Abstract
Psoriasis pathophysiology involves dysregulated neuroimmune crosstalk, yet the central mechanisms involved remain incompletely understood. Here, we show that the hypothalamic paraventricular nucleus (PVN) orchestrates cutaneous inflammation via a transsynaptic brain–skin circuit. Using neural tracing and chemogenetic approaches, we revealed functional connectivity between the PVN and both sympathetic neurons and psoriatic skin. Reactivation of imiquimod (IMQ)-induced PVN-transgenic targeted recombination in active population (TRAP) neurons (which form a specific “inflammatory memory”) is essential for psoriasis progression and can drive chronic inflammation. Single-nucleus RNA sequencing (snRNA-seq) identified ephrin receptor A7 (Epha7) as a critical mediator of synaptic plasticity in PVN inflammatory engram neurons. The inhibition of Ephrin receptor and ligand binding in the PVN normalized dendritic spine remodelling, suppressed sympathetic nerve hyperactivity, and restored the balance of Th17/Treg cells in psoriatic-like mice. Mechanistically, blockade of the Ephrin receptor attenuated sympathetic norepinephrine overflow, thereby mitigating Th17-driven inflammation. This study identifies a PVN–sympathetic–skin axis in which the inhibition of Epha7 in the PVN restores skin immune homeostasis. Furthermore, this study elucidates the central neural mechanisms of skin inflammation and promotes the transition of psoriasis treatment from single-target approaches to a synergistic neuroimmune strategy involving “brain–skin” interactions.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12974-025-03539-8.
Keywords: Psoriasis, Paraventricular nucleus, Neuroimmunity, Brain-body axis, Synaptic plasticity
Introduction
Psoriasis is a chronic inflammatory dermatological condition characterized by aberrant immune responses and the hyperproliferation of keratinocytes, affecting approximately 2–3% of the global population [1, 2]. The pathophysiological mechanisms underlying psoriasis are intricately linked to immune dysregulation, which involves the activation of T cells and the subsequent production of proinflammatory cytokines, notably tumor necrosis factor-alpha (Tnfa) and interleukin-17 (Il17) [2, 3]. Neural signals have long been recognized to play essential roles in modulating skin immunity [4]. However, the identity of the neurons involved in the regulation of the immune system in individuals with psoriasis remains unclear.
The role of the central nervous system in modulating peripheral inflammation has become an emerging area of interest [5, 6]. Recent evidence has highlighted the importance of the brain-body axis as a bidirectional communication system between the brain and peripheral immune responses [7, 8]. In particular, neuroimmune interactions involving the hypothalamus and the autonomic nervous system have been shown to influence inflammatory responses in various diseases, including cardiovascular [9], skin [10], and respiratory systems diseases [11]. Recent studies have emphasized the potential involvement of the paraventricular nucleus (PVN) in regulating systemic inflammation [12, 13]. For example, PVN activation enhances sympathetic outflow through β-adrenergic signaling, exacerbating cardiovascular inflammation [14]. PVN-mediated sympathetic activation has been shown to inhibit the proliferation of regulatory T (Treg) cells in peripheral immune organs, such as the spleen [15]. In psoriasis, diminished Treg activity facilitates the expansion of pathogenic Th17 cells [16, 17]. However, how PVN-sympathetic crosstalk between neuroimmune circuits disrupts cutaneous Treg homeostasis remains to be further investigated.
Synaptic plasticity is a key mechanism underlying adaptive changes in the nervous system [18, 19]. It enables neurons to adjust the strength of their connections and optimize neural circuit function in response to environmental stimuli and experiences [20, 21]. This process is crucial for essential physiological functions, including learning [22], memory [23], and stress responses [24]. Ephrin receptors are expressed in the hypothalamus, where they influence neuronal connectivity and plasticity [25–27]. Ephrin–Eph signaling regulates synaptic changes that can affect neural circuits involved in immune regulation [28]. Recent research has suggested that Ephrin–Eph signaling may modulate neuroimmune interactions by altering neuronal activity and influencing the release of neuropeptides and other mediators that impact immune cell function [29]. Therefore, the inhibition of Ephrin receptor to modulate PVN synaptic plasticity may mediate skin immune microenvironment remodeling.
In this study, we examine the role of Ephrin receptor A7 (Epha7) in the PVN and its potential to modulate both synaptic plasticity and psoriatic inflammation. We hypothesize that the inhibition of Ephrin ligand/receptor binding in the PVN reduces psoriasis inflammation by regulating synaptic plasticity within the brain–autonomic nervous system–skin axis, which influences immune function. By exploring the intersection of synaptic modulation and neuroimmune regulation, our findings may provide novel insights into how central nervous system signaling mitigates peripheral inflammation, thereby paving the way for the development of novel therapeutic strategies for psoriasis and other neuroimmune disorders.
Materials and methods
Animals
Eight- to ten-week-old male C57BL/6J mice were acquired from the Department of Laboratory Animal Sciences, Shandong University (Jinan, China). Eight- to ten-week-old adult male Fos-iCre-ERT2 mice (030323, Jackson Laboratory, Bar Harbor, ME, USA) and Gt (ROSA)-Ai14-tdTomato (Ai14) mice (007914, Jackson Laboratory, Bar Harbor, ME, USA) were used in this study. We crossed transgenic targeted recombination in active populations (TRAP) mice, specifically FosTRAP mice with Ai14 mice to generate FosTRAP × Ai14 mice. The mice were maintained on a light/dark cycle (12 h of light and 12 h of dark), with the lights on from 7:00 a.m. to 19:00 p.m., in a temperature-controlled environment (21–25 °C and 30–75% humidity). The mice had ad libitum access to food and water and were housed in polycarbonate cages. All animal experiments performed in this study were approved by the Institutional Animal Care and Use Committee at Shandong University (No. ECSBMSSDU2024-2-005).
Psoriasis mouse model establishment
The back hair of C57/BL6 mice was shaved to expose a skin area of 2 × 3 cm. A total of 62.5 mg 5% imiquimod (IMQ) cream (Med-shine Pharma) or Vaseline (Vas) cream (Lircon) was applied topically to the exposed skin for daily for 6 consecutive days. For exogenous administration, 1 µL ALW-II-41-27 (ALW) (MedChemExpress, #HY-18007) or PBS (Solarbio, #P1003) was microinjected injected into PVN region 1 h before IMQ treatment. The back skin thickness was measured using Vernier callipers. The severity of the psoriasis-like phenotype on the back skin was assessed daily using the IMQ-induced psoriasis area and severity index (PASI) score (the sum of the thickness, scaling, and erythema scores were scored from 0 to 4: 0, none; 1, slight; 2, moderate; 3, marked; and 4, very marked). On Day 6, the mice were sacrificed. Skin and spleen samples were harvested for analysis.
Histological and immunohistochemical assays
Skin tissue samples were first processed to obtain several 4 × 4 mm2 sections. Portions were fixed successively in 4% paraformaldehyde, embedded in paraffin and sectioned for hematoxylin and eosin staining and analysis. Other portions of skin tissue were immersed in 30% sucrose to generate frozen sections that were subjected to immunofluorescence staining. The brains were cryosectioned for immunostaining. Frozen coronal sections were cut at a thickness of 30 μm using a freezing microtome (Leica CM1860, Germany). The sections were subsequently blocked with 5% bovine serum albumin (BSA) (Solarbio, #A8020) and 0.3% Triton X-100 (Solarbio, #T8200) at room temperature for 30 min. Thereafter, the sections were incubated overnight at 4 °C with the following primary antibodies: rabbit anti-c-Fos (1: 1000; Abcam Technology) and mouse anti-tyrosine hydroxylase (TH) (1: 200; Santa Cruz Biotechnology). Next, the sections were incubated for 2 h at room temperature with the secondary antibodies: Alexa Fluor 488-conjugated goat anti-rabbit IgG, Alexa Fluor 594-conjugated goat anti-rabbit IgG, and Alexa Fluor 594-conjugated goat anti-mouse IgG (1: 200; Proteintech Technology). Finally, images were acquired using the LSM880 confocal microscope (Zeiss, Germany) and an Olympus confocal microscope (Olympus, Tokyo, Japan), and analyzed using ImageJ software (version 1.8.0).
Stereotaxic surgery and virus injection
The procedure for the virus injection and stereotaxic surgery was adapted from previously published study [30]. Specifically, mice were deeply anesthetized with 50 mg/kg narcolan (Aibei Biotechnology, #2502A) and securely positioned on a stereotaxic instrument (Model 900, Kopf). Viruses were bilaterally injected into the PVN regions (AP, − 0.94 mm from bregma; ML, ± 0.20 mm; DV, − 4.70 mm), lateral hypothalamic area (LH) regions (AP: − 1.22 mm from bregma; ML: ± 1.10 mm; DV: − 5 mm), and medial prefrontal cortex (mPFC) regions (AP: + 0.18 mm from bregma; ML: ± 0.05 mm; DV: − 0.28 mm) at a flow rate of 0.04 µL/min. The pipette was slowly withdrawn at least 20 min after injection to minimize backflow during retraction. In total, 1.5 µL of the retrograde transynaptic tracer PRV (Brain Case Co., Ltd., EGFP) was injected intradermally at three sites along the back skin of each mouse. The injection speed was controlled at 0.2 µL/min, with the needle retained for 20 min after the injection to minimize backflow. The incision was closed with sutures, and the mice were allowed to recover in their original cages and housed for 2 weeks before further experiments.
