Abstract
Background
Breast cancer is one of the most common malignancies and causes of mortality in women. Combination therapies are one of the treatment approaches that contribute to better performance, reduced dosage, and drug resistance. Hydralazine is an antihypertensive drug, but it can induce epigenetic changes such as DNA methylation reversal in cancer cells. ATRA is a type of vitamin A, and its deficiency is associated with the progression of various diseases. It can bind to receptors and regulate genes to inhibit multiple types of cancers.
Methods
According to bioinformatics studies, combining these two drugs can inhibit breast cancer cells. This study investigates various biological pathways, such as HIF-1, VEGF, and WNT, with the key genes of CCND1, VEGFA, VEGFA2, HIF1A, and HIF1A-AS. In the laboratory, MDA-MB-231, as a tumor cell line, and MCF10, as a normal cell line, were cultured, and the MTT assay was performed to obtain the IC50 of the drugs. Using these concentrations, isobologram and wound healing tests were performed. Gene expression was measured using real-time PCR.
Results
Results showed that hydralazine alone stimulated cancer cell growth, potentially inducing breast cancer. ATRA reduced cell survival in both normal and cancer cells. However, the combination treatment exhibited differential effects, causing a significant reduction in survival in cancer cells while the impact on normal cells was not significant.
Conclusions
Hydralazine/ATRA combination inhibits cancer cell proliferation and their adaptation to hypoxia. These findings suggest a potential for new treatment targeting breast cancer cells with minimized side effects.
Clinical trial registration
Clinical trial number: “not applicable”.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12885-025-14477-2.
Keywords: Breast cancer, Hydralazine, All-trans retinoic acid (ATRA), Drug synergy, Cell proliferation
Introduction
Cancer is one of the leading causes of death worldwide and a complex disease caused by genetic mutations in cells during tumor growth. Conventional cancer treatments, such as chemotherapy, radiotherapy, or immunotherapy, although often effective, have significant drawbacks, including tumor resistance and considerable side effects, such as decreased appetite, nausea, weakness, and hair loss. To address these limitations and reduce the necessary drug dosage, targeted therapies are being explored, focusing on discovering innovative compounds. Dual drug compounds offer a promising strategy to overcome these negative aspects of current treatment options [1].
Cancer drugs prevent tumor growth and metastasis by inhibiting cell proliferation, differentiation, and angiogenesis, as well as inducing programmed cell death. By targeting angiogenesis, they deprive tumors of the nutrients and oxygen necessary for their growth and spread. Despite their effectiveness, resistance to anti-angiogenic therapies can develop over time. It necessitated the development of complementary approaches to overcome this resistance and improve treatment outcomes [2]. For example, Doxorubicin (DOXO) is an effective drug for inhibiting various types of cancer, including breast and liver cancer. It induces apoptosis in cancer cells through DNA damage. However, its clinical use at higher doses is restricted due to significant cardiac toxicity [3]. Hydralazine is an antihypertensive drug that has also been investigated in cancer treatments. It can change epigenetic modifications in cancer cells [4]. On the other hand, all-trans retinoic acid (ATRA) is a retinoid with gene-regulatory and therapeutic potential in cancers [5].
Breast cancer is one of the most commonly diagnosed cancers worldwide. It originates from breast tissue and is the most common cause of cancer-related death in women. Nearly 660,000 people die from this cancer annually [6].
This study explores the effects of hydralazine and ATRA, both individually and in combination, on human breast cells through bioinformatics and in vitro methods. The goal is to introduce a promising combined approach for breast cancer treatment. Additionally, the study examines cell survival, invasion, and the expression of key genes in breast cancer signaling pathways. Notably, there have been no previous reports on the combined anticancer activity of these two drugs on cancer cells.
Methods and materials
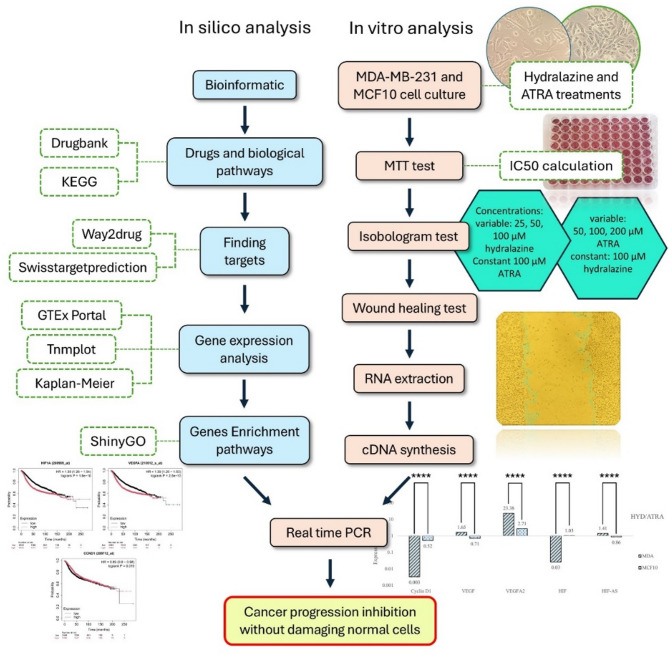
The steps of the study are shown in Fig. 1.
Fig. 1.
Schematic representation of the methods used in the present study
Drugs and biological pathways
The https://go.drugbank.com/ database provides detailed information about drugs and their targets and serves as a valuable resource for pharmaceutical research. It analyzes precise drug information, structures, interactions, mechanisms, clinical data, and molecular targets [7]. For bioinformatics analyses, hydralazine and ATRA were first examined using DrugBank. The results were then further analyzed using the KEGG database at https://www.kegg.jp/.
Targets
https://www.way2drug.com/passonline/ and http://www.swisstargetprediction.ch/ databases were used to estimate possible macromolecular targets of the drugs. This prediction is based on a combination of 2D and 3D similarity with vast libraries of known active substances on proteins of various species [8, 9].
