Abstract
Introduction
Aspiration pneumonia (AP) in older persons is associated with oropharyngeal dysphagia (OD) and is estimated to account for 5–15% of cases of community-acquired pneumonia (CAP). Artificial Intelligence Massive Screening for OD (AIMS-OD) is an algorithm for identifying OD in older patients on hospital admission using data from electronic health records (EHR). We aimed to assess the prevalence of OD among older patients hospitalized with pneumonia and thus estimate the underdiagnosis of AP based on AIMS-OD.
Materials and methods
A retrospective observational study included 15,603 patients older than 65 years who were admitted for pneumonia to a general hospital between 2013 and 2022. Clinical data were obtained from EHR. AIMS-OD is an accurate diagnostic algorithm (AUCROC > 0.79, specificity 0.92, PPV 0.86, NPV 0.58) for OD using AI and machine learning.
Results
a) AP prevalence following traditional clinical practice (ICD-10 J69.0, AP codification) on discharge was 15.57% (n=2,430, 86.73±7.43 years); b) Estimated AP prevalence related to OD identified with AIMS-OD, was 25.32% (n=3,951, 85.11±8.78 years); c) AIMS-OD identified 84.77% (n=2,060, 87.17±7.09 years) of clinically diagnosed patients (ICD-10 J69.0), and 1,891 additional cases of AP (82.87±9.84 years) undetected by clinical practice, distinguishing them from pneumonia patients without OD in seconds.
Conclusion
The prevalence of AP following traditional clinical practice among older patients hospitalized with pneumonia was 15.57%. AIMS-OD revealed a potential prevalence of AP of 25.32%. AIMS-OD allows to increase by 62.6% the detection of AP related to OD versus traditional clinical practice among older patients hospitalized with pneumonia. AIMS-OD allows massive, immediate, and accurate identification of OD on hospital admission, from which AP cases can be identified, enabling early and specific treatment to improve the poor clinical outcomes of these unrecognized patients with AP and prevent its recurrence.
Supplementary Information
The online version contains supplementary material available at 10.1186/s41479-025-00175-x.
Keywords: Oropharyngeal dysphagia, Swallowing disorders, Oropharyngeal aspiration, Aspiration pneumonia, Community-acquired pneumonia, Artificial intelligence
Introduction
Pneumonia is an infection of the alveoli and distal bronchial tree of the lungs [1], and it is a major worldwide health concern due to its high incidence and mortality [2]. It can be divided into hospital-acquired pneumonia (HAP), defined as pneumonia which occurs after a patient has been in hospital for at least two days without any previous signs of respiratory infection, and community-acquired pneumonia (CAP), defined as pneumonia which occurs outside hospital settings in people who have not been admitted to hospital for at least a month before the onset of symptoms [1]. This differentiation of pneumonia according to location originates from the idea that, according to setting, patients have different microorganisms; this differentiation is therefore important in deciding treatment and management, as stated in the American, European and Japanese pneumonia guidelines [3–6]. The general incidence of CAP ranges from 1 to 14 cases per 1,000 adult inhabitants/year in Europe but significantly increases in males, older individuals, and those with more comorbidities [1]. CAP is one of the most common acute infections which requires hospital admission [7] in an estimated 40% of cases [8].
Aspiration pneumonia (AP) is a pathophysiological condition for which there is no universally agreed definition. AP can be observed in CAP and HAP and it is described as a pulmonary infection characterized by radiological evidence of infiltrates or consolidation in patients with swallowing disorders, symptoms of oropharyngeal dysphagia (OD), or risk factors for increased oropharyngeal aspiration [9–11]. The main risk factor for AP in older persons is aspirating food or oropharyngeal secretions due to impaired swallowing physiology, mainly OD [10, 12]. The second relevant risk factor is the multiplication and invasion of pathogenic bacteria from aspirated material into lung tissue [11–13]. Poor oral hygiene facilitates the development of dental plaque and oral diseases such as dental caries and periodontitis that act as precursors to local inflammatory conditions, fostering a state of dysbiosis in the oral microbiome [12, 14]. This facilitates the dominance of gram-negative bacteria and increases their concentration [12, 15, 16]. These microorganisms in the oral cavity and saliva find a pathway to the lower respiratory tract in patients with OD and oropharyngeal aspirations [12]. The third relevant risk factor is the general health of the host, with frailty, malnutrition, and impaired immunity as indicators of host vulnerability [10, 17–19]. AP is often associated with these and other factors such as advanced age, dehydration, smoking, use of antibiotics or inhalers, impaired immune function, frequent hospital readmissions, and increased mortality [9, 10, 20].
Aspiration is a common mechanism in both CAP and AP, although AP is distinctively linked to swallowing dysfunction and the aspiration of larger, more pathogenic volumes of oral or gastric contents, particularly in vulnerable populations such as older individuals [11, 12, 21]. While up to 45% of healthy individuals may microaspirate during their sleep without developing disease, factors like pathogen virulence and weakened host defenses influence whether disease develops [21]. CAP typically involves microaspiration of upper respiratory flora, whereas AP involves oropharyngeal pathogens associated with poor oral hygiene and dysbiosis [1, 11, 12, 17, 21, 22].
The incidence of AP is closely linked to that of OD and is expected to rise with increasing life expectancy, given the high prevalence of OD in the older population, which ranges from 27% in community-dwelling older people to 75% in patients hospitalized for CAP [11, 12, 23–25]. Studies suggest that 5–15% of patients with CAP have AP, leading to recurrent pneumonia, reduced functionality, frailty, and increased mortality and healthcare costs, often because their etiology is linked to OD and the risk factors for AP are neglected [11, 22, 26, 27]. However, this prevalence may be underestimated as swallowing assessment is not usually performed on older patients admitted for CAP in many European hospitals as well as in other parts of the world [11, 12, 22, 28]. In contrast, the proportion of AP in CAP and HAP in older patients in Japan -with a more generalized assessment of swallowing disorders- has been estimated to be 60.1% and 86.7%, respectively [29]. Studies from our group using bedside clinical evaluation [30] or videofluoroscopy (VFS) [25] to evaluate older (≥ 70 years) acute hospitalized patients with CAP also showed an OD prevalence of 55% and 91.7%, respectively. Other studies have reported a prevalence of OD of 53.9% or 34.42% in patients admitted with CAP [31, 32]. Thus, OD and aspiration are much more common in older patients with pneumonia than once believed, suggesting that most of these cases of pneumonia are caused by oropharyngeal aspiration [16]. A study that made a retrospective comprehensive revision of electronic health records (EHR), found that 30.7% of older patients with suspected AP of an unknown cause had had newly diagnosed causes of aspiration [33].
Currently, there is no standard diagnostic criteria for AP [22, 28]. A recent review suggests that the diagnosis of AP depends on the presence of lung inflammation in patients with clear evidence of aspiration, documented dysphagia, or a clinical condition closely linked to either aspiration or OD [34]. A step-by-step clinical algorithm was recently developed following this definition and based on the Japanese Respiratory Society (JRS) guidelines to assist physicians in diagnosing AP in older hospitalized adults with pneumonia [28]. It includes three main steps: (1) the investigation of the causes of aspiration and pneumonia using a checklist based on the JSR guidelines; (2) the assessment of swallowing function with clinical tests such as the volume–viscosity swallowing test (V-VST) [35], as early as possible during the first 5 days of admission, and (3) the discussion of patient management, taking into account AP risk factors and aspiration, to improve patients clinical outcomes and quality of life. We have recently proposed an improvement to this algorithm to increase efficiency and screen promptly for patients at high risk of AP [11]. The improvement involves the use of artificial intelligence-based screening for OD using the Artificial Intelligence Massive Screening-OD (AIMS-OD) tool developed by our team [36], as OD is the main etiological factor for the development of AP. AIMS-OD is an accurate screening algorithm for OD that uses machine learning and allows the system to automatically and universally screen for OD in all hospitalized patients with pneumonia. If the result of the AIMS-OD is positive, clinical assessment diagnosis and management of OD follow as described above [11].
This retrospective study aims to compare the prevalence of AP among older patients hospitalized for CAP at the Mataró University Hospital, Consorci Sanitari del Maresme (CSdM), Catalonia, Spain from 2013 to 2022 using standard clinical practice versus the prevalence that AIMS-OD would have detected, and thus assess the estimated prevalence and underdiagnosis of AP.
Materials and methods
Study design and ethical considerations
A single-center retrospective observational study was conducted in a cohort that included all patients hospitalized for acute CAP at Mataró University Hospital, CSdM, from 2013 to 2022. The CSdM is a public health consortium that includes primary care, acute and sub-acute hospitalization, rehabilitation and nursing homes for a population of 278,954 citizens in 2023.
