Abstract
Objectives:
To develop a modeling tool to assess the population impact of emerging tobacco products and demonstrate its utility.
Methods:
Discrete state Markov models were developed using TreeAge Pro 2014 to operationalize and estimate current cigarette and e-cigarette use patterns in the US population (including never and former use). State transition probabilities were calculated from a longitudinal survey of US young adults and adjusted using other nationally representative data.
Results:
Over 10 years, the adjusted base case model approximated national cigarette smoking and former use prevalence and predicted e-cigarette and dual use prevalence between 1% and 2%.
Conclusions:
This proof-of-concept study provides a heuristic tool to operationalize aspects of the US Food and Drug Administration’s new public health standard regarding the impact of new tobacco products on use of existing products.
Keywords: e-cigarette, tobacco, simulation, Markov, cigarettes
Despite more than a 50% decline in cigarette smoking prevalence in the United States (US) over the past 50 years that can be attributed, in part, to a comprehensive tobacco control strategy (eg, excise taxes, policies, media campaigns, prevention and cessation services),1–3 the rate of decline has slowed over the past decade.4,5 More than 42 million Americans still use the most lethal form of tobacco, the combustible cigarette, that causes the overwhelming majority of the 480,000 preventable deaths annually.2 A predicted 5.6 million children alive today will die prematurely by becoming lifelong users of cigarettes.1,2 Avoiding or desisting use of any combustible product (cigarettes, cigars, hookah, or pipe) at as early an age as possible may dramatically minimize the cumulative morbidity, mortality and damage of combustible products.2
In addition to maintaining the full force of comprehensive tobacco control efforts, harm reduction (ie, decreasing tobacco-related morbidity and mortality with or without total elimination of nicotine/tobacco use)6–9 is another strategy that can help to accelerate reduction of combustible cigarette use.2,6,10 One form of harm reduction includes moving combustible tobacco users to less harmful forms of nicotine consumption.8,9,11 Long-term use of pharmaceutical nicotine (ie, nicotine replacement therapy-NRT), for example, has been endorsed as an acceptable strategy to reduce the death and disease resulting from combustible tobacco use.11,12 Electronic cigarettes (e-cigarettes) are a novel class of nicotine delivery systems that are usually composed of a battery, heating element, and liquid containing humectants, nicotine (typically), and flavorings. Although not conclusive, toxicological analyses generally indicate that e-cigarettes are less harmful than combustible cigarettes because they produce no carbon monoxide, contain fewer toxicants, and produce lower levels of several classes of carcinogens found in cigarette smoke.13 The dramatic increase in electronic cigarette (e-cigarette) use in the US and their impending regulation by the US Food and Drug Administration (FDA)14–20 has raised the possibility of their utility as a viable harm reduction tool.10,11,21,22
To understand the complex calculus of e-cigarettes’ overall population health impact, we must consider the multitude of influences on e-cigarette and combustible tobacco uptake, dual use of e-cigarettes and combustible tobacco, exclusive use of either product type, and cessation, including the product itself, as well as its regulation, marketing, and potential utility as an aid to combustible tobacco cessation.22,23–26 Currently, there is little definitive prospective data to inform e-cigarettes’ overall population-level effects on tobacco use behavior and health outcomes. Available data are derived predominantly from observational and cross-sectional studies, raising concerns about selection bias and unmeasured or insufficiently controlled confounders, precluding causal inference.27–31 The current state of knowledge, coupled with a diverse and evolving product range and rapid changes in e-cigarette use in the US population, has created an air of unease for regulators, public health advocates, and the scientific community concerning how best to address the complex issues surrounding the availability and use of e-cigarettes.
In the US, e-cigarettes may be regulated as tobacco products (by the FDA’s Center for Tobacco Products [CTP]) or medical devices (by the FDA’s Center for Drug Evaluation and Devices [CDER]).22 These different regulatory regimes have important implications for impending regulation of e-cigarettes and other tobacco products. The US FDA’s CTP regulates tobacco products “for the protection of the public health”32 using a variety of perspectives to inform a “public health standard”33 For regulation of new tobacco products like e-cigarettes, the public health standard requires the CTP to determine the potential harms versus benefits to individual users and to the population as a whole. In addition, this determination must consider the likelihood that these products: (1) attract non-users (especially youth who otherwise would never have used any tobacco products); (2) prevent, delay, or accelerate current combustible tobacco users from stopping use of all tobacco/nicotine products (ie, as a cessation aid), and (3) promote immediate or eventual switching to exclusive use or prolong dual use (ie, without a reduction in cigarette use). Depending on these patterns of use, e-cigarettes may or may not result in meaningful reductions in harm from combustible cigarettes at the population level.
Waiting for years to obtain data on actual population patterns of use is impractical and could result in missed opportunities to protect the public’s health.2,10 One means of addressing this problem is through simulation modeling, an approach to aid understanding of the interplay between comprehensive tobacco control strategies and the use of different emerging tobacco products by projecting patterns of tobacco use into the future.34 Longitudinal surveillance data are needed to make accurate estimates of transition probabilities between different tobacco use states (eg, exclusive cigarette use, cessation of cigarettes, dual use, or exclusive use of e-cigarettes)35 When based on actual product use patterns, simulation modeling can provide a method to understand and predict long-term outcomes to inform the public health standard.36−41
State transition models (STMs) can be used to understand changes in longitudinal data, and have been employed extensively in medical decision-making contexts.42,43 These models also have been applied to the study of behavior change processes such as smoking cessation36–38,41,44,45 and alcohol consumption.46 STMs, also known as Markov models when applied to population cohorts, can be used to assess the rate, prevalence, and stability of tobacco use patterns47 and can be used to address questions about the potential impact of a new product like e-cigarettes on population-level patterns of use of other tobacco products. Model development requires explicit specification (eg, baseline states, transitional probabilities between states over time) of all aspects of the complex patterns of poly-tobacco use behavior. Although all models are imperfect, well-constructed models can help operationalize the FDA’s public health standard33 and can be a useful tool for determining whether the introduction and use of new tobacco products may result in some benefit, some harm, or negligible impact on the death and disease primarily caused by cigarettes.
