Abstract
Purpose
To evaluate the effectiveness of histogram features from conventional diffusion weighted imaging (DWI), diffusion kurtosis imaging (DKI) and intravoxel incoherent motion (IVIM) parameters in predicting the status of glioma IDH mutation and 1p/19q codeletion based on the 2021 WHO classification of central nervous system tumors.
Methods and materials
A total of 422 participants who had DWI, DKI, and IVIM were enrolled between January 2020 and March 2024. The histogram characteristics of ADC, diffusional kurtosis(K), diffusion coefficient (Dk), pseudo-diffusion coefficient(D*), pure diffusion coefficient(D), perfusion fraction(f) in the solid component of tumors were calculated. Groups were compared by IDH genotype and 1p/19q codeletion status, utilizing logistic regression analysis and receiver operating characteristic curve to evaluate the differential diagnostic performance in predicting IDH and 1p/19q genotypes.
Results
Significant differences were observed in thirty-nine histogram-based features of diffusion parameters between IDH mutant gliomas and IDH wildtype glioblastoma. In IDH mutant gliomas, significant differences were found in thirty-six histogram-based features of DWI, DKI and IVIM parameters between those with and without 1p/19q codeletion. The IVIM model and the combined model showed superior diagnostic performance compared to the DWI model in terms of AUCs for predicting IDH mutations (0.903, 0.913 and 0.807, respectively p < 0.05), and 1p/19q codeletion in IDH mutant gliomas (0.825, 0.855, and 0.769, respectively; p < 0.05). Correlations between Ki-67 and the mean values of ADC, Dk, K, D, D*, and f were significant, with correlation coefficients from − 0.17 to 0.36 (all p < 0.05).
Conclusion
The prediction of IDH mutation status in adult diffuse glioma and the 1p/19q codeletion status in IDH mutant glioma could be improved through histogram features of IVIM-derived parameters and the combined model.
Supplementary Information
The online version contains supplementary material available at 10.1186/s40644-025-00931-8.
Keywords: Magnetic resonance imaging, Glioma, Isocitrate dehydrogenase, 1p/19q, Ki-67 antigen
Introduction
Adult diffuse glioma is prevalent primary brain tumor with a consistently poor prognosis [1]. The 2021 world health organization (WHO) classification of central nervous system (CNS) tumors emphasis on glioma genotyping, complicating accurate diagnosis [2]. Identifying glioma subtypes is improved by two key biomarkers: the isocitrate dehydrogenase (IDH) mutation status and the 1p/19q codeletion status, which offer greater precision than conventional histological characteristics [3]. Previous studies have shown a significant link between IDH mutation status and the internal diversity and biological behavior of glioma, with patients having IDH mutant glioma typically experiencing better survival rates than those with IDH wildtype glioma [4]. For gliomas with IDH mutations, maximal resection of the tumor is advised due to its strong connection with increased survival chances [5]. In addition, specific inhibitors for IDH are accessible for the treatment of gliomas with IDH mutations [6]. A more positive response to radiotherapy and chemotherapy is also seen in gliomas with 1p/19q codeletion [1]. The Ki-67 labeling index (LI) serves as a well-established biomarker that reflects cell proliferation, tumor differentiation and patient prognosis, indicating mitotic activity and cellular division [7]. As a result, the creation of noninvasive strategies for pre-surgical prediction of glioma genotypes is highly important.
For patients with glioma, magnetic resonance imaging (MRI) serves as a vital diagnostic method before surgery. Conventional MRI is commonly utilized in the preoperative evaluation of gliomas [8–11]. Nonetheless, its usefulness is restricted by its inability to offer comprehensive details about the tumor’s microstructure [8]. Advanced imaging techniques, such as diffusion, perfusion, and metabolic imaging, are extensively used for grading and genotyping gliomas [12–15]. Perfusion-weighted imaging (PWI) is beneficial but demands a contrast agent, whereas magnetic resonance spectroscopy is limited by difficulties such as personalized positioning and poor signal acquisition and complex operational procedures [12, 16]. Artificial intelligence (AI) has been widely used to identify the genetic mutation status of gliomas and have achieved satisfactory results [17]. However, AI relies on a large amount of high-quality, standardized training data, and its imaging characteristics differ from those of biological physical parameters. Diffusion MRI has been extensively utilized for glioma evaluation because it can non-invasively assess the random movement of water molecules. In addition, this method is readily available and does not necessitate contrast material [18, 19].
The apparent diffusion coefficient (ADC) is acknowledged as a sensitive imaging biomarker in the grading and genotyping of gliomas [13, 20]. The ADC values obtained from the monoexponential diffusion-weighted imaging (DWI) model tend to be overestimated because of the effects of capillary microcirculation [21]. Diffusion kurtosis imaging (DKI) enables the use of additional diffusion metrics to better reflects the microstructural complexity of the examined tissues [18]. Intravoxel incoherent motion (IVIM) allows for separate quantitative assessments of diffusion and perfusion fractions without the use of contrast agents [22]. Previous studies have indicated the value of DKI and IVIM in classifying and predicting the molecular features of gliomas [23, 24]. However, multiple studies investigating the diagnostic performance of IVIM in assessing IDH mutation status have yielded inconsistent results [25–27].
As far as we know, there are few studies comparing DWI, DKI, IVIM and combined models for predicting the IDH and 1p/19q genotype of adult diffuse glioma. Hence, the study’s aim was to evaluate the diagnostic effectiveness of DWI, DKI, and IVIM in predicting the IDH mutation and 1p/19q codeletion status of adult diffuse glioma.
Methods
Patients
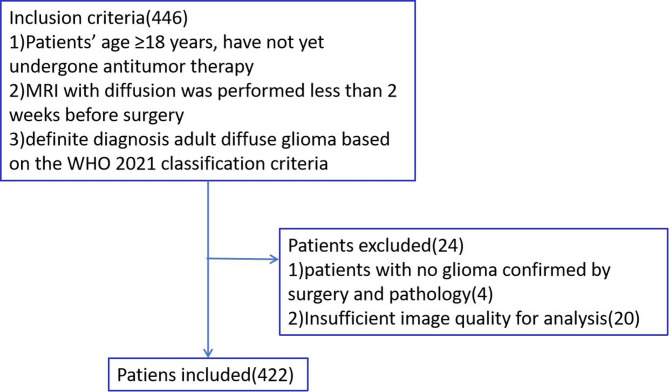
This retrospective study was approved by the Institutional Review Board, and informed consent was waived. Patients identified with glioma before undergoing their first surgery at our institution between January 2020 and March 2024 were selected. The study’s inclusion criteria included: (1) participants older than 18 years; (2) having completed diffusion MRI examinations (DWI, DKI and IVIM) within 14 days before surgery; (3) information on IDH mutation status was available for all patients; (4) no implants were present in the body that could interfere with MRI scans. Criteria for exclusion: (1) patients without glioma confirmation via surgery and pathology; (2) image quality not sufficient for analysis. Figure 1 offers a summary of the case selection procedure. At the start, participants were divided into wildtype and mutation groups according to their IDH genotype. Following this, the IDH mutation group was categorized by the presence or absence of the 1p/19q codeletion.
Fig. 1.
Participant selection flowchart
MRI parameters
The use of 3.0 Tesla MR imaging scanners (Magnetom Skyra and Prism; Siemens) with a head and neck coil was implemented. The scanning protocol included standard MR sequences such as axial T2-weighted imaging and before and after contrast T1-weighted imaging. Furthermore, imaging sequence with a single-shot echoplanar in the axial plane was used to perform DWI with multiple b values. The settings included: TR of 5000 ms, TE of 78 ms, FOV of 220 mm × 220 mm, an acquisition matrix of 150 × 135, a section thickness of 5 mm, a gap of 1 mm, a parallel acceleration factor of 2, and an acquisition time of 8 min and 25 s. In all three orthogonal diffusion directions, thirteen distinct b-values (b = 2000, 1400, 1000, 800, 700, 600, 400, 300, 200, 150, 100, 50, and 0 s/mm2) were utilized, with signal averages of 4, 4,3,3, 2, 2, 2, 2, 2, 2, 2, 2 and 2, respectively.
