Abstract
Antibiotic resistance is a threat to human health, yet recent work highlights how loss of resistance may drive pathogenesis in some bacteria. In two recent studies, we found that β-lactam antibiotics and nutrient stresses faced during infection selected for genetic inactivation of the Pseudomonas aeruginosa antibiotic efflux pump mexEFoprN. Unexpectedly, efflux pump mutations increased P. aeruginosa virulence during infection; however, neither the prevalence of mexEFoprN inactivating mutations in real human infections, nor the mechanisms driving increased virulence of efflux pump mutants are known. We hypothesized that human infection would select for virulence enhancing mutations. Using genome sequencing of clinical isolates, we show that mexEFoprN efflux pump inactivating mutations are enriched in P. aeruginosa isolates from cystic fibrosis infections relative to isolates from acute respiratory infections. Combining RNA-seq, metabolomics, genetic approaches, and infection models we show that efflux pump mutants have elevated quorum sensing driven expression of elastase and rhamnolipids which increase P. aeruginosa virulence during acute and chronic infections. Restoration of the efflux pump in a representative respiratory isolate and the notorious cystic fibrosis Liverpool epidemic strain reduced their virulence. These findings suggest that mutations inactivating antibiotic resistance mechanisms could lead to greater patient mortality and morbidity.
Subject terms: Antimicrobial resistance, Pathogens, Bacteriology, Molecular evolution
Antibiotics and nutritional stresses can inactivate Pseudomonas aeruginosa efflux pump mexEFoprN. Here, the authors demonstrate that the inactivation of this efflux pump impacts quorum sensing mediated virulence in P. aeruginosa clinical isolates.
Introduction
The evolution of antibiotic resistance during chronic bacterial infections limits treatment options, increases healthcare costs, and is linked to worse outcomes in diseases like cystic fibrosis (CF) and in patients in the intensive care unit (ICU)1–3. However, there is also evidence of possible fitness costs associated with antibiotic resistance. Mutations that inactivate antibiotic resistance mechanisms might initially seem less favorable during bacterial pathogen evolution, but emerging evidence suggests the opposite: loss of some resistance functions can increase bacterial virulence and potentially worsen disease. This phenomenon is exemplified by our recent work showing that inactivating mutations of the Pseudomonas aeruginosa mexEFoprN antibiotic efflux pump operon can increase virulence in an acute lung infection model4,5. Work from our lab and others has linked the occurrence of mexEFoprN inactivating mutations to increased resistance to aztreonam, cefiderocol, and imipenem-relebactam, which are not antibiotics transported by this efflux pump5–7. Despite these alarming data, questions remain regarding the prevalence of such mutations among real-world P. aeruginosa clinical isolates as well as how these mutations function to increase P. aeruginosa pathogenicity.
P. aeruginosa is an opportunistic pathogen that constantly adapts and mutates under antibiotic pressure, making P. aeruginosa infections difficult to treat8. Hence, mechanisms of antibiotic resistance acquisition, like overexpression of resistance/nodulation/division (RND) antimicrobial efflux pumps, have been well studied9. MexEF-OprN is a tripartite P. aeruginosa RND antibiotic efflux pump comprised of MexE, MexF, and OprN. The pump extrudes ciprofloxacin, quinolones, and chloramphenicol, and inactivation of any component of the pump abrogates its efflux function. In the clinic, P. aeruginosa isolates with activating mutations in the MexEF-OprN transcriptional activator, mexT10, or inactivating mutations in its transcriptional repressor mexS11 have been identified. These strains have historically been named nfxC mutants due to their norfloxacin resistance and have been used in several studies to characterize the functions of MexEF-OprN12–14.
In addition to increasing antibiotic resistance via overexpression of MexEF-OprN, P. aeruginosa nfxC mutants have been associated with altered quorum sensing (QS) phenotypes. Since many P. aeruginosa virulence factors are QS-regulated15, the effect of MexEF-OprN on QS is expected to have consequences on P. aeruginosa pathogenesis. One study demonstrated increased extracellular levels of the 3-oxo-C12-Homoserine Lactone (HSL) QS signaling molecule in stationary phase nfxC mutants, suggesting that 3-oxo-C12-HSL was a substrate of the efflux pump16. The 3-oxo-C12-HSL QS signal activates expression of the LasR-LasI QS system and its dependent virulence genes, like lasB, which encodes elastase. Thus, the nfxC mutant had reduced activation of LasR-LasI and lower lasB expression16. However, other studies report increased levels of Pseudomonas Quinolone Signal (PQS) precursors HHQ17 or kynurenine18 in the supernatants of nfxC mutants, suggesting that MexEF-OprN overexpression reduces the biosynthesis of the PQS QS signal by pumping out one or more of its precursors. Consequently, this could reduce virulence of nfxC mutants because PQS activates biosynthesis of an arsenal of P. aeruginosa pathogenic factors like elastase, rhamnolipid, pyocyanin, and hydrogen cyanide19,20. It is, however, worth noting that mexT, which is mutated in nfxC strains, can regulate the expression of other P. aeruginosa genes21, making it difficult to conclude if the reported effects on QS in nfxC mutants can be solely attributed to increased MexEF-OprN expression. Based on these conflicting findings relating nfxC mutants to altered QS phenotypes, questions remain: how do mexEFoprN inactivating mutations affect QS? And do mexEFoprN inactivating mutations increase P. aeruginosa virulence in the host?
P. aeruginosa is a major cause of acute respiratory infections in patients in the ICU2,22 and chronic infections in people with CF (pwCF)23, and β-lactam antibiotics are the most widely used antibiotics to treat these infections24,25. Several studies have linked β-lactam antibiotic exposure and treatment to mexEFoprN inactivating mutations and increased virulence phenotypes. We have shown that either chronic aztreonam exposure or selection in synthetic CF sputum medium with or without the antibiotic cefiderocol can select for inactivating mutations in mexEFoprN during laboratory experimental evolution5,6. Several other reports suggest that mexEFoprN inactivating mutations are also selected in vivo. In P. aeruginosa clinical isolates, the increased expression of the β-lactam-specific RND efflux pump, MexAB-OprM, has been linked to reduced expression of MexEF-OprN26. Shields et al. found that imipenem-relebactam treatment led to inactivating mutations in either mexE or mexF in 4/5 critically ill ICU patients7. Notably, in the Shields et al. study, three patients expired after detection of mexE or mexF variants, suggesting a potential connection between these variants, increased P. aeruginosa virulence, and patient mortality. Supporting the connection between mexE and mexF variants and P. aeruginosa virulence, we showed that deletion of mexEFoprN alone was sufficient to increase P. aeruginosa virulence during acute murine infections4. We found that mutations of mexE, mexF, or oprN were sufficient to increase P. aeruginosa surface swarming motility and expression of the rhlA gene involved in making the rhamnolipid surfactant that facilitates swarming4. These findings suggest that rhamnolipids may play a role in the increased virulence of mexEFoprN mutants. Despite these studies linking mexEFoprN inactivating mutations to increased P. aeruginosa virulence, it is unclear if and how often mexEFoprN inactivating mutations arise during chronic infection and whether these mutations increase QS-mediated virulence phenotypes in vivo.
In this study, we test the hypothesis that mexEFoprN inactivating mutations evolve during human infections and increase P. aeruginosa virulence. Here, we determine the prevalence of mexEFoprN variants among ICU respiratory P. aeruginosa isolates and CF P. aeruginosa isolates. We then use bacterial genetics and infection models to elucidate how inactivation of MexEF-OprN affects lung infection and airway damage. Finally, we test these identified virulence mechanisms using representative ICU and CF P. aeruginosa isolates with mexEFoprN inactivating mutations. Overall, this study highlights how mutations in antibiotic efflux pumps can have unexpected effects on P. aeruginosa pathogenicity.
Results
Inactivating efflux pump mutations are enriched in CF clinical isolates
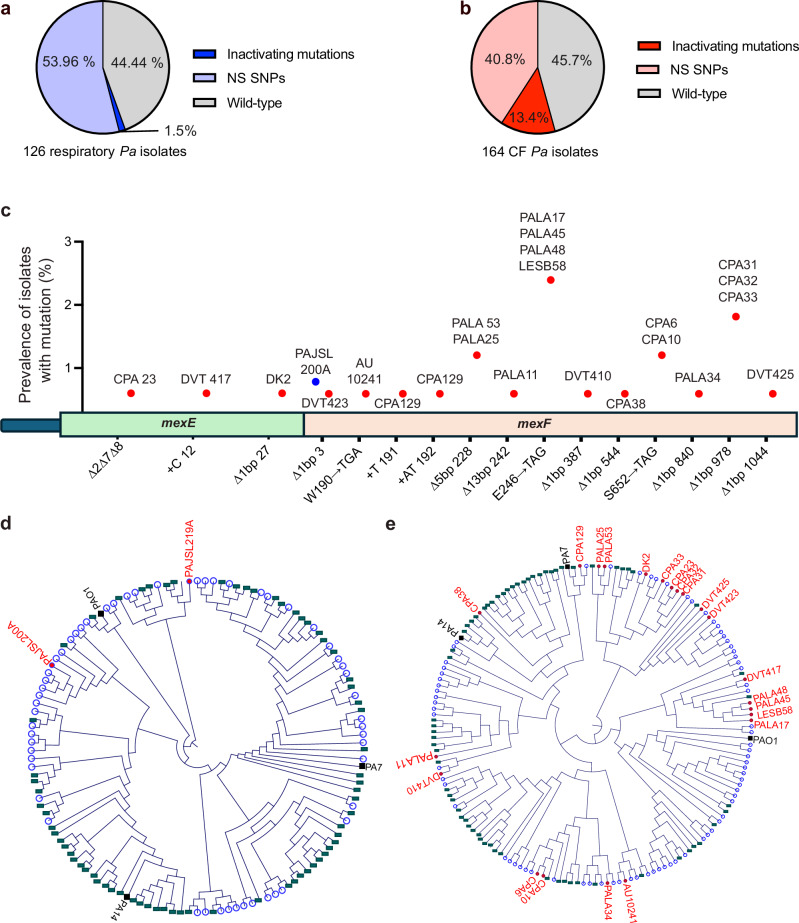
We previously reported that chronic in vitro aztreonam exposure can select for deleterious mutations in the P. aeruginosa mexEFoprN antibiotic efflux pump operon5, and others have detected mexEFoprN inactivating mutations in P. aeruginosa ICU isolates7. To determine the relative prevalence of mexEFoprN inactivating mutations in P. aeruginosa clinical isolates, genome sequences of 126 P. aeruginosa isolates (Supplementary data 1) obtained from patients in the ICU and 164 isolates (Supplementary data 2) obtained from pwCF were analyzed. Using PAO1 as a reference genome, we found that 53.96% of ICU isolates and 40.8% of CF isolates had nonsynonymous mutations in mexE, mexF, or oprN (Fig. 1a, b Supplementary data 1 and 2 and Fig. S1). Additionally, a P. aeruginosa ICU isolate, PAJSL219A lacked the entire mexEFoprN operon and another, PAJSL200A had a frameshift mutation in mexF (Fig. 1c). Compared to ICU isolates, mexE or mexF inactivating mutations (deletions, nonsense mutations, and frameshift mutations) were enriched among P. aeruginosa CF isolates (n = 22/164 CF isolates vs. 2/126 ICU isolates, p = 0.0002, Chi-square test) (Fig. 1a–c) including the highly transmissible P. aeruginosa Liverpool Epidemic Strain (LESB58) (Fig. 1c). Phylogenetic analysis of the ICU and CF P. aeruginosa strains revealed that the strains possessing inactivating mutations had evolved from diverse P. aeruginosa lineages (Fig. 1d, e). Since all the components of the tripartite MexEF-OprN efflux pump are essential for its function, deleterious frameshift, nonsense, or deletion mutations in either mexE or mexF should inactivate the efflux pump in these clinical isolates.
