Abstract
Although transduction with amphotropic murine leukemia virus (MLV) vectors has been optimized successfully for hematopoietic differentiated progenitors, gene transfer to early hematopoietic cells (stem cells) is still highly restricted. A similar restriction to gene transfer was observed in the mouse stem cell line FDC-Pmix compared with transfer in the more mature myeloid precursor cell line FDC-P1 and the human erythroleukemia cell line K562. Gene transfer was not improved when the vector was pseudotyped with gp70SU of the 10A1 strain of MLV, which uses the receptor of the gibbon ape leukemia virus (Pit1), in addition to the amphotropic receptor (Pit2). Although 10A1 and amphotropic gp70SU bound to FDC-P1, K562, and fibroblasts, no binding to FDC-Pmix cells was detected. This indicates that FDC-Pmix cells lack functional Pit2 and Pit1 receptors. Pseudotyping with the vesicular stomatitis virus G protein improved transduction efficiency in FDC-Pmix stem cells by 2 orders of magnitude, to fibroblast levels, confirming a block to retroviral infection at the receptor level.
The hematopoietic stem cell with long-term reconstitution ability is a target for gene therapy. However, the efficiency of gene transfer to stem cells with murine leukemia virus (MLV) vectors packaged as amphotropic pseudotypes is extremely poor (8). In clinical protocols and in studies on large mammals, fewer than 1% of the peripheral blood leukocytes contain the transgene after transplantation of transduced hematopoietic progenitors (6, 12, 36).
More detailed analysis of gene transfer to stem cells has been difficult. Stem cells comprise a very small population in the bone marrow, and their exact phenotype is not known. Thus, the purity of stem cell preparations is generally low and their analysis is restricted to small cell numbers. The frequency of SCID repopulating cells was estimated to be 1 in 600 CD34+ CD38− cells (21), and that of mouse repopulating cells in the Sca-1+ c-Kit+ lin− bone marrow population is about 1 in 100 (28).
To analyze the mechanisms that limit retroviral gene transfer to hematopoietic stem cells, we therefore chose the stem cell line FDC-Pmix, which shows factor-dependent growth (7, 15). These cells are the closest available in vitro equivalent to hematopoietic stem cells. As a comparison, the more mature granulocyte-macrophage precursor cell line FDC-P1 and the human erythroleukemia cell line K562 were analyzed. Gene transfer with amphotropic vectors is highly restricted in the hematopoietic stem cell line FDC-Pmix and more efficient in FDC-P1 (5, 17). This is in accordance with primary progenitor cells, where transduction efficiency is higher in more mature progenitors than in primary multipotent hematopoietic stem cells (21).
A possible explanation for the restricted gene transfer to stem cells is that MLV infects only cycling cells while the nuclear transport of the preintegration complex is inhibited in quiescent cells (34). Most stem cells do not cycle (1). However, transduction of FDC-Pmix cells, which actively cycle, is also restricted, indicating that there are additional limitations to transduction in early cells. A restriction at the level of retroviral transcription could explain the low transfer efficiency. Indeed, we have previously found that expression of vectors derived from the Moloney strain of MLV, which are most frequently used, is poor in hematopoietic progenitors. However, this restriction can be eliminated by using chimeric vectors in which the long-terminal repeat is derived from either the polycythemia strain of Friend spleen focus-forming virus or the myeloproliferative sarcoma virus and the downstream leader sequence is derived from the murine embryonal stem cell virus (MESV) (3, 13). These vectors do not limit transduction at the level of expression.
In previous studies, we have shown that infection of FDC-Pmix with amphotropic MLV is also restricted before synthesis and integration of proviral DNA, i.e., at the level of vector binding, penetration, or reverse transcription (5). Infection efficiency with ecotropic MLV could not be studied, since FDC-Pmix cells contain an interfering ecotropic virus. Here, we analyzed the restriction for amphotropic MLV in more detail. We tested if virus binding to the amphotropic receptor Pit2 was restricted and if transduction efficiency could be improved by pseudotypes that enter the cell by other receptors. A vector pseudotyped with the gp70SU of the 10A1 strain of MLV was studied. This virus utilizes the amphotropic receptor (Pit2), as well as the receptor for the gibbon ape leukemia virus (GALV) (Pit1) (25, 29). The latter receptor is highly expressed in bone marrow cells (20). Moreover, 10A1 MLV induces stem cell leukemia in mice (31). It has been suggested that pseudotyping with the 10A1 env may improve gene transfer to hematopoietic stem cells (20). Here we show that neither amphotropic nor 10A1 MLV gp70SU binds to FDC-Pmix cells at detectable levels and that gene transfer in FDC-Pmix cells with 10A1 pseudotypes is also restricted. In contrast, transduction was improved greatly with vesicular stomatitis virus (VSV) G-protein pseudotypes of MLV. These data indicate that transduction with 10A1 and amphotropic MLV is limited at the level of virus binding and possibly penetration.
MATERIALS AND METHODS
Cells and retroviral vectors.
The cell lines used for infection include the mouse factor-dependent myeloid cell line FDC-P1, the mouse factor-dependent hematopoietic stem cell lines FDC-Pmix, and the human erythroleukemia cell line K562 (7, 10). Two independent isolates of FDC-Pmix were used: A4, which has multipotent differentiation capacity in erythroid and myeloid lineages, and 15s, which has lost its differentiation capacity. Additionally, the mouse fibroblast cell lines SC-1 (ATCC CRL-1404), NIH 3T3, and L929 were used. Fibroblasts and FDC-P1 cells were maintained in minimal essential medium (Sigma, Deisenhofen, Germany) supplemented with 10% fetal calf serum (PAN Systems, Aidenbach, Germany). FDC-Pmix and FDC-P1 cells were maintained in Iscove’s modified Dulbecco’s medium (Gibco, Paisley, Great Britain) supplemented with 20% horse serum. Conditioned medium from cells transfected with a bovine papillomavirus vector carrying the interleukin-3 gene was used as a source of interleukin-3 at concentrations necessary for maximum stimulation of FDC-P1 and FDC-Pmix cells (19). K562 cells were maintained in RPMI 1640 (Gibco) supplemented with 10% fetal calf serum.
