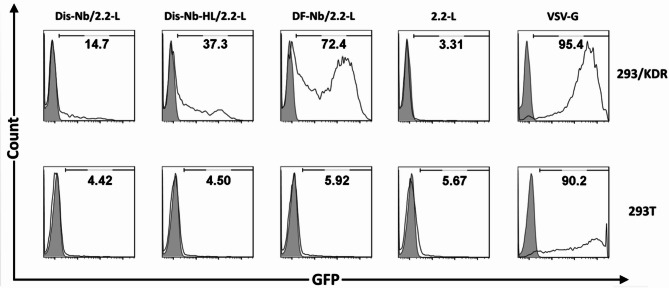
To study the transduction efficiency and specificity of LVs co-displaying both 3VGR19 nanobody and SVG (Dis-Nb/2.2-L, Dis-Nb-HL/2.2-L and DF-Nb/2.2-L viruses), VEGFR2 expressing 293/KDR cells and 293T cells were used as target and negative control cells, respectively. VSV-G LVs and 2.2-L LVs served as the positive non-targeting and negative control viruses, respectively. Moreover, DF-Nb-ΔFc/2.2-L virus was used to evaluate the effect of Fc domain on transduction efficiency. As shown in Fig. 4, VSV-G LVs were able to transduce almost all cells in both 293/KDR and 293T cells, while low levels of GFP expression in both cell types (approximately 3 and 5%, respectively) was observed for that of the 2.2-L LVs. In the case of test groups, Dis-Nb/2.2-L and Dis-Nb-HL/2.2-L viruses had background infectivity on 293T cells similar to that of the 2.2-L LVs (approximately 4%); however, when incubated with 293/KDR cells, transduction efficiency of around 14% and 37% were achieved, respectively. On the other hand, DF-Nb/2.2-L LVs reached a transduction efficiency of about 72% in 293/KDR cells and around 6% GFP expression in 293T cells. Additionally, as represented in supplementary Fig. 3, incubation of 293/KDR cells with DF-Nb-ΔFc/2.2-L virus resulted in transduction of approximately 5% of cells. Transduction assay was also performed for VEGF121-displaying LVs (Dis-VE/2.2-L, Dis-VE-HL/2.2-L, DF-VE/2.2-L, and DF-VE-ΔFc /2.2-L) (supplementary Fig. 4). Transduction efficiencies of Dis-VE/2.2-L, Dis-VE-HL/2.2-L, DF-VE-ΔFc /2.2-L, and DF-VE/2.2-L viruses on 293/KDR cells were approximately 56%, 77%, 88%, and 91%, respectively. In 293T cells, both Dis-VE/2.2-L and Dis-VE-HL/2.2-L viruses showed background transduction efficiency (≈ 3%), while a higher background infection of 293T cells was observed for DF-VE-ΔFc /2.2-L and DF-VE/2.2-L viruses (around 11% and 24%, respectively).
Fig. 4.
In vitro analysis of targeted transduction by flow cytometry. 293/KDR (expressing VEGFR2) or negative control (293T) cells were transduced by equal amounts (normalized by p24 content) of targeted LVs (Dis-Nb/2.2-L, Dis-Nb-HL/2.2-L and DF-Nb/2.2-L viruses), positive non-targeting control (VSV-G viruses) or negative control (2.2-L LVs). Four days post-infection, transduction efficiency was studied by analysis of GFP expression by flow cytometry. Shaded and open histograms represent non-transduced and transduced cells, respectively. The best transduction result was achieved by using DF-Nb/2.2-L LVs (co-displaying DF-Nb molecules and SVG)
In Silico studies
To obtain more insights into the structural features of the inserted nanobody in the constructed LVs and the impact of used linkers on nanobody display as well as their contribution to transduction efficiency, in silico studies were performed on Dis-Nb, Dis-Nb-HL, pDF-Nb, and pDF-Nb-ΔFc at the atomic level. The designed molecules contain regions from several known structures that may affect each other in the final molecular conformation. The transmembrane helix regions were predicted at residues 175 to 197 for Dis-Nb protein (total 202 residues), 221 to 243 for Dis-Nb-HL protein (total 248 residues), 178 to 200 for construct DF-Nb-ΔFc (total 224 residues), and 417 to 439 for DF-Nb protein (total 463 residues) (supplementary Fig. 5). The 3D models were successfully generated and Ramachandran analyses verified the quality of the full-length models, in which more than ∼ 97% residues in phi and psi plots were located in the favorite and allowed regions (supplementary Fig. 6). Following energy minimizations, the full-length molecules were simulated in POPC membranes at 310 K in 1 bar pressure constant condition. The SASA measurements either on the nanobody or the CDR segments revealed no meaningful difference between the designed constructs (supplementary Figs. 7, 8, 9, and 10). RMSD plot analyses of the full-length molecules elucidated that all constructs reached a plateau during MD trajectories (supplementary Fig. 11 and supplementary movie files for: Dis-Nb, Dis-Nb-HL, DF-Nb). In addition, RMSD and RMSF calculations of full-length molecules, as well as nanobody segment could not explain the observed difference in the transduction results (supplementary Figs. 11, 12, 13, and 14). However, the measured distance between the targeting moiety and the lipid membrane was significantly different for each construct. The distance between the alpha carbon of the first residue of linkers and the phosphorous atom in the closest lipid molecule was obtained as 0, 28.26, 68.40, and 82.01 Å for DF-Nb-ΔFc, Dis-Nb, Dis-Nb-HL, and DF-Nb constructs, respectively (Fig. 5).
