Abstract
Background
Noncommunicable diseases (NCDs) contribute significantly to global morbidity and mortality, accounting for 74% of all deaths worldwide. Many of these chronic diseases can be prevented or their onset mitigated through lifestyle interventions. Complementing these efforts, robust biomarkers enable early diagnosis (secondary prevention), while tertiary prevention can reduce long-term complications and improve disease management. Moreover, the importance of prevention extends beyond NCDs to infectious diseases, where lifestyle-related factors can also play a pivotal role. Innovative human-based research methods have shown suitable for modeling several diseases and advancing drug discovery. These approaches are particularly relevant in prevention research, given the inherently human nature of the lifestyle and environmental factors associated with disease risk and progression.
Methods
Here we conducted a retrospective evaluation of biomedical research projects funded under the 7th Framework Programme (FP7), Horizon 2020, and currently ongoing Horizon Europe (HE). We developed and refined a computer-based approach based on the use of Natural Language Processing (NLP) to examine three key aspects: (i) the integration of primary, secondary, and tertiary prevention in these projects, (ii) the biomedical research areas which most frequently incorporate prevention, and (iii) the use of animal-based research versus human-centric approaches.
Results
Our findings reveal a persistent gap in the percentage of prevention-focused biomedical research projects, with only 4.4%, 4.5%, and 1.9% of FP7, H2020, and HE projects, respectively, addressing prevention. This gap was particularly pronounced in certain biomedical research areas, such as age-related diseases and diabetes and metabolic syndrome research, which showed a decrease in the percentage of prevention-related projects especially under most recent framework programme (HE). While the reliance on animal-based methods has been generally modest, averaging around 26% of all prevention-related projects, tertiary prevention research, and prevention projects focused on some biomedical areas (i.e., age-related diseases, personalized medicine, antimicrobial resistance, bone disorders, and respiratory diseases) showed increased percentages in projects using animals under more recent FPs. Analysis of funding distribution revealed progressively less funding allocated to prevention-related projects focused on diabetes and metabolic syndrome, neurodegenerative diseases, age-related disorders, and AMR. In addition, the proportion of funding allocated to both secondary and tertiary prevention decreased under HE.
Conclusions
A shift toward human-centric approaches, particularly in prevention-focused research, is essential to enhance the translatability of findings. As policymakers prepare for the next EU funding framework, these insights offer critical guidance for developing targeted funding strategies that prioritize human-centric prevention research as a cornerstone of public health.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12967-025-07019-8.
Keywords: Prevention, Biomedical research, Noncommunicable diseases, Infectious diseases, Funding, Framework programme, Projects, Animals, Human-centric approaches
Introduction
Noncommunicable diseases (NCDs), including cancer, heart disease, stroke, metabolic syndrome related disorders (e.g., diabetes), and chronic lung disease, represent a significant proportion of global morbidity and mortality, collectively accounting for 74% of all deaths worldwide [1]. In addition, cognitive decline and dementia affect more than 55 million people worldwide, with nearly 10 million new cases of dementia reported each year [2].
While drug discovery and development remain crucial to tackling the burden of these diseases, it is equally important to invest in primary prevention research, considering that it is well-established that many of these diseases can be mitigated or even prevented through lifestyle-related interventions. Strategies such as adopting a varied and balanced plant-based diet [3–5] and avoiding ultra-processed foods [6], engaging in regular physical activity, avoiding tobacco use and alcohol abuse, improving sleep quality, and addressing environmental risk factors have demonstrated profound effects in reducing the incidence and severity of these conditions [7–10].
Complementing these efforts, the use of robust biomarkers for early diagnosis (secondary prevention) can inform personalized medical interventions [11, 12], while tertiary prevention can help mitigate or delay the onset of long-term complications, reduce disability, and enhance disease management [12, 13].
In some cases, preventive measures have proven to be more effective than curative approaches (pharmaceutical treatments), either in preventing disease onset or in managing associated symptoms [14, 15]. This has been particularly evident in the context of dementias, such as Alzheimer’s disease, where the development of safe and effective disease-modifying treatment options has been so far unsuccessful [16, 17].
Furthermore, many NCDs are interrelated [18, 19], emphasizing the need for research strategies that focus on comorbidities rather than individual diseases, as highlighted in a recent Humane World for Animals scoping analysis [20].
The importance of prevention extends beyond NCDs to infectious diseases, where lifestyle-related factors can also play a pivotal role. For instance, specific high-quality plant-based dietary patterns have been shown to reduce the risk of COVID-19 infection and hospitalization [21]. Such findings underline the potential of prevention as a cross-cutting strategy to improve health outcomes across disease categories.
Despite chronic diseases accounting for up to 80% of healthcare costs in the EU, preventive healthcare represented only 0.38% of GDP in 2020 [22]. Furthermore, preventive medicine and research remain underfunded across EU Member States, comprising only a small fraction of overall health budgets [23]. Evidence-based health promotion and disease prevention strategies have the potential to reduce the prevalence of NCDs by as much as 70% [24]. Increasing investment in prevention-focused, human-relevant, and patient-centered research could address pressing public health challenges, lower healthcare and public health expenditures, and help mitigate the burden of chronic degenerative diseases. Recognizing this, the European Parliament has repeatedly emphasized the need to invest more in prevention; resolutions adopted in 2023 underscored the centrality of prevention in addressing mental health challenges [25, 26] and tackling NCDs [27].
Concerning communicable diseases, in November 2020, the European Commission put forward a new health security framework designed to better protect EU citizens. Drawing on lessons from the COVID-19 pandemic, this framework strengthens the EU’s ability to prevent, prepare for, and respond to serious cross-border health threats. It does so through Regulation (EU) 2022/2371 [28] and by expanding the roles of the European Centre for Disease Prevention and Control (ECDC) [29] and the European Medicines Agency (EMA) [30] in disease prevention, surveillance, and preparedness.
A previous analysis conducted by the European Commission’s Joint Research Centre (JRC) examined the major outputs associated with Alzheimer’s disease, breast cancer, and prostate cancer research projects funded under framework programme 5 (FP5), FP6, FP7, and Horizon 2020 (H2020), and what role the selection of the methodological approach(es) (animal and/or human-based methods and technologies) played in the generation of research impact [31]. When considering prevention research, this analysis found that, on average, only 8% of the analyzed projects included a focus on prevention. These findings underscore a potential gap in research funding allocation, particularly in areas where prevention research and the implementation of preventative strategies could yield significant benefits.
The present study aimed to expand this analysis by conducting a comprehensive retrospective evaluation of biomedical research projects funded under FP7, H2020, and current Horizon Europe (HE). Utilizing data retrieved from the CORDIS database [32], and by leveraging a computer-based approach based on the use of Natural Language Processing (NLP), this analysis aimed to (i) identify the extent to which primary, secondary and tertiary prevention has been addressed in these programmes, (ii) identify the diseases (NCDs and infectious diseases) or biomedical research areas most frequently addressed in projects that incorporated prevention, and (iii) determine the percentage of projects that incorporated animal-based research and/or innovative human-based approaches in their strategies.
The results of this analysis could inform future research funding strategies by identifying specific biomedical and disease areas where prevention-related research has been underfunded. Highlighting the importance of human-centric research strategies in this context can enhance the translatability of findings and maximize the long-term impact of research investments. Relying on human-relevant research strategies is especially important when the goal of research is to explore the systemic and multi-factorial elements underlying human behavior, lifestyle, disease etiology and complexity [33].
Such insights are particularly timely as policymakers and funding bodies prepare for the next European funding programme, FP10. By highlighting trends and gaps in prevention-related research, this study seeks to guide the development of targeted calls for proposals that better align with the overarching goal of improving public health outcomes through preventive measures [34].
Methodological approach
Manual screening of CORDIS projects
Information on projects funded under the FP7 (2007–2013), H2020 (2014–2020) and currently ongoing Horizon Europe (HE) (2021 - till October 24, 2024) was retrieved from the European Commission’s CORDIS web portal [32], which provides downloadable Excel files of funded projects for each framework programme. The analysis focused on these funding frameworks because CORDIS data for these periods is more consistent and precise compared to older framework programmes. Initially, projects were filtered to include only those with the keyword ‘prevention’ in the ‘Objective’ field. This led to the identification of 286, 462, and 223 biomedical projects addressing “prevention” for FP7, H2020 and HE, respectively.
Manual screening was conducted independently by two researchers (FP and FF), following a predefined set of qualifiers and specific keywords for each qualifier to label the projects. Irrelevant entries, i.e., non-biomedical projects, were excluded from the analysis. To limit the number of biomedical research areas, a set of keywords was pre-defined and subsequently refined to enable the clustering of projects according to their main field of research. For example, projects related to different types of cancer were all labeled under “cancer”. In a similar way, disorders falling under the umbrella of metabolic syndromes (including diabetes) were labeled as “metabolic syndromes/diabetes”. The same approach was used to group projects addressing various rare diseases (labelled as “rare disease”), ageing-related complications (“ageing”), infectious diseases (“infection OR virus”), and other health issues, as reported in Table 1. To help align their assessments, the two team members first applied this approach in parallel to a set of projects (about 30). They then compared their categorizations and reached a consensus on a standard method for keyword assignment.
Table 1.