All the vectors used for these studies were based on the Friend spleen focus-forming virus/MESV (SF1N) or myeloproliferative sarcoma virus/MESV (MPEVneo) chimeric vectors carrying neo, which have been described previously (13). Replication-competent Moloney MLV recombinants, in which part of the pol gene and most of the env gene were replaced with those from either MLV 4070A (Mo-Ampho-MP, R320 [26]) or 10A1 (Mo-10A1 [30]) as a SalI-ClaI fragment, were used for binding assays and for pseudotyping of vectors. Virus was propagated in SC-1. The amphotropic packaging cell line GP+Am12 was used to generate amphotropic SF1N (22).
Transduction efficiency.
To determine transduction efficiencies, 12-h virus supernatants were collected. A total of 106 cells per well were inoculated with serial dilutions of the supernatant. Plates were centrifuged for 1 h at 900 × g at room temperature and incubated at 37°C for a further 12 h. Since Polybrene and protamine sulfate were found to improve transduction efficiency in fibroblasts but not in FDC-Pmix cells (data not shown), a polycation was not used. After infection, fibroblasts were trypsinized and hematopoietic cells were washed twice in phosphate-buffered saline (PBS). Fibroblasts were plated at concentrations ranging from 1 × 101 to 3 × 104 cells per dish and selected with medium containing G418 (0.4 mg [dry weight] per ml; GIBCO). Hematopoietic cells were plated in agar (0.3%) cultures containing G418 (1 mg [dry weight] per ml). The cell concentrations ranged from 3 × 101 to 3 × 103, and 3 × 105 cells per dish. After 10 days of incubation, duplicate cultures were scored for colony formation. In parallel, cloning efficiency was determined in the absence of G418. Transduction efficiency was calculated as the ratio of colonies grown in the presence of G418 to colonies grown without selection. The titer was calculated from the transduction efficiencies in SC-1. Only virus dilutions below the level of saturation were included in the calculation. The multiplicity of infection (MOI) was determined from the vector titer on SC-1 cells and is expressed as G418 resistance transfer units (GTU) per milliliter.
Binding assay.
The gp70SU binding assay was performed as described by Kadan et al. (18). For amphotropic virus, monoclonal antibody 83A25 (14) followed by a biotinylated goat anti-rat antibody (Pharmingen, Hamburg, Germany) was used. For the 10A1 strain, a goat antiserum to MLV gp70 (35) followed by a biotinylated donkey anti-goat antibody (Laboserv, Giessen, Germany) was used. In both cases, cells were stained with streptavidin-phycoerythrin (Pharmingen). Cells (106 cells per sample) were harvested by brief trypsinization (necessary only for fibroblasts), rinsed with serum-containing medium, and resuspended in 1 ml of virus supernatant. After an incubation of 45 min at 4°C, the cells were washed three times with 2 ml of ice-cold PBS with 5% fetal calf serum. The cells were resuspended in 50 μl of the first antibody and incubated on ice for 30 min. They were then washed three more times, resuspended in 50 μl of the secondary antibody, and incubated on ice for another 30 min. After the next washing, the cells were incubated with 50 μl of streptavidin-phycoerythrin on ice for 10 min, washed, and finally fixed in 500 μl of PBS with 1% paraformaldehyde. Following gp70SU binding and antibody staining, cell samples were analyzed for fluorescence intensity on a flow cytometer (FACScalibur; Becton Dickinson, Heidelberg, Germany).
Packaging cell line for VSV G-protein-pseudotyped retroviral vectors.
Moloney MLV gag and pol were cloned as an EcoRI-HindIII fragment into pSBC-2 under the control of the simian virus 40 promoter (11). This pSBC-gp plasmid was cotransfected with a plasmid containing the hygromycin resistance gene into 293 cells. After selection with 100 μg of hygromycin per ml, cell clones were analyzed by flow cytometry for intracellular p30 expression. The clone 293gp2 with high p30 expression was transduced with SF1N carrying the neo gene, which had been packaged in GP+Am12 (22). After selection with 200 μg of G418 per ml, clones were subjected to calcium phosphate transfection with the plasmid pSNJG expressing the G protein of the New Jersey strain of vesicular stomatitis virus (VSV) (cloned from pNJG6 into pSG [16]) under the control of the simian virus 40 promoter in pSG. Supernatants could be harvested daily for 1 week. The titers ranged from 104 to 105 GTU/ml for the cell clone SF23 used in this study.
RESULTS
Insufficient transduction of multipotent hematopoietic cells with amphotropic pseudotypes.
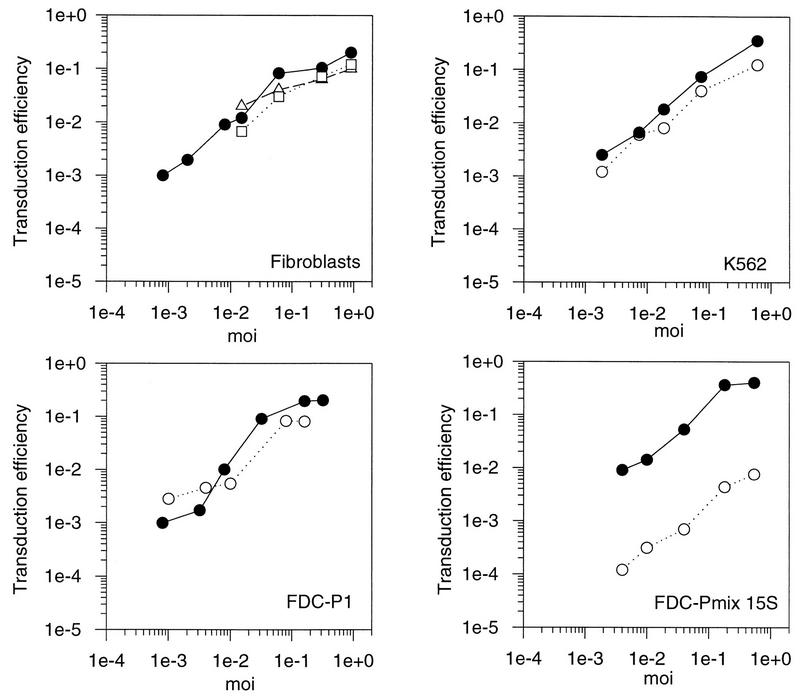
Initially, the transduction efficiencies were compared among the mouse fibroblast cell lines SC-1, NIH 3T3, and L929 cells by using a retroviral vector containing neo with an amphotropic murine leukemia virus (A-MLV) helper at different MOIs (determined on SC-1). The results are shown in Fig. 1. The portion of transduced cells (transduction efficiency) increased linearly with rising MOI and leveled off at an MOI between 0.1 and 1 GTU/cell. Although SC-1 and NIH 3T3 cells tended to be slightly more susceptible than L929 cells, the transduction efficiencies did not differ by more than sixfold between the different fibroblast cell lines. SC-1 cells were then used as a standard for transduction experiments throughout the rest of this study.