In the study, the prevalence of AP diagnosed following standard clinical practice and reported under the diagnosis code category J69.0 (Pneumonitis due to solids and liquids, WHO’s International Classification of Diseases [ICD-10]) was compared to the prevalence of AP by identifying OD with the artificial intelligence-based software AIMS-OD [36], considering an AIMS-OD score higher than 0.7 as a positive risk of AP. This cut-off was selected from the ROC curve to obtain a positive predictive value (PPV) over 0.86. During the study period at Mataró University Hospital (CSdM), standard clinical practice did not include exploring all patients with CAP for OD with the V-VST. Only patients suspected of being at high risk for OD according to clinical criteria by hospital nurses were explored. In contrast, the AIMS-OD algorithm was retrospectively applied to all patients with CAP in this study. Clinical data from the EHR of patients was used to characterize the phenotypes described in the study population and dataset section.
The study protocol was approved by the Ethics Committee (EC) of the CSdM (code: CEIm 35/23) and was conducted according to the principles and rules laid down in the Declaration of Helsinki and its amendments. Exemption from the informed consent form to use the data from the EHR of our patients was granted by the EC. All members of the research team act following the ICH E6 (R2) Good Clinical Practice - Scientific guideline and European regulations.
Study population and dataset
The study population included all older patients (> 65 years of age) admitted for acute pneumonia and discharged with CAP diagnostic ICD-10 codes at the CSdM (Mataró University Hospital -acute care hospital-) from 2013 to 2022 (Additional file 1). Patients were included in the study following a non-probabilistically consecutive sampling. The most recent CAP admission was registered for those patients having more than one admission for CAP during the study period. The sample of patients with CAP was divided into five subgroups to identify those risk factors, clinical characteristics, or comorbidities associated with higher incidence or prevalence of AP. The following five groups were created (Fig. 1):
Fig. 1.
Distribution of study groups. Group A: Patients with CAP without AP. A group of patients considered to be without risk of AP (risk of OD < 0.7 according to AIMS-OD) and no ICD-10 diagnosis codification for AP (J69.0) during the admission for CAP (AIMS-OD < 0.7 + no ICD-10 J69.0); Group B: Patients estimated to have AP. Patients with a high risk of OD (> 0.7 according to AIMS-OD) and, consequently, with estimated AP among the patients admitted for CAP (AIMS-OD > 0.7); Group C: Patients with AP according to traditional clinical practice. A group of patients with ICD codification for AP during hospital admission for CAP (ICD-10 J69.0); Group D: Patients with AP according to both AIMS-OD risk and ICD-10 diagnosis codification for AP during the admission for CAP (AIMS-OD > 0.7 + ICD-10 J69.0); Group E: Patients estimated as having AP according to AIMS-OD and not clinically diagnosed. Patients with a high risk of OD according to AIMS-OD but no ICD-10 diagnosis codification for AP (J69.0) (AIMS-OD > 0.7 + no ICD-10 J69.0); Group F: Patients not identified by the algorithm and identified by the clinicians. Patients with no risk of OD according to AIMS-OD (AIMS-OD < 0.7) but with ICD-10 diagnosis codification for AP (ICD-10 J69.0) by the clinicians (AIMS-OD < 0.7 + ICD-10 J69.0) CAP: community-acquired pneumonia
Group A: Patients without AP. A group of patients considered to be without risk of AP (risk of OD < 0.7 according to AIMS-OD) and no ICD-10 diagnosis codification for AP (J69.0) during the admission for CAP (AIMS-OD < 0.7 + no ICD-10 J69.0);
Group B: Patients estimated as having AP. A group of patients with a high risk of OD (> 0.7 according to AIMS-OD) and, consequently, with probable AP among the patients admitted for CAP (AIMS-OD > 0.7).
Group C: Patients with AP according to traditional clinical practice. A group of patients with ICD codification for AP during hospital admission for CAP independently of AIMS-OD risk (ICD-10 J69.0);
Group D: Patients with AP according to both AIMS-OD risk and ICD-10 diagnosis codification for AP during the admission for CAP (AIMS-OD > 0.7 + ICD-10 J69.0).
Group E: Patients estimated as having AP and not clinically diagnosed. Patients with a high risk of OD according to AIMS-OD (AIMS-OD > 0.7) but no ICD-10 diagnosis codification for AP (ICD-10 J69.0) (AIMS-OD > 0.7 + no ICD-10 J69.0).
GROUP F: Patients not identified as having AP by the algorithm and identified by the clinicians. This group includes the patients with no risk of OD according to AIMS-OD (AIMS-OD < 0.7) but with ICD-10 diagnosis codification for AP (ICD-10 J69.0) by the clinicians (AIMS-OD < 0.7 + ICD-10 J69.0).
Sample size
We included a total sample of 15,603 patients admitted for acute pneumonia and discharged with ICD-10 codes for CAP from the Mataró University Hospital, CSdM, during the study period.
Clinical variables and measures
We retrospectively collected a series of clinical variables through the EHR for the characterization of the older population with CAP and the comparative analysis between the subgroups mentioned above. Clinical variables are described below:
Age: measured as mean and standard deviation.
Sex: measured in percentage, as female or male.
Number of previous hospital admissions in the last 2 years before acute event of CAP: measured as mean and standard deviation.
Number of days of hospital stay: measured as mean and standard deviation.
Barthel Index value on admission: divided into four subcategories in percentages, based on the grade of dependency (independent patient, 96–100; moderate, 61–96; severe, 21–61; or total dependency, 0–21) [37]. We collected only the available Barthel data from the EHR.
Mini-nutritional assessment short form (MNA-SF): divided into three subcategories (well-nourished, 12–14; at risk of malnutrition, 8–11; and malnourished, 0–7) in percentages, regarding the MNA-SF score of each participant [38]. We collected only the available MNA-sf data from the EHR.
Charlson Comorbidity Index (CCI): measured as mean and standard deviation [39].
-
Comorbidities included were:
- Diabetes mellitus: Diabetes mellitus due to underlying condition, drug or chemical-induced diabetes mellitus, type I, type II and other types of diabetes mellitus.
- Respiratory disease: Chronic obstructive pulmonary disease (COPD), and pulmonary fibrosis.
- Cerebrovascular disease: Intracerebral or intracranial hemorrhages, cerebral infarction, transient ischemic attack, intracranial trauma, and postoperative cerebrovascular complications.
- Dementia: Associated with cerebrovascular diseases, vascular dementia, Reye syndrome, Alzheimer’s disease, and other and non-specific dementia.
- Neurodegenerative disease: Primarily, movement disorders, and demyelinating diseases.
- Delirium.
- Neoplasia: All kinds of malignant tumors.
- Heart disease: Ischemic and other cardiomyopathies.
- Hepatopathies: Acute hepatitis A, acute hepatitis B, viral acute hepatitis, alcoholic hepatitis and toxic liver disease.
- Acute renal disease.
Data processing
Preprocessing steps included verifying the availability of key variables, removing patients with incomplete or irrelevant data, and retaining only the most recent results for Barthel, and MNA-SF scores. A dichotomous risk variable for OD (absent/present) was created based on a threshold risk of 0.7. The Shapiro-Wilk test was used to assess the normality of numerical variables. None of the patients in our database had incomplete relevant data to calculate the AIMS-OD risk that needed to be removed.
The processing of the database variables was performed with the programming language Python (Python version 3.11; Python Software Foundation. (2024); https://www.python.org [40].
Risk assessment software: AIMS-OD
The risk estimation service is offered through an application programming interface (API), allowing any facility with EHR to request their patients’ OD risk. The version of AIMS-OD used in this study (V1) has an area under the receiver operating characteristic (ROC) curve of 0.79; for this study, we selected a cutoff of 0.7 providing a positive predictive value of 0.86; negative predictive value of 0.58; positive likelihood ratio (LH) of 5.28 and negative LH of 0.61 (not published). AIMS-OD is a patented software that primarily uses demographic and administrative variables, diagnostic codes (ICD codes), and dispensed medication (ATC codes), potentially associated with OD from the patient’s EHR in the 24 months preceding admission. The predictive algorithm used in this study (Version 1) was trained using a database containing the EHR of 4,751 older patients hospitalized for any cause in a general hospital. According to the V-VST, 55.74% had OD and they had multiple associated comorbidities. The web service process involves the following steps: (a) First, the healthcare facility anonymizes the clinical data of its EHR and sends a query via Hypertext Transfer Protocol Secure (HTTPS) using the JavaScript Object Notation (JSON) format, which is an international standard. (b) The API receives the query and verifies the user’s authorization and whether it contains the required fields and security measures. (c) Finally, the API sends the query to our Expert System (ES), which predicts the risk of OD. The risk is calculated as a number between 0 and 1 and returned to the consulting healthcare facility as “YES/NO” or “FAIL/PASS” whether or not it passes the established threshold (0.7) for OD risk [36].
