Abstract
Background
Chronic ankle instability (CAI) is associated with impaired balance, proprioception, and limited dorsiflexion range of motion (ROM). Whole-body vibration (WBV) and blood flow restriction (BFR) may address these deficits. This study aimed to determine whether adding BFR to WBV would produce greater immediate improvements in these parameters compared to WBV alone.
Methods
In this double-blind, randomized controlled trial, thirty-eight non-professional male athletes with CAI were randomly assigned to WBV (n = 19; age: 23.5 ± 4.7 years) or WBV + BFR group (n = 19; age: 24.1 ± 4.9 years). Both groups received a single session of WBV at 30 Hz and 2 mm amplitude; the WBV + BFR group additionally underwent BFR at 80% arterial occlusion pressure. Balance error scoring system (BESS), modified star excursion balance test (m-SEBT), ankle dorsiflexion proprioception, side hop test, dorsiflexion ROM, and perceived instability were measured before and after the intervention.
Results
Statistical tests showed no significant changes in BESS scores (p > 0.05). The WBV + BFR group showed a significant improvement in anterior reach of the m-SEBT (p = 0.04), while the WBV-only group did not (p = 0.69). Statistical analysis indicated a significant reduction in dorsiflexion repositioning error in the WBV group (p = 0.004), with between-group differences favoring WBV. Perceived instability significantly improved in the combined group (p = 0.001). Both groups showed improved dorsiflexion ROM (p < 0.05), with no significant difference between them (p = 0.76).
Conclusion
Both WBV and WBV + BFR improved dorsiflexion ROM in athletes with CAI, but neither produced immediate gains in static or dynamic balance, except for anterior reach with the combined intervention. Adding one session of BFR to WBV appeared to impair proprioception and increase perceived instability. These methods may be better used as adjuncts in rehab, not standalone treatments. Clinically, they should be applied cautiously and not immediately before activities demanding high joint stability.
Clinical trial registration
Registered at IRCT (IRCT20230203057314N1; June 2, 2023).
Supplementary Information
The online version contains supplementary material available at 10.1186/s13102-025-01309-w.
Keywords: Chronic ankle instability, Blood flow restriction, Whole body vibration, Balance, Proprioception
Introduction
Lateral ankle sprains are common in both athletes and the general population, with community surveys reporting an incidence of 19.0 to 26.6 per 1000 person-years [1] and accounting for 25–30% of sports injuries [2]. While data specific to non-professional athletes are limited, their injury patterns likely reflect both groups. Approximately 40% of individuals who experience an initial lateral ankle sprain develop chronic ankle instability (CAI) [3], characterized by recurrent episodes of the ankle giving way and persistent symptoms such as pain, swelling, weakness, instability, recurrent sprains, and functional limitations lasting for at least one year [4]. Non-professional athletes with these injuries often experience delayed care, increasing complications and prolonging recovery. In this group, CAI can significantly impair daily activities and sports performance, emphasizing the need for timely care [5].
CAI disrupts proprioceptive feedback by damaging mechanoreceptors, leading to impaired joint position sense, increased functional instability, and a heightened risk of re-injury [6]. This condition frequently causes muscle inhibition, which can persist for up to six months, further elevating the likelihood of recurrent sprains [7, 8]. Management strategies for CAI typically involve non-invasive rehabilitation programs targeting range of motion (ROM), strength, proprioception, and neuromuscular control [9]. While resistance and functional training are commonly employed to address these muscle deficits, neurological impairments may limit their effectiveness [10]. Given these persistent symptoms, effective management strategies are essential to address both functional deficits and re-injury risks.
In this regard, blood flow restriction (BFR) training, which involves applying a cuff to partially restrict blood flow during exercise, has become a novel approach for managing CAI [11]. This method enhances muscle strength and hypertrophy, simulating the effects of high-load resistance training [12]. BFR training also induces anabolic responses and optimizes muscle adaptation during low-load exercises by altering neuromuscular recruitment patterns. This is achieved through mechanisms such as muscle hypoxia and metabolic stress, which increase motor unit recruitment even with low-intensity exercises [13]. Additionally, BFR training is effective in mitigating muscle atrophy during immobilization and promoting recovery following surgery [11].
On the other hand, whole-body vibration (WBV) therapy has been proposed as an effective intervention for CAI. WBV involves exercising on a platform that oscillates at specific frequencies and amplitudes, stimulating neuromuscular and skeletal systems simultaneously [14]. This therapy has been shown to improve muscle strength, flexibility, and balance, making it a promising tool for ankle injury rehabilitation [15]. Specifically, WBV has been demonstrated to enhance joint position sense, proprioception, and balance in individuals with CAI by providing strong sensory stimulation and activating alpha motor neurons [16].
Traditional CAI interventions focus on balance and proprioception exercises [17], but WBV may provide superior proprioceptive feedback to improve balance and ankle protection [14]. Given the neurological deficits often unaddressed by traditional methods [10], integrating BFR, which promotes muscle adaptation, could improve outcomes. Previous research has shown the combined effects of WBV and BFR on muscle mass, endurance, and strength in healthy individuals, with promising results [18]. Since CAI involves both proprioceptive and neuromuscular impairments, combining WBV’s sensory stimulation with BFR’s muscle adaptation mechanisms offers a unique approach to addressing these deficits. WBV improves joint position sense and balance, while BFR increases strength through hypoxic stress, complementing traditional CAI rehabilitation. Additionally, both interventions can immediately improve neuromuscular and metabolic functions [19, 20], which may benefit balance and proprioception. Despite growing interest in BFR and WBV as rehabilitation modalities, the immediate effects of their combined application on balance, proprioception, and ankle mobility in athletes with CAI remain unclear. Investigating these acute responses is crucial because early changes can inform clinicians about the safety, effectiveness, and potential mechanisms underlying these interventions before prescribing them in full rehabilitation programs. Understanding the immediate effects also helps optimize treatment strategies aimed at improving joint stability and functional performance in this population. This study aimed to evaluate the immediate effects of combining WBV and BFR on balance, proprioception, functional performance, dorsiflexion ROM, and perceived instability in non-professional male athletes with CAI.
Methods
Participants
The study included non-professional male athletes aged 18–35 years with CAI. Participants were recruited using convenience sampling through advertisements in gyms, universities, local physiotherapy clinics, and on social media. They were randomly assigned to either the WBV + BFR group or the WBV-only group using a sealed envelope method. Group allocation was blinded to participants, and the assessor (Physiotherapist) was also blinded to group assignment.
