Abstract
Fibrosis, caused by excessive extracellular matrix (ECM) deposition, is a major contributor to organ dysfunction and mortality. Tetraspanins (TSPANs) have emerged as key regulators of fibrotic processes across various organs. This review examines the roles of TSPANs (e.g., TM4SF5, CD151, CD63, and CD9) in fibrosis in the liver, heart, lungs, kidneys, skin, and cornea, focusing on their influence on fibroblast activation, epithelial-mesenchymal transition (EMT), inflammation, and ECM remodeling through TGF-β/Smad, STAT3, and integrin signaling. Potential TSPAN-targeted therapies, such as monoclonal antibodies, RNA silencing, and exosome engineering are also evaluated. Despite promising preclinical results, challenges remain in tissue specificity, delivery, and clinical application for TSPAN-based antifibrotic therapies.
Keywords: Epithelial-Mesenchymal transition, Extracellular matrix, Organ fibrosis, Tetraspanins, Therapeutic perspectives
Introduction
Fibrosis is characterized by the excessive deposition of ECM, manifested as abnormal tissue repair and excessive scar formation [1]. This process can lead to the remodeling of tissues and organs. Scarring is an acute example of fibrosis that can occur anywhere in the body, including the skin and internal organ; this results in dense connective tissue replacing natural tissue, ultimately leading to the loss of normal tissue function [2]. Overall, fibrosis is estimated to account for 45% of all deaths in industrialized countries [3], causing widespread adverse effects on social stability and human health, and has become one of the major global health issues [4]. However, our understanding of its mechanisms and treatments remains limited.
Central to fibrotic pathogenesis are dysregulated cellular interactions and signaling, including the emerging role of tetraspanins (TSPANs), a family of transmembrane proteins that organize cell-surface microdomains (tetraspanin-enriched microdomains, TEMs) to modulate intercellular communication, adhesion, and receptor signaling [5]. Early studies primarily linked TSPANs to cancer biology, but increasing evidence has firmly established their role in fibrosis, revealing how these membrane organizers modulate cell migration, exosome secretion, and receptor trafficking—processes central to fibroblast activation and ECM homeostasis [5–7]
Fibrosis arises from the aberrant activation of fibroblasts and dysregulated ECM accumulation and is characterized by the increased deposition of fibrillar collagens (e.g., collagen I/III) and α-smooth muscle actin (α-SMA)-positive myofibroblasts. This pathological response stems from the body’s complex adaptive stress mechanisms triggered by persistent insults, including genetic predispositions, chronic infections, oxidative stress, environmental irritants, autoimmune inflammation, ischemic injury, metabolic disorders (e.g., hypercholesterolemia, obesity and diabetes), and hemodynamic overload [4]. Key drivers include inflammation with macrophage polarization and cytokine/chemokine signaling (e.g., TGF-β, IL-6 and TNF-α), EMT and fibroblast activation and dysregulated growth factor signaling (e.g., FGF and PDGF) augmenting ECM biosynthesis. Central to these mechanisms is the TGF-β/Smad axis, which orchestrates myofibroblast differentiation, matrix deposition, and the suppression of matrix metalloproteinases (MMPs) [8]. TSPANs, known for clustering with receptors and signaling molecules in TEMs, are now recognized as critical regulators of these processes, acting alongside canonical pathways such as the TGF-β/Smad pathway to orchestrate cell–matrix crosstalk[5–7, 9–16].
Fibrosis is a panorgan disorder that affects diverse systems, such as the liver (nonalcoholic steatohepatitis, NASH) [17], kidneys (diabetic nephropathy, DN) [18], lungs (idiopathic pulmonary fibrosis, IPF) [19], heart (postinfarction remodeling) [20], and pancreas, often complicating chronic autoimmune conditions [17, 21]. Notably, despite tissue-specific manifestations, fibrotic processes across organs share conserved molecular pathways. Traditional views of fibrosis as irreversible have been revised by evidence of its dynamic nature: withdrawal of injury stimuli, such as those used in CCl₄-induced liver fibrosis, enables regression via myofibroblast inactivation, immune cell reprogramming (e.g., anti-inflammatory macrophages and regulatory T cells), and ECM degradation via MMP- tissue inhibitors of metalloproteinases (TIMPs) balance shifts [22–25]. Concurrently, TSPANs emerge as pivotal nodes in this interplay: for example, TM4SF5 accelerates liver fibrosis by enhancing hepatocyte stellate cell (HSCs) crosstalk [7], whereas CD9 (TSPAN29) promotes cardiac fibrosis through GP130/STAT3-mediated inflammatory signaling [6]. Targeting TSPAN1-TGF-β1 crosstalk in pulmonary epithelial cells attenuates EMT and fibrotic remodeling [9, 10], highlighting their therapeutic potential. By integrating TSPAN-mediated mechanisms with established pathways such as the TGF-β pathway and inflammation, we gain a more comprehensive view of fibrosis as a network-driven disease, opening new avenues for targeted interventions. While progress in immune regulation and ECM turnover has led to therapeutic advances, the full spectrum of TSPAN-dependent signaling in fibrotic tissues remains unknown, making them a critical frontier for mechanistic and translational research. In summary, ins-depth investigations of the role of the TSPAN family in fibrotic diseases can contribute to elucidating the underlying mechanisms of fibrosis and pave the way for novel therapeutic strategies.
This review systematically synthesizes literature from the past 25 years, sourced through database searches (e.g., PubMed and Web of Science) using keywords such as “tetraspanins”, “fibrosis” and “organ fibrosis”. Studies were selected via a three-stage screening processes including title screening, abstract review, and full-text evaluation, resulting in the inclusion of 165 high-quality articles. The data were integrated using a thematic hierarchical approach and categorized by organ fibrosis type: hepatic, cardiac, pulmonary, renal, dermal, and corneal. This framework was used to outline the core functions, regulatory pathways, and therapeutic potential of TSPANs, with a focus on convergent evidence from mechanistic studies and advancements in clinical translation.
TSPANs in liver fibrosis
Liver fibrosis
Liver fibrosis, as a reparative response of the liver to chronic injury, has long been a subject of intense focus in medical research. Numerous studies indicate that its initiation and progression involve a complex interplay of mechanisms and interactions between various cellular signaling pathways [26–28]. From the perspective of pathogenic factors, several elements, including viral infections (such as hepatitis B and C) [29, 30], excessive alcohol consumption [31], nonalcoholic fatty liver disease (NAFLD) [32], and autoimmune liver diseases [33, 34], can serve as triggers for the onset of liver fibrosis. In the pathological process, initial hepatocyte injury rapidly induces an inflammatory response, which then activates HSCs [28]. This activation prompts the transdifferentiation of HSCs into myofibroblasts, which subsequently produce and secrete ECM components, with collagen being the most prominent. When the balance between ECM synthesis and degradation is disrupted, excessive deposition occurs, leading to the gradual development of liver fibrosis. Simultaneously, immune cells [34], cytokine networks [35], and key signaling pathways, such as those involving TGF-β and platelet-derived growth factor (PDGF) [36], are deeply involved and work synergistically to promote the fibrotic process. A comprehensive understanding of these fundamental mechanisms is crucial for further exploration of the role of the TSPAN family in the progression of liver fibrosis.
The role of TSPANs in liver fibrosis
Hepatic stellate cell (hscs) activation
HSCs play a key role in the pathogenesis and progression of liver fibrosis [37, 38]. In a normal liver, HSCs are primarily in a quiescent state, storing fat and vitamin A. However, upon liver injury, HSCs become activated and transform into myofibroblasts that synthesize ECM, leading to the development of fibrosis [37]. During HSCs activation, the cells undergo significant morphological changes, such as an increase in cytoplasmic volume and the expression of α-SMA, which serves as a marker for activated HSCs. Additionally, activated HSCs secrete MMPs and TIMPs to regulate the balance between ECM degradation and accumulation [37].
The activation of HSCs is a central event in liver fibrosis and is typically driven by liver injury, inflammation, and various signaling molecules. Cytokines and growth factors, such as TGF-β1, PDGF, TNF-α, and IL-1, are involved in the activation process [38]. In particular, TGF-β1 is considered the major driver of HSC activation [25, 39]. TGF-β1 binds to its receptor on HSCs and activates the SMAD signaling pathway, which promotes the transformation of HSCs into a fibrogenic phenotype, increasing the secretion of collagen and other ECM components and triggering the fibrotic response.
Transmembrane 4 L six family member 5 (TM4SF5) is a critical member of the TSPAN family that plays important roles in various physiological and pathological processes [40], particularly in liver fibrosis and cancer progression. TM4SF5 interacts with the EGF receptor and integrin α5 [41], and binds to the TSPANs CD151 and CD63 [42]. Its expression is linked to liver fibrosis in CCl4-treated animals [43] and hepatic cancer [44]. By regulating cell migration, adhesion, and signaling, TM4SF5 contributes to the progression of fibrosis and cancer metastasis [40].
TM4SF5 may promote EMT through the activation of the TGFβ1 signaling pathway, while TGFβ1, in turn, can increase TM4SF5 expression, creating a positive feedback loop. Activated TM4SF5 contributes to the activation of HSCs by stimulating the SMAD signaling pathway and interacting with the epidermal growth factor receptor (EGFR) and extracellular-regulated kinase (Erk) signaling pathways. This interaction drives the transformation of HSCs into myofibroblast-like cells, resulting in the production of ECM proteins, such as collagen I [7]. In a CCL4-induced mouse liver fibrosis model, TM4SF5 expression, α-SMA expression, and collagen I deposition are observed along the fibrotic zones, indicating that TM4SF5 is involved in the activation of myofibroblasts [43], ultimately promoting the generation and deposition of collagen I, a hallmark of fibrosis [45, 46].
CD151, also known as tetraspanin 24 (TSPAN24), has previously been shown to cluster at the cell membrane in TEMs and is widely expressed in several cell types, including epithelial, endothelial, muscle, HSCs, and hematopoietic cells [47–49].
CD151 is involved in the regulation of HSC migration, as well as in the process of wound healing [49, 50]. MMPs regulate the turnover of liver matrix proteins. A specific single nucleotide mutation in the MMP-7 gene (resulting in the amino acid substitution of glycine at position 137 to aspartic acid) is strongly associated with the development of liver cirrhosis. Compared with the MMP-7 (Gly-137) variant, the MMP-7 (Asp-137) variant has a stronger binding affinity for CD151, which enhances MMP-7 activation at the cell surface. The strengthened interaction between MMP-7 (Asp-137) and CD151 promotes the migration of HSCs, a crucial process in liver fibrosis progression.
Cholangiocyte activation-mediated liver fibrosis
Cholangiocytes serve as critical regulators of fibrogenesis in cholestatic liver diseases, mediating bidirectional crosstalk between the biliary epithelium and the hepatic microenvironment. Through the secretion of fibrogenic mediators (e.g., TGF-β), direct interaction with activated HSCs, and the modulation of biliary epithelial repair responses, cholangiocytes promote profibrotic signaling cascades; this involves enhancing HSC proliferation, collagen deposition, and MMP dysregulation, thereby exacerbating extracellular matrix remodeling and cirrhotic scarring [51].
According to Nicholas’ research, the expression of TSPAN8 (CO-029) is upregulated (> 2-fold) during liver cirrhosis. TSPAN8 is located primarily on the surface of hepatocytes and cholangiocytes. In cirrhotic livers, especially in proliferative bile duct structures, TSPAN8 is more immunopositive [52]. These findings suggest that TSPAN8 may play a role in the development or maintenance of liver cirrhosis, potentially related to cell proliferation and/or cholangiocyte activation. Given that TSPAN8 expression is upregulated in HCV-related cirrhosis and shares similarities with CD81 (TSPAN28), which is known to be involved in B cell proliferation and is a candidate receptor for hepatitis C virus (HCV) [53, 54], TSPAN8 might participate in the immune response and liver damage process induced by HCV infection; it may regulate cellular responses post-HCV infection, thereby influencing the progression of liver cirrhosis. As cirrhosis is a late stage of liver fibrosis, it is hypothesized that TSPAN8 plays a role in cholangiocyte activation and proliferation, which are closely associated with the progression of liver fibrosis.
Regulation of inflammatory environment
Chronic inflammation plays a crucial role in the development of liver fibrosis by triggering HSC activation and ECM deposition. Damaged hepatocytes and activated HSCs release proinflammatory cytokines and chemokines, which recruit immune cells to the liver, perpetuating a cycle of immune response and tissue repair. However, persistent inflammatory stimuli lead to uncontrolled fibrous tissue proliferation, promoting fibrosis progression, cirrhosis, and even liver cancer [55].
In addition to its role in HSC activation, TM4SF5 emerges as a critical modulator of cellular responses under pathological conditions, including chronic inflammation, tissue injury, and tumorigenesis [40]. Specifically, TM4SF5 expression is transcriptionally upregulated in hepatocytes and macrophages during fibrogenesis, driven by proinflammatory cytokines (e.g., IL-6 and TNF-α) and oncogenic signaling [56].
During the progression of nonalcoholic fatty liver disease (NAFLD), TM4SF5 plays a pivotal role in the bidirectional communication between hepatocytes and macrophages. TM4SF5 expression enhances glycolysis and glucose sensitivity in macrophages, leading to the activation of M1-type macrophages. These M1 macrophages secrete interleukin-6 (IL-6), which induces hepatocytes to release CCL20 and CXCL10, further promoting the formation of an inflammatory milieu. Prolonged exposure to these chemical signals can reprogram macrophages into M2-type cells, supporting the progression from NAFLD to liver fibrosis [56].
TM4SF5 also regulates the activity of signal transducer and activator of transcription 3 (STAT3) by modulating the levels of suppressor of cytokine signaling (SOCS) proteins, such as SOCS1 and SOCS3 [57]. During the fibrosis progression phase, TM4SF5, through the inflammatory environment, mediates the release of chemokines such as CCL20 [17], increases SIRT1 levels, and decreases SOCS levels, leading to the activation of STAT3. The activation of STAT3 promotes the accumulation of collagen I and laminin γ2 [43, 58], key ECM components that are characteristic of liver fibrosis. Additionally, the expression of TM4SF5 and the activation of STAT3 may form a positive feedback loop, wherein STAT3 activation further upregulates TM4SF5 expression, intensifying inflammatory and fibrotic processes [59].
In chronic inflammatory liver diseases and hepatocellular carcinoma, CD151(TSPAN24) expression is upregulated [60], potentially further promoting the recruitment of inflammatory cells and sustaining liver inflammatory responses. CD151 colocalizes with vascular cell adhesion molecule-1 (VCAM-1) on hepatic sinusoidal endothelial cells (HSECs) [60]. VCAM-1 is a known molecule that mediates lymphocyte adhesion to endothelial cells [61]. CD151 forms TEMs on the cell membrane, which serve as platforms to promote the clustering of other membrane proteins such as VCAM-1 and ICAM-1, to construct adhesive platforms [62]. CD151 may stabilize higher affinity interactions mediated by VCAM-1, facilitating lymphocyte arrest and firm adhesion. Cytokines released by inflammatory cells, especially TGF-β, drive the fibrosis process.
Viral infection-mediated liver fibrosis
Persistent hepatitis C virus (HCV) infection drives chronic inflammation and progressive hepatic fibrosis in most affected individuals, with CD81 (TSPAN28) emerging as a critical mediator of viral entry and fibrogenesis [63, 64]. As a core component of TEMs, CD81 forms a functional complex with integrin α4β1, EWI-F, and EWI-2, anchoring it to the actin cytoskeleton to facilitate viral receptor clustering and membrane remodeling [65, 66]. This interaction enhances the lateral mobility of CD81, enabling high-affinity binding of the HCV envelope glycoprotein E2 to the extracellular loops of CD81, a prerequisite for viral fusion and entry [67]. The subsequent formation of the CD81-claudin-1 (CLDN1) coreceptor complex is essential for HCV internalization, with CLDN1 directly interacting with viral E1/E2 glycoproteins to promote membrane penetration [68–71].