Gene expression analysis
The https://gtexportal.org database, a public resource for studying the regulation of gene expression specific to each tissue and cell, was used to analyze the expression of HIF1A, VEGFA, and CCND1 genes in different tissues, and their expression graphs were plotted. Then, using the https://tnmplot.com database, expression levels of each gene in healthy individuals, primary, and metastatic patients were analyzed [10]. The Kaplan-Meier plotter database at https://kmplot.com/ evaluates the impact of genes on survival using data from GEO, TCGA, and EGA. Patients are classified into two groups with high and low expression of the interested genes, and survival analysis was conducted on data from 4929 patients (all ER statuses and tumor types) [11].
Gene enrichment pathways
Enrichment analyses are basic molecular pathways and functional categories of genes, such as Gene Ontology. ShinyGO is a graphical web-based software at http://bioinformatics.sdstate.edu/go/, that provides insights into the desired genes [12].
Cell culture
The MDA-MB-231 and MCF10 were obtained from Tehran Pasteur Cell Bank and Stem Cell Technology Research Center (STRC), respectively. They were cultured in Dulbecco’s Modified Eagle Medium (DMEM) (Manufactured by Gibco, USA) supplemented with 10% fetal bovine serum (Gibco, USA), 100 µg/ml of amphotericin B (CIPLA LTD, India), penicillin (Jaber Ebne Hayyan Pharmaceutical, Iran), and streptomycin (Nitin Lifescience, India). They were maintained in an incubator with 5% CO2 at a temperature of 36.5 degrees Celsius. The medium in the flask was changed every 72 h.
MTT and isoblogram test
MTT test can measure cell viability and IC50 for the drugs. First, 10,000 cells and 100 µl of the medium are added to each well of the 96-well plate. The plate is placed in an incubator for 24 h for the cells’ adherence. Then, the cells were treated with hydralazine (ADIT lifescience, India), ATRA (Adonis drug, Iran), and a fresh DMEM medium. The treatment was performed with concentrations of 50, 100, 200, and 400 µM of hydralazine and 12.5, 25, 50, and 100 µM of ATRA in a 150 µL volume. The doxorubicin drug (manufactured by EBEWE, Austria) was used as a positive control at a concentration of 2 mg/ml. The cell plate was placed back in the incubator for an initial treatment period of 24 h. Due to the suspected shorter half-lives of hydralazine and ATRA compared to doxorubicin, a second dose was administered at this 24-hour time point. For the second dose, each well was charged with 15 µL of the drugs (except for doxorubicin), and the plate was incubated for another 24 h. After replacing the fresh medium, 15 µL of filtered Thiazolyl blue tetrazolium bromide (Sigma, USA) was added to each well (0.5 mg/ml). This yellow substance enters living cell mitochondria and is converted into purple-colored formazan by the succinate dehydrogenase enzyme. Next, the plate was placed for 4 h in the incubator, and 100 µL of Dimethyl sulfoxide manufactured by Sigma was added to each well, permeabilizing the cell membranes, dissolving the formazan, and causing the purple color to spread in the wells. The presence of more living cells leads to increased production of formazan, resulting in a deeper purple color in the wells. Finally, the optical density of the color was read by the ELx808 ELISA reader (BioTek, USA) at wavelengths of 570 and 630 nm. The IC50 of the drugs was then determined using the ED50 plus plugin in Excel. Isobologram test.
For the isoblogram test, 10,000 cells were placed in 100 µL of medium in each well. After 24 h of incubation, variable concentrations of 25, 50, and 100 µM of hydralazine combined with a constant concentration of 100 µM of ATRA, and variable concentrations of 50, 100, and 200 µM of ATRA combined with a constant concentration of 100 µM of hydralazine, were added in 150 µL of the new medium. Doxorubicin treatment was used as a positive control. After 24 h of incubation, the drugs (except for doxorubicin) were recharged, and the plate was incubated for an additional 24 h. Finally, as mentioned in the MTT test, the color intensity of each well was read using an ELISA reader.
Wound healing test
This test can simulate the cellular migration in the body using cell-cell connections to heal the wound. Initially, 100,000 cells were added to each well of a 24-well plate using TRED and symmetrically spread. After 24 h of incubation and observing a cell density of about 80%, a direct scratch was made in each well’s middle using the yellow pipette tip. Next, the fresh culture medium with selected concentrations of each drug (100 µM of hydralazine and 100 µM of ATRA) and their combination was treated with the cells. Photos were taken of the wound area at 0, 24, and 48 h of incubation. Finally, the image analysis was performed using the ImageJ software (wound healing plugin), and the statistical analysis was performed using Prism software (version 8.0).
Gene expression analysis using Real-time PCR
Several primers were designed for five key genes of CCND1, VEGFA, VEGFA2, HIF1A, and HIF1A-AS that play roles in various breast cancer pathways. The VEGFA gene has several mRNA isoforms, and in this study, transcription variants 2 and 7 were selected. They differ primarily in their structure and potentially their function, but they are both involved in the angiogenesis mechanism. Variant 2, lacking exons 6 and 7, is a well-studied isoform crucial for angiogenesis. In contrast, variant 7, with a unique structural arrangement, has limited research. To design specific primers, the https://www.ncbi.nlm.nih.gov/nucleotide database was used. The exons and introns of mRNA were examined for perfect sections, and using the Primer-BLAST software, several primers were designed for each gene. All forward and reverse primers were designed to be separated by at least one intron, ensuring the specific amplification of mRNA (Table 1). Primers were obtained from SinaClone Company (Tehran, Iran), and prepared according to the provided instructions. All forward and reverse primer concentrations were standardized using 1X TE buffer, so all primers have the same concentration.
Table 1.