The main purpose of this proof-of-concept study, is to develop a Markov model to operationalize (ie,, make explicit and transparent) the complex states and transitions that must be examined simultaneously and dynamically over time to determine the net public health impact of emerging tobacco products like e-cigarettes. Data collected as part of an existing 3-year longitudinal study of trajectories of tobacco use among US young adults assessed at 6-month intervals are used to illustrate the model by examining how population use of e-cigarettes and combustible cigarettes may change over the next decade if current use patterns are sustained (status quo).
METHODS
General Approach
Figure 1 depicts a linear progression in cigarette and e-cigarette use states over multiple occasions in a format akin to a decision tree. Following the figure from left to right, a never user may remain a never user or transition to a new state: cigarette use, e-cigarette use, or dual use of both cigarettes and e-cigarettes. Multiple pathways exist in the next stage of transitions, whereby individuals can maintain use of a particular product, switch to or add another product, or quit. These transitions can continue over time (not shown) for any number of sampling occasions, producing trajectories of use. Whereas this study is specific to cigarette and e-cigarette use, this conceptual approach can accommodate multiple types of tobacco use or outcomes and various definitions of state status.
Figure 1. Linear Model of Patterns of Cigarette and E-cigarette Use over Time (Grey Arrow); Assessment Time Points Indicated by the Numbered Circles (T0, T1, T2…Tn).
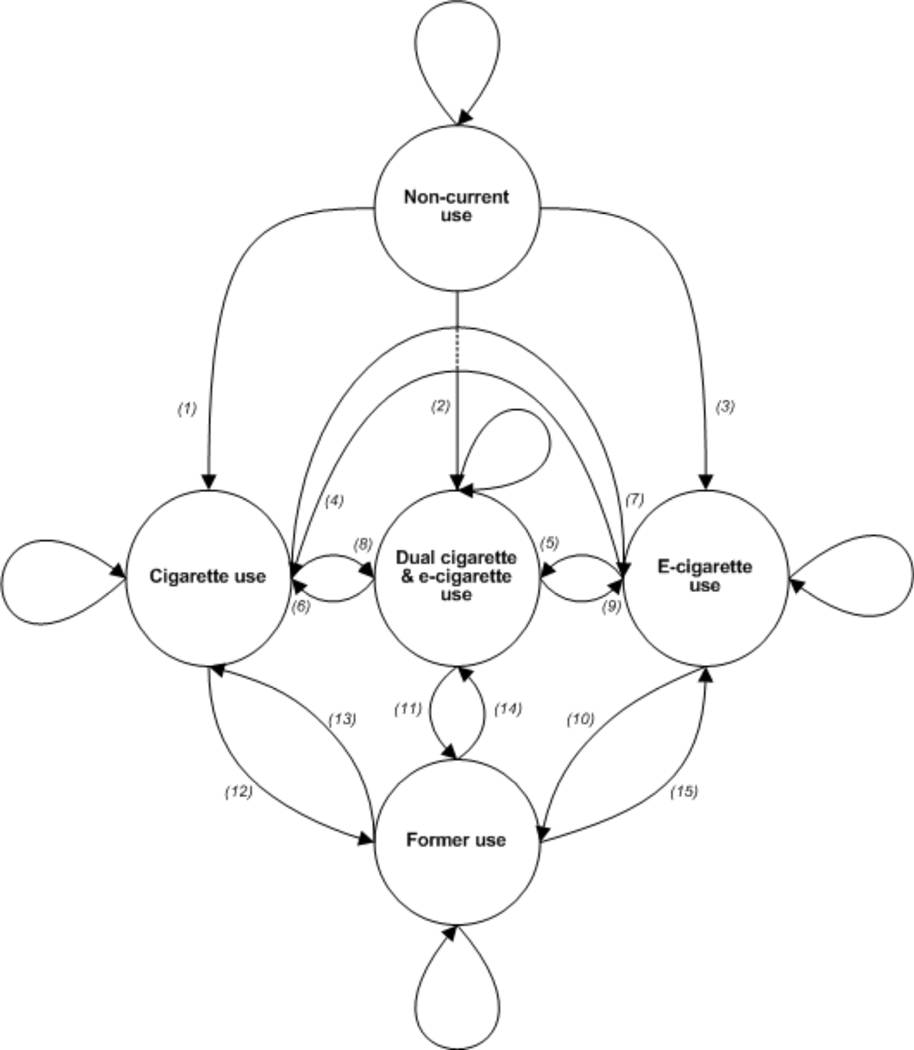
The Markov model presented in Figure 2 shows the transition process depicted in the linear model from Figure 1. Directed arrows represent transitions and looped arrows at each state represent maintenance within that state. E-cigarette and cigarette-naïve individuals begin in non-current use (a variant of never use) and can transition to current exclusive or dual use (paths 1–3). Once in a current use state, individuals can maintain use (looped arrows), transition to 1 of 2 alternative states (paths 4–9), or cease use of both products (paths 10–12). Following the transition to former use of both products, individuals can maintain cessation (looped arrow) or relapse to cigarette, e-cigarette, or dual use (paths 13–15). For the purposes of modeling in this study, we assume that transitions between states are governed by a Markov process, ie, the probability of transition to a future state is determined by the present state, and is independent of the way in which the present state arose from preceding state(s).