Sample size estimation was performed using G*Power (version 3.1.9.2) for a repeated measures ANOVA (within-between interaction). Using an effect size of 0.45 from Werasirirat et al. (2022) [21], with α = 0.05 and power = 0.95, the analysis indicated a minimum requirement of 20 participants (λ = 16.20, F = 4.41, df = 1,18). To increase detection power and account for possible dropouts, we recruited 38 participants, 19 in each group. Inclusion criteria comprised non-professional male athletes who had engaged in physical activity at least twice per week over the past six months. Participants were interviewed to verify the absence of any history of professional sports participation. Additional criteria included a history of at least one ankle sprain occurring at least one year prior, causing temporary functional limitations, a score of ≤ 24 on the Cumberland Ankle Instability Tool, and recurrent ankle sprains or instability [4]. Exclusion criteria were: Acute injuries, fractures, or lower limb surgeries within the past three months, medical conditions affecting balance (e.g., vestibular, visual, or auditory impairments), cardiovascular conditions, hypertension, blood clotting disorders, diabetes, internal prostheses, acute disc herniation, joint inflammation, recent strokes, epilepsy, or malignancies, as well as unwillingness to continue the intervention.
Procedures
This study was a parallel, double-arm, and double-blind randomized controlled trial, conducted at the physiotherapy clinic of the School of Rehabilitation, Tehran University of Medical Sciences (TUMS). The study adhered to the Consolidated Standards of Reporting Trials (CONSORT) guidelines for reporting clinical trials. The research was registered in the Iranian Registry of Clinical Trials (IRCT20230203057314N1) on June 6, 2023, under the approval ID IR.TUMS.FNM.REC.1401.162 from the Ethics Committee of TUMS.
A total of 163 participants were assessed for eligibility (Fig. 1). Among the 111 eligible participants, 73 declined to participate in the intervention for various reasons. The remaining 38 participants were randomly allocated to either the WBV group (n = 19) or the WBV + BFR group (n = 19). After being informed about the research objectives and procedures, participants voluntarily signed a written informed consent form. They were instructed to consume their last meal at least one hour prior to the intervention and to wear appropriate sports shoes and clothing. Athletes performed a standardized warm-up targeting lower limb muscle groups (including the plantar flexors, knee flexors, hip extensors, adductors, and knee extensors) through static stretching. Each stretch was held for 10 s per muscle group, with a maximum 10-second rest between stretches [22]. Following this warm-up, baseline assessments were conducted. Based on group allocation, participants underwent one of the assigned interventions. Immediately after completing the intervention, all assessments were repeated under conditions identical to the initial evaluation.
Fig. 1.
CONSORT flow diagram
Interventions
Whole-body vibration
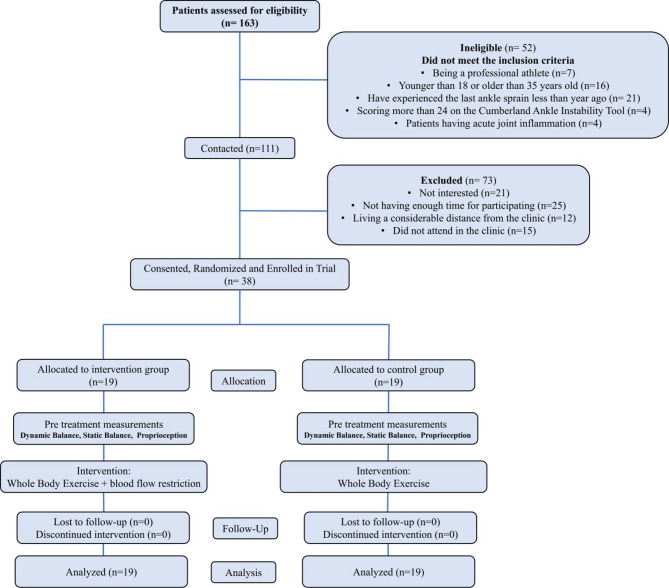
Participants were first informed about the intervention procedure and the proper positioning on the WBV device. While wearing a zero-pressure BFR cuff (Yelsa model, Arsam Farahosh Electromechanical Company, Iran), participants were positioned barefoot on the WBV platform (POWER PLATE, Next Generation, USA). They were instructed to alert the therapist immediately if they experienced pain, discomfort, or unpleasant sensations during the intervention to allow for immediate cessation. The WBV device was set to a frequency of 30 Hz and an amplitude of 2 mm. While on the platform, participants adopted a squat position with 40-degree knee flexion and maintained this position for one minute while receiving vibration. This protocol was repeated six times per participant, Between repetitions, participants rested for two minutes [15] (Fig. 2). It should be noted that for the control group receiving WBV, the BFR cuff was wrapped around the proximal thigh in the same manner as the group receiving real BFR, but without inflation.
Fig. 2.
Right: setup of blood flow restriction along with pulse oximeter. Left: participant receiving whole-body vibration in a squat position with blood flow restriction applied via the corresponding cuff. Socks were used in the figure to distinguish the healthy limb from the involved limb
Whole-body vibration along with blood flow restriction
This group underwent a WBV intervention similar to the WBV group along with BFR intervention. For blood flow restriction, we used the Yelsay model BFR device (Farahoush Electromechanic Arsam, Iran). Participants lay supine with legs extended, and a cuff measuring 10 cm in width and 75 cm in length was placed on the proximal thigh of the affected leg. Arterial occlusion pressure (AOP) was measured with a pulse oximeter, following an established procedure with acceptable agreement compared to Doppler ultrasound [23]. The big toe was cleaned with alcohol before being placed in the pulse oximeter, which was activated to track changes in blood volume. The cuff pressure was steadily increased in steps of 10 mmHg until the pulse oximeter showed no more periodic blood volume changes, indicating complete arterial occlusion. The pressure was then reduced to 80% of this AOP and maintained throughout the intervention. Next, the cuff tubes were released, and the pulse oximeter was removed to prevent disruption during treatment. The BFR protocol was applied intermittently: the cuff was inflated during the vibration periods (about 1 min) and deflated during the rest intervals (about 2 min). This intermittent approach ensured adequate tissue blood flow and minimized the risk of prolonged ischemia. The total occlusion time per session was kept between 5 and 10 min to preserve tissue safety.
Throughout the intervention, the cuff remained firmly positioned on the upper thigh to maintain consistent pressure. The inflation and deflation cycles were carefully timed to coincide with the whole-body vibration sessions, ensuring a reliable and repeatable application of BFR [24, 25]. (Fig. 2).