Primers used in the Real-Time PCR reaction, along with their sequences
| Genes | Accession numbers | Sequences | Products |
|---|---|---|---|
|
VEGF 165b isoform (long form isoform g) |
NM_001033756 | CGCAGACGTGTAAATGTTCCTG (F) | 97 bp |
| (R) TTCCTGGTGAGAGATCTGCAAG | |||
|
VEGF Isoform 20 (long form isoform b) |
NM_003376.6 | TCCTGGAGCGTTCCCTGT(F) | 160 bp |
| (R) GCCTCGGCTTGTCACATCT | |||
| HIF1-α | NM_001530.4 | GCAGCAACGACACAGAAAC (F) | 166 bp |
| (R) GCAGGGTCAGCACTACTTC | |||
| HIF1α -AS1 | NR_047116.1 | CACACGCGGAGAAGAGAAG (F) | 177 bp |
| (R) GGTTGGGGTACTGGAAGCA | |||
| GAPDH | NM_0012 | GAGTCAACGGATTTGGTCG (F) | 149 bp |
| (R) GAATTTGCCATGGGTGGA | |||
| CCND1 | NM_053056.3 | CGCTTTGTCTGTCGTGAT (F) | 197 bp |
| (R) TACATGTTGGTGCTGGGAA |
A 6-well plate, TRED, and specific solutions in the ParsTous kit (Iran) were used to extract the total RNA (rRNA, tRNA, and mRNA) of the designed treatments (ATRA, hydralazine, and their combinations) in both cell lines. Next, the DNase enzyme was used to remove potential genomic DNA, and then, it was inactivated by EDTA (manufactured by ASD, Iran). The amount of extracted RNA was measured using a Nanodrop device (Montreal Biotech, Canada) at wavelengths of 260 to 280 nm. A value in the range of 2 to 2.2 is acceptable. Then, electrophoresis on an agarose gel was done to ensure the quality of the RNA. Extracted RNA was used to synthesize cDNA using the reverse transcriptase enzyme in the ParsTous kit (Iran). Using the Rotor-Gene 3000 (Corbett, Australia), expression levels of HIF1A, HIF1A-AS, VEGFA, VEGFA2, and CCND1 genes were measured. The GAPDH gene was considered a housekeeping reference for data normalization. To determine the thresholds, the LinReg software (v. 2021.2) was used. Finally, all results were statistically analyzed using Excel (Office 16) and Prism software with the ANOVA test, and P-values less than 0.05 were considered significant.
Results
Biological pathways of hydralazine
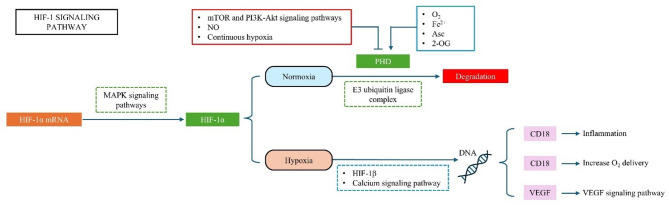
The bioinformatics analysis showed the effects of hydralazine on both healthy and cancerous cells through the HIF-1 and VEGF pathways (Fig. 2). HIFs (Hypoxia-inducible factors) can regulate epithelial-mesenchymal transition, angiogenesis, and metabolic adaptations in lung and breast cancers. They consist of three isoforms, HIF-1, HIF-2, and HIF-3 [13]. This pathway suggests that hydralazine can increase tumor angiogenesis by increasing VEGF gene expression through its effects on HIF-1 and prolyl hydroxylase domain (PHD).
Fig. 2.
The HIF-1 pathway in the KEGG database responses to cellular oxygen levels. In normoxic conditions, PHD enzymes use 2-oxoglutarate, ascorbate, iron, and oxygen to hydroxylate Pro402 and Pro564 residues in the oxygen-dependent degradation domain of the HIF-1α subunit. In hypoxic conditions, deficiency in co-factors or an increase in nitric oxide and reactive oxygen species leads to a reduction in the hydroxylation and degradation of HIF-1α. So, it will be stabilized and translocated to the nucleus, where it dimerizes with HIF-1β. This complex binds to hypoxia response elements in the promoter of the VEGF gene and causes angiogenesis. (2-oxoglutarate (2OG), ascorbate (Asc), iron (Fe2+), oxygen (O2), nitric oxide (NO), and reactive oxygen species (ROS))
Biological pathways of ATRA
The results of KEGG and Drugbank databases showed that ATRA can affect both healthy and cancerous cells through the regulation of the cell cycle, VEGF, and Wnt/β-catenin pathways. In the ATRA-RAR signaling pathway, ATRA binds to retinoic acid receptors (RARs) and retinoid X receptors (RXRs) in the cell nucleus, activating them. These activated complexes reduce VEGF levels, leading to less blood vessel formation, tumor growth, and cell proliferation. Additionally, ATRA lowers the expression of CCND1, a Wnt/β-catenin target, causing cell cycle arrest and reduced proliferation. It encourages pathway inhibitors to bind to Wnt ligands or Fz receptors, reducing pathway activation. It highlights the diverse roles of ATRA in controlling cell cycle progression, signaling pathways, and cellular differentiation (Fig. 3).
Fig. 3.
Functional pathways of ATRA in the human body. (A) ATRA in the cell cycle pathway. (B) ATRA-RAR signaling pathway in breast cancer. (C) ATRA suppresses the expression of CCND1 as a Wnt/β-catenin target, leading to cell cycle arrest and reduced proliferation
ATRA halts the cell cycle in breast cancer cells by increasing the expression of cyclin-dependent kinase (CDK) inhibitors such as p21 and p27, which reduce CDK activity and prevent cell cycle progression in breast cancer [14] (Fig. 3 Section A).
In the ATRA-RAR signaling pathway, ATRA binds to RARs and RXRs in the cell nuclei, activating them. These activated complexes decrease VEGF expression, leading to reduced angiogenesis, tumor growth, and proliferation [15] (Fig. 3 Section B).
Besides, ATRA suppresses the expression of CCND1, a Wnt/β-catenin target, leading to cell cycle arrest and reduced proliferation. The dysregulation of the Wnt/β-catenin signaling pathway leads to tumor growth, invasion, and metastasis [16] (Fig. 3 Section C). This figure illustrates the multifaceted roles of ATRA in regulating cell cycle progression, signaling pathways, and cellular differentiation, highlighting its potential therapeutic effects in breast cancer treatment.