FIG. 1.
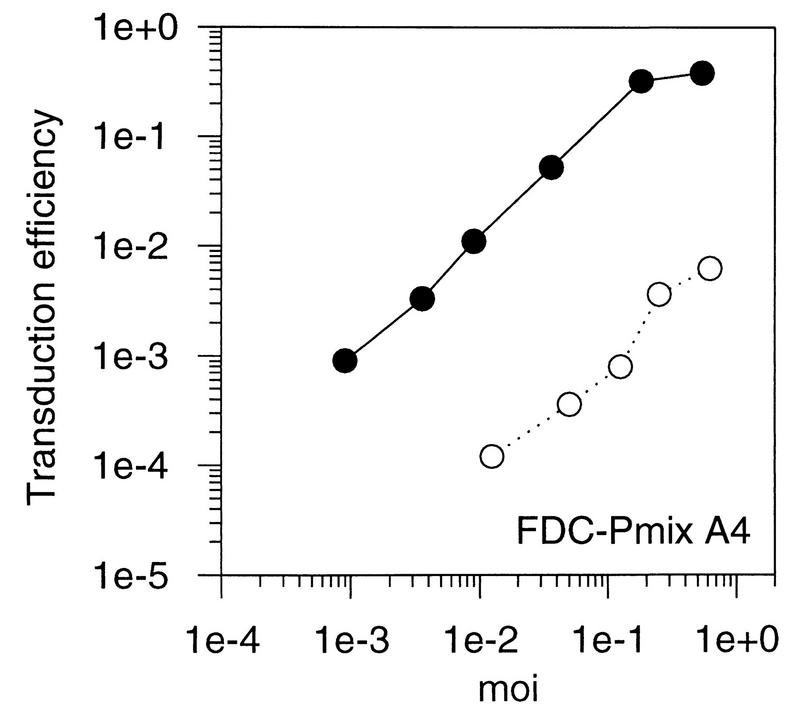
Transduction of fibroblasts and hematopoietic progenitor cell lines with an A-MLV vector. Fibroblasts and hematopoietic cell lines were transduced at different MOIs with a retroviral vector containing neo packaged by an amphotropic helper virus. Transduction efficiencies were calculated as the ratio of G418-resistant cell clones to the number of unselected clones. Solid circles, SC-1 fibroblasts; open squares, NIH 3T3 cells; open triangles, L929 cells; open circles, cell line mentioned in the figure.
The efficiency of retroviral transfer to hematopoietic progenitor cell lines was analyzed in comparison to fibroblasts. Several MOIs were tested. If cells are heterogeneous and bind virus with different affinities, a nonlinear correlation of the MOI to the portion of cells transfected would have been expected. In the mouse stem cell lines FDC-Pmix A4 and FDC-Pmix 15s, transduction efficiencies were approximately 100-fold lower than in fibroblasts at all MOIs tested. No clear subpopulation that was more susceptible to transduction could be discerned. The mouse myeloid cell line FDC-P1, representing a more mature hematopoietic precursor, could be readily transduced at low MOIs, while transduction was reduced at higher MOIs. This indicates that a fraction of cells was fully susceptible. K562, a human erythroleukemia cell line, was efciently transduced at all MOIs (Fig. 1). Thus, transduction was reduced in the immature stem cell lines compared to the more mature hematopoietic cell lines and fibroblasts. These results are in agreement with data for primary hematopoietic cells, where transduction efficiencies are lowest in the most immature hematopoietic stem cells and improve as the cells differentiate (21). We therefore conclude that FDC-Pmix cells are a useful system to develop strategies for improving gene transfer to hematopoietic stem cells.
10A1 pseudotypes do not improve transfer efficiency to FDC-Pmix cells.
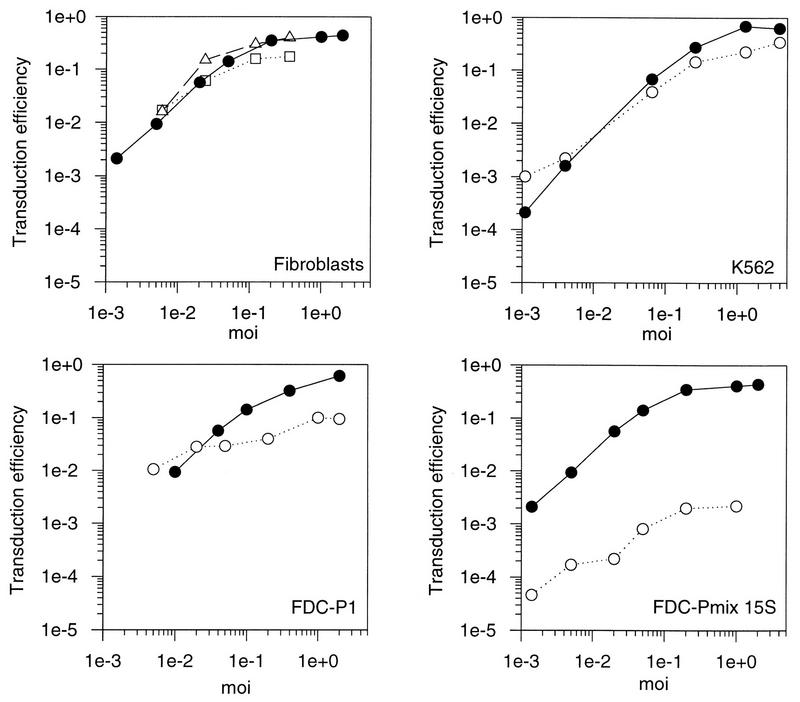
Pseudotypes of MLV that use receptors other than Pit2 were tested for their ability to overcome a possible restriction of virus entry in FDC-Pmix stem cells. The 10A1 strain of MLV uses the GALV receptor (Pit1) as well as the amphotropic receptor (Pit2) for entry into human and NIH 3T3 mouse fibroblasts (23). Using interference studies, we found that 10A1 can also enter SC-1 cells via either of these two receptors (data not shown). SC-1 cells are derived from wild mice. 10A1 also most probably uses Pit1 for entry into cells of other mouse strains including DBA/2 and B6D2F1, from which FDC-Pmix and FDC-P1 cells, respectively, were isolated.