Image analysis
The equation was used for DWI data-fitting within the DWI model: Sb/S0 = exp (–b· ADC), where S0 and Sb denote the signal intensities at a specific b-value and at b = 0 s/mm2, respectively. Two b values, 1000 and 0 s/mm2, were employed to compute the ADC map. The DKI model involved modifying diffusion signal intensities across five b values (b = 2000, 1400, 1000, 700, and 0 s/mm2) using the formula: Sb/S0 = exp (–b×Dk + b2×Dk2×K/6). Dk symbolizes the ADC free from Gaussian bias, with K indicating the diffusional kurtosis. The IVIM model calculates the parameters D, D*, and f through an equation that utilizes 10 unique b values (b = 1000, 800, 600, 400, 300, 200, 150, 100, 50 and 0 s/mm2): Sb/S0 = (1–f) exp (–b×D) + f×exp [–b× (D + D*)]. D represents the actual diffusion coefficient, D* denotes the pseudo-diffusion coefficient, and f signifies the perfusion fraction. Software for post-processing (Diffusion Toolbox; Siemens Healthcare) was utilized for DWI, DKI, and IVIM processing, resulting in quantitative parametric maps such as ADC, K, Dk, D, D* and f.
The open-source tool 3D Slicer (version 4.11.2) was used to delineate the volume of interest (VOI) for assessing the DWI, DKI, and IVIM parameters. The analysis of the images was done by a neuroradiologist (observer A Y.S., with 6 years of neuroimaging expertise) who was blinded to the clinical and histopathological information. Areas of edema, necrosis, cysts, calcification, hemorrhage or visible vessels were carefully avoided, using T2-weighted and T1-weighted with contrast enhancement images as references. VOI was defined using T2-weighted and T1-weighted with contrast enhancement images as references. Image registration was performed using Statistical Parametric Mapping (SPM8) within MATLAB. The entire tumor histogram features were extracted using the 3D Slicer, and the subsequent histogram features were selected:10th percentile, 90th percentile, entropy, kurtosis, mean, median, interquartile range (IQR) and skewness. To determine the consistency between different observers, another observer B, with a decade of neuroimaging experience, measured diffusion parameters from 30 randomly selected lesions. The intraclass correlation coefficient (ICC) was used to assess inter-observer and intra-observer agreement for histogram features, with interpretations as follows: (1) values < 0.5 indicated poor agreement; (2) values ranging from 0.5 to 0.75 indicated moderate agreement; (3) values from 0.75 to 0.9 indicated good agreement; (4) values > 0.9 indicated excellent agreement.
Pathological examination
Sanger sequencing was used to identify mutations at the hotspot codons R172 and R132 in the IDH1 and IDH2 genes from surgically removed specimens. Mutations in either codon were classified as IDH mutations, and the status of 1p/19q codeletions was identified using fluorescence in situ hybridization (FISH) analysis. The presence of a 1p/19q codeletion is shown by both 1p/1q and 19q/19p signal ratios being below 0.70. The expression of Ki-67 was assessed through immunohistochemical staining. Specifically, immunostaining of tumor specimens was performed with a monoclonal anti-human Ki-67 antibody. Afterward, the proportion of tumor cell nuclei that were positively stained with the Ki-67 antibody was measured and presented as the Ki-67 labeling index (LI). Calculations were based on the area showing the highest density of staining.
Statistical analysis
The continuous variables were displayed as mean ± standard deviation or median. The Mann-Whitney U test was employed to assess the differences in histogram features between groups with IDH mutations and IDH wildtypes, as well as between groups with 1p/19q codeletion and those without. The variables that showed statistical significance in differentiating between the IDH mutation and wildtype groups, and between the 1p/19q codeletion and non-codeletion groups, were selected for binary logistic regression analysis.
For each logistic regression model, 10-fold cross-validation was used for validation. The optimal cut-off value was determined as the point in the upper left corner, which maximized the sum of sensitivity and specificity. Receiver operating characteristic (ROC) curves were used to evaluate the diagnostic performance. Furthermore, a post-hoc test with Bonferroni correction was performed for multiple comparisons, evaluating variations in diagnostic effectiveness between diffusion models and analyzing histogram characteristics across different models. To address potential collinearity between variables and model overfitting, multicollinearity was assessed using the variance inflation factor (VIF), and model quality was evaluated using the Akaike Information Criterion (AIC). To evaluate the differences in the area under the receiver operating characteristic curve for different models, the DeLong test was applied. The statistical analysis was conducted with SPSS software (version 19.0), R software (version 4.2.2), and GraphPad Prism software (version 8.1.1). The diagnostic performance was also assessed through ROC analyses. Finally, Spearman’s correlation coefficient was used to evaluate the correlations between Ki-67 LI and diffusion parameters. Two-sided p values less than 0.05 indicated statistical significance.
Results
Patient demographics
A total of 422 patients participated in our study, comprising 252 males and 170 females, with an average age of 51.05 years ± 13.59, and an age range of 21 to 81 years. Based on the WHO 2021 classification, 108 individuals were identified with IDH mutant astrocytoma, 83 with IDH mutant and 1p/19q codeletion oligodendrogliomas, and 231 with IDH wildtype glioblastoma. Table 1 provides a summary of how participants are categorized based on IDH mutation and 1p/19q codeletion status. The ICC results indicate that all histogram feature measurements had good to excellent interobserver and intraobserver agreement, with all ICC values being 0.765 or higher (Table S1).
Table 1.
Participant demographics
| Parameter | All glioma subtypes | IDH Wildtype | IDH mutation | IDH mutation 1p/19q non-codeletion | IDH mutation 1p/19q codeletion |
|---|---|---|---|---|---|
| No. of participants | 422 | 231 | 191 | 108 | 83 |
| Mean age and SD (y) |
51.05 ± 13.59 (21–81) |
58.53 ± 11.02 (29–81) |
42.01 ± 10.59 (21–68) |
40.56 ± 10.17 (21–67) |
42.01 ± 10.59 (22–68) |
| Male participants | 252 | 147 | 105 | 65 | 40 |
| Female participants | 170 | 84 | 86 | 43 | 43 |
| WHO grade 2 | 92 | 0 | 92 | 37 | 55 |
| WHO grade 3 | 70 | 0 | 70 | 42 | 28 |
| WHO grade 4 | 260 | 231 | 29 | 29 | 0 |
Note.Unless otherwise indicated, data are the number of participants. Data in parentheses are ranges. IDH = isocitrate dehydrogenase, WHO = World Health Organization
Correlation of diffusion parameters with IDH and 1p/19q genotype in adult diffuse glioma
Table 2 summarizes the comparisons of histogram features of diffusion parameters between the IDH mutation and wildtype groups. Table 3 summarizes the comparisons of histogram features for diffusion parameters between the 1p/19q non-codeletion and codeletion groups. In the logistic regression analysis using a single diffusion model to predict IDH mutation status in adult diffuse glioma, the parameters ADC90th and ADCmean from ADC, Dk90th, Dkmedian and K10th from DKI, as well as D10th, D90th, Dentropy, Dmean, D*10th, D*90th, D*entropy, D*kurtosis, D*skewness, f90th, fentropy and fskewness from IVIM were significant (Table 4). In the logistic regression analysis, the combined model (which integrates DWI, DKI, and IVIM models) was used to predict IDH mutation status in adult diffuse glioma, comprising Dk90th, Dkmedian, Dentropy, Dmean, D*90th, f90th, fentropy and fskewness (Table 4). Figures 2 and 3 display the representative cases. Figure 4 shows comparisons of various DWI, DKI and IVIM parameters among various subgroups.
Table 2.