Data analysis and statistical methods
Prior to analysis, plausibility checks and predefined acceptable ranges were applied to key variables of the study database (e.g., age, weight, standardized clinical scores), and any values falling outside these limits were corrected or excluded. A formal statistical outlier detection procedure was not performed.
A variety of statistical tests were employed depending on the type and distribution of the variables. For numerical variables data, descriptive statistics such as median, mean, standard deviation, Interquartile range (IQR) and sample size were calculated to provide a comprehensive overview. For numerical variables, appropriate statistical tests were selected based on the normality of the data, employing either Mann-Whitney U tests for non-parametric distributions or T-tests for parametric ones. Categorical variables were analyzed using methods such as Fisher’s exact test, Cochran-Armitage test and Chi-square test to assess associations. All analyses were conducted with a significance threshold of 0.05. Barthel Index and MNA-sf values were obtained in a proportion of the total study population; thus, calculations for these variables were made according to available data recorded during hospital admission.
Prevalence rates for various diseases and geriatric syndromes were calculated for each subgroup defined by the target variable, and tests of independence were performed.
Effect sizes were calculated to provide additional context for the observed statistical differences and associations. Cliff’s Delta was used to quantify the magnitude of the difference based on the Mann-Whitney U test statistic. It measures the probability that a randomly selected value from one group will be greater than a randomly selected value from another group. Cohen’s d was used to quantify the difference between group means in parametric tests, providing a standardized measure of effect size. For binary variables, the Phi Coefficient was used to measure the strength of associations. Similarly, Cramér’s V, an extension of the Phi coefficient, was employed to measure the strength of associations between categorical variables.
Results
Description of the population with acute pneumonia
Clinical characteristics of the overall study population are depicted in Table 1. The mean age of the participants was 81.76 ± 8.91 years old. Females represented 48.42% of the population. Overall, patients presented a mean number of 3.92 ± 3.60 (median 3 (IQR 1.0–5.0) previous hospital admissions in the previous 2 years, and the average hospital stay for the current admission due to pneumonia was 7.58 ± 10.59 days (median 6.2 (IQR 4.0–9.0)). Functional status was severely impaired with a mean Barthel Index on admission of 58.96 ± 34.04 points. Barthel Index characterization showed that most participants had some degree of dependence (moderate in 32.11%, severe in 29.95%, and total in 20.00% of the sample), with only 17.93% independent for daily living activities. Only 9.58% of patients in the study population (n = 1494) received a nutritional evaluation, of whom 88.42% were malnourished according to the MNA-SF. An additional 8.63% were at risk of malnutrition, with only 2.95% being well-nourished. Finally, patients had few comorbidities with a mild mean CCI of 2.07 ± 3.16 points (median 1.0 (IQR 0.0–3.0)). The most prevalent comorbidities present in the study population were acute renal disease in 22.88%, heart disease in 18.55%, delirium in 15.64%, dementia in 14.86%, and neoplasia in 14.61%.
Table 1.
Demographics and clinical characteristics of the study population including all the subgroups studied. Statistical comparisons between study groups are represented as symbols in the p-value column above their corresponding p-values. These comparisons are detailed in the table footnote
| All | Group A (AIMS-OD < 0.7 + no ICD 10 J69.0) | Group B (AIMS OD > 0.7) | GrouP C (ICD-10 J69.0) | Group D (AIMS-OD > 0.7 + ICD-10 J69.0) | Group E (AIMS-OD > 0.7 + no ICD-10 J69.0) | P-value | |
|---|---|---|---|---|---|---|---|
| N | 15,603 | 11,282 | 3951 | 2430 | 2060 | 1891 | |
| Age (years; mean ± SD) | 81.76 ± 8.91 | 80.5 ± 8.64 | 85.11 ± 8.78 | 86.73 ± 7.43 | 87.17 ± 7.09 | 82.87 ± 9.84 |
*⋆#⧧⨳◾⧥⫦⫩ < 0.0001 |
| Sex (woman) (%, n) | 48.42 (7555/15603) | 47.91 (5405/11282) | 49.99 (1975/3951) | 51.07 (1241/2430) | 51.75 (1066/2060) | 48.07 (909/1891) |
*⫩ < 0.05 ⋆# < 0.01 |
|
Hospital admissions (episodes 2 years before admission for CAP) (median, IQR) |
3.0 (1.0–5.0) | 3.0 (1.0–6.0) | 3.0 (1.0–5.0) | 3.0 (1.0–5.0) | 3.0 (1.0–5.0) | 3.0 (1.0–5.0) |
*#⧧⫦ < 0.0001 ⋆ < 0.001 ⨳⫩ < 0.01 |
|
Days of stay (days per admission) (median, IQR) |
6.2 (4.0–9.0) | 6.0 (3.69–9.0) | 6.4 (4.0–9.0) | 6.5 (4.0–9.40) | 6.25 (4.0–9.0) | 6.5 (4.0–9.0) |
*⧧⫦⫩ < 0.001 ◾⧥ < 0.01 ⋆ < 0.05 |
| Barthel on admission (mean ± SD) (median, IQR) | 65.0 (30.0–90.0) | 80.0 (55.0–100.0 | 32.5 (5.0–6.0) | 25.0 (0.0–55.0) | 25.0 (0.0–50.0) | 45.0 (15.0–70.0) |
*⋆#⧧◾⧥⫦⫩ < 0.0001 ⨳ < 0.001 |
| - Independent (96–100) (%,n) |
17.93 (1236/6892) |
25.97 (1127/4.339) |
4.07 (95/2332) |
3.82 (59/1545) |
3.40 (45/1324) |
4.96 (50/1008) |
*⋆#◾⧧⧥⫦⫩ < 0.0001 ⨳ < 0.01 |
| - Moderate dependency (61–96) (%,n) |
32.11 (2213/6892) |
40.17 (1743/4.339) | 17.97 (419/2332) |
14.50 (224/1545) |
13.07 (173/1324) |
24.40 (246/1008) | |
| - Severe dependency (21–61) (%,n) |
29.95 (2064/6892) |
26.27 (1140/4.339) | 35.98 (839/2332) |
34.50 (533/1545) |
33.84 (448/1324) |
38.79 (391/1008) | |
| - Total dependency (0–21) (%,n) |
20.00 (1379/6892) |
7.58 (329/4.339) | 41.98 (979/2332) |
47.18 (729/1545) |
49.70 (658/1324) |
31.84 (321/1008) | |
| MNA-SF on admission (mean ± SD) | 9.15 ± 6.03 | 10.43 ± 6.46 | 8.06 ± 5.63 | 7.46 ± 5.51 | 7.25 ± 5.3 | 8.83 ± 2.82 |
*⋆# < 0.0001 ⧧⫦⫩ < 0.01 |
| - Well-nourished (12–14) (%,n) | 2.95 (44/1494) | 5.01 (39/778) | 0.91 (6/658) | 1.44 (6/416) | 1.12 (4/358) | 20.1 (40/199) |
*⋆# < 0.0001 ⧧ < 0.01 ⫦⫩ < 0.05 |
| - At risk of MN (8–12) (%,n) | 8.63 (129/1494) | 11.44 (89/778) | 7.45 (49/658) | 5.05 (21/416) | 4.47 (16/358) | 46.23 (92/199) | |
| - Malnourished (0–7) (%,n) | 88.42 (1321/1494) | 83.55 (650/778) | 91.64 (603/658) | 93.51 (389/416) | 94.41 (338/358) |
33.67 (67/199) |
|
| Charlson (median, IQR) | 1.0 (0.0–3.0) | 1.0 (0.0–3.0) | 2.0 (0.0–4.0) | 2.0 (1.0–4.0) | 2.0 (1.0–4.0) | 1.0 (0.0–4.0) |
*⋆#⧧⧥⫦⫩ < 0.0001 ◾ < 0.001 |
| Diabetes mellitus (%,n) |
28.05 (4377/15603) |
27.73 (3128/11282) |
27.41 (1083/3951) |
33.33 (810/2430) |
31.26 (644/2060) |
23.22 (439/1891) |
◾⧥ < 0.01 ⋆⧧#⫩⫦ < 0.0001 |
| Chronic Obstructive Pulmonary Disease (%,n) |
3.99 (623/15603) |
3.72 (420/11282) |
4.56 (180/3951) |
4.49 (109/2430) |
4.17 (86/2060) |
4.97 (94/1891) |
*⧧ < 0.05 |
| Pulmonary fibrosis (%,n) |
0.47 (74/15603) |
0.55 (62/11282) |
0.30 (12/3951) |
0.16 (4/2430) |
0.19 (4/2060) |
0.42 (8/1891) |
# < 0.05 ⋆ < 0.01 |
| Acute renal disease (%,n) | 22.88 (3570/15603) | 19.80 (2234/11282) | 30.09 (1189/3951) | 33.42 (812/2430) | 32.28 (665/2060) | 27.71 (524/1891) |
*⋆#⧧⫦ < 0.0001 ⨳⫩ < 0.01 |
| Cerebrovascular disease (%,n) | 2.28 (355/15603) | 1.53 (173/11282) | 4.33 (171/3951) | 4.77 (116/2430) |
5.1 (105/2060) |
3.49 (66/1891 |
*⋆#⧧ < 0.0001 ⫦⫩ < 0.05 |
| Heart disease (%,n) | 18.55 (2894/15603) | 18.74 (2114/11282) | 17.19 (679/3951) | 19.88 (483/2430) | 18.54 (382/2060) | 15.71 (297/1891) |
⫦ < 0.001 ⨳⧧ < 0.01 *⫩ < 0.05 |
| Neurodegenerative disease (%,n) |
5.1 (796/15603) |
3.63 (410/11282) | 9.11 (360/3951) | 9.75 (237/2430) | 10.24 (211/2060) | 7.87 (149/1891) |
*⋆#⧧ < 0.0001 ⫦⫩ < 0.05 |
| Dementia (%,n) | 14.86 (2319/15603) | 6.52 (736/11282) | 37.81 (1494/3951) | 43.37 (1054/2430) | 46.84 (965/2060) | 27.97 (529/1891) |
*⋆#◾⨳⧧⧥⫦⫩ < 0.0001 ∘ < 0.05 |
| Delirium (%,n) | 15.64 (2441/15603) | 10.47 (1181/11282) | 28.90 (1142/3951) | 31.65 (769/2430) | 31.60 (2441/2060) | 25.97 (491/1891) |
*⋆#⧧⫦⫩ < 0.0001 ◾⨳⧥ < 0.05 |
| Hepatopathy (%,n) |
8.41 (1312/15603) |
8.99 (1014/11282) |
6.07 (240/3951) |
7.16 (176/2430) |
5.63 (116/2060) |
6.56 (124/1891) |
*# < 0.0001 ⋆ < 0.01 ∘ < 0.05 |
| Neoplasia (%,n) | 14.61 (2279/15603) | 15.49 (1748/11282) | 11.57 (457/3951) | 12.92 (314/2430) | 11.65 (240/2060) | 11.48 (217/1891) |
*#⧧ < 0.0001 ⧧ < 0.001 ⋆ < 0.01 |
MNA-SF: mini-nutritional assessment short form; MN: malnutrition; AIMS-OD: artificial intelligence massive screening for oropharyngeal dysphagia; ICD-10 J69.0: International Classification of diseases (ICD-10) code for aspiration pneumonia; IQR: interquartile range.