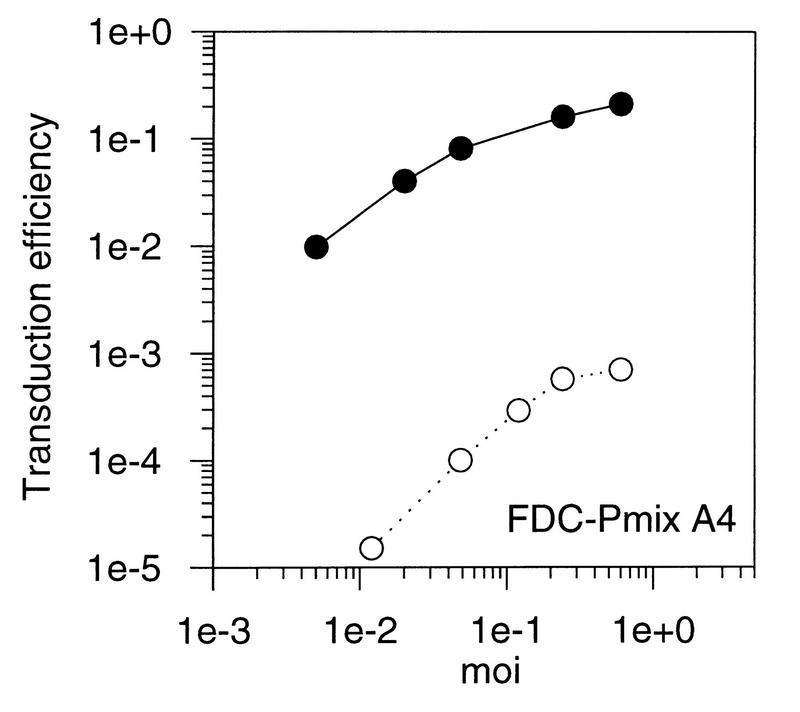
We transduced FDC-Pmix, FDC-P1, and K562 by using a neo vector packaged in 10A1 gp70SU. However, as with amphotropic pseudotypes, transduction was reduced by more than 100-fold in stem cells but was nearly equivalent to fibroblast transduction efficiency in the more mature hematopoietic cell lines (Fig. 2). Transduction of hematopoietic stem cells therefore could not be improved with the 10A1 strain of MLV. In conclusion, transduction in FDC-Pmix is restricted for both 10A1 and amphotropic pseudotyped vectors.
FIG. 2.
Transduction of fibroblasts and hematopoietic progenitor cell lines with an MLV vector, pseudotyped with the surface proteins of 10A1. Fibroblasts and hematopoietic cell lines were transduced at different MOIs with a retroviral vector containing neo packaged by a 10A1 helper virus. Transduction efficiencies were calculated as the ratio of G418-resistant cell clones to the number of unselected clones. Solid circles, SC-1 fibroblasts; open squares, NIH 3T3 cells, open circles, cell line mentioned in the figure.
No detectable binding of amphotropic and 10A1 gp70SU to FDC-Pmix.
In a previous study, we have shown that A-MLV infection of FDC-Pmix stem cells was restricted early during infection, at the level of virus binding, penetration, or reverse transcription (5). To investigate this restriction further, we analyzed the binding of amphotropic and 10A1 gp70SU to fibroblasts and different hematopoietic progenitor cell lines by a flow cytometric assay. Cells were incubated with virus supernatants, and binding of virus and soluble surface glycoprotein gp70SU was detected with an antibody to gp70 (Fig. 3). Binding to fibroblasts such as NIH 3T3 and SC-1 cells and the human hematopoietic cell line K562 was high. Binding to FDC-P1 was much lower but still detectable. In contrast, binding was never detected for the two hematopoietic stem cell lines FDC-Pmix A4 and FDC-Pmix 15s. Thus, the level of virus binding was correlated with the susceptibility of each of these cell lines to transduction. Fibroblasts and K562 cells, which could be efficiently transduced, bound high levels of MLV, while no virus binding was detected in the stem cell lines with low susceptibility to retroviral transfer.
FIG. 3.