Drug targets
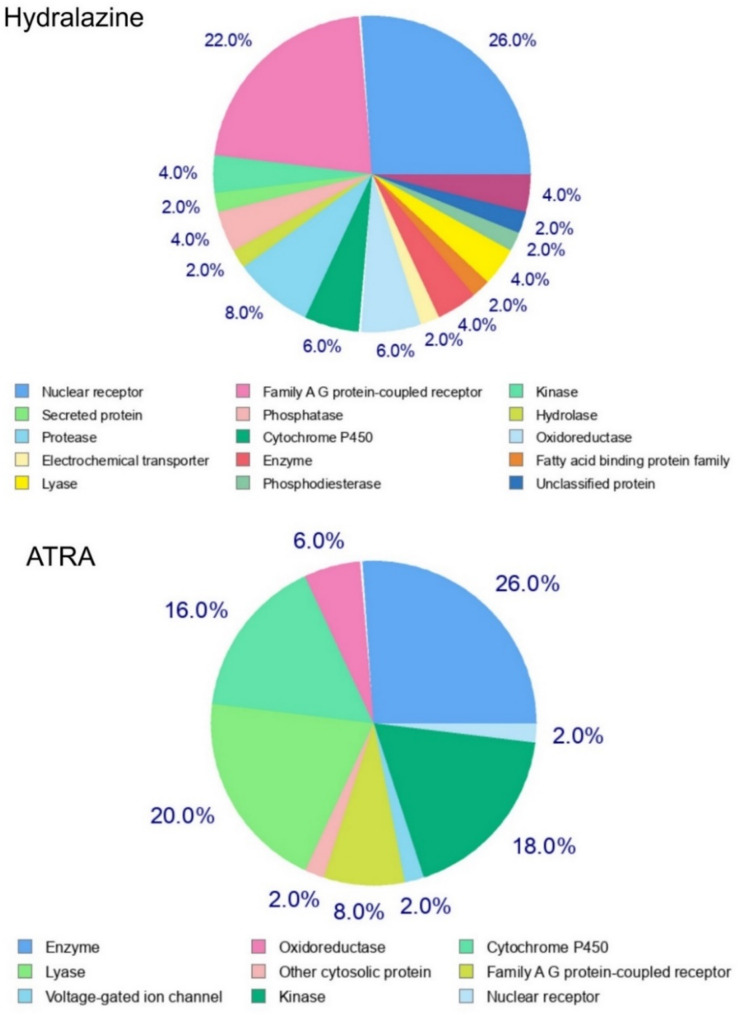
Molecular targets of the drug obtained from the way2drug database are listed in Table 2. The target classification chart from SwissTargetPrediction is shown in Fig. 4. The results indicate that these drugs have various targets in the body, so they can affect different molecules and pathways. Therefore, by combining these two drugs, different mechanisms impact cancer cells.
Table 2.
Direct and indirect targets of hydralazine and ATRA, along with the probability of binding for each drug
| Drugs | Interaction | Target names | Confidence |
|---|---|---|---|
| Hydralazine | Direct | Tyrosine-protein kinase receptor FLT3 | 0.8759 |
| Cell division cycle 2-like protein kinase 6 | 0.6967 | ||
| Mediated | Cytochrome P450 3A4 | 0.6882 | |
| Nuclear receptor subfamily 0 group B member 1 | 0.4936 | ||
| ATRA | Direct | Retinoic acid receptor | 0.7789 |
| Cytochrome P450 2C9 | 0.5903 | ||
| Mediated | Solute carrier organic anion transporter family member 1B3 | 0.5228 | |
| Nuclear receptor subfamily 0 group B member 1 | 0.5204 |
Fig. 4.
Target categorization of hydralazine and ATRA
Gene expression analysis
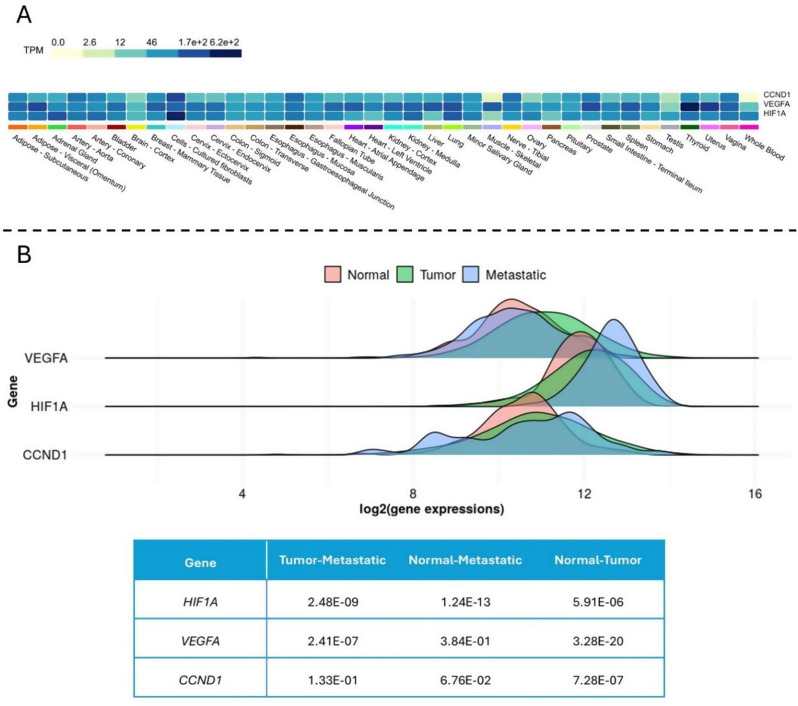
The results of gene expression analysis obtained from the GTEX and TNMplot databases show that these genes are suitable for the study (Fig. 5).