Fig. 1. Inactivating mutations in mexEFoprN are enriched among CF P. aeruginosa isolates.
a Percentage of acute respiratory ICU P. aeruginosa isolates with inactivating mutations or nonsynonymous single nucleotide polymorphisms (NS SNPs) in mexE, mexF, or oprN. b Percentage of CF P. aeruginosa isolates with inactivating mutations or NS SNPs in mexE, mexF, or oprN. c Genomic location of mexE and mexF inactivating mutations in clinical isolates. Blue indicates prevalence of mutation among ICU P. aeruginosa isolates and red indicates prevalence among CF P. aeruginosa isolates. Circular cladograms for ICU (d) and CF (e) P. aeruginosa isolates, respectively. Strains with WT mexE, mexF, and oprN are indicated by blue circles, strains with NS SNPs are indicated by green rectangles, strains with inactivating mutations are indicated by red dots, reference strains are indicated in black.
Efflux mutants cause systemic infection and increased inflammation during infection
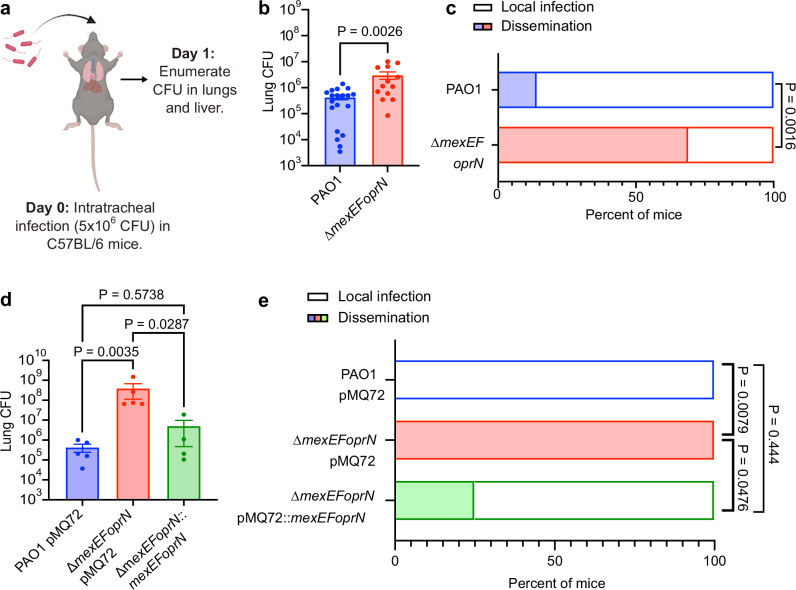
Using an acute infection model with wild-type (WT) C57BL/6 mice (Fig. 2a), we previously found that PAO1 ∆mexEFoprN was hypervirulent relative to PAO1 WT, causing 90% mortality of mice at 48 h post-infection compared to 50% mortality of PAO1 WT-infected mice at 96 h4. We sought to determine whether mortality was being driven by the bacteria directly or indirectly via the immune response to the bacterial infection. Bacterial burdens were quantified at 24 h post-infection in the lungs and livers of intratracheally infected mice. PAO1 ∆mexEFoprN-infected C57BL/6 mice had ~10-fold higher lung bacterial burdens compared to PAO1 WT-infected mice (Fig. 2b). Bacterial dissemination to the liver during P. aeruginosa lung infections are an established marker of systemic infection27–30, hence we next examined the presence of viable bacteria in the livers of PAO1 and PAO1∆mexEFoprN infected mice. Bacterial dissemination to the liver was observed in 69% of PAO1 ∆mexEFoprN-infected mice but only in 14% of PAO1 WT-infected mice (Fig. 2c), thus indicating that infection with the efflux pump mutant caused systemic infection in significantly more mice. Inflammatory cytokines were also upregulated in efflux pump mutant-infected mice (Fig. S2), consistent with worse disease during mutant infections. Importantly, both bacterial replication in the lungs (Fig. 2d) and dissemination to the liver (Fig. 2e) were reduced by complementation, comparing the PAO1 pMQ72 and PAO1 ∆mexEFoprN pMQ72 vector control strains to the PAO1 ∆mexEFoprN::mexEFoprN complemented mutant. These data showed that deletion of mexEFoprN contributes to PAO1 hypervirulence during acute infections by increasing bacterial burdens and lung inflammation.
Fig. 2. MexEF-OprN loss of function mutants cause systemic infection and increased inflammation during infection.
a Schematic representation of acute lung infections in C57BL/6 mice created using BioRender. b Bacterial CFU enumerated from the whole lung lysate of PAO1 (n = 21) or PAO1 ∆mexEFoprN (n = 16) infected C57BL/6 mice at 24 h post infection (hpi). Data show mean ± SEM. Statistical significance analyzed by two-sided unpaired t-test. c Percentage of PAO1 (n = 21) or PAO1 ∆mexEFoprN (n = 16) infected C57BL/6 mice with viable bacteria in the liver at 24 hpi. Statistical significance analyzed by Fisher’s exact test. n indicates the number of mice/group. d Bacterial CFU enumerated from the whole lung lysate of PAO1 pMQ72 (n = 5), PAO1 ∆mexEFoprN pMQ72 (n = 5) or PAO1 ∆mexEFoprN::mexEFoprN (n = 4) infected C57BL/6 mice at 24 hpi. Data show mean ± SEM. Statistical significance analyzed by ANOVA Kruskal–Wallis test. e Percentage of PAO1 pMQ72 (n = 5), PAO1 ∆mexEFoprN pMQ72 (n = 5), or PAO1 ∆mexEFoprN::mexEFoprN (n = 4) infected C57BL/6 mice with viable bacteria in the liver at 24 hpi. Statistical significance analyzed by Fisher’s exact test. n indicates the number of mice/group.
Increased elastase production drives efflux pump mutant virulence
Rhamnolipids (biosurfactant) and elastase (protease) are important P. aeruginosa virulence factors that facilitate bacterial breakdown of the respiratory epithelial barrier by targeting intercellular tight junctions and degrading the host tissue matrix31–33. Previously, we showed that mutations in mexEFoprN could increase swarming motility and related rhamnolipid gene expression of a rhamnolipid-GFP reporter construct in vitro4. Yet, it was unclear if rhamnolipid or other P. aeruginosa virulence factors were driving the mexEFoprN efflux pump mutant’s increased virulence in vivo.
First, we took a genome-wide approach to compare the transcriptome of the ΔmexEFoprN mutant to the WT strain. Transcriptomic analyses of the PAO1 ΔmexEFoprN efflux pump mutant relative to PAO1 WT grown in rich LB laboratory growth medium to mid-exponential phase (OD600 ~ 0.6) revealed few differences between the two strains. While the mexE and mexF genes were significantly downregulated in the mutant compared to WT as expected (Supplementary data 3), among 10 up-regulated genes in the efflux pump mutant relative to PAO1 WT (>2-fold increase, adjusted p < 0.05), only the mvfR gene, encoding the PQS-dependent response regulator, was consistent with our previous findings (Supplementary data 3). This was surprising, since we previously detected increased expression of the rhamnolipid biosynthesis gene, rhlA, in the efflux pump mutant relative to WT4. However, since the RNA-seq analyses were performed at a single time point in mid-exponential phase growth (OD600 ~ 0.6), and MvfR can increase rhamnolipid gene expression19, we sought to quantify the expression of rhamnolipid (rhlA) and another mvfR-regulated virulence factor, elastase (lasB), at a later growth phase when QS genes typically become induced (early stationary phase; OD600 ~ 1.0). In early stationary phase, lasB and rhlA gene expression were analyzed by RT-qPCR, and both were increased ~3-fold and ~4-fold, respectively, in the PAO1 ∆mexEFoprN efflux pump mutant compared to PAO1 WT (Fig. 3a, b). Complementation of PAO1 ∆mexEFoprN reduced elastase and rhlA gene expression (Fig. S3A, B). Phenotypic analyses were consistent with gene expression, as elastase and rhamnolipid production were increased in the mutant using a fluorogenic elastase reporter substrate (Fig. 3c) and the rhamnolipid plate assay (Fig. 3d), respectively. Further, levels of lasB and rhlA gene expression were also analyzed in PAO1 WT and PAO1 ∆mexEFoprN grown to OD600 ~ 1.0 in synthetic CF sputum medium (SCFM2), which mimics the growth conditions of P. aeruginosa in the CF airways34. The expression of both lasB and rhlA was increased ~5 fold in the PAO1 ∆mexEFoprN efflux pump mutant compared to PAO1 WT (Fig. S4A, B).
Fig. 3. QS dependent increase in levels of elastase and rhamnolipids drives hypervirulence of PAO1 ∆mexEFoprN.
a Fold change in lasB gene expression in PAO1 and PAO1 ∆mexEFoprN measured by RT-qPCR. Data show mean ± SEM, n = 3 independent experiments. Statistical significance analyzed by two-sided unpaired t-test. b Fold change in rhlA gene expression in PAO1 and PAO1 ∆mexEFoprN measured by RT-qPCR. Data show mean ± SEM, n = 3 independent experiments. Statistical significance analyzed by two-sided unpaired t-test. c Elastase activity determined from the supernatants of PAO1 and PAO1 ∆mexEFoprN using a fluorogenic substrate. Fluorescence has been normalized to total protein levels in the culture supernatants. Data show mean ± SEM, n = 6 independent experiments. Statistical significance analyzed by two-sided unpaired t-test. d Rhamnolipid production in PAO1 and PAO1 ∆mexEFoprN using cetyltrimethylammonium bromide (CTAB) methylene blue agar plates. A halo (white arrow) indicates rhamnolipid production. e Bacterial CFU enumerated from the whole lung lysate of PAO1 (n = 21), PAO1 ∆mexEFoprN (n = 16), PAO1 ∆mexEFoprN∆lasB (n = 5), PAO1 ∆mexEFoprN∆rhlA (n = 7) or PAO1 ∆mexEFoprN∆lasB∆rhlA (n = 6) infected C57BL/6 mice at 24 hpi. Data show mean ± SEM. Statistical significance analyzed by ANOVA Kruskal-Wallis test. f Percentage of PAO1 (n = 21), PAO1 ∆mexEFoprN (n = 16), PAO1 ∆mexEFoprN∆lasB (n = 5), PAO1 ∆mexEFoprN∆rhlA (n = 7) or PAO1 ∆mexEFoprN∆lasB∆rhlA (n = 6) infected C57BL/6 mice with viable bacteria in the liver at 24 hpi. Statistical significance analyzed by Fisher’s exact test. n indicates the number of mice/group. g Total PQS levels quantified in PAO1 and PAO1 ∆mexEFoprN by thin-layer chromatography (TLC). Data show mean ± SEM, n = 7 independent experiments. Statistical significance analyzed by two-sided unpaired t-test. h Intracellular PQS levels quantified in PAO1 and PAO1 ∆mexEFoprN quantified by TLC. Data show mean ± SEM, n = 7 independent experiments. Statistical significance analyzed by two-sided unpaired t-test. i Fold change in lasB gene expression in PAO1 ∆mexEFoprN and PAO1 ∆mexEFoprN∆pqsA measured by RT-qPCR. Data show mean ± SEM, n = 3 independent experiments. Statistical significance analyzed by two-sided unpaired t-test. j Fold change in rhlA gene expression in PAO1 ∆mexEFoprN and PAO1 ∆mexEFoprN∆pqsA measured by RT-qPCR. Data show mean ± SEM, n = 3 independent experiments. Statistical significance analyzed by two-sided unpaired t-test.
