Abstract
An immunological hierarchy among three H-2Db-restricted cytotoxic T lymphocyte (CTL) determinants in simian virus 40 (SV40) large T antigen (Tag) was described previously: determinants I and II/III are immunodominant, whereas determinant V is immunorecessive. To assess the immunogenicity of each determinant individually and define mechanisms that contribute to the immunorecessive nature of determinant V, we constructed a panel of recombinant vaccinia viruses (rVVs) expressing minigenes encoding these determinants in various polypeptide contexts. We found the following. (i) Immunization of mice with an rVV encoding full-length SV40 Tag resulted in priming for CTL responses to determinants I and II/III but not determinant V. (ii) rVVs encoding peptide I or II/III in the cytosol or targeted to the endoplasmic reticulum (ER) were highly antigenic and immunogenic. (iii) rVVs encoding peptide V minigenes were antigenic and immunogenic if the peptide was targeted to the ER, expressed in the cytosol with short flanking sequences, or expressed from within a self-protein, murine dihydrofolate reductase. (iv) Presentation of the nonflanked peptide V (preceded by a Met codon only) could be enhanced by using a potent inhibitor of the proteasome. (v) H-2Db–epitope V peptide complexes decayed more rapidly than complexes containing epitope I or II/III peptides. In brefeldin A blocking experiments, functional epitope V complexes were detected longer on targets expressing ER-targeted epitope V than on targets expressing forms of epitope V dependent on the transporter associated with antigen processing. Therefore, limited formation of relatively unstable cell surface H-2Db complexes most likely contributes to the immunorecessive nature of epitope V within SV40 Tag. Increasing the delivery of epitope V peptide to the major histocompatibility complex class I presentation pathway by ER targeting dramatically enhanced the immunogenicity of epitope V.
Cells infected with viruses or undergoing oncogenic transformation express new or altered self-proteins that may trigger host immune responses. CD8+ cytotoxic T lymphocytes (CTL) recognize such proteins in the form of small antigenic peptide fragments complexed with cell surface major histocompatibility complex (MHC) class I molecules (32, 86). Peptides presented by MHC class I complexes are generated largely from endogenously synthesized proteins through proteolysis in the cytosol, often by the action of proteasomes (22, 47, 63, 80), and are delivered to the endoplasmic reticulum (ER) by the transporter associated with antigen processing (TAP) (49, 83, 87), where they are assembled with nascent class I molecules. The resulting complex is then transported via the secretory pathway to the cell surface (17, 55, 81, 85). Multiple factors contribute to the ability of a given determinant in a protein to elicit a CTL response. These include the efficiency of production of the peptide and transport to the ER (7, 8, 20, 26, 54, 57), peptide affinity for class I molecules (15, 82), and the frequency of potentially reactive T cells in the repertoire (12, 18, 52, 56).
We have used simian virus 40 (SV40) large tumor, or T, antigen (SV40 Tag) to understand factors which govern the immunogenicity of CTL determinants within a tumor antigen. SV40 Tag is a multifunctional 94-kDa nuclear oncoprotein which can initiate and maintain transformation of a wide variety of cell types in vitro (28). In vivo, the Tag can induce neoplasia with a metastatic potential when expressed as a transgene under control of a tissue-specific promoter or when expressed from the viral promoter (10, 34, 37, 59). In the immunocompetent host, the Tag often induces a vigorous cellular immune response which leads to the rejection of transplanted Tag-induced tumors (reviewed in references 76 and 77). Although SV40 Tag has long served as a model tumor inducer and immunogen in experimental systems, more recent detection of SV40 virus in isolates derived from human tumors has prompted renewed interest in the potential role of the immune system in controlling tumor induction by SV40 (9, 13, 41, 46).
SV40 Tag contains four H-2b-restricted CTL determinants, of which three are H-2Db restricted (19, 43, 51, 77). These include CTL determinants I (residues 206 to 215), II/III (residues 223 to 231), and determinant V (residues 489 to 497). Determinant V was previously characterized as immunorecessive within the context of the full-length Tag (50, 69). That is, determinant V-specific CTL responses were detected only (and then with some difficulty) following immunization of mice with transformed cells expressing a mutated Tag in which the three immunodominant determinants (determinants I, II/III, and IV) were deleted or rendered nonantigenic (50, 69).
To further characterize the immunological potential of the H-2b-restricted SV40 Tag CTL epitopes, including the immunorecessive determinant V, we have extended these findings by examining the antigenicity and immunogenicity of SV40 Tag CTL determinants expressed in various polypeptide contexts from recombinant vaccinia viruses (rVVs). Our results show that the immunogenicity of the immunorecessive epitope V is significantly enhanced by targeting it to the ER.
MATERIALS AND METHODS
Plasmid construction and manipulation.
Plasmid DNA used for sequencing and transfection was routinely prepared by the QIAwell-8 procedure (QIAGEN Inc., Chatsworth, Calif.).
The shuttle vector plasmid pSC11 (14) was kindly provided by B. Moss (National Institutes of Health, Bethesda, Md.). The plasmid was modified (generating pSC-SKNN) by insertion of synthetic oligonucleotides to convert the unique SmaI site into a multiple cloning site containing recognition sites for the restriction enzymes SalI, KpnI, NcoI, and NotI so that these cloning sites are located downstream of the viral P7.5 promoter.
Minigenes encoding CTL determinants were generated by ligation of complementary synthetic oligonucleotide pairs into plasmid pSC-SKNN which had been digested with SalI and NcoI. The CTL determinant sequences were preceded by an ATG initiation codon and followed by two termination codons to ensure efficient translation termination (6, 26). The sequence CCACC was included upstream of the initiating ATG codon to promote efficient translation (38). Minigenes representing CTL determinants fused to the adenovirus type 5 E3/19K signal sequence were constructed by insertion of annealed oligonucleotide pairs into a modified pSC11 plasmid so that an additional Ala codon was inserted between the 16-residue ES sequence and the N terminus of the CTL determinant (67).
To construct pSC-I-V-II/III, a group of overlapping complementary oligonucleotides was phosphorylated with T4 polynucleotide kinase, annealed to form a longer double-stranded DNA fragment with SalI and NcoI complementary overhanging 5′ termini, and ligated into pSC-SKNN vector which had been digested with SalI and NcoI. The pSC-I-Db-II/III plasmid was constructed similarly, but oligonucleotides which encoded an H-2Db-binding motif–determinant sequence (31, 50) were used in place of the determinant V-encoding oligonucleotides. The sequence and orientation of all determinant-encoding inserts within the pSC-derivative plasmids were confirmed by DNA sequencing.
pSC-941T was constructed by cloning a modified SV40 Tag-encoding cDNA as a SalI fragment into the pSC-SKNN vector. The SV40 Tag cDNA was obtained as a BamHI fragment from plasmid p941T (39) and ligated into the BamHI site of a modified pUC19 (84) polylinker region in which the SmaI site had been converted to a SalI site. The 941T fragment was recovered by SalI digestion and ligated into plasmid pAlter for site-directed mutagenesis. Two sequences (nucleotides 4231 to 4225 and 3134 to 3128 [79]) which might serve as cryptic transcription termination signal sequences for the vaccinia virus transcriptase (TTTTTNT [23]) were mutated by the Altered Sites mutagenesis procedure (Promega Corp., Madison, Wis.) without altering the amino acid sequence of the Tag. The mutagenized 941T fragment was transferred into SalI-gapped pSC-SKNN (producing pSC-941T) for vaccinia virus construction (see below).
CTL determinants were inserted into the coding sequence for murine dihydrofolate reductase (DHFR) by ligation of synthetic oligonucleotide pairs into a unique SstI site. The DHFR derivative used had been previously altered to include the pAb 901 monoclonal antibody recognition determinant derived from SV40 Tag (29).
Animals.
Four- to six-week-old male C57BL/6 (B6/) mice were purchased from Jackson Laboratory, Bar Harbor, Maine, and routinely used between the ages of 5 and 12 weeks. All mice were maintained in the animal facility at Pennsylvania State University College of Medicine, Hershey.
Cell lines and viruses.
B6/WT-19 cells have been described previously (50, 78). B6/K-1,4,5 (H-2b) cells, which were generated by sequential cocultivation of B6/K-0 cells (B6/K-0 cells express wild-type SV40 Tag) with SV40 Tag-specific CTL clones, have been described elsewhere (71, 72). B6/K-1,4,5 cells are resistant to lysis by SV40 Tag-specific CTL clones due to a deletion which removes sequences encoding determinants I and II/III and to point mutations which alter determinants IV and V (42, 51).