Comparisons of histogram characteristics of DWI, DKI, and IVIM parameters between IDH-mutated and IDH wildtype adult diffuse glioma
| Parameters | IDH mutation | IDH wildtype | P | Parameters | IDH mutation | IDH wildtype | P |
|---|---|---|---|---|---|---|---|
| DWI model | IVIM model | ||||||
| ADC10th(10− 3mm2/s) | 1.031(0.189) | 0.840(0.152) | <0.001 | D10th(10− 3mm2/s) | 1.007 (0.197) | 0.801(0.152) | <0.001 |
| ADC90th(10− 3mm2/s) | 1.669(0.304) | 1.480(0.308) | <0.001 | D90th(10− 3mm2/s) | 1.670 (0.309) | 1.442(0.322) | <0.001 |
| ADCentropy | 5.210(0.411) | 5.140(0.513) | 0.127 | Dentropy | 5.250 (0.428) | 5.120(0.536) | 0.009 |
| ADCIQR (10− 3mm2/s) | 0.327(0.131) | 0.325(0.141) | 0.830 | DIQR (10− 3mm2/s) | 0.340(0.135) | 0.325(0.147) | 0.291 |
| ADCkurtosis | 5.990(4.654) | 7.200(8.961) | 0.091 | Dkurtosis | 5.890 (4.678) | 6.920(7.301) | 0.093 |
| ADCmean(10− 3mm2/s) | 1.349(0.247) | 1.128(0.194) | <0.001 | Dmean(10− 3mm2/s) | 1.336 (0.251) | 1.088(0.199) | <0.001 |
| ADCmedian(10− 3mm2/s) | 1.338(0.247) | 1.084(0.198) | <0.001 | Dmedian(10− 3mm2/s) | 1.324 (0.283) | 1.039(0.202) | <0.001 |
| ADCskewness | 0.620(1.023) | 1.210(1.074) | <0.001 | Dskewness | 0.600 (1.015) | 1.230(1.004) | <0.001 |
| DKI model | |||||||
| Dk10th(10− 3mm2/s) | 1.152(0.301) | 0.984(0.202) | <0.001 | D*10th(10− 3mm2/s) | 20.980(16.983) | 28.090(22.505) | <0.001 |
| Dk90th(10− 3mm2/s) | 1.879 (0.390) | 1.761(0.378) | 0.002 | D*90th(10− 3mm2/s) | 114.730 (21.569) | 143.360(29.448) | <0.001 |
| Dkentropy | 5.310(0.524) | 5.370(0.543) | 0.287 | D*entropy | 2.440(0.327) | 2.710(0.369) | <0.001 |
| DkIQR(10− 3mm2/s) | 0.376 (0.206) | 0.398(0.185) | 0.252 | D*IQR (10− 3mm2/s) | 46.320 (17.538) | 56.830(21.223) | <0.001 |
| Dkkurtosis | 5.770(4.399) | 7.290(9.511) | 0.043 | D*kurtosis | 7.880 (5.236) | 6.150(2.882) | <0.001 |
| Dkmean(10− 3mm2/s) | 1.512 (0.320) | 1.334(0.240) | <0.001 | D*mean(10− 3mm2/s) | 68.850(12.418) | 85.670(17.388) | <0.001 |
| Dkmedian(10− 3mm2/s) | 1.502 (0.337) | 1.283(0.243) | <0.001 | D*median(10− 3mm2/s) | 66.280 (13.521) | 82.200(18.877) | <0.001 |
| DKskewness | 0.460 (1.069) | 1.050 (1.224) | <0.001 | D*skewness | 1.010 (0.843) | 0.860(0.584) | 0.035 |
| K10th(10− 3mm2/s) | 0.366 (0.150) | 0.491(0.130) | <0.001 | f10th (%) | 1.015 (1.576) | 1.691(1.857) | <0.001 |
| K90th(10− 3mm2/s) | 0.728 (0.269) | 0.869(0.179) | <0.001 | f90th (%) | 7.581(3.667) | 11.065(4.055) | <0.001 |
| Kentropy | 4.240 (0.515) | 4.450(0.470) | <0.001 | fentropy | 1.870 (0.469) | 2.390(0.421) | <0.001 |
| KIQR (10− 3mm2/s) | 0.190 (0.144) | 0.199(0.093) | 0.462 | fIQR (%) | 3.229 (1.631) | 4.793(1.904) | <0.001 |
| Kkurtosis | 6.240 (12.899) | 7.650(31.763) | 0.565 | fkurtosis | 2.594 (2.706) | 1.304(1.513) | <0.001 |
| Kmean(10− 3mm2/s) | 0.543(0.194) | 0.679(0.133) | <0.001 | fmean (%) | 4.207 (2.211) | 6.172(2.455) | <0.001 |
| Kmedian(10− 3mm2/s) | 0.530 (0.205) | 0.676(0.135) | <0.001 | fmedian (%) | 3.673 (2.117) | 5.539(2.367) | <0.001 |
| Kskewness | 0.690 (1.043) | 0.220(1.327) | <0.001 | fskewness | 2.820 (1.932) | 1.790(1.477) | <0.001 |
Note.Data in parentheses is standard deviation, IDH = isocitrate dehydrogenase
Table 3.
Comparisons of histogram characteristics of DWI, DKI, and IVIM parameters between IDH mutation 1p/19q non-codeletion and IDH mutation 1p/19q codeletion adult diffuse glioma
| Parameters | 1p/19q non-codeletion | 1p/19q codeletion | P | Parameters | 1p/19q non-codeletion |
1p/19q codeletion |
P |
|---|---|---|---|---|---|---|---|
| DWI model | IVIM model | ||||||
| ADC10th(10− 3mm2/s) | 1.089(0.204) | 0.957(0.135) | <0.001 | D10th(10− 3mm2/s) | 1.066 (0.206) | 0.930(0.153) | <0.001 |
| ADC90th(10− 3mm2/s) | 1.776(0.326) | 1.529(0.201) | <0.001 | D90th(10− 3mm2/s) | 1.774 (0.335) | 1.535(0.203) | <0.001 |
| ADCentropy | 5.290(0.379) | 5.120(0.434) | 0.004 | Dentropy | 5.320 (0.392) | 5.160(0.457) | 0.011 |
| ADCIQR (10− 3mm2/s) | 0.358(0.148) | 0.289(0.092) | <0.001 | DIQR (10− 3mm2/s) | 0.368(0.149) | 0.303(0.103) | 0.001 |
| ADCkurtosis | 4.910(4.124) | 7.380(4.984) | <0.001 | Dkurtosis | 4.740 (3.928) | 7.400(5.151) | <0.001 |
| ADCmean(10− 3mm2/s) | 1.439(0.269) | 1.232(0.148) | <0.001 | Dmean(10− 3mm2/s) | 1.427 (0.278) | 1.218(0.142) | <0.001 |
| ADCmedian(10− 3mm2/s) | 1.440 (0.302) | 1.206(0.154) | <0.001 | Dmedian(10− 3mm2/s) | 1.428 (0.312) | 1.218(0.142) | <0.001 |
| ADCskewness | 0.290(0.964) | 1.050(0.940) | <0.001 | D skewness | 0.250 (0.944) | 1.050(0.926) | <0.001 |
| DKI model | |||||||
| Dk10th(10− 3mm2/s) | 1.232(0.291) | 1.048(0.284) | <0.001 | D*10th(10− 3mm2/s) | 21.380(16.683) | 20.450(17.454) | 0.707 |
| Dk90th(10− 3mm2/s) | 2.001(0.346) | 1.719(0.388) | <0.001 | D*90th(10− 3mm2/s) | 111.970 (20.396) | 118.33(22.624) | 0.043 |