Fig. 5.
The distance between the alpha carbon of the first residue of linkers and the phosphorous atom in the closest lipid molecule in (a) DF-Nb-ΔFc, (b) Dis-Nb, (c) Dis-Nb-HL, and (d) DF-Nb constructs. The secondary structure of molecules were predicted for each of designed molecules using PSIpred [40] and SPIDER2 [41] webservers. The transmembrane (TM) domain residues were predicted using TMHMM 2.0 webserver [42]. Molecular models in atomic scale were predicted by homology modeling, threading and ab-initio methods using RaptorX [43], I-TASSER [44], QUARK [45], Swiss-model [46], and MODELLER [47] webservers. The distance between the alpha carbon of the first residue of linkers and the phosphorous atom in the closest lipid molecule was obtained as 0, 28.26, 68.40, and 82.01 Å for DF-Nb-ΔFc, Dis-Nb, Dis-Nb-HL, and DF-Nb constructs, respectively
Discussion
In the present study, we explored the potential benefits of transitioning from a chimeric to a two-molecule transductional targeting strategy in enhancing the efficiency of gene delivery via lentiviral vectors (LVs). Our findings indicated that both the 3VGR19 nanobody and VEGF121 (the natural ligand) exhibit improved performance with the two-molecule approach compared to the chimeric method. Nevertheless, the degree of enhancement is influenced by the specific context in which the targeting ligand is presented, including factors such as spacer and transmembrane (TM) domains, as well as the nature of the targeting ligand itself. Additionally, our results reveal that, unlike the 3VGR19 nanobody, the spacer domain is less significant for VEGF121, which targets a membrane-distal region of its corresponding receptor. In this study, we adopted two backbones (pDisplay and pDF) to display nanobody on lentiviral particles. The pDisplay is a commercially available plasmid (containing murine Ig κ-chain leader sequence and platelet-derived growth factor receptor (PDGFR) TM domain) that was previously used to anchor targeting moieties into LVs’ envelopes [50, 51]. The pDF plasmid was constructed in an attempt to develop an optimal lentiviral display platform by combination of CD28 TM domain (an envelope incorporation motif derived from the membrane-proximal region of the HIV-1 gp41 cytoplasmic tail to enhance cell surface expression and protein incorporation onto LVs), along with the Fc region of the human IgG1 antibody to facilitate the display of scFv antibodies on the surfaces of LV particles [37].