AAV-hSyn-DIO-mCherry, AAV-hSyn-DIO-hM3D (Gq)-mCherry, AAV-hSyn-DIO-hM4D (Gi)-mCherry, and AAV-EF1α-DIO-ChR2-mCherry viruses were purchased from Genechem Co., Ltd. (Shanghai, China). AAV-hSyn-mCherry, AAV-hSyn-hM4D (Gi)-mCherry, CSSP-YFP-2E4, and pAAV-hSyn-GCaMP6s-P2A-NLS viruses were purchased from Brain Case Co., Ltd. (Wuhan, China). All viruses were stored at − 80 °C until use, and the viral titers were greater than 1012 viral particles per mL.
Optogenetics
Eight-week-old male FosTRAP mice that received bilaterally ChR2 expression in the PVN and underwent optic fiber implantation were allowed to recover for 2 weeks. 30 min before IMQ treatment, tamoxifen (TM) was injected to label the active PVN neurons in the mice. The mice were housed and allowed to rest for 4 weeks before the test. Subsequently, blue light (473 nm, 10 ms per pulse, 20 Hz) was used to stimulate labelled PVN neurons for 6 days (1 h/day). In some mice, the optogenetic activation of isolated skin nerve fibers were recorded, demonstrating synchronized alterations in skin nerve activity, whereas in the remaining mice, the levels of psoriasis-like skin manifestations and skin inflammation were assessed. Following the experiments, brain sections were prepared to confirm efficient viral labelling in the target areas.
Fiber photometry recording and analysis
Two weeks after the injection of AAV-GCaMP6s viruses into the unilateral PVN and LH, an optical fiber was implanted 300 μm above the injection sites in the PVN and LH (Half of the mice were operated on the left brain and half on the right brain. The number of these mice was equal when they were grouped). A custom titanium headpost was securely anchored to the skull using light-blocking dental acrylic to prevent interference from ambient light. The RWD Fiber Photometry System (Shenzhen, China) was utilized for data acquisition, employing fiber-coupled LEDs with wavelengths of 470 nm (GCaMP6s calcium-dependent signal) and 410 nm (background fluorescence correction). The signals were recorded at a frequency of 120 Hz, alternating between 470 nm and 410 nm light pulses, resulting in frame rates of 60 Hz for both the GCaMP6s and control signals. The GCaMP6s signal (F) during specific epochs was calculated as F470/F410, with ∆F/F values defined as (F − F0)/F0. Calcium signals exceeding 3 standard deviations (s.d.) from the baseline were classified as events. For each mouse, the average peak ∆F/F values and the number of events per minute were evaluated. The baseline area under the curve (AUC) was determined as the 5 s period preceding immobility onset and the 5 s period following immobility termination.
snRNA-seq and analysis
Groups of 20 mice were euthanized, and their fresh PVN tissues were rapidly harvested and processed for single-cell capture (Sin, China). The mRNA libraries were constructed and sequenced on the MGISEQ-2000 platform by BGI Genomics (China). Raw sequencing data in Fastq format were processed through the CeleScope pipeline to generate 10× single-cell gene expression matrices. We implemented strict quality control procedures by applying ± 2 standard deviation thresholds from the mean values to exclude outliers in UMI counts and gene numbers per cell, based on the assumption of a normal distribution. Subsequently, cells whose mitochondrial content exceeded 0.5% or whose ribosomal content surpassed 1.5% were systematically removed from the analysis. Subsequent data analysis was performed in RStudio (R 4.2.2) using the Seurat package for dimensionality reduction, clustering, and visualization; Harmony for batch effect correction; and ClusterProfiler for the Kyoto Encyclopaedia of Genes and Genomes (KEGG) enrichment analysis. Additional statistical analyses were conducted to evaluate differential gene expression and pathway activity.
Local field potential (LFP)
The mice were stably anesthetized for LFP recordings in the PVN. A single tungsten wire was implanted at the following stereotaxic coordinates: AP, − 0.94 mm from bregma; ML, ± 0.20 mm; DV, − 4.70 mm. The reference wire was positioned symmetrically relative to the tungsten wire, while the ground wire was secured on the cerebellum using miniature stainless-steel screws. The electrodes were connected to a LabChart signal amplifier (AD Instruments, Australia). Neuronal oscillations were categorized into five frequency bands: delta (1–4 Hz), theta (4–8 Hz), alpha (8–13 Hz), beta (13–30 Hz), and gamma (30–100 Hz). Data acquisition and analysis were performed using LabChart software.
In addition, LFP experiments were conducted to monitor skin nerve fiber activity in mice under stable anesthesia. Electrode placement was as follows: the positive electrode was inserted into the target recording area, specifically the skin nerve fibers; the negative electrode was placed on the skin wall, opposite the horizontal line of the positive electrode; and the reference electrode was positioned subcutaneously within the abdominal cavity. All the data were recorded and analyzed using LabChart software.
RT-qPCR analysis
Total RNA was extracted using TRIzol reagent according to the manufacturer’s protocol. The extracted RNA was reverse-transcribed into cDNA using the PrimeScript RT Master Mix. The synthesized cDNA was subsequently utilized as a template for RT-qPCR analysis with SYBR Green Pro Taq Mix according to the manufacturer’s instructions. The relative mRNA expression levels were calculated using the 2−ΔΔCt method, with GAPDH serving as the reference gene. The sequences of the primers used in this study are listed in Supplementary Table S1.
Brain slice Preparation and electrophysiology
For brain slice preparation, the mice were rapidly decapitated, and the brains were immediately removed and placed in the cutting solution containing the following reagents (in mM): 0.4 KH2PO4, 1.8 CaCl2, 3.1 KCl, 12 MgCl2, 20 glucose, 26 NaHCO3, and 112 NaCl. The brain tissue was sliced into 300 μm coronal slices using a vibratome (Leica VT1200s, Germany) vibrating at a velocity of 0.18 mm/s. PVN-containing brain slices were maintained for at least 1 h at 34℃ in artificial cerebrospinal fluid (ACSF) containing the following (in mM): 0.4 KH2PO4, 1.2 MgCl2, 1.8 CaCl2, 3.1 KCl, 20 glucose, 26 NaHCO3, and 122 NaCl. The slices were then incubated room temperature until use. All the solutions were continuously infused with 95% O2 and 5% CO2.
Patch clamp recording: We observed PVN neurons under a 40× water immersion lens, and whole-cell recordings were obtained using a MultiClamp 700B amplifier and glass electrodes (3–5 mΩ) filled with an internal solution containing the following components (in mM): 0.2 EGTA, 0.3 Na-GTP, 2 MgSO4, 4 NaCl, 4 Mg-ATP, 10 HEPES, 10 KCl, 10 phosphocreatine, and 126 K-gluconate. Data were acquired and analyzed using the Digidata 1440 A and Mini Analysis Program (Synaptosoft).
Behavioural tests of anxiety and depression
All behavioral tests were performed in a dimly lit testing room (approximately 20 lx). The mice were habituated for at least half a day before the tests.
Open field test (OFT): The OFT was conducted in a quiet environment using an open-field box measuring 25 cm × 25 cm × 45 cm. The bottom of the box was divided into 16 equal squares, with the central area consisting of the four middle squares. The mice were gently placed in one corner of the box, and their behaviour was observed for 5 min. The time spent in the central area was recorded and analyzed using the DigBehav video tracking system. After the test, the chamber was thoroughly cleaned with 75% ethanol and water to eliminate any potential olfactory cues.
Elevated plus maze (EPM): The EPM consisted of a central platform connected to two open arms and two closed arms, arranged at 90-degree angles and positioned 40 cm above the floor. The open arms, measuring 30 cm × 5 cm, lacked side or end walls. In contrast, the closed arms, measuring 30 cm × 5 cm × 15 cm, were enclosed with side and end walls but remained open at the top. The mice were individually placed on the central platform and allowed 5 min for free exploration. The duration spent in the open arms was recorded and subsequently analyzed using the TopScan-TopView Behaviour Analysis System. Following each trial, the fecal matter was removed, and the maze was cleaned with 75% ethanol to eliminate residual odors.
Forced swim test (FST): Mice were individually placed in a glass cylinder (25 cm high, 12 cm in diameter) filled with fresh water (24 ± 1℃) to a height of 20 cm from the bottom. The immobility time was defined as the time that the animals remained floating or motionless, with only their head above the water surface, and the immobility time was recorded for the last 4 min of the 6 min test period (1 min for adaptation).
Tail suspension test (TST): Mice were individually suspended approximately 20 cm above the surface of a table placed 15 cm from the distal end of their tail. The immobility time, defined as the absence of body movement, was recorded during the last 4 min of the 6 min test period (1 min for adaptation).
Transmission electron microscopy (TEM)
The mouse brains were rapidly dissected and sectioned within 3 min. Tissue blocks containing the PVN region were carefully prepared to ensure that their dimensions did not exceed 1 mm3, and they were immediately transferred into an EP tube containing fresh TEM fixative for stabilization. The tissues were subsequently postfixed with a 1% OsO4 solution for 2 h at room temperature. After this step, the samples were dehydrated at room temperature to facilitate resin penetration and embedding. Ultrathin Sect. (70 nm) were obtained using an ultramicrotome (Leica UC7, Germany). These sections were then stained with a 2% uranium acetate-saturated alcohol solution for 8 min in the dark, followed by rinses with 70% ethanol and ultrapure water. Finally, high-resolution images were acquired using TEM (HITACHI, Japan).
Norepinephrine (NE) ELISA
The dorsal skin of the mice was collected, and NE levels were measured using an Enzyme-Linked Immunosorbent Assay Kit (NE ELISA Kit; Elabscience, #E-EL-0047) according to the manufacturer’s instructions.