| Dkentropy | 5.400(0.381) | 5.200(0.651) | 0.010 | D*entropy | 2.400(0.340) | 2.490(0.303) | 0.060 |
| DkIQR(10− 3mm2/s) | 0.412(0.243) | 0.330(0.133) | 0.006 | D*IQR (10− 3mm2/s) | 44.720(15.717) | 48.400(19.560) | 0.151 |
| Dkkurtosis | 4.890(3.943) | 6.920(4.710) | 0.001 | D*kurtosis | 8.580 (5.951) | 6.970(3.977) | 0.035 |
| Dkmean(10− 3mm2/s) | 1.620(0.303) | 1.371(0.287) | <0.001 | D*mean(10− 3mm2/s) | 67.250(12.229) | 70.940(12.425) | 0.041 |
| Dkmedian(10− 3mm2/s) | 1.625 (0.303) | 1.372(0.287) | <0.001 | D*median(10− 3mm2/s) | 64.420(13.579) | 68.700(13.133) | 0.030 |
| Dkskewness | 0.230 (0.947) | 0.760(1.146) | 0.001 | D*skewness | 1.110 (0.918) | 0.870(0.719) | 0.057 |
| K10th(10− 3mm2/s) | 0.336 (0.117) | 0.405(0.177) | 0.001 | f10th (%) | 1.047(1.733) | 0.973 (1.353) | 0.748 |
| K90th(10− 3mm2/s) | 0.699 (0.272) | 0.765(0.261) | 0.092 | f90th (%) | 6.884(2.959) | 8.488 (4.272) | 0.003 |
| Kentropy | 4.210 (0.537) | 4.280(0.487) | 0.394 | fentropy | 1.74(0.412) | 2.02(0.494) | <0.001 |
| KIQR(10− 3mm2/s) | 0.197(0.165) | 0.182(0.110) | 0.478 | fIQR (%) | 2.859(1.094) | 3.711 (2.047) | <0.001 |
| Kkurtosis | 4.390 (2.453) | 8.650(19.165) | 0.023 | fkurtosis | 2.786(3.037) | 2.344(2.195) | 0.265 |
|
Kmean (10− 3mm2/s) |
0.509(0.185) | 0.587(0.197) | 0.006 | fmean (%) | 3.905 (2.129) | 4.599(2.267) | 0.031 |
| Kmedian(10− 3mm2/s) | 0.494(0.204) | 0.575(0.197) | 0.006 | fmedian (%) | 3.460 (2.135) | 3.949(2.074) | 0.114 |
| Kskewness | 0.670 (0.667) | 0.710(1.393) | 0.765 | fskewness | 2.850 (2.074) | 2.770(1.742) | 0.786 |
Note. Data in parentheses is standard deviation, IDH = isocitrate dehydrogenase, IQR = interquartile range
Table 4.
Logistic regression models for predicting IDH mutation status and 1p/19q codeletion in adult diffuse glioma
| Model | Level | P | AUC | Sen(%) | Spe(%) | Model | Level | P | AUC | Sen(%) | Spe(%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| IDH mutation vs. IDH wildtype | IDH-mutant 1p/19q codeletion vs. IDH mutant 1p/19q non-codeletion | ||||||||||
| DWI model | 0.807(0.766–0.863) | 72.25 | 77.06 | DWI model | 0.769(0.703–0.827) | 86.75 | 64.81 | ||||
| Intercept | -5.974 | <0.001 | Intercept | -4.979 | 0.249 | ||||||
| ADC90th | -0.005 | <0.001 | ADC10th | 0.01 | 0.008 | ||||||
| ADCmean | 0.012 | <0.001 | ADC90th | -0.005 | 0.089 | ||||||
| DKI model | 0.812(0.771–0.848) | 74.35 | 78.35 | ADCentropy | 2.584 | 0.022 | |||||
| Intercept | 0.273 | 0.771 | ADCmedian | -0.008 | 0.008 | ||||||
| Dk90th | -0.003 | <0.001 | DKI model | 0.793(0.728–0.848) | 89.16 | 61.11 | |||||
| Dkmedian | 0.005 | <0.001 | Intercept | 3.679 | 0.002 | ||||||
| K10th | -0.004 | <0.001 | Dk10th | -0.005 | 0.102 | ||||||
| Kskewness | 0.211 | 0.055 | Dk90th | -0.008 | 0.045 | ||||||
| IVIM model | 0.903(0.870–0.929) | 89.01 | 80.09 | Dkmean | 0.032 | 0.023 | |||||
| Intercept | -9.840 | 0.003 | DKmedian | -0.022 | 0.005 | ||||||
| D90th | -0.004 | 0.010 | Kkurtosis | 0.066 | 0.151 | ||||||
| Dentropy | 2.082 | 0.003 | IVIM model | 0.825(0.763–0.876) | 81.93 | 76.85 | |||||
| Dmean | 0.007 | <0.001 | Intercept | 2.781 | 0.132 | ||||||
| D*10th | 0.041 | 0.028 | D90th | -0.007 | 0.002 | ||||||
| D*90th | -0.044 | 0.013 | Dmean | 0.020 | 0.029 | ||||||
| D*entropy | 4.154 | 0.031 | Dmedian | -0.016 | 0.022 | ||||||
| D*median | -0.047 | 0.052 | D*90th | -0.022 | 0.022 | ||||||
| D*kurtosis | 0.245 | 0.008 | D*kurtosis | -0.062 | 0.104 | ||||||
| D*skewness | -1.656 | 0.006 | fentropy | 3.320 | <0.001 | ||||||
| f 10th | -0.040 | 0.052 | fmean | -0.038 | 0.034 | ||||||
| f 90th | 0.043 | 0.002 | Combined model | 0.855(0.797–0.901) | 81.93 | 78.70 | |||||
| fentropy | -4.686 | <0.001 | Intercept | 2.799 | 0.149 | ||||||
| fskewness | 0.403 | <0.001 | ADC10th | -0.010 | 0.003 | ||||||
| Combined model | 0.913(0.882–0.938) | 86.39 | 84.42 | ADC90th | -0.020 | 0.003 | |||||
| Intercept | -11.966 | <0.001 | Dk10th | -0.005 | 0.074 | ||||||
| Dk90th | -0.008 | <0.001 | Dkmean | 0.017 | 0.031 | ||||||
| Dkmedian | -0.009 | 0.003 | Dkmedian | -0.016 | 0.006 | ||||||
| Dentropy | 3.411 | <0.001 | Kkurtosis | 0.045 | 0.143 | ||||||
| Dmean | 0.021 | <0.001 | D90th | 0.013 | 0.019 | ||||||
| D*90th | -0.021 | 0.019 | D*90th | -0.018 | 0.075 | ||||||
| D*kurtosis | 0.124 | 0.093 | D*kurtosis | -0.078 | 0.050 | ||||||
| D*skewness | -0.766 | 0.091 | fentropy | 2.507 | <0.001 | ||||||
| f90th | 0.030 | 0.002 | |||||||||
| fentropy | -2.534 | 0.004 | |||||||||
| fskewness | 0.337 | 0.001 | |||||||||
Note. The combined model integrates the DWI, DKI, and IVIM models. AUC = the area under the curve, Sen = sensitivity, Spe = specificity, Data in parentheses are 95% confidence intervals
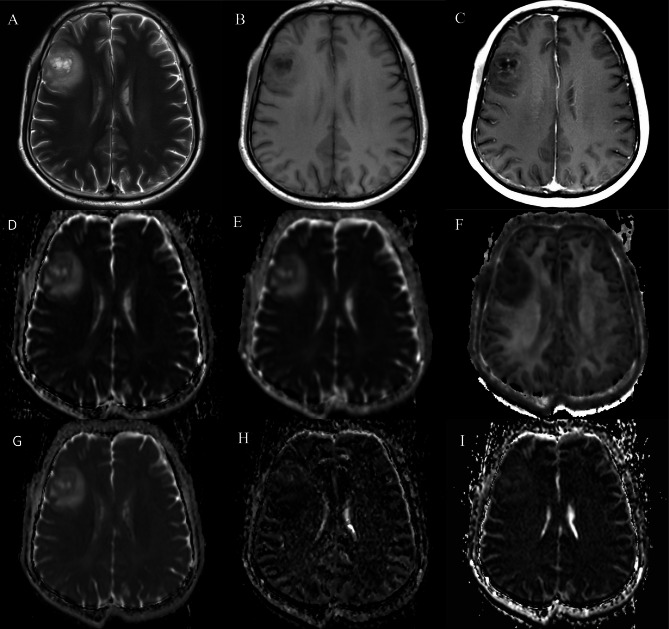
Fig. 2.