Main qualifiers considered in this analysis and keywords assigned to label projects
| Qualifier | Description and rules | Main keywords assigned to label projects (indicated in bold) |
|---|---|---|
| Disease area, biomedical research topic |
Indicate the main biomedical research area (e.g., cancer, diabetes, obesity, HIV, virus, infectious disease, hypertension, metabolic syndrome, cardiovascular diseases, heart disease, Alzheimer’s disease, dementia, ageing, diet, healthcare, antimicrobial resistance (AMR), etc.). Be consistent with the use of keywords. Combinations of keywords can be used to label each project, as appropriate. For example, the same project may have covered ‘inflammation’ and ‘cancer’, or ‘ageing’ and ‘dementia’, or ‘rare disease’ and ‘development’, etc |
‘Cancer’: any cancer-related projects; indicate the type of cancer in bracket, e.g.: ‘cancer (cervical)’. ‘Metabolic syndrome’: projects addressing metabolism, hypertension, stroke, obesity, overweight, dyslipidemia, hyperglycemia, insulin resistance, diabetes, or analogous disorders. ‘Alzheimer’ and/or ‘dementia’: projects addressing Alzheimer’s disease or other forms of dementia. ‘Neurodegenerative’: projects addressing other neurodegenerative disorders, such as Parkinson’s disease, Multiple Sclerosis. ‘Mental health’: projects addressing psychological or mental health-related disorders, including depression, violence, suicide, etc. Indicate the type of disorder, e.g., ‘mental health, depression’. ‘Heart’ and/or ‘Cardiovascular’: projects addressing heart and cardiac issues, including thrombosis, coronary disease, atrial disease, atherosclerosis, etc. ‘Infection’: projects addressing sepsis, staphylococcus, tuberculosis, ulcers, etc. ‘Virus’: projects addressing viral infections, e.g., HIV, malaria, SARS-CoV-2, etc. ‘Rare disease’: project addressing any rare disease, e.g. amyotrophic lateral sclerosis (ALS), scleroderma, sickle cell disease, etc. ‘Elderly’ and/or ‘ageing’: any projects specifically addressing age-related disorders/diseases or conditions. ‘Child’ and/or ‘infant’: any projects addressing childhood- or infancy-related disorders/diseases or conditions. ‘Development’: any projects addressing development-related disorders/diseases or conditions, e.g., Autism spectrum disorders, attention deficit hyperactivity disorder (ADHD). ‘AMR’: projects referring to antimicrobial resistance. ‘Healthcare’: any projects addressing healthcare. ‘Osteo’ and/or ‘bone’: any projects addressing bone-related disorders/diseases or conditions. ‘Autoimmune’: any projects addressing autoimmune disorders/diseases. ‘Respiratory’: any projects addressing respiratory-related disorders/diseases or conditions. ‘Inflammation’ and/or ‘inflammatory’: any projects addressing inflammation-related processes, disorders/diseases or conditions. ‘Personalized medicine’ and/or ‘precision medicine’: any projects addressing personalized or precision medicine |
| Type of prevention |
Indicate what type of prevention was considered in the project. Combinations of keywords can be used to label each project, as appropriate. For example, the same project may have covered ‘primary’ and ‘secondary’ prevention, or ‘secondary’ and ‘tertiary’ prevention, or all three prevention categories, etc. If a project is a false positive (i.e., the word ‘prevention’ appears in the Objective field, but the project does not focus on biomedical science), label it as ‘not relevant.’ Similarly, projects addressing non-human animal diseases (e.g., porcine pneumonia or sheep toxoplasmosis) should also be labeled as ‘not relevant’ |
‘Primary’: projects addressing diet, lifestyle risk factors and activities or analyses aimed at preventing or delaying the onset of a disease; also vaccines development/testing fall under this category. ‘Secondary’: projects addressing early detection and diagnosis, biomarkers discovery, diagnostic tools, etc. ‘Tertiary’: projects addressing any strategies aimed at stopping or delaying disease progression and/or managing side effects; some drugs may fall under this category |
| Use of animals |
Indicate whether live animals (in bred or transgenic) were considered in the project. Species like Drosophila m. and Zebrafish embryos were not considered (as they are not included under Directive 2010/63/EU [35]). If the information is unclear (as it is not explicitly mentioned in the objective), make your best guess by indicating “yes” or “no” followed by “unclear”, |
‘Yes’: projects that accounted for the use of live animals, collection of animal organs, tissues and/or cells. ‘No’: projects that did not account for any of the above |
| Use of human methods/models |
Indicate whether human-centered approaches, methods or models were considered. After indicating ‘yes’ or ‘no’, specify what approach was used. Combination of keywords can be used to label each project, as appropriate. For example, the same project may have considered ‘humans’ and ‘in vitro’, or ‘humans’ and ‘in silico’, or a combination of all three approaches etc. If the information is unclear (as it is not explicitly mentioned under the objective), make your best guess by indicating “yes” or “no” followed by “unclear” |
‘Yes’: projects that incorporated any human-derived/based approaches, such as: “humans” (e.g., patient cohorts, clinical trials, volunteers, human biological samples such as blood, plasma, biopsies, etc.); “in vitro” (e.g., human cell cultures, cell lines, 3D models, organ-on-chip, microphysiological systems, etc.), “in silico” (e.g. human health data sets (GWAS, genomics, other ‘omics’ data set repositories, AI, digital twin, digital cohorts, computational models, portable/wearable devices, eHealth, imaging/PET/MRI data sets, etc.). ‘No’: projects that did not incorporate any of these approaches |
This preliminary (manual) data set was used to design a computational-based approach based on the use of Natural Language Processing (NLP)/Large Language Model (LLM) to identify additional biomedical research projects addressing prevention, as described in Sect. 2.2.
Natural language processing-based approach for data analysis
The initial (manual) pilot data set was extended by using a Large language model (LLM) approach. The DeepSeek-V3 LLM [36] was used via its programmatic interface to infer whether the projects were prevention-specific, which types of prevention were proposed (primary, secondary or tertiary) and what was the research area of the project that best fitted our curated categories. The LLM queries used for every type of inference are available in Supplementary Table S4.
We used precision, recall, and F1-score (their harmonic mean) to evaluate how the NLP approach performed. Using the manually annotated pilot as a gold standard, Table 2 shows the predictive performance of the LLM query approach.
Table 2.
LLM-based inference performance evaluation
| Criteria evaluated | Precision | Recall | F1-score |
|---|---|---|---|
| Is prevention-related? | 1.000 | 0.873 | 0.932 |
| Primary prevention | 0.737 | 0.762 | 0.750 |
| Secondary prevention | 0.788 | 0.639 | 0.706 |
| Tertiary prevention | 0.563 | 0.724 | 0.634 |
The LLM screening led to the identification of 595 FP7, 1,120 H2020, and 36 HE additional prevention-related projects that were combined with the initial manual data set to generate an extended annotated data set (see Supplementary Excel “FP7-H2020-HE_Prevention Projects_EXTENDED DATA SET”). The extended data set was considered in the final analysis (Table 3).
Table 3.
Total number of biomedical research projects (and funding) addressing prevention considered in this retrospective analysis
| Projects | FP7 | H2020 | HE (as of Oct 24, 2024) |
|---|---|---|---|
| Total number of projects | 19,814 | 35,386 | 13,865 |
| Biomedical projects addressing “prevention” | 881 (4.4%) | 1,582 (4.5%) | 259 (1.9%) |
| Total budget for the framework programme (Euro) | 55.7 billion | 83.1 billion | 41.2 billion |
| Budget allocated to projects addressing “prevention” | 3.45 billion (6.2%) | 3.52 billion (4.2%) | 1.71 billion (4.2%) |
The Excel filter function was used to group projects together based on their keywords. The grouping considered the following qualifiers:
prevention type (primary, secondary, tertiary, alone or in combination),
the biomedical or disease area,
the use of animals,
the use of human-centered methods and approaches also defined as non-animal new approach methods or NAMs (considering ‘humans’, ‘in vitro’, and/or ‘in silico’).
as shown in Figs. 1, 2, 3, 4, 5 and 6; Table 4, Supplementary Figs. 1–3 and Tables S1–S3. In addition to project numbers, also funding (project total cost) distribution across (i) disease/biomedical research areas, (ii) prevention type (primary, secondary, or tertiary), and (iii) methodological approach (animal use and overall NAMs’ use) was analysed and reported in Tables 5, 6 and 7. All graphs were generated using Excel.
Fig. 1.
Overall numbers and percentages of prevention-related projects. A Biomedical research projects funded under FP7, H2020 and Horizon Europe (HE) that considered any type of prevention (primary, secondary and/or tertiary) are shown, with corresponding percentages of prevention-related projects for FP7, H2020 and HE, respectively. B The percentages of projects related to primary, secondary and tertiary prevention (either exclusively, or in combination with other prevention categories) funded under FP7, H2020 and HE. C The percentages of projects with an exclusive focus on primary, secondary and tertiary prevention are shown for each framework programme (FP7, H2020 and HE). The values in B and C refer to the percentages of all prevention-related projects under each framework programme
Fig. 2.
Main biomedical research areas addressed in prevention-related projects. The diseases and/or biomedical research areas that were most frequently addressed in projects funded under FP7, H2020 and Horizon Europe (HE) that considered prevention (primary, secondary or tertiary, alone or in combination) are shown. The values refer to the percentages of prevention-related projects under each framework programme
Fig. 3.
Type of prevention addressed in projects on the nine most covered biomedical research areas. The percentages of projects that considered primary, secondary, and tertiary prevention (either alone or in combination), are shown, considering the seven most prevalent biomedical areas: cancer (A), infectious diseases and/or virus (B), diabetes and/or metabolic syndrome (C), cardiovascular diseases (D), ageing-related diseases (E), mental health disorders (F), neurodegeneative diseases (G), healthcare (H), along with personalized/precision medicine-related projects (I). The values represent the percentages of disease-specific projects that considered prevention, funded under FP7, H2020 and HE
Fig. 4.
Overall use of animals in prevention-related projects. A Percentage of all prevention-related projects funded under FP7, H2020 and HE that utilized animals (orange bars) (either alone or in combination with other methods), or did not utilize animals (blue bars). B-J Percentages of projects focusing on the nine most covered biomedical areas: cancer (B), infectious diseases and/or virus (C), diabetes and/or metabolic syndrome (D), cardiovascular diseases (E), ageing-related diseases (F), mental health disorders (G), neurodegenerative diseases (H), healthcare (I), along with personalized/precision medicine-related projects (J), that considered or did not consider the use of animals (either alone or in combination with other methods) and were funded under FP7, H2020 and HE. The values in B-J refer to the percentages of disease specific projects-related to prevention under each framework programme
Fig. 5.
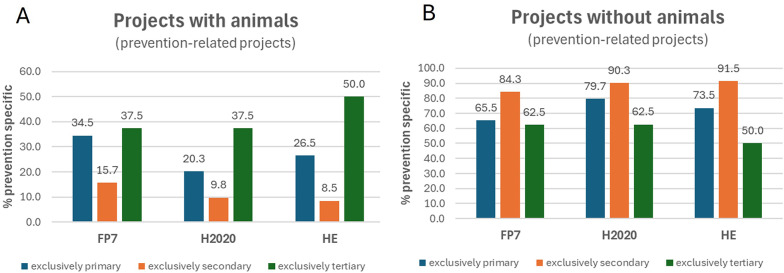
Animal use considering the type of prevention. A, B Percentages of projects that utilized animals A and those that did not use them (B), across projects focused exclusively on either primary, secondary or tertiary prevention funded under FP7, H2020 and HE. The values represent the percentages of all projects within each prevention-specific group across the three analyzed framework programmes
Fig. 6.
Overall use of human-centric methods and approaches in prevention-related projects. A Percentages of prevention-related projects that utilized any human-centered methods (ALL, dark blue bars), and specific subcategories [in vitro (orange bars), humans (including patients/cohorts and biological samples) (dark green), in silico (including human data, omics and other computational approaches (light blue)] either alone or combined with other (animal) methods funded under FP7, H2020 and HE. The values represent the percentages of all prevention-related projects under each framework programme. B-J Percentages of projects on the seven most covered biomedical areas: cancer (B), infectious and/or viral diseases (C), diabetes and/or metabolic syndrome (D), cardiovascular diseases (E), ageing-related diseases (F), mental health (G), neurodegenerative diseases (H), healthcare (I), and personalized/precision medicine related projects (J), that accounted for the use of human-centered methods (as shown in A), either alone or combined with other (animal) methods, funded under FP7, H2020 and HE. The values in B-J refer to the percentages of disease specific prevention-related projects under each framework programme
Table 4.