To investigate if increased levels of elastase and rhamnolipids were driving hypervirulence of PAO1 ∆mexEFoprN, we constructed lasB or rhlA deletion mutants in the PAO1 ∆mexEFoprN mutant background and performed acute infections in C57BL/6 mice. Eliminating elastase biosynthesis reduced bacterial lung burdens 100-fold but eliminating rhamnolipid biosynthesis did not affect lung burdens (Fig. 3e). However, significantly fewer mice infected with PAO1 ∆mexEFoprN∆lasB or PAO1 ∆mexEFoprN∆rhlA showed bacterial dissemination to the liver than those infected with the PAO1 ∆mexEFoprN mutant, indicating that both elastase and rhamnolipids contribute to systemic infection (Fig. 3f). Increased expression of inflammatory genes in the lungs of PAO1 ∆mexEFoprN infected mice also indicated more severe lung damage compared to PAO1 ∆mexEFoprN∆lasB and PAO1 ∆mexEFoprN∆rhlA infected mice (Fig. S2). Further, deletion of both lasB and rhlA did not have an additive effect on lung infection as bacterial lung burdens of the PAO1 ∆mexEFoprN∆lasB∆rhlA triple mutant were equivalent to the PAO1 ∆mexEFoprN∆lasB double mutant (Fig. 3e), suggesting that bacterial burdens were primarily driven by increased elastase production. However, both lasB and rhlA contributed to increased dissemination of PAO1 ∆mexEFoprN, because systemic infection was observed in fewer mice infected with the PAO1 ∆mexEFoprN∆lasB∆rhlA triple mutant, as well as the PAO1 ∆mexEFoprN∆lasB and PAO1 ∆mexEFoprN∆rhlA double mutants (Fig. 3f).
Increased QS drives virulence of efflux pump mutants
In addition to elastase and rhamnolipid biosynthesis genes being regulated by MvfR, both virulence factors are regulated by two other P. aeruginosa quorum-sensing (QS) systems. Specifically, the elastase gene lasB is regulated by the LasR-LasI QS system and the 3-oxo-C12-HSL QS signal35, and the rhamnolipid gene rhlA is regulated by the RhlR-RhlI QS system and the C4-HSL QS signal35. As noted above, both can also be regulated by the third QS system, where PQS is the signaling molecule that binds the transcription factor MvfR increasing lasB and rhlA gene expression19,20. Previous studies have associated increased mexEFoprN expression with changes in QS signals and precursors, but have conflicted. Some studies link increased mexEFoprN expression to increased extracellular levels of 3-oxo-C12-HSL, while others have associated increased expression of the efflux pump with increased extracellular levels of the PQS precursor molecules HHQ or kynurenine16–18. Additionally, increased mexEFoprN expression has been associated with reduced expression of 3-oxo-C12-HSL-dependent and PQS-dependent genes16–18. Based on these complicated previous results, we next wanted to determine if deletion of MexEF-OprN could affect the intracellular levels of 3-oxo-C12-HSL, PQS, or PQS precursors and explain increased elastase and rhamnolipid biosynthesis.
Metabolomic analyses were performed on cells and supernatants of PAO1 pMQ72 (WT vector control), PAO1 ∆mexEFoprN pMQ72 (mutant vector control), and PAO1 ∆mexEFoprN::mexEFoprN (complemented strain). Intracellular and extracellular PQS levels were higher in the PAO1 ∆mexEFoprN strain compared to PAO1 WT (Fig. S5C, F), while neither intracellular nor extracellular levels of 3-oxo-C12-HSL or C4-HSL were significantly different (Fig. S5A, B, D, E). In agreement with our findings from RNA-seq analysis, mvfR gene expression was also higher in PAO1 ∆mexEFoprN compared to PAO1 WT (Fig. S5G). The intracellular level of the PQS precursor kynurenine was notably more abundant in the PAO1 ∆mexEFoprN pMQ72 strain compared to PAO1 pMQ72, but complementation of PAO1 ∆mexEFoprN::mexEFoprN did not reduce intracellular kynurenine to WT levels (Fig. S5H). Since in vivo complementation studies restored bacterial burdens to WT levels (Fig. 2), these metabolomic data suggest that increased levels of intracellular kynurenine are not the driving factor for the mutant’s increased virulence because intracellular kynurenine was not restored by complementation.
To further test effects of loss of mexEFoprN on PQS signaling, both total (Fig. 3g) and intracellular PQS (Fig. 3h) were quantified by thin-layer chromatography, and PQS was more abundant in PAO1 ∆mexEFoprN compared to PAO1 WT and the complemented mutant strain (Fig. S5I, J). To test whether increased lasB and rhlA expression was dependent on PQS, we generated a PAO1 ∆mexEFoprN∆pqsA strain and determined the expression of these genes in the generated mutant strain. Deletion of pqsA in the ∆mexEFoprN mutant background resulted in a 2-fold reduction in lasB and rhlA expression (Fig. 3i, j). Surprisingly, in SCFM2 cultures of PAO1 WT and PAO1 ∆mexEFoprN, mvfR gene expression was similar (Fig. S5K), indicating that in SCFM2, increased lasB and rhlA expression in PAO1 ∆mexEFoprN could be driven by increased intracellular PQS levels, which have not been quantified. This could lead to increased mvfR target gene activation, although the levels of mvfR do not differ, or the mechanism of lasB and rhlA upregulation could be dependent on other QS systems in SCFM2.
Our metabolomics analyses suggested that kynurenine was likely not involved in the increased PQS biosynthesis and subsequent increased lasB and rhlA expression. However, because intracellular levels of the PQS precursor kynurenine were also higher in PAO1 ∆mexEFoprN compared to PAO1 WT, and kynurenine was previously shown to accumulate in the supernatants of nfxC mutants18, we wanted to further test the hypothesis that increased PQS production and subsequent elastase and rhamnolipid gene expression could be dependent on kynurenine biosynthesis. To test this hypothesis, we deleted kynU in the PAO1 ∆mexEFoprN background and measured PQS levels, lasB, and rhlA gene expression in this strain. Since kynU encodes kynureninase, which is required for PQS biosynthesis from kynurenine, we would expect PAO1 ∆mexEFoprN∆kynU to produce reduced levels of PQS, lasB, and rhlA compared to PAO1 ∆mexEFoprN. Unexpectedly, the PAO1 ∆mexEFoprN and PAO1 ∆mexEFoprNΔkynU mutants produced equal levels of PQS, with equivalent lasB and rhlA gene expression (Fig. S6). These findings confirm that accumulation of intracellular kynurenine was not responsible for increased PQS levels and rhlA or lasB gene expression in the efflux pump mutant.
P. aeruginosa efflux mutants cause increased infection and airway damage in CF infection models
Genomic analyses showed that mexEFoprN inactivating mutations were enriched in CF clinical isolates relative to ICU isolates, leading us to hypothesize that these mutations would be beneficial to P. aeruginosa during CF infection. To test this hypothesis, we used a human CF airway epithelial cell infection model, which recapitulates dysfunctional cystic fibrosis transmembrane regulator (CFTR) related epithelial defects and an Scnn1b-transgenic mouse infection model that mimics mucus and inflammatory signatures of human CF infection36–38.
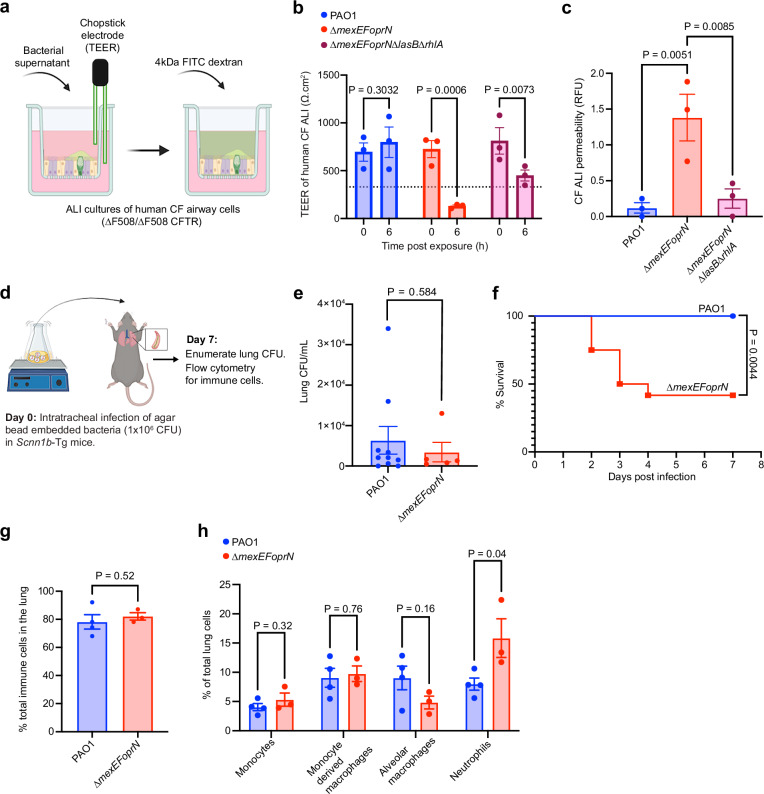
Air liquid interface (ALI) cultures derived from human CF (∆F508/∆F508 CFTR) airway progenitor cells were treated with the secreted products in supernatants from PAO1, PAO1 ∆mexEFoprN, and PAO1 ∆mexEFoprN∆lasB∆rhlA cultures to test whether increased levels of elastase and rhamnolipids in the efflux pump mutant cause epithelial barrier damage. CF airway damage was quantified by measuring the transepithelial electrical resistance (TEER) of CF cultures before (0 h) and after exposure to bacterial culture supernatants until the TEER decreased below 330 Ω.cm2, indicating a loss in barrier integrity (Fig. 4a). At 6 h post-exposure, the TEER of CF cultures exposed to PAO1 ∆mexEFoprN supernatant was lower than 330.Ω cm2 (Fig. 4b), indicating loss of epithelial barrier function. However, no loss of barrier function was observed in CF epithelia exposed to PAO1 or PAO1 ∆mexEFoprN∆lasB∆rhlA supernatants (Fig. 4b). Epithelial barrier permeability was also assessed using fluorescein isothiocyanate (FITC) labeled 4 kDa dextran (Fig. 4a). At 6 h, only CF epithelia exposed to PAO1 ∆mexEFoprN supernatants showed increased dextran permeability (Fig. 4c). Together, these data indicate that increased elastase and rhamnolipids in PAO1 ∆mexEFoprN can cause damage to the CF airway epithelium.