Fig. 5.
Gene expression analysis in healthy and cancerous conditions. (A) Gene expression Heat map in normal tissues obtained from GTEX, which indicates that all three genes have high expression levels in breast tissue. (B) The gene expression graphs in three states of healthy, primary tumor, and metastatic, along with the p-value of the graphs indicating their significance
Patients survival
The survival rate of patients using the Kaplan-Meier plotter database showed that the aberrant expression of HIF1A, VEGFA, and CCND1 genes was associated with reduced survival in breast cancer patients (Fig. 6).
Fig. 6.
Kaplan-Meier plots showing patients’ survival differences with gene expression changes due to mutations, including statistical analysis for survival time
Enrichment pathways
ShinyGO enrichment pathways analysis for the genes of interest is listed in Table 3. Results show their involvement in various types of cancers.
Table 3.
Biological pathways of HIF1A, VEGFA, and CCND1 genes that are ordered from smallest to largest enrichment FDR. This analysis indicates the importance of these genes in various disease pathways and cancer types
| Pathways | Enrichment FDR | nGenes | Genes in the Pathway |
|---|---|---|---|
| Bladder cancer | 2.0E-04 | 2 | 41 |
| Pathways in cancer | 2.0E-04 | 3 | 530 |
| Kaposi sarcoma-associated herpesvirus infection | 2.2E-05 | 3 | 194 |
| Proteoglycans in cancer | 2.2E-05 | 3 | 202 |
| Renal cell carcinoma | 3.4E-04 | 2 | 68 |
| Pancreatic cancer | 3.6E-04 | 2 | 76 |
| AGE-RAGE signaling pathway in diabetic complications | 5.3E-04 | 2 | 100 |
| HIF-1 signaling pathway | 5.5E-04 | 2 | 109 |
| Thyroid hormone signaling pathway | 6.1E-04 | 2 | 121 |
| MicroRNAs in cancer | 9.7E-04 | 2 | 161 |
MTT method
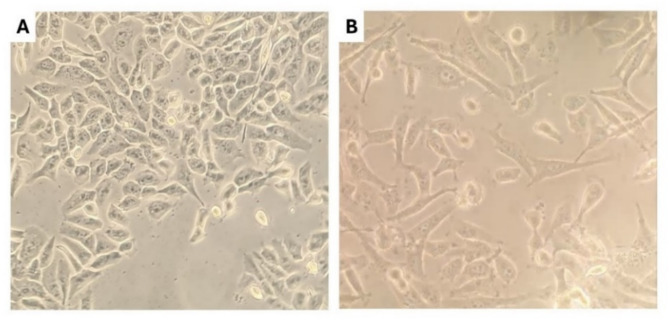
After the cell culture steps (Fig. 7), the MTT test results were obtained. The optical density read by the ELISA reader is shown in Fig. 8. The IC50 for ATRA in the MDA-MB-231 cells was about 130 µM, and in the MCF10 cells, it was 456 µM. The IC50 for hydralazine could not be calculated due to its stimulatory effect on cell growth; therefore, a concentration of 100 µM of this drug was considered for the final combined drug formulation. These results show decreased survival in ATRA-treated cells and increased survival in hydralazine-treated cells by dose. At similar concentrations, MCF10 cells’ survival in ATRA treatment was higher compared to MDA-MB-231 cells, indicating the higher cytotoxicity of this drug on malignant tumor cells. Hydralazine can stimulate breast cancer cell growth in both tumorigenic and non-tumorigenic states, but its effect on MCF10 cells was greater at similar concentrations.
Fig. 7.
Morphology of breast cell lines. (A) Non-tumorigenic, island-like MCF10 cells, which are epithelial-like and tend to aggregate in the flask. (B) Tumorigenic MDA-MB-231 cells, which are spindle-shaped and more dispersed in the flask
Fig. 8.
MTT test results show the cytotoxicity of ATRA and the growth stimulation of hydralazine in both cell lines with concentration increase. (A) MDA-MB-231 cells. (B) MCF10 cells
Isobologram test
The results obtained from the reduction of formazan crystals after 48 h are shown in Fig. 9. A constant concentration of 100 µM of hydralazine and a variable concentration of 200 µM of ATRA were used solely to ensure the reducing effect on cell viability in case of increased concentration.
Fig. 9.
The results of the isobologram test in both cell lines show cell viability in treatments with selected constant concentration of 100 µM for hydralazine, and a variable concentration close to the IC50 of ATRA at 100 µM has a very strong inhibitory effect similar to the control drug doxorubicin. By increasing the concentration of ATRA in the combination, viability decreases. The different effects of this combined treatment on breast cells in MDA-MB-231 and MCF10 are significant (P-values < 0.05), indicating the specific impact of this combination on tumor cells (unlike doxorubicin). (A) Treatment in MDA-MB-231 cells. (B) Treatment in MCF10 cells
Wound healing test
Statistical analysis graphs for both cell lines are shown in Fig. 10. MDA-MB-231 control cells were not treated with any drug, and after 48 h, the gap was almost filled. In the doxorubicin treatment, the morphology of the cells changed, and cell migration was completely inhibited. Treatment with hydralazine alone showed that the cell migration was the same as the control cells. ATRA treatment changed cell morphology and decreased the invasion rate of cells compared to the control. The hydralazine and ATRA combination had a greater impact on preventing cell migration in this line compared to the individual drugs. This combination reduced the wound closure rate in 24 h, and after 48 h, in addition to the reduced rate, the cells were apoptosis, and the wound even expanded (Fig. 11).
Fig. 10.
Wound healing graphs show a decrease in migration rate in the combined drug treatment (P-values < 0.05 are significant). (A) Results in MDA-MB-231 cells. (B) Results in MCF10 cells. After 48 h following treatment with doxorubicin, the cells were so damaged that the scratch completely disappeared, and calculation with the ImageJ software was not possible; however, to identify the result significance, a value of 200 was assumed
Fig. 11.