Total numbers of disease-specific research projects funded under FP7, H2020 and horizon Europe (HE), along with the percentages of those that are prevention-related
| Keyword/group of keywords | FP7 | H2020 | HE | |||
|---|---|---|---|---|---|---|
| ALL disease specific projects | Prevention-related (18.5% average |
ALL disease specific projects | Prevention-related (19% average |
ALL disease specific projects | Prevention-related (7.8% average |
|
| Cancer | 1184 | 141 | 2184 | 235 | 939 | 54 |
| % | 11.9 | 10.8 | 5.8 | |||
| Diabet* OR metaboli* | 756 | 125 | 1511 | 227 | 688 | 29 |
| % | 16.5 | 15.0 | 4.2 | |||
| Heart OR cardio* | 606 | 68 | 1178 | 134 | 461 | 36 |
| % | 11.2 | 11.4 | 7.8 | |||
| Alzheimer OR dementia | 215 | 29 | 361 | 62 | 155 | 11 |
| % | 13.5 | 17.2 | 7.1 | |||
| Neurodegen* | 248 | 53 | 405 | 102 | 154 | 14 |
| % | 21.4 | 25.2 | 9.1 | |||
| Ageing OR elderl* | 370 | 70 | 696 | 126 | 187 | 13 |
| % | 18.9 | 18.1 | 7.0 | |||
| Mental health | 60 | 44 | 189 | 111 | 118 | 22 |
| % | 73.3 | 58.7 | 18.6 | |||
| Infect* OR virus | 936 | 153 | 1587 | 232 | 663 | 56 |
| % | 16.3 | 14.6 | 8.4 | |||
| Child* OR infant* | 565 | 31 | 988 | 73 | 368 | 23 |
| % | 5.5 | 7.4 | 6.3 | |||
| Healthcare | 353 | 55 | 1275 | 117 | 512 | 22 |
| % | 15.6 | 9.2 | 4.3 | |||
| AMR OR antimicrobial | 107 | 13 | 292 | 34 | 132 | 7 |
| % | 12.1 | 11.6 | 5.3 | |||
| Osteo* OR bone | 353 | 21 | 639 | 29 | 266 | 7 |
| % | 5.9 | 4.5 | 2.6 | |||
| Autoimmune | 107 | 24 | 137 | 31 | 46 | 7 |
| % | 22.4 | 22.6 | 15.2 | |||
| Rare disease | 35 | 10 | 66 | 30 | 45 | 3 |
| % | 28.6 | 45.5 | 6.7 | |||
| Respiratory | 108 | 21 | 216 | 54 | 89 | 10 |
| % | 19.4 | 25.0 | 11.2 | |||
| Inflammat* | 423 | 13 | 662 | 43 | 284 | 17 |
| % | 3.1 | 6.5 | 6.0 | |||
The total numbers were extracted by searching for reported keywords in the “objectives” section of the project descriptions (data sourced from CORDIS)
Table 5.
Funding distribution across biomedical research areas
| Prevention-related projects | FP7 | H2020 | HE |
|---|---|---|---|
| Total funding (€) | Total funding (€) | Total funding (€) | |
| 3,446,101,731.32 | 3,515,757,578.18 | 1,714,215,247.86 | |
| Cancer | 346,611,532.38 | 428,445,590.25 | 219,184,122.48 |
| % | 10.1 | 12.2 | 12.8 |
| Diabet* OR metaboli* | 505,622,494.61 | 487,251,599.51 | 151,183,011.50 |
| % | 14.7 | 13.9 | 8.8 |
| Heart OR cardio | 228,270,178.37 | 324,673,690.76 | 264,606,032.15 |
| % | 6.6 | 9.2 | 15.4 |
| Alzheimer OR dementia | 124,083,154.48 | 152,333,746.53 | 51,164,092.00 |
| % | 3.6 | 4.3 | 3.0 |
| Neurodegen* | 227,340,842.35 | 277,972,785.86 | 49,174,365.00 |
| % | 6.6 | 7.9 | 2.9 |
| Ageing OR elderl* | 281,165,498.60 | 353,492,481.79 | 79,647,449.25 |
| % | 8.2 | 10.1 | 4.6 |
| Mental health | 147,699,912.06 | 206,056,054.64 | 60,048,376.20 |
| % | 4.3 | 5.9 | 3.5 |
| Infect* OR virus | 480,369,933.56 | 610,886,422.30 | 210,236,370.47 |
| % | 13.9 | 17.4 | 12.3 |
| Child* OR infant* | 132,698,359.33 | 168,650,766.90 | 110,152,714.29 |
| % | 3.9 | 4.8 | 6.4 |
| *Development | 18,945,900.53 | 101,081,131.82 | 4,743,411.24 |
| % | 0.5 | 2.9 | 0.3 |
| Healthcare | 190,871,965.02 | 305,876,023.09 | 210,181,867.92 |
| % | 5.5 | 8.7 | 12.3 |
| AMR | 527,481,137.19 | 142,798,309.63 | 14,506,860.50 |
| % | 15.3 | 4.1 | 0.8 |
| Osteo OR bone | 55,892,771.67 | 39,709,127.89 | 16,836,334.50 |
| % | 1.6 | 1.1 | 1.0 |
| Autoimmune | 144,969,125.04 | 102,234,799.12 | 45,740,563.71 |
| % | 4.2 | 2.9 | 2.7 |
| Rare disease | 40,108,163.97 | 35,472,591.39 | 153,003,285.47 |
| % | 1.2 | 1.0 | 8.9 |
| Respiratory | 42,051,510.16 | 150,701,905.23 | 16,780,716.75 |
| % | 1.2 | 4.3 | 1.0 |
| Inflammat* | 76,819,989.33 | 39,997,686.24 | 66,473,160.63 |
| % | 2.2 | 1.1 | 3.9 |
| Personali* OR precision | 360,431,816.70 | 576,570,194.77 | 158,673,007.73 |
| % | 10.5 | 16.4 | 9.3 |
Both absolute numbers and relative percentages are reported
Table 6.
Funding distribution across prevention type
| Prevention-related | FP7 | H2020 | HE |
|---|---|---|---|
| Total funding (€) | Total funding (€) | Total funding (€) | |
| 3,446,101,731.32 | 3,515,757,578.18 | 1,714,215,247.86 | |
| All primary | 1,561,077,167.09 | 1,662,880,189.61 | 884,207,604.09 |
| % | 45.3 | 47.3 | 51.6 |
| All secondary | 2,233,481,402.70 | 1,922,236,751.13 | 862,535,627.00 |
| % | 64.8 | 54.7 | 50.3 |
| All tertiary | 1,860,752,393.78 | 1,713,453,059.74 | 563,465,506.75 |
| % | 54.0 | 48.7 | 32.9 |
Both absolute numbers and relative percentages are reported
Table 7.
Funding distribution across methods (animals and NAMs)
| Prevention-related | FP7 | H2020 | HE |
|---|---|---|---|
| Total funding (€) | Total funding (€) | Total funding (€) | |
| 3,446,101,731.32 | 3,515,757,578.18 | 1,714,215,247.86 | |
| Projects with animals | 1,008,047,406.61 | 727,300,987.01 | 201,958,862.80 |
| 29.3 | 20.7 | 11.8 | |
| Projects without animals | 2,438,054,324.71 | 2,788,456,591.17 | 1,072,024,866.09 |
| 70.7 | 79.3 | 62.5 | |
| Projects with NAMs | 3,199,394,825.86 | 3,293,710,050.20 | 1,256,575,719.89 |
| 92.8 | 93.7 | 73.3 |
Both absolute numbers and relative percentages are reported
Results
Overall proportions of prevention-related biomedical research projects
We first evaluated the overall representation of prevention-related biomedical research projects funded under the EU’s framework programmes FP7, H2020, and HE (Table 3). Prevention-related projects comprised only a small fraction of the total projects funded, with percentages of 4.4%, 4.5%, and 1.9% for FP7, H2020, and HE, respectively (Fig. 1A). When considering funding, these projects were supported with 3.45 billion euro under FP7, 3.52 billion euro under H2020, and 1.71 billion euro under HE (as of October 24, 2024), corresponding to approximately 6.2%, 4.2%, and 4.2% of the overall budget for these three framework programmes, respectively (Table 3).
Further categorization of these projects by the type of prevention– primary, secondary, and tertiary – showed fluctuating distributions across the three funding programmes (Fig. 1B). The majority of prevention-related projects considered combinations of different prevention types, with secondary prevention having slightly higher representation in FP7 and H2020 (50–51%, orange bars), while projects addressing primary prevention were more represented under HE (nearly 58%, blue bars). In contrast, tertiary prevention accounted for a smaller but relatively constant proportion, ranging from 41 to 48% across all programmes.
When examining projects with an exclusive focus on a single prevention type, primary prevention was the most prevalent under FP7 and HE, while projects that focused exclusively on secondary prevention were more common under H2020. In contrast, tertiary prevention projects consistently represented the smallest category (Fig. 1C).
A closer examination of the primary prevention-related projects revealed that ‘vaccines’ and ‘nutrition/diet’ related projects accounted for 5.4–9.6% and 8.8–10.3%, respectively, of all primary prevention-related projects funded under FP7, H2020, and HE, with vaccine showing a decline across the analyzed framework programmes (Suppl. Figure 1).
Biomedical research areas mostly addressed in prevention-related projects
Next, we assessed what biomedical or disease areas were most frequently addressed in prevention-related projects funded under these three funding programmes (FP7, H2020 and HE). The data revealed that cancer, infectious diseases or virus-related research, diabetes and/or other metabolic syndrome-related disorders, and heart and/or cardiovascular diseases, were the top four biomedical research areas addressed in prevention-related projects (Fig. 2). In particular, the percentage of prevention projects focusing on cancer and infectious diseases/viruses showed an increase under the current HE (16%, 14.9% and 20.8%, and 17.4%, 14.7% and 21.6%, under the three FPs for cancer and infectious/virus projects, respectively).
Notably, personalized/precision medicine-related projects addressing prevention also showed a remarkable increase starting from H2020 (7.9%, 15.1% and 24.3%, under FP7, H2020 and HE, respectively) (Fig. 2).
An increase in the percentage of projects addressing prevention was observed for inflammation-related research, where the percentage of these projects increased from 1.5% under FP7, to 2.7% under H2020 and 6.6% under HE (Fig. 3).
When considering the total numbers of projects that focused on the aforementioned diseases/biomedical areas (regardless of prevention) globally funded under these three FPs, the average percentage of those that incorporated prevention shifted from about 19% (under FP7 and H2020) to a lower 7.8% under HE considering all diseases on average (Table 4). One noticeable exception was in the field of mental health, with overall higher percentages of prevention-related projects (73.3%, 58.7% and 18.6% for FP7, H2020 and HE, respectively). However, these percentages also suggest a decrease over time when comparing the three FPs. It is noteworthy that lower percentages of disease related projects addressing prevention were generally noticed under HE for almost all analysed diseases, except for pediatric and inflammation-related disease projects: the percentage of those addressing preventions remained more stable across the three funding programmes (Table 4).
When considering percentages of funding allocations, we noticed that while some areas — such as cardiovascular diseases, pediatric disorders, healthcare, rare diseases, inflammation-related diseases, and personalized/precision medicine — showed higher percentages of funding under most recent FPs (H2020 and HE) compared to FP7, other areas, including diabetes/metabolic syndrome, neurodegenerative diseases, age-related disorders, and AMR, exhibited declined percentages under current HE, whilst others (e.g., cancer, Alzheimer/dementia, and osteo/bone diseases) remained more stable across the three FPs (Table 5). It should be noted that comparing absolute funding distributions across the three framework programmes is challenging, as HE was still ongoing at the time this analysis was conducted, and the reported total funding amounts (in euros) only covered projects funded up to October 24, 2024.
Type of prevention addressed in analyzed projects
We further examined the type of prevention addressed by projects covering the nine most represented biomedical areas (i.e., cancer, infectious and/or viral diseases, diabetes and other metabolic syndrome disorders, cardiovascular diseases, ageing-related disorders, mental health issues, neurodegenerative disorders, healthcare, and the general field of personalized/precision medicine) (Fig. 3 and Suppl. Table S1).
In the fields of both cancer and cardiovascular disease research, a higher percentage of projects addressing secondary prevention could be noticed (Fig. 3A and D, orange bars). In contrast, projects related to infectious and/or viral diseases (Fig. 3B) and diabetes/metabolic syndrome disorders (Fig. 3C) more frequently addressed primary prevention, although the percentages of infectious/viral disease research projects under this prevention category were lower under more recent FPs (71.2%, 56% and 58.9% of FP7, H2020 and HE prevention projects, respectively) (Fig. 3B, blue bars).