In our study, the insertion of a helical linker between the transmembrane domain and the nanobody within the pDisplay-based constructs resulted in 2.5 fold increase in the transduction of 293/KDR cells (Fig. 4). This increase in transduction efficiency can be partially attributed to a 1.5-fold rise in surface expression and the integration of Dis-Nb-HL on both virus-producing cells and virions (Figs. 2 and 3a, respectively). The noted enhancement in the expression of Dis-Nb-HL aligns with prior study, which demonstrated that the addition of a similar helical linker in Tf-fusion proteins led to elevated expression levels in HEK293 cells compared to the same fusion proteins lacking the helical linker [52]. The enhanced transduction efficiency may be elucidated by findings from Rasbach et al., who reported an approximate 5-fold increase in the transduction of Her2/neu positive cells through the use of the same linker [32]. They proposed that the insertion of the spacer domain (in their case, the helical linker) may have mitigated the distance between the Her2/neu-specific DARPin and its corresponding binding site located in a membrane-proximal domain of Her2/neu, thereby rendering the displayed DARPin more sterically favorable for binding to its target binding domain [53]. Following the same rationale, it can be inferred that the 3VGR19 nanobody may interact with one of the more membrane-proximal regions of the seven extracellular Ig-like domains of VEGFR2 [54]. Additionally, VEGF121 was employed as an alternative targeting moiety, which binds to the membrane-distal domains of VEGFR2 (domains II and III). The Dis-VE/2.2-L viruses demonstrated a 3.8-fold increase in transduction efficiency in KDR cells, along with a more pronounced histogram shift in the virus-cell binding assay when compared to Dis-Nb/2.2-L LVs. The observed results may be explained by the greater affinity of VEGF (Kd = 75–760 pM) [55–57] compared to 3VGR19 nanobody (Kd = 5.4 nM [34]) as well as potential differences in their accessibility to the respective binding sites. Conversely, the introduction of the helical linker had a negligible effect on the transduction efficiency of lentiviral vectors (LVs) presenting VEGF121, resulting in only a 1.38-fold increase (supplementary Fig. 4). This finding aligns with the membrane distal binding sites of VEGF121 within VEGFR2.
Utilizing DF-Nb, we observed an improved transduction rate relative to pDisplay-based mNbs (refer to Fig. 4). The enhanced transductional targeting efficacy of DF-Nb/2.2-L in comparison to Dis-Nb-HL/2.2-L viruses may be attributed to variations in the transmembrane domain (CD28 versus PDGFR), differences in the spacer domain (Fc domain versus HL), and/or the incorporation of the gp41 motif [37]. It has been previously established that the appropriate transmembrane domain and the inclusion of a gp41 incorporation motif play crucial roles in enhancing the cell surface display of heterologous proteins and their integration into lentiviral (LV) particles. Furthermore, it has been shown that the insertion of Fc region from human IgG1 can significantly boost the cell surface expression of recombinant membrane-bound proteins [58]. Moreover, spacer domains such as IgG Fc region have been utilized to enhance antigen binding and T-cell signaling of chimeric antigen receptors (CARs), especially in the case of membrane-proximal epitopes [59, 60]. This enhancement is likely linked to increased accessibility of the targeting moieties. While it is challenging to determine the specific contribution of each component to the observed higher transduction efficiency of DF-Nb/2.2-L compared to Dis-Nb-HL/2.2-L viruses, the IgG1 Fc domain appears to be a critical factor. Indeed, the removal of the Fc domain from DF-Nb resulted in a substantial decline in transduction efficiency, dropping from 72% in DF-Nb/2.2-L to 5% in DF-Nb-ΔFc/2.2-L (supplementary Fig. 3). On the contrary, no significant difference was observed in the transduction efficiency between DF-VE/2.2-L (88%) and DF-VE-ΔFc/2.2-L (91%) (supplementary Fig. 4). These findings are in agreement with the minimal impact of the helical linker on the transduction efficiency of LVs containing pDisplay-based mVEs (Dis-VE and Dis-VE-HL) and contrary to the findings related to pDisplay-based mNbs (Dis-Nb and Dis-Nb-HL), which can be attributed to the membrane-distal binding site of VEGF.
It is noteworthy that, unlike the chimeric approach, which demonstrated comparable transduction efficiency (approximately 30% of 293/KDR cells) for both 3VGR19 nanobody and VEGF121 [20], the present study revealed that the transduction efficiencies of all mVE-based lentiviral vectors (LVs) surpassed those of their mNb counterparts. One possible explanation for this observation might be structural limitations imposed on VEGF121 within the chimeric sindbis E2 GP, which are alleviated when VEGF121 is displayed independently in the two-molecule targeting approach. Conversely, the sindbis GP, which is used as a fusogenic molecule in the two-molecule targeting system (2.2-L), showed a higher level of non-specific transduction (≈ 3–6%) compared to the chimeric sindbis (≈ 1%). This suggests that the insertion of 3VGR19 nanobody or VEGF121 between residues 71–74 of E2 is more effective in detargeting 9reducing the targetting) of sindbis GP than the 20 amino acid linker used in the case of 2.2-L. Accordingly, the nonspecific transduction rates of all LVs using 2.2-L as fusogenic molecule were also in the range of 3–6%, with the exception of instances where VEGF121 was presented in pDF, which showed 11% for DF-VE-ΔFc/2.2-L and 24% for DF-VE/2.2 L LVs. The unexpectedly elevated nonspecific transduction observed in DF-VE/2.2 L and DF-VE-ΔFc/2.2-L LVs appears to be attributable to the nonspecific binding of mVEs displayed by pDF.