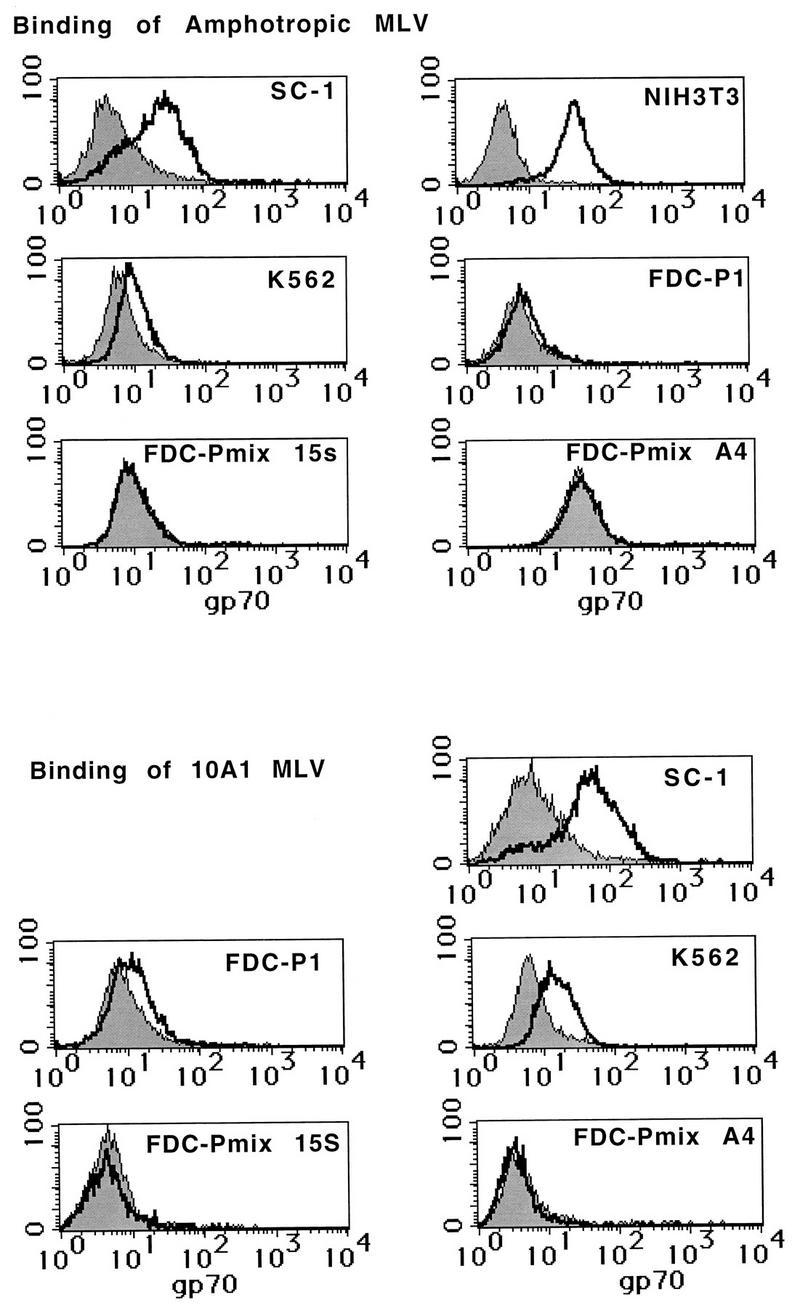
Binding of amphotropic and 10A1 pseudotyped MLV to fibroblasts and hematopoietic cell lines. Cells were incubated with virus supernatants. Total virions, as well as the viral glycoprotein gp70SU, bound to the cell surface were then stained with an anti-gp70 antibody, followed by binding of a biotinylated secondary antibody and streptavidin-phycoerythrin. The cells were then analyzed on a flow cytometer.
VSV G-protein-pseudotyped retroviral vectors efficiently transduce multipotent hematopoietic progenitor cells.
If the lack of functional Pit1 and Pit2 retroviral receptors on FDC-Pmix was indeed limiting retroviral transfer in stem cells, pseudotyping with VSV G protein may improve the transduction efficiency, since VSV has a broad host range (38). First, we demonstrated that FDC-Pmix cells were susceptible to infection with wild-type VSV and therefore must display the VSV receptor. We infected FDC-Pmix cells with wild-type VSV and stained the cells with an anti-VSV G protein antiserum after 2 days. All the cells were positive and showed a massive cytopathic effect (data not shown). As expected, FDC-Pmix cells were fully susceptible to infection with VSV.
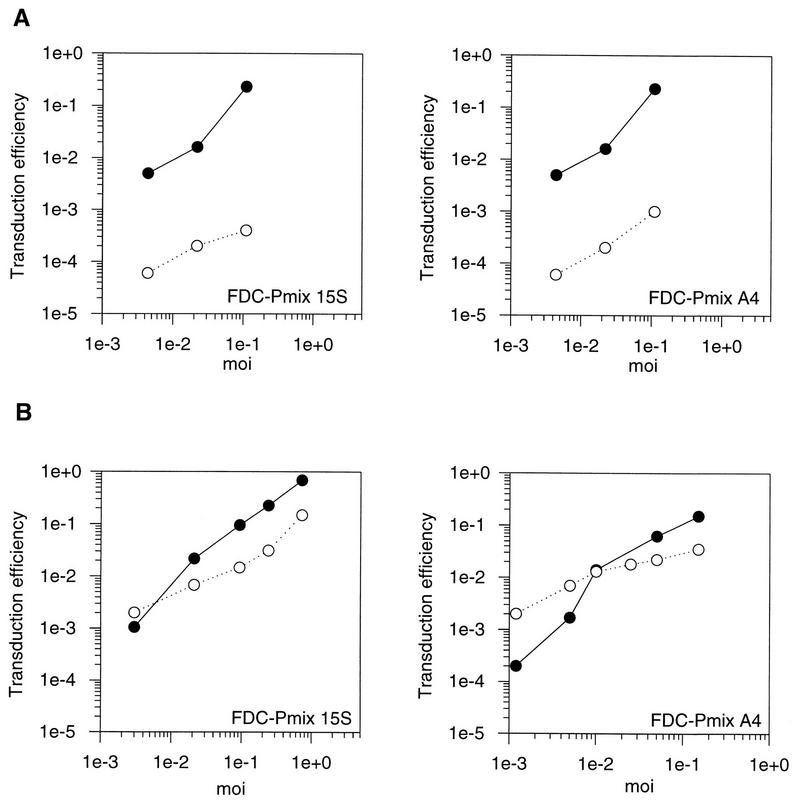
A packaging cell line for VSV G-protein-pseudotyped SF1N retroviral vector [MLV(VSV)] was established (see Materials and Methods). As a control, the same retroviral vector was packaged in the amphotropic packaging cell line GP+Am12. Transduction efficiency was determined with the amphotropic and MLV(VSV) vectors. Approximate equivalent efficiencies of transfer to both stem cells and fibroblasts were observed with MLV(VSV), in contrast to the 100-fold reduction in stem cell transduction when the same vector was pseudotyped in amphotropic retroviral particles (Fig. 4). At lower MOIs, transduction with MLV(VSV) was generally found to be even more efficient in stem cells than in fibroblasts. The minor reduction of transfer efficiency with MLV(VSV) vectors at higher MOIs was accompanied by an up to fourfold reduction in the cloning efficiency of cells incubated with the pseudotyped vector (data not shown). This is most likely to be due to cytotoxicity of the VSV G protein in concentrated inoccula.