Fig. 4. PAO1 ∆mexEFoprN is hypervirulent in CF infection models.
a Schematic representation of CF airway barrier dysfunction assay created using Biorender. b Transepithelial electrical resistance (TEER) of air liquid interface (ALI) cultures derived from human cystic fibrosis (CF) airways (∆F508/∆F508 CFTR) measured using STX2 chopstick electrodes. TEER < 330 Ω.cm2 (dotted line) indicates a loss in epithelial barrier function. TEER was recorded before (0 h) and at 6 h after exposure to PAO1, PAO1 ∆mexEFoprN, or PAO1 ∆mexEFoprN∆lasB∆rhlA supernatants. Data show mean ± SEM, n = 3 independent experiments. Statistical significance analyzed by ANOVA Fisher’s LSD test. c Permeability of the CF airway epithelial barrier determined using 4 kDa fluorescein isothiocyanate (FITC) labeled dextran at 6 h post exposure to PAO1, PAO1 ∆mexEFoprN, or PAO1 ∆mexEFoprN∆lasB∆rhlA supernatants. Data show mean ± SEM, n = 3 independent experiments. Statistical significance analyzed by ANOVA Fisher’s LSD test. d Schematic representation of chronic lung infections in Scnn1b-Tg mice created using Biorender. e Bacterial CFU enumerated on day 7 from the lung homogenates of Scnn1b-Tg mice infected with PAO1 (n = 10) or PAO1 ∆mexEFoprN (n = 13) embedded in agar beads. Data show mean ± SEM. Statistical significance analyzed by two-sided unpaired t-test. f Survival curves of Scnn1b-Tg mice infected with PAO1 (n = 10) or PAO1 ∆mexEFoprN (n = 13) embedded in agar beads. Statistical significance analyzed by Mantel-Cox test. n indicates the number of mice/group. g, h Flow cytometry for total immune cells, monocyte, macrophages, and neutrophils in the lungs of PAO1 (n = 4) or PAO1 ∆mexEFoprN (n = 3) infected mice at day 7 post infection. Data show mean ± SEM. Statistical significance analyzed by two-sided unpaired t-test.
Next, we tested the virulence of PAO1 ∆mexEFoprN using a low-dose chronic lung infection model in Scnn1b-Tg mice. The Scnn1b-Tg mice overexpress the epithelial sodium channel, resulting in airway mucus accumulation, reduced mucociliary clearance, and neutrophil infiltration similar to lung airways in pwCF37. Scnn1b-Tg mice were infected with a low dose (1 × 106 colony-forming units [CFUs]) of PAO1 WT or PAO1 ∆mexEFoprN embedded in SCFM2 agar beads (Fig. 4d). Using SCFM2 makes the model more CF-like because P. aeruginosa gene expression patterns in SCFM2 mirror P. aeruginosa gene expression patterns in CF sputum39,40. Although we did not observe a difference in the lung burden of PAO1 WT or PAO1 ∆mexEFoprN infected mice that survived through day 7 (Fig. 4e), the mutant caused lethality of 50% infected mice by day 3 post infection, but no lethality was observed in PAO1 infected mice (Fig. 4f). Moreover, in the surviving mutant infected mice, there were significantly more neutrophils in the lungs compared to WT PAO1 infected mice (Fig. 4g, h). This indicates that PAO1 ∆mexEFoprN is hypervirulent and causes increased lung inflammation compared to PAO1 during chronic infections in Scnn1b-Tg mice.
Restoration of mexEFoprN reduces virulence of P. aeruginosa clinical isolates
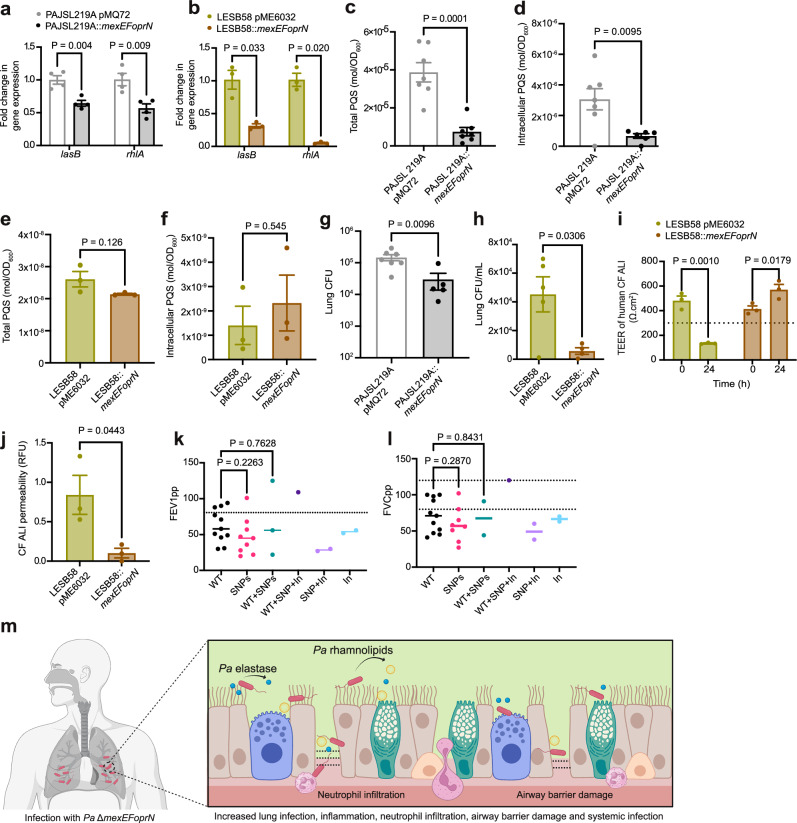
So far, our findings using the laboratory strain PAO1 indicate that inactivation of mexEFoprN was sufficient to increase P. aeruginosa virulence through increased elastase and rhamnolipids. Because strain PAO1 has a mexS mutation that affects mexEFoprN expression41, we wanted to test effects of mexEFoprN inactivating mutations in other strain backgrounds, and especially on the virulence of P. aeruginosa clinical isolates. For this, we genetically restored WT copies of the mexEFoprN operon in P. aeruginosa PAJSL219A, an acute respiratory ICU isolate that lacked the mexEFoprN operon, and P. aeruginosa LESB58, a CF isolate with a nonsense mutation in mexF. Since lasB and rhlA were important virulence factors in the PAO1 ∆mexEFoprN mutant, we hypothesized that the mexEFoprN mutations in the two clinical isolates would also increase expression of these important virulence factors. We analyzed lasB and rhlA gene expression in the complemented clinical isolates by RT-qPCR. As predicted, restoration of the WT mexEFoprN operon in both P. aeruginosa PAJSL219A and P. aeruginosa LESB58 reduced lasB and rhlA gene expression (Fig. 5a, b).
Fig. 5. Restoration of mexEFoprN expression reduces virulence of P. aeruginosa clinical isolates.
a RT-qPCR fold-change in lasB and rhlA expression in PAJSL219A pMQ72 and PAJSL219A::mexEFoprN (mean ± SEM, n = 3; two-sided unpaired t-test). b RT-qPCR fold-change in lasB and rhlA expression in LESB58 pME6032 and LESB58::mexEFoprN (mean ± SEM, n = 3; two-sided unpaired t-test). c Total PQS in PAJSL219A pMQ72 and PAJSL219A::mexEFoprN by TLC (mean ± SEM, n = 7, two-sided unpaired t-test). d Intracellular PQS in PAJSL219A pMQ72 (n = 7) and PAJSL219A::mexEFoprN (n = 6) quantified by TLC (mean ± SEM, two-sided unpaired t-test). e Total PQS in LESB58 pME6032 and LESB58::mexEFoprN by TLC (mean ± SEM, n = 3, two-sided unpaired t-test). f Intracellular PQS in LESB58 pME6032 and LESB58::mexEFoprN by TLC (mean ± SEM, n = 3, two-sided unpaired t-test). g CFU in C57BL/6 mouse whole lung lysates at 24 hpi with PAJSL219A pMQ72 (n = 7) and PAJSL219A::mexEFoprN (n = 5; mean ± SEM, two-sided unpaired t-test). h CFU on day 7 from lung homogenates of Scnn1b-Tg mice infected with LESB58 pME6032 (n = 5) or LESB58::mexEFoprN (n = 4) embedded in agar beads (mean ± SEM, two-sided unpaired t-test). i TEER of ALI cultures of human CF airways (∆F508/∆F508) before (0 h) and 24 h after exposure to LESB58 pME6032 or LESB58::mexEFoprN supernatants. Dotted line (TEER < 330 Ω.cm2): loss in epithelial barrier function (mean ± SEM, n = 3, ANOVA Fisher’s LSD test). j CF airway epithelial barrier permeability using 4 kDa FITC labeled dextran at 24 h post exposure to LESB58 pME6032 or LESB58::mexEFoprN supernatants (mean ± SEM, n = 3, two-sided unpaired t-test). k, l Percent predicted forced expiratory volume in 1 s (FEV1pp, k) and percent predicted forced vital capacity (FVCpp, l) of pwCF infected with P. aeruginosa strains with wild-type mexEFoprN (WT) (n = 11), non-synonymous SNPs (n = 9), inactivating mutations (In) (n = 2), or a mixed population (WT + SNP (n = 3), WT + SNP+In (n = 1), SNP+In (n = 2)). Dotted lines indicate FEV1pp = 80% (k) and FVCpp (l, i.e., 80–100%), the lower limits of normal FEV1pp and FVCpp (k, l: ANOVA Fisher’s LSD test). m Model: mexEFoprN efflux pump mutants increase elastase and rhamnolipid, leading to increased epithelial damage, replication during infection, dissemination, and lethality in vivo (created using Biorender).
To further test if the reduced virulence factor expression was due to reduced PQS levels in the mexEFoprN restored clinical isolates, total and intracellular PQS levels were determined in P. aeruginosa PAJSL219A pMQ72 (vector control), P. aeruginosa PAJSL219A::mexEFoprN (restored strain), P. aeruginosa LESB58 pME6032 (vector control), and P. aeruginosa LESB58::mexEFoprN (restored strain). Restoration of mexEFoprN in P. aeruginosa PAJSL219A significantly reduced total and intracellular levels of PQS (Fig. 5c, d). Surprisingly, restoring mexEFoprN expression in P. aeruginosa LESB58 did not have any effect on total and intracellular PQS levels (Fig. 5e, f), indicating that PQS-independent mechanisms are responsible for increased virulence factor expression in P. aeruginosa LESB58.
Because PAJSL219A is an acute respiratory infection ICU P. aeruginosa isolate, acute infections of C57BL/6 mice were carried out with P. aeruginosa PAJSL219A pMQ72 (vector control) and P. aeruginosa PAJSL219A::mexEFoprN (restored strain). Restoration of the WT mexEFoprN operon resulted in ~10-fold decrease in the lung bacterial burden of P. aeruginosa PAJSL219A::mexEFoprN infected mice compared to P. aeruginosa PAJSL219A pMQ72 infections (Fig. 5g). However, no bacterial dissemination was observed in either P. aeruginosa PAJSL219A pMQ72 or P. aeruginosa PAJSL219A::mexEFoprN infected mice, demonstrating that invasiveness varies in different P. aeruginosa strain backgrounds.
To test if introducing a WT copy of the mexEFoprN operon would also reduce the virulence of the CF isolate P. aeruginosa LESB58, Scnn1b-Tg mice were infected with P. aeruginosa LESB58 pME6032 (vector control) or P. aeruginosa LESB58::mexEFoprN (restored strain). On day 7 post-infection, the lung bacterial burden in P. aeruginosa LESB58 pME6032 infected mice was 4-fold higher than P. aeruginosa LESB58::mexEFoprN infected mice (Fig. 5h). To test if the mexEFoprN inactivating mutation in P. aeruginosa LESB58 increased CF airway damage, CF human airway epithelial cultures were treated with supernatants from P. aeruginosa LESB58 pME6032 (vector control) or P. aeruginosa LESB58::mexEFoprN (restored strain). Epithelial damage was determined by reduced TEER and increased dextran permeability. At 24 h, CF epithelial cultures exposed to P. aeruginosa LESB58 pME6032 vector control supernatant showed a significant reduction in barrier integrity (Fig. 5i) and increased dextran permeability (Fig. 5j), but the integrity of CF epithelial cultures exposed to the supernatant of P. aeruginosa LESB58::mexEFoprN restored strain was not compromised (Fig. 5i, j). These data indicate that mexEFoprN inactivating mutations contribute to the increased virulence of P. aeruginosa clinical isolates, including abilities to infect and damage CF airway epithelial barriers.