Outcome measures
Static and dynamic balance, along with ankle proprioception, were assessed at baseline and immediately post-intervention as follows:
Dynamic balance
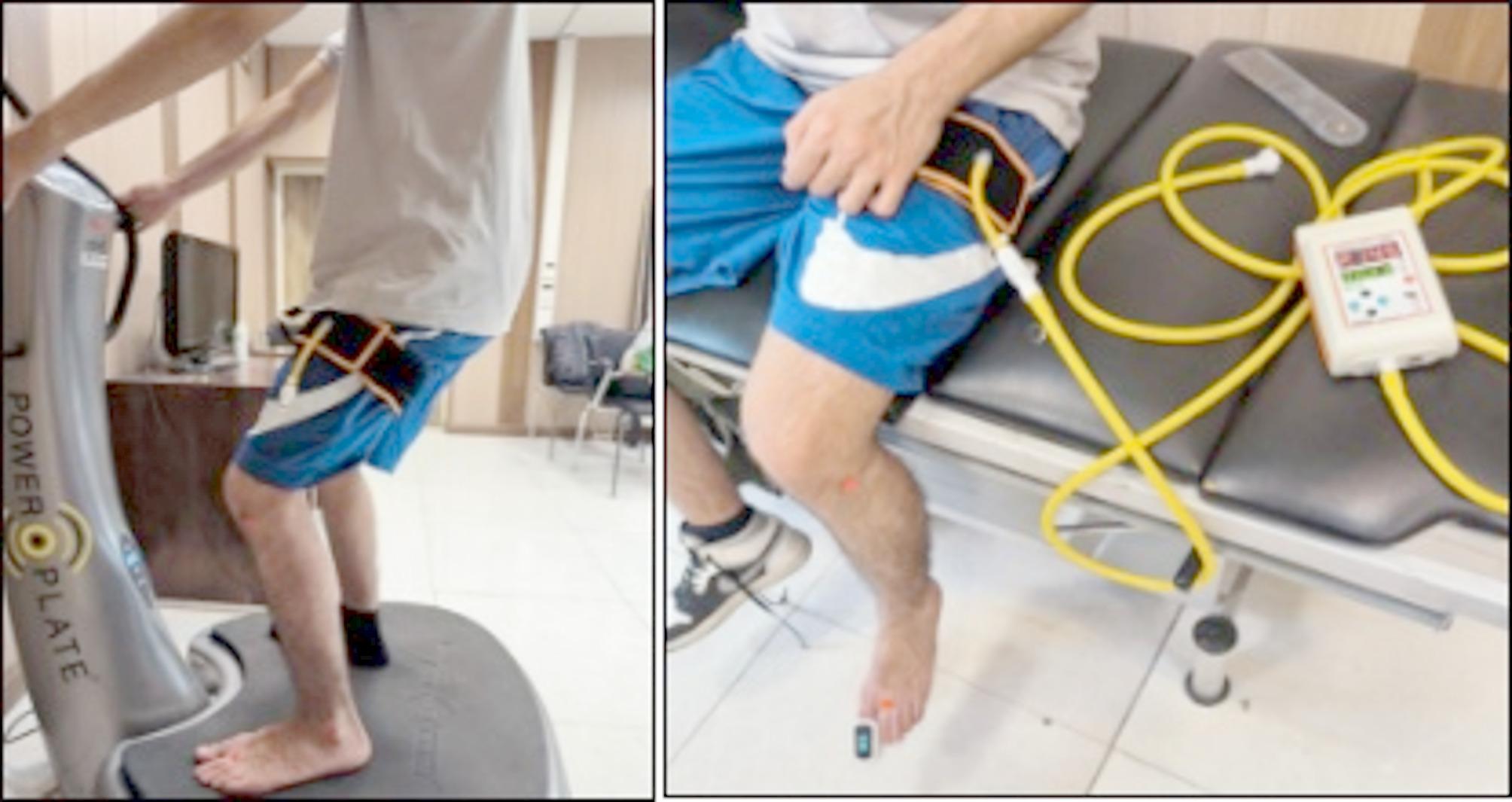
Dynamic balance was measured using the modified Star Excursion Balance Test (m-SEBT) [17]. Participants placed the injured foot at the center of a 120 cm-diameter circle, with three marked directions: anterior, posteromedial, and posterolateral. The angles between the anterior and each posterior direction was 135° and between the two posterior directions were 90°. Participants, instructed to look straight ahead, stood on the injured foot and reached with the opposite foot to the farthest point along the marked lines. The distance reached was measured in centimeters from the circle’s center (Fig. 3). Each participant performed three trials in each direction. The average of these distances was calculated and normalized by dividing the reach distance by the lower limb length (measured from the anterior superior iliac spine to the medial malleolus) and multiplying by 100 for adjustment [26].
Fig. 3.
Leftmost three images: dynamic balance assessment using the modified Star Excursion Balance Test. Rightmost six images: static balance assessment using the Balance Error Scoring System. Socks were used in the figure to distinguish the healthy limb from the involved limb
Static balance
Static balance was assessed using the Balance Error Scoring System (BESS) [27]. This test evaluates performance in three stances: double-leg stance (standing with feet together and hands on hips), single-leg stance (standing on the non-dominant foot with hands on hips), and tandem stance (standing heel-to-toe with the non-dominant foot behind the dominant foot). Each stance was performed twice—once on a firm surface and once on a foam surface—with eyes closed.
Errors were recorded during each 20-second trial and included actions such as opening eyes, lifting hands off hips, stepping out of position, falling, lifting the forefoot or heel, excessive hip abduction (> 30°), or failing to return to the starting position within 5 s (Fig. 3). Participants performed 1–3 practice trials before completing three main trials, and the average error score across these trials was used for analysis [27].
Proprioception
Proprioception of the injured foot was assessed by measuring joint repositioning errors for dorsiflexion [28]. Participants sat with their trunk, thigh, and lower leg at 90-degree angles, and their feet off the ground to eliminate tactile feedback. A black blindfold was used to avoid visual interference.
For the dorsiflexion test, the examiner passively moved the ankle to 10 degrees, instructing the participant to actively reproduce the target angle. Three markers were placed on the lateral malleolus, lateral tibial condyle, and second metatarsal head, and movements were recorded with a camera. The recorded images were analyzed using Kinovea software (version 2023.1.2), measuring the reconstructed ankle angle by connecting marker centers (Fig. 4). The procedure was repeated three times. The average of the three trials was used as the joint repositioning error. The absolute error (the magnitude of the difference irrespective of direction) was determined using the following formula:
(1) Absolute error=
.
Fig. 4.