Cutaneous sympathetic nerve ablation
A solution of 6 mg/mL 6-hydroxydopamine (6-OHDA) (Sigma, #H4381) was freshly prepared by dissolving 6-OHDA in 0.1% ascorbic acid (MedChemExpress, #HY-B0166) in PBS, which was intradermally injected into mouse back skin 2 days before IMQ treatment. The control animals were injected with vehicle (0.1% ascorbic acid in PBS).
Isolation of immune cells from mouse skin
At the end of experiment, the skin was fully minced and digested in DMEM containing 40 mg/mL BSA (Solarbio, #A8020), 4 mg/mL type 1 collagenase (Solarbio, #C8140), 2 mg/mL type 4 collagenase (Sigma, #C4-BIOC), and 2 mg/mL hyaluronidase (Sigma, #H3506) at 37 °C and 225 rpm for 1.5 h. Then, 5 µg/mL DNase I (Sigma, #9003-98-9) was added and the digestion continued for 30 min. The cell suspension was subsequently filtered through a 70 μm cell strainer, and the skin immune cells were isolated with 40% and 80% lymphocyte separation solutions by centrifugation at 2000 rpm for 20 min. The cell suspension was subsequently filtered through a 70 μm cell strainer. The resulting single-cell suspensions were counted, and 1 × 106 cells were prepared for subsequent experiments. eBioscience™ Foxp3 (Thermo Fisher Scientific, #126409) was used for cell membrane breakdown.
Flow cytometry
Single-cell suspensions in PBS were pre-incubated with Fc Block anti-mouse CD16/32 (1: 100, Biolegend, #156603). The Zombie Aqua™ Fixable Viability Kit (1: 100, Biolegend, #423101) and fluorochrome-conjugated surface antigens used for mouse skin immune cells were the Alexa Fluor® 700 anti-mouse CD45.2 (1: 100, Biolegend, #109821), fluorescein isothiocyanate (FITC) anti-mouse CD3 (1: 100, Biolegend, #100203), R-phycoerythrin (PE) anti-mouse CD4 (1: 100, eBioscience, #100407), Pacific Blue™ anti-mouse FoxP3 (1: 100, Biolegend, #126409), and allophycocyanin (APC) anti-mouse Il17a (1: 100, Elabscience, #E-AB-F1199E). The samples were analyzed using a CytoFLEX flow cytometer (Beckman Coulter, USA) and CytExpert software (version 2.4).
Statistical analysis
GraphPad Prism 9.0 software was used for all statistical analyses. The data were presented as means ± SEMs. Two-tailed Student’s t tests and one-way or two-way ANOVA followed by the Tukey or Holm‒Sidak post hoc test were used to perform comparisons between groups. Differences with p < 0.05 were considered statistically significant.
Results
Identification of activated brain nuclei associated with IMQ-induced psoriasis-like dermatitis
We used TRAP mice to capture neurons that were active during skin inflammation. We further crossed these TRAP mice with a Cre-dependent tdTomato reporter line, Ai14, to visualize activity-dependent excitatory neurons (Fig. 1A).
Fig. 1.
Identification of the brain regions involved in psoriasis-like dermatitis. (A) Experimental setup. FosTRAP × Ai14 mice were treated with TM to label active neurons. (B) Experimental timeline of the skin inflammation mouse model. (C) Representative photos of mouse back skin. (D-F) Scoring curves of back skin thickness, scaling, and erythema (n = 8). (G) Representative H&E-stained back skin sections from mice on Day 6 of IMQ treatment. Scale bars, 200 μm. (H) Statistical analysis of the epidermal thickness (n = 6). (I) Representative photos of mouse spleens. (J-K) Spleen weight and the ratio of the spleen weight to body weight (n = 4). (L-M) Representative images and numbers of c-Fos+ cells (during Vas and IMQ treatment) among several types of brain neurons (n = 6). Scale bars, 100 μm. (N) Schematic and experimental timeline of the PRV-EGFP intradermal injection and TM labelling of neurons. (O) Representative images of PRV-infected (green) and TM-labelled (red) neurons. Scale bars, 200 μm. (P) Quantification of the average percentage of colabelled neurons in the Fos+ population (n = 4). The data are representative of three independent experiments and are presented as the means ± SEMs. The p-value was calculated using two‐way ANOVA followed by the Tukey or Holm‒Sidak post hoc test (D, E, F, M) or two-tailed unpaired Student’s t test (H, J, K). *p < 0.05, **p < 0.01, ***p < 0.001 compared with the Vas group
The FosTRAP × Ai14 mice were injected with TM (to induce Cre recombination and, as a result, tdTomato expression) 2 h before initiation of IMQ treatment. As controls, we used another group of FosTRAP × Ai14 mice that were injected with TM at the same time but subjected to Vas treatment. These groups were used to compare inflammation-related activity and brain activity during healthy homeostasis (Fig. 1B). Six days after Vas or IMQ treatment, the mice in the IMQ groups developed psoriasis-like lesions characterized by increased skin thickness, widespread erythema, and scaling (Fig. 1C-F). We performed H&E staining of 5 μm paraffin sections of skin to further evaluate the histological changes in IMQ-induced psoriasis-like skin lesions. As shown in Fig. 1G, H, the skin from IMQ group presented significant epidermal layer thickening. In addition, the enlargement of the spleen indicates excessive activation of the immune system. It is also an index of the severity of the inflammatory response. The spleen volume and weight of the mice in the IMQ groups were significantly greater than those in the vehicle (Fig. 1I-K). These results indicated that the psoriasis-like dermatitis model was successfully established in these mice.
Whole mice brain sections were observed under an Olympus fluorescence microscope. Compared with the Vas group, the IMQ group presented increased tdTomato expression (indicative of neuronal activity) in several brain areas (Fig. 1L, Fig. S1A), including the primary somatosensory cortex (S1) (involved in tactile, thermal, and nociceptive processing), the periventricular nucleus (PV) (autonomic regulation and endocrine integration), the PVN (neuroendocrine and autonomic nervous function regulation), the LH (motivational drive and energy homeostasis), and the substantia nigra pars compacta (SNc) (dopamine-mediated motor coordination and reward processing) (Fig. 1M). As a method to identify neurons that commonly participate in skin control and inflammation, we first performed intradermal injections of PRV encoding EGFP in FosTRAP × Ai14 mice to retrogradely and transsynaptically label upstream neurons. Furthermore, neurons activated during IMQ treatment were labelled (tdTomato) upon the TM injection (Fig. 1N). The results showed substantial overlap between skin control neurons (EGFP) and skin inflammatory neurons (tdTomato) in the PVN, LH, and SNC regions (Fig. 1O, P). In addition, the same manipulation in the Vas group did not show significant differences in each brain areas (Fig. S1B-D). These results indicate that PVN, LH, and SNC may induce dermatitis and have a direct path of communication with the skin. Thus, consistent with previous studies, the brain, including the PVN and LH, responds to peripheral immune challenge.
Chemogenetic Inhibition of the PVN and LH region attenuates IMQ-induced skin inflammation
The development of dermatitis involves multiple brain nuclei/regions. Here, we examined whether the PVN and LH were involved in IMQ-induced neuronal activation using fiber photometry. The pAAV-hSyn-GCaMP6s-P2A-NLS virus was injected into the C57BL/6J mice’s PVN and LH to specifically monitor neural activity (Fig. 2A, J). The results revealed that the PVN and LH neuronal activity of IMQ-treated mice increased and plateaued, whereas the Vas-treated mice presented a much lower increase in the sensitivity of the activity of PVN and LH neurons, which faded in a very short time (Fig. 2B, K). The PVN and LH neurons of the IMQ group of mice showed a higher peak (larger response) and area under the curve (AUC) (Fig. 2C, L). These results showed that the dermatitis group presented significantly increased neural activity in PVN and LH neurons compared with the Vas group.
Fig. 2.
PVN and LH neuronal activities are correlated with psoriasis-like dermatitis, and their inhibition alleviates symptoms. (A) Schematic diagram illustrating the recording of calcium signals in the PVN. Scale bar, 200 μm. (B) Left panel: Average calcium signals of PVN neurons (∆F/F in %) synchronized during control or psoriasiform dermatitis. Thick lines, means; shaded areas, SEMs. Right panel: Heatmap of calcium signals in each mouse. (C) The peak and AUC of calcium signals recorded from PVN neurons (n = 4). (D) Left panel: Representative image and diagram of the injection of AAV-hM4Di virus into the PVN. Scale bar, 500 μm. Right panel: Experimental timeline. (E-F) PASI score and representative photographs (n = 4). (G) Spleen weight and the ratio of spleen weight to body weight (n = 4). (H) Representative H&E-stained back skin sections and statistical analysis of the epidermal thickness. Scale bars, 200 μm (n = 6). (I) The mRNA expression levels of Il6, Il1b, Tnfa, Il23, and Il17a in the skin were quantified using qRT‒PCR (n = 3). (J) Schematic diagram illustrating the recording of calcium signals in the LH. Scale bar, 200 μm. (K) Left panel: Average calcium signals (∆F/F in %) of LH neurons synchronized during control or psoriasiform dermatitis. (L) The peak and AUC of calcium signals recorded from LH neurons (n = 4). (M) Left panel: Representative image and diagram of the injection of AAV-hM4Di virus into the LH. Scale bar, 500 μm. Right panel: Experimental timeline. (N-O) PASI score and representative photographs (n = 4). (P) Spleen weight and the ratio of spleen weight to body weight (n = 4). (Q) Representative H&E-stained back skin sections and statistical analysis of epidermal thickness. Scale bars, 200 μm (n = 6). (R) The mRNA expression levels of Il6, Il1b, Tnfa, Il23, and Il17a in the skin were quantified using qRT‒PCR (n = 3). The data are representative of three independent experiments and are presented as the means ± SEMs. The p-value was calculated using two-tailed unpaired Student’s t test. **p < 0.01, ***p < 0.001 compared with the Vas group (C, L); *p < 0.05, **p < 0.01, and ***p < 0.001 compared with the Saline group (E, G, H, I, N, P, Q, R)
In addition, we further investigated the roles of the PVN and LH in dermatitis by employing a chemogenetic approach through the injection of AAV-hSyn-hM4Di (Gi)-mCherry into the C57BL/6J mice’s PVN and LH regions. The mice were injected with CNO (5 mg/kg) or saline 30 min before IMQ treatment. We intraperitoneally injected CNO into mice to silence the PVN and LH neuronal activity (Fig. 2D, M). The results showed that the inhibition of PVN neurons during dermatitis reversed IMQ-induced changes in the PASI score (Fig. 2E, F) and spleen size (Fig. 2G), epidermal thickness (Fig. 2H) and skin inflammation levels (Fig. 2I). Similarly, Inhibition of LH neurons also reduced PASI scores (Fig. 2N, O) and spleen size (Fig. 2P), epidermal thickness (Fig. 2Q) and skin inflammation levels (Fig. 2R) in mice. In summary, these data indicate that PVN and LH activity are involved in IMQ-induced dermatitis and that their inhibition can attenuate psoriasis-like symptoms.We utilized FosTRAP mice in which the control group was administered a virus expressing the mCherry fluorescent reporter but devoid of the DREADD-coding sequence. Saline and CNO were administered during the induction of dermatitis (Fig. S2A). By employing a TM-based control strategy to specifically inhibit PVNFos neurons, we observed attenuated dermatitis-like skin pathology and splenomegaly, consistent with the effects seen in the PVN-hM4Di treatment (Fig. S2B, C). Therefore, we hypothesized that the PVN and LH neurons are key brain regions involved in central immune regulation.