B6/T117A1 cells were derived by isolation of a Tag-expressing, immortalized focus following calcium phosphate-mediated transfection of mouse embryo fibroblasts with the plasmid pSLM361-13 as described elsewhere (50, 73). Plasmid pSLM361-13 was generated from plasmid pLM247 (50) by site-directed mutagenesis using the Altered Sites procedure. B6/T117A1 cells express a Tag derivative in which only determinants I (Δ207-215) and II/III (Δ223-231) are deleted and in which three residues within determinant IV (residues 406, 408, and 411) have been replaced by Ala residues.
Tag-transformed cell lines were routinely maintained in closed vessels in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10 or 5% (vol/vol) heat-inactivated fetal bovine serum (FBS) (HyClone, Logan, Utah), 2 mM l-glutamine, 100 U of penicillin per ml, 100 μg of streptomycin per ml, 25 μg of kanamycin per ml, 20 mM HEPES, and 0.0225% sodium bicarbonate.
HuTK−143 cells were derived from human osteosarcoma cells (ATCC CRL-8303) (62). The CV-1 cell line is a continuous cell line derived from African green monkey kidney cells (ATCC CCl-70) (45). Ltk/Db cells have been described previously (3); these cells were generated by transfecting Ltk− cells (H-2k) with the H-2Db gene, along with the pSV2-neo plasmid as a selectable marker.
T2/Db cells (2) were generously provided by P. Cresswell (Yale University, New Haven, Conn.). The cells were maintained in Iscove’s modified Dulbecco’s medium (IMDM) supplemented with 10% FBS, 0.0225% sodium bicarbonate, 25 mM HEPES, 2 mM l-glutamine, 100 U of penicillin per ml, and 100 μg of streptomycin per ml, as well as 5 × 10−5 M β-mercaptoethanol and 100 μg of G418 per ml to maintain selection for the transfected H-2Db gene. The RMA cell line and the antigen presentation-defective, TAP2-deficient RMA/s cell line have been described elsewhere (36, 44).
SV40 strain VA45-54 (74) was prepared as described previously (75). The wild-type vaccinia virus strain (Western Reserve isolate [WR]) was propagated in HuTK−143 cells. The vaccinia virus recombinant TM-1 (referred to in this study as rVV-Dhfr/) has been described elsewhere (29).
SV40-specific CTL clones.
SV40-specific CTL clones Y-1 or K-11, Y-2 or K-19, and Y-5 or H-1 recognize, respectively, SV40 Tag CTL determinants I (Tag amino acid residues 206 to 215), II/III (residues 223 to 231), and V (residues 489 to 497) (11, 19, 43, 50, 69, 70). The CTL clones were maintained in vitro as described previously (19, 50).
Generation of rVVs.
The procedure for generating rVVs was modified from a protocol described elsewhere (24). CV-1 cells in 25-cm2 cell culture flasks were infected with wild-type vaccinia virus (WR strain; multiplicity of infection [MOI] of 0.1) in 1 ml of PBS/BSA (phosphate-buffered saline [PBS] [pH 7.4] supplemented with 0.1% [wt/vol] bovine serum albumin [BSA]). After 2 h, the virus solution was aspirated and replaced with 5 ml of complete DMEM and calcium phosphate-DNA precipitates, which contained 10 μl of pSC-derivative recombinant plasmid DNA (20 μg) and were prepared by using a CellPhect transfection kit (Pharmacia LKB Biotechnology Inc., Uppsala, Sweden). The cultures were incubated at 37°C in 5% CO2 for 2 to 3 days, a time at which cytopathic effects were evident. The cells were harvested by trypsinization, lysed in 1 ml of PBS/BSA by three cycles of freeze-thawing, and sonicated for 1 min. The cell lysate was diluted and used to infect HuTK-143 cells in a six-well plate in the presence of 25 μg of 5-bromodeoxyuridine (to select thymidine kinase-negative [TK−] progeny viruses) per ml in plaquing medium (equal-volume mixture of 1.8% Noble agar and 2× Eagle’s medium supplemented with 10% FBS) at 37°C in 5% CO2. Potential recombinants were further screened for β-galactosidase expression. After 48 to 72 h, the wells were overlaid with 2 ml of plaquing medium supplemented with 0.025% 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) and incubated until blue plaques appeared (usually overnight). Blue plaques were picked and subjected to three rounds of plaque purification. Viral stocks were prepared by reinfection of HuTK−143 cells.
Cytotoxicity assay.
Cytotoxicity assays were performed as described previously (19, 50, 70). Vaccinia virus-infected targets were prepared by one of two procedures for use in CTL assays. Fibroblasts were harvested by trypsinization and washed with media and PBS/BSA, and 5 × 105 cells were infected in 15-ml conical tubes at an MOI of 10 in a final volume of 0.5 ml of PBS/BSA for 1 h at 37°C. Infection suspensions were then diluted with 5 ml of complete DMEM and rocked at 37°C for 3 h. The infected cells were then labeled with Na51CrO4 for an additional 30 min, washed three times, and combined with effectors. Alternatively, fibroblast monolayers were labeled overnight in culture with Na51CrO4, harvested by trypsinization, and infected as described above. For some experiments, infections were performed in T/B, which is PBS/BSA supplemented with an equal volume of incomplete DMEM prepared as described previously (50) but without FBS and sodium bicarbonate.
Targets were also prepared for CTL assays by incubation of fibroblasts (B6/K-1,4,5) or nonadherent cells (RMA) in media containing synthetic peptides corresponding to CTL determinants. Either the targets were incubated in suspension with both peptide and Na51CrO4, or peptide was added after the cells had been labeled and washed free of unincorporated 51Cr. Unbound peptides and unincorporated Na51CrO4 were subsequently removed by a minimum of two washes.
In experiments involving proteasome inhibitors, 51Cr-labeled targets were incubated in T/B containing inhibitor for at least 30 min prior to addition of virus. Targets were maintained in the presence of inhibitor throughout the infection period but were washed free of inhibitor before being combined with effectors in the cytotoxicity assay.
51Cr-labeled targets infected with vaccinia virus recombinants were incubated in the presence of 4 mg of brefeldin A (BFA) per ml for various times to monitor the relative rates of H-2Db-peptide complex decay (50, 85). Briefly, Ltk/Db cells were labeled in culture overnight with 51Cr, harvested by trypsinization, washed in media and T/B, combined with virus at an MOI of 10 in 1 ml of T/B, and incubated at 37°C. After 4 h, infected cells were diluted with T/B containing BFA for a final concentration of 4 mg/ml, and incubation was continued at 37°C. Targets were washed in T/B containing 2 mg of BFA per ml, resuspended in RPMI containing 2 mg of BFA per ml, combined with equal volumes of effector suspension in 96-well plates, centrifuged, and incubated for 4 h at 37°C in 5% CO2.
Generation of bulk culture vaccinia virus-specific CTL for in vitro cytotoxicity assays.
Bulk culture vaccinia virus-specific secondary in vitro-stimulated CTL (Vac-CTL) were generated by a procedure modified from that described by Eisenlohr and coworkers (25). Briefly, a single-cell suspension was prepared from the spleens of two mice which had been immunized intravenously (i.v.) with wild-type vaccinia virus (rVV-WR; 2 × 106 PFU/mouse) for at least 2 weeks prior to sacrifice. One-third of the splenocytes were infected with 108 PFU of rVV-WR for 1 h at 37°C in 1 ml of PBS/BSA. After three washes with complete IMDM, the infected splenocytes were mixed with the remaining splenocytes and seeded into 12-well plates in complete IMDM. The cultures were maintained at 37°C in 5% CO2 for 5 to 7 days before being used in cytotoxicity assays.
Generation of bulk culture SV40 Tag-specific CTL from mice immunized with vaccinia virus recombinants.
Bulk culture SV40 Tag-specific CTL were generated from splenic lymphocytes isolated at least 3 weeks following immunization of groups of four C57BL/6 mice with 0.5 × 107 to 1 × 107 PFU of rVV (i.v.). Splenic lymphocytes were prepared and restimulated in vitro with gamma-irradiated B6/WT-19 cells as described elsewhere (50). To verify vaccinia virus infection, 2 × 107 splenocytes from the same groups were restimulated for 6 days in loosely capped, upright T25 flasks containing 12 ml of RPMI medium supplemented with 1 × 106 naive C57BL/6 splenocytes which had been infected for 4 h with rVV-WR (MOI, 10), washed free of virus, and gamma irradiated on ice (60,000 rads).
Peptide transport assays utilizing SLO-permeabilized Ltk/Db cells.