Mechanistically, HCV infection triggers EGFR/MAPK signaling in hepatocytes, phosphorylating ERK to activate activator protein-2 (AP-2). This transcription factor upregulates the expression of matrix metalloproteinase-2 (MMP-2), degrading basement membrane components and promoting HSC migration and activation [72, 73]. The results of a study by Antonio Mazzocca further demonstrated that CD81 knockdown in HSCs abrogates E2-induced MMP-2 expression and fibrogenic responses, underscoring the role of CD81 in HCV-driven fibrosis [67]. Collectively, these findings establish CD81 as a multifunctional hub that integrates viral pathogenesis, cytoskeletal remodeling, and profibrotic signaling cascades in HCV-infected livers.
Anti-fibrotic protective mechanism
Adiponectin protects against liver fibrosis by mediating the activity of TIMP-1 and CD63 (TSPAN30) [74]. Adiponectin activates the AMP-activated protein kinase (AMPK) signaling pathway, leading to the increased expression of TIMP-1 at both the gene and protein levels. In HSCs, TIMP-1 forms a complex with CD63 and αvβ1 integrin, creating a signaling pathway that does not rely on the activity of MMPs [74]. This complex formation reduces the activation of focal adhesion kinase (FAK), a nonreceptor protein tyrosine kinase involved in regulating cell adhesion, migration, and proliferation. As a result of reduced FAK activity, the migratory ability of HSCs is inhibited [75], which is of critically important for delaying the progression of liver fibrosis.
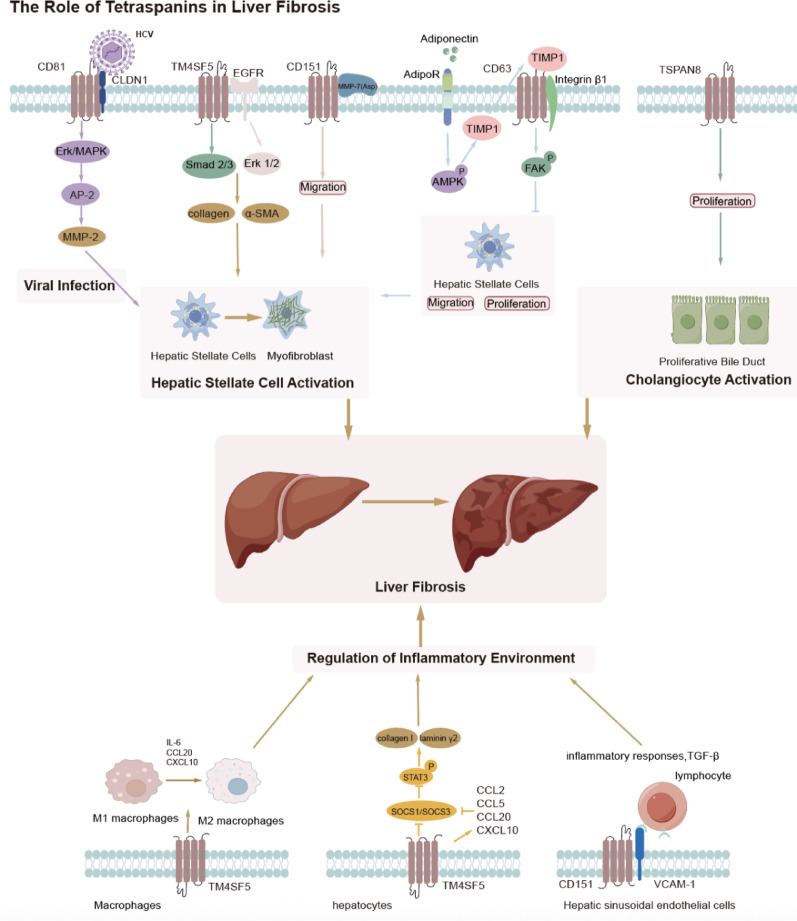
In summary, liver fibrosis is a complex, dynamic process driven by chronic liver injury, inflammation, and the excessive accumulation of ECM components. TSPANs, such as TM4SF5, CD151, and CD81, play key roles in mediating cellular communication and signaling pathways that regulate HSC activation and ECM deposition. These TSPANs influence processes such as HSCs migration, immune cell recruitment, and fibrosis progression, while also contributing to the inflammatory environment that drives fibrosis. Notably, TSPANs may act not only individually but also cooperatively. Kang et al. (2014) provided one of the first mechanistic insights into inter-TSPAN interactions in fibrosis. In a CCl4-induced liver fibrosis model, TM4SF5 was shown to interact with CD151 and modulate CD63 internalization, thereby facilitating hepatic stellate cell migration and ECM remodeling [42]. This suggests that TM4SF5 may function as a central regulator within TEMs, coordinating the activities of other TSPANs in a profibrotic context. In parallel, TSPAN8 expression is upregulated during liver cirrhosis, particularly in cholangiocytes, and may contribute to fibrosis through its involvement in cell proliferation and cholangiocyte activation. In addition to their role in promoting fibrosis, TSPANs such as CD63 are involved in protective mechanisms that counteract fibrotic development. Altogether, a deeper understanding of the diverse and sometimes opposing roles of TSPANs in liver fibrosis offers promising opportunities for targeted therapeutic strategies and precision delivery approaches. (Fig. 1).
Fig. 1.
Role of tetraspanins in liver fibrosis
Complex and dynamic processes, chronic liver injury, inflammation, and the excessive accumulation of ECM components play pivotal roles in liver fibrosis. Tetraspanins, including TM4SF5, CD151, and CD81, are crucial in mediating the cellular communication and signaling pathways that govern the activation of HSCs and the deposition of ECM. These tetraspanins modulate crucial processes, including the migration of HSCs, the recruitment of immune cells, and the regulation of the inflammatory environment, leading to the advancement of fibrosis. Notably, CO − 029 (TSPAN8) expression is upregulated during liver cirrhosis, especially in cholangiocytes, and might contribute to fibrosis through its participation in cell proliferation and cholangiocyte activation. In addition to their profibrotic functions, certain tetraspanins, such as CD63, participate in protective mechanisms through the inhibition of the migration and proliferation of HSCs.
TSPANs in cardiac fibrosis
Cardiac fibrosis
Cardiac fibrosis is a common pathological condition characterized by the excessive accumulation of ECM components, leading to impaired cardiac function. This process involves complex mechanisms including various signaling pathways and cellular interactions [76]. At the macro level, prolonged hypertension, myocardial infarction, cardiomyopathy, valvular heart diseases, and metabolic disorders such as diabetes and obesity can lead to myocardial injury, initiating the fibrotic process. At the micro level, myocardial injury triggers the apoptosis or necrosis of cardiomyocytes, releasing cytokines and chemokines that promote inflammatory cell infiltration. These inflammatory cells secrete profibrotic factors, further exacerbating fibrosis.
Concurrently, cardiac fibroblasts are activated and transform into myofibroblasts, which synthesize and secrete ECM components, particularly collagen [77]. When collagen synthesis surpasses degradation, excessive accumulation results in cardiac fibrosis. Multiple signaling pathways are involved in this process. Among the signaling pathways involved in cardiac fibrosis, the TGF-β pathway is considered one of the most critical profibrotic factors [77]. By binding to its receptor, TGF-β activates downstream signaling pathways, promoting fibroblast proliferation and differentiation, and increasing ECM synthesis. TGF-β also indirectly enhances fibrosis by modulating the expression of other cytokines [78].
Additionally, the activation of the renin–angiotensin–aldosterone system (RAAS) increases the level of angiotensin II, which not only increasees blood pressure and exacerbates the myocardial load but also stimulates fibroblast proliferation and collagen secretion [79]. Noncoding RNAs, particularly microRNAs (miRNAs), also play significant roles in regulating the expression of gene associated with cardiac fibrosis, influencing the progression of fibrosis. Some miRNAs upregulate or downregulate the expression of fibrosis-related genes, thereby affecting cellular functions and behavior [80]. Research indicates that TSPAN family members may influence the formation and remodeling of the ECM, thereby affecting the onset and progression of cardiac fibrosis [81, 82]. Therefore, understanding these fundamental mechanisms will help elucidate the role of the TSPAN family in cardiac fibrosis.
The role of TSPANs in cardiac fibrosis
Cardiac fibroblast activation
Cardiac fibroblast activation is a key event in the development of cardiac fibrosis. In response to injury, such as myocardial infarction or chronic hypertension, cardiac fibroblasts become activated, proliferate, and transdifferentiate into myofibroblasts. These activated fibroblasts secrete excessive ECM proteins, such as collagen, leading to fibrosis, which disrupts normal cardiac structure and function, ultimately contributing to heart failure and reduced contractility [77].
In the context of myocardial fibrosis, CD63 has been shown to interact with TIMP-1 and integrin β1 on cardiac fibroblasts. This interaction activates the Smad2/3 and β-catenin signaling pathways, leading to increased collagen synthesis and deposition. Timp1 knockout mice exhibit reduced cardiac fibrosis, improved diastolic function, and alleviated cardiac hypertrophy [20]. CD63 (TSPAN30), a member of the TSPAN family, is widely expressed on the cell surface and within endosomes [83, 84]; it plays a crucial role in various cellular processes, including cell adhesion, migration, signal transduction, and immune responses [84]. Although the detailed mechanism of CD63 in this process is not fully understood, CD63 contributes to the activation of cardiac fibroblasts and the progression of myocardial fibrosis, ultimately affecting the structure and function of the heart.
Additionally, CD63 has recently emerged as a novel target for nanoemulsion-based19F MRI imaging and drug delivery to activated cardiac fibroblasts, presenting new diagnostic and therapeutic opportunities for assessing and treating cardiac fibrosis [85].
Myocardial cell injury and inflammation
When cardiomyocytes are injured, often as a result of myocardial infarction, hypertension, or chronic stress, they release proinflammatory cytokines and other signaling molecules. These signals trigger the activation of cardiac fibroblasts, causing them to proliferate, migrate, and differentiate into myofibroblasts. The myofibroblasts then produce excessive amounts of ECM proteins, which leads to the development of fibrotic tissue [76].
CD9 (TSPAN29) is a 24 kDa TSPAN protein found in various cell types, such as endothelial cells, smooth muscle cells, and myocardial cells [6, 86]; it functions primarily as an organizer of multimolecular complexes, including integrins and other TSPANs [87]. CD9 is crucial for cell adhesion and migration [88], metastasis [89], and immune response [90]. Recently, TSPAN29 was also shown to be involved in cardiovascular disease [91].
With respect to cardiac fibrosis, in a transverse aortic constriction (TAC) model CD9 expression increased (> twofold), and the overexpression of CD9 exacerbated cardiac fibrosis, as evidenced by increased collagen deposition in cardiac tissues [6]. In cardiomyocytes, CD9 knockdown reduced GP130 protein levels, suggesting that CD9 may function by stabilizing GP130. The immunoprecipitation results indicated that CD9 directly interacted with GP130, with upregulated CD9 expression leading to increased GP130 protein levels and the increased phosphorylation of Signal Transducer and Activator of Transcription 3 (STAT3). GP130 is a transmembrane protein and a signal-transducing receptor in the IL-6 family that plays a critical role in cardiomyocyte survival, hypertrophy, and cardiac fibrosis [92–94]. STAT3 is a transcription factor that, once phosphorylated, translocates to the nucleus to activate the expression of downstream genes; this results in the upregulation of the expression of fibrotic markers such as collagen Ia, collagen III, and CTGF at the mRNA level. These findings suggest that CD9 promotes cardiac fibrosis through the GP130/STAT3 signaling pathway [6].
In addition, CD9 overexpression in cardiac tissue promoted the expression of inflammatory factor mRNAs. Conversely, CD9 knockdown suppressed their expression [6]. These findings suggest that CD9 may also regulate inflammatory responses involved in cardiac fibrosis [77, 95].
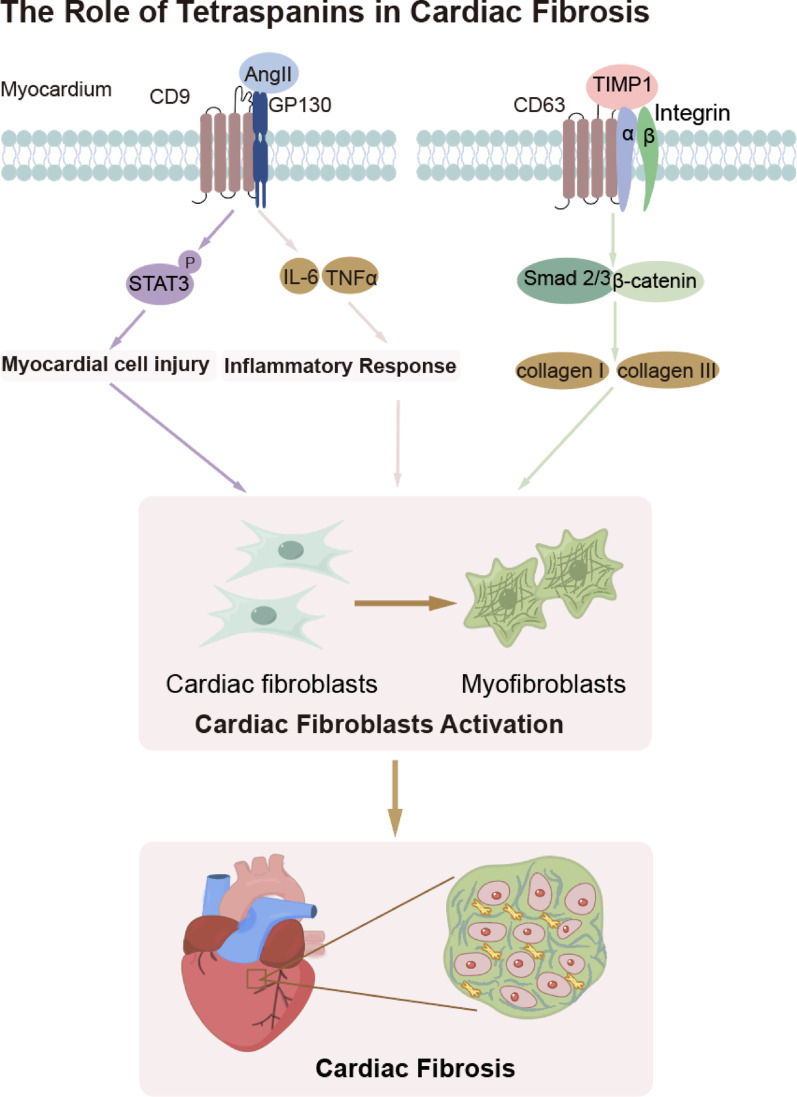
In summary, cardiac fibrosis, a key pathological process leading to impaired heart function, involves complex mechanisms driven by myocardial injury, inflammation, and excessive ECM deposition. TSPANs, such as CD63 and CD9, play crucial roles in the activation of cardiac fibroblasts. CD63 interacts with TIMP-1 and integrin β1, activating profibrotic signaling pathways such as the Smad2/3 and β-catenin pathways to promote collagen synthesis. Additionally, CD9 stabilizes the GP130 protein, triggering the STAT3 pathway, which upregulates the expression of fibrosis-related markers and inflammatory factors. These findings suggest that TSPANs are integral to both the activation of fibroblasts and the inflammatory response in cardiac fibrosis. Therefore, in-depth research into these mechanisms holds promise for the development of novel therapeutic strategies to prevent and reverse cardiac fibrosis (Fig. 2).