Similarly, primary prevention-related projects addressing ageing/elderly disease research decreased under H2020 (68.6%, 54% and 61.5% for the three FPs, respectively) (Fig. 3E, blue bars). For the same research area, the percentage of secondary prevention projects increased in more recent FPs (32.9%, 38.9% and 46.2% for FP7, H2020 and HE, respectively) (Fig. 3E, orange bars).
Noteworthy, in the field of mental health and neurodegenerative diseases, the percentage of primary prevention projects increased under H2020 and HE, with more than 77% and nearly 43% of HE projects addressing primary prevention, respectively (Fig. 3F and G, blue bars).
The proportion of primary, secondary and tertiary prevention projects focusing on healthcare varied across the three FPs, but generally showed a predominance of primary and secondary prevention activities especially under FP7 and HE (Fig. 3H).
The percentage of personalized medicine projects considering primary prevention decreased under H2020 (36%) compared to FP7 (45.7%) and HE (60.3%) (Fig. 3I, blue bars), while those covering tertiary prevention saw a slight increase under H2020 (50.6%) compared to FP7 (48.6%) and HE (39.7%) (Fig. 3I, green bars). On the other hand, the percentage of projects addressing secondary prevention decreased a bit over time (68.6%, 64% and almost 62% for the three FPs, respectively (Fig. 3I, orange bars).
When considering other diseases or biomedical research fields, we found some increased percentages in HE projects addressing primary prevention specifically in the areas of Alzheimer’s disease/dementia (31%, 29% and 54.5% for the three FPs, respectively) (Suppl. Table S1).
Similar figures were observed also in the field of development-related disorders (47.4%, 57.8% and 87.5% of FP7, H2020 and HE projects addressing primary prevention, respectively), as well as respiratory diseases (33.3%, 63% and 80%, respectively) (Suppl. Table S1).
In contrast, the percentage of osteo-/bone-related diseases addressing primary prevention showed a decrease under H2020 (33.3%, 20.7% and 42.9% for the three FPs, respectively). Similarly, the proportion of antimicrobial resistance (AMR) research projects addressing primary prevention decreased under HE (53.8%, 52.9%, and 42.9% of FP7, H2020 and HE primary prevention projects, respectively), while the percentage of AMR-related secondary prevention/diagnostics research projects decreased under H2020 (46.2%, 32.4% and 57.1%, respectively).
On the other hand, the percentage of projects on childhood/infancy-related disorders as well as autoimmune diseases that addressed secondary prevention increased under more recent FPs (from 32.3% in FP7, to 53.4% in H2020, and 47.8% in HE, for childhood/infancy, and from 50% in FP7, to 67.7% in H2020 and 71.4% in HE, for autoimmune disorders) (Suppl. Table S1).
Analysis of funding distribution showed that projects considering primary prevention (exclusively or in combination with other prevention types), while being relatively stable under both FP7 (about 45% of total prevention-related funding) and H2020 (about 47%), increased under HE (nearly 52%) (Table 6). On the other hand, the proportion of funding allocated to both secondary and tertiary prevention progressively decreased under H2020 and HE (Table 6).
Use of animals in prevention-related biomedical projects
We examined the proportion of prevention-related projects that utilized animals (alone or combined with other methods/approaches) vs. those that did not (Fig. 4). On average, across the 18 main biomedical disease areas addressed in these projects, we found that the majority of prevention-related projects (74% on average) that received funding during the three analyzed cycles did not involve the use of animals in their research strategies, with an increase starting from H2020 (67.8%, 75.6%, and 79.2% of FP7, H2020 and HE prevention-related projects without animals, respectively) (Fig. 4A, light blue bars). On the other hand, the percentage of prevention projects involving the use of animals decreased from 32.1% in FP7 to 24.4% under H2020 and 20.8% under HE (Fig. 4A, orange bars).
When considering the nine most represented biomedical areas (i.e., cancer, infectious and/or viral diseases, diabetes and other metabolic syndrome disorders, cardiovascular diseases, ageing- related disorders, mental health issues, neurodegenerative disorders, healthcare, and personalized/precision medicine) (Fig. 4 and Suppl. Table S2), similar percentage distributions were confirmed for cancer (Fig. 4B), infectious/viral diseases (Fig. 4C), diabetes/metabolic syndrome (Fig. 4D), heart/cardiovascular diseases (Fig. 4E), mental health-related projects (Fig. 4G), and neurodegenerative diseases (Fig. 4H). Concerning healthcare projects, on average, 96% of the projects addressing prevention did not consider the use of animals, and this percentage remained quite stable across the three FPs (Fig. 4I, blue bars).
On the other hand, prevention-related projects focused on ageing-related diseases showed an increase over time in the percentage of those accounting for the use of animals, rising from 21.4% in FP7, to 31% in H2020, and 38.5% in HE (Fig. 4F, orange bars). This was accompanied by a progressive decrease in the percentage of projects that did not involve animal use, from 78.6% in FP7, to 69% in H2020 and 61.5% in HE (Fig. 4F, blue bars).
Similarly, also personalized/precision medicine-related projects linked to prevention that utilized animals increased under the most recent funding programme (14.3%, 9.2%, and 23.8% under FP7, H2020, and HE, respectively) (Fig. 4J, orange bars).
When considering less-represented biomedical research fields, we noticed decreasing percentages of projects accounting for animals, particularly in the area of Alzheimer’s disease and dementia (48.3%, 24.2% and 9.1% for the three FPs, respectively) (Suppl. Figure 2 A), child/infant related diseases (25.8%, 31.5%, and 8.7%, respectively) (Suppl. Figure 2B), developmental diseases (36.8%, 26.7%, and 0%, respectively) (Suppl. Figure 2 C), autoimmune diseases (54.2%, 25.8% and 14.3%, respectively) (Suppl. Figure 2 F), rare diseases (50%, 50% and 0%, respectively) (Suppl. Table S2), and inflammatory diseases (76.9%, 46.5%, and 35.3%, for FP7, H2020 and HE, respectively) (Suppl. Figure 2 H and Suppl. Table S2).
In contrast, other research fields exhibited an increase in the percentage of prevention projects with animals under most recent funding programme, such as AMR (15.4% of FP7, 8.8% of H2020, and 42.9% of HE projects) (Suppl. Figure 2D and Suppl. Table S2); osteo/bone-related disorders (28.6% of FP7, 31% of H2020, and 57.1% of HE prevention projects)(Suppl. Figure 2E and Suppl. Table S2); and respiratory diseases (9.5% of FP7, 5.6% of H2020, and 40% of HE projects) (Suppl. Figure 2G and Suppl. Table S2).
Noteworthy, when considering the use of animal-based models in relation to the type of prevention research (primary, secondary or tertiary), we observed that the percentage of exclusively tertiary prevention-related projects that accounted for animals increased under HE, from 37.5% in both FP7 and H2020, to and 50% in HE (Fig. 5A, green bars). On the contrary, the percentage of exclusively primary or exclusively secondary prevention projects that utilized animals decreased under HE (26.5% and 8.5% of HE projects on primary and secondary prevention, respectively) (Fig. 5A, dark blue and orange bars). These figures were confirmed when examining the percentages of prevention-related projects that did not consider the use of animals (Fig. 5B), which showed a progressive increase in secondary prevention projects without animals (84.3%, 90.3% and 91.5%, across the three FPs) (orange bars in Fig. 5B), and decreasing percentages for tertiary prevention projects (green bars in Fig. 5B).
Use of human-centric approaches in prevention-related biomedical projects
We further explored the proportion of projects that considered the use of human-centered approaches, either alone or in combination with other methods (Fig. 6). For the purpose of this analysis, we considered any human-centric or human-based research strategies, including human-based in vitro models (labelled as ‘in vitro’), human clinical/cohort studies and human-derived biological samples (labelled as ‘humans’), large human data sets and in silico/computational approaches and technologies (labelled as ‘in silico’), as detailed in Table 1.
Our findings revealed that the vast majority of all prevention-related projects made use of such approaches (85.7%, 88.2%, and 93.1%, for FP7, H2020 and HE, respectively), with increasing percentages across the three FPs (Fig. 6A, dark blue bars).
When examining specific subcategories, we noticed that most of these projects focused on patients/participants’ cohorts, clinical trials and/or biological samples (‘humans’) (72.7%, 76.6% and 70.5% for the three FPs, respectively) (Fig. 6A, orange bars).
The percentage of projects utilizing in vitro methods/models slightly decreased under most recent FP (35%, 31%, and 26.1%, for FP7, H2020 and HE, respectively) (Fig. 6A, green bars), whilst, on the contrary, the percentage of those considering the use of big data and other computational approaches (‘in silico’) increased starting from H2020 (43.7%, 49.7%, 64.3%, respectively) (Fig. 6A, light blue bars).
When considering the nine most represented biomedical areas, similar percentage distributions could be observed, particularly for cancer (Fig. 6B), infectious diseases (Fig. 6C), diabetes and other metabolic syndrome disorders (Fig. 6D), cardiovascular diseases (Fig. 6E), age-associated diseases (Fig. 6F), and healthcare (Fig. 6I).
In the area of mental health, prevention-related projects that utilized in vitro methods were relatively underrepresented (between 0.9 and 6.8% across all FPs, green bars), while the percentage of those considering computational approaches increased over time (45.7%, 42.9% and 61.5%, respectively, see light blue bars in Fig. 6G). In the area of personalized medicine, the use of both in vitro and in silico approaches increased with more recent FPs (Fig. 6J, see green and light blue bars).
On the other hand, the percentage of prevention projects on neurodegenerative diseases that considered the use of any human-based methods decreased under H2020 and the most recent HE (90.6%, 85.3%, and 78.6%, for FP7, H2020, and HE, respectively) (Fig. 6H, dark blue bars).
When considering other less represented disease areas, under HE, an increase in the percentage of projects that considered the use of computational/in silico approaches was noted in the field of Alzheimer’s disease and dementia (44.8%, 59.7%, and 90.9%, under FP7, H2020, and HE, respectively) (Suppl. Figure 3 A and Suppl. Table S3), childhood-related diseases (41.9%, 42.5%, and 78.3%, under FP7, H2020, and HE, respectively) (Suppl. Figure 3B and Suppl. Table S3), developmental disorders (26.3%, 48.9%, and 83.3%, respectively) (Suppl. Figure 3 C and Suppl. Tables S3), osteo/bone related diseases (28.6%, 48.3%, and 57.1%, respectively) (Suppl. Figure 3E and Suppl. Table S3), autoimmune disorders (25%, 45.2%, and 57.1%, respectively) (Suppl. Figure 3 F and Suppl. Table S3), and inflammatory diseases (23.1%, 18.6%, and 70.6%, respectively) (Suppl. Figure 3 H and Suppl. Table S3).
Despite the generally high percentage of prevention projects that addressed AMR and utilized human-based approaches (78.5% across all three FPs, on average), a decrease in the percentage of such projects could be seen over time, from 84.6% under FP7, to 79.4% under H2020, and 71.4% under HE (considering all human-based approaches altogether) (Suppl. Figure 3D and Suppl. Table S3).
An analysis of funding distribution across projects, based on their use of animal or human-based methods (NAMs), shows an overall decrease in funding percentages under the most recent HE programme (Table 7). However, this may be partly explained by incomplete reporting of total funding in CORDIS, as several projects have no value listed under ‘total cost.‘
Discussion
How much does the EU invest in prevention within biomedical research?