A 53-year-old male with WHO grade 3 oligodendroglioma with IDH mutant and 1p/19q codeletion. The lesion displayed hyperintensity on T2WI (A) and hypo-intensity on T1WI (B), and patchy enhancement on post contrast T1WI (C), with (D) ADC value of 1.222 × 10− 3mm2/s, (E) Dk value of 1.403 × 10− 3mm2/s, (F) K value of 0.568 × 10− 3 mm2/s, (G) D value of 1.202 × 10− 3mm2/s, (H) D* value of 70.51 × 10− 3mm2/s, and (I) f value of 4.132%
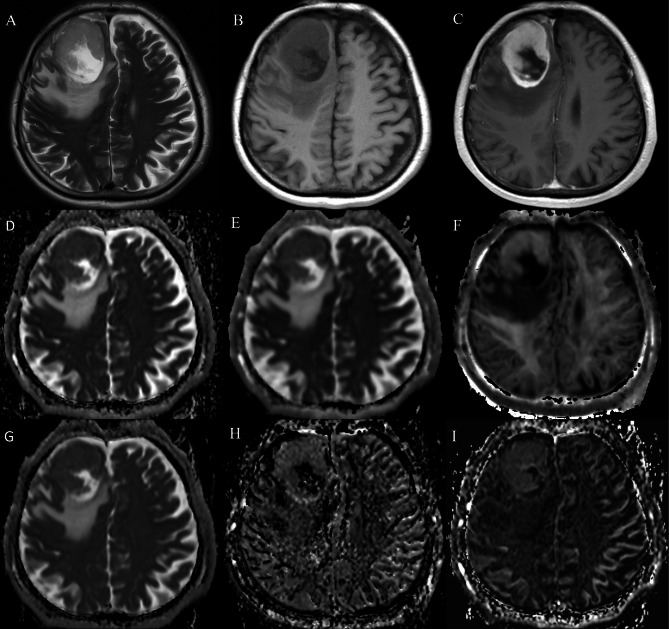
Fig. 3.
A 76-year-old male with isocitrate dehydrogenase (IDH) wildtype glioblastoma. The lesion showed hyperintensity on T2WI (A) and hypo-intensity on T1WI (B), and enhancement on post contrast T1WI (C), with (D) ADC value of 0.916 × 10− 3mm2/s, (E) Dk value of 1.147 × 10− 3mm2/s, (F) K value of 0.659 × 10− 3mm2/s, (G) D value of 0.863 × 10− 3mm2/s, (H) D* value of 77.04 × 10− 3mm2/s, and (I) f value of 6.756%
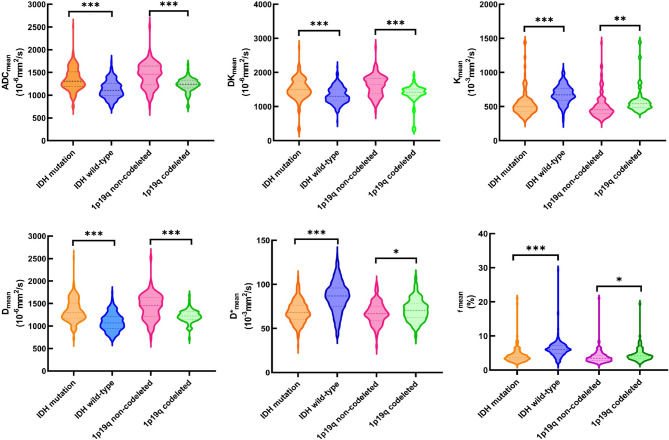
Fig. 4.
Violin plots illustrate typical histogram characteristics of diffusion parameters in IDH wildtype and IDH mutant adult diffuse glioma, as well as in groups with IDH mutation and 1p/19q codeletion, and IDH mutation with 1p/19q non-codeletion. *p < 0.05; **p < 0.01; ***p < 0.001
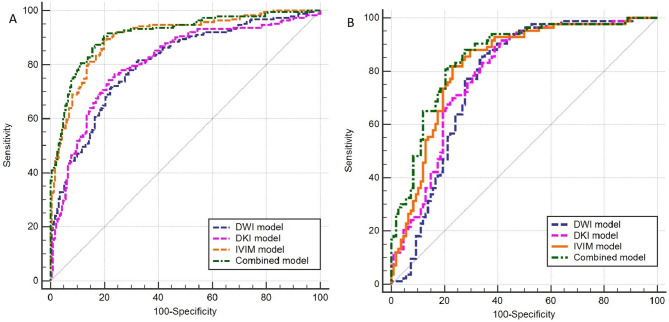
As shown in Table 5, there was no significant between DWI model and DKI model for predicting IDH mutation status in adult diffuse gliomas. In contrast, the AUC of combined models showed a significant difference when compared to DWI and DKI model (p<0.05). For the combined model, we incorporated DWI, DKI, and IVIM parameters that demonstrated univariate predictive significance (p < 0.1) for IDH mutation and 1p/19q codeletion status. We used a forward stepwise selection procedure with p-to-enter and p-to-remove value thresholds of p < 0.05 and p > 0.1. For the IDH mutation prediction model: The VIF values for Dk90th, Dentropy, Dmean, D*90th, fentropy, and fskewness were all < 5. The model incorporating these parameters yielded the lowest AIC value. The combined model achieved the top AUC of 0.913, with sensitivity at 86.39% and specificity at 84.42%. Subsequently, the IVIM model demonstrated an AUC of 0.903, with sensitivity at 89.01% and specificity at 80.09%. The DKI model achieved an AUC of 0.812, with sensitivity at 74.35% and specificity at 78.35%, while the DWI model had an AUC of 0.807, with sensitivity at 72.25% and specificity at 77.06% (Figure. 5 A).
Table 5.
Comparison of logistic regression models regarding mutation status and 1p/19q codeletion in adult diffuse glioma
| Parameter | IDH genotype | IDH mutant 1p/19q genotype |
|---|---|---|
| Combined model vs. DWI model | <0.001 | <0.001 |
| Combined model vs. DKI model | <0.001 | 0.005 |
| Combined model vs. IVIM model | 0.085 | 0.053 |
| DWI model vs. DKI model | 0.754 | 0.202 |
| DWI model vs. IVIM model | <0.001 | 0.032 |
| DKI model vs. IVIM model | <0.001 | 0.200 |
Note. Data are P values; P values less than 0.05 indicate a statistically significant difference. The DeLong test is used to compare the difference in the area under the ROC curve
Fig. 5.
Areas under the receiver operating characteristic curve of DWI, DKI, IVIM and combined model. The logistical models predict (A) IDH mutation in adult diffuse glioma and (B) 1p/19q codeletion in IDH-mutated adult diffuse glioma
In the logistic regression analysis using a single diffusion model to predict 1p/19q codeletion in IDH mutant adult diffuse gliomas, the parameters ADC10th, ADCentropy and ADCmedian from ADC, Dk90th, Dkmean, Dkmedian and K10th from DKI and D90th, Dmedian, D*90th, fentropy and fmean from IVIM were significant (Table 4). To predict the 1p/19q codeletion status, a logistic regression analysis was conducted using a combined model that included ADC10th, ADC90th, Dkmean, Dkmedian, D90th, D*90th and fentropy. As shown in Table 5, there was significant difference between DWI model and combined model for predicting 1p/19q codeletion status in IDH mutant adult diffuse gliomas. For the 1p19q codeletion prediction model: The VIF values for ADC90th, Dkmean, Kkurtosis, and fentropy were all < 3. Likewise, the model containing these parameters achieved the lowest AIC value. The combined model obtained the highest AUC (0.855) with 81.93% of sensitivity and 78.70% of specificity, followed by the IVIM model (AUC, 0.830) with 81.93% of sensitivity and 76.85% of specificity, DKI model (AUC, 0.790) with 89.16% of sensitivity and 61.11% of specificity, and DWI model (AUC, 0.770) with 86.75% of sensitivity and 64.81% of specificity (Figure.5B). The AUCs of IVIM model and combined model outperformed than the DWI model for predicting IDH and 1p/19q genotyping (all corrected p < 0.05).