Figure 2. Markov Model of Cigarette and E-cigarette Use (Non-current Use, Cigarette Use, E-cigarette Use, Dual Use, and Former Use States) with Transitions Labeled Consecutively (Paths 1–15).
The FDA’s public health standard could be supported by changes in patterns of tobacco use that result in transitions to less harmful patterns of use that then can be translated into reduced risk and reduced exposure outcomes. These changes might include limited movement from non-current use to cigarette and e-cigarette use (paths 1–3), increased movement away from cigarette use (paths 7, 9, 11–12), increased transition to former use (paths 10–12), and reduced relapse to cigarette and e-cigarette use (paths 13–15). By using data to estimate rates of e-cigarette use or the effects of e-cigarette use on cigarette use, researchers and regulators may be able to make better estimates of whether the introduction (or removal) of these products is beneficial, has little impact on, or is harmful to the public’s health.
Sample
The current study uses data from the Legacy Young Adult Cohort Study, a longitudinal survey of tobacco use behavior in a national sample of young adults aged 18–34 drawn from GfK’s Knowledge-Panel®. Complete details of survey methods have been published previously.48 Briefly, the sample was recruited via address-based sampling to provide a statistically valid representation of the US population, including cell phone-only households. African-American and Hispanic young adults were oversampled to ensure sufficient sample sizes for subgroup analyses, and the online survey was administered in English and Spanish. The current study uses longitudinal data from N = 643 young adults aged 18–24 who completed the first 4 waves of data collection (June 2011, January 2012, July 2012, and January 2013) and provided complete data on tobacco use at each time point.
Measures
Baseline demographic measures (sex, race/ethnicity, education) were obtained to characterize the sample. Tobacco use was assessed with measures of ever tobacco/nicotine use and past 30-day use. Non-current users were defined as those who were never users/smokers or had never used a tobacco/nicotine product more than monthly and had used neither cigarettes nor e-cigarettes in the past 30 days (Table 1). Current use of cigarettes and e-cigarettes was defined as having used the product on at least one occasion during the past 30 days; participants reporting past 30-day use of both products were classified as dual users. Due to intermittent patterns of use and experimentation among young adults, participants did not have to meet a threshold (eg, 100 cigarettes) to be considered a current user. Former users were defined as those participants who reported no past 30-day use of either product at the current wave but reported past 30-day use of either product at the prior wave.
Table 1.
Definitions for Initial Baseline Probabilities in the Markov Models
| Tobacco Use States | Definition |
|---|---|
|
| |
| Non-current use | Individuals met criteria (1) or (2): 1) never use, which included never used a tobacco product OR smoked no tobacco products in their lifetime (including cigarettes, cigars, pipes, little cigars, e-cigarettes, and hookah) 2) non-current use, which included tried a tobacco or nicotine product only once OR never used a tobacco or nicotine product monthly AND had used neither cigarettes nor e-cigarettes in the past 30 days. |
| Cigarette use | Individuals used cigarettes during the past 30 days at the current wave. |
| Dual cigarette and e-cigarette use | Individuals used cigarettes and e-cigarettes during past 30 days at the current wave. |
| E-cigarette use | Individuals used e-cigarettes during the past 30 days at current wave. |
| Former use | Individuals reported no past 30-day use of either product at the current wave but reported past 30-day use of either product at a prior wave. |
Initial and Transition Probabilities
Longitudinal surveillance data were used to provide the prevalence rates for the initial states as well as to estimate the transition probabilities over multiple occasions (Table 2) that underlie each of the paths in Figure 2. Current cigarette, e-cigarette, and dual use were probabilities estimated in the sample at Wave 2 (Note: population-based weights were not applied prior). To classify individuals as non-current or former users more accurately, participants in the “non-current use” state were tracked from Wave 1 to Wave 2. Those who remained non-current users across the 2 waves were classified as such; those who were current cigarette, e-cigarette, or dual users at Wave 1 and reported no use at Wave 2 were classified as “former users.” Transition probabilities for all paths were estimated as the unweighted probabilities of maintenance or transition among the 5 states over a one-year period between Waves 2 and 4; this timeframe was chosen to be consistent with a one-year cycle length in model analyses. Consistent with decision tree modeling, transitions from a single state were mutually exclusive; thus, the sum of all transition probabilities from a single state was equal to 1.
Table 2.
Initial and Transition Probabilities for Projected Markov Models
| Initial | Transition | |||
|---|---|---|---|---|
|
|
||||
| Probability | Path Number | Wave 2 Jan-2012 (N = 643) | Wave 2 to Wave 4 Jan-2012 to Jan-2013 (1 year; N = 643) | |
|
| ||||
| Non-current use | 0.7947 | |||
| to non-current use | - | - | 0.9295 | |
| to cigarette use | 1 | - | 0.0587a | |
| to dual use | 2 | - | 0.0039 | |
| to e-cigarette use | 3 | - | 0.0079 | |
| Cigarette use | 0.1477 | |||
| to cigarette use | - | - | 0.7158b | |
| to dual use | 8 | - | 0.0211 | |
| to e-cigarette use | 7 | - | 0.0315 | |
| to former use | 12 | - | 0.2316 | |
| Dual use | 0.0218 | |||
| to dual use | - | - | 0.1429 | |
| to cigarette use | 6 | - | 0.5714 | |
| to e-cigarette use | 9 | - | 0.0714 | |
| to former use | 11 | - | 0.2143 | |
| E-cigarette use | 0.0093 | |||
| to e-cigarette use | - | - | 0 | |
| to cigarette use | 4 | - | 0.1667 | |
| to dual use | 5 | - | 0 | |
| to former use | 10 | - | 0.8333 | |
| Former use | 0.0264 | |||
| to former use | - | - | 0.5882 | |
| to cigarette use | 13 | - | 0.4118 | |
| to dual use | 14 | - | 0 | |
| to e-cigarette use | 15 | - | 0 | |
Note.