The capacity of TAP to transport peptides was determined as described by Androlewicz et al. (4). Briefly, TYNRTRALV synthetic peptide (20 μg) was iodinated with 1 mCi of Na125I by using chloramine T to a specific activity of 6 × 104 to 9 × 104 cpm/pmol (125I-TYN). Ltk/Db cells were washed once in serum-free medium and incubated for 10 min on ice with streptolysin O (SLO) (Murex, Norcross, Ga.) at 1 U/ml. The cells were then washed three times in serum free-medium and resuspended in intracellular transport buffer (ICT) (50 mM HEPES [pH 7.0], 78 mM KCl, 4 mM MgCl2, 8.37 mM CaCl2, 10 mM EGTA, 1 mM dithiothreitol, 10 mM ATP) containing BSA (4 mg/ml). SLO-permeabilized cells (106/sample) were incubated with 125I-TYN (125 ng) mixed with different amounts of unlabeled peptides at 37°C for 30 min. The cells were then lysed with 2% Triton X-100–0.15 M NaCl–10 mM Tris (pH 7.4)–5 mM phenylmethylsulfonyl fluoride, and the lysate was incubated with 50 μl of packed concanavalin A (ConA)-Sepharose to recover glycosylated 125I-TYN. The amount of bound peptide was quantitated by gamma counting.
Relative decay rates of H-2Db complexes stabilized on RMA/s cells by synthetic peptides corresponding to SV40 Tag CTL epitopes.
Relative rates of H-2Db-peptide complex decay were monitored by flow cytometric analysis of H-2Db complexes remaining on the surface of RMA/s cells following incubation at 37°C. Briefly, RMA/s cells were incubated overnight at 29°C (5% CO2) in the presence of synthetic peptides corresponding to SV40 Tag CTL epitopes as described previously (43, 50). The cells were washed free of peptide and resuspended in complete RPMI medium lacking peptide, and incubation was continued at 37°C for up to 6 h. Aliquots were removed at appropriate intervals and stored on ice to stabilize the remaining complexes. Samples were prepared for flow cytometric analysis of H-2Db molecules using the monoclonal antibody 28-14-8 (60) as described elsewhere (43, 50).
RESULTS
CTL recognition of SV40 Tag determinants expressed from rVVs.
To study the immunogenicity and antigenicity of three H-2Db-restricted SV40 Tag determinants, rVVs (Fig. 1A) that express each determinant as a cytosolic peptide with an NH2-terminal Met to enable efficient translation initiation (25) or with an NH2-terminal 16-amino-acid ER insertion sequence from the adenovirus type 5 E3/19K glycoprotein (plus an additional Ala) to allow for TAP-independent presentation were constructed (1, 26, 67). We also constructed rVVs expressing full-length Tag or a polydeterminant minigene consisting of determinants I, V, and II/III fused without intervening residues (again with an initiating NH2-terminal Met) to examine CTL recognition and immunogenicity for these epitopes when processed and presented from within more complex substrates.
FIG. 1.
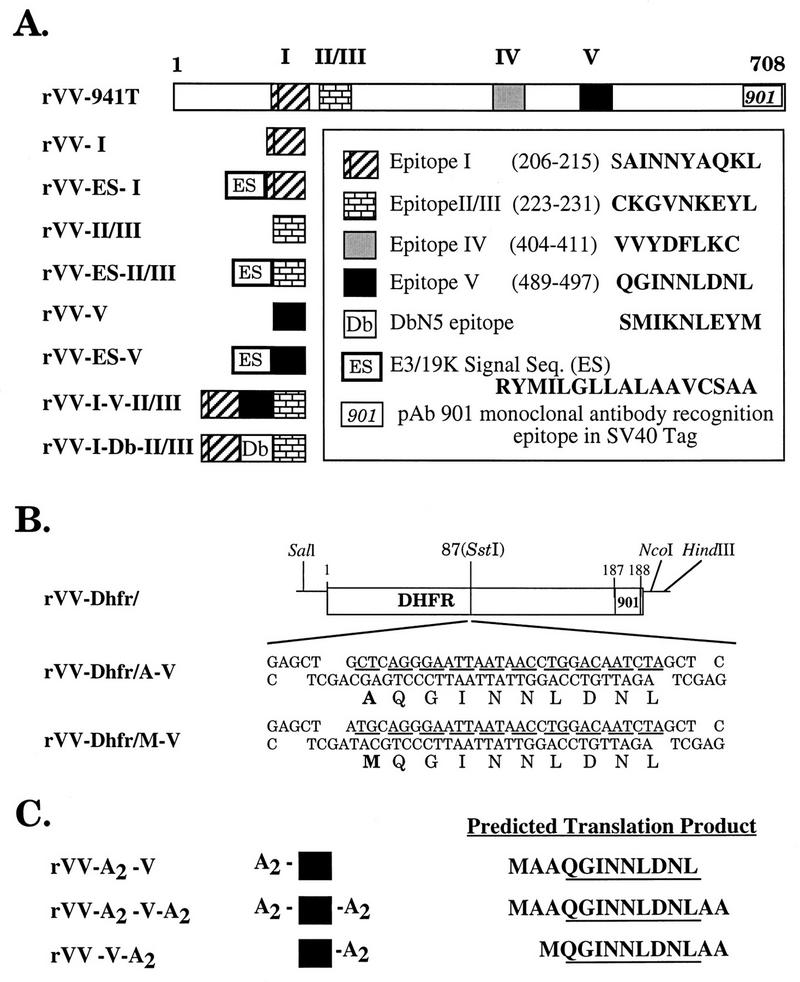
rVVs which express SV40 Tag CTL determinants. Amino acid sequences are shown as single letters. Initiating Met codons were included in the construction of all minigene constructs but are not indicated in the diagrams. (A) Full-length SV40 Tag cDNA (941T) and CTL determinant minigenes constructed with or without an ES sequence. (B) Determinant V derivatives inserted into a murine DHFR derivative bearing the SV40 Tag 901 monoclonal antibody determinant. (C) (Ala)2-flanked determinant V minigenes. Initiating Met residues are included in the predicted translation products.
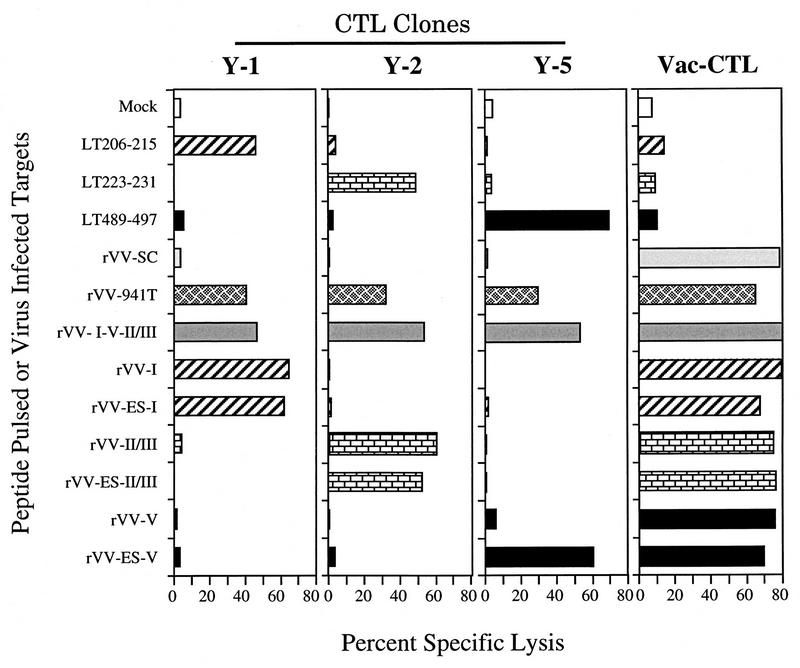
The antigenicity of the Tag determinants produced by the various rVVs was analyzed by infecting B6/K-1,4,5 cells and testing for lysis by CTL clones with a standard 51Cr release assay. The effectors used for the initial assays were CTL clones Y-1, Y-2, and Y-5, which recognize SV40 Tag determinant I, II/III, or V, respectively. Vac-CTL were used to verify infection of target cells.
As shown in Fig. 2, B6/K-1,4,5 cells pulsed with the synthetic determinant peptides were specifically recognized by the respective Tag-specific CTL clones Y-1, Y-2, and Y-5 but not by the Vac-CTL, confirming the specificity of the clones. Cells infected with rVV-941T or rVV-I-V-II/III were lysed by each of the Tag-specific CTL clones, as well as by the Vac-CTL. Comparison of CTL lysis suggested that determinants I, II/III, and V were presented from rVV-I-V-II/III at least as efficiently as from rVV-941T. Target cells infected with rVV-I and rVV-ES-I were specifically recognized by clone Y-1, whereas the cells infected with rVV-II/III and rVV-ES-II/III were lysed by clone Y-2. Target cells infected with rVV-ES-V were recognized by clone Y-5, but, surprisingly, cells expressing the cytosolic determinant V peptide were lysed at very low levels. This is unlikely to be due to poor infection, since all the rVVs sensitized cells for comparable lysis by the Vac CTL.
FIG. 2.