Longitudinal variables were taken from the two years prior to admission for community-acquired pneumonia (CAP). Transversal variables from the admission for CAP.
Statistical comparisons:
* Statistically significant Group A (AIMS-OD < 0.7 + no ICD-10 J69.0) vs. Group B (AIMS-OD > 0.7).
⋆ Statistically significant Group A (AIMS-OD < 0.7 + no ICD-10 J69.0) vs. Group C (ICD-10 J69.0).
# Statistically significant Group A (AIMS-OD < 0.7 + no ICD-10 J69.0) vs. Group D (AIMS-OD > 0.7 + ICD-10 J69.0).
⧧ Statistically significant Group A (AIMS-OD < 0.7 + no ICD-10 J69.0) vs. Group E (AIMS-OD > 0.7 + no ICD-10 J69.0).
⨳ Statistically significant Group B (AIMS-OD > 0.7) vs. Group C (ICD-10 J69.0).
◾ Statistically significant Group B (AIMS-OD > 0.7) vs. Group D (AIMS-OD > 0.7 + ICD-10 J69.0).
⧥ Statistically significant Group B (AIMS-OD > 0.7) vs. Group E (AIMS-OD > 0.7 + no ICD-10 J69.0).
∘ Statistically significant Group C (ICD-10 J69.0) vs. Group D (AIMS-OD > 0.7 + ICD-10 J69.0).
⫦ Statistically significant Group C (ICD-10 J69.0) vs. Group E (AIMS-OD > 0.7 + no ICD-10 J69.0).
⫩ Statistically significant Group D (AIMS-OD > 0.7 + ICD-10 J69.0) vs. Group E (AIMS-OD > 0.7 + no ICD-10 J69.0).
Description of the prevalence of clinical diagnosis of AP and AP identification through AIMS-OD
According to the standard clinical practice at CSdM leading to a ICD-10 diagnosis code J69.0 (AP), 2,430 (15.57%) patients were diagnosed with AP (Group C) in the entire study sample of older patients admitted for CAP during the study period. However, only 6.27% (n = 978) of the study population underwent a formal clinical assessment of OD via the V-VST, of whom 548 showed clinical signs of OD. Among those assessed with V-VST, 376 had an AIMS-OD value > 0.7, and 272 patients had both a positive V-VST and an AIMS-OD value > 0.7. When we considered the prevalence of AP by identifying patients with OD according to AIMS-OD (Group B), we found that up to 3,951 patients were estimated to have AP (25.32%). AIMS-OD identified 84.77% of patients (n = 2060) with AP already clinically diagnosed by the clinicians with the ICD-10 J69.0 code (Group D) and an additional group of 1,891 patients from Group E with AP unrecognized by the clinicians and without the J69.0 codification. Thus, AIMS-OD increased the detection of AP related to OD by 62.6% versus standard clinical practice at our institution. Finally, we found that only 370 patients of 15,603 (2.37%) were clinically identified with the J69.0 ICD-10 code for AP but had a low risk according to AIMs-OD (< 0.7) (Group F).
Phenotypic characteristics of study groups
Table 1 shows the comparisons between sub-groups defined in this study. We found three main groups of results when analyzing the comparisons performed between all patients admitted for CAP: (1) Differences between Group A without AP (Patients with CAP without AP) and groups with AP, Group B (Patients having AP according to AIMS-OD), Group C (Patients with AP diagnosis according to standard clinical practice), Group D (Patients with estimated AP according to both AIMS-OD risk and ICD-10 diagnosis codification for AP during the admission for CAP) or Group E (Patients having AP according to AIMS-OD and not clinically diagnosed); (2) Differences between Groups B and C; and (3) Differences between Groups B, C and D versus Group E.
We found clear phenotypic differences when comparing patients in Group A (CAP patients without AP or its risk) with those in the other four groups. Although the comparison between all groups shows statistically significant differences due to the large number of patients included in the study (p-values in the table), when we evaluated the effect size of these differences mathematically, we found significant differences only between patients without AP or its risk and all subgroups of patients with AP or its risk, with less effect when comparing Group A versus E (patients with OD diagnosis according to AIMS-OD and potential AP according to the AIMS-OD algorithm, but not clinically detected) (Additional file 2). CAP patients without AP or its risk (Group A) were younger, with better functional status with only 7.58% of patients being totally dependent, better nutritional status, fewer comorbidities (lower Charlson Comorbidity Index), and a lower prevalence of acute renal disease, delirium, dementia, and neurodegenerative diseases.
Patients in Group B (patients with AP according to AIMS-OD) and C (patients with clinical diagnosis of AP according to ICD-10 J69.0 code) had similar clinical characteristics, as shown in Table 1. Some significant differences were found when comparing the two subgroups of patients. However, they had a negligible effect when we assessed the effect size mathematically of these differences for all variables included in Table 1 (Additional file 2). This indicates the similarity between the two groups and that AIMS-OD can identify the same phenotype of patients with AP as those clinically diagnosed with the ICD-10 J69.0 code. The combination of Groups B and C (Group D) determines those patients with estimated AP according to both AIMS-OD risk and ICD-10 diagnosis during the admission for CAP. As a result, Group D shows a reliable phenotype of patients with probable AP. These are patients of advanced age (87.17 ± 7.09 years), with an equal gender distribution, severely impaired functional capacity (BI 30.19 ± 30.28; median 25.0 (0.0–50.0)), with almost half classified as totally dependent (49.70%), and moderate comorbidities (CCI 2.69 ± 2.73; median 2.0 (1.0–4.0)), highlighting a high prevalence of dementia and neurodegenerative diseases compared to patients in Group A.
Patients in Group E, identified by AIMS-OD but not by clinical practice, had intermediate clinical severity compared with Group A (no AP, better clinical status group) and Groups B, C and D (patients with clinical + AIMS-OD diagnosis of AP, worse clinical status groups). Compared with Group A, patients in Group E were older (small effect), had better nutritional status (MNA-sf) and higher prevalence of delirium (small effect size), and lower functional capacity (large effect size). Compared with Groups B and C and their sum, those in Group E were younger, had better functional and nutritional status and lower prevalence of dementia, showing small or medium effect sizes in the mentioned variables (Additional file 2).