Adjusted model includes 10% decay in transition at each stage.
Adjusted model accounts for ratio of young adults reporting current regular smoking to past 30-day smoking (0.544) with the remainder of past 30-day smokers assumed to quit (path 12).
Status Quo and Adjusted Models
In the status quo (base case) model, transition probabilities were assumed to be the same across all cycles with no decay. To calibrate this model to current population tobacco use better, 2 adjustments were made to the base case to account for: (1) the likelihood that past 30-day young adult smokers become regular smokers; and (2) decreasing initiation by age. A ratio of current smoking (smoking ≥ 100 cigarettes and current every day or someday smoking; 17.3% among 18–24 year olds) to past 30-day smoking among young adults (31.8% among 18–25 year olds) was calculated from 2012 data provided by the National Health Interview Survey1 and the National Survey on Drug Use and Health,49 respectively. The probability of maintaining cigarette smoking was multiplied by this ratio (0.544) at each stage with the remainder of past 30-day smokers moving via the “former users” pathway (path 12). The adjusted model also included 10% decay in the probability of initiating cigarette use per year (path 1), assuming there was a decline in initiation with increasing age. This 10% decay was derived empirically to adjust projected estimates to be aligned better with other national data sources.
Data Analysis
Frequencies of demographic characteristics and bivariate analyses to estimate initial and transition probabilities were conducted in Stata IC, version 13.1. Markov transition models were generated in TreeAge Pro 2014 (TreeAge Software, Inc., Williamstown, Massachusetts, US), and cohort analysis was run to determine the projected percentage of the cohort in each of the 5 states over a 10-year time horizon.
RESULTS
Sample Characteristics
Among individuals included in the current analysis (N = 643) at baseline all were aged 18–24 years (mean = 21.3 years, standard error = 0.1), 58% (N = 373) were female, 61% (N = 389) were non-Hispanic Whites, 13% (N = 81) were non-Hispanic Blacks, 7% (N = 45) were other non-Hispanics, and 20% (N = 128) were Hispanic (any race). The majority had completed some college education or higher (75%; N = 482), 19% (N = 122) completed high school only, and 6% (N = 39) had less than a high school education.
The base case model (Table 3) begins with 79% of the sample in non-current cigarette/e-cigarette use, 15% in cigarette-exclusive use, 2% in dual use, 1% in e-cigarette-exclusive use, and 3% in former cigarette/e-cigarette use. At one year, non-current use prevalence decreases, with the majority transitioning to cigarette use. Dual and e-cigarette use fluctuate slightly (± 1%) at one year. At 5 years, non-current users comprise 55% of the cohort, and former users are at 15%. Cigarette use continues to increase, and there is a small increase in dual and e-cigarette use. At 10 years, 38% and 37% are in non-current use and cigarette use, respectively, and 22% are in former use. The proportion within dual and e-cigarette use is less than 5% of the total sample.
Table 3.
Status Quo Markov Models of Tobacco Use Behavior among US Young Adults Aged 18–24 from Baseline Projected over 10 Years
| Non-current users | Cigarette users | Dual users | E-cigarette users | Former users | |
|---|---|---|---|---|---|
|
| |||||
| Base case model | |||||
| Baseline | 79% | 15% | 2% | 1% | 3% |
| 1 year | 74% | 18% | 1% | 1% | 6% |
| 5 years | 55% | 28% | 1% | 1% | 15% |
| 10 years | 38% | 37% | 1% | 2% | 22% |
| Adjusted base case model | |||||
| Baseline | 79% | 15% | 2% | 1% | 3% |
| 1 year | 74% | 13% | 1% | 1% | 11% |
| 5 years | 58% | 18% | 1% | 1% | 22% |
| 10 years | 48% | 21% | 1% | 1% | 29% |
Note.
% = prevalence
The adjusted base case model begins with the same initial prevalence levels as the base case model. At one year, non-current use decreases slightly, as does cigarette use, with an 8% increase in former use. There is little change in dual and e-cigarette use. At 5 years, non-current use decreases to 58%, with 18% in cigarette use, and 22% in former use; there is no change from one year estimates for dual or e-cigarette use (both at 1%). At 10 years, trends continue with 48% remaining non-current users, 21% cigarette users, 29% former users, and 2% total among dual and e-cigarette use.
DISCUSSION
This proof-of-concept study provides a heuristic model to operationalize aspects of the FDA’s public health standard with respect to the impact of emerging tobacco products on use of existing products. The Markov model was tested for illustrative purposes using longitudinal data from a cohort of US young adults followed over 18 months to estimate the potential public health impact of e-cigarette use on patterns of cigarette use. The model provides a means to quantify the extent to which current patterns of e-cigarette and cigarette use might produce changes in population prevalence that are harm-reducing, harm-inducing, or not influential. As new longitudinal data become available, our estimates of initial and transitional probabilities can be updated and adjusted to fit the entire population so that the model can provide better approximations to real word patterns of use that will inform regulation and policy.