Previously, Shields et al. linked mexEFoprN inactivating mutations with poor patient outcomes. Thus, we were interested whether infection with P. aeruginosa with inactivating mutations in mexEFoprN could be linked to worse outcomes in the isolates we analyzed. For 60/164 CF P. aeruginosa clinical isolates, we were able to retrospectively analyze links between lung function and P. aeruginosa mexEFoprN genotypes. Two lung function measurements, percent predicted forced expiratory volume in 1 s (FEV1pp) and percent predicted forced vital capacity (FVCpp), were compared for pwCF infected with P. aeruginosa strains encoding WT mexEFoprN operons or P. aeruginosa strains with SNPs or inactivating mutations in mexEFoprN (Supplementary data 2 and Fig. 5k, l). There were several patients that were also co-infected with strains that had mexEFoprN inactivating mutations and strains with WT or nonsynonymous SNPs in mexEFoprN. Since P. aeruginosa infections are associated with accelerated loss in lung function, it was unsurprising that most of the patients showed lower than normal FEV1pp and FVCpp. However, notably, 4 out of 5 patients infected with at least one P. aeruginosa strain with inactivating mutations in mexEFoprN had lower than normal lung function parameters (Supplementary data 2 and Fig. 5k, l), further supporting a potential link between inactivating mutations in mexEFoprN and poor patient outcomes.
Discussion
P. aeruginosa infects greater than 60% of adults with CF23 and is one of the most common causes of acute respiratory infections in patients in the ICU. In both pwCF and patients in the ICU, P. aeruginosa infections are associated with lung function decline and increased morbidity2,42–44. Hence, identifying mutations that contribute to P. aeruginosa hypervirulence is important. Here, we demonstrate that inactivating mutations in the mexEFoprN operon, which were identified in ~1.5% of ICU P. aeruginosa isolates and ~13.4% of CF P. aeruginosa isolates analyzed in this study, can increase the virulence of P. aeruginosa during chronic CF infections and acute lung infections. While it is not known if any host factors select for these mutations, prolonged β-lactam exposure can select for mexEFoprN inactivating mutations5,7, which is concerning because β-lactam antibiotics are used to treat both chronic CF P. aeruginosa infections and acute respiratory P. aeruginosa infections.
Increased P. aeruginosa elastase activity correlates with a higher 30-day mortality in P. aeruginosa-infected patients in the ICU45. In the acute murine infection model, we observed increased lethality, increased systemic infection, and pulmonary inflammation in PAO1 ∆mexEFoprN infected mice, which was associated with elevated elastase and rhamnolipid levels. This indicates that patients infected with P. aeruginosa ∆mexEFoprN strains may be at a higher risk of sepsis, morbidity, and mortality.
While the ICU isolate P. aeruginosa PAJSL219A lacked the mexEFoprN operon, it did not disseminate like PAO1 ∆mexEFoprN. At first, this result may seem unexpected; however, it is well appreciated that virulence can vary greatly from one P. aeruginosa strain to another46. Thus, it is perhaps unsurprising that systemic infection was not observed during acute infections with the P. aeruginosa PAJSL219A because the presence of other genomic mutations could be responsible for reduced dissemination, which are absent in the PAO1 genomic background. Consistent with our experiments with the PAO1 ΔmexEFoprN mutant, restoring a wild-type copy of mexEFoprN in P. aeruginosa PAJSL219A reduced its elastase and rhamnolipid expression and lung bacterial burden in infected mice. These data indicate that despite other confounding factors, the mexEFoprN mutation increased virulence factor expression of P. aeruginosa PAJSL219A.
We observed that restoring mexEFoprN in the CF isolate P. aeruginosa LESB58 reduced elastase and rhamnolipid expression and human CF airway damage. Unlike PAO1 ∆mexEFoprN chronic infections, we did not see any lethality of Scnn1b-Tg mice infected with P. aeruginosa LESB58. This could again be due to other genomic mutations in P. aeruginosa LESB58 relative to PAO1, but restoring mexEFoprN expression in the strain resulted in reduced lung bacterial burdens, which is consistent with our PAO1 findings. LES was the first CF epidemic strain identified, having infected 79% of pwCF in a Liverpool adult CF center47. Besides the UK, the LES strain has been detected in North America48, highlighting its high transmissibility. Further, LESB58 infections have been associated with pulmonary and extrapulmonary exacerbations in pwCF49–51, and LES was associated with higher 3 year mortality in Canadian pwCF compared to pwCF in Canada infected with other P. aeruginosa strains48. While P. aeruginosa LESB58 is undoubtedly an aggressive strain, its mechanisms of enhanced virulence remained unclear52. In this study, we demonstrate that the virulence of P. aeruginosa LESB58 could be driven by mexF inactivation, leading to increased elastase and rhamnolipids. Since few strains are as readily transmissible as LES, it is tempting to hypothesize that the mexF mutation may also contribute to its transmissibility from person-to- person. Unfortunately, while infection transmission models are frequently used for viruses like influenza53, such models do not yet exist for P. aeruginosa. Adapting transmission models for P. aeruginosa analyses could help address such questions in future studies. Alternatively, it would be interesting to examine other epidemic clones of P. aeruginosa54,55 in CF and other infection types to determine whether mexEFoprN mutations are more common among transmissible lineages of P. aeruginosa compared to P. aeruginosa that do not spread from person-to-person.
Modulator therapies that correct CFTR dysfunction have been a breakthrough in the management of CF disease, improving outcomes and prolonging life. As modulator therapies reduce airway mucus accumulation, which makes pwCF susceptible to chronic P. aeruginosa infections, the rate of P. aeruginosa detection in CF sputum by traditional culture has decreased56. However, using sensitive sequencing-based detection methods, several studies reveal that P. aeruginosa infections generally persist following CFTR modulator therapy57–60. Notably, in the study by Ledger et al. 3 pwCF who were infected with mexE and mexF variants received CFTR modulators yet had persistent lung colonization by these variants even up to 12 months post initiation of CFTR corrector therapy, indicating that these strains are difficult to eradicate60. While it is unknown if mutations in mexE and mexF may play a role in adaptation to the CF airways, our findings suggest that persistent infections with these strains could result in severe lung damage. From our retrospective analysis of lung function measurements from a cross-sectional study, we found that 4/5 pwCF who were infected with at least one P. aeruginosa strain with inactivating mutations had low FEV1pp and FVCpp. Among the 5 pwCF infected with P. aeruginosa strains with mexEFoprN inactivating mutations, the only patient showing normal FEV1pp and FVCpp values was infected with a mixture of 6 different P. aeruginosa strains-2 with WT mexEFoprN, 2 with mexEFoprN SNPs, and 2 with inactivating mutations in mexEFoprN. Since we do not know the relative abundance of these strains in this individual, it is possible that having a mixture of mexEFoprN genotypes could lead to better outcomes. These retrospective analyses of human clinical isolates and lung function data are admittedly limited by a small sample size and a lack of longitudinal samples. In the future, longitudinal sampling from patient cohorts could be used to determine if P. aeruginosa strains with inactivating mutations in mexEFoprN outcompete the other strains with WT mexEFoprN and if pwCF infected with mexEFoprN mutants show prolonged exacerbations and increased hospitalizations.
P. aeruginosa virulence factors are necessary to sequester nutrients from the host to enable P. aeruginosa survival, but it can be costly. Hence, P. aeruginosa has evolved a stringent response system, which is activated only under nutrient deprivation to control the activation of QS and QS-dependent virulence factor expression. However, in a recent study, it has been shown that inactivating mutations in mexT, which are prevalent in CF P. aeruginosa isolates, are able to activate P. aeruginosa QS systems and downstream virulence factors independent of the stringent response system61. Since mexT is the transcriptional activator of mexEFoprN, our findings indicate that Figueroa et al. may have observed increased QS activation and virulence in their mexT mutants due to the absence of the MexEF-OprN efflux pump. In our study, we focus only on inactivating mutations in mexEFoprN; however, there may be several strains with mexT inactivating mutations, like Figueroa et al. have indicated in their study, that do not express the MexEF-OprN efflux pump and could also be hypervirulent. Additionally, the effects of missense mutations in mexEFoprN that are found in ~50% of P. aeruginosa clinical isolates have yet to be tested.
Likewise, our findings using the PAO1 strain indicate that increased elastase and rhamnolipid expression was PQS dependent in the deletion mutant. This mechanism was also recapitulated in the ICU P. aeruginosa isolate PAJSL219A; however, elastase and rhamnolipid expression were PQS independent in the CF isolate P. aeruginosa LESB58. It is possible that the QS signaling pathway regulating expression of virulence factors is strain dependent. As clinical isolates adapt to the nutritional environment and other stresses present during infection, these factors could influence the signaling pathways involved in virulence upregulation in the mutant. Future studies will address these open questions through metabolomic analyses and dual-RNA-seq analyses of infected animals.
Our study has some limitations that should be acknowledged. We measured P. aeruginosa dissemination by quantifying CFU in the liver. This is an accepted marker for dissemination, but future work could examine dissemination in blood, spleen, and other tissues. As noted above, we performed a limited retrospective analysis of patient lung function and associations with mexEFoprN variants. To fully understand the potential effects of mexEFoprN variants on disease progression, a larger longitudinal study would be necessary. However, these preliminary, limited data presented here are consistent with others suggesting that loss of mexEFoprN may be linked to poor patient outcomes.
Finally, P. aeruginosa has 12 RND antibiotic efflux pumps, and the antimicrobial substrates of these pumps have been well characterized9. However, many of these pumps are also expressed in the absence of antibiotics, indicating they may have functions beyond antibiotic efflux. Although identification of alternative non-antibiotic substrates of MexEF-OprN was beyond the scope of this study, we use multiple infection models to show that mutations leading to the loss of MexEF-OprN function alone are sufficient to increase P. aeruginosa virulence (Fig. 5m). Our findings also caution against the clinical use of broad-spectrum efflux pump inhibitors, which are currently in clinical development, to tackle P. aeruginosa antimicrobial resistance, as inhibition of some efflux pumps like MexEF-OprN could increase P. aeruginosa pathogenesis.
Methods
Animals
C57BL/6 and Scnn1b-Tg mice were obtained from The Jackson Laboratory and were housed in a specific-pathogen-free animal facility with 12:12 light: dark cycle, 21 ± 2 °C, ~ 40% humidity at the Cedars-Sinai vivarium. Mice were 8–12 weeks old at the time of experiments. Animal protocols were approved by the Cedars-Sinai Institutional Animal Care and Use Committee (IACUC protocols #8115). Male and female mice were used for the infection experiments, as no sex-based differences in CFU were observed.