Reactivation of PVN neuronal ensembles captured during dermatitis recapitulates an inflammatory state in dermatitis
We reactivated the TRAPed neurons and evaluated their effects on skin inflammation to clarify the relationship between the brain and the body’s immune response. For the neuronal reactivation, we co-expressed a stimulatory DREADD (designer receptor exclusively activated by designer drugs; Gq-coupled) in the captured neurons. DREADDs are engineered muscarinic G-protein-coupled receptors (GPCRs) activated by the synthetic ligand CNO. Thus, CNO administration enables the reactivation of captured neurons at will. We used FosTRAP mice, the control group was injected with a virus encoding the mCherry fluorescent reporter but lacking the DREADD coding sequence (Sham group). This Sham group allowed us to control for the surgical procedure, viral vector expression, amount of IMQ consumed, and application of CNO. As in the previous experiment, we injected TM during the IMQ treatment to capture active PVN and LH neurons and enable their expression of both Gq-DREADDs and a fluorescent reporter. After 4 weeks of recovery, we reactivated the captured PVN and LH neuronal ensembles (Fig. 3A) (by CNO injection) and evaluated their effects on skin inflammation. Especially, the accuracy of TRAPed cells was evidenced by substantial overlap between reactivated cells in the PVN and LH brain regions and neuronal cells activated during re-induced skin inflammation (validated by c-Fos staining; Fig. 3B, C). Our findings demonstrate that reactivation of PVN TRAPed neurons during dermatitis recapitulated psoriasis-like skin appearance (Fig. 3D, Fig. S3A), epidermal hyperplasia (Fig. 3E, F), splenomegaly (Fig. 3G, Fig. S3B), hyperactive skin nerves (Fig. 3H), and skin inflammation (Fig. 3I). In contrast, reactivation of LH TRAPed neurons induced only mild alterations in the skin appearance (Fig. 3J, Fig. S3C), epidermal thickness (Fig. 3K, L), spleen weight (Fig. 3M, Fig. S3D), skin nerves (Fig. 3N), and skin inflammation levels (Fig. 3O). These data suggest that PVN-associated neural circuits play a more pronounced role in driving dermatitis-related immune dysregulation than LH-mediated pathways.
Fig. 3.
PVN reactivation induces brain–skin axis-mediated psoriasiform inflammation. (A) Experimental timeline for the reactivation of PVN and LH labelled neurons during IMQ treatment through chemogenetics. (B-C) Representative images and statistical diagrams of the PVN and LH showing colabelled neurons (white arrow) with reactivation neurons (green) and hM3Dq neurons (red) (n = 6). Scale bar, 50 μm. (D-I) PASI score (n = 8), epidermal hyperplasia (n = 6), and spleen/bady weight ratio (n = 4), skin nerve LFPs (n = 4), and Il23 and Il17a mRNA levels (n = 3) after chemogenetic PVN reactivation. Scale bar, 200 μm. (J-O) PASI score (n = 8), epidermal hyperplasia (n = 6), and spleen/bady ratio (n = 4), skin nerve LFP (n = 4), Il23 and Il17a mRNA level (n = 3) after chemogenetic LH reactivation. Scale bar, 200 μm. (P) Experimental timeline for the reactivation of labelled PVN neurons during IMQ treatment through optogenetics. (Q-R) Schematic diagram illustrating ChR2-EGFP virus expression in the PVN and synchronous skin nerve signal recording. Scale bar, 500 μm. (S-X) PASI score (n = 8), epidermal hyperplasia (n = 6), and spleen/bady weight ratio (n = 4), skin nerve LFPs (n = 4), and Il23 and Il17a mRNA level (n = 3) after optogenetic PVN reactivation. Scale bar, 200 μm. The data are representative of three independent experiments and are presented as the means ± SEMs. The p-value was calculated using two-tailed unpaired Student’s t test. *p < 0.05, **p < 0.01, ***p < 0.001 compared with the Sham group
We reactivated the TRAPed neurons during dermatitis using optogenetics (15 min/day) to address this question further (Fig. 3P). Moreover, we assessed the LFP of skin nerve fibers, revealing direct association between PVN TRAPed neurons and skin nerve fibers (Fig. 3Q, R). Consistent with aforementioned findings, optogenetic activation of PVN TRAPed neurons during dermatitis induction recapitulated psoriasis-like cutaneous manifestations in mice, including characteristic skin lesions (Fig. 3S, Fig. S3E), significant epidermal thickening (Fig. 3T, U), hyperactive skin nerves (Fig. 3W), and skin inflammation (Fig. 3X). Notably, no overt splenomegaly was observed in these animals (Fig. 3V, Fig. S3F), which was potentially attributable to the relatively mild systemic inflammatory response compared with that in classical psoriasis models. Next, we investigated the regulation of neural inputs received by the PVN in dermatitis. We observed greater than 50% overlap between neurons projecting to the PVN (EGFP) and neurons labelled during dermatitis (mCherry) in the mPFC and posterior subthalamic nucleus (PSTh) brain regions, as identified by retrograde tracer virus injection into the PVN of mice (Fig. S4A-C). Chemogenetic inhibition of neural projections targeting the mPFC-PVN circuit during dermatitis (Fig. S4D) mitigated psoriasis-like skin manifestations (Fig. S4E) and splenomegaly (Fig. S4F, G) in mice. These results indicate that PVN neuronal activity modulates cutaneous inflammation through brain-skin axis signalling, providing functional evidence for central neural regulation of peripheral inflammatory cascades in psoriasiform dermatopathology. Nevertheless, the neural inputs and outputs involved in this process warrant further investigation.
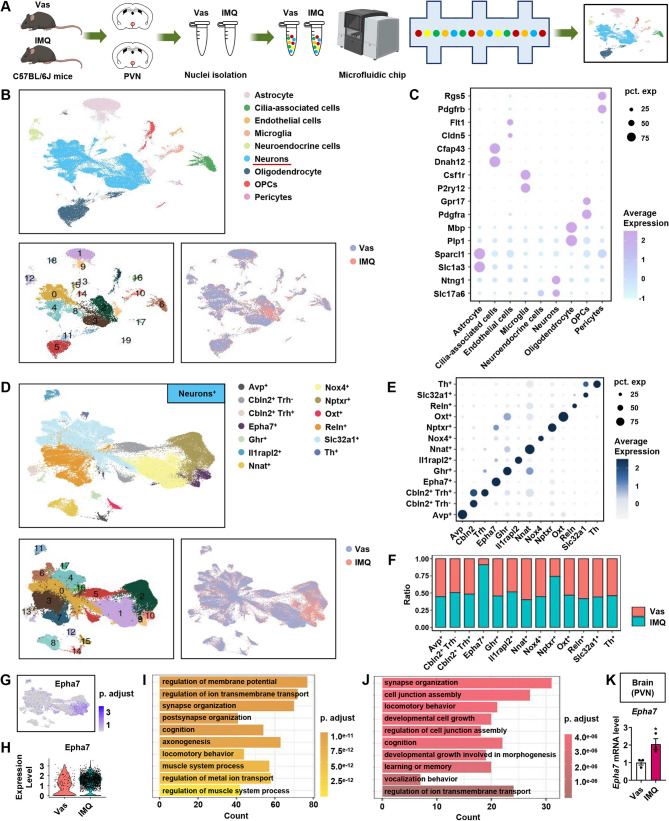
The molecular characteristics of PVN neuronal hyperactivity mediated by dermatitis based on single-nucleus RNA sequencing (snRNA-seq)
We performed snRNA-seq on 123,414 cells from Vas control and IMQ-treated C57BL/6J mice to characterize the cellular heterogeneity in the PVN comprehensively (Fig. 4A). Unsupervised clustering revealed nine major cell populations—astrocytes, cilia—associated cells, endothelial cells, microglia, neuroendocrine cells, neurons, oligodendrocytes, oligodendrocyte precursor cells (OPCs), and pericytes—with significant compositional differences between the treatment groups (Fig. 4B). Each cluster was accurately annotated through unique expression patterns of signature marker genes, as visualized in a dot plot highlighting representative markers (Fig. 4C). Subsequent reclustering of neuronal populations (enriched for Slc17a6 and Ntng1 expression) resolved 17 transcriptionally distinct subclusters that were consolidated into 13 neuronal subtypes based on marker gene profiles (Fig. 4D), and the characteristic expression patterns systematically illustrated (Fig. 4E).