As expected, in both the base case and adjusted models, the largest changes over the 10-year time horizon occurred in the non-current use, cigarette use, and former use states, with the dual use and e-cigarette use states remaining at low prevalence in all years. In developing new models, it is important to calibrate them against existing or historical data as part of a cross validation process. In our base case model, cigarette use overestimated current national estimates of past 30-day cigarette use in young adults between 5 and 10 years after baseline. Adjustments to 2 pathways in the base case model resulted in a cigarette smoking prevalence in line with the national estimate of current smoking in the next age group (25–44 year olds) in 2012,1 and a similar estimate of the one-year prevalence of quitting among 18–24 year olds reported by the Centers for Disease Control and Prevention (8.2% in 2010).50
Overall, based on the illustrative longitudinal survey used in this study, the status quo models demonstrated limited impact of e-cigarettes on patterns of current and former cigarette use in this young adult population. However the transition probabilities matrix (Table 2) identified 2 null pathways from e-cigarette use: (1) e-cigarette use to dual use (Figure 2, path 5); and (2) remaining in e-cigarette use (Figure 2, e-cigarette use looped arrow). Transition probabilities from former use of both products to dual use and e-cigarette use alone were also zero (Figure 2, paths 14–15). Thus, the low probabilities of e-cigarette and dual use over time were influenced by the low prevalence of e-cigarette and dual use derived from the Legacy Young Adult Cohort survey, as well as these zero-probability pathways. Therefore, the model helps to operationalize and identify the key transitions that may drive future outcomes. This demonstrates the heuristic usefulness of modeling in determining how future scenarios might change if these 2 pathways used different parameter estimates.
Limited movement between e-cigarette, dual, and former use states suggests that there may be insufficient data or that it may be too early to make useful inferences concerning the public health impact of e-cigarettes, but does reflect the current, real-world patterns of tobacco use as assessed in the Legacy Young Adult Cohort Study between June 2011 to January 2013. When estimating population impact, use of data from this limited cohort study illustrates the critical importance of having recent data from a sample with the full range of ages and socio-demographics. Ideally, surveys also should have questions specifically designed from the outset to inform the model. A related issue is that with rapid changes in products, marketing, prices and in patterns of e-cigarette use, calibration of this behavior in the model and in future work will be challenging. Model calibration will require surveillance tools that are nationally representative and capable of rapid data collection. Mixed methods will be needed to ensure that data collected are sufficiently rigorous (reliable and valid) and detailed to describe changing patterns of nicotine/tobacco product use and how those patterns map on to exposure to harmful constituents and poor health outcomes.
Data from the cross-sectional Monitoring the Future (MTF) study suggest the occurrence of large reductions in combustible cigarette use as well as greater uptake of e-cigarettes among adolescents.20 These trends may not be surprising considering the rapid advances in e-cigarette technology, advertising, and sales during the past several years.51–53 Whereas the majority of uptake appears to be among vulnerable youth who are at risk for experimentation with any number of behaviors (eg, marijuana, alcohol, new products) or would likely eventually use cigarettes anyway, much larger increase in uptake may be of concern, especially if it results in more youth who otherwise would have never used any tobacco product starting with e-cigarettes and progressing to combustible cigarettes.27,54 However, the large drop in combustible cigarette use suggests that an opposite pathway may be just as likely. Given the cross-sectional nature of the MTF survey, it is also possible that uptake of e-cigarettes in youth might deter progression to combustible cigarette use. Our STM presented here is agnostic to the direction of the presumed causal pathway of these 2 trajectories (ie, into or out of cigarette use). Importantly, making an explicit model improves the transparency of assumptions and improves the demand for the types of scientific evidence needed to inform policymaking and regulation. Cross-sectional data alone cannot address questions of causality in a model of the changes in e-cigarette use and in cigarette use that occur contemporaneously.27
Limitations
The approach and models presented are simple and deterministic and should be construed as a starting point. The status quo results demonstrate the potential utility of one particular method – Markov/STM – among many others.55 As with other studies, interpretation of these findings is subject to limitations of the data used. In particular, our sample size was small, used only 3 data points to estimate trajectories, focused on young adult initiation and not on adult cessation, and covered a short time period (18 months). We calibrated the status quo initial probabilities to other nationally-representative data, but this is not a substitute for accurately estimating initial and state transition probabilities obtained from a comprehensive population sample over multiple waves of data collection. We did not model use of other tobacco products, but the basic structure of our model could be extended to accommodate other products (eg, smokeless tobacco, hookah/shisha/waterpipe tobacco smoking) and/or poly-tobacco use patterns. Of note, among the sample recruited, over three-fourths had completed some college education which is higher than the national average.56 Future work should strive for a more representative sample.