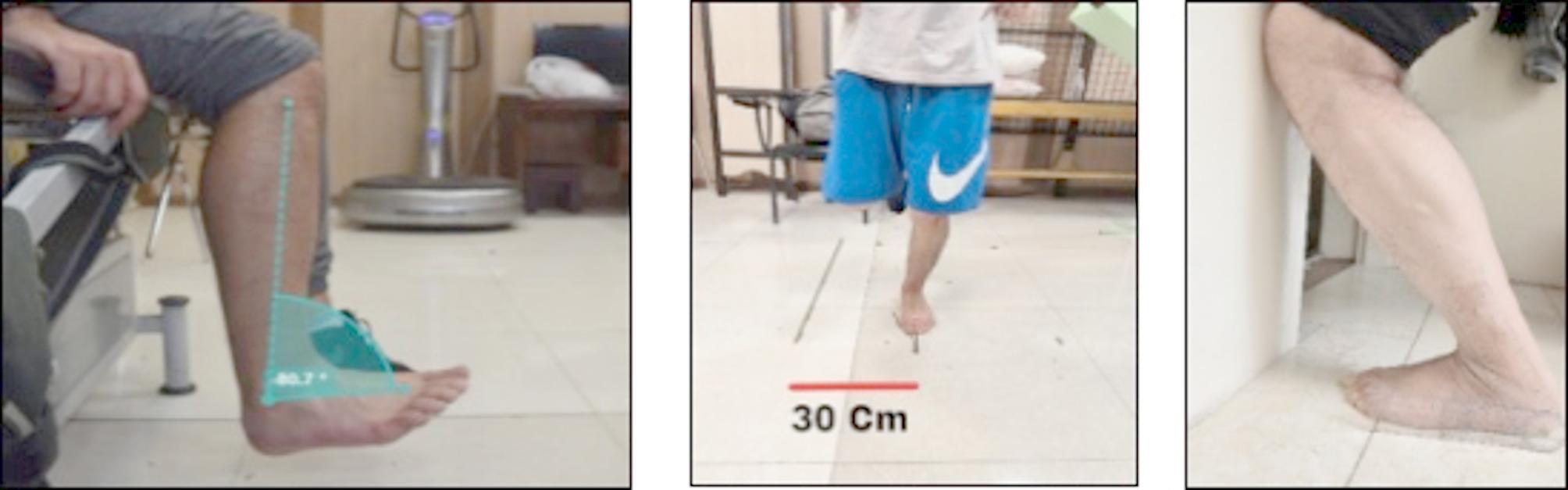
Left: Participant constructing the target dorsiflexion angle using Kinovea software with markers on the lateral malleolus, lateral tibial condyle, and second metatarsal head. Middle: Side hop test. Right: Active dorsiflexion angle measurement
Where X represents the target angle, C denotes the estimated angle, and K indicates the number of repetitions.
Side hop test
The side hop test was performed by hopping laterally on the injured limb between two lines spaced 30 cm apart (Fig. 4). One repetition was defined as successfully hopping laterally and returning to the starting position. Participants were required to complete 10 repetitions as quickly as possible. The test was considered invalid if the opposite foot touched the ground or if the 30 cm distance was not maintained. An electronic stopwatch was used to record the fastest time. Participants performed 1 to 3 practice trials, followed by 3 main trials, and the average time of the three main trials was used for statistical analysis [29].
Perceived instability
Participants were asked to indicate their perceived ankle instability during the side hop test by marking a 10 cm vertical line with no numbers. They were asked, “How unstable did your ankle feel during the test?” Higher marks on the scale represented greater perceived instability during the test. The perceived instability level was then recorded by placing a ruler along the 10 cm line. This data was collected three times, following each repetition of the side hop test [30].
Active dorsiflexion range of motion measurement
Dorsiflexion ROM was assessed using the weight-bearing lunge test against a wall (Fig. 4). Participants stood with their heel on the ground, the big toe and second toe positioned 10 cm from the wall, and balanced themselves by lightly touching the wall with two fingers from each hand. They were instructed to lunge forward, guiding their knee toward the wall in line with the second toe until it touched the wall. If the knee could not reach the wall without lifting the heel, the participant moved the foot closer to the wall in 1 cm increments and repeated the lunge until the knee could touch the wall while keeping the heel grounded. The farthest distance between the big toe and the wall, where the knee maintained contact without lifting the heel, was recorded as the maximum dorsiflexion range. A standard goniometer was used to measure the angle, with the stationary arm aligned parallel to the ground and the movable arm along the fibula. Participants performed three practice trials followed by three main trials, and the average of the main trials was used for data analysis [31].
Statistical analysis
Statistical analysis was conducted using SPSS version 27 with a significance level of p ≤ 0.05. Data normality was assessed using the Shapiro-Wilk test. Independent samples t-tests were used for between-group comparisons, considering mean improvements after the intervention for each group, while paired t-tests analyzed within-group changes for normally distributed data. For non-normal data, the Mann-Whitney test was used for between-group comparisons, and the Wilcoxon test for within-group comparisons. Effect sizes were calculated using Cohen’s d and classified as negligible (< 0.20), small (0.20–0.49), medium (0.50–0.79), or large (≥ 0.80) [32].
Results
The Shapiro-Wilk test showed that all variables followed a normal distribution (p > 0.05), except for the absolute error in dorsiflexion. Baseline characteristics are presented in Table 1.
Table 1.
Participants characteristics
| Variables | WBV (n = 19) | WBV + BFR (n = 19) | P value |
|---|---|---|---|
| Age (year) | 23.47 ± 4.70 | 24.05 ± 4.88 | 0.71 |
| Height (cm) | 178.5 ± 3.76 | 179 ± 6.03 | 0.82 |
| Weight (kg) | 73.63 ± 8.01 | 73.05 ± 9.49 | 0.82 |
| BMI (kg/m2) | 22.75 ± 1.46 | 23.02 ± 1.66 | 0.57 |
| Lifetime recurrence rates (median) | 3 | 3 | 1.00 |
Abbreviation: body mass index. Data are expressed as means ± standard deviation
The characteristics of participants in the WBV and WBV with BFR groups are shown in Table 1. None of the characteristics were significantly different between the groups. Also, the groups were similar in terms of their recurrence rates before the intervention.
Static and dynamic balance
For the BESS scores measuring static balance, the WBV group showed a slight increase from 12.31 ± 4.06 before the intervention to 12.84 ± 2.75 after the intervention; however, this change was not statistically significant (p = 0.41). Similarly, the combined group experienced a small increase from 11.00 ± 4.1 to 11.7 ± 2.97, which also did not reach statistical significance (p = 0.16). Between-group comparison showed no statistically significant difference (p = 0.75).