Results of the wound healing test in MDA-MB-231 cells, showing the effectiveness of the ATRA/hydralazine combination on the cells’ migration
MCF10 control cells were not treated with any drug, and after 48 h, the gap was almost closed. Doxorubicin Treatment showed complete inhibition of cell migration, apoptosis, and morphological changes in these non-tumor cells in a way that analysis with ImageJ software was not possible in the images taken 48 h later. The treatment with hydralazine alone showed cell migration as the control cells. Treatment with ATRA indicated complete inhibition of cell migration, degradation, destruction, and morphological changes in this cell line. The hydralazine and ATRA combination did not show significant effects on cell migration compared to the individual drugs, indicating the non-cytotoxicity and lack of inhibition of this combination on these healthy breast tissue cells (Fig. 12).
Fig. 12.
Results of the wound healing test in MCF10 cells show the combination’s lesser impact compared to doxorubicin
Gene expression analysis by real-time PCR
According to Fig. 13, the expression of each gene was directly examined in both cell lines. Using the Tukey test, the significance comparison of the graphs for each comparison was measured. All of the p-values were < 0.0001.
Fig. 13.
Comparative analysis of RT-PCR. The combination of ATRA and hydralazine can be selected as the most effective treatment with the greatest effect on MDA-MB-231 cells and the least effect on the MCF10 cells. This effect appears on key genes related to cell cycle regulation (CCND1), angiogenesis (VEGFA, VEGFA2), and hypoxia adaptation (HIF1A, HIF1A-AS). The reduction rate is generally higher in MDA-MB-231 cells, indicating a potentially greater therapeutic efficacy of this compound in breast cancer cells compared to non-tumor cells
In the MDA-MB-231 cell line, all three treatments (Hydralazine, ATRA, and hydralazine/ATRA) significantly reduce the expression of CCND1, with ATRA showing the greatest reduction. In MCF10 cells, CCND1 expression is also reduced by all three treatments. Hydralazine significantly reduces the expression of this gene in these cells, which can have negative effects on the body. The reduction in MCF10 is much less affected compared to MDA-MB-231.
Hydralazine significantly increased the expression of VEGFA in MDA-MB-231 cells, but it decreased in MCF10 cells. Treatment with ATRA has shown little effect on this gene, and few changes were observed in the combined treatment. The harmful effects of hydralazine are deactivated when it is combined with ATRA, so angiogenesis is inhibited.
The VEGFA2 gene expression is similar in all three treatments. In all treatments, MDA-MB-231 shows a significant increase compared to the control sample, while a slight increase is observed in the MCF10 cell line. The increased expression of this gene is more significant in MDA-MB-231 cells compared to MCF10 cells.
In MDA-MB-231 cells, HIF1A expression is reduced by the combination drug and slightly by ATRA, while hydralazine did not affect these cells alone. In MCF10 cells, hydralazine reduced HIF1A expression, affecting normal body cells under hypoxic conditions. ATRA and the combination treatment were ineffective on this gene’s expression in these cells. The negative effects of hydralazine were eliminated when combined with ATRA.
In MDA-MB-231 cells, ATRA and hydralazine significantly reduced the expression of HIF1A-AS. However, in the combined treatment, the expression of this gene slightly increased. The negative effects of hydralazine were eliminated in combination with ATRA. In MCF10 cells, none of the treatments showed any significant effect on HIF1A-AS expression.
Discussion
Breast cancer is a highly complex and heterogeneous disease, characterized by diverse molecular subtypes and intricate signaling networks that drive tumor progression and therapeutic resistance. While significant advancements have been made with single-agent targeted therapies and chemotherapy, the development of acquired resistance and the presence of intrinsic resistance mechanisms often limit their long-term efficacy. Consequently, combination therapy, which aims to simultaneously target multiple pathways or overcome resistance mechanisms, represents a crucial strategy to improve treatment outcomes and enhance durable responses in breast cancer patients.
Previous studies have shown that combining hydralazine and valproic acid can synergistically cause significant DNA damage, cell cycle arrest, and apoptosis in breast cancer cell lines [17]. Additionally, the combination of hydralazine, valproic acid, and the chemotherapy agent cisplatin has shown synergistic cytotoxic results for cervical cancer treatment [18]. The combination of hydralazine and decitabine (a demethylating agent) can reactivate silenced tumor suppressor genes through epigenetic modulation, leading to increased apoptosis in colorectal cancer cells [19].
A combination of ATRA and vorinostat (a histone deacetylase inhibitor) showed synergistic effects in inducing cellular differentiation and apoptosis in breast cancer cells [20]. A study on the treatment of acute promyelocytic leukemia was conducted using a combination of ATRA and arsenic trioxide for newly diagnosed patients, which increased remission and survival [21]. In a phase II clinical trial, adding ATRA to paclitaxel and cisplatin improved response and long-term survival in patients with advanced non-small cell lung cancer [22].
This present study is in line with previous research and uses bioinformatics studies, MDA-MB-231, and MCF10 cell culture to analyze the in vitro behavior of these cells, so it provides a much up-to-date and valid analysis of breast cancer. MDA-MB-231 cells are considered cancer cells, while MCF10 cells are considered normal body cells. While specialized media are often recommended for normal breast epithelial cells, our use of DMEM supplemented with 10% FBS is supported by previous work demonstrating successful maintenance of normal breast epithelial morphology and function in this medium, particularly for short-term experiments [23].
Based on the bioinformatics results, hydralazine and ATRA have a high potential for combination therapy due to their multiple molecular targets. Also, HIF1A, VEGFA, and CCND1 are key genes in breast cancer progression, and their expression dysregulation in breast tissue is associated with cancer. Additionally, the survival results indicate that this dysregulation reduces patient survival, which is useful in clinical decisions and designing personalized treatment plans. Enrichment pathways analysis indicates the genes’ high activity in various biological pathways and cancers. By understanding the functions of these pathways, the precise role of the genes in biological processes can be clarified.