Fig. 2.
Role of tetraspanins in cardiac fibrosis
Cardiac fibrosis, a key pathological process that compromises heart function, involves intricate mechanisms driven by myocardial injury, inflammation, and excessive ECM deposition. Tetraspanins, including CD63 and CD9, play critical roles in the activation of cardiac fibroblasts, myocardial injury and inflammation. CD63 interacts with TIMP-1 and integrin β1, thereby activating profibrotic signaling pathways such as Smad2/3 and β-catenin, which promote collagen synthesis. CD9 stabilizes the GP130 protein, initiating the STAT3 pathway, which ultimately results in myocardial injury and subsequent activation of cardiac fibroblasts. Additionally, CD9 participates in inflammation-mediated cardiac fibrosis by activating cardiac fibroblasts. These fibroblasts contribute to fibrosis progression by synthesizing collagen and other ECM components.
TSPANs in pulmonary fibrosis
Pulmonary fibrosis
Pulmonary fibrosis (PF) is a highly challenging research topic in the field of respiratory diseases, and its pathogenesis involves a complex network of multifactorial and multilayered interactions. Various factors can trigger pulmonary fibrosis, such as the prolonged inhalation of harmful dust (e.g., silica or asbestos) [96], recurrent lung infections [97], autoimmune diseases affecting the lungs [98], certain drug side effects [99], and genetic susceptibility [100]. In terms of pathophysiological processes, early alveolar epithelial cell (AEC) injury is considered a critical initiating event, potentially caused directly or indirectly by the aforementioned pathogenic factors [101].
Damaged AECs release multiple profibrotic factors, such as TGF-β and PDGF, which recruit and activate fibroblasts [102, 103]. Additionally, these cells undergo EMT, transforming into myofibroblasts that, along with activated fibroblasts, synthesize and secrete ECM components, including collagen and fibronectin [104]. When ECM synthesis exceeds degradation, lung tissue is progressively replaced by scar tissue, leading to fibrosis.
The abnormal activation of the Wnt/β-catenin pathway is also closely associated with the development of pulmonary fibrosis, affecting cell proliferation, differentiation, and EMT [105]. Additionally, oxidative stress plays a crucial role in the progression of pulmonary fibrosis, with excessive reactive oxygen species (ROS) production disrupting the cellular redox balance, damaging cell structure and function, and further exacerbating fibrosis development [106, 107]. Understanding these fundamental mechanisms lays the foundation for accurately interpreting the role of the TSPAN family in pulmonary fibrosis.
The role of TSPANs in pulmonary fibrosis
Alveolar epithelial cell homeostasis and regulation
Idiopathic pulmonary fibrosis (IPF), a form of interstitial pneumonia characterized by chronic and progressive pulmonary scarring, was initially considered an inflammatory disease. However, recent studies have shown that anti-inflammatory treatments are largely ineffective in IPF patients, indicating that inflammation is not the primary cause. Instead, the abnormal behavior of AECs has been identified as a key trigger for the development of fibrosis [108, 109].
CD151 (TSPAN24) plays a crucial and complex role in the progression of pulmonary fibrosis, particularly in maintaining AEC homeostasis. Multiple studies have demonstrated that CD151 is indispensable for maintaining epithelial integrity in AECs [110, 111]. In the absence of CD151, AECs undergo mesenchymal-like changes, activating the TGF-β signaling pathway, which ultimately leads to pulmonary fibrosis [19]. CD151 ensures epithelial integrity by tightly binding to the basement membrane (BM) [111]. Kazuyuki Tsujino and colleagues reported that CD151 deficiency impairs cell adhesion, causing cells to detach more easily from the BM. After repeated damage to AECs, these cells become activated and interact with migrating immune cells and fibroblasts, leading to the upregulation of the expression of p-Smad2 and other profibrotic factors. The increased deposition of these ECM components disrupts tissue structure, promoting the differentiation of fibroblasts and AECs into myofibroblasts, and leading to excessive ECM production in the lung. Furthermore, in CD151-deficient mice, spontaneous pulmonary fibrosis is observed at 30 weeks, and in a bleomycin-induced lung injury model, fibrosis is significantly more pronounced, confirming the protective role of CD151 in IPF [19].
CD9 (TSPAN29) also has protective effects on AECs, with its mechanisms being dual-faceted. On the one hand, the absence of CD9 inhibits the key TGF-β signaling pathway involved in tissue repair and fibrosis progression [112]. This study suggested that CD9 deletion likely impedes the binding of TGF-β to TgR1 and TgR2, leading to a reduction in and impeding fibrosis progression. On the other hand, Lokesh P. Tripathi’s research [112] suggested that CD9 shares some functional similarities with CD151. In the absence of CD9, downstream TNF signaling pathways may be activated, particularly through the upregulation of Fas and Mapk10, triggering the assembly of the Fas receptor-induced death-inducing signaling complex (DISC), and accelerating AEC damage and apoptosis. Additionally, the loss of CD9 affects ECM composition, potentially through the regulation of ECM-related genes such as Lox, Mkx, Eln, and Mmp13, thereby altering alveolar structure and function. CD9 deficiency also leads to the hyperactivation of macrophages, resulting in elevated levels of proinflammatory cytokines such as TNF-α and MMPs, which play pivotal roles in creating an inflammatory environment and driving fibrosis progression [113, 114]. Overall, these mechanisms are interconnected, potentially disrupting alveolar structure, enhancing inflammatory responses, promoting cell apoptosis, and ultimately accelerating pulmonary fibrosis development.
Tetraspanin 1 (TSPAN1) plays a significant role in regulating key signaling pathways involved in pulmonary fibrosis. Studies have shown that TSPAN1 expression is markedly downregulated (> twofold) in the lung tissues of patients with idiopathic IPF and in bleomycin-induced PF mice [10, 115]. Further research suggested that TSPAN1 inhibits the phosphorylation of IκBα, reducing nuclear NF-κB translocation and activation, and thereby preventing apoptosis. These findings suggest that TSPAN1 could be a potential therapeutic target for treating pulmonary fibrosis or acute lung injury by regulating cell apoptosis and inflammation [115]. Furthermore, another study reported that the downregulation of TSPAN1 expression is associated with EMT in AECs [10, 115]. EMT, a central process in tissue fibrosis, is characterized by the upregulation of the expression of EMT-related proteins such as α-SMA, vimentin, and N-cadherin, which are associated with IPF progression [117–118]. Silencing TSPAN1 promotes cell migration and the expression of fibrosis markers, whereas overexpressing TSPAN1 has the opposite effect. Additionally, TSPAN1 influences the Smad2/3 and beta-catenin pathways, which are crucial in the EMT process. Overall, TSPAN1 acts as a key regulator in the development of PF by modulating EMT and related signaling pathways. These findings highlight TSPAN1 as a potential therapeutic target for IPF treatment.
In addition to regulating EMT, TM4SF5 is involved in the regulation of cellular oxidative stress, influencing the progression of pulmonary fibrosis [119]. In the context of fibrosis, TM4SF5 in lung epithelial cells induces the CD44v8-10 splice variant through an inverse relationship with ZEB2 and ESRPs. This variant forms complexes with the xc − system (comprising of CD98hc and xCT), increasing intracellular glutathione levels and modulating ROS for cell survival. By modulating oxidative stress, TM4SF5 helps mitigate the progression of pulmonary fibrosis.
Novel mechanisms
In addition to its role in maintaining AEC homeostasis, CD151 is deeply involved in the regulation of fibrosis-associated signaling pathways. Research by Lokesh et al. demonstrated that the loss of CD151 could induce changes in the expression of circadian rhythm-related genes, including the downregulation of Cry1, Per2, and Per3 expression and the upregulation of Arntl (Bmal1) and Npas2 expresion [112]. This disruption of biological rhythms may affect the cell cycle and proliferation, thereby promoting the progression of fibrosis. Furthermore, CD151 deficiency may enhance fibrosis by upregulating the expression of cell cycle regulators such as Ccnd (cyclin D) and downregulating apoptosis inhibitors such as X-linked inhibitor of apoptosis protein (Xiap), leading to increased cell proliferation and decreased apoptosis, contributing to the accumulation of cells during fibrosis.
Conversely, Fujishima et al. provided a contrasting perspective, suggesting that CD151 interacts with MMP-7, potentially exacerbating IPF pathology [120]; it acts as a docking molecule for proMMP-7, binding to its propeptide on cell surfaces, which leads to its activation [121]. This interaction is particularly prominent in hyperplastic alveolar and metaplastic bronchiolar epithelial cells, as well as in alveolar macrophages. The activation of proMMP-7 through CD151 contributes to the pathology of IPF by promoting epithelial cell migration, apoptosis, myofibroblast growth, and neutrophil accumulation. As such, the precise role of CD151 in pulmonary fibrosis signaling pathways remains controversial and warrants further investigation to clarify its mechanisms.
Research by Duan et al. revealed that in lung adenocarcinoma (ADC) and squamous cell carcinoma (SCC), the expression of TIMP-1 and CD63 is significantly elevated in tumor-associated fibroblasts (TAFs) [122]. This overexpression is mediated by abnormal hyperactivity of the TGF-β1/SMAD3 signaling pathway, which plays a crucial role in fibroblast activation and the fibrotic response. Upon secretion by TAFs, TIMP-1 interacts with the CD63 receptor on the surface of lung adenocarcinoma cells, enhancing cancer cell proliferation and invasiveness and thereby contributing significantly to tumor progression [122]. Indeed, CD63 plays a crucial role in lung fibrosis by forming a complex with TIMP1 and integrin β1 on the surface of fibroblasts. This complex formation is significantly induced by multiwalled carbon nanotube (MWCNT) exposure, leading to the activation of the ERK signaling pathway. The activation of this pathway promotes fibroblast proliferation and contributes to the development of lung fibrosis [123]. CD63, therefore, is integral to the molecular mechanism through which TIMP1 modulates the fibrotic response in the lungs.
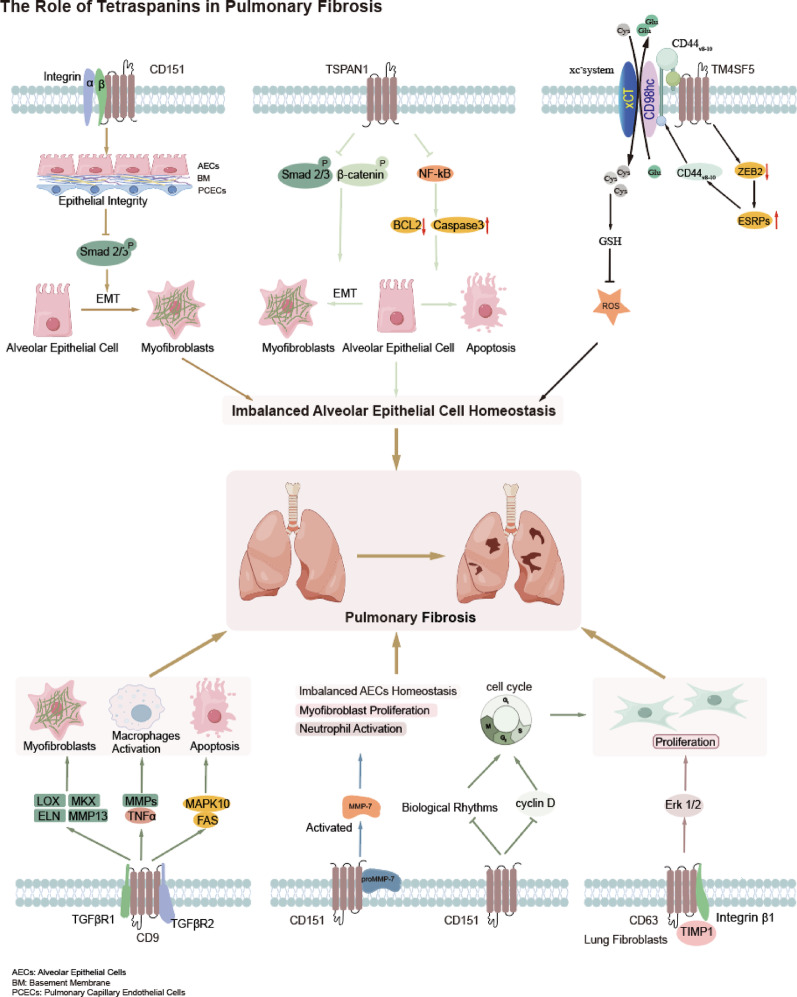
In summary, pulmonary fibrosis is a progressive respiratory disease characterized by ECM deposition, leading to lung tissue scarring and impaired function. AEC injury leads to the activation of fibroblasts, which differentiate into myofibroblasts that produce ECM components, such as collagen, contributing to the fibrotic process. Tetraspanins, including CD151, CD9, and TSPAN1, are central in regulating AECs homeostasis and fibroblast activation. For example, CD151 is essential for maintaining epithelial integrity, and its deficiency disrupts cell adhesion, exacerbating fibrosis by promoting the activation of TGF-β signaling and ECM production. On the other hand, CD9 has a dual role in regulating fibrosis, with its absence impairing TGF-β signaling and promoting AECs damage, while also affecting ECM composition and inflammation. Additionally, TSPAN1 has been shown to influence the development of PF by regulating apoptosis, inflammation, and EMT. Other TSPANs, such as TM4SF5 and CD63, also contribute to PF through their involvement in oxidative stress regulation and ECM remodeling, highlighting their potential as therapeutic targets for this disease. Overall, understanding the roles of TSAPNs in PF provides valuable insights into their molecular mechanisms and identifies new avenues for targeted therapeutic interventions (Fig. 3).
Fig. 3.
Role of tetraspanins in pulmonary fibrosis
Pulmonary fibrosis (PF) is a progressive respiratory disease characterized by ECM deposition, causing lung tissue scarring and impaired function. In PF, tetraspanins such as CD151, CD9, and TSPAN1 are key in regulating AEC homeostasis and fibroblast activation. CD151 maintains epithelial integrity; its deficiency disrupts cell adhesion, exacerbating fibrosis via TGF-β pathway activation. CD9 has a dual role in fibrosis; its absence impairs TGF-β signaling, increasing AEC damage and affecting the ECM and inflammation. TSPAN1 influences PF development through modulating apoptosis, inflammation, and EMT.TM4SF5 and CD63 also contribute to PF by regulating oxidative stress and ECM remodeling.
TSPANs in renal fibrosis
Renal fibrosis
Renal fibrosis has become a focal point and a challenging issue in kidney disease research, garnering widespread attention in recent years [124]. The pathogenesis of kidney fibrosis is highly complex and involves interactions across multiple levels. Various factors can induce kidney fibrosis, including metabolic disturbances caused by diabetes [125]. Chronic hyperglycemia not only leads to alterations in renal hemodynamics, placing the glomerulus in a state of hyperfiltration and hyperperfusion, but also activates several intracellular signaling pathways, such as protein kinase C pathways, resulting in renal cell injury [126, 127]. Hypertension, which causes elevated intraglomerular pressure, exerts continuous stress on the glomerular capillaries, stimulating mesangial cell proliferation and enhancing ECM synthesis [128]. Additionally, autoimmune diseases, such as IgA nephropathy, involve the deposition of immune complexes in the mesangial area of the glomerulus, triggering local inflammatory responses and initiating fibrosis [129]. Furthermore, the glomerular basement membrane (GBM) plays a crucial role in kidney function, and abnormalities in this membrane can have a significant impact on the development of renal fibrosis, such as that observed in Alport syndrome, resulting in structural and functional abnormalities of intrinsic renal cells due to gene mutations, which act as inherent drivers of renal fibrosis [130, 131].