In 2021, EU Member States invested approximately €95.3 billion in preventive healthcare, accounting for 0.65% of GDP. This marked an 88.2% increase from the previous year, largely influenced by COVID-19-related immunization programmes [22]. Whilst this may seem an encouraging figure, investment in prevention research remains limited. In the area of cancer, only 16% of European cancer research funding has been directed towards prevention initiatives, highlighting a significant gap between the real-world need for primary prevention and the actual funding priorities [37, 38].
Low preventive healthcare expenditure in EU countries can lead to several long-term health impacts, in particular, a higher prevalence of NCDs [39], which account for approximately 90% of deaths in Western Europe, straining healthcare budgets significantly [40]. This can lead to diminished quality of life and increase in disability-adjusted life years (DALYs) among populations [41].
In addition, poor health from preventable diseases costs the European economy approximately 15% of GDP annually due to lost productivity and reduced quality of life, creating a cycle of economic decline [41]. Countries with low preventive spending face escalating healthcare costs due to the need for more extensive treatment of preventable conditions, which could potentially lead to an estimated excess spending of €350 billion by 2050 [40].
As populations age, the demand for healthcare increases, yet low preventive spending fails to effectively address the underlying health issues [42]. Insufficient funding restricts the development and implementation of innovative healthcare solutions and programmes aimed at prevention, resulting in reactive rather than proactive healthcare systems [43]. Greater investment in prevention-focused, human-relevant research could significantly reduce NCDs’ prevalence, reduce the risk of infectious diseases, alleviate public health burdens, and lower healthcare expenditures.
In this retrospective analysis we sought to investigate the extent to which the EU has invested in prevention-related biomedical research during the last three framework programmes (FP7, H2020 and HE). Our findings reveal a persistent underinvestment in prevention-focused research, with only 4.4%, 4.5%, and 1.9% of life sciences-related projects addressing prevention under the entire FP7, H2020, and HE funding cycles, respectively. These projects were supported with 6.2% of the overall budget under FP7, and an equal 4.2% of the overall budget under both H2020 and HE.
When considering the overall number of biomedical research projects covering a specific disease, we found that the percentage of those that accounted for prevention ranged between 7.8 and 19% over the three FPs (on average, about 15% of all biomedical-related projects). When looking at cancer, in particular, the average of projects addressing prevention was about 9.5%, while those focused on Alzheimer’s were on average 12.6% (see Table 4). These figures are broadly consistent with our previous analysis, which found that, on average, about 8% of projects on Alzheimer’s disease, breast cancer and prostate cancer addressed prevention research [31]. It is noteworthy that, under most recent HE, a general decline in the percentage of any disease related projects addressing prevention could be observed, except for pediatric diseases and inflammatory diseases, whose percentages remained more similar comparing the three FPs (see Table 4).
When considering percentages of funding distribution, some areas, including diabetes and metabolic syndrome, neurodegenerative diseases, age-related disorders, and AMR exhibit a decrease across the three analysed FPs. Due to the potential impact of preventative measures on overall health, greater investment in prevention research is essential, aligning with recent policy recommendations [24–27].
Notably, while primary and secondary prevention were well-represented in these projects, a concerning figure emerged: the percentage of primary prevention projects specifically addressing nutrition research represented less than 10% on average. This is particularly alarming given the critical role of diet in preventing NCDs such as cardiovascular disease [44], diabetes [45], cancer [46], and Alzheimer’s disease [47], as well as infectious diseases [21].
Several factors may contribute to the underfunding of nutrition research, which has significant public health implications. One reason could be that nutrition research often lacks the clear, tangible outcomes and quick returns on investment that are typically associated with pharmaceutical or therapeutic interventions [48, 49]. Additionally, the complex, multifactorial nature of nutrition and its relationship to disease can make it challenging to design and evaluate effective preventative strategies, potentially deterring funders. Furthermore, the food industry’s significant influence on nutrition research and policy may also play a role, as some companies may be hesitant to support research that could lead to recommendations limiting certain types of foods or beverages. Finally, the fact that preventative nutrition approaches often require long-term investments and may not yield immediate, measurable results may lead funders to prioritize more short-term, treatment-focused research initiatives. As a result, despite its potential to yield significant public health benefits, nutrition research remains underfunded.
To address this gap, future funding frameworks should prioritize lifestyle-based prevention strategies, including nutrition and diet research, to enhance the prevention and delay the onset of major diseases. By doing so, we can capitalize on the potential of preventative nutrition approaches to improve public health outcomes and reduce the burden of NCDs.
Projects on infectious and/or viral disease research, as well as those on ageing and elderly diseases that addressed primary prevention showed a decline starting from H2020. Considering the beneficial protective impact of primary preventative interventions and the role of lifestyle factors, such as diet in preventing infectious diseases [50, 51] or their long-lasting symptoms (e.g. those of COVID19 [52]), or in delaying age-related complications [53], more calls for proposals should be designed to adequately support these research activities.
On a positive note, mental health-related projects that considered primary prevention showed an increase in percentage comparing the three FPs, which is in line with current EP recommendations [25] and the call by the EC to Member States to submit best practices on mental health promotion, prevention, early detection, and early intervention [54].
Lifestyle-related interventions, including dietary and physical activity, have been shown promising to prevent osteoporosis and the risk of bone fractures [55–57]. However, our findings indicate a decrease in the percentage of osteo/bone-related research projects focused on primary prevention particularly under H2020, while considering that the figure for currently ongoing HE is still incomplete (with only 7 osteo/bone-related research projects addressing prevention). This highlights the need to allocate additional resources and develop targeted calls for proposals to prioritize primary prevention in bone health research.
It is noteworthy to mention that AMR has been recognized as one of the most pressing global health and development threats [58], with 35,000 people dying every year in Europe due to AMR [59]. This has prompted the need for multisectoral coordinated actions, including the development of new antimicrobial medicines, vaccines, and diagnostic tools [58]. Our findings reveal that, on average, less than 10% of AMR-related projects funded under FP7, H2020 and HE addressed prevention (Table 4). Notably, among these projects, the proportion of those addressing primary prevention declined from about 54% under FP7 to less than 43% under HE, while the proportion of secondary prevention projects in this area resulted lower in H2020 (32.4%) compared to FP7 (46.2%) (Suppl. Table S1). These figures prompt the need to invest more resources in primary prevention and diagnostics in the field of AMR research.
The use of animals in biomedical research related to prevention
Traditionally, conducting research in areas such as neuroscience, ageing, immune system function, infectious disease research (especially for emerging pathogens), systemic effects, and developmental biology has been particularly challenging without the use of animal models or entire living organisms. However, remarkable progress has been made in recent years to overcome these limitations. Advances now enable researchers to study systemic effects, model immune responses [60], predict human pharmacokinetics [61], and connect multiple organs using technologies such as multi-organ-on-chip platforms [62]. In parallel, computer-based simulations of disease mechanisms and systemic effects of both pharmaceutical and non-pharmaceutical interventions are becoming increasingly sophisticated, further reducing reliance on animal models (see e.g [63]). Therefore, it is becoming increasingly difficult to identify specific areas of biomedical research where the use of animal models can still be considered indispensable.
However, despite the European Medicines Agency (EMA) implementing special support measures through initiatives like the Innovation Task Force [64] to encourage the replacement, reduction, and refinement (3Rs) of animal use in the development, manufacturing, and testing of human and veterinary medicines, the submission of data from existing animal models remains a mandatory requirement for preclinical testing in the EU [65].
The current regulatory landscape often lags behind technological innovation, particularly regarding the use of NAMs such as advanced in vitro models (e.g., organ-on-chip, 3D tissue constructs), and computational models. The regulatory landscape should keep pace with scientific progress in the field of NAMs, enabling the integration of advanced, human-specific technologies that can improve biomedical research, enhance safety assessments, and reduce reliance on animal models. Without regulatory alignment, scientific advancements risk being underutilized, delaying both safer product development and more ethical research practices.
It should also be considered that the use of animals in basic or translational research aimed at understanding human diseases is not a regulatory requirement. In fact, human-based models, tools, and approaches are now widely available and increasingly used in these areas [66]. These models are proving instrumental in unraveling disease mechanisms, fostering human-centric research, and potentially improving translational outcomes [31, 67].
Our analysis revealed an increase in the percentages of projects accounting for the use of computational/in silico approaches, including ‘omics’ and large human datasets, under more recent FPs. This reflects a growing recognition of the importance of human health data-driven approaches in elucidating the complexities of disease pathogenesis and developing targeted interventions. Human health-derived data, including electronic health records (EHRs), biobank and patients’ registries, medical imaging and multi-omics studies, have emerged as a vital resource for identifying disease risk factors, discovering novel biomarkers, and devising personalized prevention strategies [12]. In contrast to traditional animal-based models, human datasets provide a direct representation of human pathophysiology, thereby enhancing the translational relevance and applicability of research findings [33]. The integration of artificial intelligence (AI) and machine learning with human health datasets has further enhanced the predictive modeling capabilities, enabling more accurate analysis of disease progression patterns and informing evidence-based healthcare interventions.
A crucial step towards optimizing the utilization of human health data is the establishment of the European Health Data Space (EHDS), which aims to standardize and facilitate secure access to health data across EU member states [68]. By supporting the secondary use of health data, the EHDS will enable large-scale epidemiological studies, cross-border research collaborations, and AI-driven real-world evidence generation, ultimately informing the development of more effective prevention strategies and public health policies. As a result, as the EHDS evolves, its role in advancing human-centric biomedical research will grow, reducing reliance on traditional experimental models and promoting the development of data-driven, personalized healthcare solutions.
Our analysis revealed that the majority of prevention-related projects (almost 90% on average) utilized human-centered approaches (alone or combined with other animal methods), with percentages increasing over time.
Nevertheless, animal use in basic and translational research remains disproportionately high, accounting for 72% of animal use in these areas, as reported in the 2022 ALURES statistics [69]. This continued reliance on animal models is juxtaposed against the growing number of failed clinical trials, particularly for chronic and complex diseases, where preclinical results from animal studies often fail to translate to humans [70]. This disconnect raises a critical question: do animal models reliably predict human health outcomes, especially for conditions influenced by lifestyle factors?
Our analysis of EU-funded prevention-related biomedical research projects funded under the three analyzed FPs indicates that almost 26% involved the use of animals. While this may be interpreted as a positive finding indicative of reduced animal use in prevention science, it underscores a potentially missed opportunity. Prevention research, by its very nature, deals with systemic and multifaceted elements of human behavior, lifestyle, disease etiology, and complexity—areas that are intrinsically challenging, if not impossible, to model in non-human species [33]. This highlights the pressing need to further leverage and prioritize human-centric approaches in biomedical research to avoid these limitations and improve translational outcomes.
Moreover, our analysis highlights a higher percentage in the use of animal-based models within tertiary prevention research, with an increase from 37.5% in both FP7 and H2020 to 50% in HE. Tertiary prevention strategies have generally relied on pharmaceutical interventions to reduce long-term complications and improve disease management. The approval and marketing of these interventions have historically been contingent on animal data for safety and efficacy testing. However, in recent years the legislative landscape has evolved, with increasing support for the integration of non-animal testing approaches in the development and evaluation of pharmaceuticals in the EU [71], and at global level (e.g., in the US, with the recent FDA Modernization Act 3.0 H.R.7248 [72], and the recent FDA roadmap to reducing animal testing in preclinical safety studies [73]). As regulatory frameworks evolve, there is an opportunity to better align tertiary prevention research with modern, human-centric approaches, reducing reliance on animal models and advancing both the safety and efficacy of interventions for long-term disease management.