CTL recognition of rVVs. B6/K-1,4,5 cells were pulsed with synthetic peptides corresponding to determinant I (LT206-215), determinant II/III (LT223-231), or determinant V (LT489-497) or infected with rVVs expressing the full-length SV40 Tag (rVV-941T), minigenes corresponding to Tag CTL determinants (rVV−), or bearing no Tag insert (rVV-SC). ES, the 16-residue E3/19K signal sequence derived from adenovirus (Fig. 1). Targets were combined with SV40 Tag-specific CTL clone Y-1, Y-2, or Y-5 at effector-to-target cell (E/T) ratios of 10:1 or with Vac-CTL) at an E/T ratio of 40:1 for 4 h.
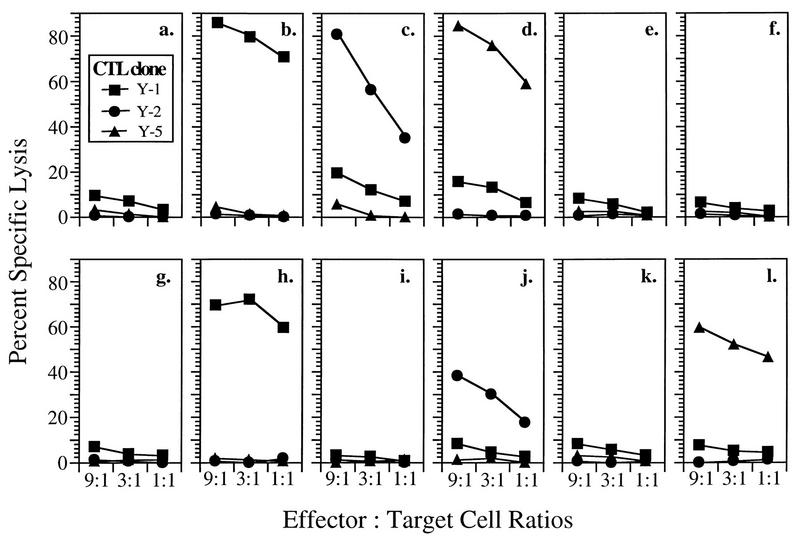
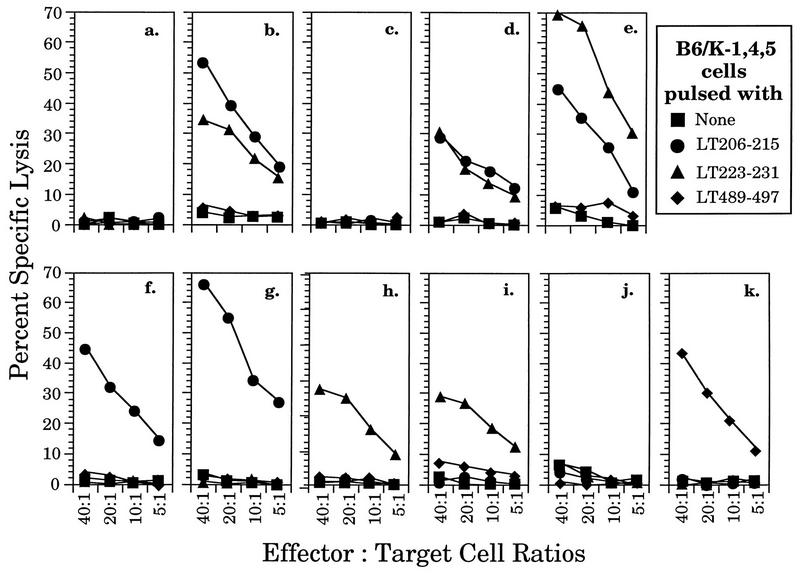
The TAP dependence of presentation of the rVV-encoded peptides was determined by using T2 cells expressing a Db transgene (T2/Db cells). T2 cells are defective in presentation of MHC class I-restricted antigens due to a deletion of the region of the MHC which encodes TAP (2). T2/Db cells were specifically lysed by the appropriate Tag-specific CTL clones when infected with rVVs expressing ER-targeted peptides but not with any of the rVVs expressing cytosolic peptides or proteins (Fig. 3). Thus, as with other antigenic peptides (6, 67), Tag determinants expressed from rVVs are presented in a TAP-dependent manner unless they are specifically targeted to the ER by an NH2-terminal leader sequence.
FIG. 3.
The ES leader sequence allows presentation of Tag CTL epitopes in TAP1/2-deficient T2/Db cells. T2/Db cells were either pulsed with synthetic peptides corresponding to epitope I, II/III, or V (designated by LT) or infected with rVVs expressing the Tag CTL epitope minigenes and incubated with the CTL clone Y-1, Y-2, or Y-5 at the indicated E/T ratios for 4 h. (a) Mock infection; (b) LT206-215; (c) LT223-231; (d) LT489-497; (e) rVV-SC; (f) rVV-941T; (g) rVV-I; (h) rVV-ES-I; (i) rVV-II/III; (j) rVV-ES-II/III; (k) rVV-V; (l) rVV-ES-V. See Fig. 1 for further details.
CTL recognition of determinant V is influenced by short flanking sequences.
A trivial explanation for the failure of rVV-V to sensitize target cells for lysis by determinant V-specific CTL was that this recombinant failed to produce the proper peptide. Since the only means of identifying such minigene products is by CTL recognition, it was not possible to completely eliminate this possibility. Three additional rVVs independently produced from the original shuttle plasmid similarly failed to sensitize infected targets for lysis by determinant V-specific CTL clones (data not shown).
Studies conducted with Saccharomyces cerevisiae suggested that the initiating Met was likely to be retained by the determinant V peptide expressed from rVV-V (48). It was unclear whether the Met extension would interfere with presentation of the determinant V peptide. To explore this possibility, we synthesized peptide derivatives of determinant V extended at the amino terminus with a single Met and/or Ala residue and examined these peptides for Db binding and recognition by determinant V-specific CTL clones. Our results indicated that single Met or Ala NH2-terminal extensions reduced the ability of determinant V synthetic peptides to sensitize target cells for lysis by Y-5 by at least 10- to 100-fold (in assays performed in the presence or absence of FBS, respectively). We also compared H-2Db binding of the Met- or Ala-extended 10-mer peptides and the determinant V 9-mer. Consistent with results obtained from the CTL lysis assays, the ability of the NH2-extended peptides to stabilize H-2Db molecules on tap2-deficient RMA/s cells was also reduced greater than 10-fold (data not shown). These results implied that extension of the determinant V peptide by a single residue (Ala or Met) might significantly reduce peptide presentation in vivo.
To determine whether an NH2-terminal Met would always interfere with presentation of determinant V, derivatives of mouse DHFR into which the sequence MQGINNLDNL (rVV-Dhfr/M-V) or AQGINNLDNL (rVV-Dhfr/A-V) was inserted were generated (Fig. 1B). The DHFR derivative utilized serves as an efficient carrier for heterologous CTL determinants in vitro and in vivo (29). Ltk/Db cells infected with rVV-Dhfr/M-V or rVV-Dhfr/A-V were recognized by the determinant V-specific CTL clone H-1 (Table 1); in fact, the Met-flanked determinant V peptide appeared to be presented better than the Ala-flanked peptide. These results demonstrate that processing and presentation of determinant V are not prevented by the presence of a Met residue immediately adjacent to the NH2 terminus of determinant V when the determinant is located in the context of a larger polypeptide.
TABLE 1.
Lysis by SV40-specific CTL clones of targets infected with rVVs expressing murine DHFR derivatives bearing epitope V inserts
| rVV or peptidea | % Specific target cell lysis by CTL cloneb:
|
|
|---|---|---|
| K-19 | H-1 | |
| rVV-I-V-II/III | 74 | 38 |
| rVV-V(cl2) | 2 | 9 |
| rVV-ES-V | 4 | 62 |
| rVV-ES-I | 1 | 0 |
| rVV-Dhfr/A-V | ||
| c11 | 2 | 23 |
| c12 | 1 | 19 |
| rVV-Dhfr/M-V | ||
| cl1 | 2 | 49 |
| cl2 | 2 | 39 |
| LT489-497 | 1 | 53 |
| None | 1 | 0 |
51Cr-labeled Ltk/Db cells were incubated with 1 mM LT489-497 or infected with the indicated vaccinia virus recombinant (MOI = 10) (Fig. 1) for 5 h.
SV40 Tag-specific CTL clones K-19 (epitope II/III specific) and H-1 (epitope V specific) were used at E/T ratios of 12:1 and 15:1, respectively.