The results of the m-SEBT test for dynamic balance are presented in Table 2. The combined group was the only group to demonstrate a statistically significant improvement in anterior reach distance (p = 0.04, Cohen’s d = 0.51). No statistically significant changes were observed for posteromedial and posterolateral reach distances within or between groups. Although the BFR + WBV group showed an increase in composite reach distance scores, this change was not statistically significant (p > 0.05).
Table 2.
Whitin- and between-group comparison of dynamic balance performance (n = 19 per group)
| Reach distance | Group | Pre-intervention | Post-intervention | Within-group | Between-group | ||
|---|---|---|---|---|---|---|---|
| Sig. | Cohen’s d | Sig. | Cohen’s d | ||||
| Anterior (%) | WBV | 69.45 ± 5.06 | 69.65 ± 5.32 | 0.69 | 0.09 | 0.35 | 0.30 |
| BFR + WBV | 69.18 ± 5.27 | 69.95 ± 5.14 | 0.04 | 0.51 | |||
| Posteromedial (%) | WBV | 100.14 ± 4.57 | 100.22 ± 5.17 | 0.82 | 0.05 | 0.81 | 0.07 |
| BFR + WBV | 100.72 ± 3.78 | 100.68 ± 4.01 | 0.91 | 0.25 | |||
| Posterolateral (%) | WBV | 97.16 ± 5.04 | 97.42 ± 5.49 | 0.37 | 0.21 | 0.34 | 0.31 |
| BFR + WBV | 98.54 ± 4.13 | 98.40 ± 3.95 | 0.64 | 0.11 | |||
| Composite score (%) | WBV | 88.92 ± 4.63 | 89.10 ± 4.91 | 0.43 | 0.18 | 0.96 | 0.02 |
| BFR + WBV | 89.48 ± 3.48 | 89.68 ± 3.99 | 0.48 | 0.17 | |||
Abbreviations: WBV, whole body vibration; BFR, blood flow restriction. Values in bold indicate statistically significant differences (p < 0.05). Data are expressed as means ± standard deviation
Side hop test, perceived instability, dorsiflexion range of motion, and proprioception
In the side hop test, no statistically significant improvements were observed within or between groups. Perceived instability significantly increased in the BFR + WBV group (p = 0.001, Cohen’s d = 0.87), with a significant between-group difference favoring this group (p = 0.01, Cohen’s d = 0.87). Both groups showed statistically significant improvements in dorsiflexion ROM (WBV: p = 0.013, Cohen’s d = 0.63; BFR + WBV: p = 0.028, Cohen’s d = 0.55), but there were no significant differences between groups (p = 0.76). The WBV group showed a reduction in joint position sense error from pre- to post-intervention, though this change did not reach statistical significance (p = 0.08, Cohen’s d = 0.42). Conversely, the BFR + WBV group showed a statistically significant increase in error (p = 0.02, Cohen’s d = 0.60). Between groups, the WBV group demonstrated a significantly greater improvement compared to the BFR + WBV group (p = 0.004, Cohen’s d = 1.00), indicating that WBV alone may be more effective in improving joint position sense (Table 3).
Table 3.
Whitin- and between-group comparison of (n = 19 per group)
| Variable | Group | Pre-intervention | Post-intervention | Within-group | Between-group | |||
|---|---|---|---|---|---|---|---|---|
| Sig. | Cohen’s d | Sig. | Cohen’s d | |||||
| Side hop test (second) | WBV | 10.84 | 10.90 | 0.70 | 0.09 | 0.99 | 0.002 | |
| BFR + WBV | 10.74 | 10.80 | 0.59 | 0.12 | ||||
| Perceived instability | WBV | 3.89 | 3.90 | 0.97 | 0.007 | 0.01 | 0.874 | |
| BFR + WBV | 3.67 | 4.10 | 0.001 | 0.87 | ||||
| Dorsiflexion ROM (deg) | WBV | 40.66 | 41.10 | 0.013 | 0.63 | 0.76 | 0.100 | |
| BFR + WBV | 40.42 | 40.78 | 0.028 | 0.55 | ||||
| Joint repositioning error (deg) | WBV | 3.34 ± 1.86 | 2.54 ± 1.52 | 0.08 | 0.42 | 0.004 | 1.00 | |
| BFR + WBV | 3.25 ± 2.31 | 4.24 ± 2.09 | 0.02 | 0.60 | ||||
Abbreviations: WBV, whole body vibration; BFR, blood flow restriction; ROM, range of motion. Values in bold indicate statistically significant differences (p < 0.05)
Discussion
This study aimed to evaluate the immediate effects of combining BFR with WBV on balance, ankle dorsi flexion ROM and proprioception in non-professional athletes with CAI. The findings showed significant changes in some measures both in WBV and WBV combined with BFR. However, the changes were not consistently similar between the groups.
Balance performance
Static balance, measured by the BESS, did not improve significantly in either group. Collectively, this indicates that static balance may be less sensitive to acute vibratory stimulation. Also, the similar results in both groups show that adding BFR does not seem to increase the effect of WBV on static balance in people with CAI.
To our knowledge, no previous study has examined the effects of combining WBV with BFR on static balance in people with CAI. However, some relatively relevant studies. For example, Karim et al. (2019) found that 75 s of WBV at 30 Hz improved static balance in healthy dancers [33]. Their results differ from ours, likely because they studied healthy people and used triplanar vibration, while we studied individuals with CAI and used vertical vibration. These differences in participants and vibration type may affect how the body responds to WBV. Similarly, Rendos et al. (2017) found no immediate improvement in static balance after WBV. They used 30 Hz vertical vibration with high amplitude. Balance was measured by center of pressure during single-leg stance. The findings were consistent in both recreational athletes and individuals with CAI [16]. Additionally, Pollock et al. [34]. examined the acute effects of WBV at 30 Hz using both high and low amplitudes with rotational vibrations. They found no significant changes in static balance, assessed by center of mass behavior during both double-leg and single-leg stances [34].
The limited improvement in our study may be due to the nature of static balance tasks. These tasks rely heavily on proprioception, muscle stiffness, and neuromuscular coordination. Short-term WBV, even when combined with BFR, might not be enough to produce noticeable changes in these systems. Also, the BESS test may not be sensitive enough to detect small changes in neuromuscular function after just one session of WBV.