The results of our two-molecule targeting strategy for transduction demonstrated efficiencies that were comparable to, or even exceeded, those reported by Yang et al. [28] and Lei et al. [31]. Specifically, transduction efficiencies of 52% and 30% were achieved in 293T cells that stably expressed the target antigen, respectively. Of note, both of these studies used detargeted forms of SVG as fusogen and the full form of anti-CD20 antibody as targeting ligand which required additional accessory proteins such as Igα and Igβ in contrast to our display platform.
In our in silico study, we found no correlation between transduction efficiency and either solvent accessibility or the fluctuations of the nanobody within the constructs. This suggests that variations in the nanobody segment did not influence its accessibility to VEGFR2. However, the simulation results indicated a correlation between the distance of the nanobody from the lipid bilayer (measured at 0 Å, 28.26 Å, 68.40 Å, and 82.01 Å for DF-Nb-ΔFc, Dis-Nb, Dis-Nb-HL, and DF-Nb, respectively) and the observed transduction efficiencies (5%, 14%, 37%, and 72%, respectively) as illustrated in Fig. 5. This correlation implies that the distance between the targeting moiety and the surface of the enveloping lipid bilayer may serve as a significant predictor of ligand accessibility and binding efficiency for lentiviral vectors and their targets.
In our study, the factors affecting the transduction efficiency of targeted LVs were investigated in 293/KDR cells. To further expand these findings in clinical settings, it is essential to evaluate the identified factors in primary endothelial cells as well. Additionally, when using the Fc domain as a spacer, it is crucial to consider that Fc spacer domains may interact with Fc gamma receptors (FcγRs), potentially leading to non-specific activation and/or transduction of immune cells. This challenge must be resolved prior to advancing to in vivo studies, as previously indicated by the deletion or mutation of regions critical for Fc receptor binding in CARs [61–63]. Moreover, the improved transduction efficiency observed with the two-molecule strategy, coupled with the reduced non-specific transduction of chimeric SVGs [20] provides an opportunity to investigate the combination of both approaches (i.e., co-enveloping LVs with chimeric SVG and targeting molecule) to increase the avidity of LVs for the targeted cells, while benefiting from the diminished background transduction associated with chimeric SVG.
Conclusion
Collectively, to our knowledge, we reported the first attempt to generate targeted LVs via independent co-display of detargeted SVG and nanobody. This dual-molecular targeting strategy surpassed the chimeric approach in terms of transduction efficiency. The extent of improvement in transduction efficiency appears to be influenced by both the context of the targeting ligand’s display, including factors such as spacer and transmembrane (TM) domains, as well as the specific targeting ligand utilized. Notably, the removal of the Fc domain from nanobody-displaying LVs resulted in a significant reduction in transduction efficiency, decreasing from 72 to 5%, while the efficiency of VEGF121-containing LVs remained unaffected. Similarly, insertion of a helical linker between the transmembrane domain and nanobody into the pDisplay-based constructs led to 2.5 fold increase in the transduction of 293/KDR cells. We also found that higher transduction efficiencies for the two-molecule targeting system compared to chimeric were accompanied with slightly higher non-specific transduction (3–6% versus 1%). Finally, results of the in silico studies indicated a direct correlation for the transduction efficiency and the distance between the nanobody and lipid bilayer implying its role for the ligand accessibility and binding efficiency of LVs and their targets. Taken together, the modular nature of this strategy offers great possibilities to optimize transductional targeting of LVs via independent modification of targeting molecules which relieves the potential restrain on functionality of targeting ligands. In addition, it provides the possibility of individual or concurrent alteration in different domains of the display platform (such as targeting ligand, TM domain, and spacer). This strategy expedites the development of novel targeted LVs by allowing a diverse range of targeting ligands to be readily tested.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by the graduate school of Pasteur Institute of Iran in fulfillment of the Ph.D. thesis of R. Ahani in the “Medical Biotechnology” program. Authors appreciate all the employees of Pasteur Institute of Iran for their great supports and helps.