Discussion
This research assessed the underdiagnosis of AP in older hospitalized patients with acute pneumonia in a general hospital. The main findings of this study show a prevalence of AP of 15.57% according to standard clinical diagnosis reported with the ICD-10 codes among older patients with acute CAP. However, when using AIMS-OD, an AI-based tool that analyses EHR to identify patients at high risk of OD, and consequently AP, the estimated prevalence of AP increased to 25.32%. This potentially leaves 12.12% (Group E, 1890 patients) of older patients with acute CAP underdiagnosed and undertreated for the main risk factors associated with AP, as this study clearly suggests we may be missing patients with our current clinical risk assessment. This specific group of clinically underdiagnosed AP patients presents less severe clinical characteristics as they are younger, with better functional and nutritional status than those clinically identified by standard clinical AP diagnosis. The identification of this new phenotype of patients as having AP according to the OD risk determination by AIMS-OD, provides an opportunity to identify these patients early and avoid potential complications by treating OD and therefore improving their clinical outcomes and QoL through simple interventions such as the minimal-massive intervention (MMI) [41]. MMI is a multimodal intervention focused on preventing the main risk factors associated with AP (OD and aspiration, impaired health status and vulnerability and poor oral health and hygiene) [11, 41, 42]. The MMI is a based on: (1) fluid rheological adaptation to avoid deglutition safety impairments; (2) textural adaptation of solid food and nutritional supplementation to improve nutritional status; and (3) oral hygiene recommendations to reduce oral bacterial load and potential colonization by respiratory pathogens [41]. A previous study showed that MMI reduced 6-month hospitalizations for lower respiratory tract infections and improved survival [41]. In addition, we found that AIMS-OD identified up to 84.77% of patients classified by standard clinical practice with ICD-10 J69.0 code, indicating that AIMS-OD can accurately identify the same phenotype of patients with AP as those diagnosed by clinicians using clinical tools. Notably, only 370 patients (2.37% of the total cohort; Group F) were clinically diagnosed with AP but had a low risk according to AIMS-OD (score < 0.7), suggesting a relatively small proportion of cases not captured by the algorithm. This reinforces the potential of AIMS-OD as a reliable screening tool for detecting AP-related risk based on OD. The AIMS-OD software allows massive, immediate and accurate identification of OD -and therefore AP cases- on hospital admission, allowing early and specific treatment with directed antibiotics and early treatment for OD to improve the poor clinical outcomes of these patients.
In this retrospective study, we decided to apply an AIMS-OD threshold of 0.7 to prioritize specificity (0.92) and PPV (0.86), ensuring that those identified as at risk were highly likely to have OD and therefore AP. This allowed us to estimate a prevalence of 25.3%, which already exceeds that of routine clinical practice (15.6%). However, due to the low sensitivity at this threshold (0.43), the true prevalence might be even higher. Notably, prior studies using videofluoroscopy (the gold standard for OD) in older CAP patients at our institution have reported OD prevalence rates up to 75% (53% impaired safety; 16.7 silent aspirations) [25], supporting this interpretation. In future prospective applications, a lower cut-off (0.46) will be used to enhance sensitivity and allow AIMS-OD to function as an effective screening tool for early risk detection and intervention.
The overall study population consisted of older patients (81.76 ± 8.91 years) who had been admitted approximately 4 times in the previous 2 years with an average of 7.58 admission days. In terms of functionality, the Barthel Index was severely impaired, with the majority of patients having some degree of dependency. Regarding nutritional status, a nutritional evaluation using the Mini-Nutritional Assessment Short Form (MNA-SF) was conducted on a subset of patients referred for nutritional assessment. The prevalence of malnutrition (MN) was 88.42%, while 8.63% were at risk of MN and only 2.95% of the patients had a proper nutritional status. The sample, however, is biased as it only included patients who had already been identified by healthcare professionals as potentially having malnutrition or at risk for it and thus, the resulting results on MN should be interpreted with caution. In a similar cohort of patients admitted for acute illness and properly assessed for MN, the prevalence of MN was 30.6% [24]. In our study, patients had a mild CCI score of 2.07 ± 3.16 points. The most prevalent comorbidities were acute renal disease (22.88%), heart disease (18.55%), delirium (15.64%), dementia (14.86%), and neoplasia (14.61%). These phenotypic characteristics of our population are consistent with those observed in our previous studies and in other populations of older patients admitted with pneumonia, demonstrating a faithful representation of this group within the context of pneumonia-related hospital admissions [25, 30, 43–46]. The primary population affected by pneumonia, particularly CAP, in hospitalized patients in southern Europe is of older adults, particularly those aged 65 years and older, showing the potential clinical utility of an AI-based algorithm, developed for this specific population, for the identification of OD, the main risk factor for AP [43–46].
The study population was divided into five subgroups to analyze risk factors and clinical characteristics associated with AP. Patients in Group A (without OD) were generally younger, with better functional and nutritional status and fewer comorbidities. In contrast, Groups B and C (AP risk or AP diagnosis) shared similar clinical features, demonstrating an 84.77% overlap between AIMS-OD identification and standard ICD-10 J69.0 coding. Group D, which combined these two, represented a well-defined AP phenotype with advanced age, severe functional impairment, and a high prevalence of dementia and neurodegenerative diseases. Meanwhile, Group E (patients identified by AIMS-OD but not by clinical diagnosis) exhibited an intermediate profile, suggesting that these individuals—often overlooked due to their milder health status—could benefit from early identification and targeted interventions to prevent complications including mortality.
These findings highlight the importance of a systematic and multidimensional assessment in all older patients hospitalized with pneumonia. Beyond diagnosing OD and identifying AP risk using tools like AIMS-OD, clinicians should routinely assess patients’ functionality, nutritional status, and oral hygiene using validated clinical tools. Implementing this structured assessment approach in routine care would allow for early and individualized interventions, such as the MMI, improving patient outcomes, preventing complications such as recurrent pneumonia, and optimizing healthcare resource use.
Underdiagnosis of AP has significant clinical implications, as it can lead to inadequate treatment (inappropriate antibiotic treatment or interventions to address underlying risk factors like OD), recurrent pneumonia due to failure to address the underlying causes of aspiration, lost opportunities to treat the underlying causes, increased morbidity and mortality, and higher healthcare costs (recurrent hospitalizations and prolonged hospital stays) [33, 47, 48]. An important consideration to keep in mind is that some patients with OD can also develop pneumonia unrelated to aspiration. For example, infections caused by microorganisms such as influenza virus, SARS-CoV-2, Legionella pneumophila, or Mycoplasma pneumoniae are not classified as AP. However, based on the current underdiagnosis of AP, we and others suggest that future clinical practice should prioritize the classification of pneumonia occurring in patients with OD as AP [11, 20, 49]. While this may contribute to marginal overdiagnosis, it is a pragmatic approach to managing this complex problem.
Though the common practice of treating all AP with antibiotics that cover for anaerobics is now being reconsidered, and the newest European and American guidelines recommend against this practice, it should still be considered depending on clinical information including history, comorbidities, oral hygiene status, past culture results and severity [3, 4, 49]. Formerly, anaerobic bacteria were considered predominant pathogens in AP. Recent studies, however, indicate a decreasing role of anaerobes and an increasing prevalence of Gram-negative bacteria (GNB) such as Escherichia coli, Klebsiella pneumoniae, and Pseudomonas aeruginosa [12]. Frail older patients with OD were found to have impaired health status, poor oral health, high oral bacterial load, and prevalence of oral colonization by gram negative bacteria and respiratory pathogens and VFS signs of impaired safety of swallow, and were therefore at risk for contracting AP [15, 50]. Studies have shown no difference in outcomes between standard CAP therapy and those specifically targeting anaerobic bacteria, although anaerobic coverage may be considered in cases of poor oral hygiene [2]. Overuse of broad-spectrum antibiotics with anti-anaerobic activity can lead to Clostridioides difficile infection, development of multi-drug-resistant organisms, adverse drug reactions, and higher costs. Therefore, the international guidelines (such as European and American guidelines) recommend the use of standard CAP therapy in cases of AP [2–4]. In other words, in patients with CAP and aspiration risk factors, the guidelines issue an ungraded, good practice statement advising the prescription of a standard CAP therapy regimen and not specific therapy targeting anaerobic bacteria. The rationale provided is that most standard antibiotic regimens for sCAP (e.g., beta-lactam/beta-lactamase inhibitors, carbapenems, moxifloxacin) already contain some anti-anaerobic coverage, making specifically targeted anaerobic therapies not more effective. This approach aims to simplify therapy and reduce the risk of antibiotic resistance and C. difficile infection.
On the other hand, treatment directed at compensating for swallowing impairments has also to be taken into account as OD and aspiration is the main risk factor for the development of AP. Several studies have recommended the use of rheological adaptation of fluids and textural adaptation of solid food to avoid aspiration together with nutritional and oral hygiene recommendations [41, 51–53]. Multimodal strategies, like MMI, compared with the usual care, have shown clinical improvements in older patients admitted for acute disease at mid term by improving nutritional status, functionality and reducing mortality and hospital admissions for lower respiratory tract infections [41]. Patients with AP have the right to receive the best treatment, which in this case will be timely antibiotic and compensatory treatment for their swallowing and nutritional deficiencies.