On the other hand, dynamic balance results measured by the m-SEBT showed a significant increase in anterior reach distance within the BFR + WBV group, while the between-group difference was not statistically significant. Many studies investigating the effects of BFR on dynamic balance in individuals with CAI have primarily examined its use alongside rehabilitation exercises, rather than as a standalone intervention. We found only one study by Mahmoud et al. (2023) reporting that BFR alone did not improve dynamic balance in women with CAI, which aligns with our findings [35]. However, Clark et al. (2024) investigated whether adding BFR to dynamic balance exercises might impair balance in individuals with CAI due to fatigue effects [36]. They reported a decrease in the SEBT composite score when BFR was applied. In our study, scores of the m-SEBT test significantly improved only in the anterior direction. Unlike our study, Clark et al. used BFR during balance exercises similar to the SEBT, which may have made the effects of fatigue stronger. This shows that combining BFR with WBV may help reduce the negative effects of BFR on balance. This could be relevant for actions like walking, jogging, or certain types of landing mechanics often performed by athletes with CAI. Finally, it should be noted that this study used just a single intervention session. Repeated exposure to the interventions might improve balance in these patients [37].
Proprioception
We assessed proprioception using dorsiflexion because reduced dorsiflexion is linked to a higher risk of re-injury in people with CAI, playing an important role in daily and sports activities [38]. Measuring other angles like plantar flexion or using multiple dorsiflexion positions would have made the test longer and possibly caused fatigue, which could affect the immediate results. Regarding ankle proprioception, the results were more distinct. The absolute repositioning error for dorsiflexion improved slightly after WBV, but this change was only marginally significant. In contrast, the combined WBV with BFR significantly increased the error. This led to a significant between-group difference with a large effect size (Cohen’s d = 1.00). This suggests that WBV may increase the accuracy of detecting and correcting joint position errors during movement, possibly by influencing sensory receptors in the muscles and joint capsules. The improvement may be due to WBV’s ability to activate muscles, thereby increasing the sensitivity of the nerves controlling joint position.
Currently, no studies have directly investigated the effects of WBV or BFR on proprioception in individuals with CAI or specifically in the ankle joint. However, research on other joints in healthy individuals provides some insights. For instance, Yamada et al. (2021) found that BFR did not worsen absolute knee joint position error after BFR interventions [39]. Their results are different from ours, probably because their study used BFR while walking and tested joint position sense while standing. Our finding is also in agreement with Kusienicka (2023) study, which found a negative effect of BFR on wrist proprioception, leading to increased absolute repositioning error [40]. This decline in accuracy may be attributed to the heightened stress on the neuromuscular system resulting from the combined use of BFR and WBV.
An interesting finding is that combining BFR with WBV improved both ankle dorsiflexion ROM and dynamic balance in the anterior direction of the m-SEBT. This suggests potential benefits for improving sagittal-plane function in athletes with CAI, such as better forward lunge mechanics and more stable single-leg landings.
The combined use of BFR and WBV reduced joint repositioning accuracy, possibly because BFR lowers oxygen delivery, causing temporary fatigue in muscles and sensory structures. Although regular BFR training can improve muscle growth, one session of BFR with WBV may cause discomfort, reduce blood flow, and temporarily lower performance by affecting proprioception and limiting the benefits of WBV [41]. Muscle fatigue may also release substances like blood lactate [42] that further impair proprioception. Additionally, the squat position on the WBV platform might have placed the dorsiflexor muscles in a slackened state, decreasing spindle sensitivity and reducing proprioceptive accuracy [43].
Side hop test, perceived instability, and dorsiflexion range of motion
Neither group showed substantial improvement in the side hop test, which contrasts with the findings of Werasirirat et al. (2022), who reported greater improvements in CAI participants following a four-week rehabilitation program combined with BFR [21]. This means that WBV alone or combined with BFR may not produce immediate benefits for lower limb function and agility. The side hop test requires quick, lateral movements, which may not be directly trained by WBV or BFR. A longer program or other functional tests might show more clearly if these methods are helpful. Also, the absence of changes in the side hop test shows that people with CAI may need specific exercises to improve function and agility.
The big drop in perceived stability in the BFR + WBV group, along with a strong effect size (0.87) and a clear difference between groups, suggests that adding BFR to WBV might make the joint feel less stable. This could be due to muscle fatigue and reduced blood flow caused by BFR [36]. Muscle fatigue caused by BFR likely disrupted the body’s ability to sense joint position and stability. This may be because fatigue affects how well the sensory receptors in muscles and joints work. Also, the build-up of waste products from fatigue could make this worse, leading to poorer proprioception and a greater feeling of instability. Fatigue caused by BFR worsens the proprioceptive problems from WBV, leading to increased feelings of instability when both are used together. This recommends caution is needed when combining these treatments, especially for people who need better joint stability, like those with CAI.
Dorsiflexion ROM showed significant improvements within both groups, with moderate effect sizes. However, the between-group analysis showed no significant differences, suggesting that both interventions are similarly effective in improving dorsiflexion mobility. This improvement may result from WBV’s mechanical vibrations increasing flexibility through reflex muscle relaxation, increased local circulation, thermoregulation, and neural effects [44]. The absence of significant between-group differences suggests that WBV alone is sufficient to improve dorsiflexion ROM. Notably, the effect size for dorsiflexion improvement was smaller in the combined BFR + WBV group compared to WBV alone. This may be due to ischemic conditions caused by BFR temporarily limiting flexibility, which could offset the benefits of WBV in the combined group. An interesting finding is the simultaneous improvement in dorsiflexion ROM and anterior dynamic balance, which are closely related [45]. Thus, gains in one often contribute to improvements in the other.
Limitations and future directions
This study has several limitations that future research should address. First, the immediate effects reported here do not reflect potential long-term benefits or drawbacks of the interventions. Longitudinal studies are necessary to determine whether combining BFR with WBV leads to sustained improvements in balance and proprioception in athletes with CAI. Second, although WBV was applied at a fixed frequency and amplitude, individual responses to vibration may vary and affect outcomes. Future research should evaluate varying WBV frequencies and amplitudes to identify optimal settings for improving dynamic balance and proprioception. Additionally, incorporating a wider range of functional performance measures, including sport-specific tasks, would offer a more complete understanding of how these interventions impact real-world athletic performance. In addition, a parallel-group design was used in this study. Future studies could consider a crossover design with adequate washout periods to facilitate a more detailed statistical analysis. Also, although we included non-professional male athletes who engaged in physical activity at least twice a week for six months to maintain sample consistency, we did not assess their baseline physical activity levels using validated tools like the International Physical Activity Questionnaire. Future research should use these standardized measures to better account for the effects of physical activity on study outcomes. Finally, including only male participants limits the generalizability of our findings. This decision was made to minimize hormonal and neuromuscular differences that might affect short-term outcomes. However, future studies should include women to examine possible gender-specific responses and improve the broader applicability of the results.