Among analyzed biomedical research areas, ageing-associated diseases showed an increase over time in the percentage of prevention-related projects that utilized animals, from FP7 (21.4%) to HE (38.5%). Preference for using animals such as mice in ageing studies may stem, apart from their genetic similarity to humans, also from convenience, as their relatively short lifespan allows researchers to observe the progression of aging and age-related diseases within a much shorter timeframe than in humans [74, 75]. However, while the fundamental aspects of the ageing process are the same in all mammals [76], laboratory animals often fail to capture the variability present in natural populations or the interactions between cellular mechanisms of ageing and complex external factors. Consequently, findings derived from these models may not directly translate to the genetic polymorphisms associated with health and lifespan in humans, who live in environments vastly different from controlled laboratory settings [77]. Additionally, certain genes involved in central nervous system development are either unique to primates or exclusively found in humans, further limiting the relevance of some animal models for studying human-specific traits [78]. Moreover, the most used animal models, such as rodents and non-mammalian species, do not naturally develop age-related neurodegenerative disorders [77].
In addition to studying ageing directly in humans through observational cohort studies, patient-derived induced pluripotent stem cells (iPSCs) and their differentiated derivatives have also shown promising for studying cellular ageing and the underlying molecular mechanisms [79]. Recent efforts have focused on overcoming the potential limitations of these models, by optimizing reprogramming and differentiation protocols with the aim of producing more reliable and robust patient-derived cells capable of accurately modeling aging and age-related diseases [80]. The reprogramming technology nowadays allows for the generation of multiple isogenic cell types sharing the same genetic background [81]. By differentiating iPSCs into brain cell types and leveraging co-culture platforms, researchers can gain critical insights into cell-type-specific dysfunctions associated with aging [82–84], and neurodegenerative diseases, such as Alzheimer’s disease [85], and Parkinson’s disease [86, 87]. However, our findings suggest that the percentage of prevention projects on neurodegenerative diseases that considered the use of human-based methods decreased under most recent HE (78.6%) as compared to FP7 (90.6%) and H2020 (85.3%).
Our analysis also showed that prevention-related projects focusing on personalized and precision medicine and utilizing animals increased from FP7 (14.3%) to HE (23.8%). Personalized medicine represents a paradigm shift from the “one-size-fits-all” approach, focusing on understanding the individual differences among patients with the same disease. This approach enables the selection of therapies tailored to specific patient groups, predicting the likelihood of a treatment’s effectiveness for each individual [88]. In this context, the reliance on non-human animal models is increasingly questionable. These models fail to account for the genetic, epigenetic, and interpersonal variability that is central to the personalized medicine’s principles. Human-based, patient-derived in vitro models (e.g., iPSC-derived models generated from individuals with specific genetic mutations, iPSC-derived organ-on-chips) [89, 90], and advanced in silico approaches (e.g., AI) [91] provide tools that directly reflect human biology, paving the way for personalized treatments tailored to the genetic profile of the patient. Attempting to model these uniquely human complexities in other species not only yields limited insights but also contradicts the core principles of personalized and precision medicine, making such efforts scientifically unfounded and counterproductive.
Our analysis also showed that under current HE, the percentage of prevention-related projects addressing AMR, osteo/bone-related disorders, as well as respiratory diseases, that accounted for the use of animals is higher than in previous FPs (FP7 and H2020). In the context of AMR, this increase is accompanied by a decline in the percentage of prevention projects that incorporate human-centered approaches.
Concerns have been raised regarding the comparison of rodent preclinical models to the human skeleton, particularly in the context of cortical bone, highlighting key differences that limit the translational relevance of rodent models in skeletal research [92]. On the other hand, innovative computational technologies are emerging as a promising approach to effectively and efficiently study AMR [93, 94]. Also, human-based complex in vitro models have been successfully employed to study bone [95, 96], as well as respiratory diseases [97, 98]. These approaches should be leveraged to support human-centered prevention research in these fields of biomedical science.
Study limitations and future directions
When considering potential limitations, this study focused solely on CORDIS as the project repository, as one of its primary objectives was to conduct a comparative temporal analysis of the three most recent EU framework programmes (FP7, H2020, and HE). However, it is important to acknowledge that prevention-related projects have also been funded under the EU4Health programme, launched in 2021. These projects, accessible via the EU Funding & Tenders Portal, were highlighted in a recent analysis conducted within the PROPHET project [99, 100]. That study identified 67 prevention-related projects (48% of which targeted cancer) that focused more prominently on secondary and tertiary prevention.
Future research should aim to expand this analysis by incorporating additional project repositories, such as EU4Health. This could ultimately lead to a more comprehensive understanding of prevention-related projects and their impact on European healthcare policies.
Furthermore, further studies should focus on integrating the EHDS with emerging technologies, such as AI-driven analytics and digital health tools, to improve real-time disease monitoring and predictive modeling. The effectiveness of the EHDS in improving human-centered healthcare policies and reducing reliance on animal-based research methodologies will be crucial in shaping the future of European biomedical research.
Conclusions and potential recommendations
Investing more in prevention-focused research is imperative, particularly through the adoption of human-centric technologies and strategies. The complex interplay of lifestyle-related factors, as well as the etiopathology and physiopathology of diseases in humans, cannot be accurately reproduced in animal models, underscoring the need for approaches that directly reflect human biology.
Under HE, an increased use and deployment of human-centric approaches could be observed, compared to previous framework programmes. However, the overall percentage of biomedical research projects addressing prevention remains critically low. Our analysis also reveals an increase in the percentage of projects that accounted for the use of animals specifically in the area of tertiary prevention (from 37.5% in FP7 and H2020 to 50% in HE), ageing-related diseases, and personalized or precision medicine. Similarly, the proportion of HE projects using animals in areas such as AMR, bone, and respiratory diseases is higher than in previous framework programmes. These patterns reflect a counter-productive funding strategy. Prevention research inherently involves complex, human-specific factors—such as behavior, lifestyle, and environmental influences—that are extremely difficult, if not impossible, to model in non-human species. It could be argued that research project planning and funding strategies may default to animal-based approaches out of habit, rather than being guided by a thoughtful, evidence-based assessment of their suitability. Continuing to pour resources into animal-based prevention research—especially for primary and secondary prevention—is not only scientifically questionable, it’s a significant misallocation of public and private funds. Animal models fail to replicate the intricate, multifactorial nature of human health and disease, particularly the dynamic interplay between lifestyle, environment, psychosocial factors, and genetic predisposition. The shift toward human-based research is essential to generate actionable results that can effectively inform and shape public health policies. By prioritizing such approaches, we can bridge the gap between research outputs and their real-world application, leading to improved health outcomes and more sustainable healthcare systems.
Based on the main findings of this analysis, some recommendations are proposed (Table 8) for consideration by policymakers and funding bodies in shaping the next EU funding programme, FP10, and evidence-based research-to-policy translation. By identifying trends and gaps in prevention-focused research, this study aims to inform the design of targeted calls for proposals that align more effectively with the overarching goal of enhancing public health outcomes through preventive strategies. In addition to targeted funding allocation, practical measures to enhance the effectiveness, feasibility, and societal impact of prevention research aligned with EU policy priorities are presented.
Table 8.
Possible recommendations for shaping FP10 to enhance prevention-focused research and enable research-to-policy translation
| Area | Recommendation |
|---|---|
| Prevention research, policy alignment, and research impact | 1. Increase investment in human-centric prevention research, prioritizing areas where conditions are largely preventable (e.g., diabetes and metabolic disorders), to align with policy recommendations and maximize public health impact |
| Primary prevention | 2. Prioritize lifestyle-based prevention (e.g., nutrition/diet) strategies in future funding frameworks to strengthen efforts against major NCDs |
| Human-centered approaches | 3. Prioritize and expand the use of human-centric approaches in prevention research to address systemic and multifactorial aspects of human health that cannot be modeled in non-human species |
| 4. Prioritize human-centric approaches to enhance the safety and efficacy of pharmaceutical interventions in long-term disease management (tertiary prevention) | |
| Infectious diseases | 5. Increase funding and design calls for proposals for research on primary prevention in infectious diseases, recognizing the protective impact of lifestyle-based interventions, such as diet, in preventing infections and hospitalization |
| Bone-related diseases | 6. Allocate additional resources and design targeted calls for proposals to prioritize primary prevention in bone health research |
| 7. Prioritize funding for prevention projects that emphasize human-centered approaches in bone-related disorders | |
| Antimicrobial resistance (AMR) | 8. Increase investment in primary prevention and diagnostics within AMR research to address this pressing global health challenge |
| 9. Prioritize funding for prevention projects that emphasize human-centered approaches in AMR | |
| Age-related diseases | 10. Increase funding and design calls for proposals for research on primary prevention in age-related conditions, recognizing the protective impact of lifestyle-based interventions in delaying age-related complications |
| 11. Invest in patient-derived cells (e.g., iPSCs and their differentiated derivatives) alongside observational cohort studies to advance the understanding of cellular aging and its molecular mechanisms | |
| Respiratory diseases | 12. Prioritize funding for prevention projects that emphasize human-centered approaches in respiratory diseases |
| Personalized/precision prevention | 13. Allocate more funding to personalized (or precision) prevention research based on human-centered approaches |
| Collaboration and stakeholder engagement | 14. Foster multi-sector collaboration by designing calls that explicitly require partnerships between academia, healthcare providers, policymakers, patients, and patient associations to improve prevention uptake and translation |
| 15. Support cross-border prevention initiatives, particularly for addressing shared public health challenges such as AMR, metabolic diseases, or age-related disorders, ensuring alignment with broader EU health and sustainability policies | |
| Training and workforce development | 16. Establish targeted training programmes on human-centric prevention research, including the use of patient-derived models, advanced in vitro technologies, and systems approaches, to build expertise across the EU |
| 17. Promote interdisciplinary education by integrating prevention research, human-specific methods, and public health systems thinking into relevant curricula (biomedical, public health, engineering, etc.) | |
| Policy alignment and research uptake | 18. Incentivize research that explicitly demonstrates potential for policy uptake or real-world implementation |
| 19. Establish EU-level monitoring of prevention research impact, developing indicators that track how funded projects contribute to measurable health improvements, reduced disease burden, or policy implementation |
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
Conceptualisation: FP; Writing—Original Draft Preparation: FP, FF; Writing—Review and Editing: FP, FF, AG, EP, RP, IT, HC; Final Review and Editing: FP, FF, AG, EP, RP, IT, HC; Data analysis: FP, FF, IT; Project Administration: FP, HC. All authors have read and agreed to the published version of the manuscript.
Data availability
The dataset supporting the conclusions of this article is included within the article, its supplementrary file, and the xls file ‘FP7-H2020-HE_Prevention Projects_EXTENDED DATA SET’.