Several factors contribute to the underdiagnosis of AP when relying solely on standard clinical methods. These include the suboptimal “clinical judgement” criteria for OD from our clinicians, and the lack of routine swallowing screening and assessments in older CAP patients, a universally agreed definition of AP, standardized diagnostic criteria for AP [11, 22, 28], and an established set of clinical competencies on the management of AP to be taught to medical professionals [54]. These factors can be mitigated with education of healthcare professionals on OD and its nutritional and respiratory complications and with the use of new diagnostic algorithms of AP taking into account OD, including massive screening AI tools that allow clinical decision support and more awareness among healthcare professionals.
AIMS-OD offers a potential solution to the problem of AP underdiagnosis. AIMS-OD can analyze EHR and improve the diagnosis of AP in older patients by identifying a larger number of patients with a high risk of OD, and therefore at risk of AP, than traditional diagnostic methods. AIMS-OD is able to achieve these results by utilizing AI and a machine learning approach, showing the risk of OD on clinicians’ desktops for all patients admitted to the hospital and allowing the screening of thousands of patients in a few seconds [36].
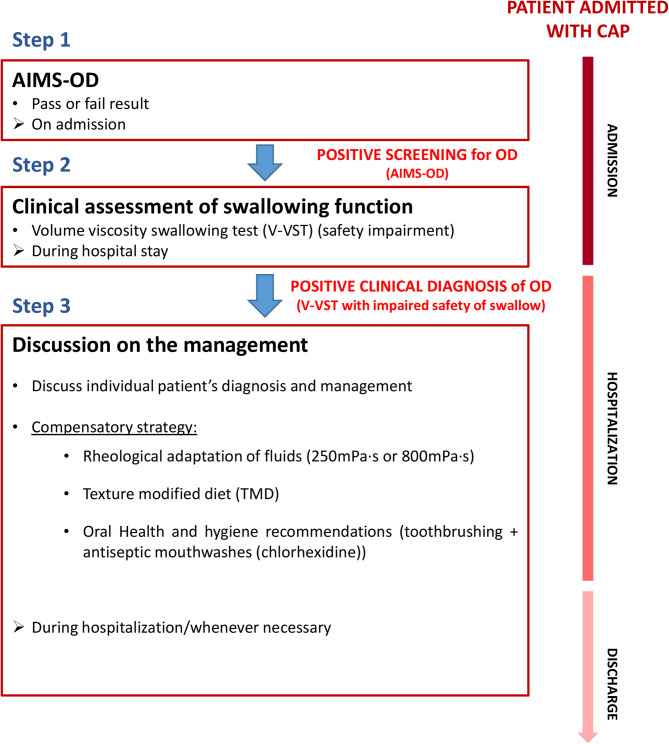
Currently, there is a lack of standardized criteria for the diagnosis of AP [22, 28]. However, AP is a significant health concern, particularly among older adults, and is often underdiagnosed in standard clinical practice [11, 22, 28]. The underdiagnosis of AP can lead to poor clinical outcomes, including recurrent pneumonia and readmissions, reduced functionality, frailty, and increased mortality [11]. According to recent literature, OD and tracheobronchial aspiration should be taken into account as relevant risk factors in older patients admitted with pneumonia [11, 22, 28, 49], and thus, an algorithm for the detection of AP should include this aspect. On the basis of our previous publication on the development of a clinical algorithm for AP diagnosis [49], that included aspiration risk assessment and OD clinical evaluation, and the development of AI-screening assisted tools [36], we propose to develop and implement an AP diagnosis algorithm including the AI-assisted screening tool AIMS-OD [11]. This algorithm (Fig. 2) will help to systematically screen all hospitalized patients with CAP for OD risk that would indicate a high risk of having AP. The algorithm leverages machine learning and AI to enhance the efficiency of diagnosing AP and can be utilized on patient admission. An older patient admitted with pneumonia and identified as at risk for OD by AIMS-OD on admission should undergo further evaluation using a clinical test, such as the V-VST, conducted by trained healthcare professionals. If the V-VST indicates swallowing safety impairments, diagnosis of AP is discussed and confirmed with the patient’s physician and, once agreed, proper antibiotic treatment and the MMI is implemented alongside the use of postures or maneuvers, as needed (Fig. 2) [11].
Fig. 2.
Proposed diagnosis algorithm for AP in patients hospitalized with community-acquired pneumonia (CAP). AIMS-OD: artificial intelligence massive screening for oropharyngeal dysphagia; +: positive Modified from Ortega O 2024 [11]
The study’s findings have several important implications for clinical practice. First of all, it emphasizes the need for increased awareness of AP, its diagnosis and treatment by a multidisciplinary team. The study also supports the implementation of a routine systematic swallowing screening for older patients admitted with CAP to identify those at risk of OD and AP. Finally, it demonstrates the value of integrating automatic AI-based tools like AIMS-OD into clinical practice to improve the accuracy and timeliness of AP diagnosis, even identifying those patients who might be missed by standard clinical methods alone. These clinical decision support tools present an essential value proposition for clinicians by increasing the number of patients with OD detected automatically (currently done by questionnaires and interviews) in a few seconds. This offers an opportunity to perform more valuable actions on older patients, taking into account our ageing society, where healthcare professionals will encounter very old patients with AP in various settings. This new diagnosis algorithm can lead to improved patient outcomes, including reduced readmissions for recurrent pneumonia or lower tract respiratory infections, decreased morbidity and mortality, and lower healthcare costs. Proactive approaches to AP diagnosis and management ultimately enhance patient safety and improve the overall quality of care delivered to older patients hospitalized with CAP.
The study has some limitations, the first being that the data obtained are retrospective and, although from a large database, from a single center. The second is that although there is no other ICD-10 code for AP, the established one (J69.0) may not be accurate enough to identify the true prevalence of AP in older patients with CAP. This is because the code includes AP resulting from regurgitated food, gastric secretions, or vomit, which are not the primary mechanisms observed in our patient cohort. These mechanisms are more commonly associated with patients in unconscious states, such as those in the ICU or those under the influence of alcohol. Additionally, the codification of AP is mainly performed when the physician observes overt aspiration (i.e., ‘Pneumonitis due to solids and liquids’), and AP caused by silent aspiration may be overlooked if this is considered. Moreover, the assignment of the ICD-10 J69.0 code ultimately depends on the clinician’s judgment and may vary depending on their awareness of AP and the thoroughness of their assessment. The authors advocate for improvement and specialization of the ICD-10 codification and for the use of AI-based tools, alongside routine swallowing assessments, to improve the identification of patients at risk of AP and provide a more comprehensive assessment. However, there is still a need for external prospective validation of our AIMS-OD tool with the gold standard (VFS of fiberoptic endoscopic evaluation of swallow) before the model is adopted in clinical practice. Another limitation of the study is that some clinical tests were not systematically performed on all hospitalized patients included and the data available was limited (MNA, Barthel or Charlson) showing some bias through the more affected patients who had the tests performed and, therefore, the characterization of the study sample was tailored by data available in the EHR of patients. Finally, patient follow up (readmissions and respiratory infections, among others) was unavailable because the data provided is pseudo-anonymous and non-identifying according to current legal regulations (LOPD 3/2018). The same occurs with mortality, which is not recorded in the hospital’s EHR unless the patient’s death occurs during hospital admission. These variables would have given us a better idea of clinical evolution in the different subgroups.
Future research on implementing the proposed diagnostic algorithm for AP that includes the systematic screening of OD in clinical practice could have several important directions. One key area of investigation is the impact of the diagnostic algorithm implementation on CAP patient outcomes, including the incidence of recurrent pneumonia, readmissions, length of hospital stay, and both morbidity and mortality rates. Understanding how it influences these outcomes will provide valuable insights into its effectiveness in improving patient care. Additionally, evaluating the cost-effectiveness of using AIMS-OD within the AP diagnostic algorithm for older patients with CAP could help determine its economic viability and potential for widespread adoption. Another essential avenue for future research is the development and validation of standardized diagnostic criteria for AP. This would ensure greater diagnostic accuracy and consistency across various healthcare settings, ultimately improving clinical decision-making and patient outcomes.
Conclusions
AP is an underdiagnosed entity among hospitalized older patients with CAP according to standard clinical practice at Mataró University Hospital. The introduction of the use of AI-based tools in clinical practice for the systematic screening of OD to assist clinicians in its diagnosis (such as AIMS-OD) will increase the number of patients diagnosed with AP, ensure specific treatment, and allow early and specific clinical management of OD to avoid further aspirations, and nutritional and respiratory complications including hospital readmissions for the same cause. This improvement will ensure a safe and effective deglutition, proper nutrition, and the reduction of oral bacterial load by improving patients’ oral health and hygiene, potentially improving the poor health outcomes associated with AP in these patients. However, clinical validation of these AI-based tools against the gold standard for swallowing evaluation is still needed in order to apply the algorithm in real clinical practice.