Fig. 4.
snRNA-seq reveals that the Eph receptor in the PVN mediates brain–skin neuroimmune crosstalk. (A) Schematic of the experimental strategy applied for the single-cell transcriptome analysis. (B) Uniform manifold approximation and projection (UMAP) plot illustrating the integrated analysis of 125,049 cells isolated from 40 mice brain treated with Vas or IMQ. (C) Dot plot showing the expression of cell markers identified in each population. (D-E) UMAP plot and dot plot showing the expression of markers identified in each neuronal population. (F) Group difference plot of positive neurons. (G) UMAP plot showing the expression of Epha7. (H) Epha7 expression level in the Vas and IMQ groups. (I) Comparison of differences between neurons expressing Epha7 and other subpopulations. (J) Comparison of differences between the Vas and IMQ groups. (K) The mRNA level of Epha7 in the brain (PVN) was determined by qRT-PCR (n = 3). The data are representative of three independent experiments and are presented as the means ± SEMs. The p-value was calculated using two-tailed unpaired Student’s t test. *p < 0.05 compared with the Vas group
The bar graphs show differential neuronal proportions between the groups, revealing that IMQ-induced population dynamics were marked by a significant expansion of Epha7+ and Nptxr+ neuronal subtypes (Fig. 4F, G). Nptxr is involved in the mediation of synaptic material uptake during synapse remodeling. Compared with the Vas group, the mRNA expression level of Nptxr in the PVN of the IMQ group was increased (Fig. S5A). Notably, IMQ treatment specifically upregulated Epha7 expression in Epha7+ neurons, with no statistically significant difference observed in Epha7− neurons (Fig. 4H, Fig. S5B). The Gene Ontology analysis revealed that Epha7+ neurons were preferentially associated with synaptic plasticity pathways, particularly “synapse organization” and “postsynapse organization” (Fig. 4I). Furthermore, IMQ-treated Epha7+ neurons presented increased enrichment of “synapse organization” processes compared with their Vas-treated counterparts (Fig. 4J). The expression levels of Epha7 in the PVN were further verified by RT‒qPCR (Fig. 4K). Collectively, these results suggest that a key role for the Eph signaling pathway in the PVN in mediating neuroimmune crosstalk between the brain and skin.
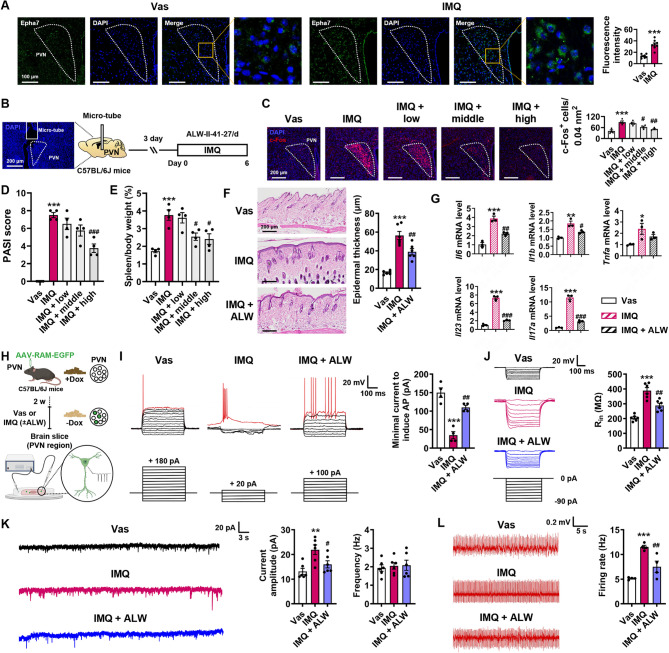
Inhibition of Ephrin receptor ameliorates dermatitis and the abnormal excitability of labelled neurons in the PVN during dermatitis
We collected mouse brain tissue immediately after six consecutive days of Vas or IMQ treatment. We first verified the expression of Epha7 in the PVN brain region of the Vas and IMQ groups using immunofluorescence staining, and the results were consistent with the snRNA-seq results (Fig. 5A). The pan-Ephrin receptor inhibitor ALW (0.5, 2.5, and 5 µg/µL) was injected into the C57BL/6J mice’s PVN 1 h prior to IMQ treatment through a stereotaxic microinfusion to verify the functional role of the Ephrin receptor in the PVN brain region involved in dermatitis. Mouse brain tissues were collected immediately after six consecutive days of treatment (Fig. 5B). Dose-dependent suppression of Ephrin receptor activity significantly ameliorated IMQ-induced dermatitis severity, demonstrating the therapeutic potential of central Eph signaling modulation in psoriasiform inflammation. The data showed that middle- and high-dose ALW treatments decreased the excitability of PVN neurons (Fig. 5C), reversing the PASI score (Fig. 5D), and splenomegaly (Fig. 5E). In subsequent investigations, we employed the maximal effective concentration of ALW (5 µg/µL) to test whether pharmacological Ephrin receptor inhibition ameliorated psoriasiform pathology. ALW treatment ameliorated IMQ-induced epidermal hyperplasia (Fig. 5F) and substantially downregulated the mRNA expression of psoriasis-associated cytokines, including Il6, Il1b, Tnfa, Il23, and Il17a (Fig. 5G).
Fig. 5.
Eph receptor inhibition in the PVN ameliorates psoriasiform dermatitis and PVNFos neuronal excitability. (A) Representative images and statistical diagrams of Epha7 expression in the PVN (n = 8). (B) Experimental timeline for the ALW stereotaxic microinfusion targeted to the PVN. Scale bar, 200 μm. (C) Representative images and statistical diagrams of c-Fos+ neurons in the PVN (n = 4). Scale bar, 200 μm. (D-F) ALW alleviated epidermal hyperplasia (n = 4), splenomegaly (n = 4), and psoriasiform skin manifestations (n = 6). Scale bar, 200 μm. (G) The mRNA expression levels of Il6, Il1b, Tnfa, Il23, and Il17a in the PVN were quantified using qRT‒PCR (n = 3). (H) Schematic diagram of patch clamp recordings from PVNFos neurons during dermatitis. (I) Representative traces and statistical diagrams showing minimal current injections required to evoke APs (red traces) from PVNFos neurons (n = 6). (J) Representative traces and input resistance statistics of PVNFos neurons in response to hyperpolarizing step current injections (n = 6). (K) Representative traces and statistical diagrams showing average amplitude and frequency of spontaneous discharges in PVNFos neurons (n = 6). (L) Representative traces and statistics of hyperactive skin nerves (n = 4). The data are representative of three independent experiments and are presented as the means ± SEMs. The p-value was calculated using one‐way ANOVA followed by the Tukey or Holm‒Sidak post hoc test (C, D, E, F, G, I, J, K, L) or two-tailed unpaired Student’s t test (A). *p < 0.05, **p < 0.01, and ***p < 0.001 compared with the Vas group; #p < 0.05, ##p < 0.01, and ###p < 0.001 compared with the IMQ group
In combination with the Tef-off system (Fig. S6), PVNFos neurons were labelled during dermatitis, and the patch clamp technique was used to record PVNFos neuronal excitability to reflect activation-dependent changes in synaptic plasticity (Fig. 5H).
Using slice electrophysiology, we found that the amount of current required to evoke the first action potentials (AP) (the excitation threshold of neuron) in neurons from the ALW group was strikingly greater than that in neurons from the IMQ group (Fig. 5I). Consistently, ALW-treated PVNFos neurons presented significantly decreased membrane input resistance, suggesting that their activity was restored (a larger current is required to depolarize the membrane potential to the APs) (Fig. 5J). Furthermore, ALW reduces the amplitude of spontaneous discharges in PVNFos neurons (Fig. 5K) and ameliorates the hyperexcitability of skin nerves (Fig. 5L) caused by dermatitis.
In addition, we test the abnormal behavioural changes in psoriasis-like dermatitis model mice under stress using the OFT, EPM, FST and TST. The results showed that ALW rescued the anxiety-like behaviours caused by dermatitis (Fig. S7A, B), but no significant change in depression-like behaviour was observed among the groups (Fig. S7C, D). In conclusion, these findings suggest a pivotal role for Eph signaling in the PVN in bridging skin inflammation and neuropsychiatric comorbidities.
Inhibition Ephrin receptor reduces synaptic structural plasticity and abnormal oscillations in PVN neurons
The GO enrichment analysis of snRNA-seq data revealed significant differences in synaptic function between naive and dermatitis model mice. Structural modifications of synapses are important for synaptic plasticity and transmission. Thus, we injected the CSSP-YFP-2E4 virus into the PVN in FosTRAP mice and injected TM intraperitoneally to label active synapses (Fig. 6A). The images were visualized using a 980 confocal microscope, and 3D confocal images were reconstructed. The IMQ group presented an approximately 2-fold increase in the number of dendritic branch intersections in PVN neurons, which was effectively attenuated by ALW preconditioning (Fig. 6B). High-resolution confocal imaging (63 × oil immersion) enables a quantitative spine subtype analysis, classifying dendritic protrusions into mushroom, thin, and stubby morphotypes. Psoriasiform inflammation significantly increased the total spine density, particularly the densities of mushroom and thin spines, whereas the density of stubby spines changed little. ALW treatment normalized the redistribution of these pathologically altered spines to near-baseline levels (Fig. 6C, D).