Author contributions
R. A. and M. H. E. performed the main parts of the experiments and prepared the first draft of the manuscript, N. M., R. A. C., M. R. K. and N. M were involved in conceptualization of the project, design of some experiments and performing various in silico studies and assisting the preparation of the first draft and editing of the in silico parts of the submitted manuscript, M.B. provided the VEGFR2-specific nanobody constructs. F. R. and K. A. conceptualized, designed and supervised the project and edited the submitted manuscript. All authors have studied the final submitted manuscript and agreed for its submission to Virology journal.
Funding
This work was supported by the graduate school of Pasteur Institute of Iran in fulfillment of the Ph.D. thesis of R. Ahani [grant number BP-8918] in the “Medical Biotechnology” program.
Data availability
The data that support the findings of this study are available from the corresponding authors upon reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors reviewed and approved the final version of the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Roshank Ahani and Mohammad Hossein Etemadzadeh have contributed equally to this study as co-first authors.
Contributor Information
Farzin Roohvand, Email: farzin.roohvand3@gmail.com.
Kayhan Azadmanesh, Email: azadmanesh@pasteur.ac.ir.
References
- 1.Waehler R, Russell SJ, Curiel DT. Engineering targeted viral vectors for gene therapy. Nat Rev Genet. 2007;8:573–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hadi M, Qutaiba B, Allela O, Jabari M, Jasoor AM, Naderloo O, Yasamineh S, Gholizadeh O, Kalantari L. Recent advances in various adeno-associated viruses (AAVs) as gene therapy agents in hepatocellular carcinoma. Virol J. 2024 12;21(1):17. [DOI] [PMC free article] [PubMed]
- 3.Das SK, Menezes ME, Bhatia S, Wang XY, Emdad L, Sarkar D, Fisher PB. Gene therapies for cancer: strategies, challenges and successes. J Cell Physiol. 2015;230:259–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones M, Kovacevic B, Ionescu CM, Wagle SR, Quintas C, Wong EYM, Mikov M, Mooranian A, Al-Salami H. The applications of targeted delivery for gene therapies in hearing loss. J Drug Target. 2023;31(6):585–95. [DOI] [PubMed] [Google Scholar]
- 5.Deng L, Liang P, Cui H. Pseudotyped lentiviral vectors: ready for translation into targeted cancer gene therapy? Genes Dis. 2022;10(5):1937–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Sanctis F, Ugel S, Facciponte J, Facciabene A. The dark side of tumor-associated endothelial cells. Semin Immunol. 2018;35:35–47. [DOI] [PubMed] [Google Scholar]
- 7.Xu M, Zhang T, Xia R, Wei Y, Wei X. Targeting the tumor stroma for cancer therapy. Mol Cancer. 2022;21:208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aird WC. Endothelial cell heterogeneity. Cold Spring Harb Perspect Med. 2012;2:a006429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dong Z, Nor JE. Transcriptional targeting of tumor endothelial cells for gene therapy. Adv Drug Deliv Rev. 2009;61:542–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dhanabal M, Ramchandran R, Waterman MJ, Lu H, Knebelmann B, Segal M, Sukhatme VP. Endostatin induces endothelial cell apoptosis. J Biol Chem. 1999;274:11721–6. [DOI] [PubMed] [Google Scholar]
- 11.Trepel M, Stoneham CA, Eleftherohorinou H, Mazarakis ND, Pasqualini R, Arap W, Hajitou A. A heterotypic bystander effect for tumor cell killing after adeno-associated virus/phage-mediated, vascular-targeted suicide gene transfer. Mol Cancer Ther. 2009;8:2383–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Falcon BL, Chintharlapalli S, Uhlik MT, Pytowski B. Antagonist antibodies to vascular endothelial growth factor receptor 2 (VEGFR-2) as anti-angiogenic agents. Pharmacol Ther. 2016;164:204–25. [DOI] [PubMed] [Google Scholar]
- 13.Ferrara N, Adamis AP. Ten years of anti-vascular endothelial growth factor therapy. Nat Rev Drug Discov. 2016;15:385–403. [DOI] [PubMed] [Google Scholar]
- 14.Hida K, Maishi N, Sakurai Y, Hida Y, Harashima H. Heterogeneity of tumor endothelial cells and drug delivery. Adv Drug Deliv Rev. 2016;99:140–7. [DOI] [PubMed] [Google Scholar]
- 15.Musumeci F, Radi M, Brullo C, Schenone S. Vascular endothelial growth factor (VEGF) receptors: drugs and new inhibitors. J Med Chem. 2012;55:10797–822. [DOI] [PubMed] [Google Scholar]