FIG. 4.
Transduction of fibroblasts and FDC-Pmix cells with an amphotropic and a VSV G protein-pseudotyped vector. Cells were transduced at different MOIs with a retroviral vector containing neo packaged in the amphotropic packaging cell line GP+Am12 (A) or as VSV G-protein pseudotypes in the packaging line 293gp2 (B). Transduction efficiencies were calculated as the ratio of G418-resistant cell clones to the number of unselected clones. Solid circles, SC-1 fibroblasts; open circles, cell line mentioned in the figure.
DISCUSSION
Results obtained from current gene marking and gene therapy protocols with human hematopoietic cells have shown that the early hematopoietic stem cell is restrictive to efficient retroviral transduction (21;; reviewed in reference 4). By analogy, a similar restriction is observed when multipotent FDC-Pmix stem cells are infected with amphotropic retrovirus, in contrast to more mature murine and human hematopoietic progenitors, which are permissive for efficient infection (5). We show here that this restriction is extended to 10A1-pseudotyped vectors that use the related Pit1 receptor, while vectors pseudotyped by VSV G protein transduced FDC-Pmix efficiently. Moreover, binding of 10A1 and A-MLV gp70SU to FDC-Pmix could not be detected. Therefore, retroviral gene transfer with 10A1 or amphotropic pseudotyped MLV vectors was found to be restricted at the receptor level.
Although no binding to FDC-Pmix was detected, gp70SU bound to the more mature FDC-P1 and K562. These findings are in agreement with data for primary progenitor cells. Human bone marrow CD34+ CD38− cells are enriched for long-term repopulating cells and can bind amphotropic MLV only after stimulation with growth factors. Nevertheless, binding is readily detected in the more mature CD34+ CD38+ cells (9). Moreover, a fraction of mouse bone marrow cells enriched for long-term repopulating cells were shown to express low levels of mRNA encoding amphotropic receptor (Pit2) (27). Although these studies suggest that the lack of virus binding to FDC-Pmix could be due to a low level of receptor, data so far have been circumstantial. Several studies have shown that low MLV receptor expression does not necessarily correlate with a low susceptibility to infection (39). Our observation that FDC-P1 cells bind less gp70SU but are transduced almost as efficiently as fibroblasts is in agreement with those studies. An additional limiting factor may be necessary for efficient transduction. Although cells that do not bind virus are expected to be resistant to infection, receptor expression of FDC-Pmix could be below detection limit but sufficiently high to support virus entry. However, the improvement of transfer efficiency with VSV G-protein pseudotypes shown here is a clear proof that lack of a functional receptor on stem cells indeed restricts transduction.
Our data indicate that the lack of a functional retrovirus receptor may include not only Pit2 but also other members of this receptor family, such as the GALV receptor Pit1. The lack of transduction of the hematopoietic stem cell lines was, however, unexpected, because previous studies suggested that retroviral pseudotypes, which use Pit1, might have improved transfer efficiency in hematopoietic stem cells. Pit1 is highly expressed in bone marrow and induces stem cell leukemia in mice (20). This indicates that 10A1 may have a preferential tropism for stem cells (31). Moreover, infection of CD34+ progenitor and long-term culture initiating cells with MLV vectors pseudotyped with the gp70SU protein of GALV led to a slight (two- to fourfold) improvement of transduction efficiency, but these studies failed to determine if hematopoietic stem cells were transduced (37). In contrast, we could not detect binding of 10A1 to FDC-Pmix cells, and transduction was not improved by pseudotyping with 10A1 surface proteins. These data clearly show that FDC-Pmix cells lack not only functional amphotropic receptor (Pit2) but also a functional mouse homolog of the GALV receptor (Pit1). These two receptors have over 60% amino acid identity and are presumed to have very similar topology in the membrane. Both function as phosphate symporters and are homologous to the phosphate permease of Neurospora crassa (20, 24, 25). Little is known about the transcriptional regulation of this family; however, the expression levels of Pit2 have been shown to be regulated in a tissue- and developmental stage-specific manner (20, 33). It would not be unexpected if Pit1 and Pit2 are regulated similarly in certain cell types.
In contrast to 10A1 pseudotypes, VSV pseudotypes of MLV efficiently transduced FDC-Pmix. This clearly shows that transduction of FDC-Pmix by 10A1 (or A-MLV) pseudotypes is reduced due to a lack of functional receptor. Accordingly, binding of virus to the receptor was found to be reduced. Since te VSV G protein also mediates penetration of virus, we cannot exclude that virus penetration is also restricted in the case of 10A1 or A-MLV pseudotypes. At lower MOIs, transduction by VSV G pseudotypes tended to be even more efficient in FDC-Pmix than in fibroblasts, but at MOIs above 10−3, the transduction efficiency of FDC-Pmix was slightly lower. A possible explanation for this reduction may be a cytotoxic effect of the VSV G protein in concentrated vector preparations. In support of this theory, cloning efficiency was reduced after transduction with MLV(VSV) pseudotypes but not with amphotropic vectors.
In agreement with our study, a higher transduction efficiency was achieved in human CD34+ progenitor cells with a VSV G protein-pseudotyped lentivirus vector than with the amphotropic pseudotype, although transfer to early progenitors was not studied separately (2). In one study, CD34+ CD38− cells, which are enriched for stem cells, could not be transduced with a VSV G protein-pseudotyped vector (1). Although this seemingly contradicts the results of our study, it may have a simple explanation. The CD34+ CD38− cells did not cycle during transduction, and retroviral gene transfer is known to be restricted in quiescent cells, due to an inhibition of nuclear transport of the preintegration complex (34). In contrast, FDC-Pmix cells propagate readily in vitro and are not subject to this preintegration block.
The hematopoietic stem cell is the target of several gene therapy protocols. The work presented here demonstrates that the currently used packaging systems may be insufficient to transduce this cell population but that VSV(MLV) pseudotypes can. To date, the development of efficient packaging systems for the VSV G-protein pseudotypes has been impeded by the cytotoxicity of the G protein. We are therefore testing other viral surface proteins that pseudotype MLV for their ability to mediate virus entry into hematopoietic cells. This work, together with the development of culture conditions under which primary human hematopoietic stem cells can be expanded (32), will considerably improve gene transfer and thus allow successful gene therapy in hematopoietic cells.