Supplementary Information
Acknowledgements
The authors would like to thank Jane Lewis for the English revision of the manuscript. This document is a component of Clàudia Sitges-Milà’s doctoral dissertation. This work has been carried out within the framework of the Doctorate in Medicine Program of the Universitat Autònoma de Barcelona (UAB). The authors, particularly Sitges-Milà, express their gratitude to the Department of Medicine and the Doctoral School at UAB for providing a valuable opportunity for professional development.
Authors’ contributions
Conceptualization: AMM, OO, PC; Data gathering: CA, JM; Data analysis and statistics: CA, JM; Interpretation of results: AMM, CSM, OO, PC, RB, YY, DM; Writing– original draft: AMM, CSM, OO; Writing– review & editing: AMM, CSM, OO, PC, RB, YY, DM. All authors read and approved the final manuscript.
Funding
The following research grants supported this paper:
- Instituto de Salud Carlos III (ISCIII), Investigación Clínica Independiente: ICI20/00117; and co-funded by the European Union (FEDER funds).
- Instituto de Salud Carlos III (ISCIII), Fondo de Investigaciones Sanitarias: PI22/01101; and co-funded by the European Union (FEDER funds).
- The transformative project “Implantació d’un protocol d’innovació assistencial pel maneig de disfàgia orofaríngia: cribratge sistemàtic i protocol·lització del tractament compensatori (Disfàgia Orofaríngia)”, with code PT-092023-CSC, is promoted and funded by Servei Català de la Salut and is part of the transformative projects of SISCAT with non-finalist affected funds (FANF). Departament de Recerca i Universitats de la Generalitat de Catalunya. Agència de Gestió d'Ajuts Universitaris i de Recerca. Generalitat de Catalunya. Suport a la Gestió de la Recerca: 2021 SGR 01344. The content of this publication reflects only the opinions of the authors. CatSalut is not responsible for the use that may be made of the information contained herein.
Data availability
The datasets generated and/or analyzed during the current study are not publicly available due to institutional policies and ethical regulations regarding patient data management. However, the anonymized dataset may be available from the corresponding author upon reasonable request and subject to institutional approval.
Declarations
Ethics approval and consent to participate
The study protocol was approved by the Ethics Committee (EC) of the CSdM (code: CEIm 35/23), which waived the need for informed consent as this was a retrospective study.
Consent for publication
Not applicable.
Competing interests
- Martín-Martínez and P. Clavé are co-founders of AIMS-medical, the spin-off that developed AIMS-OD. -J. Miró and C. Amadó are employees of AIMS-medical.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Alberto Martín-Martínez and Clàudia Sitges-Milà contributed equally to this work.
Contributor Information
Pere Clavé, Email: pere.clave@ciberehd.org.
Omar Ortega, Email: oortega@csdm.cat.
References
- 1.Torres A, Cilloniz C, Niederman MS, Menéndez R, Chalmers JD, Wunderink RG, et al. Pneumonia Nat Rev Dis Primers. 2021;7(1):25. [DOI] [PubMed] [Google Scholar]
- 2.Calabretta D, Martìn-Loeches I, Torres A. New guidelines for severe Community-acquired pneumonia. Semin Respir Crit Care Med. 2024;45(2):274–86. [DOI] [PubMed] [Google Scholar]
- 3.Metlay JP, Waterer GW, Long AC, Anzueto A, Brozek J, Crothers K, et al. Diagnosis and treatment of adults with Community-acquired pneumonia. An official clinical practice guideline of the American thoracic society and infectious diseases society of America. Am J Respir Crit Care Med. 2019;200(7):e45–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martin-Loeches I, Torres A, Nagavci B, Aliberti S, Antonelli M, Bassetti M, et al. ERS/ESICM/ESCMID/ALAT guidelines for the management of severe community-acquired pneumonia. Eur Respir J. 2023;61(4):2200735. [DOI] [PubMed] [Google Scholar]
- 5.Lim WS, Baudouin SV, George RC, Hill AT, Jamieson C, Le Jeune I, et al. BTS guidelines for the management of community acquired pneumonia in adults: update 2009. Thorax. 2009;64(Suppl 3):iii1–55. [DOI] [PubMed] [Google Scholar]
- 6.Yatera K, Yamasaki K. Management of the diagnosis and treatment of pneumonia in an aging society. Intern Med. 2025;64(4):503–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trotter CL, Stuart JM, George R, Miller E. Increasing hospital admissions for pneumonia, England. Emerg Infect Dis. 2008;14(5):727–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martin-Loeches I, Timsit JF, Kollef MH, Wunderink RG, Shime N, Nováček M, et al. Clinical and Microbiological outcomes, by causative pathogen, in the ASPECT-NP randomized, controlled, phase 3 trial comparing ceftolozane/tazobactam and meropenem for treatment of hospital-acquired/ventilator-associated bacterial pneumonia. J Antimicrob Chemother. 2022;77(4):1166–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Almirall J, Yoshimatsu Y, Scannapieco FA. Chapter 6.4 - Respiratory infections and aspiration pneumonia. In: Clavé P, Ortega O, editors. A Multidisciplinary Approach to Managing Swallowing Dysfunction in Older People. Academic Press, Elsevier; 2024. p. 169–77. 10.1016/B978-0-323-91686-8.00046-X. Cited 10 Feb 2025.
- 10.Ortega O. Doctoral Thesis. Study of oral microbiota and respiratory complications of oropharyngeal dysphagia: physiopathology, diagnosis and treatment of risk factors of oropharyngeal dysphagia and aspiration pneumonia in older patients. Universitat Autònoma de Barcelona; 2016. ISBN: 9788449065613. http://hdl.handle.net/10803/393975.
- 11.Ortega O, Guidotti L, Yoshimatsu Y, Sitges C, Martos J, Miró J, et al. Swallowing and aspiration: how to evaluate and treat swallowing disorders associated with aspiration pneumonia in older persons. Semin Respir Crit Care Med. 2024;45(6):678–93. [DOI] [PubMed] [Google Scholar]
- 12.Fadell F, Saliba R, El-Solh AA. Bacteriology of aspiration pneumonia: the lung Microbiome and the changing microbial etiology. Semin Respir Crit Care Med. 2024;45(6):626–33. [DOI] [PubMed] [Google Scholar]
- 13.Almirall J, Boixeda R, de la Torre MC, Torres A. Aspiration pneumonia: A renewed perspective and practical approach. Respir Med. 2021;185(February):106485. [DOI] [PubMed] [Google Scholar]
- 14.Mira A, Simon-Soro A, Curtis MA. Role of microbial communities in the pathogenesis of periodontal diseases and caries. J Clin Periodontol. 2017;44(Suppl 18):S23–38. [DOI] [PubMed] [Google Scholar]
- 15.Ortega O, Sakwinska O, Combremont S, Berger B, Sauser J, Parra C, et al. High prevalence of colonization of oral cavity by respiratory pathogens in frail older patients with oropharyngeal dysphagia. Neurogastroenterol Motil. 2015;27(12):1804–16. [DOI] [PubMed] [Google Scholar]
- 16.Connolly MJ. Of proverbs and prevention: aspiration and its consequences in older patients. Age Ageing. 2009;39(1):2–4. [DOI] [PubMed] [Google Scholar]
- 17.Yoshimatsu Y, Smithard DG. A paradigm shift in the diagnosis of aspiration pneumonia in older adults. J Clin Med. 2022;11(17):5214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smithard DG, Yoshimatsu Y. Pneumonia, Aspiration Pneumonia, or Frailty-Associated Pneumonia? Geriatr (Basel). 2022;7(5):115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoshimatsu Y, Thomas H, Thompson T, Smithard DG. Prognostic factors of poor outcomes in pneumonia in older adults: aspiration or frailty? Eur Geriatr Med. 2024;15(2):481–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.The Japanese Respiratory Society. The JRS guidelines for the management of pneumonia in adults. Tokyo: Medical Review Co; 2024. [DOI] [PubMed] [Google Scholar]
- 21.Rodriguez AE, Restrepo MI. New perspectives in aspiration community acquired pneumonia. Expert Rev Clin Pharmacol. 2019;12(10):991–1002. [DOI] [PubMed] [Google Scholar]
- 22.Mandell LA, Niederman MS. Aspiration pneumonia. N Engl J Med. 2019;380(7):651–63. [DOI] [PubMed] [Google Scholar]