Fig. 6.
ALW-mediated synaptic pruning normalizes PVN hyperactivity. (A) Representative confocal Z-stack images and reconstructions of the PVN neurons of mice injected with the CSSP-YFP-2E4 virus. Scale bar, 50 μm. (B) Traces of neuronal processes and the number of dendritic intersections (n = 4). (C) Representative confocal Z-stack images of the dendritic segments of PVN neurons. Scale bar, 50 μm. (D) Quantification of all spines, mushroom spines, thin spines, and stubby spines on PVN neurons (n = 10). (E) Representative images of the synaptic ultrastructure; red arrows indicate synapses in the PVN. Scale bar, 500 nm. Magnified images show the length and thickness of the synaptic PSD. (F) Quantification of synapses and the length and thickness of the synaptic PSD (n = 6). (G) Diagram illustrating the localization of LFP electrode implants. (H-I) Representative images of LFP electrode implants and LFP power in the different frequency bands in the PVN (n = 4). The data are representative of three independent experiments and are presented as the means ± SEMs. The p-value was calculated using two-way ANOVA (B, I) or one‐way ANOVA (D, F) followed by the Tukey or Holm‒Sidak post hoc test. *p < 0.05, **p < 0.01, ***p < 0.001 compared with the Vas group; #p < 0.05, ##p < 0.01, ###p < 0.001 compared with the IMQ group
Ultrastructural validation via TEM revealed that ALW administration resulted in the following effects in C57BL/6J mice: a 42% reduction in the postsynaptic density (PSD) length and a 12% decrease in synaptic vesicle density per bouton within the PVN neuropil (Fig. 6E, F). We further investigated neuronal functional by recording the local field potentials (LFPs) in the PVN region using a LabChart detection device and analysed changes in neuronal oscillations. As shown in Fig. 6G, LFPs were recorded in the PVN. The data showed that theta, alpha, beta, and gamma oscillations increased in the PVN of IMQ-treated mice. After pretreatment with ALW, beta and gamma oscillations decreased significantly to levels similar to those in the Vas-treated group (Fig. 6H, I). These multiscale observations, ranging from nanoscale synaptic architectures to circuit-level plasticity, confirm that the inhibition of Eph signalling mediated by ALW normalizes PVN hyperactivity through synaptic pruning mechanisms. These results provide a structural basis for its immunomodulatory effects via the brain-skin neural circuitry.
Inhibition Ephrin receptor alleviates the skin inflammation by suppressing excessive excitation of the skin sympathetic nerve
We evaluated cutaneous TH staining and NE release in C57BL/6J mice across the experimental groups to investigate the PVN–sympathetic nerves–skin immune axis. ALW treatment significantly reversed dermatitis-induced sympathetic hyperactivation, as shown by normalized TH+ staining (Fig. 7A, B) and NE levels (Fig. 7C). We tested whether ALW restored Treg homeostasis in psoriatic skin by performing flow cytometry with lineage-specific markers (CD45.2+CD3+CD4+Foxp3+) (Fig. S8A), and identified a functionally impaired Treg population in psoriatic lesions. The results show that ALW mitigated the dermatitis-associated decrease in cutaneous Treg numbers (Fig. S8B, C) and increase in cutaneous Th17 numbers (Fig. S9A). We hypothesized that Treg/Th17 imbalance may be involved in regulation of immune homeostasis by ALW.
Fig. 7.
ALW suppresses sympathetic nerve hyperactivity to resolve psoriasiform dermatitis. (A, B) Representative images of immunofluorescence staining and statistical analysis of mouse skin sections stained for TH (sympathetic nerves, red) (n = 4). (C) NE concentrations in the skin (n = 6). (D) The flow chart shows that the sympathetic nerve mediates the feedback between psoriasis and the immune system. (E) NE concentrations in the skin after sympathetic nerve blockade (n = 6). (F-J) Sympathetic blockade reversed ALW-ameliorated psoriasiform skin manifestations (n = 6), epidermal hyperplasia (n = 6), and splenomegaly (n = 4). (K) The mRNA expression levels of Il6, Il1b, Tnfa, Il23, and Il17a in the PVN were quantified using qRT‒PCR (n = 3). The data are representative of three independent experiments and are presented as the means ± SEMs. The p-value was calculated by one‐way ANOVA followed by the Tukey or Holm‒Sidak post hoc test (B, C) or two-tailed unpaired Student’s t test (E, G, I, J, K). ***p < 0.001 compared with the Vas group; #p < 0.05, ##p < 0.01 compared with the IMQ group (B, C). *p < 0.05, **p < 0.01 compared with the IMQ + ALW group (E, G, I, J, K)
We performed systemic administration of 6-OHDA for sympathetic nerve ablation in C57BL/6J mice to further validate the central role of sympathetic signalling (Fig. 7D). After denervation, changes in skin NE levels (Fig. 7E) was observed, and ALW no longer reversed the psoriatic skin changes induced by IMQ in the mice (Fig. 7F, G), including skin epidermal thickening (Fig. 7H, I) and splenomegaly (Fig. 7J). Notably, sympathetic blockade reversed the anti-inflammatory effects of ALW, with significant rebound increases in the Il6, Il1b, Tnfa, Il23, and Il17a mRNA levels (Fig. 7K). Furthermore, suppression of central sympathetic outflow restores peripheral Treg cell numbers (Fig. S9B, C). Collectively, these findings indicate that ALW alleviates psoriasiform dermatitis by targeting the PVN-sympathetic-skin axis.
Discussion
Research has indicated that the nervous and immune systems are intricately interconnected [31–33]. From the perspective of central regulation, investigations of neural–immune interactions have revealed that the brain constructs a dynamic neural representation network by integrating peripheral immune signals with autonomic nervous system outputs to modulate immune responses [31, 32, 34]. In this study, we observed the specific activation of the central nervous system during dermatitis, particularly in the PVN and LH regions. Furthermore, the inhibition of these neurons exerted a protective effect on skin tissue, as evidenced by five distinct measures: skin appearance, histological analysis of skin tissue, assessment of splenomegaly, skin nerve excitability, and skin levels of inflammatory factor mRNAs. Our findings establish the PVN as a critical mediator of psoriasiform inflammation, extending prior observations regarding hypothalamic involvement in systemic immune regulation [35]. Additionally, recent studies have shown that reactivation of specific brain regions during peripheral inflammation can reproduce inflammatory responses. For example, the insular cortex receives information from the gastrointestinal tract via the brainstem and thalamus, where it stores immune memory associated with DSS-induced colitis [36]. Notably, in the context of skin inflammation, the dynamic encoding mechanism of the neuroimmune axis underlying hypothalamic–skin interaction remains unclear. Our results demonstrate that the reactivation of captured PVN neurons during dermatitis is sufficient to induce a robust inflammatory response in the skin. These findings indicate the close regulatory relationship between the brain and skin, revealing bidirectional communication mediated by neuroimmune pathways. This crosstalk underscores the dual role of the PVN as both a target and a source of neuromodulators, suggesting potential therapeutic targets for psoriasis.
The PVN of the hypothalamus is a critical hub for coordinating neuroendocrine and autonomic responses to stress, fluid homeostasis, and cardiovascular regulation, primarily through its projections to the pituitary gland and brainstem nuclei [37–39]. Previous studies have established that synaptic plasticity within the PVN, including dynamic changes in dendritic spine density, docked vesicle numbers, and PSD length, is essential for adaptive neuronal responses to physiological challenges [40]. Notably, structural plasticity in PVN neurons correlates with the expression of functional activation markers such as Fos, a widely used indicator of neuronal activity. For example, chronic stress induces dendritic remodelling in PVN corticotropin-releasing hormone (CRH) neurons, accompanied by increased Fos expression, suggesting a bidirectional relationship between the synaptic architecture and neuronal excitability [41, 42]. Previous studies have established roles for Ephrin/Eph interactions in regulating spine morphogenesis and synaptic vesicle clustering. For example, Epha4 ablation preserves synapse function and improves the cognitive performance of the APPPS1 transgenic mouse model of Alzheimer’s disease [43]. Our findings reveal that the ALW-mediated disruption of Epha7 signalling attenuates synaptic plasticity in the PVN. Specifically, inhibition Ephrin receptor treatment significantly reduced the dendritic spine density (by ~ 23%), number of docked vesicles (by ~ 12%), and PSD length (by ~ 42%). The observed decrease in Fos expression after ALW treatment further supports the premise that impaired synaptic connectivity reduces neuronal activation thresholds. Notably, our data support prior work by showing that Epha7 is indispensable for maintaining PVN immune engram cells excitability under basal conditions [44, 45]. Previous studies reported that the genetic loss of Efna5 ligand and its receptors, Epha4 and Epha7, in conjunction with the Akt pathway in mice inhibits the growth and progression of medulloblastoma tumors [46]. Collectively, these findings underscore Epha7/Efna5 as therapeutic targets for disorders involving maladaptive PVN plasticity, such as inflammation or chronic stress syndromes.
The PVN serves as a central regulator of sympathetic outflow to peripheral organs and cutaneous tissues, modulating systemic physiological responses through direct and indirect projections to preganglionic sympathetic neurons in the spinal cord and brainstem [47–49]. Emerging evidence highlights that PVN-driven sympathetic hyperactivity, characterized by excessive NE release, exacerbates inflammatory disorders and immune dysregulation by altering adrenergic receptor (AR)-mediated signalling [50, 51]. Our data further support the pathological link between PVN-mediated sympathetic overdrive and skin inflammation.