We next examined whether addition of only short extensions [(Ala)2] to the NH2 and/or COOH terminus of determinant V sequence (Fig. 1C) might allow for presentation of determinant V from an rVV-encoded minigene. Results presented in Fig. 4 reveal that addition of (Ala)2 to the COOH terminus had the greatest enhancing effect on presentation, but addition to the NH2 terminus also allowed for recognition of the determinant V minigene by CTL clone Y-5. Addition of (Ala)2 to both termini resulted in presentation at levels similar to that observed for the NH2-terminally extended derivative. Similar results were obtained with H-1, the other determinant V-specific CTL clone (data not shown). These results suggest that short (Ala)2 extensions overcome a defect which prevents efficient presentation of the cytosolic determinant V from rVV-V. Since the amino termini of the determinant V derivatives encoded by rVV-V and rVV-V-A2 recombinants are identical, it was of interest to determine how the COOH (Ala)2 extension might enhance presentation of the determinant V peptide.
FIG. 4.
CTL recognition of rVVs expressing (Ala)2-extended determinant V derivatives. Ltk/Db cells were mock infected (a), pulsed with a synthetic peptide corresponding to determinant V (LT489-497 [b]), or infected with rVVs expressing the full-length SV40 Tag (rVV-941T [d]), a minigene corresponding to fused determinants I, V, and II/III (rVV-I-V-II/III [e]), or various determinant V derivatives (rVV-V, -ES-V, -A2-V, -V-A2, and -A2-V-A2 [f to j, respectively]) or an rVV bearing no Tag insert (rVV-SC [c]). See Fig. 1 for descriptions. Targets were combined with SV40 Tag-specific CTL clone Y-5 or Vac-CTL at the indicated E/T ratios for 4 h.
Synthetic peptides corresponding to the determinant V sequence and extended at the amino and/or carboxy terminus with two alanine residues were synthesized and assayed for CTL recognition and Db binding (stabilization). The results of these assays were consistent with results obtained from the analysis of the Met-extended 10-mer determinant V derivative (see above) and revealed that (Ala)2 extensions at the amino and/or carboxy terminus reduced both CTL recognition and Db binding (stabilization) relative to those of the 9-mer determinant peptide (>100-fold reduction; data not shown). These results implied that rescued presentation of the cytosolic determinant V peptide from (Ala)2-appended rVV-V-like recombinants most likely did not result from recognition of an extended determinant V peptide by the determinant V-specific CTL clones. Clearly, however, addition of flanking sequences did overcome a presentation defect apparently unique to the Met-extended determinant V peptide.
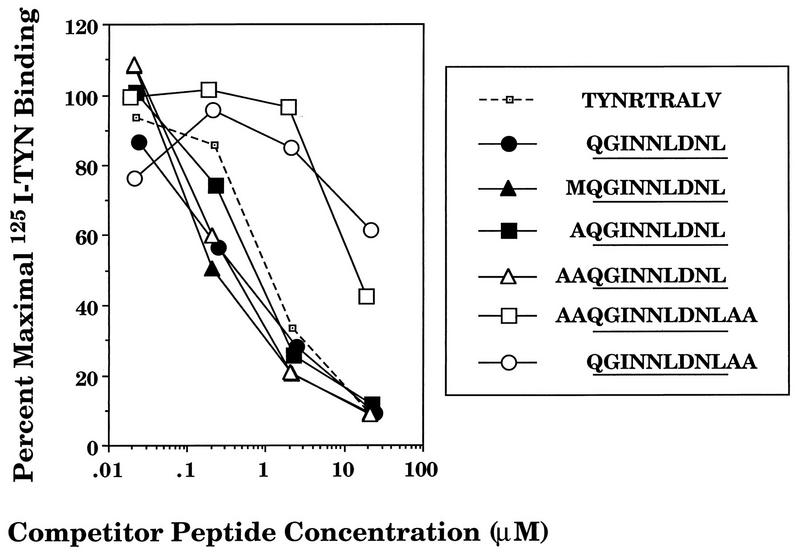
Neisig and coworkers have previously reported that synthetic peptides corresponding to SV40 Tag minimal determinants V and I are efficiently transported by TAP in permeabilized RMA cells (53). We examined the capacity of TAP to transport the minimal and extended determinant V peptides into the ER. Ltk/Db cells were permeabilized with SLO and incubated with an iodinated peptide from influenza virus nucleoprotein (NP) containing an N-linked glycosylation site (T125IYNRTALV [4]). The transport of the T125IYNRTALV peptide into the ER is monitored by its binding to ConA-Sepharose, which is dependent on the addition of an N-linked oligosaccharide. The capacity of TAP to transport Tag peptides was inferred by their abilities to block glycosylation of the indicator peptide. In preliminary experiments using T2 cells and an rVV expressing both TAP subunits, we confirmed that glycosylation of the radioiodinated peptide under the same experimental conditions is strictly dependent upon TAP expression (data not shown). As seen in Fig. 5, in Ltk/Db cells the determinant V synthetic peptide competed with an efficiency similar to that of the unlabeled nucleoprotein peptide, demonstrating that it is efficiently transported by mouse TAP. Extension of the amino terminus by a single Met or Ala or two Ala residues had no significant effect on TAP-mediated transport. By contrast, extension of the carboxy terminus with two Ala residues reduced TAP-mediated transport. This is consistent with reports that mouse TAP prefers peptides which terminate with a hydrophobic residue (66). These findings indicate that TAP should not be a limiting factor in the presentation of the determinant V peptide encoded by rVV-V.
FIG. 5.
Relative transport efficiencies of determinant V peptide derivatives. SLO-permeabilized Ltk/Db cells were used to assay inhibition of transport of a radiolabeled peptide (125I-TYN) corresponding to the NP determinant TYNRTRALV. The permeabilized cells were supplemented with 125I-TYN in the presence of synthetic peptides corresponding to determinant V derivatives. Inhibition of transport is indicated by reduction in percentage of 125I-TYN peptide recovered by lectin affinity chromatography.
A proteasome inhibitor allows limited recognition of rVV-V by a determinant V-specific CTL clone.
Our results indicate that a COOH (Ala)2 extension apparently overcomes a defect which blocks presentation of the cytosolic rVV-V determinant V peptide. Since the NH2 coding sequences of the two minigenes (rVV-V and rVV-V-A2) are identical (Fig. 1A and C), it is unclear how the COOH extension could increase the efficiency with which the initiating Met residue is removed. TAP does not appear to represent a pertinent limitation, as the (Ala)2-extended determinant V synthetic peptides are transported with 100-fold-decreased efficiency (Fig. 5). Alternately, addition of two COOH-terminal Ala residues may simply stabilize the Met-extended determinant V peptide against rapid destruction by cytosolic proteases.
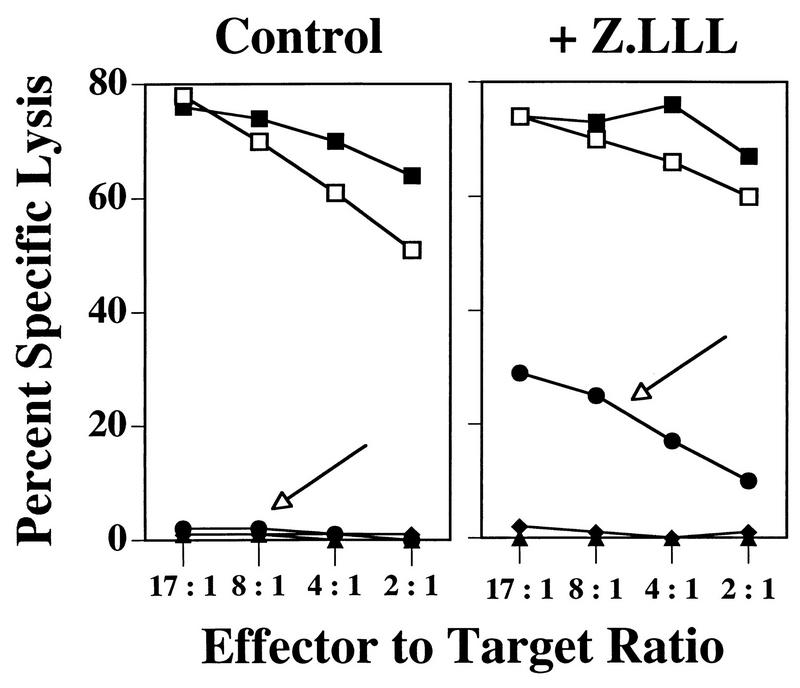
To examine the stability of the rVV-V-encoded product, we utilized the peptide aldehyde proteasome inhibitor cbz-LLL-CHO (MG132), which has been shown to reduce protein degradation within intact cells and to interfere with the generation of antigenic peptides from proteins (33, 58, 88). Incubation with cbz-LLL-CHO did indeed enhance presentation of the determinant V minigene product to CTL clone H-1 (Fig. 6). This effect was specific, as H-1 recognition of targets infected with an irrelevant vaccinia virus recombinant (rVV-ES-I) was not induced; inappropriate recognition of rVV-V by a determinant I-specific CTL clone was not induced under the same conditions (data not shown).
FIG. 6.