Correlation of diffusion parameters with adult diffuse glioma and Ki-67 labeling index
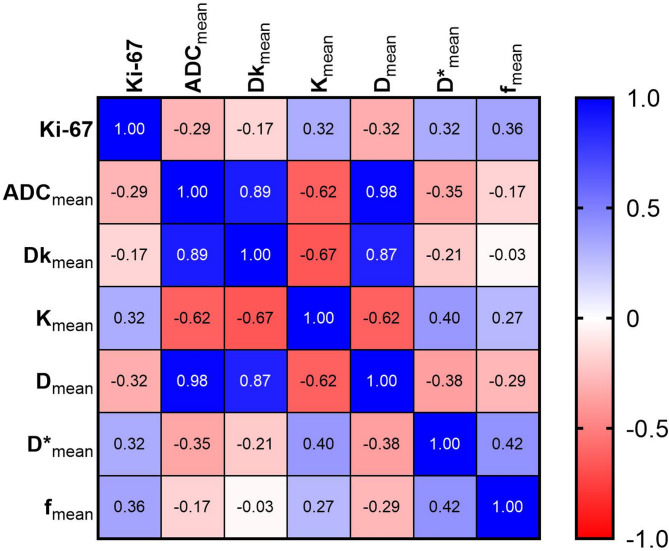
Correlations between Ki-67 LI and the diffusion parameters are shown in Fig. 6. Significant correlations were found between the Ki-67 LI and most histogram features for the DWI, DKI, and IVIM parameters. The values of Kmean, D*mean and fmean showed a positive correlated with the Ki-67 LI, with correlation coefficients of 0.32, 0.32, and 0.36, respectively (all p < 0.05), In contrast, the ADCmean, Dkmean and Dmean values were negatively correlated with the Ki-67 LI, with correlation coefficients of -0.29, -0.17, and − 0.32, respectively (all p < 0.05).
Fig. 6.
The heat map illustrating the correlations between Ki-67 LI and the parameters of DWI, DKI, and IVIM
Discussion
Our results revealed that all models (DWI, DKI, IVIM, and the combined model) are suitable for predicting adult diffuse glioma IDH genotyping and 1p/19q genotyping. The current study’s findings indicated that the IVIM model and combined model outperformed the DWI model in diagnosing IDH mutation status or 1p/19q codeletion status.
This research differentiated adult diffuse gliomas based on tumor subtype according to the 2021 WHO classification. IDH mutant gliomas showed notably higher values in several ADC features, including median, mean, the 10th and 90th percentiles, compared to IDH wildtype glioblastoma. Sun et al. [28]found that ADC P10th can be used to identify the IDH mutation status of gliomas, with an AUC of 0.757. Wang et al. [13]found that by using different b values, ADC was able to distinguish the IDH mutation status of gliomas, with AUCs ranging from 0.754 to 0.851. Our results are consistent with those of previous studies. One potential reason is that IDH mutant gliomas often exhibit fewer diffusion barriers, less complex tissue, and more uniform tumors. On the other hand, IDH wildtype glioblastoma is a tumor with high mitotic activity, marked by microstructural alterations, increased cell density, cellular diversity, and hemorrhage, which can significantly limit water molecule movement [29]. In IDH mutant glioma, reduced aggressiveness is associated with decreased protein levels and cell proliferation compared to IDH wildtype glioblastoma, which can be identified using diffusion metrics that reflect cellular microstructure [30].
IDH mutated gliomas have more uniform tumor populations compared to IDH wildtype glioblastomas. The study found that most K and Dk features of DKI effectively differentiate between these two types. Our study consistent with Chu et al., who found that the K values were correlated with IDH mutation status in gliomas [19]. Dk is associated with dense cellularity, and like ADC values, high cellularity in gliomas might result in reduced Dk values [31]. The parameter K may serve as a quantitative indicator of the tissue microstructural heterogeneity [32]. Therefore, the elevated nuclear-to-cytoplasmic ratios, increased cellular density, and complex microarchitectural organization of IDH-wildtype glioblastoma collectively contribute to significantly higher K values compared to IDH-mutant counterparts.
IVIM, employing a bi-exponential model, is more advanced than DWI in quantifying tumor microcirculation and cellularity. Researchers have widely investigated the role of IVIM-derived parameters, including D, D*, and f, in characterizing glioma. Our study demonstrated that except for Dentropy and DIQR, most IVIM-derived parameters of related features were helpful in distinguishing between IDH mutant glioma and IDH wildtype glioblastoma, which is consistent with previous studies [27]. The f-value measures the fraction of capillary blood flow in each voxel, correlating with normal angiogenesis features like intact vasculature and full pericyte coverage. It may serve as a hemodynamic biomarker for assessing changes in vascular permeability, especially concerning microvascular integrity and endothelial barrier dysfunction [33, 34]. Therefore, the f-value may function as a hemodynamic biomarker for quantitatively assessing vascular permeability alterations, particularly in relation to compromised microvascular integrity and endothelial barrier dysfunction. Our study revealed that histogram features obtained from f, such as fmean, fmedian, and f90th, were significantly higher in IDH wildtype glioblastomas than that of IDH mutant gliomas, a finding that aligns with previous research [25]. The positive findings of our study suggest that the histogram features of the IVIM parameters may be attributed to the characterization of adult diffuse glioma.
Moon et al. [17]utilized deep generative AI technology to predict the IDH mutation status in gliomas on both internal and external test sets, with AUC values of 0.938 and 0.833, respectively. Our results were comparable to theirs. Although the IDH mutation prediction has significant clinical implications, the research on deep learning has been hindered by the small and imbalanced size of the datasets. Song et al. [16]found that the mean normalized cerebral blood volume value could be used to distinguish the IDH mutation status of gliomas, with an AUC of 0.815. Our results showed superior diagnostic performance to theirs. This might be because our measurement method is more direct, enabling a clearer depiction of the most fundamental histological differences (cell density, microstructure complexity). They are less affected by the significant blood-brain barrier leakage characteristics of aggressive wild-type tumors, which greatly impede the accuracy and reliability of PWI.
Glioma with 1p/19q codeletion has been associated with a higher tumor cellularity [35]. The increase of tumor cells may impede water molecule diffusion, resulting in decreased ADC and Dk values [36]. Our study suggests that ADC and Dk values could act as indicators for preoperative classification of 1p/19q codeletion status in IDH mutant gliomas, aligning with findings by Jenkinson et al. [37] and Park et al. [38]. Their research revealed that tumors with 1p/19q codeletion exhibited lower mean histogram-derived ADC and Dk value compared with 1p/19q non-codeletion glioma. K was used to measure how tissue water molecule diffusion deviated from a Gaussian distribution, suggesting a more intricate tissue microstructure [39]. Additionally, the K values were influenced by tumor cell density, with increased density leading to restricted or hindered diffusion of water molecules, thereby resulting in elevated K values [40]. Our study also found that K was capable of predicting the 1p/19q codeletion status in IDH mutant gliomas. However, inconsistent with the result by Chu et al., they found K was not helpful in the identification of 1p/19q codeletion status [19]. This discrepancy may be attributed to the small sample size and instability of their study. Our study showed that the D and D* parameter such as Dmean, Dskewnes, and D*median values were useful in predicting 1p/19q codeletion of gliomas with IDH mutations, which matches the results of a previous study [23]. Additionally, our study demonstrated that histogram features of IVIM model and combined model could provide additional value in identification of 1p/19q codeletion status of IDH mutant glioma compared to the conventional DWI model. The positive results of our study indicate that the IVIM parameters may contribute to the 1p/19q genotyping of IDH mutant glioma.