Previous studies have reported that Treg cells suppress excessive immune responses and maintain immune homeostasis by secreting Il10 and Tgfb in a contact-dependent manner under physiological conditions [52]. High levels of Tnfa, Il1b and other inflammatory factors in the microenvironment of psoriatic skin induce Treg apoptosis or promote their transformation into Th17 cells, which differentiate into effector cells and secrete proinflammatory cytokines such as Il17a and Il22 [53, 54]. Previous studies have indicated that NE binding to α1-AR on macrophages enhances NLRP3 inflammasome activation, driving Il1b release in individuals with metabolic syndrome, whereas β2-AR agonism directly inhibits Treg proliferation [55–57]. This result aligns with our findings that skin inflammation stimulation increased sympathetic hyperactivity, which was accompanied by a 2.5-fold increase in plasma NE levels, paralleled by reduced skin Treg populations and increased Th17 populations (Th17/Treg imbalance). Importantly, ALW decreased NE levels and alleviated both inflammation and Treg depletion, suggesting that ALW-mediated PVN neuronal excitability plays a critical role in the modulation of organ sympathetic function. Notably, the aforementioned effects were significantly mitigated following sympathetic nerve blockade, thereby underscoring the critical role of ALW in the PVN–sympathetic nerve–NE signalling pathway for immune suppression. Collectively, our findings underscore the PVN as a therapeutic target for IMQ-induced skin disorders involving sympathetic–immune crosstalk, particularly those driven by dysregulation of the PVN–NE–skin axis. Similarly, previous studies have reported that other skin inflammations are also regulated by brain nuclei. Future studies will explore this therapeutic potential in alternative models of psoriasis to validate its broader applicability.
In summary, our findings suggest that blocking ephrin ligand/receptor binding in the PVN, which affects synaptic plasticity, can restore the skin inflammation. Our results reveal the potential of ALW-mediated inhibition of PVN activity via the brain–body circuit as a strategy to suppress skin inflammation. Consequently, this study provides an additional perspective on these pathological conditions and potentially opens new avenues for therapeutic intervention.
Supplementary Information
Acknowledgements
We thank the Translational Medicine Core Facility of Shandong University for consultation and instrument availability that supported this work.
Authors’ contributions
Qingyu Ren: Conceptualization, Methodology, Software, Data curation, Formal analysis, Writing-original draft. Zhanpeng Gao: Conceptualization, Methodology, Investigation. Weikai Han: Conceptualization, Methodology, Investigation. Yaqi Tang: Methodology, Validation. Mengdong Shi: Methodology, Investigation. Yanan Yue: Validation. Xijia Xin: Validation. Chenyu Zhang: Validation. E Liu: Investigation. Bo Dong: Validation. Qingwei Yue: Investigation. Jinhao Sun: Conceptualization, Methodology, Supervision, Writing-review & editing, Project administration, Funding acquisition.
Funding
This work was supported by the National Natural Science Foundation (grant number: U2202211, 82471517, 82371574), and the Tai-Shan scholar program (ts20190979). We thank the Anatomy and Neurobiology Department of Shandong University for consultation and instrument availability that supported this work.
Data availability
The snRNA-Seq datasets specifically focusing on brain PVN cells following 6 days of Vas and IMQ treatment have been deposited in the NCBI GEO database and are publicly accessible as of the publication date (Accession Numbers: GSE296273, GSE296150). All data generated or analyzed during this study are included in this published article.
Declarations
Ethics approval and consent to participate
All animal experiments in this study were approved by the Institutional Animal Care and Use Committee at the Shandong University (No. ECSBMSSDU2024-2-005).
Consent for publication
All listed authors consent to the submission, and all data are used with the consent of the person generating the data.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ghoreschi K, Balato A, Enerbäck C, Sabat R. Therapeutics targeting the IL-23 and IL-17 pathway in psoriasis. Lancet (London England). 2021;397(10275):754–66. [DOI] [PubMed] [Google Scholar]
- 2.Germán B, Wei R, Hener P, Martins C, Ye T, Gottwick C et al. Disrupting the IL-36 and IL-23/IL-17 loop underlies the efficacy of calcipotriol and corticosteroid therapy for psoriasis. JCI Insight. 2019;4(2):e123390. [DOI] [PMC free article] [PubMed]
- 3.Ogawa E, Sato Y, Minagawa A, Okuyama R. Pathogenesis of psoriasis and development of treatment. J Dermatol. 2018;45(3):264–72. [DOI] [PubMed] [Google Scholar]
- 4.Marek-Jozefowicz L, Czajkowski R, Borkowska A, Nedoszytko B, Żmijewski MA, Cubała WJ et al. The Brain-Skin axis in Psoriasis-Psychological, psychiatric, hormonal, and dermatological aspects. Int J Mol Sci. 2022;23(2):669. [DOI] [PMC free article] [PubMed]
- 5.Talley S, Valiauga R, Anderson L, Cannon AR, Choudhry MA, Campbell EM. DSS-induced inflammation in the colon drives a Proinflammatory signature in the brain that is ameliorated by prophylactic treatment with the S100A9 inhibitor paquinimod. J Neuroinflamm. 2021;18(1):263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Süß P, Hoffmann A, Rothe T, Ouyang Z, Baum W, Staszewski O, et al. Chronic peripheral inflammation causes a Region-Specific myeloid response in the central nervous system. Cell Rep. 2020;30(12):4082–e956. [DOI] [PubMed] [Google Scholar]
- 7.Xu H, Hehnly C, Lehtinen MK. The choroid plexus: a command center for brain-body communication during inflammation. Curr Opin Immunol. 2025;93:102540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kraynak TE, Marsland AL, Wager TD, Gianaros PJ. Functional neuroanatomy of peripheral inflammatory physiology: A meta-analysis of human neuroimaging studies. Neurosci Biobehav Rev. 2018;94:76–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zheng H, Katsurada K, Nandi S, Li Y, Patel KP. A critical role for the paraventricular nucleus of the hypothalamus in the regulation of the volume reflex in normal and various cardiovascular disease States. Curr Hypertens Rep. 2022;24(7):235–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pondeljak N, Lugović-Mihić L. Stress-induced interaction of skin immune cells, hormones, and neurotransmitters. Clin Ther. 2020;42(5):757–70. [DOI] [PubMed] [Google Scholar]
- 11.de Mello AH, Costa AB, Engel JDG, Rezin GT. Mitochondrial dysfunction in obesity. Life Sci. 2018;192:26–32. [DOI] [PubMed] [Google Scholar]
- 12.Ogundele OM, Lee CC, Francis J. Age-dependent alterations to paraventricular nucleus insulin-like growth factor 1 receptor as a possible link between sympathoexcitation and inflammation. J Neurochem. 2016;139(5):706–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pham GS, Mathis KW. Lipopolysaccharide challenge reveals Hypothalamic-Pituitary-Adrenal axis dysfunction in murine systemic lupus erythematosus. Brain Sci. 2018;8(10):184. [DOI] [PMC free article] [PubMed]
- 14.Garrott K, Dyavanapalli J, Cauley E, Dwyer MK, Kuzmiak-Glancy S, Wang X, et al. Chronic activation of hypothalamic Oxytocin neurons improves cardiac function during left ventricular hypertrophy-induced heart failure. Cardiovascular Res. 2017;113(11):1318–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bellinger DL, Millar BA, Perez S, Carter J, Wood C, ThyagaRajan S, et al. Sympathetic modulation of immunity: relevance to disease. Cell Immunol. 2008;252(1–2):27–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Furiati SC, Catarino JS, Silva MV, Silva RF, Estevam RB, Teodoro RB, et al. Th1, Th17, and Treg responses are differently modulated by TNF-α inhibitors and methotrexate in psoriasis patients. Sci Rep. 2019;9(1):7526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li X, Jiang M, Chen X, Sun W. Etanercept alleviates psoriasis by reducing the Th17/Treg ratio and promoting M2 polarization of macrophages. Immun Inflamm Dis. 2022;10(12):e734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bannerman DM, Sprengel R, Sanderson DJ, McHugh SB, Rawlins JN, Monyer H, et al. Hippocampal synaptic plasticity, Spatial memory and anxiety. Nat Rev Neurosci. 2014;15(3):181–92. [DOI] [PubMed] [Google Scholar]
- 19.Zheng ZH, Tu JL, Li XH, Hua Q, Liu WZ, Liu Y, et al. Neuroinflammation induces anxiety- and depressive-like behavior by modulating neuronal plasticity in the basolateral amygdala. Brain Behav Immun. 2021;91:505–18. [DOI] [PubMed] [Google Scholar]
- 20.Fares J, Bou Diab Z, Nabha S, Fares Y. Neurogenesis in the adult hippocampus: history, regulation, and prospective roles. Int J Neurosci. 2019;129(6):598–611. [DOI] [PubMed] [Google Scholar]
- 21.Bains JS, Wamsteeker Cusulin JI, Inoue W. Stress-related synaptic plasticity in the hypothalamus. Nat Rev Neurosci. 2015;16(7):377–88. [DOI] [PubMed] [Google Scholar]
- 22.Favuzzi E, Marques-Smith A, Deogracias R, Winterflood CM, Sánchez-Aguilera A, Mantoan L, et al. Activity-Dependent gating of parvalbumin interneuron function by the perineuronal net protein Brevican. Neuron. 2017;95(3):639–e5510. [DOI] [PubMed] [Google Scholar]