- 16.Anthony Simmons RP, Whitehead AA, Kolokoltsov RA, Davey. Use of Recombinant lentivirus pseudotyped with vesicular stomatitis virus glycoprotein G for efficient generation of human anti-cancer chimeric T cells by transduction of human peripheral blood lymphocytes in vitro. Virol J. 2006;28:38. [DOI] [PMC free article] [PubMed]
- 17.Patel M, Giddings AM, Sechelski J, Olsen JC. High efficiency gene transfer to airways of mice using influenza hemagglutinin pseudotyped lentiviral vectors. J Gene Med. 2013;15:51–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deng L, Liang P, Cui H. Pseudotyped lentiviral vectors: Ready for translation into targeted cancer gene therapy? Genes & Diseases, Genes Dis. 2022;10(5):1937–1955. [DOI] [PMC free article] [PubMed]
- 19.Leung JY, Ng MM, Chu JJ. Replication of alphaviruses: a review on the entry process of alphaviruses into cells. Adv Virol 2011;249640. [DOI] [PMC free article] [PubMed]
- 20.Ahani R, Roohvand F, Cohan RA, Etemadzadeh MH, Mohajel N, Behdani M, Shahosseini Z, Madani N, Azadmanesh K. Sindbis Virus-Pseudotyped lentiviral vectors carrying VEGFR2-Specific nanobody for potential transductional targeting of tumor vasculature. Mol Biotechnol. 2016;58(11):738–47. [DOI] [PubMed] [Google Scholar]
- 21.Michels A, Ho N, Buchholz CJ. Precision medicine: in vivo CAR therapy as a showcase for receptor-targeted vector platforms. Mol Ther. 2022;30:2401–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morizono K, Pariente N, Xie Y, Chen IS. Redirecting lentiviral vectors by insertion of integrin-tageting peptides into envelope proteins. J Gene Med. 2009;11:549–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Situ K, Chua BA, Bae SY, Meyer AS, Morizono K. Versatile targeting system for lentiviral vectors involving biotinylated targeting molecules. Virology. 2018;525:170–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parker CL, Jacobs TM, Huckaby JT, Harit D, Lai SK. Efficient and Highly Specific Gene Transfer Using Mutated Lentiviral Vectors Redirected with Bispecific Antibodies. mBio 2020, 21;11(1):e02990-19. [DOI] [PMC free article] [PubMed]
- 25.Kasaraneni N, Chamoun-Emanuelli AM, Wright G, Chen Z. Retargeting Lentiviruses via SpyCatcher-SpyTag Chemistry for Gene Delivery into Specific Cell Types. mBio 2017, 12;8(6):e01860-17. [DOI] [PMC free article] [PubMed]
- 26.Kasaraneni N, Chamoun-Emanuelli AM, Wright GA, Chen Z. A simple strategy for retargeting lentiviral vectors to desired cell types via a disulfide-bond-forming protein-peptide pair. Sci Rep. 2018;8:10990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lei Y, Joo KI, Wang P. Engineering fusogenic molecules to achieve targeted transduction of enveloped lentiviral vectors. J Biol Eng. 2009;3:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang L, Bailey L, Baltimore D, Wang P. Targeting lentiviral vectors to specific cell types in vivo. Proc Natl Acad Sci U S A. 2006;103:11479–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang XY, Kutner RH, Bialkowska A, Marino MP, Klimstra WB, Reiser J. Cell-specific targeting of lentiviral vectors mediated by fusion proteins derived from Sindbis virus, vesicular stomatitis virus, or avian sarcoma/leukosis virus. Retrovirology. 2010;25:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee CL, Dang J, Joo KI, Wang P. Engineered lentiviral vectors pseudotyped with a CD4 receptor and a fusogenic protein can target cells expressing HIV-1 envelope proteins. Virus Res. 2011;160:340–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lei Y, Joo KI, Zarzar J, Wong C, Wang P. Targeting lentiviral vector to specific cell types through surface displayed single chain antibody and fusogenic molecule. Virol J. 2010;11:7:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rasbach A, Abel T, Munch RC, Boller K, Schneider-Schaulies J, Buchholz CJ. The receptor attachment function of measles virus hemagglutinin can be replaced with an autonomous protein that binds Her2/neu while maintaining its fusion-helper function. J Virol. 2013;87:6246–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lecocq Q, De Vlaeminck Y, Hanssens H, D’Huyvetter M, Raes G, Goyvaerts C, Keyaerts M, Devoogdt N, Breckpot K. Theranostics in immuno-oncology using nanobody derivatives. Theranostics. 2019;9:7772–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Behdani M, Zeinali S, Khanahmad H, Karimipour M, Asadzadeh N, Azadmanesh K, Khabiri A, Schoonooghe S, Habibi Anbouhi M, Hassanzadeh-Ghassabeh G, Muyldermans S. Generation and characterization of a functional nanobody against the vascular endothelial growth factor receptor-2; angiogenesis cell receptor. Mol Immunol. 2012;50:35–41. [DOI] [PubMed] [Google Scholar]