Bacterial strains and growth conditions
Bacterial strains and plasmids are listed in Supplementary data 4. Deletion mutants were derived from P. aeruginosa PAO1, which was obtained from Colin Manoil’s laboratory (University of Washington, USA). All strains were grown at 37 °C in lysogeny broth (LB) or synthetic CF sputum medium (SCFM2, Synthbiome Inc, USA) or LB agar (Becton, Dickinson [BD] Biosciences, USA), unless otherwise specified. When necessary, mutants were selected by adding the following antibiotics to the growth medium: gentamicin (Gm): 10 μg/mL for E. coli and 30 μg/mL for P. aeruginosa; tetracycline: 10 μg/mL for E. coli and 200 μg/mL for P. aeruginosa LESB58.
Mutant construction
Gene deletion mutants were generated using the suicide plasmid vector, pEX18Gm, as described previously in ref. 62. Briefly, ~1 kb of the upstream and downstream regions of each gene were amplified from the PAO1 chromosomal DNA (primers specified in Supplementary data 5). The amplified upstream and downstream fragments were then assembled into pEX18Gm using NEBuilder HiFi DNA Assembly Cloning Kit (New England Biolabs, USA). The pEX18Gm plasmids were transformed into E. coli DH5α as specified in the NEB protocol and later verified by Sanger sequencing. P. aeruginosa strains were transformed with the verified pEX18Gm plasmids by electroporation followed by selection on LB agar plates containing Gm 30 μg/mL at 37 °C overnight. Isolated clones were then counter-selected on no-sodium LB agar plates containing 15% sucrose at room temperature (RT) for 48 h. Deletion mutants were confirmed by PCR and whole-genome sequencing (WGS). WGS data are available in BioProject PRJNA1184772.
To complement PAO1 ∆mexEFoprN and the clinical isolates P. aeruginosa PAJSL219A and P. aeruginosa LESB58, the entire mexEFoprN operon was amplified from PAO1 chromosomal DNA using primers specified in Supplementary data 5. Amplified fragments were assembled into the expression vectors pMQ72 or pME6032 (NovoPro Bioscience, China) using NEBuilder HiFi DNA Assembly Cloning Kit (New England Biolabs, USA) and transformed into E. coli DH5α as specified in the NEB protocol. After verification by Sanger sequencing, P. aeruginosa strains were transformed with the plasmids by electroporation, and mutants were selected on LB plates containing gentamicin (pMQ72) or tetracycline (pME6032). Strains electroporated with the empty vector indicated as PAO1 pMQ72, PAO1 ∆mexEFoprN pMQ72, PAJSL219A pMQ72, and LESB58 pME6032 were used as negative controls for comparison to the complemented strains.
DNA extraction, purification, and PCR
Plasmid DNA was isolated using Monarch Plasmid Miniprep Kit (New England Biolabs, USA). Genomic DNA was isolated using the DNeasy Blood & Tissue Kit (Qiagen, Germany). PCR was performed using either KAPA HIFI 2X ready mix (KAPA Biosystem, USA). Primers used are listed in Supplementary data 5.
Genome analysis of P. aeruginosa clinical isolates
P. aeruginosa ICU respiratory strains45 were a generous gift from the Pulmonary Translational Research Core at University of Pittsburgh. Genomic DNA was isolated from these strains using the DNeasy Blood and Tissue Kit (Qiagen, Germany) and was sequenced by Microbial Genome Sequencing Center (MiGs, Pittsburgh, USA). Genome sequences are available in BioProject PRJNA1184772 and PRJNA934930.
The CF P. aeruginosa isolates were collected from June 2020 to June 2023 at the Stanford Cystic Fibrosis Center from routine sputum cultures performed in the clinical laboratory. Isolates were collected with patient consent for biobanking and clinical data capture under protocol approved by the Stanford University IRB (Protocol # 11197)63. Genome sequences of 97 other P. aeruginosa CF strains and the PAO1 strain were downloaded from GenBank. Nucleotide sequences of all strains were systematically sampled at every position and on each strand to create 200-bp sequencing reads tiling the entire genome. Variants were called, annotated, and compared by examining how these simulated read datasets aligned to the P. aeruginosa PAO1 reference genome. The breseq pipeline64 (v0.38.3) and its associated gdtools utility program were used to conduct this analysis.
CLC Genomics v24.0.1 (Qiagen, Germany) was used to generate circular cladograms for P. aeruginosa ICU and CF isolates.
RNA-seq analyses
Overnight LB cultures of PAO1 WT and PAO1 ∆mexEFoprN were diluted in LB broth and grown at 37 °C and shaking at 250 RPM until cultures reached a density of OD600 ~ 0.6. Cultures were then centrifuged at 12,000 × g for 5 min, and cell pellets were resuspended in RNA later and stored in –80 °C until RNA extraction. RNA was isolated using the RNeasy mini-Kit (Qiagen, Germany), and residual DNA was removed using a DNA-free DNA Removal Kit (Invitrogen, USA). Ribosomal RNA was depleted using Ribo-Zero rRNA Removal Kit (Illumina). RNA sequencing libraries were prepared using the Illumina Stranded Total RNA Prep kit (Illumina Inc., USA) and sequenced using an Illumina NovaSeq6000 at the Cedars-Sinai Cancer Applied Genomics Core, targeting 30 M reads per sample. RNA-seq reads were trimmed to remove adapter sequences using Flexbar65. Trimmed reads were aligned to the PAO1 reference genome using Bowtie and samtools66,67. Raw read counts per PAO1 gene were calculated using HT-Seq68. Read counts per gene were normalized, and differential gene expression was calculated using DESeq269. RNA sequencing data are available in BioProject PRJNA1184772.
RNA isolation and RT-qPCR
To determine the expression of lasB and rhlA, overnight LB or SCFM2 cultures were diluted in fresh LB or SCFM2 and incubated at 37 °C and shaking at 250 RPM till cultures attained OD600 of ~1.0. SCFM2 cultures were thoroughly vortexed and pipetted to make a homogeneous bacterial suspension for OD600 measurements. RNA was isolated using the RNeasy mini-Kit (Qiagen, Germany), and residual DNA was removed using a DNA-free DNA Removal Kit (Invitrogen, USA). SuperScript III First-Strand Synthesis SuperMix (Invitrogen, USA) was used to synthesize cDNA, and PowerUp SYBR Green master mix (Applied Biosystems, USA) was used for qPCR. The primers used are listed in Supplementary data 5. rpoD was used as the housekeeping gene, and gene expression was calculated using the 2−ΔΔCt method.
Elastase activity measurement
To determine the levels of secreted elastase, bacterial strains were incubated overnight in LB at 37 °C and shaking at 250RPM. Cell-free supernatants were then obtained by filtering the overnight culture through 0.22-μm syringe filters. After estimating the total protein amounts in the supernatants using bicinchoninic acid (BCA) assay kit (Thermo Fisher Scientific, USA), supernatants were diluted in assay buffer (10 mM of Tris-HCl pH 7.2, 10 mM CaCl2). In a single well of a 96-well clear-bottom black plate, 10 μL of diluted supernatant (containing 20–50 μg total protein) was mixed with 90 μL assay buffer containing 200 µM Abz-Ala-Gly-Leu-Ala-p-Nitro-benzyl-amide (Biosynth Ltd., UK). All the reactions were prepared on ice. Fluorescence (Ex: 330 nm, Em: 460 nm) from the wells was then measured in a kinetic mode every minute for 30 min using a Varioskan Lux Microplate Reader (Thermo Fisher Scientific, USA). Elastase activity (RFU/min/μg protein) was determined as a ratio of the slope obtained from the curve plotted between fluorescence and time (RFU/min) and the total protein amount (μg) in each sample.
Rhamnolipid plate assay
Rhamnolipid plates were prepared according to a previously described protocol70 with some modifications in the medium composition. The rhamnolipid plates were prepared with M9 salts (BD Biosciences, USA), 0.2% glucose, 0.02% methylene blue, 0.05% cetyltrimethylammonium bromide, and 1.5% agar. Overnight LB cultures of bacterial strains grown at 37 °C and shaking at 250 RPM were then spotted onto the rhamnolipid plates. Plates were incubated at 37 °C for 24 h and then at RT until the appearance of a blue halo, indicating the production of rhamnolipids.
Metabolomics analyses
PAO1 pMQ72, PAO1 ∆mexEFoprN pMQ72, and PAO1 ∆mexEFoprN::mexEFoprN cultures were grown in LB broth to OD600 of 0.8. Cultures were centrifuged at 4000 × g for 15 min, and the supernatant was separated from the cell pellet. The cell pellet was washed in PBS, flash frozen, and then stored in –80 °C till metabolite extraction. The culture supernatant was filtered through a 0.22-μm filter to remove any remaining live bacteria, flash frozen, and stored in –80 °C until metabolite extraction. Metabolite extraction and metabolomic analysis were performed at the UCLA metabolomics core. Briefly, dried metabolites were resuspended in 50 μL 50% ACN:water, and 5 μL was loaded onto a Luna NH2 3 μm 100 A (150 × 2.0 mm) column (Phenomenex) using a Vanquish Flex UPLC (Thermo Scientific). The chromatographic separation was performed with mobile phases A (5 mM NH4AcO pH 9.9) and B (ACN) at a flow rate of 200 μL/min. A linear gradient from 15% A to 95% A over 18 min was followed by 7 min isocratic flow at 95% A and re-equilibration to 15% A. Metabolites were detected with a Thermo Scientific Q Exactive mass spectrometer run with polarity switching in full scan mode using a range of 70–975 m/z and 70.000 resolution. Maven (v 8.1.27.11) was used to quantify the targeted polar metabolites by AreaTop, using expected retention time and accurate mass measurements (<5 ppm) for identification. Data analysis, including principal component analysis and heat map generation was performed using in-house R scripts. The normalized data represented is the metabolite intensity (area under the curve) normalized to the median intensity of all the metabolites in the respective sample. Metabolomics data have been uploaded to MassIVE and can be accessed using the MassIVE ID MSV000096391.
PQS quantification by thin-layer chromatography (TLC)
Cultures were grown overnight at 37 °C at 250 RPM. Afterwards, the strains were inoculated to an OD600 of 0.01 in 25 mL of fresh LB media supplemented with 30 μg/mL gentamicin and 0.2% arabinose. Cultures were grown for 8 h at 37 °C and shaking at 250 RPM reach peak PQS production in early stationary phase. Two milliliters of culture were extracted using acidified ethyl acetate (0.1 mL acetic acid/1 L ethyl acetate) at a 1:1 ratio. The organic phase was removed and dried under nitrogen gas. Dried samples were resuspended using 15 μL of Optima grade methanol, and 5 μL of samples were spotted onto a straight-phase phosphate-impregnated TLC plate after being activated for 1 h at 100 °C. PQS standards (100 μM–500 μM) were also spotted on the same plate. After spotting, samples were allowed to run using a mobile phase consisting of 95:5 dichloromethane-methanol. PQS was visualized by intrinsic fluorescence after excitation using long-wave UV light. Digital images were captured and analyzed using the UVP, Inc., Gel Doc-It imaging system. PQS concentrations were determined using the VisionWorks LS Image Acquisition and Analysis software.
Acute murine lung infection
Twelve-week-old male or female C57BL/6 mice were used for intratracheal infection experiments as described previously4. For the clinical isolate P. aeruginosa PAJSL 219 A, 1 × 107 bacteria were intratracheally instilled per mouse, but for acute infections of all other P. aeruginosa strains, 5 × 106 bacteria were intratracheally instilled per mouse. At day 1 post-infection, mice were euthanized by CO2 inhalation, and lung and liver tissues were harvested. The harvested tissues were homogenized in PBS, and dilutions of the homogenates were plated on LB agar to enumerate viable bacteria. Whole lung homogenates were also used for RNA isolation and RT-qPCR to determine the expression of inflammatory genes.