CTL clone H-1 recognition of Ltk/Db targets infected with rVV-V is rescued by incubation in the presence of the proteasome inhibitor cbz-LLL-CHO. 51Cr-labeled Ltk/Db cells were infected with vaccinia virus recombinants or pulsed with the peptide LT489-497 in the presence (+Z.LLL) or absence (Control) of 60 μM cbz-LLL-CHO for 5 h. The targets were washed and combined with the CTL clone H-1 at the E/T ratios indicated. Lysis of the rVV-V-infected targets is indicated (arrows). Vaccinia virus recombinants are described in Fig. 1. ▪, rVV-ES-V; •, rVV-V; ▴, rVV-ES-I; ⧫, no rVV; □, LT489-497.
These results are consistent with the idea that the poor antigenicity of the determinant V minigene product (expressed from rVV-V) results from rapid cytosolic proteolysis. Inefficient removal of the NH2-terminal Met extension may contribute indirectly by interfering with H-2Db binding and thereby reduce the amount of determinant V complexes available for CTL recognition.
Immunogenicity of rVVs expressing Tag determinants.
To correlate the findings from these in vitro assays to antigen presentation in vivo, we determined the capacity of the rVVs to prime splenocytes for secondary in vitro restimulation by Tag-expressing tissue culture cells. The lytic activity of restimulated splenocyte cultures was determined by using target cells pulsed with appropriate synthetic peptides (Fig. 7).
FIG. 7.
Induction of CTL responses in vivo by vaccinia virus recombinants expressing SV40 Tag or Tag CTL determinant minigenes. Splenocytes from naive or immunized mice were restimulated in vitro with B6/WT-19 cells and were combined at the indicated E/T ratios with 51Cr-labeled B6/K-1,4,5 cells which had been incubated in media only (None) or media supplemented with synthetic peptides corresponding to determinant I (LT206-215), II/III (LT223-231), or V (LT489-497). The following viruses were used for immunizations: none (a), SV40 (b), rVV-SC (c), rVV-941T (d), rVV-I-V-II/III (e), rVV-I (f), rVV-ES-I (g), rVV-II/III (h), rVV-ES-II/III (i), rVV-V (j), and rVV-ES-V (k).
Immunization of C57BL/6 mice with SV40 (Fig. 7b) or a vaccinia virus recombinant expressing full-length SV40 Tag (rVV-941T [Fig. 7d]) led to induction of CTL specific for determinants I and II/III but not determinant V. rVVs expressing minigenes encoding cytosolic or ER-targeted determinants I (Fig. 7f and g) or II/III (Fig. 7h and i) were effective in inducing CTL specific for the corresponding peptides. In the case of determinant I, responses were at least as vigorous as those induced by rVV-941T (full-length Tag). The rVV encoding the I-V-II/III polydeterminant minigene was the most efficient primer of a site II/III response, which was similar in magnitude to that induced by rVV-I. CTL generated under similar conditions specifically recognized determinants processed and presented on SV40 Tag-transformed cell lines (data not shown). Splenocytes obtained from mice which received no virus or mice immunized with a vaccinia virus recombinant lacking a Tag-derived insert (rVV-SC) did not show specific cytolytic activity towards targets pulsed with SV40 CTL determinant peptides (Fig. 7a and c).
Immunization with SV40 or rVV-941T failed to induce CTL specific for determinant V (Fig. 7b and d). Similarly, rVV-I-V-II/III failed to induce detectable levels of determinant V-specific CTL in these experiments. As expected from its poor antigenicity, rVV-V failed to induce determinant V-specific CTL in vivo (Fig. 7j). Most importantly, rVV-ES-V induced determinant V-specific CTL (Fig. 7k), and indeed the lytic activity of the cultures was similar to that induced by rVVs expressing cytosolic determinant I or II/III. These results clearly indicate that CTLs specific for determinant V can be expanded upon appropriate immunization, in this case with rVV-ES-V.
During the course of this study, we found that the sensitivity of CTL assays utilizing bulk-cultured CTL as effectors could be increased by using RMA cells in place of B6/K-1,4,5 cells as peptide-pulsed targets. This enabled detection of determinant V-specific CTL following immunization with rVV-I-V-II/III (Table 2 and data not shown). CTL precursor frequency measurements (35, 50, 51) performed under similar conditions revealed that rVV-ES-V was at least fourfold more efficient in inducing determinant V-specific CTL than was rVV-I-V-II/III (data not shown). In numerous experiments, determinant V-specific CTL were not detected following immunization with SV40 Tag-transformed cells (wild-type T), rVV-941T, or rVV-V, regardless of the target cell type used for the subsequent cytolysis assay (data not shown).
TABLE 2.
Induction of SV40-specific CTL by immunization of C57BL/6 mice with vaccinia virus recombinants expressing epitope V minigene derivatives
| Expt and rVV used for immunizationa | % Specific target cell lysisb
|
|||
|---|---|---|---|---|
| RMA + LT206-215 | RMA + LT489-497 | RMA + DbN5 | B6/T117A1 | |
| 1 | ||||
| rVV-V (cl4) | 3 | 3 | 3 | 2 |
| rVV-ES-V | 6 | 76 | 5 | 42 |
| rVV-A2-V | 4 | 35 | 5 | 18 |
| rVV-A2-V-A2 | 4 | 13 | 5 | 5 |
| rVV-V-A2 | 4 | 46 | 3 | 17 |
| rVV-I-V-II/III | 87 | 54 | 7 | 42 |
| rVV-I-Db-II/III | 82 | 4 | 3 | 2 |
| 2 | ||||
| rVV-V (cl2) | 3 | 2 | 2 | NDc |
| rVV-ES-V | 8 | 50 | 4 | ND |
| rVV-A2-V | 8 | 47 | 6 | ND |
| rVV-A2-V-A2 | 11 | 47 | 6 | ND |
| rVV-V-A2 | 7 | 29 | 3 | ND |
| rVV-I-V-II/III | 100 | 44 | 9 | ND |
| rVV-I-Db-II/III | 100 | 14 | 13 | ND |
Groups of two or four C57BL/6 mice were immunized i.v. with 107 PFU of vaccinia virus recombinants. Splenocytes were restimulated in vitro with B6/WT-19 cells for 6 days and used in a 51Cr release assay at an E/T ratio of 50:1.
51Cr-labeled RMA cells were incubated in RPMI medium containing peptide at a final concentration of 1 μM. B6/T117A1 cells express a derivative of Tag lacking epitopes I, II/III, and IV and were induced with gamma interferon prior to the assay. LT206-215, epitope I; LT489-497, epitope V; DbN5, SMIKNLEYM.
ND, not done.
Since rVV-V failed to induce determinant V-specific CTL in vivo, the rVVs expressing (Ala)2-flanked determinant V peptides were examined as an alternate means of determining the immunogenicity of a cytosolic form of determinant V expressed from a minigene. As shown in Table 2, determinant V-specific CTL were induced by immunization with rVV-A2-V, rVV-A2-V-A2, or rVV-V-A2. Determinant V-specific CTL induced by each recombinant recognized both RMA targets pulsed with the synthetic determinant V peptide LT489-497 and B6/T117A1 cells which present determinant V peptide derived from an endogenously processed SV40 Tag derivative lacking determinants I, II/III, and IV. We note that, on the basis of comparison of the efficiency of target cell lysis in several experiments, CTL induction by the (Ala)2-flanked determinant V minigene constructs was consistently weaker than CTL responses induced by rVV-ES-V (Table 2).
Finally, we examined the immunogenicity of rVVs which expressed murine DHFR derivatives bearing a determinant V insert (Table 3). Both the Met- and the Ala-flanked determinant V inserts were able to induce determinant V-specific responses when located within the DHFR polypeptide context. The composite results of several experiments revealed that the levels of lysis of determinant V-pulsed targets by CTL induced by the rVV-Dhfr/X-V derivatives (where X is A or M) were weak in comparison to lysis of targets by CTL induced by the rVV-ES-V minigene construct. A weak but detectable immunogenicity of determinant V from within the context of the rVV-Dhfr/X-V derivatives is reminiscent of previous results obtained with Tag-transformed cell lines; epitope V is weakly immunogenic from within nearly full-length Tag derivatives which bear mutations that inactivate epitopes I, II/III, and IV (50, 51a, 69).
TABLE 3.
Induction of epitope V-specific CTL by murine DHFR derivatives bearing an epitope V insert flanked at the amino terminus by an alanine or methionine residue
| rVV used for immunizationa | % Specific target cell lysis
|
|||
|---|---|---|---|---|
| RMA cells pulsed withb:
|
SV40 Tag-expressing cells
|
|||
| LT489-497 | DbN5 | B6/T117A1 | B6/K-1,4,5 | |
| rVV-ES-V | 62 | 3 | 44 | 3 |
| rVV-Dhfr/A-V | ||||
| cl1 | 20 | 9 | 20 | 5 |
| cl2 | 46 | 13 | 24 | 8 |
| rVV-Dhfr/M-V | ||||
| cl1 | 44 | 12 | 36 | 4 |
| cl2 | 29 | 5 | 24 | 3 |
| rVV-Dhfr/ | 9 | 9 | 10 | 5 |
Groups of four C57BL/6 mice were immunized i.v. with 107 PFU of vaccinia virus recombinant. Splenocytes were restimulated in vitro for 6 days with γ-irradiated B6/WT-19 cells and used as effectors in a 51Cr release assay at a 50:1 E/T ratio.