ACKNOWLEDGMENTS
We thank B. Schaefer and L. H. Evans for providing the anti-gp70 goat serum and the MAb 83A25, respectively. The SF1N vector was supplied by C. Baum, whom we also thank for critical discussions.
This work was supported by grant 0310718 from the Bundesministerium für Bildung und Familie. It was also supported in part by the National Cancer Institute, DHHS, under contract to ABL. The Heinrich-Pette-Institut is financially supported by Freie und Hansestadt Hamburg and Bundesministerium für Gesundheit.
REFERENCES
- 1.Agrawal Y P, Agrawal R S, Sinclair A M, Young D, Maruyama M, Levine F, Ho A D. Cell-cycle kinetics and VSV-G pseudotyped retroviral-mediated gene transfer in blood-derived CD34+ cells. Exp Hematol. 1996;24:738–747. [PubMed] [Google Scholar]
- 2.Akkina R K, Walton R M, Chen M L, Li Q-X, Planelles V, Chen I S Y. High-efficiency gene transfer into CD34+ cells with a human immunodeficiency virus type 1-based retroviral vector pseudotyped with vesicular stomatitis virus envelope glycoprotein G. J Virol. 1996;70:2581–2585. doi: 10.1128/jvi.70.4.2581-2585.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baum C, Hegewisch Becker S, Eckert H G, Stocking C, Ostertag W. Novel retroviral vectors for efficient expression of the multidrug resistance (mdr-1) gene in early hematopoietic cells. J Virol. 1995;69:7541–7547. doi: 10.1128/jvi.69.12.7541-7547.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baum, C., C. Stocking, T. Wagener, H.-G. Eckert, and W. Ostertag. Gene transfer and transgene expression in hematopoietic cells. In M. Strauss and W. Barranger (ed.), Concepts of gene therapy, in press. Walter de Gruyter, Berlin, Germany.
- 5.Beck-Engeser G, Stocking C, Just U, Albritton L, Dexter M, Spooncer E, Ostertag W. Retroviral vectors related to the myeloproliferative sarcoma virus allow efficient expression in hematopoietic stem and precursor cell lines, but retroviral infection is reduced in more primitive cells. Hum Gene Ther. 1991;2:61–70. doi: 10.1089/hum.1991.2.1-61. [DOI] [PubMed] [Google Scholar]
- 6.Bodine D M, Moritz T, Donahue R E, Luskey B D, Kessler S W, Martin D I, Orkin S H, Nienhuis A W, Williams D A. Long-term in vivo expression of a murine adenosine deaminase gene in rhesus monkey hematopoietic cells of multiple lineages after retroviral mediated gene transfer into CD34+ bone marrow cells. Blood. 1993;82:1975–1980. [PubMed] [Google Scholar]
- 7.Boettiger D, Anderson S, Dexter T M. Effect of src infection on long-term marrow cultures: increased self-renewal of hemopoietic progenitor cells without leukemia. Cell. 1984;36:763–773. doi: 10.1016/0092-8674(84)90356-8. [DOI] [PubMed] [Google Scholar]
- 8.Brenner M K, Cunningham J M, Sorrentino B P, Heslop H E. Gene transfer into human hemopoietic progenitor cells. Br Med Bull. 1995;51:167–191. doi: 10.1093/oxfordjournals.bmb.a072945. [DOI] [PubMed] [Google Scholar]
- 9.Crooks G M, Kohn D B. Growth factors increase amphotropic retrovirus binding to human CD34+ bone marrow progenitor cells. Blood. 1993;82:3290–3297. [PubMed] [Google Scholar]
- 10.Dexter T M, Garland J, Scott D, Scolnick E, Metcalf D C. Growth of factor-dependent hemopoietic precursor cell lines. J Exp Med. 1980;152:1036–1047. doi: 10.1084/jem.152.4.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dirks W, Wirth M, Hauser H. Dicistronic transcription units for gene expression in mammalian cells. Gene. 1993;128:247–249. doi: 10.1016/0378-1119(93)90569-o. [DOI] [PubMed] [Google Scholar]
- 12.Dunbar C E, Cottler Fox M, O’Shaughnessy J A, Doren S, Carter C, Berenson R, Brown S, Moen R C, Greenblatt J, Stewart F M, et al. Retrovirally marked CD34-enriched peripheral blood and bone marrow cells contribute to long-term engraftment after autologous transplantation. Blood. 1995;85:3048–3057. [PubMed] [Google Scholar]
- 13.Eckert H-G, Stockschläger M, Just U, Hegewisch-Becker S, Grez M, Uhde A, Zander A, Ostertag W, Baum C. High-dose multidrug resistance in primary human hematopoietic progenitor cells transduced with optimized retroviral vectors. Blood. 1996;88:3407–3415. [PubMed] [Google Scholar]
- 14.Evans L H, Morrison R P, Malik F G, Portis J, Britt W J. A neutralizable epitope common to the envelope glycoproteins of ecotropic, polytropic, xenotropic, and amphotropic murine leukemia viruses. J Virol. 1990;64:6176–6183. doi: 10.1128/jvi.64.12.6176-6183.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ford A M, Bennett C A, Healy L E, Navarro E, Spooncer E, Greaves M F. Immunoglobulin heavy-chain and CD3 delta-chain gene enhancers are DNase I-hypersensitive in hemopoietic progenitor cells. Proc Natl Acad Sci USA. 1992;89:3424–3428. doi: 10.1073/pnas.89.8.3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gallione C J, Rose J K. Nucleotide sequence of a cDNA clone encoding the entire glycoprotein from the New Jersey serotype of vesicular stomatitis virus. J Virol. 1983;46:162–169. doi: 10.1128/jvi.46.1.162-169.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Just U, Stocking C, Spooncer E, Dexter T M, Ostertag W. Expression of the GM-CSF gene after retroviral transfer in hematopoietic stem cell lines induces synchronous granulocyte-macrophage differentiation. Cell. 1991;64:1163–1173. doi: 10.1016/0092-8674(91)90271-y. [DOI] [PubMed] [Google Scholar]