Hypoxia in solid tumors leads to increased angiogenesis, metastasis, and death. Hydralazine induces HIF-1 and VEGF signaling by inhibiting PHD activity, stabilizing HIF-1α, and activating VEGF, which promotes tumor angiogenesis and cancer progression [24].
The MTT test shows ATRA has a high cytotoxic effect on tumor cells (IC50 of 130 µM) compared to non-tumor cells (IC50 of 456 µM), making it a potential breast cancer treatment but harmful to normal cells. Hydralazine increases cell growth without causing apoptosis and may promote angiogenesis and metastasis through the HIF-1 pathway, requiring further studies. Using the isobologram test, various combination of hydralazine/ATRA includes a consistent and variable concentration of the drugs treated cultured cells. The results of the treatment-viability graph indicate significant apoptosis in MDA-MB-231 compared to MCF10 cells, suggesting a specific induction of apoptosis in malignant tumor cells. Therefore, this therapeutic combination reduces required dosage, drug resistance, and side effects while increasing treatment efficacy due to its lack of impact on non-cancerous cells.
In wound healing results, hydralazine and ATRA alone cannot reduce cell migration in MDA-MB-231 cells, and even hydralazine increases cancer cell numbers on the plate and closes the scratch. While the hydralazine/ATRA combination reduces cell migration significantly, the reduction is the same as with doxorubicin. In MCF10 cells, ATRA and doxorubicin not only inhibited cell migration but also had significant cytotoxic effects. However, the combination did not show cytotoxicity and only stopped migration. So, the hydralazine/ATRA combination can inhibit the cancer cells’ metastasis without damaging normal cells.
Gene expression findings show that ATRA and the combination inhibit cancer cell proliferation by regulating CCND1. Hydralazine affects the cell cycle and angiogenesis differently in normal and cancer cells. Combined treatment reduces HIF1A expression in cancer cells, limiting their adaptation to hypoxia, and has the best effect in inhibiting cancer cells with minimal impact on normal cells. These findings suggest that hydralazine may inhibit critical pathways for normal cell survival and create conditions for tumor growth. Therefore, using this drug for blood pressure reduction in patients may induce breast cancer progression pathways and damage healthy tissue. On the other hand, ATRA alone reduces the cell cycle progression in MDA-MB-231 cells, but it cannot limit tumor adaptation under hypoxic conditions. It can also stimulate hypoxic conditions by affecting the anti-sense gene. However, by combining these two drugs, the negative effects of each treatment alone are eliminated, and a synergistic effect on specific genes is observed. This combination has shown potential for inhibiting cell proliferation, angiogenesis, and hypoxia adaptation while showing significantly fewer side effects, making it an effective therapeutic option for the treatment and inhibition of breast cancer.
While this study demonstrates the therapeutic potential of hydralazine and ATRA co-administration in breast cancer models, some limitations warrant acknowledgment. First, our findings rely exclusively on in vitro analyses using MCF-7 and MDA-MB-231 cell lines, which may not fully recapitulate the complexity of human tumor microenvironments or interpatient heterogeneity. Second, the effective concentrations of hydralazine (5–20 µM) and ATRA (10–40 µM) in cellular assays require rigorous pharmacokinetic validation to assess translational feasibility in vivo. Third, comprehensive multi-omics profiling (e.g., single-cell RNA-seq) would better resolve subtype-specific drug responses. Finally, combination efficacy was benchmarked against monotherapies but not compared to standard clinical regimens (e.g., paclitaxel or doxorubicin), necessitating future comparative studies.
Conclusion
The combination of hydralazine and ATRA effectively reduces cancer cell migration and metastasis without affecting normal cells. This treatment also inhibits cancer cell proliferation by regulating CCND1 and reducing HIF1A expression, limiting cancer cells’ adaptation to hypoxia. Overall, the hydralazine/ATRA combination shows promise in targeting breast cancer cells while minimizing impact on normal cells. Further in vivo studies are needed to understand the molecular mechanisms involved. These findings suggest the potential for new treatments with fewer side effects compared to current therapies.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This research was supported by the Graduate University of Advanced Technology, Kerman, Iran.
Abbreviations
- DOXO
Doxorubicin
- ATRA
All-trans retinoic acid
- STRC
Stem Cell Technology Research Center
- DMEM
Dulbecco's Modified Eagle Medium
- PHD
Prolyl hydroxylase domain
- RARs
Retinoic acid receptors
- RXRs
retinoid X receptors
- CDK
Cyclin-dependent kinase
Author contributions
Mohammad mehdi Yaghoobi contributed to design and implementation of the research; Amirhesan Yahyapour and Nahid Askari participated in the collection of data and contributed substantially to the drafting, writing and revising of the manuscript; all authors have approved the final version of the manuscript.
Funding
This study was not funded by any specific grants from public, commercial, or non-profit funding organizations.
Data availability
All data essential to replicate this study are included in this article and its supplementary material. Additional raw data are available from the corresponding author upon request.