51Cr-labeled RMA cells were incubated in the presence of 1 μM peptide in RPMI medium. LT489-497, epitope V, QGINNLDNL; DbN5, SMIKNLEYM.
H-2Db complexes containing epitope V peptides are relatively unstable.
A simple explanation for the increased potency of rVV-ES-V may be related to increased production of the determinant V peptide by this recombinant (5). It has previously been reported that incubation of SV40 Tag-transformed cells in the presence of BFA leads to a rapid reduction in the susceptibility of targets to lysis by epitope V-specific CTL clones (50). These results were taken to indicate that the abundance of epitope V complexes was low and/or that functional cell surface H-2Db–epitope V complexes were relatively unstable. Both intrinsic instability of H-2Db–epitope V complexes and low abundance of available epitope V peptide could contribute to low cell surface densities of H-2Db–epitope V complexes on Tag-transformed cells in vitro or on professional antigen-presenting cells in vivo. Limited production of the determinant V peptide in vivo and/or instability of H-2Db–epitope V complexes could influence the immunogenicity of epitope V.
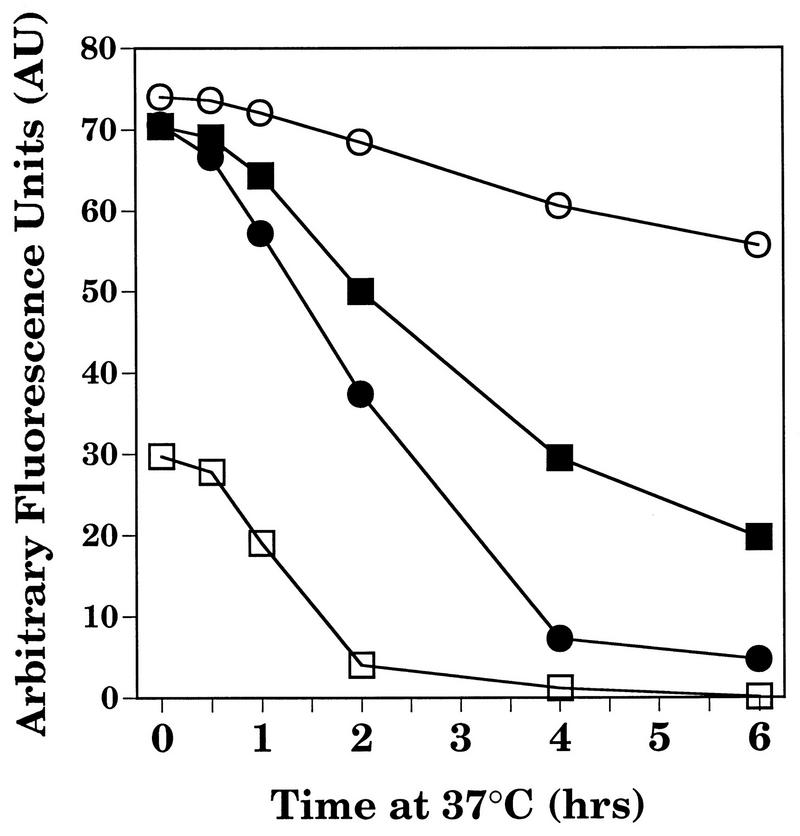
To determine whether H-2Db–epitope V complexes are intrinsically unstable, peptide-pulsed RMA/s cells were used to examine the relative stability of H-2Db complexes containing the SV40 Tag epitopes I, II/III, and V. Incubation of RMA/s cells overnight in the presence of each of these peptides (10 mM each) induced stabilization of similar levels of H-2Db complexes on the cell surface as determined by flow cytometry (Fig. 8) (50). To evaluate the stability of such complexes, RMA/s cells incubated overnight in the presence of epitope peptides were washed free of peptide, and the decay of cell surface H-2Db expression was analyzed by flow cytometry (60). The results presented in Fig. 8 reveal that complexes formed with the synthetic epitope V peptide (LT489-497) decayed more rapidly at 37°C than complexes formed with peptides corresponding to epitope I (LT206-215) or II/III (LT223-231). These results suggest that H-2Db–epitope V complexes formed by exogenously added synthetic peptide with cell surface H-2Db molecules are relatively unstable.
FIG. 8.
Relative decay of H-2Db complexes from the surface of RMA/s cells preincubated in the presence of synthetic peptides corresponding to H-2Db-restricted SV40 Tag CTL epitopes. RMA/s cells were incubated overnight at 28°C in RPMI medium supplemented with no peptide (□) or 10 mM synthetic peptides corresponding to SV40 Tag CTL epitopes I (LT206-215) (○), II/III (LT223-231) (▪), or V (LT489-497) (•). The cells were washed free of peptide, and incubation was continued at 37°C. Samples were withdrawn at the times indicated and held on ice, and the relative abundance of cell surface H-2Db complexes was determined by flow cytometry using the conformation-sensitive, H-2Db-specific monoclonal antibody 28-14-8.
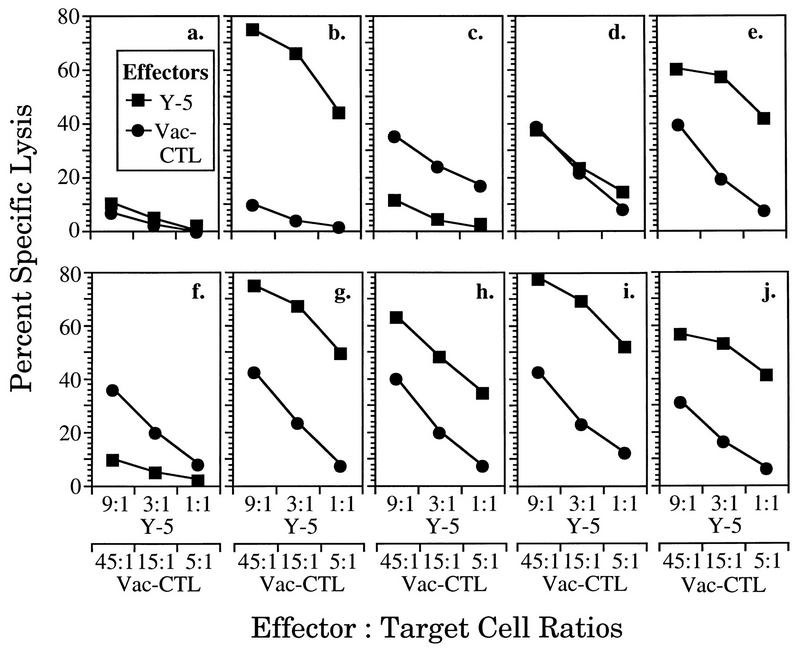
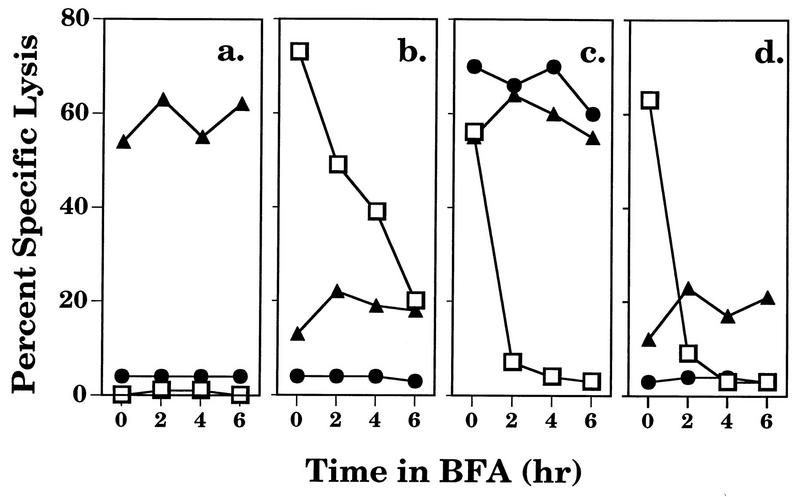
Since the immunogenicity of epitope V appears to be enhanced by fusion to the ES leader sequence (see above), we wanted to determine whether delivery of epitope V directly into the ER of rVV-ES-V-infected targets might increase the abundance or stability of cell surface H-2Db–epitope V complexes. For this purpose, BFA treatment was used to investigate whether lysis of target cells infected with rVV-ES-V and other epitope V-containing vaccinia virus recombinants by the CTL clone H-1 would diminish at similar rates. 51Cr-labeled Ltk/Db targets were infected for 4 h prior to the addition of BFA to allow for optimal expression of H-2Db–epitope complexes at the cell surface. SV40 Tag-transformed cells (B6/T5Aa) were included as a control (data not shown).