Chronic P. aeruginosa lung infection in Scnn1b-Tg mice
The protocol by Facchini et al.71 was adapted to embed bacteria in SCFM2 agar beads. Briefly, strains were grown in SCFM2 overnight at 37 °C and shaking at 250 RPM. Overnight cultures were then diluted 1:400 in fresh SCFM2 and grown to mid-log phase at 37 °C and shaking at 250 RPM. For P. aeruginosa LESB58 pME6032 and pME6032::mexEFoprN, strains were grown in SCFM2 containing 200 μg/mL tetracycline and 0.1 mM IPTG. Bacterial cultures were pelleted and resuspended in 3 mL SCFM2 containing 1.5% agar. The bacterial-SCFM2 agar suspension was then added to prewarmed (50 °C) heavy mineral oil and immediately stirred at RT for 6 min followed by another 30 min in iced water. The agar beads were washed in phosphate buffered saline (PBS) four times before final resuspension in PBS. The agar bead suspension was then homogenized, and serial dilutions of the homogenate were plated on LB agar to determine the volume of suspension required for infection.
For chronic infections, 12-week-old male or female Scnn1b-Tg mice were intratracheally instilled with 1 × 106 bacteria embedded in SCFM2 agar beads. Mice were monitored for survival three times daily until day 7 post-infection. At day 7 post-infection or at earlier time points if mice were found to be moribund, mice were euthanized by CO2 inhalation, lungs were harvested in PBS, and homogenized. Dilutions of the lung lysate were plated on LB agar to enumerate viable bacteria in the lungs.
Flow cytometry
Infected mice were euthanized by CO2 inhalation. The lungs were perfused with PBS to flush out blood. Perfused lungs were then harvested, minced, and digested using 0.2% collagenase II in RPMI containing 10% FBS. Red blood cells (RBC) were then removed from the digested tissue using RBC lysis buffer (Invitrogen, USA). 5 × 106 cells from each sample were then resuspended in staining buffer (PBS + 3% FBS + 0.01% sodium azide) containing Fc block and LIVE/DEAD stain (Invitrogen, USA) and incubated at 4 °C for 30 min. Cells were then washed and stained with the following antibodies: CD45, Siglec F, CD11b, CD11c, TCRβ, Ly6C, and Ly6G (BD, USA) at 4 °C in dark for 30 min. Fluorescence was then determined using a Cytek Aurora flow cytometer (Cytek Biosciences, USA) and analyzed using the BD FlowJo software. The gating strategy for analysis has been depicted in Fig. S7.
CF airway barrier dysfunction assay
Primary human epithelial cells from CF (∆F508/∆F508) airways were obtained under Cedars-Sinai Medical Center IRB approval STUDY00001735 from a single CF donor and were used for experiments until passage 5. Epithelial cells were cultured in PneumaCult-Ex Plus medium (Stemcell technologies, USA) on polystyrene trans-well inserts (pore size, 0.4 μm) (Corning, USA). Once a confluent monolayer was established, cells were cultured at air-liquid interface (ALI) in PneumaCult-ALI medium (Stemcell Technologies, USA) to induce differentiation into ciliated and mucous epithelial cells. The formation of tight junctions was confirmed by measuring the transepithelial electrical resistance (TEER) using STX2 chopstick electrodes (World Precision Instruments, USA). Since ALI cultures with TEER < 330.Ω cm2 showed high permeability to 4 kDa FITC dextran, only ALI cultures with TEER > 330 Ω.cm2 were used for the experiment.
To determine the effect of secreted virulence factor expression on CF airway barrier permeability, bacterial strains were first grown overnight in RPMI 1640 (Gibco, USA) containing 10% fetal bovine serum (Omega Scientific Inc, USA) at 37 °C and shaking at 250 RPM. Cell-free supernatants containing secreted virulence factors (bacterial supernatant) were harvested by filtering overnight cultures through a 0.22-μm syringe filter. The bacterial supernatants were then added to the apical surface of differentiated CF epithelia, and integrity was microscopically observed every other hour until any of the treated samples showed epithelial monolayer defects. TEER of all the exposed CF epithelia was then measured to ascertain loss of barrier function. Further, at the end point, RPMI 1640 containing 4 kDa Fluorescein Iso-thiocyanate (FITC) labeled dextran was added to the apical surface and incubated for 60 min at 37 °C, 5% CO2. The basal medium was then transferred to a 96-well clear-bottom black plate to measure FITC fluorescence (Ex: 390 nm, Em: 420 nm) using a Varioskan Lux Microplate Reader (Thermo Fisher Scientific, USA).
Statistics
All statistical analysis was performed using GraphPad Prism v10. All experiments were performed with at least three biological replicates, and data have been represented as mean ± standard error of mean (SEM). Normality of variance was assessed by the Shapiro–Wilk test. Significant outliers determined by the Graph Pad Outlier Calculator were removed from statistics. Student t-test or Mann–Whitney test was performed to analyze the significance between two unpaired groups based on data distribution. ANOVA followed by Tukey’s test and Kruskal–Wallis or Dunnett’s multiple comparisons tests were performed for parametric and non-parametric analysis of significance between three or more groups. All testing was considered significant at the two-tailed p value < 0.05.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Source data
Acknowledgements
We would like to thank members of the Jorth Lab for helpful discussions. This work was supported by grants from the Cystic Fibrosis Foundation (JORTH17F5, JORTH19P0, JORTH23I0) and the NIH (K22AI127473, R01AI146425) to PJ, a grant from the NIH (R01HL136143) to JSL, and a grant to SEF from the Cystic Fibrosis Foundation (FERNAN24F0).
Author contributions
Conceptualization, S.E.F. and P.J.; methodology, S.E.F. and P.J.; formal analysis, S.E.F., H.O., J.E.B., J.W.S., A.S. and P.J.; investigation, S.E.F., H.O., M.V., D.A., A.C.M.G., and P.J.; resources, Y.D., J.S.L., K.S.M., E.B.B. and P.J.; data curation, S.E.F., and P.J.; writing—original draft preparation, S.E.F. and P.J.; writing—review and editing, S.E.F., H.O., M.V., D.A., A.C.M.G., A.S., K.S.M., Y.D., J.S.L., E.B.B., J.E.B. and P.J.; supervision, S.E.F. and P.J.; project administration, S.E.F. and P.J.; funding acquisition, S.E.F., J.S.L. and P.J. All authors have read and agreed to the published version of the manuscript.
Peer review
Peer review information
Nature Communications thanks Catherine Llanes, Ruggero La Rosa, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Data availability
Genome sequences of all strains generated in the study and clinical isolates can be obtained from BioProject PRJNA1184772, PRJNA934930, and PRJNA1188603. RNA sequencing data are also available in BioProject PRJNA1184772. Metabolomics data can be accessed using the MassIVE ID MSV000096391. Source data has been provided with this paper, and primary data have been uploaded to Figshare (10.6084/m9.figshare.29298269). Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-025-63284-7.
References
- 1.Carmeli, Y., Troillet, N., Karchmer, A. W. & Samore, M. H. Health and economic outcomes of antibiotic resistance in Pseudomonas aeruginosa. Arch. Intern. Med.159, 1127–1132 (1999). [DOI] [PubMed] [Google Scholar]
- 2.Nathwani, D., Raman, G., Sulham, K., Gavaghan, M. & Menon, V. Clinical and economic consequences of hospital-acquired resistant and multidrug-resistant Pseudomonas aeruginosa infections: a systematic review and meta-analysis. Antimicrob. Resist Infect. Control3, 32 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aloush, V., Navon-Venezia, S., Seigman-Igra, Y., Cabili, S. & Carmeli, Y. Multidrug-resistant Pseudomonas aeruginosa: risk factors and clinical impact. Antimicrob. Agents Chemother.50, 43–48 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vaillancourt, M., Limsuwannarot, S. P., Bresee, C., Poopalarajah, R. & Jorth, P. Pseudomonas aeruginosa mexR and mexEF antibiotic efflux pump variants exhibit increased virulence. Antibiotics10, 1164 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jorth, P. et al. Evolved aztreonam resistance is multifactorial and can produce hypervirulence in Pseudomonas aeruginosa. mBio8, e00517–17 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galdino, A. C. M. et al. Siderophores promote cooperative interspecies and intraspecies cross-protection against antibiotics in vitro. Nat Microbiol. 1–16. 10.1038/s41564-024-01601-4 (2024). [DOI] [PMC free article] [PubMed]
- 7.Shields, R. K., Stellfox, M. E., Kline, E. G., Samanta, P. & Van Tyne, D. Evolution of imipenem-relebactam resistance following treatment of multidrug-resistant Pseudomonas aeruginosa pneumonia. Clin. Infect. Dis.75, 710–714 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huemer, M., Mairpady Shambat, S., Brugger, S. D. & Zinkernagel, A. S. Antibiotic resistance and persistence—Implications for human health and treatment perspectives. EMBO Rep.21, e51034 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lorusso, A. B., Carrara, J. A., Barroso, C. D. N., Tuon, F. F. & Faoro, H. Role of efflux pumps on antimicrobial resistance in Pseudomonas aeruginosa. Int. J. Mol. Sci.23, 15779 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Juarez, P., Broutin, I., Bordi, C., Plésiat, P. & Llanes, C. Constitutive activation of MexT by amino acid substitutions results in MexEF-OprN overproduction in clinical isolates of Pseudomonas aeruginosa. Antimicrob. Agents Chemother.62, e02445–17 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Richardot, C. et al. Amino acid substitutions account for most MexA alterations in clinical NfxC mutants of Pseudomonas aeruginosa. Antimicrob. Agents Chemother.60, 2302–2310 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Köhler, T., Epp, S. F., Curty, L. K. & Pechère, J.-C. Characterization of MexT, the regulator of the MexE-MexF-OprN multidrug efflux system of Pseudomonas aeruginosa. J. Bacteriol.181, 6300–6305 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fukuda, H., Hosaka, M., Hirai, K. & Iyobe, S. New norfloxacin resistance gene in Pseudomonas aeruginosa PAO. Antimicrob. Agents Chemother.34, 1757–1761 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Köhler, T. et al. Characterization of MexE–MexF–OprN, a positively regulated multidrug efflux system of. Mol. Microbiol.23, 345–354 (1997). [DOI] [PubMed] [Google Scholar]
- 15.Miranda, S. W., Asfahl, K. L., Dandekar, A. A. & Greenberg, E. P. Pseudomonas aeruginosa quorum sensing. Adv. Exp. Med. Biol.1386, 95–115 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Köhler, T., van Delden, C., Curty, L. K., Hamzehpour, M. M. & Pechere, J.-C. Overexpression of the MexEF-OprN multidrug efflux system affects cell-to-cell signaling in Pseudomonas aeruginosa. J. Bacteriol.183, 5213–5222 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lamarche, M. G. & Déziel, E. MexEF-OprN efflux pump exports the Pseudomonas quinolone signal (PQS) precursor HHQ (4-hydroxy-2-heptylquinoline). PLoS ONE6, e24310 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olivares, J. et al. Overproduction of the multidrug efflux pump MexEF-OprN does not impair Pseudomonas aeruginosa fitness in competition tests, but produces specific changes in bacterial regulatory networks. Environ. Microbiol.14, 1968–1981 (2012). [DOI] [PubMed] [Google Scholar]
- 19.Diggle, S. P. et al. The Pseudomonas aeruginosa quinolone signal molecule overcomes the cell density-dependency of the quorum sensing hierarchy, regulates rhl-dependent genes at the onset of stationary phase and can be produced in the absence of LasR. Mol. Microbiol.50, 29–43 (2003). [DOI] [PubMed] [Google Scholar]
- 20.McKnight, S. L., Iglewski, B. H. & Pesci, E. C. The Pseudomonas quinolone signal regulates Rhl quorum sensing in Pseudomonas aeruginosa. J. Bacteriol.182, 2702–2708 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fargier, E. et al. MexT functions as a redox-responsive regulator modulating disulfide stress resistance in Pseudomonas aeruginosa. J. Bacteriol.194, 3502–3511 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harris, A. D. et al. Pseudomonas aeruginosa colonization in the ICU: prevalence, risk factors and clinical outcomes. Infect. Control Hosp. Epidemiol.37, 544–548 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bruce, M. et al. CF Foundation Patient Registry Annual Data Report (CF Foundation, 2022).