Conclusion
This study investigated the immediate effects of combining WBV and BFR on balance, function, ankle dorsiflexion ROM, and proprioception in non-professional athletes with CAI. The findings suggest that both WBV alone and WBV combined with BFR improved dorsiflexion ROM. However, neither intervention led to significant immediate improvements in function, static balance, or dynamic balance. The only exception was anterior dynamic balance, which improved with the combined intervention. Given these results, WBV and BFR—whether used alone or together—should not be viewed as standalone treatments for CAI. Rather, they may be useful as complementary approaches within a comprehensive rehabilitation program. Particularly, adding BFR to WBV seemed to worsen proprioception and increase feelings of instability. This may be caused by fatigue or disrupted sensory feedback. As such, this combined approach may not be suitable immediately before tasks that require high levels of neuromuscular control, such as athletic competition or intense training. These findings emphasize caution when combining WBV and BFR, especially for those needing joint stability. Future studies should examine longer treatments, personalized protocols, and include balance or agility exercises to achieve greater functional improvements in athletes with CAI.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors gratefully acknowledge the financial support from the Research Deputy of Tehran University of Medical Sciences.
Author contributions
A.B., N.G., and Z.A. contributed to the conception and design of the study. A.B. performed the experiments. A.B., N.G., and Z.A. provided reagents and technical guidance for the experiments. A.B., N.G., Z.A., and K.M. analyzed the data. A.B., N.G., Z.A., and K.M. wrote the manuscript. A.B., N.G., Z.A., and K.M. edited the manuscript. All authors read and approved the final version of the manuscript.
Funding
Not applicable.
Data availability
The data sets used and/or analyzed during the current study will be made available upon reasonable request directed to the corresponding author.
Declarations
Ethics approval and consent to participate
All procedures were conducted in accordance with the principles of the Helsinki Declaration. Ethical approval was obtained from the Ethics Committee of the School of Nursing, Midwifery, and Rehabilitation, Tehran University of Medical Sciences (No: IR.TUMS.FNM.REC.1401.162. February 1, 2023). Written informed consent was obtained from all participants prior to participation in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kemler E, van de Port I, Valkenberg H, Hoes AW, Backx FJ. Ankle injuries in the netherlands: trends over 10–25 years. Scand J Med Sci Sports. 2015;25(3):331–7. [DOI] [PubMed] [Google Scholar]
- 2.Hong CC, Tan KJ, Calder J. Chronic lateral ankle ligament instability - Current evidence and recent management advances. J Clin Orthop Trauma. 2024;48:102328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doherty C, Bleakley C, Hertel J, Caulfield B, Ryan J, Delahunt E. Recovery from a First-Time lateral ankle sprain and the predictors of chronic ankle instability: A prospective cohort analysis. Am J Sports Med. 2016;44(4):995–1003. [DOI] [PubMed] [Google Scholar]
- 4.Gribble PA, Delahunt E, Bleakley C, Caulfield B, Docherty C, Fourchet F, et al. Selection criteria for patients with chronic ankle instability in controlled research: a position statement of the international ankle consortium. J Orthop Sports Phys Therapy. 2013;43(8):585–91. [DOI] [PubMed] [Google Scholar]
- 5.Montoye HJ, Fox G, Van Huss W. Characteristics of professional and amateur athletes. Phys Educ. 1960;17(1):3. [Google Scholar]
- 6.Hertel J, Corbett RO. An updated model of chronic ankle instability. J Athl Train. 2019;54(6):572–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Willems T, Witvrouw E, Verstuyft J, Vaes P, De Clercq D. Proprioception and muscle strength in subjects with a history of ankle sprains and chronic instability. J Athl Train. 2002;37(4):487–93. [PMC free article] [PubMed] [Google Scholar]
- 8.Perron M, Moffet H, Nadeau S, Hébert LJ, Belzile S. Persistence of long term isokinetic strength deficits in subjects with lateral ankle sprain as measured with a protocol including maximal preloading. Clin Biomech (Bristol). 2014;29(10):1151–7. [DOI] [PubMed] [Google Scholar]
- 9.Donovan L, Hart JM, Saliba SA, Park J, Feger MA, Herb CC, et al. Rehabilitation for chronic ankle instability with or without destabilization devices: a randomized controlled trial. J Athl Train. 2016;51(3):233–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hertel J. Sensorimotor deficits with ankle sprains and chronic ankle instability. Clin Sports Med. 2008;27(3):353–70. vii. [DOI] [PubMed] [Google Scholar]
- 11.Faltus J, Owens J, Hedt C. Theoretical applications of blood flow restriction training in managing chronic ankle instability in the basketball athlete. Int J Sports Phys Therapy. 2018;13(3):552. [PMC free article] [PubMed] [Google Scholar]
- 12.Cook SB, Scott BR, Hayes KL, Murphy BG. Neuromuscular adaptations to low-load blood flow restricted resistance training. J Sports Sci Med. 2018;17(1):66. [PMC free article] [PubMed] [Google Scholar]
- 13.Duchateau J, Stragier S, Baudry S, Carpentier A. Strength training: in search of optimal strategies to maximize neuromuscular performance. Exerc Sport Sci Rev. 2021;49(1):2–14. [DOI] [PubMed] [Google Scholar]
- 14.Sierra-Guzmán R, Jiménez-Diaz F, Ramírez C, Esteban P, Abián-Vicén J. Whole-body–vibration training and balance in recreational athletes with chronic ankle instability. J Athl Train. 2018;53(4):355–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adelman DL. Acute effects of whole-body vibration on dynamic postural control in subjects with functional ankle instability. The University of North Carolina at Chapel Hill; 2013.