- 18.Kadan M J, Sturm S, Andersen W F, Eglitis M A. Detection of receptor-specific murine leukemia virus binding to cells by immunofluorescence analysis. J Virol. 1992;66:2281–2287. doi: 10.1128/jvi.66.4.2281-2287.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karasuyama H, Melchers F. Establishment of mouse cell lines which constitutively secrete large quantities of interleukin 2,3,4 or 5, using modified cDNA expression vectors. Eur J Immunol. 1988;18:97–104. doi: 10.1002/eji.1830180115. [DOI] [PubMed] [Google Scholar]
- 20.Kavanaugh M P, Miller D G, Zhang W, Law W, Kozak S L, Kabat D, Miller A D. Cell-surface receptors for gibbon ape leukemia virus and amphotropic murine retroviruses are inducible sodium-dependent phosphate symporters. Proc Natl Acad Sci USA. 1996;91:7071–7075. doi: 10.1073/pnas.91.15.7071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Larochelle A, Vormoor J, Hanenberg H, Wang J C Y, Bhatia M, Lapidot T, Moritz T, Murdoch B, Xiao X L, Kato I, Williams D A, Dick J E. Identification of primitive human hematopoietic cells capable of repopulating NOD/SCID mouse bone marrow: implications for gene therapy. Nat Med. 1996;2:1329–1337. doi: 10.1038/nm1296-1329. [DOI] [PubMed] [Google Scholar]
- 22.Markowitz D, Goff S, Bank A. Construction and use of a safe and efficient amphotropic packaging cell line. Virology. 1988;167:400–406. [PubMed] [Google Scholar]
- 23.Miller A D, Cheng F. Retrovirus packaging cells based on 10A1 murine leukemia virus for production of vectors that use multiple receptors for cell entry. J Virol. 1996;70:5564–5571. doi: 10.1128/jvi.70.8.5564-5571.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller D G, Edward R H, Miller A D. Cloning of the cellular receptor for amphotropic murine retroviruses reveals homology to that for gibbon ape leukemia virus. Proc Natl Acad Sci USA. 1994;91:78–82. doi: 10.1073/pnas.91.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller D G, Miller A D. A family of retroviruses that utilize related phosphate transporters for cell entry. J Virol. 1994;68:8270–8276. doi: 10.1128/jvi.68.12.8270-8276.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Münk C, Löhler J, Prassolov V, Just U, Stockschläger M, Stocking C. Amphotropic murine leukemia viruses induce spongiform encephalopathy. Proc Natl Acad Sci USA. 1997;94:5837–5842. doi: 10.1073/pnas.94.11.5837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Orlic D, Girard L J, Jordan C T, Andersen S M, Cline A P, Bodine D M. The level of mRNA encoding the amphotropic retrovirus receptor in mouse and human hematopoietic stem cells is low and correlates with the efficiency of retrovirus transduction. Proc Natl Acad Sci USA. 1996;93:11097–11102. doi: 10.1073/pnas.93.20.11097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Osawa M, Hanada K, Hamada H, Nakauchi H. Long-term lymphohematopoietic reconstitution by a single CD34-low/negative hematopoietic cell. Science. 1996;273:242–245. doi: 10.1126/science.273.5272.242. [DOI] [PubMed] [Google Scholar]
- 29.Ott D, Friedrich R, Rein A. Sequence analysis of amphotropic and 10A1 murine leukemia viruses: close relationship to mink cell focus-inducing viruses. J Virol. 1990;64:757–766. doi: 10.1128/jvi.64.2.757-766.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ott D, Rein A. Basis for receptor specificity of nonecotropic murine leukemia virus surface glycoprotein gp70SU. J Virol. 1992;66:4632–4638. doi: 10.1128/jvi.66.8.4632-4638.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ott D E, Keller J, Rein A. 10A1 MULV induces leukemia that expresses hematopoietic stem cell markers by a mechanism that includes fli-1 integration. Virology. 1994;205:563–568. doi: 10.1006/viro.1994.1680. [DOI] [PubMed] [Google Scholar]
- 32.Petzer A L, Hogge D E, Lansdorp P M, Reid D S, Eaves C J. Self-renewal of primitive human hematopoietic cells (long-term-culture-initiating cells) in vitro and their expansion in defined medium. Proc Natl Acad Sci USA. 1996;93:1470–1474. doi: 10.1073/pnas.93.4.1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Richardson C, Bank A. Developmental-stage-specific expression and regulation of amphotropic retroviral receptor in hematopoietic cells. Mol Cell Biol. 1996;16:4240–4247. doi: 10.1128/mcb.16.8.4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roe T, Reynolds T, Yu G, Brown P O. Integration of murine leukemia virus DNA depends on mitosis. EMBO J. 1993;12:2099–2108. doi: 10.1002/j.1460-2075.1993.tb05858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simon I, Löhler J, Jaenisch R. Virus-specific transcription and translation in organs of BALB/Mo mice: comparative study using quantitative hybridization, in situ hybridization, and immunocytochemistry. Virology. 1982;120:106–121. doi: 10.1016/0042-6822(82)90010-1. [DOI] [PubMed] [Google Scholar]
- 36.van Beusechem V W, Kukler A, Heidt P J, Valerio D. Long-term expression of human adenosine deaminase in rhesus monkeys transplanted with retrovirus-infected bone-marrow cells. Proc Natl Acad Sci USA. 1992;89:7640–7644. doi: 10.1073/pnas.89.16.7640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.von Kalle C, Kiem H-P, Goehle S, Darovsky B, Heimfeld S, Torok-Storb B, Storb R, Schuening F G. Increased gene transfer into human hematopoietic progenitor cells by extended in vitro exposure to a pseudotyped retroviral vector. Blood. 1994;84:2890–2897. [PubMed] [Google Scholar]
- 38.Wagner R R, Rose J K. Rhabdoviridae: the viruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 1121–1136. [Google Scholar]
- 39.Wang H, Paul R, Burgeson R E, Keene D R, Kabat D. Plasma membrane receptors for ecotropic murine retroviruses require a limiting accessory factor. J Virol. 1991;65:6468–6477. doi: 10.1128/jvi.65.12.6468-6477.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]