Declarations
Competing interests
The authors have no competing interests to declare.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Noncommunicable diseases [Internet]. [cited 2025 Jan 13]. https://www.who.int/health-topics/noncommunicable-diseases#tab=tab_1
- 2.Dementia [Internet]. [cited 2025 Jan 13]. https://www.who.int/news-room/fact-sheets/detail/dementia
- 3.Kim H, Caulfield LE, Garcia-Larsen V, Steffen LM, Coresh J, Rebholz CM. Plant-Based diets are associated with a lower risk of incident cardiovascular disease, cardiovascular disease mortality, and all-cause mortality in a general population of middle-aged adults. J Am Heart Assoc. 2019;8:e012865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim H, Caulfield LE, Rebholz CM. Healthy plant-based diets are associated with lower risk of all-cause mortality in US adults. J Nutr. 2018;148:624–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tuso PJ, Ismail MH, Ha BP, Bartolotto C. Nutritional update for physicians: plant-based diets. Perm J. 2013;17:61–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jardim MZ, Costa BV, de Pessoa L, Duarte MC. Ultra-processed foods increase noncommunicable chronic disease risk. Nutr Res. 2021;95:19–34. [DOI] [PubMed] [Google Scholar]
- 7.CDC. Preventing chronic diseases. what you can do now [Internet]. Chronic Disease. 2024 [cited 2025 Jan 13]. https://www.cdc.gov/chronic-disease/prevention/index.html
- 8.Promoting physical activity. and healthy diets for healthy ageing in the WHO European Region [Internet]. [cited 2025 Jan 13]. https://www.who.int/europe/publications/i/item/WHO-EURO-2023-8002-47770-70520
- 9.Combination of healthy lifestyle traits may substantially reduce Alzheimer’s [Internet]. National Institutes of Health (NIH). 2020 [cited 2025 Jan 13]. https://www.nih.gov/news-events/news-releases/combination-healthy-lifestyle-traits-may-substantially-reduce-alzheimers
- 10.Breast Cancer Prevention - NCI [Internet]. 2024 [cited 2025 Jan 13]. https://www.cancer.gov/types/breast/patient/breast-prevention-pdq
- 11.Plans-Beriso E, Babb-de-Villiers C, Petrova D, Barahona-López C, Diez-Echave P, Hernández OR, et al. Biomarkers for personalised prevention of chronic diseases: a common protocol for three rapid scoping reviews. Syst Rev. 2024;13:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pastorino R, Loreti C, Giovannini S, Ricciardi W, Padua L, Boccia S. Challenges of prevention for a sustainable personalized medicine. J Personal Med. 2021;11:311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.AbdulRaheem Y. Unveiling the significance and challenges of integrating prevention levels in healthcare practice. J Prim Care Community Health. 2023;14:21501319231186500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Babatunde AO, Shobanke HA, Akinade AA, Michael AJ, Osadare M, Akanbi OK, et al. Enhancing preventive medicine over curative medicine: role of telemedicine. Public Health Pract (Oxf). 2021;2:100130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prevention Is Still the Best Medicine -. News & Events | odphp.health.gov [Internet]. [cited 2025 Jan 13]. https://odphp.health.gov/news/202401/prevention-still-best-medicine#
- 16.Perneczky R. Dementia treatment versus prevention. Dialog Clin Neurosci. 2019;21:43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeWeerdt S. Prevention: activity is the best medicine. Nature. 2011;475:S16–7. [DOI] [PubMed] [Google Scholar]
- 18.Cho SB. Comorbidity genes of Alzheimer’s disease and type 2 diabetes associated with memory and cognitive function. Int J Mol Sci. 2024;25:2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harborg S, Kjærgaard KA, Thomsen RW, Borgquist S, Cronin-Fenton D, Hjorth CF. New horizons: epidemiology of obesity, diabetes mellitus, and cancer prognosis. J Clin Endocrinol Metabolism. 2024;109:924–35. [DOI] [PubMed] [Google Scholar]
- 20.Pistollato F, Burkhart G, Deceuninck P, Bernasconi C, Di Virgilio S, Emili L et al. What public health challenges and unmet medical needs would benefit from interdisciplinary collaboration in the EU? A survey and multi-stakeholder debate. Front Public Health [Internet]. 2024 [cited 2025 Jan 13];12. https://www.frontiersin.org/journals/public-health/articles/10.3389/fpubh.2024.1417684/full [DOI] [PMC free article] [PubMed]
- 21.Rahmati M, Fatemi R, Yon DK, Lee SW, Koyanagi A, Il Shin J, et al. The effect of adherence to high-quality dietary pattern on COVID‐19 outcomes: a systematic review and meta‐analysis. J Med Virol. 2023;95:e28298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Preventive health care expenditure statistics [Internet]. [cited 2025 Jan 13]. https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Preventive_health_care_expenditure_statistics
- 23.Barnfield A, Savolainen N. Health promotion and primary prevention in 21 European countries. Eur J Pub Health. 2019;29:ckz185219. [Google Scholar]
- 24.Healthier together – EU non-communicable diseases initiative - European Commission [Internet]. 2024 [cited 2025 Jan 13]. https://health.ec.europa.eu/non-communicable-diseases/healthier-together-eu-non-communicable-diseases-initiative_en
- 25.Parliament calls for action to protect mental health [Internet]. Topics | European Parliament. 2022 [cited 2025 Jan 13]. https://www.europarl.europa.eu/topics/en/article/20220624STO33809/parliament-calls-for-action-to-protect-mental-health
- 26.communication-from-the-commission-to-the-european-parliament-and-the-council [Internet]. [cited 2024 Dec 20]. https://primarysources.brillonline.com/browse/human-rights-documents-online/communication-from-the-commission-to-the-european-parliament-and-the-council;hrdhrd46790058
- 27.Texts adopted - Non. -communicable diseases - Wednesday, 13 December 2023 [Internet]. [cited 2025 Jan 13]. https://www.europarl.europa.eu/doceo/document/TA-9-2023-0467_EN.html
- 28.Regulation – 2022/2371 - EN. - EUR-Lex [Internet]. [cited 2025 Jun 17]. https://eur-lex.europa.eu/eli/reg/2022/2371/oj/eng
- 29.Regulation – 2022/2370 - EN. - EUR-Lex [Internet]. [cited 2025 Jun 17]. https://eur-lex.europa.eu/eli/reg/2022/2370/oj/eng
- 30.Regulation (EU). 2022/123 of the European Parliament and of the Council of 25 January 2022 on a reinforced role for the European Medicines Agency in crisis preparedness and management for medicinal products and medical devices (Text with EEA relevance) [Internet]. OJ L Jan 25, 2022. http://data.europa.eu/eli/reg/2022/123/oj/eng
- 31.Deceuninck P, Gastaldello A, Mennecozzi M, Pistollato F. Exploring the connection between EU-funded research and methodological approaches: insights from a retrospective analysis. J Transl Med. 2024;22:891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Projects & results | CORDIS | European Commission [Internet]. [cited 2025 Jan 13]. https://cordis.europa.eu/projects
- 33.Frangogiannis NG. Why animal model studies are lost in translation. J Cardiovasc Aging. 2022;2:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.About. Health promotion and disease prevention through population-based interventions, including action to address social determinants and health inequity [Internet]. World Health Organization - Regional Office for the Eastern Mediterranean. [cited 2025 Jan 13]. http://www.emro.who.int/about-who/public-health-functions/health-promotion-disease-prevention.html
- 35.Directive – 2010/63 - EN. - EUR-Lex [Internet]. [cited 2025 Jan 13]. https://eur-lex.europa.eu/eli/dir/2010/63/oj/eng
- 36.DeepSeek-AI, Liu A, Feng B, Xue B, Wang B, Wu B et al. DeepSeek-V3 Technical Report [Internet]. arXiv; 2025 [cited 2025 Jul 3]. http://arxiv.org/abs/2412.19437
- 37.Schmutz A, Matta M, Cairat M, Espina C, Schüz J, Kampman E, et al. Mapping the European cancer prevention research landscape: a case for more prevention research funding. Eur J Cancer. 2023;195:113378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Only an urgent shift towards prevention-based healthcare. can secure economic stability for EU health systems, experts say [Internet]. IFPMA. [cited 2025 Jan 13]. https://www.ifpma.org/news/only-an-urgent-shift-towards-prevention-based-healthcare-can-secure-economic-stability-for-eu-health-systems-experts-say/
- 39.Health policies to. tackle chronic diseases can have positive impacts within 5 years [Internet]. [cited 2025 Apr 3]. https://www.who.int/europe/news/item/24-03-2025-health-policies-to-tackle-chronic-diseases-can-have-positive-impacts-within-5-years
- 40.Goryakin Y, Thiébaut SP, Cortaredona S, Lerouge MA, Cecchini M, Feigl AB, et al. Assessing the future medical cost burden for the European health systems under alternative exposure-to-risks scenarios. PLoS ONE. 2020;15:e0238565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ahlawat H, Boldt-Christmas O, Dash P, Dewhurst M, Dorling G, Remes J et al. McKinsey & Company [Internet]. McKinsey Global Institute. How keeping health a priority is a prescription for European prosperity. https://www.mckinsey.com/industries/healthcare/our-insights/how-keeping-health-a-priority-is-a-prescription-for-european-prosperity
- 42.The Future of Health in Europe. | Deloitte Switzerland [Internet]. [cited 2025 Jan 13]. https://www.deloitte.com/ch/en/Industries/life-sciences-health-care/perspectives/the-future-of-health-in-europe.html
- 43.The Future of Health in Europe [Internet]. Deloitte Insights. [cited 2025 Jan 13]. https://www2.deloitte.com/us/en/insights/industry/health-care/future-of-healthcare-in-europe.html
- 44.8 steps to. a heart-healthy diet [Internet]. Mayo Clinic. [cited 2025 Jan 13]. https://www.mayoclinic.org/diseases-conditions/heart-disease/in-depth/heart-healthy-diet/art-20047702
- 45.Diabetes prevention. 5 tips for taking control [Internet]. Mayo Clinic. [cited 2025 Jan 13]. https://www.mayoclinic.org/diseases-conditions/type-2-diabetes/in-depth/diabetes-prevention/art-20047639
- 46.Reducing Cancer Risk [Internet]. [cited 2025 Jan 13]. https://stanfordhealthcare.org/medical-clinics/cancer-nutrition-services/reducing-cancer-risk.html
- 47.Fekete M, Varga P, Ungvari Z, Fekete JT, Buda A, Szappanos Á et al. The role of the Mediterranean diet in reducing the risk of cognitive impairement, dementia, and Alzheimer’s disease: a meta-analysis. GeroScience [Internet]. 2025. 10.1007/s11357-024-01488-3 [DOI] [PMC free article] [PubMed]
- 48.Weaver CM, Miller JW. Challenges in conducting clinical nutrition research. Nutr Rev. 2017;75:491–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.NIH. 2020–2030 Strategic plan for nih nutrition research—a report of the NIH nutrition research task force [Internet]. [cited 2025 May 16]. https://dpcpsi.nih.gov/sites/default/files/2020NutritionStrategicPlan_508.pdf
- 50.Cassotta M, Forbes-Hernández TY, Calderón Iglesias R, Ruiz R, Elexpuru Zabaleta M, Giampieri F, et al. Links between nutrition, infectious diseases, and microbiota: emerging technologies and opportunities for human-focused research. Nutrients. 2020;12:1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.The vicious cycle of undernutrition and infectious disease. How does it work and what role do vaccines play? – VoICE [Internet]. [cited 2025 Jan 13]. https://immunizationevidence.org/the-vicious-cycle-of-undernutrition-and-infectious-disease-how-does-it-work-and-what-role-do-vaccines-play/