At the pathophysiological level, early renal cell injury leads to phenotypic changes in renal tubular epithelial cells, mesangial cells, and other renal cells, which transdifferentiate into myofibroblasts [132]. This process is accompanied by the upregulation of the expression of markers such as α-SMA, and the myofibroblasts subsequently synthesize and secrete ECM components, including collagen and fibronectin. In parallel, inflammation in the local renal microenvironment involves infiltration by immune cells, which release fibrosis-promoting cytokines such as TNF-α and IL-6, further driving the fibrotic process. Additionally, multiple signaling pathways interact, with the TGF-β pathway playing a central role [133]. The activation of TGF-β directly regulates the expression of fibrosis-related genes, promotes ECM synthesis, and mediates intercellular signaling via the Smad protein family, affecting cell proliferation, differentiation, and apoptosis. The aberrant activation of the Wnt/β-catenin pathway is also closely linked to kidney fibrosis, influencing the fate of renal cells and advancing the fibrotic process [134].
The role of the TSPANs in renal fibrosis
Renal cell injury
In the context of diabetic nephropathy (DN), TSPAN8 has been shown to play a crucial protective role in renal fibrosis. TSPAN8 expression was shown to be significantly increased (> 1.5-fold) in the kidney tissue of DN mice [135]. The incidence of diabetes is increasing in younger populations, and although the exact etiology remains unclear, kidney cells in DN are particularly vulnerable to fibrosis, which becomes a key factor in kidney damage and eventual loss of function [18]. Zhuang et al. demonstrated that, on the one hand, the overexpression of TSPAN8 significantly reduces the expression of fibrosis-associated proteins, such as fibronectin and type IV collagen, in high glucose-induced HK2 cells, thereby inhibiting the fibrotic process at its onset. On the other hand, in a high glucose-induced HK2 cell model, TSPAN8 overexpression upregulates the expression of autophagy markers, such as microtubule-associated protein 1A/1B light chain 3 (LC3-II/LC3-I) and the autophagy-related protein Beclin-1, indicating that TSPAN8 strongly promotes autophagy, helping cells cope with the stress imposed by hyperglycemia. Furthermore, in high glucose-induced HK2 cells, the miR-543 mimic weakens cell proliferation, but the overexpression of TSPAN8 partially reverses the cell proliferation inhibition induced by the miR-543 mimic, effectively protecting renal cells from the negative effects of hyperglycemia [135].
Inflammatory signaling regulation
CD37(TSPAN26) plays an essential protective role in renal fibrosis associated with IgA nephropathy [136, 137]. IgA nephropathy, a primary glomerular disease, is characterized by the deposition of IgA in the mesangial area of the glomerulus and is often accompanied by or without the presence of other immunoglobulin deposits. Rops et al. reported that in CD37-deficient mice, the deposition of IgA in the glomerulus is closely linked to mesangial proliferation and fibrosis. The deposition of IgA immune complexes likely activates local inflammatory responses, leading to excessive ECM accumulation during tissue repair, thereby accelerating the fibrotic process [136]. CD37 helps protect the kidneys by precisely regulating the formation of IgA immune complexes and the deposition of IgA in the glomerulus, thus establishing a protective barrier against IgA nephropathy. Although the exact mechanisms of the role of CD37 remain to be fully elucidated, its potential value in preventing IgA-related kidney fibrosis is clearly suggested.
Glomerular basement membrane (GBM) defects
In the context of kidney fibrosis induced by Alport syndrome, CD151(TSPAN24) plays a critical protective role. Alport syndrome, a type of hereditary nephritis, often leads to the development of chronic kidney disease and kidney fibrosis, which can ultimately progress to renal failure [131]. A study by Delimont et al. [138] highlighted that, owing to the key role of CD151 in podocyte adhesion and signaling processes [139], its loss or dysfunction could significantly contribute to the destruction of GBM, and ultimately lead to kidney fibrosis. In CD151-null mice, there is notable invasion of mesangial cell foot processes into the glomerular capillaries, which strongly suggests abnormal mesangial cell activity. This abnormality likely leads to excessive ECM deposition and glomerulosclerosis. The study also reported the activation of FAK and the dramatic increase in the expression of the proinflammatory cytokine IL-6 expression in CD151-null mice. These two factors, which act in concert, could further amplify the inflammatory response and exacerbate the progression of kidney fibrosis.
In summary, renal fibrosis is a critical factor in the progression of kidney diseases, particularly in conditions such as DN and IgA nephropathy. TSPANs play important roles in regulating fibrotic processes and inflammatory responses in the kidney. TSPAN8 has been identified as a protective molecule in DN, where it modulates fibrosis-related protein expression and promotes autophagy in response to high-glucose conditions. CD37 plays a crucial role in regulating IgA deposition and inflammation in IgA nephropathy, offering protection against kidney fibrosis by modulating immune complex formation. In contrast, CD151 (TSPAN24) is involved in podocyte adhesion and signaling, and its dysfunction in Alport syndrome contributes significantly to GBM defects and kidney fibrosis. These findings underscore the diverse roles of TSPANs in regulating kidney fibrosis and highlight their potential as therapeutic targets for managing renal diseases (Fig. 4).
Fig. 4.
Role of Tetraspanins in Renal Fibrosis
Renal fibrosis is crucial in kidney disease progression, notably in DN and IgA nephropathy. Tetraspanins are key in regulating fibrotic and inflammatory processes in the kidney. TSPAN8 protects against DN. Under high-glucose conditions, TSPAN8 modulates fibrosis-related protein expression and triggers autophagy, countering renal fibrosis. CD37 is vital in IgA nephropathy; it controls IgA deposition and inflammation, modulating immune complexes to safeguard against kidney fibrosis. CD151 (TSPAN24) is involved in podocyte adhesion and signaling. In Alport syndrome, TSPAN24 malfunction leads to GBM defects, which are linked to kidney fibrosis.
TSPANs in dermal fibrosis
Dermal fibrosis
In addition to being involved in pulmonary, liver, kidney, and cardiac fibrosis, the TSPAN family is involved in the fibrotic processes such as dermal fibrosis and systemic sclerosis (scleroderma). Dermal fibrosis refers to the excessive accumulation of ECM components, primarily collagen, in the skin, leading to thickening, stiffening, and scarring; it occurs when there is an imbalance between the production and degradation of the ECM, typically driven by abnormal fibroblast activation and proliferation [2]. Dermal fibrosis can arise because of chronic inflammation, or autoimmune diseases such as systemic sclerosis (scleroderma) [140]. The pathogenesis of dermal fibrosis involves complex interactions among fibroblasts, immune cells, and the ECM. In particular, TGF-β signaling, in particular, plays a central role in stimulating fibroblast activity, promoting fibrosis, and inhibiting ECM degradation [140]. Myofibroblasts, which arise from activated fibroblasts, are the primary cell type responsible for ECM deposition and fibrosis in the skin [140].
The role of TSPANs in dermal fibrosis
In the process of wound healing related to dermal fibrosis, CD9 (TSPAN29) plays a crucial role [141]. When the skin is injured and enters a hypoxic microenvironment, hypoxia upregulates the expression of CD9 (up to 3.9-fold) in human skin fibroblasts (HSFs). As a member of the TSPAN superfamily, CD9 directly acts on the TGF-β1 receptors TβR1 and TβR2, promoting their association; this activates the TGF-β1/Smad2/3 signaling pathway, thereby facilitating the transition of fibroblasts to myofibroblasts. This transition is manifested by increased expression of α-SMA, type I collagen (COL-I), type III collagen (COL-Ш), and other proteins, as well as excessive ECM deposition, ultimately driving the progression of skin fibrosis.
In skin fibrosis associated with SSc, CD63 (TSPAN30), a common marker of exosomes, plays a significant role [142]. Compared with that in normal fibroblasts, the expression levels of CD63, along with the other exosome markers CD9 and CD81, are upregulated (up to 2–fivefold) in SSc dermal fibroblasts both in vivo and in vitro. The increased number of exosomes in the culture medium of SSc fibroblasts, which contain elevated levels of CD63, can stimulate type I collagen expression in normal fibroblasts. This effect may be related to the dysregulation of collagen-related microRNAs in SSc exosomes. Although the exact mechanism by which CD63-rich exosomes promote collagen expression has not been fully elucidated, it is likely that CD63-positive exosomes act as a means of cell–cell communication. They transfer certain bioactive substances, such as dysregulated miRNAs, to normal fibroblasts, thereby influencing their function and leading to enhanced type I collagen production, which is a key feature of skin fibrosis.
NAG-2 (TSPAN4), also known as TM4SF7, is a TSPAN belonging to the transmembrane 4 superfamily; it forms complexes with integrins alpha3beta1 and alpha6beta1 and is highly expressed on endothelial cells and fibroblasts, playing a role in cell mobility and adhesion [143]. In skin fibrosis associated with SSc, TSPAN4 serves as a major autoantigen target [144]. Serum IgG antibodies against TSPAN4, which are present in nearly all SSc patients, can bind to endothelial cells and fibroblasts. These antibodies induce endothelial cell apoptosis and fibroblast proliferation, which are key features of SSc-related skin fibrosis. The induction of fibroblast proliferation is likely related to the activation of signaling pathways such as the AKT pathway, as demonstrated by increased AKT phosphorylation in fibroblasts incubated with anti- TSPAN4 antibodies; this leads to increased ECM production and ultimately contributes to the development of skin fibrosis. In contrast, antibodies depleted of anti- TSPAN4 reactivity do not have such effects, indicating that TSPAN4 and its corresponding autoantibodies play crucial roles in promoting the fibrotic process in SSc skin.
CD151(TSPAN24) is a transmembrane protein crucial for the development of hemidesmosomes by stabilizing laminin-integrin binding. In skin-related disorders such as syndromic epidermolysis bullosa simplex (EBS) associated with CD151 defects, CD151 malfunction has significant impacts [145]. In the cutaneous basement membrane, CD151 binds with α6β4 integrin and stabilizes its interaction with laminin-332, which is essential for the formation of the hemidesmosomal complex. When CD151 is defective due to mutations, such normal interactions are disrupted. This leads to skin fragility, as seen in patients with CD151-associated EBS, who present with blistering, onychodystrophy, and other skin manifestations. Although the present study does not directly discuss skin fibrosis, the fragility and abnormal basement membrane function caused by CD151 defects might be related to disrupted tissue integrity, which could be a factor in the development of long-term fibrotic-like changes.
In summary, dermal fibrosis, characterized by excessive collagen deposition and ECM imbalance, is a hallmark of various fibrotic skin disorders particularly in autoimmune diseases such as SSc. TSPANs have emerged as key modulators in this process, orchestrating complex interactions among fibroblasts, immune cells, and the ECM through their influence on profibrotic signaling pathways, notably those mediated by TGF-β. In particular, TSPANs such as CD9, CD63, TSPAN4, and CD151 play critical roles in maintaining cellular homeostasis, mediating cell-to-cell communication via exosomes, and regulating fibroblast activation and proliferation. These collective actions underscore the therapeutic potential of targeting TSPAN-mediated pathways to modulate dermal fibrosis and restore tissue integrity (Fig. 5).
Fig. 5.
Role of Tetraspanins in Dermal Fibrosis
Dermal fibrosis, manifested by excessive collagen and ECM deposition, is a characteristic feature of diverse fibrotic skin disorders. Tetraspanins have emerged as pivotal regulators in this process. The expression of CD9, upon skin injury-induced hypoxia, is upregulated in human skin fibroblasts. CD9 drives the fibroblast-to-myofibroblast transition and skin fibrosis by activating the TGF-β1/Smad2/3 pathway. CD63-rich exosomes may transfer dysregulated collagen-related miRNAs to normal fibroblasts, stimulating type I collagen expression and contributing to skin fibrosis. TSPAN4 serves as a major autoantigen target in SSc-related skin fibrosis. Anti-TSPAN4 IgG antibodies induce endothelial apoptosis and fibroblast proliferation via AKT activation and promote fibrosis. CD151 stabilizes laminin-integrin binding for hemidesmosome formation in the cutaneous basement membrane. In CD151-defective syndromic epidermolysis bullosa simplex, CD151 malfunction causes skin fragility leading to fibrosis.
The role of TSPANs in corneal fibrosis
Corneal fibrosis refers to the abnormal accumulation of ECM components, such as collagen, within the corneal stroma, leading to scarring, thickening, and reduced transparency of the cornea [146]. This condition impairs vision and can result from various causes, including trauma, infection, inflammation [146], and diseases such as keratoconus [147], herpetic keratitis [148], or Fuchs' dystrophy [149]. The fibrosis process in the cornea involves the activation of corneal fibroblasts, which proliferate and differentiate into myofibroblasts [150]. These myofibroblasts secrete ECM components, leading to the formation of fibrotic tissue that disrupts the normal structure of the cornea and decreases its transparency.
TSPANs, such as CD9, CD63, and CD81, are important components of exosomes and play significant roles in corneal fibrosis [151, 152]. TSPANs are essential for the biogenesis and function of exosomes, which are small vesicles that facilitate intercellular communication. They regulate the sorting of cargo into exosomes and influence their interaction with target cells [153]. Exosomes carrying TSPANs such as CD9, CD63, and CD81 can affect corneal stromal cell migration and the expression of fibrosis-related proteins. For example, in human corneal fibroblasts from individuals with type I and type II diabetes mellitus, stimulation with these exosomes led to changes in the expression of thrombospondin 1 (TSP-1) and fibronectin, both of which are associated with ECM remodeling and fibrosis [153]. Han et al. reported that corneal epithelial cell-derived exosomes, which also express TSPANs such as CD63, are involved in corneal wound healing and neovascularization [152]. These exosomes can fuse with corneal keratocytes and induce their transformation into myofibroblasts, a key event in fibrosis.
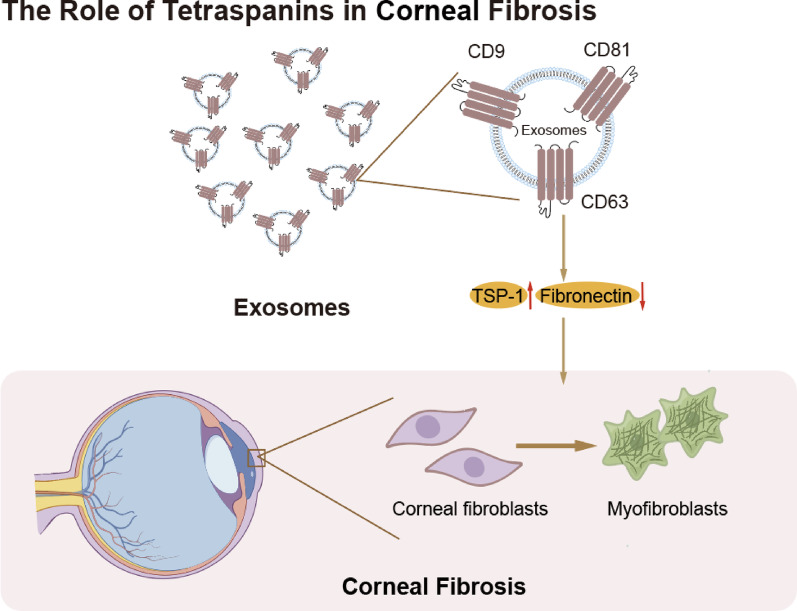
In summary, TSPANs in exosomes participate in corneal fibrosis-related processes by influencing cell–cell communication, cell transformation, and the expression of fibrosis-related proteins. While they may not directly drive fibrosis, their presence in exosomes is crucial for the proper progression of these processes. Although this profibrotic effect can induce diseases such as corneal damage to a certain degree, from another perspective, it also promotes corneal wound healing. Further investigation is needed to fully understand the mechanisms by which TSPANs contribute to corneal fibrosis (Fig. 6).