In principle, uncertainty (ie, distribution estimates) could be incorporated into the model via techniques such as individual-level microsimulation.57 Our analysis assumed a first-order Markov process, but lagged effects can be incorporated. More complex models that incorporate individual-level information, such as time spent in a state (possibly influencing the transition probabilities), also can be constructed. Whereas the models also assume discrete states, continuous time process models can be envisioned that may reflect mixing of behavioral states over time more accurately. The 10-year time horizon is arbitrary, as is the number of cycles (iterations), although we cycled the status quo models based on the temporal spacing (6-month intervals) in the Legacy Young Adult Cohort Study. Our results do not scale up to the entire population because of the limited age range of the cohort. Our cohort neither accurately estimates cigarette cessation patterns among the entire age range of the population nor does it account for population-level influx and outflow of individuals over time (eg, birth, aging, and death). The current models only attend to behavioral states of tobacco product use; they cannot yet be generalized to exposure reduction and health outcomes, although we maintain that a thorough understanding of variation in product use behaviors over time is required to ensure the accuracy of more complex models that link patterns of behavior to toxic exposures leading to disease states and death. With the availability of the longitudinal Population Assessment of Tobacco and Health (PATH) Study data gathered from approximately 46,000 individuals (adolescents and adults; tobacco users and nonusers) more elaborate models can be developed and validated.58
Future Directions
The basic structure of our models could be construed as specifying the behavior change component of a more comprehensive dynamic population model. Specifically, the Markov model presented (Figure 2) could be expanded to address other tobacco products, a greater number of tobacco products, or other categories of tobacco products (eg, combustible and noncombustible, FDA-approved cessation or harm reduction aids). Future studies modeling trajectories among population subgroups (eg, age, race/ethnicity, sex, education) could provide important information on differential uptake of products among vulnerable populations. This flexibility in collapsing or expanding states is one of the benefits of this modeling approach. Scenarios related to e-cigarette use among the entire population hinge upon the probabilities related to initiation of cigarette use via e-cigarettes (paths 4 and 5) and e-cigarette use alone (path 3);59 transitions between e-cigarette use, dual use, and cigarette use (paths 4–917); quitting all cigarette use or all nicotine/tobacco use via e-cigarettes (paths 9, 10–11);23–26 and relapse to cigarette use/dual use (paths 13–14).60 With appropriate data sources (eg, adult cessation patterns as well as youth uptake patterns), such as PATH, hypothetical what-if scenarios could be examined in sensitivity analyses, or used to identify important transition probabilities that significantly reduce or increase cigarette smoking prevalence. This model could help guide the evaluation of the introduction of modified risk tobacco products using post-market surveillance61 or policies related to availability of other tobacco products in the marketplace.62 A particular need for future modeling efforts is the standardization of e-cigarette measures and definitions to facilitate comparability of items across surveys and other assessment methods and to map patterns of use (alone, dual, or poly-use with other products) onto biomarkers of exposure to harm.63
Conclusions
This paper demonstrates the value and potential of simulation models to guide science-informed regulatory policy. Future work that builds on the Markov model conceptualized here, as well as rapid and representative longitudinal surveillance, will be crucial to address the US FDA’s CTP public health standard.
IMPLICATIONS FOR TOBACCO REGULATION.
Projected patterns of use of e-cigarettes and cigarettes using a Markov model demonstrate the utility of this approach to evaluate changes in tobacco use behavior.
Judicious use of this model by regulators can be used to evaluate current policies and guide future recommendations.
Human Subjects Statement
The current study was approved by the Independent Investigational Review Board, Inc (now Schulman Associates Institutional Review Board, Inc; Cincinnati, Ohio, and Ft. Lauderdale, Florida, US); approval dates by Wave were June 3, 2011 (Wave 1), December 9, 2011 (Wave 2), May 21, 2012 (Wave 3), and December 26, 2012 (Wave 4).
Acknowledgment
This work was supported by Legacy and the National Cancer Institute (P30CA051008).
Conflict of Interest Statement
All authors were or are currently affiliated with or employed by Legacy, a non-profit public health foundation.
ALG serves as a consultant on FDA BAA-13-00119 Enhancing tobacco surveillance through online monitoring awarded to Epidemico, Inc., a privately held company which conducts research and development of public health software projects.
Cassandra Stanton is an employee at Westat, an employee-owned research organization that conducts behavioral health research. Westat holds the initial contract to conduct the NIH/FDA sponsored Population Assessment of Tobacco and Health (PATH) Study (HHSN 27120110027C) in which Drs Stanton, Niaura, Abrams, Pearson, Villanti collaborate. Westat also holds an FDA funded award, Center for Evaluation and Coordination of Training and Research in Tobacco Regulatory Science (1U54CA189222-01) in which Drs Stanton, Niaura, Abrams, Villanti collaborate. The content is solely the responsibility of the authors and does not necessarily represent the official views of Westat, NIH or the FDA.
References
- 1.Agaku IT, King BA, Dube SR. Current cigarette smoking among adults - United States, 2005–2012. MMWR Morb Mortal Wkly Rep. 2014;63(2):29–34. [PMC free article] [PubMed] [Google Scholar]
- 2.US Department of Health and Human Services (USFHHS). The Health Consequences of Smoking – 50 Years of Progress: a Report of the Surgeon General. Atlanta, GA: USDHHS, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. [Google Scholar]
- 3.Holford T, Levy DT, McKay LA, et al. Birth cohort-specific smoking histories: 1965–2009. Am J Prev Med. 2014;46(2):e31–e37. doi: 10.1016/j.amepre.2013.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention (CDC). Vital signs: current cigarette smoking among adults aged >/=18 years --- United States, 2005−-2010. MMWR Morb Mortal Wkly Rep. 2011;60:1207–1212. [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention (CDC). CDC grand rounds: current opportunities in tobacco control. MMWR Morb Mortal Wkly Rep. 2010;59(16):487–492. [PubMed] [Google Scholar]
- 6.McNeill A, Munafo MR. Reducing harm from tobacco use. J Psychopharmacol. 2013;27(1):13–18. [DOI] [PubMed] [Google Scholar]
- 7.Kozlowski LT, Strasser AA, Giovino GA, et al. Applying the risk/use equilibrium: use medicinal nicotine now for harm reduction. Tob Control. 2001;10(3):201–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zeller M, Hatsukami D. The strategic dialogue on tobacco harm reduction: a vision and blueprint for action in the US. Tob Control. 2009;18:324–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stratton K, Shetty P, Wallace R, Bondurant SE. Clearing the Smoke: Assessing the Science Base for Tobacco Harm Reduction. Washington, DC: National Academy Press; 2001. [Google Scholar]
- 10.Abrams DB. Promise and peril of e-cigarettes: can disruptive technology make cigarettes obsolete? JAMA. 2014;311(2):135–136. [DOI] [PubMed] [Google Scholar]
- 11.Fiore MC, Schroeder SA, Baker TB. Smoke, the chief killer – strategies for targeting combustible tobacco use. N Engl J Med. 2014;370(4):297–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Food and Drug Administration. Nicotine Replacement Therapy Labels May Change. Available at: http://www.fda.gov/ForConsumers/ConsumerUpdates/ucm345087.htm. Accessed January 10, 2015.