- 23.Chiu SL, Diering GH, Ye B, Takamiya K, Chen CM, Jiang Y, et al. GRASP1 regulates synaptic plasticity and learning through endosomal recycling of AMPA receptors. Neuron. 2017;93(6):1405–e198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Joo Y, Xue Y, Wang Y, McDevitt RA, Sah N, Bossi S, et al. Topoisomerase 3β knockout mice show transcriptional and behavioural impairments associated with neurogenesis and synaptic plasticity. Nat Commun. 2020;11(1):3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Szepietowska B, Zhu W, Czyzyk J, Eid T, Sherwin RS. EphA5-EphrinA5 interactions within the ventromedial hypothalamus influence counterregulatory hormone release and local glutamine/glutamate balance during hypoglycemia. Diabetes. 2013;62(4):1282–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Szepietowska B, Horvath TL, Sherwin RS. Role of synaptic plasticity and EphA5-ephrinA5 interaction within the ventromedial hypothalamus in response to recurrent hypoglycemia. Diabetes. 2014;63(3):1140–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Freyburger M, Pierre A, Paquette G, Bélanger-Nelson E, Bedont J, Gaudreault PO, et al. EphA4 is involved in sleep regulation but not in the electrophysiological response to sleep deprivation. Sleep. 2016;39(3):613–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhatia S, Oweida A, Lennon S, Darragh LB, Milner D, Phan AV, et al. Inhibition of EphB4-Ephrin-B2 signaling reprograms the tumor immune microenvironment in head and neck cancers. Cancer Res. 2019;79(10):2722–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nie D, Di Nardo A, Han JM, Baharanyi H, Kramvis I, Huynh T, et al. Tsc2-Rheb signaling regulates EphA-mediated axon guidance. Nat Neurosci. 2010;13(2):163–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ren Q, Han W, Yue Y, Tang Y, Yue Q, Comai S, et al. Melatonin regulates neuronal synaptic plasticity in the supramammillary nucleus and attenuates Methamphetamine-Induced conditioned place preference and sensitization in mice. J Pineal Res. 2024;76(6):e13006. [DOI] [PubMed] [Google Scholar]
- 31.Shi DD, Zhang YD, Zhang S, Liao BB, Chu MY, Su S, et al. Stress-induced red nucleus Attenuation induces anxiety-like behavior and lymph node CCL5 secretion. Nat Commun. 2023;14(1):6923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vainchtein ID, Chin G, Cho FS, Kelley KW, Miller JG, Chien EC, et al. Astrocyte-derived interleukin-33 promotes microglial synapse engulfment and neural circuit development. Sci (New York NY). 2018;359(6381):1269–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tian L, Ma L, Kaarela T, Li Z. Neuroimmune crosstalk in the central nervous system and its significance for neurological diseases. J Neuroinflamm. 2012;9:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu J, Xiao K, Chen X, Deng L, Zhang L, Li Y, et al. Neuron-derived neuropeptide Y fine-tunes the Splenic immune responses. Neuron. 2022;110(8):1327–e396. [DOI] [PubMed] [Google Scholar]
- 35.Morris G, Anderson G. Hypothalamic-Pituitary-Adrenal hypofunction in myalgic encephalomyelitis (ME)/Chronic fatigue syndrome (CFS) as a consequence of activated Immune-Inflammatory and oxidative and nitrosative pathways. Mol Neurobiol. 2017;54(9):6806–19. [DOI] [PubMed] [Google Scholar]
- 36.Koren T, Yifa R, Amer M, Krot M, Boshnak N, Ben-Shaanan TL, et al. Insular cortex neurons encode and retrieve specific immune responses. Cell. 2021;184(24):5902–e1517. [DOI] [PubMed] [Google Scholar]
- 37.Weiser MJ, Osterlund C, Spencer RL. Inhibitory effects of corticosterone in the hypothalamic paraventricular nucleus (PVN) on stress-induced adrenocorticotrophic hormone secretion and gene expression in the PVN and anterior pituitary. J Neuroendocrinol. 2011;23(12):1231–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chaves T, Fazekas CL, Horváth K, Correia P, Szabó A, Török B et al. Stress adaptation and the brainstem with focus on Corticotropin-Releasing hormone. Int J Mol Sci. 2021;22(16):9090. [DOI] [PMC free article] [PubMed]
- 39.Schunke KJ, Rodriguez J, Dyavanapalli J, Schloen J, Wang X, Escobar J, et al. Outcomes of hypothalamic Oxytocin neuron-driven cardioprotection after acute myocardial infarction. Basic Res Cardiol. 2023;118(1):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Itzev DE, Lolov SR, Usunoff KG. Aging and synaptic changes in the paraventricular hypothalamic nucleus of the rat. Acta Physiol Pharmacol Bulg. 2003;27(2–3):75–82. [PubMed] [Google Scholar]
- 41.Salter EW, Sunstrum JK, Matovic S, Inoue W. Chronic stress dampens excitatory synaptic gain in the paraventricular nucleus of the hypothalamus. J Physiol. 2018;596(17):4157–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Qin XY, Shan QH, Fang H, Wang Y, Chen P, Xiong ZQ, et al. PSD-93 up-regulates the synaptic activity of corticotropin-releasing hormone neurons in the paraventricular nucleus in depression. Acta Neuropathol. 2021;142(6):1045–64. [DOI] [PubMed] [Google Scholar]
- 43.Poppe L, Rué L, Timmers M, Lenaerts A, Storm A, Callaerts-Vegh Z, et al. EphA4 loss improves social memory performance and alters dendritic spine morphology without changes in amyloid pathology in a mouse model of alzheimer’s disease. Alzheimers Res Ther. 2019;11(1):102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang W, Zhao J, Fan X, Chen S, Wang R. Targeted demethylation of the EphA7 promoter inhibits tumorigenesis via the SP1/DNMT1 and PI3K/AKT axes and improves the response to multiple therapies in cervical cancer. Cell Death Dis. 2025;16(1):324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Y, Cheng H, Zeng T, Chen S, Xing Q, Zhu B. A novel 17 apoptosis-related genes signature could predict overall survival for bladder cancer and its associations with immune infiltration. Heliyon. 2022;8(11):e11343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bhatia S, Hirsch K, Baig NA, Rodriguez O, Timofeeva O, Kavanagh K, et al. Effects of altered ephrin-A5 and EphA4/EphA7 expression on tumor growth in a Medulloblastoma mouse model. J Hematol Oncol. 2015;8:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Buijs RM, Chun SJ, Niijima A, Romijn HJ, Nagai K. Parasympathetic and sympathetic control of the pancreas: a role for the Suprachiasmatic nucleus and other hypothalamic centers that are involved in the regulation of food intake. J Comp Neurol. 2001;431(4):405–23. [DOI] [PubMed] [Google Scholar]
- 48.Milanez MIO, Silva AM, Perry JC, Faber J, Nishi EE, Bergamaschi CT, et al. Pattern of sympathetic vasomotor activity induced by GABAergic Inhibition in the brain and spinal cord. Pharmacol Rep. 2020;72(1):67–79. [DOI] [PubMed] [Google Scholar]
- 49.Xu XY, Wang JX, Chen JL, Dai M, Wang YM, Chen Q et al. GLP-1 in the hypothalamic paraventricular nucleus promotes sympathetic activation and hypertension. J Neuroscience: Official J Soc Neurosci. 2024;44(21):e2032232024. [DOI] [PMC free article] [PubMed]
- 50.Cardoso F, Klein Wolterink RGJ, Godinho-Silva C, Domingues RG, Ribeiro H, da Silva JA, et al. Neuro-mesenchymal units control ILC2 and obesity via a brain-adipose circuit. Nature. 2021;597(7876):410–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang J, Sun L, You J, Peng H, Yan H, Wang J, et al. Role and mechanism of PVN-sympathetic-adipose circuit in depression and insulin resistance induced by chronic stress. EMBO Rep. 2023;24(12):e57176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Palomares O, Martín-Fontecha M, Lauener R, Traidl-Hoffmann C, Cavkaytar O, Akdis M, et al. Regulatory T cells and immune regulation of allergic diseases: roles of IL-10 and TGF-β. Genes Immun. 2014;15(8):511–20. [DOI] [PubMed] [Google Scholar]
- 53.Shen Z, Jiang J, Zhou X, Tan Q, Yan S, Wu X, et al. Melatonin attenuates Imiquimod-Induced Psoriasis-Like inflammation and restores the Th17/Treg immune balance. Inflammation. 2024;47(6):2027–40. [DOI] [PubMed] [Google Scholar]
- 54.Ramón-Vázquez A, Flood P, Cashman TL, Patil P, Ghosh S. T lymphocyte plasticity in chronic inflammatory diseases: the emerging role of the Ikaros family as a key Th17-Treg switch. Autoimmun Rev. 2025;24(3):103735. [DOI] [PubMed] [Google Scholar]
- 55.Heidt T, Sager HB, Courties G, Dutta P, Iwamoto Y, Zaltsman A, et al. Chronic variable stress activates hematopoietic stem cells. Nat Med. 2014;20(7):754–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu D, Xu X, Dai Y, Zhao X, Bao S, Ma W et al. Blockade of AIM2 inflammasome or α1-AR ameliorates IL-1β release and macrophage-mediated immunosuppression induced by CAR-T treatment. J Immunother Cancer 2021;9(1):e001466. [DOI] [PMC free article] [PubMed]
- 57.Wang XQ, Wang TT, Fang XX, Shen WX, Peng YP, Qiu YH. Intervention of tyrosine hydroxylase expression alters joint inflammation and Th17/Treg imbalance in Collagen-Induced arthritis. Neuro-Signals. 2021;29(1):1–13. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The snRNA-Seq datasets specifically focusing on brain PVN cells following 6 days of Vas and IMQ treatment have been deposited in the NCBI GEO database and are publicly accessible as of the publication date (Accession Numbers: GSE296273, GSE296150). All data generated or analyzed during this study are included in this published article.