Fig. 6.
Role of Tetraspanins in Corneal Fibrosis
Corneal fibrosis is characterized by the abnormal deposition of extracellular matrix (ECM), particularly collagen, within the corneal stroma. Tetraspanins (CD9, CD63, and CD81) in exosomes are crucial for corneal fibrosis. These exosomes can regulate corneal stromal cell migration and fibrosis-related protein expression and can transform keratocytes into myofibroblasts.
Translational potential and clinical applications
The TSPAN family represents a promising target for antifibrotic therapy because of its central role in mediating the cellular interactions and signaling pathways that drive fibrosis. In liver fibrosis, for example, TM4SF5 has been shown to promote HSCs activation and ECM deposition via the TGF-β/SMAD and EGFR pathways. Preclinical studies have indicated that the inhibition of TM4SF5 with small-molecule inhibitors can reduce collagen I and α-SMA expression in CCl₄-induced fibrosis models [43]. Similarly, the blockade of CD151 has shown potential in reducing lymphocyte adhesion, thereby proposing a strategy to attenuate inflammation-driven fibrosis [60], suggesting that the selective inhibition of specific TSPANs may effectively attenuate liver fibrosis.
In cardiac and pulmonary fibrosis, the therapeutic strategies also highlight the function of tissue-specific TSPANs. In the heart, interfering with the CD9/GP130 interaction may help dampen STAT3-driven hypertrophic responses [6]. In contrast, in pulmonary fibrosis, restoring epithelial integrity is critical; for example, CD151 may can help reestablish alveolar epithelial adhesion and prevent TGF-β–induced EMT [19], whereas the upregulation of TSPAN1 expression has been shown to suppress NF-κB signaling and EMT in lung epithelial cells [115].
Although direct clinical trials targeting TSPANs in fibrotic diseases are not yet available, existing and emerging clinical studies offer translational insights. For instance, the anti-CD37 monoclonal antibody BI 836826 has undergone phase I/II trials in chronic lymphocytic leukemia (NCT01296932, NCT02624486), demonstrating the feasibility of targeting TSPAN family members in human patients [154, 155]. Meanwhile, exosome-based therapies—which inherently involve TSPANs such as CD63, CD81, and CD9—are being tested in early-phase trials on β-cell mass in type 1 diabetes mellitus (T1DM) are underway (NCT02138331). These platforms may eventually serve as delivery vehicles for organ- or cell-specific antifibrotic agents, although challenges remain in terms of scalability, vesicle purity, and target specificity.
In parallel, emerging studies suggest that TSPAN family members may serve as diagnostic or prognostic biomarkers in fibrosis-related conditions and cancers. For example, CD151 is significantly downregulated in the alveolar epithelium of patients with IPF, and its loss correlates with increased EMT and fibrotic remodeling, suggesting its potential as a disease severity indicator [19]. Similarly, CD63 has shown biomarker potential in multiple contexts: its expression correlates with disease progression in ovarian cancer [156] and non-small cell lung cancer (NSCLC), and its negative expression in lung adenocarcinoma is associated with poorer prognosis [157]. In pancreatic ductal adenocarcinoma (PDAC), serum levels of CD63-positive exosomes have been identified as early diagnostic indicators with high sensitivity and specificity [158]. Together, these findings indicate that TSPANs are not only promising therapeutic targets but also have value as clinical biomarkers. Their membrane localization and presence in biofluids (e.g., exosomes) make them attractive candidates for non-invasive diagnostic assays.
To advance these candidates toward the clinic, we must first establish comprehensive PK/PD profiles across delivery routes (intravenous, subcutaneous, and nanoparticle-encapsulated) and complete GLP-compliant toxicology in both rodent and nonrodent species to define half-lives, tissue distributions, clearances and safety margins. Simultaneously, we need to validate humanized mouse strains, transgenic rodents and large-animal fibrosis models that faithfully recapitulate human pathology [159], identify circulating (e.g., Pro-C3) [160] and imaging (e.g., MR elastography) [161] biomarkers, and then map reductions in murine collagen I/α-SMA staining directly onto these human endpoints. Effective animal doses must be converted to safe starting levels in humans via body-surface-area scaling and preclinical PK/PD data, guiding a two-stage clinical design: an initial healthy-volunteer PK/PD and safety assessment followed by biomarker-driven, dose-escalation studies in patients with early-stage fibrosis (such as NASH, IPF or post-MI cardiac fibrosis). Moreover, leveraging lipid nanoparticles or engineered exosomes to deliver siRNAs, antisense oligonucleotides or small-molecule inhibitors directly to fibrogenic cells (e.g., HSCs, and activated fibroblasts) enhances on-target efficacy while minimizing off-target effects [162]. Finally, given the diverse and tissue-specific functions of individual TSPANs, combination approaches (e.g., TSPAN inhibitors with approved antifibrotic agents) and adaptive trial designs are likely be needed to demonstrate meaningful clinical benefits [163]. This roadmap ensures that the therapeutic benefits observed in animal models can be predictably and safely translated into human fibrosis trials.
In summary, by integrating mechanistic insights with advanced delivery technologies and biomarker-guided patient selection, TSPAN-targeted therapies have the potential to transform fibrosis treatment from symptomatic relief to true disease modification.
Limitations, research gaps, and future directions
Although TSPANs represent compelling molecular targets for antifibrotic therapy, realizing their clinical potential will require overcoming key methodological shortcomings, filling critical biological knowledge gaps, addressing substantial translational challenges, and pursuing rigorous future studies.
Methodological limitations
Current insights into TSPAN functions rely heavily on a few animal models (e.g., CCl₄-induced liver injury) and on overexpression or knockout cell lines. Commercial antibodies often cross-react among paralogs, RNAi/CRISPR approaches can introduce off-target effects, and there is no standardized assay to quantify TEMs. Moreover, most studies are static, end-point only experiments, preventing us from understanding how efficacy and safety change over the course of fibrosis.
Research gaps
Although TM4SF5, CD151, CD63, and CD9 have all been linked to fibrosis in specific organs, we still lack a clear, stagewise picture of their individual roles and how they cooperate with integrins, TGF-β/Smad components, and exosome cargo. Additionally, most studies to date have focused on individual TSPANs in isolation, there is growing interest in their cooperative dynamics. TM4SF5–CD151–CD63 interactions in liver fibrosis represent one of the few mechanistically characterized cases of TSPAN–TSPAN crosstalk in organ fibrosis. However, comparable evidence in other organs such as the lung, kidney, or heart is still lacking. Similarly, correlations between circulating biomarkers (e.g., Pro-C3) or imaging readouts (e.g., MR elastography) and tissue-specific TSPAN expression have yet to be validated in clinical cohorts.
Challenges in TSPAN-based therapies
One of the central challenges in developing TSPAN-based antifibrotic therapies is the organ- and cell-type-specific heterogeneity of TSPAN functions. For example, CD151 plays a protective role by maintaining epithelial integrity in the lung, whereas in the liver, it can promote immune cell adhesion and inflammation, contributing to fibrosis. Similarly, CD9 has shown pro-fibrotic effects in the heart but may exert anti-inflammatory effects in pulmonary tissues. This context-dependent functional divergence poses significant risks for systemic delivery strategies and demands therapeutic approaches that can preserve normal physiological functions while selectively targeting disease-driving pathways. Furthermore, current delivery platforms—such as lipid nanoparticles or siRNA-loaded carriers—lack sufficient tissue specificity and cell-type resolution, leading to off-target effects and suboptimal pharmacokinetics [162]. RNA interference therapies, like those targeting TSPAN8, require chemical modifications (e.g., PEGylation) to improve stability and uptake but still face challenges in achieving selective tissue uptake. Exosome-based drug delivery, which naturally leverages TSPANs like CD63 and CD81, holds promise due to inherent biocompatibility. However, batch variability, purification challenges, and potential paradoxical effects (e.g., pro-fibrotic roles of CD63⁺ vesicles) remain concerns for clinical application. In addition, inter-species differences in TSPAN expression and function—such as TM4SF5 inhibitors working in rodents but not yet in human systems—underscore the necessity of humanized disease models.
Future directions
To bridge current gaps and enable clinical translation of TSPAN-based therapies, future research must integrate multi-scale modeling, high-resolution mapping, and biomarker-guided design.
First, organ- and stage-specific dissection of TSPAN function should be prioritized using spatial omics [164], single-cell profiling, and 3D in vitro systems such as organ-on-chip platforms [165]. These tools will help uncover how TSPANs interact with integrins, TGF-β/Smad signaling, and exosome cargo across fibrotic timelines and tissue contexts. Importantly, inter-TSPAN crosstalk, observed mechanistically only in the liver to date, should be investigated in other organs using co-IP, proximity labeling, or CRISPR-based perturbation systems.
Second, therapeutic development must embrace precision delivery technologies that accommodate the context-specific roles of TSPANs. Strategies may include antibody-guided nanoparticles, ligand-functionalized exosomes, or dual-marker delivery systems that restrict effects to disease-relevant cells. Animal studies should incorporate humanized or large-animal models [159] to overcome species-specific discrepancies, especially for molecules like TM4SF5.
Third, biomarker development should advance in parallel. Correlating circulating markers (e.g., Pro-C3, CD63⁺ exosomes) and non-invasive imaging tools (e.g., MR elastography) with tissue-specific TSPAN expression will enable patient stratification. This framework will also support adaptive, biomarker-enriched clinical trial designs [160, 161], beginning with phase I PK/PD studies and progressing to small, well-characterized patient cohorts [163].
Ultimately, a coordinated systems-level approach—linking molecular mechanism, delivery strategy, biomarker validation, and clinical trial design—will be essential to move TSPAN-targeted antifibrotic therapies from bench to bedside.
Conclusions and Outlook
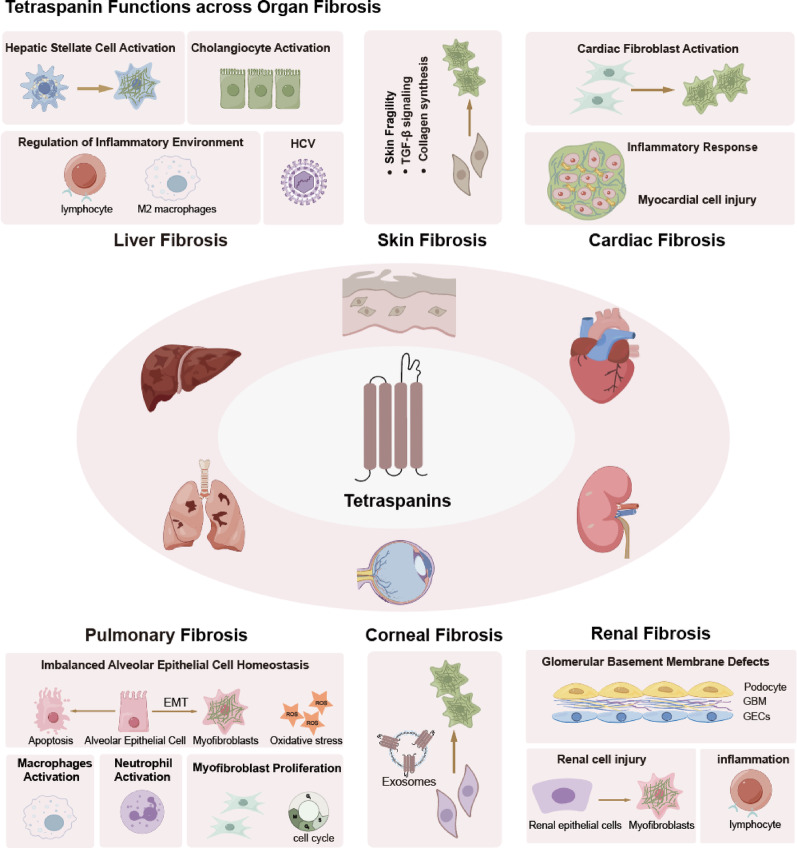
In this review, we highlighted TSPANs—especially TM4SF5, CD151, CD63 and CD9—as pivotal regulators of fibrosis across multiple organs, integrating fibroblast activation, EMT, immune–stromal crosstalk and ECM remodeling via integrin, TGF-β/Smad and STAT3 signaling (Table 1). This common pathway is pivotal across various organs and contributes to fibrosis. However, the functional contributions of individual TSPAN members also exhibit significant tissue-specific differences. For example, in pulmonary fibrosis, CD151 is critically involved in regulating epithelial cell adhesion, EMT and even the cell cycle, whereas in cardiac fibrosis, CD63 modulates fibroblast behavior and collagen synthesis, highlighting a divergence in the molecular mechanisms across organ systems (Fig. 7). By synthesizing evidence from in vitro studies and in vivo models, we demonstrate that TSPANs act as molecular hubs at the intersection of key fibrotic pathways.
Table 1.
Summary of tetraspanin aliases and their role in corresponding organ fibrosis
| Tetraspanins | Alternative name | Organ fibrosis | Effects | Specific roles | Reference |
|---|---|---|---|---|---|
| TSPAN1 | TSP-1, NET-1, TM4C, TM4SF, C4.8 | Pulmonary fibrosis | Repress | Inhibit AECs apoptosis and EMT | [10, 115] |
| TSPAN4 | NAG-2, TSP-4, TM4SF7 | Dermal fibrosis | Promote | Autoantigen target | [144] |
| TSPAN8 | CO-029, TM4SF3 | Liver fibrosis | Promote | Cholangiocytes activation | [52] |
| Renal fibrosis | Repress | Promote Autophagy | [135] | ||
| TSPAN24 | EBS7, MER2, RAPH, hemidesmosomal tetraspanin CD151, membrane glycoprotein SFA-1, platelet surface glycoprotein GP27, platelet-endothelial cell tetraspan antigen 3, PETA-3 | Liver fibrosis | Promote | HSCs activation | [49, 50] |
| Inflammatory response | [60] | ||||
| Pulmonary fibrosis | Repress | Basement membrane integrity | [19, 110, 111] | ||
| Inhibit proliferation | [112] | ||||
| Promote | AECs activation, fibroblast proliferation, Inflammatory response | [120, 121] | |||
| Renal fibrosis | Repress | Basement membrane integrity | [138, 139] | ||
| Dermal fibrosis | Repress | Basement membrane integrity | [145] | ||
| TSPAN26 | CD37, CD37 molecule, leukocyte Antigen CD37, tetraspanin-26, CD3 7antigen, tspan-26, cell differentiation antigen 37,lLeukocyte surface antigen CD37, GP52-40 | Renal fibrosis | Repress | Inhibit inflammatory responses | [136, 137] |
| TSPAN28 | S5.7, CD81, CVID6, TAPA-1 | Liver fibrosis | Promote | Promote viral infection | [65–68, 73] |
| TSPAN29 | BTCC-1, DRAP-27, GIG2, MIC3, MRP-1, CD9, 5H9, BA-2/p24 antigen, cell growth-inhibiting gene 2 protein, leukocyte antigen MIC3, motility related protein-1 (MRP-1) | Cardiac Fibrosis | Promote | Myocardial cell injury and inflammation | [6] |
| Pulmonary fibrosis | Promote | AECs apoptosis, Myofibroblasts transition, Inflammatory responses | [112] | ||
| Dermal fibrosis | Promote | Myofibroblasts transition by exosomes | [141] | ||
| Corneal fibrosis | Promote | Myofibroblasts transition by exosomes | [151, 152] | ||
| TSPAN30 | CD63, melanoma 1 antigen, granulophysin, lysosomal-associated membrane protein 3, melanoma-associated antigen ME491, melanoma-associated antigen MLA1, ocular melanoma-associated antigen | Liver fibrosis | Repress | Inhibit HSCs activation | [74] |
| Cardiac Fibrosis | Promote | Myofibroblasts transition | [20] | ||
| Pulmonary fibrosis | Promote | Myofibroblasts proliferation | [122] | ||
| Dermal fibrosis | Promote | Myofibroblasts transition by exosomes | [142] | ||
| Corneal fibrosis | Promote | Myofibroblasts transition by exosomes | [151, 152] | ||
| TM4SF5 | Liver fibrosis | Promote | HSCs activation | [7, 43] | |
| Inflammatory response | [17, 57, 59] | ||||
| Pulmonary fibrosis | Repress | Mitigate oxidative stress | |||
| [119] |
AECs: alveolar epithelial cells; HSCs: hepatic stellate cells; EMT: epithelial-mesenchymal transition
Fig. 7.