- 23.Serra-Prat M, Hinojosa G, Lõpez D, Juan M, Fabré E, Voss DS, et al. Prevalence of oropharyngeal dysphagia and impaired safety and efficacy of swallow in independently living older persons. J Am Geriatr Soc. 2011;59(1):186–7. [DOI] [PubMed] [Google Scholar]
- 24.Carrión S, Cabré M, Monteis R, Roca M, Palomera E, Serra-Prat M, et al. Oropharyngeal dysphagia is a prevalent risk factor for malnutrition in a cohort of older patients admitted with an acute disease to a general hospital. Clin Nutr. 2015;34(3):436–42. [DOI] [PubMed] [Google Scholar]
- 25.Almirall J, Rofes L, Serra-Prat M, Icart R, Palomera E, Arreola V, et al. Oropharyngeal dysphagia is a risk factor for community-acquired pneumonia in the elderly. Eur Respir J. 2013;41(4):923–6. [DOI] [PubMed] [Google Scholar]
- 26.El-Ghamrawy A, Shokeir MH, Esmat AA. Effects of low-dose hydrocortisone in icupatients with severe community-acquired pneumonia. Egypt J Chest Dis Tuberc. 2006;2006(55):91–9. [Google Scholar]
- 27.Marik PE, Kaplan D. Aspiration pneumonia and dysphagia in the elderly. Chest. 2003;124(1):328–36. [DOI] [PubMed] [Google Scholar]
- 28.Yoshimatsu Y, Melgaard D, Westergren A, Skrubbeltrang C, Smithard DG. The diagnosis of aspiration pneumonia in older persons: a systematic review. Eur Geriatr Med. 2022;13(5):1071–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Teramoto S, Fukuchi Y, Sasaki H, Sato K, Sekizawa K, Matsuse T. High incidence of aspiration pneumonia in community- and hospital acquired pneumonia in hospitalized patients: A multicenter, prospective study in Japan. Jornal Am Geriatr Soc. 2008;56(3):577–9. [DOI] [PubMed] [Google Scholar]
- 30.Cabre M, Serra-Prat M, Palomera E, Almirall J, Pallares R, Clavé P. Prevalence and prognostic implications of dysphagia in elderly patients with pneumonia. Age Ageing. 2010;39(1):39–45. [DOI] [PubMed] [Google Scholar]
- 31.Márquez-Sixto A, Navarro-Esteva J, Batista-Guerra LY, Simón-Bautista D, Rodríguez-de Castro F. Prevalence of oropharyngeal dysphagia and its value as a prognostic factor in Community-Acquired pneumonia: A prospective Case-Control study. Cureus. 2024;16(3):e55310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Melgaard D, Baandrup U, Bøgsted M, Bendtsen MD, Hansen T. The prevalence of oropharyngeal dysphagia in Danish patients hospitalised with Community-Acquired pneumonia. Dysphagia. 2017;32(3):383–92. [DOI] [PubMed] [Google Scholar]
- 33.Yoshimatsu Y, Tobino K, Ko Y, Yasuda M, Ide H, Oku Y. Careful history taking detects initially unknown underlying causes of aspiration pneumonia. Geriatr Gerontol Int. 2020;20(8):785–90. [DOI] [PubMed] [Google Scholar]
- 34.Teramoto S. The current definition, epidemiology, animal models and a novel therapeutic strategy for aspiration pneumonia. Respir Investig. 2022;60(1):45–55. [DOI] [PubMed] [Google Scholar]
- 35.Riera SA, Marin S, Serra-Prat M, Tomsen N, Arreola V, Ortega O, et al. A systematic and a scoping review on the psychometrics and clinical utility of the Volume-Viscosity swallow test (V-VST) in the clinical screening and assessment of oropharyngeal dysphagia. Foods. 2021;10(8):1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martin-Martinez A, Miró J, Amadó C, Ruz F, Ruiz A, Ortega O, et al. A systematic and universal artificial intelligence screening method for oropharyngeal dysphagia: improving diagnosis through risk management. Dysphagia. 2023;38(4):1224–37. [DOI] [PubMed] [Google Scholar]
- 37.Mahoney FI, Barthel DW. Functional evaluation: the Barthel index. Maryland State Med J. 1965;14:56–61. [PubMed] [Google Scholar]
- 38.Kaiser MJ, Bauer JM, Ramsch C, Uter W, Guigoz Y, Cederholm T, et al. Validation of the Mini nutritional assessment short-form (MNA-SF): a practical tool for identification of nutritional status. J Nutr Health Aging. 2009;13(9):782–8. [DOI] [PubMed] [Google Scholar]
- 39.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83. [DOI] [PubMed] [Google Scholar]
- 40.Virtanen P, Gommers R, Oliphant TE, Haberland M, Reddy T, Cournapeau D, et al. SciPy 1.0: fundamental algorithms for scientific computing in Python. Nat Methods. 2020;17(3):261–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martín A, Ortega O, Roca M, Arús M, Clavé Civit P. Effect of a Minimal-Massive intervention in hospitalized older patients with oropharyngeal dysphagia: A proof of concept study. J Nutr Health Aging. 2018;22(6):739–47. [DOI] [PubMed] [Google Scholar]
- 42.Ortega O, Clavé P. Oral hygiene, aspiration, and aspiration pneumonia: from pathophysiology to therapeutic strategies. Curr Phys Med Rehabilitation Rep. 2013;1(4):292–5. [Google Scholar]
- 43.Vila-Corcoles A, Ochoa-Gondar O, Rodriguez-Blanco T, Raga-Luria X, Gomez-Bertomeu F, EPIVAC Study Group. Epidemiology of community-acquired pneumonia in older adults: a population-based study. Respir Med. 2009;103(2):309–16. [DOI] [PubMed] [Google Scholar]
- 44.Froes F, Diniz A, Mesquita M, Serrado M, Nunes B. Hospital admissions of adults with community-acquired pneumonia in Portugal between 2000 and 2009. Eur Respir J. 2013;41(5):1141–6. [DOI] [PubMed] [Google Scholar]
- 45.Navarro-Torné A, Montuori EA, Kossyvaki V, Méndez C. Burden of Pneumococcal disease among adults in Southern Europe (Spain, portugal, italy, and Greece): a systematic review and meta-analysis. Hum Vaccin Immunother. 2021;17(10):3670–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Welte T, Torres A, Nathwani D. Clinical and economic burden of community-acquired pneumonia among adults in Europe. Thorax. 2012;67(1):71–9. [DOI] [PubMed] [Google Scholar]
- 47.Marin S, Serra-Prat M, Ortega O, Audouard Fericgla M, Valls J, Palomera E, et al. Healthcare costs of post‐stroke oropharyngeal dysphagia and its complications: malnutrition and respiratory infections. Eur J Neurol. 2021;28(11):0–1. [DOI] [PubMed] [Google Scholar]
- 48.Marin S, Serra-Prat M, Ortega O, Clavé P. Healthcare-related cost of oropharyngeal dysphagia and its complications pneumonia and malnutrition after stroke: a systematic review. BMJ Open. 2020;10(8):e031629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yoshimatsu Y, Tobino K, Ortega O, Oda H, Ota H, Kawabata T, et al. Development and implementation of an aspiration pneumonia cause investigation algorithm. Clin Respir J. 2023;17(1):20–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ortega O, Parra C, Zarcero S, Nart J, Sakwinska O, Clavé P. Oral health in older patients with oropharyngeal dysphagia. Age Ageing. 2014;43(1):132–7. [DOI] [PubMed] [Google Scholar]
- 51.Newman R, Vilardell N, Clavé P, Speyer R. Effect of Bolus Viscosity on the Safety and Efficacy of Swallowing and the Kinematics of the Swallow Response in Patients with Oropharyngeal Dysphagia: White Paper by the European Society for Swallowing Disorders (ESSD) (Dysphagia. Dysphagia. 2016;31(5):719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bolivar-Prados M, Rofes L, Arreola V, Guida S, Nascimento WV, Martin A, et al. Effect of a gum-based thickener on the safety of swallowing in patients with poststroke oropharyngeal dysphagia. Neurogastroenterol Motil. 2019;31(11):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ortega O, Bolívar-Prados M, Arreola V, Nascimento W, Tomsen N, Gallegos C, et al. Therapeutic Effect, Rheological Properties and Xanthan Gum Thickener on Four Different Phenotypes of Patients with Oropharyngeal Dysphagia. Nutrients. 2020;12(6):1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yoshimatsu Y, Ohtake Y, Ukai M, Miyagami T, Morikawa T, Shimamura Y, et al. Diagnose, treat, and SUPPORT. Clinical competencies in the management of older adults with aspiration pneumonia: a scoping review. Eur Geriatr Med. 2024;15(1):57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analyzed during the current study are not publicly available due to institutional policies and ethical regulations regarding patient data management. However, the anonymized dataset may be available from the corresponding author upon reasonable request and subject to institutional approval.