Ki-67 LI, an indicator of tumor cell proliferation, shows a negative correlation with ADC, Dk, and D values, and a positive correlation with K values in our study. High Ki-67 LI signifies active cell growth, restricted water diffusion, and complex tumor structures, leading to low ADC, Dk, and D values, and high K values [41, 42]. Wang et al. [13] also found that standard ADC ratios were negatively correlated with Ki-67 LI, which is consistent with our study. Our results are in agreement with an earlier IVIM study on pediatric intracranial tumors, which demonstrated a correlation between the D value and Ki-67 LI [21]. Moreover, we discovered that the K value derived from DKI had a positive correlation with Ki-67, aligning with recent research on adult intracranial tumors [43]. In our recent research, we discovered that D* and f values had a positive correlation with Ki-67 LI, which is consistent with the results of an earlier study [44]. However, in our study, the D* and f values for adult diffuse glioma did not align with the findings of She et al. [21] for pediatric intracranial tumors and Chen et al. [45] for parotid gland tumors. The difference might be due to D*’s low signal-to-noise ratio and it has relatively low reproducibility in measurements. Additionally, variations in the selection of tumor cases, their structural differences, and the choice of b value range in other studies may contribute to these inconsistencies, as low b values are particularly valuable for pseudo-diffusion calculations.
Our study had some limitations. First, this is a single-center design and retrospective study, the diffusion model outcomes might have been influenced by the uneven distribution of different glioma subtypes. Second, in this study, diffusion encoding was conducted using only three perpendicular directions. By employing additional directions, anisotropic diffusion in brain tissues can be realized through diffusion tensor imaging, thereby improving spatial resolution. Nevertheless, diffusion-weighted imaging averaged over three directions is commonly utilized in clinical diagnostic procedures. Third, elevated b-values in DKI and IVIM led to an increased degree of distortion, causing minor discrepancies in the delineation of VOI among DWI, DKI, and IVIM. Thus, further research with larger sample sizes is warranted to verify our results and ensure reproducibility. Fourth, Due to the fact that IDH-wildtypes and 1p/19q codeleted tumors fall into separate branches of the diagnostic decision tree as per the 2021 WHO classification’s redefinition of glioma subtypes, our study did not conduct a direct comparison between IDH wild-type gliomas and 1p/19q codeleted oligodendrogliomas, which is a limitation. Finally, in the current radiological-pathological study, MRI images and histopathological sections were not exactly matched on a site-by-site basis. A strong MRI-pathology control method might be needed to better confirm the relationship between diffusion parameters and the Ki-67 LI.
In conclusion, analyzing whole-tumor histograms from DWI, DKI and IVIM is a promising method for predicting glioma IDH mutation and 1p/19q codeletion status. The histogram features of IVIM-derived parameters and the combined diffusion model could improve the diagnostic performance in identifying IDH mutation and 1p/19q codeletion status. Compared with AI and PWI, our approach may offer a supplementary method for assessing the IDH mutation and 1p19q co-deletion status of gliomas.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
Xiefeng Yang: Writing – original draft, Investigation, Formal analysis. Yu Lin: Writing – review & editing, Validation. Yan Su: Writing – review & editing, Data curation. Lan Yu: Methodology, Data curation. Feng Wang: Software, Methodology, Formal analysis. Xingfu Wang: Data curation, Methodology, Formal analysis. Zhen Xing: Supervision, Conceptualization. Dairong Cao: Funding acquisition, Conceptualization.All authors reviewed the manuscript.Final approval of manuscript: all authors.
Funding
This work was funded by the National Natural Science Foundation of China (No. 82371905); Joint Funds for the Innovation of Science and Technology, Fujian Province (No.2024Y9210); Joint Funds for the Innovation of Science and Technology, Fujian Province (No.2021Y9093); Natural Science Foundation of Fujian Province (No.2023J01578).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethical approval and consent to participate
Our retrospective study was authorized by the Institutional Ethics Committee, which consented to waive informed consent because the data used in this study has no personally identifiable information of patients.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Zhen Xing, Email: anight306@126.com.
Dairong Cao, Email: dairongcao@163.com.
References
- 1.Weller M, van den Bent M, Preusser M, et al. EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat Reviews Clin Oncol. 2021;18:170–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gritsch S, Batchelor TT, Gonzalez Castro LN. Diagnostic, therapeutic, and prognostic implications of the 2021 world health organization classification of tumors of the central nervous system. Cancer. 2022;128:47–58. [DOI] [PubMed] [Google Scholar]
- 3.Brat DJ, Verhaak RG, Aldape KD, et al. Comprehensive, integrative genomic analysis of diffuse Lower-Grade gliomas. N Engl J Med. 2015;372:2481–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suh CH, Kim HS, Jung SC, Choi CG, Kim SJ. Perfusion MRI as a diagnostic biomarker for differentiating glioma from brain metastasis: a systematic review and meta-analysis. Eur Radiol. 2018;28:3819–31. [DOI] [PubMed] [Google Scholar]
- 5.Beiko J, Suki D, Hess KR, et al. IDH1 mutant malignant Astrocytomas are more amenable to surgical resection and have a survival benefit associated with maximal surgical resection. Neurooncology. 2014;16:81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mellinghoff IK, Lu M, Wen PY, et al. Vorasidenib and Ivosidenib in IDH1-mutant low-grade glioma: a randomized, perioperative phase 1 trial. Nat Med. 2023;29:615–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zeng A, Hu Q, Liu Y, et al. IDH1/2 mutation status combined with Ki-67 labeling index defines distinct prognostic groups in glioma. Oncotarget. 2015;6:30232–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou Q, Xue C, Ke X, Zhou J. Treatment response and prognosis evaluation in High-Grade glioma: an imaging review based on MRI. J Magn Reson Imaging: JMRI. 2022;56:325–40. [DOI] [PubMed] [Google Scholar]
- 9.Hu LS, Hawkins-Daarud A, Wang L, Li J, Swanson KR. Imaging of intratumoral heterogeneity in high-grade glioma. Cancer Lett. 2020;477:97–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kang KM, Song J, Choi Y, et al. MRI scoring systems for predicting isocitrate dehydrogenase mutation and chromosome 1p/19q codeletion in Adult-type diffuse glioma lacking contrast enhancement. Radiology. 2024;311:e233120. [DOI] [PubMed] [Google Scholar]
- 11.Azizova A, Prysiazhniuk Y, Wamelink I, et al. Preoperative prediction of diffuse glioma type and grade in adults: a gadolinium-free MRI-based decision tree. Eur Radiol. 2025;35:1242–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pons-Escoda A, Garcia-Ruiz A, Naval-Baudin P, et al. Differentiating IDH-mutant Astrocytomas and 1p19q-codeleted oligodendrogliomas using DSC-PWI: high performance through cerebral blood volume and percentage of signal recovery percentiles. Eur Radiol. 2024;34:5320–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang X, Shu X, He P, et al. Ultra-high b-value DWI accurately identifies isocitrate dehydrogenase genotypes and tumor subtypes of adult-type diffuse gliomas. Eur Radiol. 2024;34:6751–62. [DOI] [PubMed] [Google Scholar]