Role of TSPANs across Organ Fibrosis
The identification of TSPANs as critical molecular regulators in fibrotic diseases may represent a pivotal advance: it redirects the field from symptom-focused strategies to the precise modulation of distinct signaling nodes. Targeting TSPANs presents opportunities for organ specific, mechanism-based therapies that may overcome the nonspecific off-target effects of current antifibrotic agents. Additionally, in-depth studies of TSPAN-associated biomarkers (e.g., serum Pro-C3, elastography imaging) and delivery systems (e.g., lipid nanoparticles, engineered exosomes) enable the realization of personalized medical approaches.
Moving forward, the path to clinical impact will require (1) the comprehensive validation of the role of TSPAN in humanized and large-animal fibrosis models; (2) the refinement of organ-targeted delivery systems and robust PK/PD and toxicology profiling; and (3) early-phase, biomarker-driven trials to mitigate safety risks and demonstrate on-target engagement. By addressing these challenges in a coordinated, multidisciplinary effort, TSPAN-based therapies have the potential to deliver the first disease-modifying treatments for fibrosis, offering renewed hope for patients suffering from chronic fibrotic disorders across the liver, heart, lung, kidney, skin and cornea.
TSPANs regulate organ fibrosis through a conserved profibrotic axis—fibroblast activation and ECM deposition. Moreover, these molecules perform analogous functions across multiple organs, including direct injury to key cell populations such as myocardial cells, renal parenchymal cells, and cholangiocytes, as well as the modulation of immune microenvironments and inflammatory responses. Furthermore, when acting in specific organs, TSPANs display distinct organ-specific functions that underscore the divergence in their molecular mechanisms across tissue contexts.
AECs: alveolar epithelial cells; HSCs: hepatic stellate cells; EMT: epithelial-mesenchymal transition
Acknowledgements
Not applicable.
Abbreviations
- AECs
Alveolar epithelial cells
- Arntl
Aryl hydrocarbon receptor nuclear translocator-like
- BM
Basement membrane
- CD151
Tetraspanin 24
- CD37
Tetraspanin 37
- CD63
Tetraspanin 30
- CD81
Tetraspanin 28
- CD9
Tetraspanin 29
- CLDN1
Claudin-1
- CO-029
Tetraspanin 8
- Cry1
Cryptochrome 1
- Ccnd
Cyclin D
- CLL
Chronic lymphocytic leukemia
- DN
Diabetic nephropathy
- ECM
Extracellular matrix
- EGFR
Epidermal growth factor receptor
- EMT
Epithelial-mesenchymal transition
- Erk
Extracellular-regulated kinase
- EBS
Epidermolysis bullosa simplex
- FAK
Focal adhesion kinase
- GBM
Glomerular basement membrane
- GP130
Glycoprotein 130
- HCV
Hepatitis C virus
- HSCs
Hepatic stellate cells
- HK2
Human kidney 2
- HSF
Human skin fibroblasts
- IgA
Immunoglobulin A
- IPF
Idiopathic pulmonary fibrosis
- LC3-II/LC3-I
Microtubule-associated protein 1A/1B light chain 3 II/I
- MMPs
Matrix metalloproteinases
- MWCNTs
Multiwalled carbon nanotubes
- NAFLD
Nonalcoholic fatty liver disease
- NAG-2
TM4SF7
- NASH
Nonalcoholic steatohepatitis
- Npas2
Neuronal PAS domain protein 2
- NSCLC
Non-small cell lung cancer
- PDAC
Pancreatic ductal adenocarcinoma
- PDGF
Platelet-derived growth factor
- Per2
Period circadian protein homolog 2
- Per3
Period circadian protein homolog 3
- PK/PD
Pharmacokinetics/pharmacodynamics
- ROS
Reactive oxygen species
- SOCS
Suppressor of cytokine signaling
- SSc
Systemic sclerosis
- STAT3
Signal transducer and activator of transcription 3
- TAFs
Tumor-associated fibroblasts
- TAC
Transverse aortic constriction
- TEMs
Tetraspanin-enriched microdomains
- TGF-β
Transforming growth factor-beta
- TIMPs
Tissue inhibitors of metalloproteinases
- TIMP-1
Tissue inhibitor of metalloproteinases1
- TM4SF5
Transmembrane 4 L six family member 5
- TSPANs
Tetraspanins
- TSPAN1
Tetraspanin 1
- TSPAN8
Tetraspanin 8
- TSPAN30
Tetraspanin 30
- TSPAN37
Tetraspanin 37
- TNF
Tumor necrosis factor
- T1DM
Type 1 Diabetes Mellitus
- Xiap
X-linked inhibitor of apoptosis protein
Author contributions
Shengbiao Li, Mi Li, and Yi Zhang made equal contributions to the preparation of the original manuscript draft. Shengbiao Li and Mi Li were responsible for preparing the figures. Jiapan Li, Ji Chen, Shubo Fu, Jingyan Yi, Rong Li, Gan Qiao, and Yang Yu revised the manuscript. Chunxiang Zhang, Shengbiao Li, and Qiuhong Li provided guidance for the entire study. All the authors read and approved to the final manuscript.
Funding
This work was supported by the Sichuan Science and Technology Program [grant numbers 2024NSFSC0580 and 2023NSFSC1840], the National Natural Science Foundation of China [grant number U23A20398], the National Natural Science Foundation of China [grant number 82030007], the Central Government Guides Local Science and Technology Development Project [grant number 2022ZYD0057], the Sichuan Science and Technology Program [grant numbers 2022YFS0578, 2022YFS0614], the Science and Technology Project of the Luzhou Government [grant numbers 2022YFS0630-B1 and 2022YFS0627-A1], and Southwest Medical University [grant number 2021ZKMS005].
Availability of data materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
We give our consent for the manuscript to be published in Cell Communication and Signaling.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Shengbiao Li, Mi Li and Yi Zhang contributed equally to this work.
Contributor Information
Chunxiang Zhang, Email: zhangchx2021@163.com.
Qiuhong Li, Email: li-qiuhong@swmu.edu.cn.
References
- 1.Weiskirchen R, Weiskirchen S, Tacke F. Organ and tissue fibrosis: molecular signals, cellular mechanisms and translational implications. Mol Aspects Med. 2019;65:2–15. [DOI] [PubMed] [Google Scholar]
- 2.Talbott HE, Mascharak S, Griffin M, Wan DC, Longaker MT. Wound healing, fibroblast heterogeneity, and fibrosis. Cell Stem Cell. 2022;29:1161–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Henderson NC, Rieder F, Wynn TA. Fibrosis: from mechanisms to medicines. Nature. 2020;587:555–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rockey DC, Longo DL, Bell PD, Hill JA. Fibrosis — A common pathway to organ injury and failure. N Engl J Med. 2015;372:1138–49. [DOI] [PubMed] [Google Scholar]
- 5.Chen K, Li Q, Li Y, Jiang D, Chen L, Jiang J, et al. Tetraspanins in digestive–system cancers: expression, function and therapeutic potential (review). Mol Med Rep. 2024;30:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Y, Fan S, Kong L, Hao Z, Zhou Y, Shangguan J, et al. CD9 exacerbates pathological cardiac hypertrophy through regulating GP130/STAT3 signaling pathway. iScience. 2023;26:108070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ryu J, Lee JW. TM4SF5-Mediated roles in the development of fibrotic phenotypes. Mediat Inflamm. 2017;2017:5108525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang L, Huang C, Fan S. Mangiferin and organ fibrosis: A mini review. BioFactors. 2020;47:59–68. [DOI] [PubMed] [Google Scholar]
- 9.Masszi A, Speight P, Charbonney E, Lodyga M, Nakano H, Szászi K, et al. Fate-determining mechanisms in epithelial–myofibroblast transition: major inhibitory role for Smad3. J Cell Biol. 2010;188:383–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu G, Wang Y, Yang L, Zou B, Gao S, Song Z, et al. Tetraspanin 1 as a mediator of fibrosis inhibits EMT process and Smad2/3 and beta-catenin pathway in human pulmonary fibrosis. J Cell Mol Med. 2019;23:3583–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lang T, Hochheimer N, Tetraspanins. Curr Biol. 2020;30:R204–6. [DOI] [PubMed] [Google Scholar]
- 12.Saiz ML, Rocha-Perugini V, Sánchez-Madrid F. Tetraspanins as organizers of antigen-presenting cell function. Front Immunol. 2018;9:1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang X, Zhang J, Huang Y. Tetraspanins in cell migration. Cell Adh Migr. 2015;9:406–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jankovičová J, Sečová P, Michalková K, Antalíková J. Tetraspanins, more than markers of extracellular vesicles in reproduction. Int J Mol Sci. 2020;21:7568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kummer D, Steinbacher T, Schwietzer MF, Thölmann S, Ebnet K. Tetraspanins: integrating cell surface receptors to functional microdomains in homeostasis and disease. Med Microbiol Immunol. 2020;209:397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maecker HT, Todd SC, Levy S. The tetraspanin superfamily: molecular facilitators. FASEB J. 1997;11:428–42. [PubMed] [Google Scholar]
- 17.Ryu J, Kim E, Kang M-K, Song D-G, Shin E-A, Lee H, et al. Differential TM4SF5-mediated SIRT1 modulation and metabolic signaling in nonalcoholic steatohepatitis progression. J Pathol. 2021;253:55–67. [DOI] [PubMed] [Google Scholar]
- 18.Gross JL, de Azevedo MJ, Silveiro SP, Canani LH, Caramori ML, Zelmanovitz T. Diabetic nephropathy: diagnosis, prevention, and treatment. Diabetes Care. 2005;28:164–76. [DOI] [PubMed] [Google Scholar]
- 19.Tsujino K, Takeda Y, Arai T, Shintani Y, Inagaki R, Saiga H, et al. Tetraspanin CD151 protects against pulmonary fibrosis by maintaining epithelial integrity. Am J Respir Crit Care Med. 2012;186:170–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takawale A, Zhang P, Patel VB, Wang X, Oudit G, Kassiri Z. Tissue inhibitor of matrix Metalloproteinase-1 promotes myocardial fibrosis by mediating CD63-Integrin β1 interaction. Hypertension. 2017;69:1092–103. [DOI] [PubMed] [Google Scholar]
- 21.Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med. 2012;18:1028–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iredale JP, Thompson A, Henderson NC. Extracellular matrix degradation in liver fibrosis: biochemistry and regulation. Biochimica et biophysica acta (BBA) Mol Basis Dis. 2013;1832:876–83. [DOI] [PubMed]
- 23.Mazzarino. Matrix metalloproteinases and their inhibitors as markers of inflammation and fibrosis in chronic liver disease (Review). Int J Mol Med. 2009;24:143-152. [DOI] [PubMed]
- 24.Iredale J, Campana L. Regression of liver fibrosis. Semin Liver Dis. 2017;37:1–10. [DOI] [PubMed] [Google Scholar]
- 25.Zhang X, Sharma P, Maschmeyer P, Hu Y, Lou M, Kim J, et al. GARP on hepatic stellate cells is essential for the development of liver fibrosis. J Hepatol. 2023;79:1214–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parola M, Marra F, Pinzani M. Myofibroblast - like cells and liver fibrogenesis: emerging concepts in a rapidly moving scenario. Mol Aspects Med. 2008;29:58–66. [DOI] [PubMed] [Google Scholar]
- 27.Pellicoro A, Ramachandran P, Iredale JP, Fallowfield JA. Liver fibrosis and repair: immune regulation of wound healing in a solid organ. Nat Rev Immunol. 2014;14:181–94. [DOI] [PubMed] [Google Scholar]
- 28.Seki E, Schwabe RF. Hepatic inflammation and fibrosis: functional links and key pathways. Hepatology. 2015;61:1066–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Najafi N, Razavi A, Jafarpour H, Raei M, Azizi Z, Davoodi L, et al. Evaluation of hepatic injury in chronic hepatitis B and C using APRI and FIB-4 indices compared to fibroscan results. Ann Med Surg (Lond). 2024;86:3841–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sebastiani G, Gkouvatsos K, Pantopoulos K. Chronic hepatitis C and liver fibrosis. World J Gastroenterol. 2014;20:11033–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Osna NA, Tikhanovich I, Ortega-Ribera M, Mueller S, Zheng C, Mueller J, et al. Alcohol-Associated liver disease outcomes: critical mechanisms of liver injury progression. Biomolecules. 2024;14:404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tu T, Calabro SR, Lee A, Maczurek AE, Budzinska MA, Warner FJ, et al. Hepatocytes in liver injury: victim, bystander, or accomplice in progressive fibrosis? J Gastroenterol Hepatol. 2015;30:1696–704. [DOI] [PubMed] [Google Scholar]
- 33.Penz-Österreicher M, Österreicher CH, Trauner M. Fibrosis in autoimmune and cholestatic liver disease. Best Pract Res Clin Gastroenterol. 2011;25:245–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang D, Zhang Y, Sun B. The molecular mechanisms of liver fibrosis and its potential therapy in application. Int J Mol Sci. 2022;23:12572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karsdal MA, Manon-Jensen T, Genovese F, Kristensen JH, Nielsen MJ, Sand JMB, et al. Novel insights into the function and dynamics of extracellular matrix in liver fibrosis. Am J Physiol Gastrointest Liver Physiol. 2015;308:G807–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu Y, Wen XM, Lui ELH, Friedman SL, Cui W, Ho NPS, et al. Therapeutic targeting of the PDGF and TGF-β-signaling pathways in hepatic stellate cells by PTK787/ZK22258. Lab Invest. 2009;89:1152–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kamm DR, McCommis KS. Hepatic stellate cells in physiology and pathology. J Physiol. 2022;600:1825–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Higashi T, Friedman SL, Hoshida Y. Hepatic stellate cells as key target in liver fibrosis. Adv Drug Deliv Rev. 2017;121:27–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dewidar B, Meyer C, Dooley S, Meindl-Beinker AN. TGF-β in hepatic stellate cell activation and liver fibrogenesis-updated 2019. Cells. 2019;8:1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee JW. Transmembrane 4 L six family member 5 (TM4SF5)-mediated epithelial-mesenchymal transition in liver diseases. Int Rev Cell Mol Biol. 2015;319:141–63. [DOI] [PubMed] [Google Scholar]
- 41.Kim H, Kwon S, Nam SH, Jung JW, Kang M, Ryu J, et al. Dynamic and coordinated single-molecular interactions at TM4SF5‐enriched microdomains guide invasive behaviors in 2‐ and 3‐dimensional environments. FASEB J. 2017;31:1461–81. [DOI] [PubMed] [Google Scholar]