- 13.Orr MS. Electronic cigarettes in the USA: a summary of available toxicology data and suggestions for the future. Tob Control. 2014;23(Suppl 2):ii18–ii22. [Google Scholar]
- 14.Food and Drug Administration. Deeming Tobacco Products to be Subject to the Federal Food, Drug, and Cosmetic Act, as Amended by the Family Smoking Prevention and Tobacco Control Act; Regulations on the Sale and Distribution of Tobacco Products and Required Warning Statements for Tobacco Products. In Department of Health and Human Services FaDA, ed. Vol Docket No. FDA-2014-N-0189. Federal Register; 2014. [Google Scholar]
- 15.King BA, Patel R, Nguyen KH, Dube SR. Trends in awareness and use of electronic cigarettes among U.S. adults, 2010–2013. Nicotine Tob Res. 2014;17(2):219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pearson JL, Richardson A, Niaura RS, et al. e-cigarette awareness, use, and harm perceptions in US adults. Am J Public Health. 2012;102(9):1758–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu SH, Gamst A, Lee M, et al. The use and perception of electronic cigarettes and snus among the U.S. population. PLoS One. 2013;8(10):e79332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giovenco DP, Lewis MJ, Delnevo CD. Factors associated with e-cigarette use: a national population survey of current and former smokers. Am J Prev Med. 2014;47(4):476–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention (CDC). Tobacco product use among middle and high school students - United States, 2011 and 2012. MMWR Morb Mortal Wkly Rep. 2013;62(45):893–897. [PMC free article] [PubMed] [Google Scholar]
- 20.University of Michigan News. Use of alcohol, cigarettes, and a number of illicit drugs declines among U.S. teens. Available at: http://monitoringthefuture.org//pressreleases/14drugpr_complete.pdf. Accessed December 22, 2014.
- 21.Benowitz NL, Goniewicz ML. The regulatory challenge of electronic cigarettes. JAMA. 2013;310(7):685–686. [DOI] [PubMed] [Google Scholar]
- 22.Cobb NK, Abrams DB. The FDA, e-cigarettes, and the demise of combusted tobacco. N Engl J Med. 2014;371(16):1469–1471. [DOI] [PubMed] [Google Scholar]
- 23.Brown J, Beard E, Kotz D, et al. Real-world effectiveness of e-cigarettes when used to aid smoking cessation: a cross-sectional population study. Addiction. 2014;109(9):1531–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bullen C, Howe C, Laugesen M, et al. Electronic cigarettes for smoking cessation: a randomised controlled trial. Lancet. 2013;382(9905):1629–1637. [DOI] [PubMed] [Google Scholar]
- 25.Biener L, Hargraves JL. A longitudinal study of electronic cigarette use in a population-based sample of adult smokers: association with smoking cessation and motivation to quit. Nicotine Tob Res. 2014;17(2):127–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McRobbie H, Bullen C, Hartmann-Boyce J, Hajek P. Electronic cigarettes for smoking cessation and reduction. Cochrane Database Syst Rev. 2014;12:CD010216. [Google Scholar]
- 27.Niaura RS, Glynn TJ, Abrams DB. Youth experimentation with e-cigarettes: another interpretation of the data. JAMA. 2014;312(6):641–642. [DOI] [PubMed] [Google Scholar]
- 28.Callahan-Lyon P. Electronic cigarettes: human health effects. Tob Control. 2014;23(Suppl 2):ii36–ii40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walton KM, Abrams DB, Bailey WC, et al. NIH electronic cigarette workshop: developing a research agenda. Nicotine Tob Res. 2015;17(2):259–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schroeder MJ, Hoffman AC. Electronic cigarettes and nicotine clinical pharmacology. Tob Control. 2014;23(Suppl 2):ii30–ii35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pearson JL, Stanton CA, Cha S, et al. E-cigarettes and smoking cessation: insights and cautions from a secondary analysis of data from a study of online treatment-seeking smokers. Nicotine Tob Res. 2014; Dec 26. pii: ntu269. [Epub ahead of print]. [Google Scholar]
- 32.H.R. 1256 – 111th Congress: Family Smoking Prevention and Tobacco Control Act HR 1256. Vol 2012: Gov-Track.us (database of federal legislation); 2009. Available at: https://www.govtrack.us/congress/bills/111/hr1256/text. Accessed March 27, 2015. [Google Scholar]
- 33.Villanti AC, Vargyas EJ, Niaura RS, et al. Food and drug administration regulation of tobacco: integrating science, law, policy, and advocacy. Am J Public Health. 2011;101(7):1160–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abrams DB. Comprehensive smoking cessation policy for all smokers: systems integration to save lives and money. In Bonnie RJ, Stratton K, Wallace RB, eds. Ending the Tobacco Problem: A Blueprint for the Nation. Washington, DC: National Academy Press; 2007. [Google Scholar]
- 35.Division of Program Coordination P, and Strategic Initiatives,. FDA Center for Tobacco Products Research Interest Areas. 2014. Available at: https://prevention.nih.gov/tobacco-regulatory-science-program/research-priorities. Accessed April 30, 2014.