- 14.Maynard J, Okuchi S, Wastling S, et al. World health organization grade II/III glioma molecular status: prediction by MRI morphologic features and apparent diffusion coefficient. Radiology. 2020;296:111–21. [DOI] [PubMed] [Google Scholar]
- 15.Gao A, Zhang H, Yan X, et al. Whole-Tumor histogram analysis of multiple diffusion metrics for glioma genotyping. Radiology. 2022;302:652–61. [DOI] [PubMed] [Google Scholar]
- 16.Song S, Wang L, Yang H, et al. Static (18)F-FET PET and DSC-PWI based on hybrid PET/MR for the prediction of gliomas defined by IDH and 1p/19q status. Eur Radiol. 2021;31:4087–96. [DOI] [PubMed] [Google Scholar]
- 17.17.Moon HH, Jeong J, Park JE, et al. Generative AI in glioma: ensuring diversity in training image phenotypes to improve diagnostic performance for IDH mutation prediction. Neurooncology. 2024;26:1124–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qi XX, Shi DF, Ren SX, et al. Histogram analysis of diffusion kurtosis imaging derived maps May distinguish between low and high grade gliomas before surgery. Eur Radiol. 2018;28:1748–55. [DOI] [PubMed] [Google Scholar]
- 19.Chu JP, Song YK, Tian YS, et al. Diffusion kurtosis imaging in evaluating gliomas: different region of interest selection methods on time efficiency, measurement repeatability, and diagnostic ability. Eur Radiol. 2021;31:729–39. [DOI] [PubMed] [Google Scholar]
- 20.Guo J, Fu X, Li Y, et al. Ultra high b-value diffusion weighted imaging enables better molecular grading stratification over histological grading in adult-type diffuse glioma. Eur J Radiol. 2023;168:111140. [DOI] [PubMed] [Google Scholar]
- 21.She D, Lin S, Guo W, Zhang Y, Zhang Z, Cao D. Grading of pediatric intracranial tumors: are intravoxel incoherent motion and diffusional kurtosis imaging superior to conventional DWI? AJNR Am J Neuroradiol. 2021;42:2046–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Federau C, Maeder P, O’Brien K, Browaeys P, Meuli R, Hagmann P. Quantitative measurement of brain perfusion with intravoxel incoherent motion MR imaging. Radiology. 2012;265:874–81. [DOI] [PubMed] [Google Scholar]
- 23.Luo H, He L, Cheng W, Gao S. The diagnostic value of intravoxel incoherent motion imaging in differentiating high-grade from low-grade gliomas: a systematic review and meta-analysis. Br J Radiol. 2021;94:20201321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gao A, Zhang H, Yan X, et al. Whole-Tumor histogram analysis of multiple diffusion metrics for glioma genotyping. Radiology. 2022;302:E16. [DOI] [PubMed] [Google Scholar]
- 25.Yu M, Ge Y, Wang Z, et al. The diagnostic efficiency of integration of 2HG MRS and IVIM versus individual parameters for predicting IDH mutation status in gliomas in clinical scenarios: A retrospective study. J Neurooncol. 2024;167:305–13. [DOI] [PubMed] [Google Scholar]
- 26.Sheng Y, Dang X, Zhang H, et al. Correlations between intravoxel incoherent motion-derived fast diffusion and perfusion fraction parameters and VEGF- and MIB-1-positive rates in brain gliomas: an intraoperative MR-navigated, biopsy-based histopathologic study. Eur Radiol. 2023;33:5236–46. [DOI] [PubMed] [Google Scholar]
- 27.Cao M, Wang X, Liu F, Xue K, Dai Y, Zhou Y. A three-component multi-b-value diffusion-weighted imaging might be a useful biomarker for detecting microstructural features in gliomas with differences in malignancy and IDH-1 mutation status. Eur Radiol. 2023;33:2871–80. [DOI] [PubMed] [Google Scholar]
- 28.28.Sun Y, Yang Z, Deng K, et al. Histogram analysis of quantitative susceptibility mapping and apparent diffusion coefficient for identifying isocitrate dehydrogenase genotypes and tumor subtypes of adult-type diffuse gliomas. Quant Imaging Med Surg. 2023;13:8681–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Le Rhun E, Preusser M, Roth P, et al. Molecular targeted therapy of glioblastoma. Cancer Treat Rev. 2019;80:101896. [DOI] [PubMed] [Google Scholar]
- 30.Berens ME, Sood A, Barnholtz-Sloan JS, et al. Multiscale, multimodal analysis of tumor heterogeneity in IDH1 mutant vs wild-type diffuse gliomas. PLoS ONE. 2019;14:e0219724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang Y, Li C, Liu Y, et al. Histogram analysis in prostate cancer: a comparison of diffusion kurtosis imaging model versus monoexponential model. Acta Radiol (Stockholm Sweden: 1987). 2020;61:1431–40. [DOI] [PubMed] [Google Scholar]
- 32.Xiao Z, Tang Z, Zhang J, et al. Whole-tumor histogram analysis of monoexponential and advanced diffusion-weighted imaging for sinonasal malignant tumors: correlations with histopathologic features. J Magn Reson Imaging: JMRI. 2020;51:273–85. [DOI] [PubMed] [Google Scholar]
- 33.Bihan DL, Turner RJMRM. The capillary network: a link between IVIM and classical perfusion. 2010;27:171–8. [DOI] [PubMed]
- 34.Le Bihan D. What can we see with IVIM MRI? NeuroImage. 2019;187:56–67. [DOI] [PubMed] [Google Scholar]
- 35.Kim SH, Kim H, Kim TS. Clinical, histological, and immunohistochemical features predicting 1p/19q loss of heterozygosity in oligodendroglial tumors. Acta Neuropathol. 2005;110:27–38. [DOI] [PubMed] [Google Scholar]
- 36.Surov A, Meyer HJ, Wienke A. Correlation between apparent diffusion coefficient (ADC) and cellularity is different in several tumors: a meta-analysis. Oncotarget. 2017;8:59492–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jenkinson MD, Smith TS, Brodbelt AR, Joyce KA, Warnke PC, Walker C. Apparent diffusion coefficients in oligodendroglial tumors characterized by genotype. J Magn Reson Imaging: JMRI. 2007;26:1405–12. [DOI] [PubMed] [Google Scholar]
- 38.Park YW, Han K, Ahn SS, et al. Prediction of IDH1-Mutation and 1p/19q-Codeletion status using preoperative MR imaging phenotypes in lower grade gliomas. AJNR Am J Neuroradiol. 2018;39:37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.39.Jensen JH, Helpern JA, Ramani A, Lu H, Kaczynski K. Diffusional kurtosis imaging: the quantification of non-gaussian water diffusion by means of magnetic resonance imaging. Magn Reson Med. 2005;53:1432–40. [DOI] [PubMed] [Google Scholar]
- 40.Li F, Shi W, Wang D, et al. Evaluation of histopathological changes in the microstructure at the center and periphery of glioma tumors using diffusional kurtosis imaging. Clin Neurol Neurosurg. 2016;151:120–7. [DOI] [PubMed] [Google Scholar]
- 41.Fisher BJ, Naumova E, Leighton CC, et al. Ki-67: a prognostic factor for low-grade glioma? Int J Radiat Oncol Biol Phys. 2002;52:996–1001. [DOI] [PubMed] [Google Scholar]
- 42.Koral K, Mathis D, Gimi B et al. Common pediatric cerebellar tumors: correlation between cell densities and apparent diffusion coefficient metrics. 2013;268:532–7. [DOI] [PubMed]
- 43.Zhao J, Wang YL, Li XB, et al. Comparative analysis of the diffusion kurtosis imaging and diffusion tensor imaging in grading gliomas, predicting tumour cell proliferation and IDH-1 gene mutation status. J Neurooncol. 2019;141:195–203. [DOI] [PubMed] [Google Scholar]
- 44.Chaudhary N, Zhang G, Li S, Zhu W. Monoexponential, biexponential and stretched exponential models of diffusion weighted magnetic resonance imaging in glioma in relation to histopathologic grade and Ki-67 labeling index using high B values. Am J Translational Res. 2021;13:12480–94. [PMC free article] [PubMed] [Google Scholar]
- 45.Chen Y, Huang N, Zheng Y, Wang F, Cao D, Chen T. Characterization of Parotid gland tumors: Whole-tumor histogram analysis of diffusion weighted imaging, diffusion kurtosis imaging, and intravoxel incoherent motion - A pilot study. Eur J Radiol. 2024;170:111199. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.