- 42.Kang M, Ryu J, Lee D, Lee M-S, Kim H-J, Nam SH et al. Correlations between transmembrane 4 L6 family member 5 (TM4SF5), CD151, and CD63 in liver fibrotic phenotypes and hepatic migration and invasive capacities. Ray RB, editor. PLoS ONE. 2014;9:e102817. [DOI] [PMC free article] [PubMed]
- 43.Kang M, Jeong S-J, Park SY, Lee HJ, Kim HJ, Park KH, et al. Antagonistic regulation of transmembrane 4 L6 family member 5 attenuates fibrotic phenotypes in CCl(4) -treated mice. FEBS J. 2012;279:625–35. [DOI] [PubMed] [Google Scholar]
- 44.Lee S-A, Lee S-Y, Cho I-H, Oh M-A, Kang E-S, Kim Y-B, et al. Tetraspanin TM4SF5 mediates loss of contact Inhibition through epithelial-mesenchymal transition in human hepatocarcinoma. J Clin Invest. 2008;118:1354–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meindl-Beinker NM, Dooley S. Transforming growth factor-beta and hepatocyte transdifferentiation in liver fibrogenesis. J Gastroenterol Hepatol. 2008;23(Suppl 1):S122–127. [DOI] [PubMed] [Google Scholar]
- 46.Gressner AM, Weiskirchen R. Modern pathogenetic concepts of liver fibrosis suggest stellate cells and TGF-beta as major players and therapeutic targets. J Cell Mol Med. 2006;10:76–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bailey RL, Herbert JM, Khan K, Heath VL, Bicknell R, Tomlinson MG. The emerging role of tetraspanin microdomains on endothelial cells. Biochem Soc T. 2011;39:1667–73. [DOI] [PubMed] [Google Scholar]
- 48.Kwon MJ, Park S, Choi JY, Oh E, Kim YJ, Park Y-H, et al. Clinical significance of CD151 overexpression in subtypes of invasive breast cancer. Br J Cancer. 2012;106:923–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mazzocca A, Carloni V, Sciammetta S, Cordella C, Pantaleo P, Caldini A, et al. Expression of transmembrane 4 superfamily (TM4SF) proteins and their role in hepatic stellate cell motility and wound healing migration. J Hepatol. 2002;37:322–30. [DOI] [PubMed] [Google Scholar]
- 50.Hung T-M, Chang SC, Yu W-H, Wang Y-W, Huang C, Lu S-C, et al. A novel nonsynonymous variant of matrix metalloproteinase-7 confers risk of liver cirrhosis. Hepatology. 2009;50:1184–93. [DOI] [PubMed] [Google Scholar]
- 51.Banales JM, Huebert RC, Karlsen T, Strazzabosco M, LaRusso NF, Gores GJ. Cholangiocyte pathobiology. Nat Rev Gastroenterol Hepatol. 2019;16:269–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shackel NA, McGuinness PH, Abbott CA, Gorrell MD, McCaughan GW. Novel differential gene expression in human cirrhosis detected by suppression subtractive hybridization. Hepatology. 2003;38:577–88. [DOI] [PubMed] [Google Scholar]
- 53.Itakura J, Nagayama K, Enomoto N, Sakamoto N, Tazawa J, Izumi N, et al. CD81 nucleotide mutation in hepatocellular carcinoma and lack of CD81 polymorphism in patients at stages of hepatitis C virus infection. J Med Virol. 2001;63:22–8. [PubMed] [Google Scholar]
- 54.Pileri P, Uematsu Y, Campagnoli S, Galli G, Falugi F, Petracca R, et al. Binding of hepatitis C virus to CD81. Science. 1998;282:938–41. [DOI] [PubMed] [Google Scholar]
- 55.Zhang N, Yao H, Zhang Z, Li Z, Chen X, Zhao Y, et al. Ongoing involvers and promising therapeutic targets of hepatic fibrosis: the hepatic immune microenvironment. Front Immunol. 2023;14:1131588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim E, Um H, Park J, Jung JW, Kim JE, Lee H, et al. TM4SF5-dependent crosstalk between hepatocytes and macrophages to reprogram the inflammatory environment. Cell Rep. 2021;37:110018. [DOI] [PubMed] [Google Scholar]
- 57.Jung JW, Macalino SJY, Cui M, Kim JE, Kim H-J, Song D-G, et al. Transmembrane 4 L six family member 5 senses arginine for mTORC1 signaling. Cell Metabol. 2019;29:1306–e13197. [DOI] [PubMed] [Google Scholar]
- 58.Agarwal R, Agarwal P. Targeting extracellular matrix remodeling in disease: could Resveratrol be a potential candidate? Experimental Biology Med. 2016;242:374–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee H, Kim E, Shin E-A, Shon JC, Sun H, Kim JE, et al. Crosstalk between TM4SF5 and GLUT8 regulates Fructose metabolism in hepatic steatosis. Mol Metab. 2022;58:101451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wadkin JCR, Patten DA, Kamarajah SK, Shepherd EL, Novitskaya V, Berditchevski F, et al. CD151 supports VCAM-1-mediated lymphocyte adhesion to liver endothelium and is upregulated in chronic liver disease and hepatocellular carcinoma. Am J Physiol Gastrointest Liver Physiol. 2017;313:G138–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lalor PF, Edwards S, McNab G, Salmi M, Jalkanen S, Adams DH. Vascular adhesion protein-1 mediates adhesion and transmigration of lymphocytes on human hepatic endothelial cells. J Immunol. 2002;169:983–92. [DOI] [PubMed] [Google Scholar]
- 62.Ley K, Zhang H. Dances with leukocytes: how tetraspanin-enriched microdomains assemble to form endothelial adhesive platforms. J Cell Biol. 2008;183:375–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Westbrook RH, Dusheiko G. Natural history of hepatitis C. J Hepatol. 2014;61:S58–68. [DOI] [PubMed] [Google Scholar]
- 64.Mazzocca A, Sciammetta SC, Carloni V, Cosmi L, Annunziato F, Harada T, et al. Binding of hepatitis C virus envelope protein E2 to CD81 Up-regulates matrix Metalloproteinase-2 in human hepatic stellate cells. J Biol Chem. 2005;280:11329–39. [DOI] [PubMed] [Google Scholar]
- 65.Sala-Valdés M, Ursa Á, Charrin S, Rubinstein E, Hemler ME, Sánchez-Madrid F, et al. EWI-2 and EWI-F link the tetraspanin web to the actin cytoskeleton through their direct association with Ezrin-Radixin-Moesin proteins. J Biol Chem. 2006;281:19665–75. [DOI] [PubMed] [Google Scholar]
- 66.Boucheix C, Rubinstein E, Tetraspanins. Cell Mol Life Sci. 2001;58:1189–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kumar A, Hossain RA, Yost SA, Bu W, Wang Y, Dearborn AD, et al. Structural insights into hepatitis C virus receptor binding and entry. Nature. 2021;598:521–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Evans MJ, von Hahn T, Tscherne DM, Syder AJ, Panis M, Wölk B, et al. Claudin-1 is a hepatitis C virus co-receptor required for a late step in entry. Nature. 2007;446:801–5. [DOI] [PubMed] [Google Scholar]
- 69.Krieger SE, Zeisel MB, Davis C, Thumann C, Harris HJ, Schnober EK, et al. Inhibition of hepatitis C virus infection by anti-claudin-1 antibodies is mediated by neutralization of E2-CD81-Claudin-1 associations. Hepatology. 2010;51:1144–57. [DOI] [PubMed] [Google Scholar]
- 70.Harris HJ, Farquhar MJ, Mee CJ, Davis C, Reynolds GM, Jennings A, et al. CD81 and Claudin 1 coreceptor association: role in hepatitis C virus entry. J Virol. 2008;82:5007–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Harris HJ, Davis C, Mullins JGL, Hu K, Goodall M, Farquhar MJ, et al. Claudin association with CD81 defines hepatitis C virus entry. J Biol Chem. 2010;285:21092–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lupberger J, Zeisel MB, Xiao F, Thumann C, Fofana I, Zona L, et al. EGFR and EphA2 are host factors for hepatitis C virus entry and possible targets for antiviral therapy. Nat Med. 2011;17:589–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zona L, Lupberger J, Sidahmed-Adrar N, Thumann C, Harris HJ, Barnes A, et al. HRas signal transduction promotes hepatitis C virus cell entry by triggering assembly of the host tetraspanin receptor complex. Cell Host Microbe. 2013;13:302–13. [DOI] [PubMed] [Google Scholar]
- 74.Ramezani-Moghadam M, Wang J, Ho V, Iseli TJ, Alzahrani B, Xu A, et al. Adiponectin reduces hepatic stellate cell migration by promoting tissue inhibitor of metalloproteinase-1 (TIMP-1) secretion. J Biol Chem. 2015;290:5533–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Reif S, Lang A, Lindquist JN, Yata Y, Gabele E, Scanga A, et al. The role of focal adhesion kinase-phosphatidylinositol 3-kinase-akt signaling in hepatic stellate cell proliferation and type I collagen expression. J Biol Chem. 2003;278:8083–90. [DOI] [PubMed] [Google Scholar]
- 76.Maruyama K, Imanaka-Yoshida K. The pathogenesis of cardiac fibrosis: A review of recent progress. Int J Mol Sci. 2022;23:2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang T, Jiang D, Zhang X, Chen L, Jiang J, Zhang C, et al. The role of nonmyocardial cells in the development of and the protective effects of FGF21: A current diabetic cardiomyopathyunderstanding. Cell Commun Signal. 2024;22:446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang L, Huang C, Fan S. Mangiferin and organ fibrosis: A mini review. BioFactors. 2021;47:59–68. [DOI] [PubMed] [Google Scholar]
- 79.Rodriguez-Gonzalez M, Lubian-Gutierrez M, Cascales-Poyatos HM, Perez-Reviriego AA, Castellano-Martinez A. Role of the Renin-Angiotensin-Aldosterone system in Dystrophin-Deficient cardiomyopathy. Int J Mol Sci. 2020;22:356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ghafouri-Fard S, Abak A, Talebi SF, Shoorei H, Branicki W, Taheri M, et al. Role of MiRNA and LncRNAs in organ fibrosis and aging. Biomed Pharmacother. 2021;143:112132. [DOI] [PubMed] [Google Scholar]
- 81.Sun G, Chen J, Ding Y, Wren JD, Xu F, Lu L, et al. A bioinformatics perspective on the links between Tetraspanin-Enriched microdomains and cardiovascular pathophysiology. Front Cardiovasc Med. 2021;8:630471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Termini CM, Gillette JM. Tetraspanins Function as Regulators of Cellular Signaling. Front Cell Dev Biol [Internet]. 2017 [cited 2025 Jan 14];5. Available from: https://www.frontiersin.org/journals/cell-and-developmental-biology/articles/10.3389/fcell.2017.00034/full [DOI] [PMC free article] [PubMed]
- 83.Schröder J, Lüllmann-Rauch R, Himmerkus N, Pleines I, Nieswandt B, Orinska Z, et al. Deficiency of the tetraspanin CD63 associated with kidney pathology but normal lysosomal function. Mol Cell Biol. 2009;29:1083–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pols MS, Klumperman J. Trafficking and function of the tetraspanin CD63. Exp Cell Res. 2009;315:1584–92. [DOI] [PubMed] [Google Scholar]
- 85.Euan Martínez AA, Bergmann AK, Tellkamp F, Schott-Verdugo S, Bouvain P, Steinhausen J, et al. CD63 as novel target for nanoemulsion-based 19F MRI imaging and drug delivery to activated cardiac fibroblasts. Theranostics. 2025;15:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang F, Kotha J, Jennings LK, Zhang XA. Tetraspanins and vascular functions. Cardiovascular Res. 2009;83:7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hemler ME. Targeting of tetraspanin proteins — potential benefits and strategies. Nat Rev Drug Discov. 2008;7:747–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Powner D, Kopp PM, Monkley SJ, Critchley DR, Berditchevski F. Tetraspanin CD9 in cell migration. Biochem Soc Trans. 2011;39:563–7. [DOI] [PubMed] [Google Scholar]
- 89.Zöller M, Tetraspanins. Push and pull in suppressing and promoting metastasis. Nat Rev Cancer. 2009;9:40–55. [DOI] [PubMed] [Google Scholar]
- 90.van Spriel AB. Tetraspanins in the humoral immune response. Biochem Soc Trans. 2011;39:512–7. [DOI] [PubMed] [Google Scholar]
- 91.Cho JH, Kim E-C, Son Y, Lee D-W, Park YS, Choi JH, et al. CD9 induces cellular senescence and aggravates atherosclerotic plaque formation. Cell Death Differ. 2020;27:2681–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yasukawa H, Hoshijima M, Gu Y, Nakamura T, Pradervand S, Hanada T, et al. Suppressor of cytokine signaling-3 is a Biomechanical stress–inducible gene that suppresses gp130-mediated cardiac myocyte hypertrophy and survival pathways. J Clin Invest. 2001;108:1459–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ancey C. Human cardiomyocyte hypertrophy induced in vitro by gp130 stimulation. Cardiovascular Res. 2003;59:78–85. [DOI] [PubMed] [Google Scholar]
- 94.Fischer P, Hilfiker-Kleiner D. Survival pathways in hypertrophy and heart failure: the gp130-STAT3 axis. Basic Res Cardiol. 2007;102:279–97. [DOI] [PubMed] [Google Scholar]
- 95.Kurose H, Mangmool S. Myofibroblasts and inflammatory cells as players of cardiac fibrosis. Arch Pharm Res. 2016;39:1100–13. [DOI] [PubMed] [Google Scholar]
- 96.Gandhi SA, Min B, Fazio JC, Johannson KA, Steinmaus C, Reynolds CJ, et al. The impact of occupational exposures on the risk of idiopathic pulmonary fibrosis: A systematic review and meta-analysis. Ann Am Thorac Soc. 2024;21:486–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Odashima K, Kagiyama N, Kanauchi T, Ishiguro T, Takayanagi N. Incidence and etiology of chronic pulmonary infections in patients with idiopathic pulmonary fibrosis. PLoS ONE. 2020;15:e0230746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Castelino FV, Varga J. Interstitial lung disease in connective tissue diseases: evolving concepts of pathogenesis and management. Arthritis Res Ther. 2010;12:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Skeoch S, Weatherley N, Swift AJ, Oldroyd A, Johns C, Hayton C, et al. Drug-induced interstitial lung disease: A systematic review. J Clin Med. 2018;7:356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fingerlin TE, Murphy E, Zhang W, Peljto AL, Brown KK, Steele MP, et al. Genome-wide association study identifies multiple susceptibility loci for pulmonary fibrosis. Nat Genet. 2013;45:613–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Serrano-Mollar A. [alveolar epithelial cell injury as an etiopathogenic factor in pulmonary fibrosis]. Arch Bronconeumol. 2012;48(Suppl 2):2–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kelly M, Kolb M, Bonniaud P, Gauldie J. Re-evaluation of fibrogenic cytokines in lung fibrosis. CPD. 2003;9:39–49. [DOI] [PubMed] [Google Scholar]
- 103.Bonner JC. Regulation of PDGF and its receptors in fibrotic diseases. Cytokine Growth Factor Rev. 2004;15:255–73. [DOI] [PubMed] [Google Scholar]