- 36.Abrams DB, Graham AL, Levy DT, et al. Boosting population quits through evidence-based cessation treatment and policy. Am J Prev Med. 2010;38(3 Suppl):S351–S363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Levy DT, Mabry PL, Graham AL, et al. Exploring scenarios to dramatically reduce smoking prevalence: a simulation model of the three-part cessation process. Am J Public Health. 2010;100(7):1253–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levy DT, Bauer JE, Lee HR. Simulation modeling and tobacco control: creating more robust public health policies. Am J Public Health. 2006;96(3):494–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mendez D, Warner KE. Setting a challenging yet realistic smoking prevalence target for Healthy People 2020: learning from the California experience. Am J Public Health. 2008;98(3):556–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Warner KE, Mendez D. Tobacco control policy in developed countries: yesterday, today, and tomorrow. Nicotine Tob Res. 2010;12(9):876–887. [DOI] [PubMed] [Google Scholar]
- 41.Levy DT, Villanti AC, Pearson JP, et al. Modeling the effects of a menthol ban on smoking-attributable deaths in the United States. Am J Public Health. 2010;101(7):1236–1240. [Google Scholar]
- 42.Beck JR, Pauker SG. The Markov process in medical prognosis. Med Decis Making. 1983;3(4):419–458. [DOI] [PubMed] [Google Scholar]
- 43.Sonnenberg FA, Beck JR. Markov models in medical decision making: a practical guide. Med Decis Making. 1993;13(4):322–338. [DOI] [PubMed] [Google Scholar]
- 44.Killeen PR. Markov model of smoking cessation. Proc Natl Acad Sci U S A. 2011;108(Suppl 3):15549–15556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yeh HW, Ellerbeck EF, Mahnken JD. Simultaneous evaluation of abstinence and relapse using a Markov chain model in smokers enrolled in a two-year randomized trial. BMC Medical Research Methodology. 2012;12:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Delucchi KL, Weisner C. Transitioning into and out of problem drinking across seven years. J Stud Alcohol Drugs. 2010;71(2):210–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.White HR, Bray BC, Fleming CB, Catalano RF. Transitions into and out of light and intermittent smoking during emerging adulthood. Nicotine Tob Res. 2009;11(2):211–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rath JM, Villanti AC, Abrams DB, Vallone DM. Patterns of tobacco use and dual use in US young adults: the missing link between youth prevention and adult cessation. J Environ Public Health. 2012;2012:679134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Substance Abuse and Mental Health Services Administration. Results from the 2012 National Survey on Drug Use and Health: Summary of National Findings. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2013. [Google Scholar]
- 50.Centers for Disease Control and Prevention (CDC). Quitting smoking among adults –United States, 2001–2010. MMWR Morb Mortal Wkly Rep. 2011;60(44):1513–1519. [PubMed] [Google Scholar]
- 51.Richardson A, Ganz O, Vallone D. Tobacco on the web: surveillance and characterisation of online tobacco and e-cigarette advertising. Tob Control. 2014; Feb 14. doi: 10.1136/tobaccocontrol-2013-051246. [Epub ahead of print]. [DOI] [Google Scholar]
- 52.Giovenco DP, Hammond D, Corey CG, et al. E-cigarette market trends in traditional US retail channels, 2012–2013. Nicotine Tob Res. 2014; Dec 26. pii: ntu282. [Epub ahead of print]. [Google Scholar]
- 53.Zhu SH, Sun JY, Bonnevie E, et al. Four hundred and sixty brands of e-cigarettes and counting: implications for product regulation. Tob Control. 2014;23(Suppl 3):iii3–iii9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vanyukov MM, Tarter RE, Kirillova GP, et al. Common liability to addiction and “gateway hypothesis”: theoretical, empirical and evolutionary perspective. Drug Alcohol Depend. 2012;123(Suppl 1):S3–S17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Luke DA, Stamatakis KA. Systems science methods in public health: dynamics, networks and agents. Annu Rev Public Health. 2012;33:357–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.US Department of Education, National Center for Education Statistics. Fast Facts: Enrollment. Digest of Education Statistics, 2012 (NCES 2014–015), Chapter 3. Available at: http://nces.ed.gov/fastfacts/display.asp?id=98. Accessed March 10, 2015.
- 57.Siebert U, Alagoz O, Bayoumi AM, et al. State-transition modeling. Med Decis Making. 2012;32(5):690–700. [DOI] [PubMed] [Google Scholar]
- 58.Population Assessment of Tobacco and Health (PATH) Study. Available at: https://pathstudyinfo.nih.gov/UI/HomeMobile.aspx. Accessed February 5, 2015.
- 59.Centers for Disease Control and Prevention (CDC). Notes from the field: electronic cigarette use among middle and high school students - United States, 2011–2012. MMWR Morb Mortal Wkly Rep. 2013;62(35):729–730. [PMC free article] [PubMed] [Google Scholar]
- 60.Etter J-F, Bullen C. A longitudinal study of electronic cigarette users. Addict Behav. 2014;39(2):491–494. [DOI] [PubMed] [Google Scholar]
- 61.O’Connor RJ. Postmarketing surveillance for “modified-risk” tobacco products. Nicotine Tob Res. 2012;14(1):29–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.O’Connor RJ. Non-cigarette tobacco products: what have we learnt and where are we headed? Tob Control. 2012;21(2):181–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Delnevo CD, Bauer UE. Monitoring the tobacco use epidemic III: the host: data sources and methodological challenges. Prev Med. 2009;48(1 Suppl):S16–S23. [DOI] [PubMed] [Google Scholar]