Declarations
Ethics approval and consent to participate
Ethical approval (Code: 02.83760; Date: 12 January 2024) was granted by the Ethics Committee of KGUT’s Institute of Sciences and High Technology and Environmental Sciences, confirming adherence to institutional ethical standards for research.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Victoir B, Croix C, Gouilleux F, Prié G. Targeted therapeutic strategies for the treatment of Cancer. Cancers. 2024;16(2):461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vafopoulou P, Kourti M. Anti-angiogenic drugs in cancer therapeutics: A review of the latest preclinical and clinical studies of anti-angiogenic agents with anticancer potential. J Cancer Metastasis Treat. 2022;8:18. [Google Scholar]
- 3.Mohammad N, Vikram Singh S, Malvi P, Chaube B, Athavale D, Vanuopadath M, et al. Strategy to enhance efficacy of doxorubicin in solid tumor cells by methyl-β-cyclodextrin: involvement of p53 and Fas receptor ligand complex. Sci Rep. 2015;5(1):11853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Romão IC, Siqueira SMC, Silva Abreu, FOMd. Santos hsd. Hydralazine and hydrazine derivatives: properties, applications, and repositioning potential. Chemistry & Biodiversity; 2024. n/a(n/a):e202401561. [DOI] [PubMed]
- 5.Jin Y, Teh SS, Lau HLN, Xiao J, Mah SH. Retinoids as anti-cancer agents and their mechanisms of action. Am J Cancer Res. 2022;12(3):938. [PMC free article] [PubMed] [Google Scholar]
- 6.Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J Clin. 2024;74(3):229–63. [DOI] [PubMed] [Google Scholar]
- 7.Knox C, Wilson M, Klinger CM, Franklin M, Oler E, Wilson A, et al. DrugBank 6.0: the drugbank knowledgebase for 2024. Nucleic Acids Res. 2024;52(D1):D1265–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daina A, Michielin O, Zoete V. SwissTargetPrediction: updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 2019;47(W1):W357–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Filimonov DA, Lagunin AA, Gloriozova TA, Rudik AV, Druzhilovskii DS, Pogodin PV, et al. Prediction of the biological activity spectra of organic compounds using the pass online web resource. Chem Heterocycl Compd. 2014;50(3):444–57. [Google Scholar]
- 10.Bartha Á, Győrffy B. TNMplot.com: A web tool for the comparison of gene expression in normal, tumor and metastatic tissues. Int J Mol Sci. 2021;22(5):2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Győrffy B. Integrated analysis of public datasets for the discovery and validation of survival-associated genes in solid tumors. Innov. 2024;5(3). [DOI] [PMC free article] [PubMed]
- 12.Ge SX, Jung D, Yao R. ShinyGO: a graphical gene-set enrichment tool for animals and plants. Bioinformatics. 2019;36(8):2628–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qannita RA, Alalami AI, Harb AA, Aleidi SM, Taneera J, Abu-Gharbieh E, et al. Targeting hypoxia-inducible Factor-1 (HIF-1) in cancer: emerging therapeutic strategies and pathway regulation. Pharmaceuticals. 2024;17(2):195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Álvarez-Fernández M, Malumbres M. Mechanisms of sensitivity and resistance to CDK4/6 Inhibition. Cancer Cell. 2020;37(4):514–29. [DOI] [PubMed] [Google Scholar]
- 15.Hamad M, Mehana RA, Abd-Al haseeb MM, Houssen M. Potential antitumour effect of all-trans retinoic acid on regorafenib-treated human colon cancer cell lines. Contemp Oncology/Współczesna Onkologia. 2023;27(3):198–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xi H-M, Lu H, Weng X-Q, Sheng Y, Wu J, Li L et al. Combined application of salinomycin and ATRA induces apoptosis and differentiation of acute myeloid leukemia cells by inhibiting WNT/β-Catenin pathway. Anti-Cancer agents in medicinal chemistry (Formerly current medicinal chemistry-Anti-Cancer agents). 2023;23(9):1074–84. [DOI] [PubMed]
- 17.Arce C, Perez-Plasencia C, Gonzalez-Fierro A, de la Cruz-Hernandez E, Revilla-Vazquez A, Chavez-Blanco A, et al. A proof-of-principle study of epigenetic therapy with hydralazine and magnesium valproate plus doxorubicin cyclophosphamide as neoadjuvant therapy for locally advanced breast cancer. BMC Cancer. 2007;7(1):A23. [Google Scholar]
- 18.Coronel J, Cetina L, Pacheco I, Trejo-Becerril C, González-Fierro A, de la Cruz-Hernandez E, et al. A double-blind, placebo-controlled, randomized phase III trial of chemotherapy plus epigenetic therapy with hydralazine valproate for advanced cervical cancer. Preliminary results. Med Oncol. 2011;28(1):540–6. [DOI] [PubMed] [Google Scholar]
- 19.Zambrano P, Segura-Pacheco B, Perez-Cardenas E, Cetina L, Revilla-Vazquez A, Taja-Chayeb L, et al. A phase I study of hydralazine to demethylate and reactivate the expression of tumor suppressor genes. BMC Cancer. 2005;5(1):44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Munster PN, Troso-Sandoval T, Rosen N, Rifkind R, Marks PA, Richon VM. The histone deacetylase inhibitor suberoylanilide hydroxamic acid induces differentiation of human breast cancer cells. Cancer Res. 2001;61(23):8492–7. [PubMed] [Google Scholar]
- 21.Lo-Coco F, Avvisati G, Vignetti M, Thiede C, Orlando SM, Iacobelli S, et al. Retinoic acid and arsenic trioxide for acute promyelocytic leukemia. N Engl J Med. 2013;369(2):111–21. [DOI] [PubMed] [Google Scholar]
- 22.Arrieta O, González-De la Rosa CH, Aréchaga-Ocampo E, Villanueva-Rodríguez G, Cerón-Lizárraga TL, Martínez-Barrera L, et al. Randomized phase II trial of all-trans-retinoic acid with chemotherapy based on Paclitaxel and cisplatin as first-line treatment in patients with advanced non–small-cell lung cancer. J Clin Oncol. 2010;28(21):3463–71. [DOI] [PubMed] [Google Scholar]
- 23.Petersen OW, Rønnov-Jessen L, Howlett AR, Bissell MJ. Interaction with basement membrane serves to rapidly distinguish growth and differentiation pattern of normal and malignant human breast epithelial cells. Proceedings of the National Academy of Sciences. 1992;89(19):9064-8. [DOI] [PMC free article] [PubMed]
- 24.Mehrabani M, Nematollahi MH, Tarzi ME, Juybari KB, Abolhassani M, Sharifi AM, et al. Protective effect of hydralazine on a cellular model of parkinson’s disease: a possible role of hypoxia-inducible factor (HIF)-1α. Biochem Cell Biol. 2020;98(3):405–14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data essential to replicate this study are included in this article and its supplementary material. Additional raw data are available from the corresponding author upon request.