- 24.Zakhour, J., Sharara, S. L., Hindy, J.-R., Haddad, S. F. & Kanj, S. S. Antimicrobial treatment of Pseudomonas aeruginosa severe sepsis. Antibiotics11, 1432 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCoy, K. S. et al. Inhaled aztreonam lysine for chronic airway pseudomonas aeruginosa in cystic fibrosis. Am. J. Respir. Crit. Care Med.178, 921–928 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horna, G., López, M., Guerra, H., Saénz, Y. & Ruiz, J. Interplay between MexAB-OprM and MexEF-OprN in clinical isolates of Pseudomonas aeruginosa. Sci. Rep.8, 16463 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sawa, T. et al. IL-10 improves lung injury and survival in Pseudomonas aeruginosa pneumonia. J. Immunol.159, 2858–2866 (1997). [PubMed] [Google Scholar]
- 28.Rangel, S. M., Diaz, M. H., Knoten, C. A., Zhang, A. & Hauser, A. R. The role of ExoS in dissemination of Pseudomonas aeruginosa during Pneumonia. PLoS Pathog.11, e1004945 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bouillot, S. et al. Pseudomonas aeruginosa Exolysin promotes bacterial growth in lungs, alveolar damage and bacterial dissemination. Sci. Rep.7, 2120 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kikuchi, Y. et al. Hyperoxia exaggerates bacterial dissemination and lethality in Pseudomonas aeruginosa pneumonia. Pulm. Pharm. Ther.22, 333–339 (2009). [DOI] [PubMed] [Google Scholar]
- 31.Cigana, C. et al. Pseudomonas aeruginosa elastase contributes to the establishment of chronic lung colonization and modulates the immune response in a murine model. Front. Microbiol. 11, 10.3389/fmicb.2020.620819 (2021). [DOI] [PMC free article] [PubMed]
- 32.Zulianello, L. et al. Rhamnolipids are virulence factors that promote early infiltration of primary human airway epithelia by Pseudomonas aeruginosa. Infect. Immun.74, 3134–3147 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nomura, K. et al. Pseudomonas aeruginosa elastase causes transient disruption of tight junctions and downregulation of PAR-2 in human nasal epithelial cells. Respir. Res.15, 21 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lewin, G. R. et al. Application of a quantitative framework to improve the accuracy of a bacterial infection model. Proc. Natl. Acad. Sci. USA120, e2221542120 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pearson, J. P., Pesci, E. C. & Iglewski, B. H. Roles of Pseudomonas aeruginosa las and rhl quorum-sensing systems in control of elastase and rhamnolipid biosynthesis genes. J. Bacteriol.179, 5756–5767 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brao, K. J. et al. Scnn1b-transgenic BALB/c mice as a model of Pseudomonas aeruginosa infections of the cystic fibrosis lung. Infect. Immun.88, e00237–20 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou, Z. et al. The ENaC-overexpressing mouse as a model of cystic fibrosis lung disease. J. Cyst. Fibros.10, S172–S182 (2011). [DOI] [PubMed] [Google Scholar]
- 38.Vaillancourt, M., Fernandes, S. E., Aguilar, D., Milesi Galdino, A. C. & Jorth, P. A chronic Pseudomonas aeruginosa mouse lung infection modeling the mucus obstruction, lung function, and inflammation of human cystic fibrosis. Infect. Immun.93, e0023025 (2025). [DOI] [PMC free article] [PubMed]
- 39.Palmer, K. L., Aye, L. M., Whiteley, M., Al, P. E. T. & Acteriol, J. B. Nutritional cues control pseudomonas aeruginosa multicellular behavior in cystic fibrosis sputum □✝. J. Bacteriol.189, 8079–8087 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Duncan, R. P. et al. Improvement of a mouse infection model to capture Pseudomonas aeruginosa chronic physiology in cystic fibrosis. Proc. Natl. Acad. Sci. USA121, e2406234121 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee, S., Gallagher, L. & Manoil, C. Reconstructing a Wild-Type Pseudomonas aeruginosa Reference Strain PAO1. J. Bacteriol.203, e00179 (2021). [DOI] [PMC free article] [PubMed]
- 42.Rosenfeld, M. et al. Association of Pseudomonas aeruginosa infection stage with lung function trajectory in children with cystic fibrosis. J. Cyst. Fibros.22, 857–863 (2023). [DOI] [PubMed] [Google Scholar]
- 43.Kosorok, M. R. et al. Acceleration of lung disease in children with cystic fibrosis after Pseudomonas aeruginosa acquisition. Pediatr. Pulmonol.32, 277–287 (2001). [DOI] [PubMed] [Google Scholar]
- 44.Vincent, J.-L. et al. International study of the prevalence and outcomes of infection in intensive care units. JAMA302, 2323–2329 (2009). [DOI] [PubMed] [Google Scholar]
- 45.Zupetic, J. et al. Elastase activity from Pseudomonas aeruginosa respiratory isolates and ICU mortality. Chest160, 1624–1633 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bragonzi, A. et al. Pseudomonas aeruginosa microevolution during cystic fibrosis lung infection establishes clones with adapted virulence. Am. J. Respir. Crit. Care Med.180, 138–145 (2009). [DOI] [PubMed] [Google Scholar]
- 47.Panagea, S. et al. PCR-based detection of a cystic fibrosis epidemic strain of Pseudomonas aeruginosa. CNS Drugs7, 195–200 (2003). [DOI] [PubMed] [Google Scholar]
- 48.Aaron, S. D. et al. Infection with transmissible strains of Pseudomonas aeruginosa and clinical outcomes in adults with cystic fibrosis. JAMA304, 2145–2153 (2010). [DOI] [PubMed] [Google Scholar]
- 49.Al-Aloul, M., Miller, H., Stockton, P., Ledson, M. J. & Walshaw, M. J. Acute renal failure in CF patients chronically infected by the Liverpool epidemic Pseudomonas aeruginosa strain (LES). J. Cyst. Fibros.4, 197–201 (2005). [DOI] [PubMed] [Google Scholar]
- 50.Al-Aloul, M. et al. Increased morbidity associated with chronic infection by an epidemic Pseudomonas aeruginosa strain in CF patients. Thorax59, 334–336 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mohan, K. et al. Empyema due to a highly transmissible Pseudomonas aeruginosa strain in an adult cystic fibrosis patient. J. Med. Microbiol.59, 614–616 (2010). [DOI] [PubMed] [Google Scholar]
- 52.Gagné-Thivierge, C. et al. A multi-host approach to identify a transposon mutant of Pseudomonas aeruginosa LESB58 lacking full virulence. BMC Res. Notes11, 198 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maher, J. A. & DeStefano, J. The ferret: an animal model to study influenza virus. Lab. Anim.33, 50–53 (2004). [DOI] [PubMed] [Google Scholar]
- 54.Somayaji, R. et al. Long-term clinical outcomes of “Prairie Epidemic Strain” Pseudomonas aeruginosa infection in adults with cystic fibrosis. Thorax72, 333–339 (2017). [DOI] [PubMed] [Google Scholar]
- 55.Fothergill, J. L., Walshaw, M. J. & Winstanley, C. Transmissible strains of Pseudomonas aeruginosa in cystic fibrosis lung infections. Eur. Respir. J.40, 227–238 (2012). [DOI] [PubMed] [Google Scholar]
- 56.Nichols, D. P. et al. Pharmacologic improvement of CFTR function rapidly decreases sputum pathogen density, but lung infections generally persist. J. Clin. Investig.133, 10.1172/JCI167957 (2023). [DOI] [PMC free article] [PubMed]
- 57.Harris, J. K. et al. Changes in airway microbiome and inflammation with ivacaftor treatment in patients with cystic fibrosis and the G551D mutation. Ann. Am. Thorac. Soc.17, 212–220 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Armbruster, C. R. et al. Persistence and evolution of Pseudomonas aeruginosa following initiation of highly effective modulator therapy in cystic fibrosis. mBio15, e00519–24 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nichols, D. P. et al. PROMISE: working with the CF community to understand emerging clinical and research needs for those treated with highly effective CFTR modulator therapy. J. Cyst. Fibros.20, 205–212 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ledger, E. L. et al. Impact of CFTR modulation on Pseudomonas aeruginosa infection in people with cystic fibrosis. J. Infect. Dis. jiae051. 10.1093/infdis/jiae051 (2024). [DOI] [PMC free article] [PubMed]
- 61.Figueroa, W. et al. Mutations in mexT bypass the stringent response dependency of virulence in Pseudomonas aeruginosa. Cell Rep.44, 115079 (2025). [DOI] [PubMed] [Google Scholar]
- 62.Hmelo, L. R. et al. Precision-engineering the Pseudomonas aeruginosa genome with two-step allelic exchange. Nat. Protoc.10, 1820–1841 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pourtois, J. D. et al. New Pseudomonas infections drive Pf phage transmission in CF airways. JCI Insight. 10, e188146 (2025). [DOI] [PMC free article] [PubMed]
- 64.Deatherage, D. E. & Barrick, J. E. Identification of mutations in laboratory evolved microbes from next-generation sequencing data using breseq. Methods Mol. Biol.1151, 165–188 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dodt, M., Roehr, J. T., Ahmed, R. & Dieterich, C. FLEXBAR—flexible barcode and adapter processing for next-generation sequencing platforms. Biology1, 895–905 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li, H. et al. The Sequence alignment/map format and SAMtools. Bioinformatics25, 2078–2079 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Langmead, B. & Salzberg, S. L. Fast gapped-read alignment with Bowtie 2. Nat. Methods9, 357–359 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Putri, G. H., Anders, S., Pyl, P. T., Pimanda, J. E. & Zanini, F. Analysing high-throughput sequencing data in Python with HTSeq 2.0. Bioinformatics38, 2943–2945 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol.15, 550 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Armbruster, C. R. et al. Adaptation and genomic erosion in fragmented Pseudomonas aeruginosa populations in the sinuses of people with cystic fibrosis. Cell Rep.37, 109829 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Facchini, M., De Fino, I., Riva, C. & Bragonzi, A. Long-term chronic Pseudomonas aeruginosa airway infection in mice. J. Vis. Exp. 51019. 10.3791/51019 (2014). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
Genome sequences of all strains generated in the study and clinical isolates can be obtained from BioProject PRJNA1184772, PRJNA934930, and PRJNA1188603. RNA sequencing data are also available in BioProject PRJNA1184772. Metabolomics data can be accessed using the MassIVE ID MSV000096391. Source data has been provided with this paper, and primary data have been uploaded to Figshare (10.6084/m9.figshare.29298269). Source data are provided with this paper.