- 52.Motti ML, Tafuri D, Donini L, Masucci MT, De Falco V, Mazzeo F. The role of nutrients in prevention, treatment and post-coronavirus disease-2019 (COVID-19). Nutrients. 2022;14:1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roberts SB, Silver RE, Das SK, Fielding RA, Gilhooly CH, Jacques PF, et al. Healthy aging—nutrition matters: start early and screen often. Adv Nutr. 2021;12:1438–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.BP Portal [Internet]. [cited 2025 Jan 13]. https://webgate.ec.europa.eu/dyna/bp-portal/submission
- 55.Tucker KL. Osteoporosis prevention and nutrition. Curr Osteoporos Rep. 2009;7:111–7. [DOI] [PubMed] [Google Scholar]
- 56.Rizzoli R, Chevalley T. Nutrition and osteoporosis prevention. Curr Osteoporos Rep. 2024;22:515–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pinheiro MB, Oliveira J, Bauman A, Fairhall N, Kwok W, Sherrington C. Evidence on physical activity and osteoporosis prevention for people aged 65 + years: a systematic review to inform the WHO guidelines on physical activity and sedentary behaviour. Int J Behav Nutr Phys Activity. 2020;17:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.amr-factsheet. pdf [Internet]. [cited 2025 Jan 13]. https://www.who.int/docs/default-source/antimicrobial-resistance/amr-factsheet.pdf
- 59.Breaking the deadlock. to develop new antibiotics to fight antimicrobial resistance | BEUC [Internet]. [cited 2025 Jan 13]. https://www.beuc.eu/news/breaking-deadlock-develop-new-antibiotics-fight-antimicrobial-resistance
- 60.Ramadan Q, Hazaymeh R, Zourob M. Immunity-on-a-chip: integration of immune components into the scheme of organ-on-a-chip systems. Adv Biol (Weinh). 2023;7:e2200312. [DOI] [PubMed] [Google Scholar]
- 61.Keuper-Navis M, Walles M, Poller B, Myszczyszyn A, van der Made TK, Donkers J, et al. The application of organ-on-chip models for the prediction of human pharmacokinetic profiles during drug development. Pharmacol Res. 2023;195:106853. [DOI] [PubMed] [Google Scholar]
- 62.Picollet-D’hahan N, Zuchowska A, Lemeunier I, Le Gac S. Multiorgan-on-a-chip: a systemic approach to model and decipher inter-organ communication. Trends Biotechnol. 2021;39:788–810. [DOI] [PubMed] [Google Scholar]
- 63.Computer simulations move. step closer to reducing animal use in drug testing | University of Oxford [Internet]. 2018 [cited 2025 Jul 3]. https://www.ox.ac.uk/news/2018-03-12-computer-simulations-move-step-closer-reducing-animal-use-drug-testing
- 64.Supporting innovation | European Medicines Agency (EMA) [Internet]. 2020 [cited 2025 Jan 13]. https://www.ema.europa.eu/en/human-regulatory-overview/research-development/supporting-innovation
- 65.European Medicines Agency (EMA). Committee for Medicinal Products for Human Use (CHMP), Committee for Medicinal Products for Veterinary Use (CVMP). Guideline on the principles of regulatory acceptance of 3Rs (replacement, reduction, refinement) testing approaches. EMA/CHMP/CVMP/JEG-3Rs/450091/2012, editor. 2016 [cited 2025 Jan 13];https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-principles-regulatory-acceptance-3rs-replacement-reduction-refinement-testing-approaches_en.pdf
- 66.Loewa A, Feng JJ, Hedtrich S. Human disease models in drug development. Nat Rev Bioeng. 2023;1:545–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Marshall LJ, Bailey J, Cassotta M, Herrmann K, Pistollato F. Poor translatability of biomedical research using animals—a narrative review. Altern Lab Anim. 2023;51:102–35. [DOI] [PubMed] [Google Scholar]
- 68.Regulation, -. EU – 2025/327 - EN - EUR-Lex [Internet]. [cited 2025 Apr 3]. https://eur-lex.europa.eu/eli/reg/2025/327/oj/eng
- 69.ALURES statistics - Sect. 2. : Uses of animals for research, testing, routine production and education and training purposes - European Commission [Internet]. Environment Alures statistics. [cited 2025 Jan 13]. https://webgate.ec.europa.eu/envdataportal/content/alures/section2_number-of-uses.html
- 70.Seifirad S, Haghpanah V. Inappropriate modeling of chronic and complex disorders: how to reconsider the approach in the context of predictive, preventive and personalized medicine, and translational medicine. EPMA J. 2019;10:195–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.European Medicines Agency. Concept paper on the revision of the Guideline on the principles of regulatory acceptance of 3Rs (replacement, reduction, refinement) testing approaches (EMA/CHMP/CVMP/JEG-3Rs/450091/2012) [Internet]. [cited 2025 Jan 17]. https://assets-us-01.kc-usercontent.com/49d837e7-4c3e-0071-59f0-cb4344fbf121/ba67af0c-9723-4e2e-b025-c21d924e9c10/concept-paper-revision-guideline-principles-regulatory-acceptance-3rs-replacement-7-reduction_en.pdf
- 72.Text -. H.R.7248–118th Congress (2023–2024): FDA Modernization Act 3.0 | Congress.gov | Library of Congress [Internet]. [cited 2025 Jan 17]. https://www.congress.gov/bill/118th-congress/house-bill/7248/text
- 73.FDA. Roadmap to reducing animal testing in preclinical safety studies [Internet]. https://www.fda.gov/media/186092/download?attachment
- 74.Vanhooren V, Libert C. The mouse as a model organism in aging research: usefulness, pitfalls and possibilities. Ageing Res Rev. 2013;12:8–21. [DOI] [PubMed] [Google Scholar]
- 75.Santulli G, Borras C, Bousquet J, Calzà L, Cano A, Illario M, et al. Models for preclinical studies in aging-related disorders: one is not for all. Transl Med UniSa. 2016;13:4–12. [PMC free article] [PubMed] [Google Scholar]
- 76.Hollander CF. Pathophysiology of ageing in animal models. In: Crooks J, Stevenson IH, editors. Drugs and the elderly: perspectives in geriatric clinical pharmacology [Internet]. London: Palgrave Macmillan; 1979. pp. 15–22. 10.1007/978-1-349-03813-8_2 [Google Scholar]
- 77.Kubinyi E. Biologia Futura: four questions about ageing and the future of relevant animal models. Biol Futura. 2022;73:385–91. [DOI] [PubMed] [Google Scholar]
- 78.Bitar M, Barry G. Multiple innovations in genetic and epigenetic mechanisms cooperate to underpin human brain evolution. Mol Biol Evol. 2018;35:263–8. [DOI] [PubMed] [Google Scholar]
- 79.Pitrez PR, Monteiro LM, Borgogno O, Nissan X, Mertens J, Ferreira L. Cellular reprogramming as a tool to model human aging in a dish. Nat Commun. 2024;15:1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jothi D, Kulka LAM. Strategies for modeling aging and age-related diseases. Npj Aging. 2024;10:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sen T, Thummer RP. CRISPR and iPSCs: recent developments and future perspectives in neurodegenerative disease modelling, research, and therapeutics. Neurotox Res. 2022;40:1597–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vera E, Bosco N, Studer L. Generating late-onset human iPSC-based disease models by inducing neuronal age-related phenotypes through telomerase manipulation. Cell Rep. 2016;17:1184–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Harley J, Santosa MM, Ng CY, Grinchuk OV, Hor J-H, Liang Y, et al. Telomere shortening induces aging-associated phenotypes in hiPSC-derived neurons and astrocytes. Biogerontology. 2024;25:341–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Oyefeso FA, Goldberg G, Opoku NYPS, Vazquez M, Bertucci A, Chen Z, et al. Effects of acute low-moderate dose ionizing radiation to human brain organoids. PLoS ONE. 2023;18:e0282958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jung M, Hartmann C, Ehrhardt T, Peter L-M, Abid CL, Harwardt B, et al. Generation of a set of induced pluripotent stem cell lines from two alzheimer disease patients carrying APOE4 (MLUi007-J; MLUi008-A) and healthy old donors carrying APOE3 (MLUi009-A; MLUi010-B) to study APOE in aging and disease. Stem Cell Res. 2023;69:103072. [DOI] [PubMed] [Google Scholar]
- 86.Fiore NJ, Ganat YM, Devkota K, Batorsky R, Lei M, Lee K, et al. Bioengineered models of Parkinson’s disease using patient-derived dopaminergic neurons exhibit distinct biological profiles in a 3D microenvironment. Cell Mol Life Sci. 2022;79:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Devine MJ, Ryten M, Vodicka P, Thomson AJ, Burdon T, Houlden H, et al. Parkinson’s disease induced pluripotent stem cells with triplication of the α-synuclein locus. Nat Commun. 2011;2:440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Stefanicka-Wojtas D, Kurpas D. Personalised medicine—implementation to the healthcare system in Europe (focus group discussions). J Pers Med. 2023;13:380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Palasantzas VEJM, Tamargo-Rubio I, Le K, Slager J, Wijmenga C, Jonkers IH, et al. iPSC-derived organ-on-a-chip models for personalized human genetics and pharmacogenomics studies. Trends Genet. 2023;39:268–84. [DOI] [PubMed] [Google Scholar]
- 90.Ingber DE. Human organs-on-chips for disease modelling, drug development and personalized medicine. Nat Rev Genet. 2022;23:467–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Taherdoost H, Ghofrani A. AI’s role in revolutionizing personalized medicine by reshaping pharmacogenomics and drug therapy. Intell Pharm. 2024;2:643–50. [Google Scholar]
- 92.Preclinical Rodent Models for Human Bone Disease. Including a Focus on Cortical Bone | Endocrine Reviews | Oxford Academic [Internet]. [cited 2025 Jan 13]. https://academic.oup.com/edrv/article/45/4/493/7601054 [DOI] [PMC free article] [PubMed]
- 93.Booton RD, Meeyai A, Alhusein N, Buller H, Feil E, Lambert H, et al. One health drivers of antibacterial resistance: quantifying the relative impacts of human, animal and environmental use and transmission. One Health. 2021;12:100220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Allel K, Day L, Hamilton A, Lin L, Furuya-Kanamori L, Moore CE, et al. Global antimicrobial-resistance drivers: an ecological country-level study at the human–animal interface. Lancet Planet Health. 2023;7:e291–303. [DOI] [PubMed] [Google Scholar]
- 95.Huang J, Zhang L, Lu A, Liang C. Organoids as innovative models for bone and joint diseases. Cells. 2023;12:1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kong Y, Yang Y, Hou Y, Wang Y, Li W, Song Y. Advance in the application of organoids in bone diseases. Front Cell Dev Biol [Internet]. 2024 [cited 2025 Jan 13];12. https://www.frontiersin.org/journals/cell-and-developmental-biology/articles/10.3389/fcell.2024.1459891/full [DOI] [PMC free article] [PubMed]
- 97.Goldsteen PA, Yoseif C, Dolga AM, Gosens R. Human pluripotent stem cells for the modelling and treatment of respiratory diseases. Eur Respir Rev [Internet]. 2021 [cited 2025 Jan 13];30. https://publications.ersnet.org/content/errev/30/161/210042 [DOI] [PMC free article] [PubMed]
- 98.Dagher R, Moldobaeva A, Gubbins E, Clark S, Madel Alfajaro M, Wilen CB, et al. Human iPSC-Based model of COPD to investigate disease mechanisms, predict SARS-COV-2 outcome, and test preventive immunotherapy. Stem Cells. 2024;42:230–50. [DOI] [PubMed] [Google Scholar]
- 99.Home - PROPHET [Internet]. [cited 2024 Jan 16]. https://prophetproject.eu/
- 100.Maio A, Farina S, Osti T, Di Grande S, Pastorino R, Boccia S.Scanning the horizon of personalized prevention research: an overview of ongoing Europeanfunded initiatives. Front Public Health. 2025;13:1561328. 10.3389/fpubh.2025.1561328. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset supporting the conclusions of this article is included within the article, its supplementrary file, and the xls file ‘FP7-H2020-HE_Prevention Projects_EXTENDED DATA SET’.