- 104.Niayesh-Mehr R, Kalantar M, Bontempi G, Montaldo C, Ebrahimi S, Allameh A, et al. The role of epithelial-mesenchymal transition in pulmonary fibrosis: lessons from idiopathic pulmonary fibrosis and COVID-19. Cell Commun Signal. 2024;22:542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Königshoff M, Kramer M, Balsara N, Wilhelm J, Amarie OV, Jahn A, et al. WNT1-inducible signaling protein-1 mediates pulmonary fibrosis in mice and is upregulated in humans with idiopathic pulmonary fibrosis. J Clin Invest. 2009;119:772–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gao F, Kinnula VL, Myllärniemi M, Oury TD. Extracellular superoxide dismutase in pulmonary fibrosis. Antioxid Redox Signal. 2008;10:343–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Otoupalova E, Smith S, Cheng G, Thannickal VJ. Oxidative stress in pulmonary fibrosis. Compr Physiol. 2020;10:509–47. [DOI] [PubMed] [Google Scholar]
- 108.Richeldi L, Collard HR, Jones MG. Idiopathic pulmonary fibrosis. Lancet. 2017;389:1941–52. [DOI] [PubMed] [Google Scholar]
- 109.Crosby LM, Waters CM. Epithelial repair mechanisms in the lung. Am J Physiol Lung Cell Mol Physiol. 2010;298:L715–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shigeta M, Sanzen N, Ozawa M, Gu J, Hasegawa H, Sekiguchi K. CD151 regulates epithelial cell–cell adhesion through PKC- and Cdc42-dependent actin cytoskeletal reorganization. J Cell Biol. 2003;163:165–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Moribe H, Yochem J, Yamada H, Tabuse Y, Fujimoto T, Mekada E. Tetraspanin protein (TSP-15) is required for epidermal integrity incaenorhabditis elegans. J Cell Sci. 2004;117:5209–20. [DOI] [PubMed] [Google Scholar]
- 112.Tripathi LP, Itoh MN, Takeda Y, Tsujino K, Kondo Y, Kumanogoh A, et al. Integrative analysis reveals common and unique roles of tetraspanins in fibrosis and emphysema. Front Genet. 2020;11:585998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Takeda Y, He P, Tachibana I, Zhou B, Miyado K, Kaneko H, et al. Double deficiency of tetraspanins CD9 and CD81 alters cell motility and protease production of macrophages and causes chronic obstructive pulmonary disease-like phenotype in mice. J Biol Chem. 2008;283:26089–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Suzuki M, Tachibana I, Takeda Y, He P, Minami S, Iwasaki T, et al. Tetraspanin CD9 negatively regulates lipopolysaccharide-induced macrophage activation and lung inflammation. J Immunol. 2009;182:6485–93. [DOI] [PubMed] [Google Scholar]
- 115.Yang L, Wang Y, Pan Z, Gao S, Zou B, Lin Z, et al. Tetraspanin 1 inhibits TNFα-induced apoptosis via NF-κB signaling pathway in alveolar epithelial cells. Inflamm Res. 2018;67:951–64. [DOI] [PubMed] [Google Scholar]
- 116.Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest. 2003;112:1776–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Willis BC, Borok Z. TGF-β-induced EMT: mechanisms and implications for fibrotic lung disease. Am J Physiology-Lung Cell Mol Physiol. 2007;293:L525–34. [DOI] [PubMed] [Google Scholar]
- 118.Kasai H, Allen JT, Mason RM, Kamimura T, Zhang Z. TGF-β1 induces human alveolar epithelial to mesenchymal cell transition (EMT). Respir Res. 2005;6:56. [DOI] [PMC free article] [PubMed]
- 119.Kim JE, Kim H-J, Jung JW, Song D-G, Park D, Lee H, et al. TM4SF5-mediated CD44v8-10 splicing variant promotes survival of type II alveolar epithelial cells during idiopathic pulmonary fibrosis. Cell Death Dis. 2019;10:645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Fujishima S, Shiomi T, Yamashita S, Yogo Y, Nakano Y, Inoue T, et al. Production and activation of matrix metalloproteinase 7 (matrilysin 1) in the lungs of patients with idiopathic pulmonary fibrosis. Arch Pathol Lab Med. 2010;134:1136–42. [DOI] [PubMed] [Google Scholar]
- 121.Shiomi T, Inoki I, Kataoka F, Ohtsuka T, Hashimoto G, Nemori R, et al. Pericellular activation of proMMP-7 (promatrilysin-1) through interaction with CD151. Lab Invest. 2005;85:1489–506. [DOI] [PubMed] [Google Scholar]
- 122.Duch P, Díaz-Valdivia N, Ikemori R, Gabasa M, Radisky ES, Arshakyan M, et al. Aberrant TIMP-1 overexpression in tumor-associated fibroblasts drives tumor progression through CD63 in lung adenocarcinoma. Matrix Biol. 2022;111:207–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Dong J, Ma Q. TIMP1 promotes multi-walled carbon nanotube-induced lung fibrosis by stimulating fibroblast activation and proliferation. Nanotoxicology. 2017;11:41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Jha V, Garcia-Garcia G, Iseki K, Li Z, Naicker S, Plattner B, et al. Chronic kidney disease: global dimension and perspectives. Lancet. 2013;382:260–72. [DOI] [PubMed] [Google Scholar]
- 125.Gupta S, Dominguez M, Golestaneh L. Diabetic kidney disease: an update. Med Clin North Am. 2023;107:689–705. [DOI] [PubMed] [Google Scholar]
- 126.Forbes JM, Cooper ME. Mechanisms of diabetic complications. Physiol Rev. 2013;93:137–88. [DOI] [PubMed] [Google Scholar]
- 127.Tuttle KR, Agarwal R, Alpers CE, Bakris GL, Brosius FC, Kolkhof P, et al. Molecular mechanisms and therapeutic targets for diabetic kidney disease. Kidney Int. 2022;102:248–60. [DOI] [PubMed] [Google Scholar]
- 128.Mennuni S, Rubattu S, Pierelli G, Tocci G, Fofi C, Volpe M. Hypertension and kidneys: unraveling complex molecular mechanisms underlying hypertensive renal damage. J Hum Hypertens. 2014;28:74–9. [DOI] [PubMed] [Google Scholar]
- 129.Rajasekaran A, Julian BA, Rizk DV. IgA nephropathy: an interesting autoimmune kidney disease. Am J Med Sci. 2021;361:176–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hudson BG, Tryggvason K, Sundaramoorthy M, Neilson EG. Alport’s syndrome, goodpasture’s syndrome, and type IV collagen. N Engl J Med. 2003;348:2543–56. [DOI] [PubMed] [Google Scholar]
- 131.Torra R, Furlano M. New therapeutic options for Alport syndrome. Nephrol Dial Transpl. 2019;34:1272–9. [DOI] [PubMed] [Google Scholar]
- 132.Liu Y. Cellular and molecular mechanisms of renal fibrosis. Nat Rev Nephrol. 2011;7:684–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chen L, Yang T, Lu D-W, Zhao H, Feng Y-L, Chen H, et al. Central role of dysregulation of TGF-β/smad in CKD progression and potential targets of its treatment. Biomed Pharmacother. 2018;101:670–81. [DOI] [PubMed] [Google Scholar]
- 134.He W, Dai C, Li Y, Zeng G, Monga SP, Liu Y. Wnt/beta-catenin signaling promotes renal interstitial fibrosis. J Am Soc Nephrol. 2009;20:765–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhuang L, Ge X, Hu X, Yang Q, Pei X, Jin G. miR-543 regulates high glucose-induced fibrosis and autophagy in diabetic nephropathy by targeting TSPAN8. BMC Nephrol. 2022;23:89. [DOI] [PMC free article] [PubMed]
- 136.Rops AL, Figdor CG, van der Schaaf A, Tamboer WP, Bakker MA, Berden JH, et al. The tetraspanin CD37 protects against glomerular IgA deposition and renal pathology. Am J Pathol. 2010;176:2188–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.van Spriel AB, Sofi M, Gartlan KH, van der Schaaf A, Verschueren I, Torensma R, et al. The tetraspanin protein CD37 regulates IgA responses and anti-fungal immunity. PLoS Pathog. 2009;5:e1000338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Delimont D, Dufek BM, Meehan DT, Zallocchi M, Gratton MA, Phillips G, et al. Laminin α2-mediated focal adhesion kinase activation triggers Alport glomerular pathogenesis. PLoS ONE. 2014;9:e99083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Naylor RW, Watson E, Williamson S, Preston R, Davenport JB, Thornton N, et al. Basement membrane defects in CD151-associated glomerular disease. Pediatr Nephrol. 2022;37:3105–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Moretti L, Stalfort J, Barker TH, Abebayehu D. The interplay of fibroblasts, the extracellular matrix, and inflammation in Scar formation. J Biol Chem. 2022;298:101530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Huang W, Zhang Z, Li X, Zheng Q, Wu C, Liu L, et al. CD9 promotes TβR2-TβR1 association driving the transition of human dermal fibroblasts to myofibroblast under hypoxia. Mol Med. 2024;30:162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Nakamura K, Jinnin M, Harada M, Kudo H, Nakayama W, Inoue K, et al. Altered expression of CD63 and exosomes in scleroderma dermal fibroblasts. J Dermatol Sci. 2016;84:30–9. [DOI] [PubMed] [Google Scholar]
- 143.Tachibana I, Bodorova J, Berditchevski F, Zutter MM, Hemler ME. NAG-2, a novel Transmembrane-4 superfamily (TM4SF) protein that complexes with integrins and other TM4SF proteins. J Biol Chem. 1997;272:29181–9. [DOI] [PubMed] [Google Scholar]
- 144.Traggiai E, Lunardi C, Bason C, Dolcino M, Tinazzi E, Corrocher R, et al. Generation of anti-NAG-2 mAb from patients’ memory B cells: implications for a novel therapeutic strategy in systemic sclerosis. Int Immunol. 2010;22:367–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Dunn C, Ambur A, Foss M, Nathoo R. Expanding the spectrum of epidermolysis Bullosa simplex: syndromic epidermolysis Bullosa simplex with nephropathy and epilepsy secondary to CD151 tetraspanin defect—a case report and review of the literature. JAAD Case Rep. 2022;23:136–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.McKay TB, Hutcheon AEK, Zieske JD. Biology of corneal fibrosis: soluble mediators, integrins, and extracellular vesicles. Eye (Lond). 2020;34:271–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Caruso C, D’Andrea L, Troisi M, Rinaldi M, Piscopo R, Troisi S, et al. Corneal collagen cross-linking in patients with keratoconus from the Dresden protocol to customized solutions: theoretical basis. Int J Ophthalmol. 2024;17:951–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Amador C, Shah R, Ghiam S, Kramerov AA, Ljubimov AV. Gene therapy in the anterior eye segment. Curr Gene Ther. 2022;22:104–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.de Oliveira RC, Wilson SE. Descemet’s membrane development, structure, function and regeneration. Exp Eye Res. 2020;197:108090. [DOI] [PubMed] [Google Scholar]
- 150.Wilson SE. Corneal myofibroblasts and fibrosis. Exp Eye Res. 2020;201:108272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Escandon P, Liu A, Nicholas SE, Khan A, Riaz KM, Karamichos D. Unravelling novel roles of salivary exosomes in the regulation of human corneal stromal cell migration and wound healing. Int J Mol Sci. 2022;23:4330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Han K-Y, Tran JA, Chang J-H, Azar DT, Zieske JD. Potential role of corneal epithelial cell-derived exosomes in corneal wound healing and neovascularization. Sci Rep-uk. 2017;7:40548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Yeung V, Boychev N, Farhat W, Ntentakis DP, Hutcheon AEK, Ross AE, et al. Extracellular vesicles in corneal fibrosis/scarring. Int J Mol Sci. 2022;23:5921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Stilgenbauer S, Aurran Schleinitz T, Eichhorst B, Lang F, Offner F, Rossi J-F, et al. Phase 1 first-in-human trial of the anti-CD37 antibody BI 836826 in relapsed/refractory chronic lymphocytic leukemia. Leukemia. 2019;33:2531–5. [DOI] [PubMed] [Google Scholar]
- 155.Danilov AV, Spurgeon SE, Siddiqi T, Quinson A-M, Maier D, Smith D, et al. A phase ib, open label, dose escalation trial of the anti-CD37 monoclonal antibody, BI 836826, in combination with ibrutinib in patients with relapsed/refractory chronic lymphocytic leukemia. Invest New Drugs. 2021;39:1099–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Nakamura K, Ida N, Hirasawa A, Okamoto K, Vu TH, Hai Ly DT, et al. CD63 as a potential biomarker for patients with ovarian cancer. Eur J Obstet Gynecol Reprod Biol. 2025;306:87–93. [DOI] [PubMed] [Google Scholar]
- 157.Koh H, An H, Jung J, Song D. The prognostic significance of CD63 expressionin patients with non-small cell lung cancer. Pol J Pathol. 2019;70:183–8. [DOI] [PubMed] [Google Scholar]
- 158.Odaka H, Hiemori K, Shimoda A, Akiyoshi K, Tateno H. CD63-positive extracellular vesicles are potential diagnostic biomarkers of pancreatic ductal adenocarcinoma. BMC Gastroenterol. 2022;22:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Qing J, Ren Y, Zhang Y, Yan M, Zhang H, Wu D, et al. Dopamine receptor D2 antagonism normalizes profibrotic macrophage-endothelial crosstalk in non-alcoholic steatohepatitis. J Hepatol. 2022;76:394–406. [DOI] [PubMed] [Google Scholar]
- 160.Nielsen MJ, Veidal SS, Karsdal MA, Ørsnes-Leeming DJ, Vainer B, Gardner SD, et al. Plasma Pro-C3 (N-terminal type III collagen propeptide) predicts fibrosis progression in patients with chronic hepatitis C. Liver Int. 2015;35:429–37. [DOI] [PubMed] [Google Scholar]
- 161.Singh S, Venkatesh SK, Loomba R, Wang Z, Sirlin C, Chen J, et al. Magnetic resonance elastography for staging liver fibrosis in non-alcoholic fatty liver disease: a diagnostic accuracy systematic review and individual participant data pooled analysis. Eur Radiol. 2016;26:1431–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zhang J, Shen H, Xu J, Liu L, Tan J, Li M, et al. Liver-targeted SiRNA lipid nanoparticles treat hepatic cirrhosis by dual antifibrotic and anti-inflammatory activities. ACS Nano. 2020;14:6305–22. [DOI] [PubMed] [Google Scholar]
- 163.Richeldi L, Fernández Pérez ER, Costabel U, Albera C, Lederer DJ, Flaherty KR, et al. Pamrevlumab, an anti-connective tissue growth factor therapy, for idiopathic pulmonary fibrosis (PRAISE): A phase 2, randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2020;8:25–33. [DOI] [PubMed] [Google Scholar]
- 164.Ståhl PL, Salmén F, Vickovic S, Lundmark A, Navarro JF, Magnusson J, et al. Visualization and analysis of gene expression in tissue sections by Spatial transcriptomics. Science. 2016;353:78–82. [DOI] [PubMed] [Google Scholar]
- 165.Huh D, Matthews BD, Mammoto A, Montoya-Zavala M, Hsin HY, Ingber DE. Reconstituting organ-level lung functions on a chip. Science. 2010;328:1662–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.