- 16.Rendos NK, Jun H-P, Pickett NM, Lew Feirman K, Harriell K, Lee SY, et al. Acute effects of whole body vibration on balance in persons with and without chronic ankle instability. Res Sports Med. 2017;25(4):391–407. [DOI] [PubMed] [Google Scholar]
- 17.Hall EA, Chomistek AK, Kingma JJ, Docherty CL. Balance- and Strength-Training protocols to improve chronic ankle instability deficits, part I: assessing clinical outcome measures. J Athl Train. 2018;53(6):568–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cai ZY, Wang WY, Lin JD, Wu CM. Effects of whole body vibration training combined with blood flow restriction on muscle adaptation. Eur J Sport Sci. 2021;21(2):204–12. [DOI] [PubMed] [Google Scholar]
- 19.Lauber B, König D, Gollhofer A, Centner C. Isometric blood flow restriction exercise: acute physiological and neuromuscular responses. BMC Sports Sci Med Rehabil. 2021;13(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Z, Wei Z, Li X, Lai Z, Wang L. Effect of whole-body vibration on neuromuscular activation and explosive power of lower limb: A systematic review and meta-analysis. PLoS ONE. 2022;17(12):e0278637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Werasirirat P, Yimlamai T. Effect of supervised rehabilitation combined with blood flow restriction training in athletes with chronic ankle instability: a randomized placebo-controlled trial. J Exerc Rehabil. 2022;18(2):123–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hammami A, Slimani M, Yousfi N, Bouhlel E. The impact of short-duration static stretching or combined static stretching with dynamic stretching on sprint performance in moderately trained subjects. J Athletic Enhancement. 2015;4(3):19–20. [Google Scholar]
- 23.Lima-Soares F, Pessoa KA, Torres Cabido CE, Lauver J, Cholewa J, Rossi F, et al. Determining the arterial occlusion pressure for blood flow restriction: pulse oximeter as a new method compared with a handheld doppler. J Strength Cond Res. 2022;36(4):1120–4. [DOI] [PubMed] [Google Scholar]
- 24.Killinger B, Lauver JD, Donovan L, Goetschius J. The effects of blood flow restriction on muscle activation and hypoxia in individuals with chronic ankle instability. J Sport Rehabilitation. 2019;29(5):633–9. [DOI] [PubMed] [Google Scholar]
- 25.Zeng Z, Centner C, Gollhofer A, König D. Blood-flow-restriction training: validity of pulse oximetry to assess arterial occlusion pressure. Int J Sports Physiol Perform. 2019;14(10):1408–14. [DOI] [PubMed] [Google Scholar]
- 26.Gribble PA, Hertel J. Considerations for normalizing measures of the star excursion balance test. Meas Phys Educ Exerc Sci. 2003;7(2):89–100. [Google Scholar]
- 27.Bell DR, Guskiewicz KM, Clark MA, Padua DA. Systematic review of the balance error scoring system. Sports Health. 2011;3(3):287–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McKeon JM, McKeon PO. Evaluation of joint position recognition measurement variables associated with chronic ankle instability: a meta-analysis. J Athl Train. 2012;47(4):444–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Caffrey E, Docherty CL, Schrader J, Klossner J. The ability of 4 single-limb hopping tests to detect functional performance deficits in individuals with functional ankle instability. J Orthop Sports Phys Ther. 2009;39(11):799–806. [DOI] [PubMed] [Google Scholar]
- 30.Suttmiller AMB, Cavallario JM, Baez SE, Martinez JC, McCann RS. Perceived instability, pain, and psychological factors for prediction of function and disability in individuals with chronic ankle instability. J Athl Train. 2022;57(11–12):1048–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Konor MM, Morton S, Eckerson JM, Grindstaff TL. Reliability of three measures of ankle dorsiflexion range of motion. Int J Sports Phys Ther. 2012;7(3):279–87. [PMC free article] [PubMed] [Google Scholar]
- 32.Middel B, van Sonderen E. Statistical significant change versus relevant or important change in (quasi) experimental design: some conceptual and methodological problems in estimating magnitude of intervention-related change in health services research. Int J Integr Care. 2002;2:e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karim A, Roddey T, Mitchell K, Ortiz A, Olson S. Immediate effect of whole body vibration on sauté height and balance in female professional contemporary dancers A randomized controlled trial. J Dance Med Sci. 2019;23(1):3–10. [DOI] [PubMed] [Google Scholar]
- 34.Pollock RD, Provan S, Martin FC, Newham DJ. The effects of whole body vibration on balance, joint position sense and cutaneous sensation. Eur J Appl Physiol. 2011;111(12):3069–77. [DOI] [PubMed] [Google Scholar]
- 35.Mahmoud WS, Radwan NL, Ibrahim MM, Hasan S, Alamri AM, Ibrahim AR. Effect of blood flow restriction as a stand-alone treatment on muscle strength, dynamic balance, and physical function in female patients with chronic ankle instability. Med (Baltim). 2023;102(44):e35765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clark K, Trickett J, Donovan L, Dawson J, Goetschius J. Effects of blood flow restriction on balance performance during dynamic balance exercises in individuals with chronic ankle instability. J Sport Rehabilitation. 2024;33(3):181–8. [DOI] [PubMed] [Google Scholar]
- 37.Hoveidaei AH, Hashemi SM, Pazoki S, Nakhostin-Ansari A, Maleki Ghorbani Z, Eghdami S, et al. Effects of whole-body vibration on chronic ankle instability: a systematic review. Ann Med Surg (Lond). 2024;86(1):401–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Willems TM, Witvrouw E, Delbaere K, Mahieu N, De Bourdeaudhuij I, De Clercq D. Intrinsic risk factors for inversion ankle sprains in male subjects: a prospective study. Am J Sports Med. 2005;33(3):415–23. [DOI] [PubMed] [Google Scholar]
- 39.Yamada Y, Kasprzak R, Shotten S, Miller-Brown A, Mathew A, Loenneke J, et al. Brisk walking with practical blood flow restriction did not induce impairment of knee proprioception and fatigue. J Trainology. 2021;10:16–9. [Google Scholar]
- 40.Kusienicka K. Effect of blood flow restriction cuffs on joint proprioception with the example of the wrist. Physiotherapy Rev. 2023;27(3):13–9. [Google Scholar]
- 41.Lin YH, Li CW, Tsai LY, Liing R-J. The effects of muscle fatigue and proprioceptive deficits on the passive joint senses of ankle inversion and Eversion. Isokinet Exerc Sci. 2008;16(2):101–5. [Google Scholar]
- 42.Zhang J, Zhou R, Zhao N, Li Y, Liu H, Zhang W et al. Acute effects of blood flow restriction with whole-body vibration on sprint, muscle activation and metabolic accumulation in male sprinters. Front Physiol. 2023;Volume 14–2023. [DOI] [PMC free article] [PubMed]
- 43.Inglis JT, Frank JS, Inglis B. The effect of muscle vibration on human position sense during movements controlled by lengthening muscle contraction. Exp Brain Res. 1991;84(3):631–4. [DOI] [PubMed] [Google Scholar]
- 44.Fowler BD, Palombo KTM, Feland JB, Blotter JD. Effects of Whole-Body vibration on flexibility and stiffness: A literature review. Int J Exerc Sci. 2019;12(3):735–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Basnett CR, Hanish MJ, Wheeler TJ, Miriovsky DJ, Danielson EL, Barr JB, et al. Ankle dorsiflexion range of motion influences dynamic balance in individuals with chronic ankle instability. Int J Sports Phys Ther. 2013;8(2):121–8. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data sets used and/or analyzed during the current study will be made available upon reasonable request directed to the corresponding author.