- 35.Salmon P, Oberholzer J, Occhiodoro T, Morel P, Lou J, Trono D. Reversible immortalization of human primary cells by lentivector-mediated transfer of specific genes. Mol Ther. 2000;2:404–14. [DOI] [PubMed] [Google Scholar]
- 36.Ahani R, Roohvand F, Mohajel N, Etemadzadeh MH, Behdani M, Shahosseini Z, Azadmanesh K. Surface display of vascular endothelial growth factor receptor-2 specific nanobody on 293T cells – A potential targeting moiety for lentiviral vector-based cancer therapy. Romanian Biotechnol Lett. 2016;21:11816–24. [Google Scholar]
- 37.Taube R, Zhu Q, Xu C, Diaz-Griffero F, Sui J, Kamau E, Dwyer M, Aird D, Marasco WA. Lentivirus display: stable expression of human antibodies on the surface of human cells and virus particles. PLoS ONE. 2008;3:e3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arai R, Ueda H, Kitayama A, Kamiya N, Nagamune T. Design of the linkers which effectively separate domains of a bifunctional fusion protein. Protein Eng. 2001;14:529–32. [DOI] [PubMed] [Google Scholar]
- 39.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9:676–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ward JJ, McGuffin LJ, Buxton BF, Jones DT. Secondary structure prediction with support vector machines. Bioinformatics. 2003;19:1650–5. [DOI] [PubMed] [Google Scholar]
- 41.Heffernan R, Dehzangi A, Lyons J, Paliwal K, Sharma A, Wang J, Sattar A, Zhou Y, Yang Y. Highly accurate sequence-based prediction of half-sphere exposures of amino acid residues in proteins. Bioinformatics. 2016;32:843–9. [DOI] [PubMed] [Google Scholar]
- 42.Krogh A, Larsson B, von Heijne G, Sonnhammer EL. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J Mol Biol. 2001;305:567–80. [DOI] [PubMed] [Google Scholar]
- 43.Ma J, Wang S, Zhao F, Xu J. Protein Threading using context-specific alignment potential. Bioinformatics. 2013;29:i257–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang J, Yan R, Roy A, Xu D, Poisson J, Zhang Y. The I-TASSER suite: protein structure and function prediction. Nat Methods. 2015;12:7–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu D, Zhang Y. Ab initio protein structure assembly using continuous structure fragments and optimized knowledge-based force field. Proteins. 2012;80:1715–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bordoli L, Kiefer F, Arnold K, Benkert P, Battey J, Schwede T. Protein structure homology modeling using SWISS-MODEL workspace. Nat Protoc. 2009;4:1–13. [DOI] [PubMed] [Google Scholar]
- 47.Webb B, Sali A. Protein structure modeling with MODELLER. Methods Mol Biol. 2014;1137:1–15. [DOI] [PubMed] [Google Scholar]
- 48.Phillips JC, Braun R, Wang W, Gumbart J, Tajkhorshid E, Villa E, Chipot C, Skeel RD, Kale L, Schulten K. Scalable molecular dynamics with NAMD. J Comput Chem. 2005;26:1781–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Munch RC, Muhlebach MD, Schaser T, Kneissl S, Jost C, Pluckthun A, Cichutek K, Buchholz CJ. DARPins: an efficient targeting domain for lentiviral vectors. Mol Ther. 2011;19:686–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goyvaerts C, De Groeve K, Dingemans J, Van Lint S, Robays L, Heirman C, Reiser J, Zhang XY, Thielemans K, De Baetselier P, et al. Development of the nanobody display technology to target lentiviral vectors to antigen-presenting cells. Gene Ther. 2012;19:1133–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goyvaerts C, Dingemans J, De Groeve K, Heirman C, Van Gulck E, Vanham G, De Baetselier P, Thielemans K, Raes G, Breckpot K. Targeting of human antigen-presenting cell subsets. J Virol. 2013;87:11304–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Amet N, Lee HF, Shen WC. Insertion of the designed helical linker led to increased expression of tf-based fusion proteins. Pharm Res. 2009;26:523–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Muth A. Receptor-targeted viral vectors for basic and medical research. Technische Universität; 2015.
- 54.Brozzo MS, Bjelic S, Kisko K, Schleier T, Leppanen VM, Alitalo K, Winkler FK, Ballmer-Hofer K. Thermodynamic and structural description of allosterically regulated VEGFR-2 dimerization. Blood. 2012;119:1781–8. [DOI] [PubMed] [Google Scholar]
- 55.Sawano A, Takahashi T, Yamaguchi S, Aonuma M, Shibuya M. Flt-1 but not KDR/Flk-1 tyrosine kinase is a receptor for placenta growth factor, which is related to vascular endothelial growth factor. Cell Growth Differ. 1996;7:213–21. [PubMed] [Google Scholar]
- 56.Terman BI, Dougher-Vermazen M, Carrion ME, Dimitrov D, Armellino DC, Gospodarowicz D, Bohlen P. Identification of the KDR tyrosine kinase as a receptor for vascular endothelial cell growth factor. Biochem Biophys Res Commun. 1992;187:1579–86. [DOI] [PubMed] [Google Scholar]
- 57.Waltenberger J, Claesson-Welsh L, Siegbahn A, Shibuya M, Heldin CH. Different signal transduction properties of KDR and Flt1, two receptors for vascular endothelial growth factor. J Biol Chem. 1994;269:26988–95. [PubMed] [Google Scholar]
- 58.Cheng TL, Roffler S. Membrane-tethered proteins for basic research, imaging, and therapy. Med Res Rev. 2008;28:885–928. [DOI] [PubMed] [Google Scholar]
- 59.Abate-Daga D, Davila ML. CAR models: next-generation CAR modifications for enhanced T-cell function. Mol Therapy — Oncolytics. 2016;3:16014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guest RD, Hawkins RE, Kirillova N, Cheadle EJ, Arnold J, O’Neill A, Irlam J, Chester KA, Kemshead JT, Shaw DM, et al. The role of extracellular spacer regions in the optimal design of chimeric immune receptors: evaluation of four different ScFvs and antigens. J Immunother. 2005;28:203–11. [DOI] [PubMed] [Google Scholar]
- 61.Hombach A, Hombach AA, Abken H. Adoptive immunotherapy with genetically engineered T cells: modification of the IgG1 Fc /`spacer/‘ domain in the extracellular moiety of chimeric antigen receptors avoids /`off-target/‘ activation and unintended initiation of an innate immune response. Gene Ther. 2010;17:1206–13. [DOI] [PubMed] [Google Scholar]
- 62.Hudecek M, Sommermeyer D, Kosasih PL, Silva-Benedict A, Liu L, Rader C, Jensen MC, Riddell SR. The nonsignaling extracellular spacer domain of chimeric antigen receptors is decisive for in vivo antitumor activity. Cancer Immunol Res. 2015;3:125–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jonnalagadda M, Mardiros A, Urak R, Wang X, Hoffman LJ, Bernanke A, Chang WC, Bretzlaff W, Starr R, Priceman S, et al. Chimeric antigen receptors with mutated IgG4 Fc spacer avoid Fc receptor binding and improve T cell persistence and antitumor efficacy. Mol Ther. 2015;23:757–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors upon reasonable request.