As expected, BFA-treated B6/T5Aa (wild-type Tag-transformed) cells became resistant to lysis by the epitope V-specific CTL clone H-1 within 2 h, whereas little reduction in lysis by CTL clones K-11 and K-19 (specific for epitopes I and II/III, respectively) was seen (data not shown) (50). Similar results were obtained when Ltk/Db cells infected with vaccinia virus recombinants were used (Fig. 9). Like the Tag-transformed B6/T5Aa cells, Ltk/Db cells infected with the vaccinia virus recombinants rVV-I-V-II/III and rVV-Dhfr/M-V became resistant to lysis by the epitope V-specific CTL clone H-1 within 2 h in the presence of BFA (Fig. 9c and d); Ltk/Db cells infected with rVV-I-V-II/III were efficiently lysed by CTL clones K-11 and K-19 after 6 h of BFA treatment (Fig. 9c). By contrast, CTL clone H-1 recognition of cells infected with rVV-ES-V persisted longer, even though it did diminish dramatically over the course of the experiment (Fig. 9b). Since epitope V complexes appear to be inherently unstable (Fig. 8), these results imply that fusion of epitope V to the ES leader sequence results in an increase in the number of H-2Db–epitope V complexes at the cell surface. Taken together, our results correlate increased cell surface occupancy by H-2Db–epitope V complexes with improved immunogenicity of the rVV-ES-V recombinant. These experiments support the notion that fusion to the ES sequence enhances the immunogenicity of the immunorecessive epitope V by increasing the abundance of H-2Db–epitope V complexes in vivo.
FIG. 9.
Lysis of BFA-treated targets by SV40 Tag-specific CTL clones. 51Cr-labeled Ltk/Db cells infected with vaccinia virus recombinants expressing epitope V-containing minigenes (rVV-ES-I [a], rVV-ES-V [b], and rVV-I-V-II/III [c]) or a DHFR derivative bearing a Met-extended epitope V insert (rVV-Dhfr/M-V [d]) were treated with BFA for various times and combined with the SV40-specific CTL clone K-11 (▴), K-19 (•), or H-1 (□) at E/T ratios of 1:1, 15:1, and 15:1, respectively, in a 4-h cytotoxicity assay.
DISCUSSION
A principal factor which regulates CTL responses is the quantity of peptide-MHC complexes displayed on the cell surface (16, 86). This could be limited at any of the steps in the antigen processing pathway. Recent findings with rVVs expressing cytosolic or ER-targeted peptides suggest that proteolysis is commonly the major limiting factor in peptide production. Expression of CTL determinants as rVV-encoded cytosolic or ER-targeted peptides results in the expression of enormous amounts of peptide class I complexes at the cell surface, with more than 50,000 complexes being expressed within the first 6 h of infection. In general, this represents a 10- to 100-fold increase in the amount of peptide relative to that recovered from cells expressing the corresponding full-length proteins (5).
This improvement in generating functional peptide-class I complexes makes the use of peptide-encoding vaccinia virus vectors an appealing strategy for enhancing the immunogenicity of weak CTL determinants. We tested this vaccine strategy by using three H-2Db-restricted CTL determinants from SV40 Tag, among which an immunological hierarchy has been demonstrated (50, 77). Our results demonstrate that rVVs expressing the immunodominant CTL determinants I and II/III as minigenes (rVV-I and rVV-II/III) were recognized by the respective determinant-specific CTL clones in vitro and were effective immunogens.
The most important finding of this study is that the immunogenicity of determinant V was dramatically improved by fusion to the ES sequence. Targeting determinant I and II/III peptides to the ER provided little, if any, enhancement of immunogenicity or antigenicity, suggesting that peptide translocation into the ER for these immunodominant determinants is not a rate-limiting step in antigen presentation. Similar results have been obtained by others using immunologically potent CTL epitopes (6, 40, 61). On the other hand, the rVV-ES-V recombinant consistently induced more-potent epitope V-specific CTL responses than any of the other epitope V-containing derivatives tested in this study. While other determinant V-containing vaccinia virus recombinants were immunogenic, the responses induced were weaker, and reliable detection of those responses required the use of peptide-pulsed targets which allowed for increased sensitivity in the cytotoxicity assay.
ER targeting contributed to enhanced antigenicity and immunogenicity of determinant V in TAP-expressing cells. It is unlikely that the ES sequence improved the immunogenicity of epitope V by functioning simply as an amino-terminal flanking sequence, for the following reasons: (i) the ES sequence did allow presentation of epitope V from within TAP-deficient T2/Db cells, while, as expected, none of the other epitope V-containing vaccinia virus recombinants were recognized under similar conditions (data not shown); and (ii) the ES sequence did increase the quantity of functional H-2Db–epitope V complexes on the surface of TAP1/2+ cells, as indicated in BFA blocking experiments (Fig. 9). These results demonstrate that the ES sequence can enhance the immunogenicity of a weak CTL epitope, such as epitope V, most likely because increasing the delivery of the appropriately preprocessed peptide directly into the ER enhances MHC class I-restricted antigen presentation. However, other factors, such as T-cell repertoire, will determine whether enhanced delivery of weak epitopes in general will induce an efficient CTL response.
Consistent with our previous results obtained from the analysis of the immunogenicity of SV40 Tag-transformed cells, expression of the full-length SV40 Tag from a vaccinia virus recombinant produced targets which could be recognized by determinant V-specific CTL clones in vitro but did not induce detectable levels of determinant V-specific CTL in vivo, even when cytotoxicity assays with improved sensitivity were used. The poor immunogenicity of rVV-941 Tag may result from relatively low levels of determinant V produced from the Tag protein. It is also likely that T cells responding to determinants I, II/III, and IV suppress determinant V-specific CTLs. Such immunodomination has been observed repeatedly in immunization experiments which utilize cells expressing full-length Tag or SV40 virus (50). Such a domination model is consistent with our demonstration that immunization with vaccinia virus recombinants which express derivatives of a self-protein (murine DHFR) bearing an epitope V insert do induce epitope V-specific CTL. Similar results have been obtained with influenza virus NP expressed by rVV (21) or from plasmid DNA (30); CTL specific for weak determinants in NP were induced only when the immunodominant determinant was destroyed by mutation of a crucial class I binding residue(s). If so, results presented here, namely, the induction of determinant V-specific CTL by rVV-I-V-II/III, imply that efficient suppression may require that the immunorecessive determinant be expressed in limiting amounts. Perhaps differences in the amount of epitope V peptide generated by differing immunization procedures account for the ability of others to detect epitope V-specific CTL in immunization experiments involving purified, full-length SV40 Tag protein or SV40 Tag expressed following immunization with plasmid DNA (65). Immunization with SV40 Tag-transformed cells consistently fails to result in induction of detectable levels of epitope V-specific CTL (50).
A second important finding resulting from this study is that the antigenicity and immunogenicity of determinant V, when expressed as a minigene product, are dramatically influenced by the presence of short flanking sequences. The rVV-V recombinant (encoding MQGINNLDNL) failed to produce sufficient amounts of the appropriate determinant V peptide to sensitize infected targets for lysis by two independently derived determinant V-specific CTL clones. This failure could imply that the minimal determinant V peptide is unstable in cells or that a property intrinsic to the determinant V peptide may prevent efficient presentation of the Met-extended form of the determinant V peptide expressed by rVV-V. Although our observations imply that the determinant V peptide is intrinsically unstable in cells, the defect in rVV-V recognition may also result from an artifact imposed by the construction of the epitope V-encoding minigene.
Findings for yeast have shown that an N-terminal Met is efficiently removed by Met aminopeptidase only if the radius of gyration of the penultimate residue is less than 1.29 Å (48). If similar constraints apply to murine cells, the initiating Met preceding determinant V is likely to be retained since the penultimate residue in the rVV-V-encoded minigene peptide is a Gln (1.75 Å). As addition of an NH2-terminal Met to the corresponding nonamer determinant synthetic peptide reduces its antigenicity by 100-fold, the inability to remove Met may contribute to this poor antigenicity. Insertion of an (Ala)2 spacer between the initiating Met and determinant V (MAAQGINNLDNL as in rVV-A2-V) would be expected to, and did, overcome such a defect. It is clear that amino acid sequences predicted to result in inefficient removal of an NH2 Met extension do not necessarily prevent efficient recognition of minigene products. It was previously found that an H-2Kb-binding peptide from vesicular stomatitis virus nucleocapsid is efficiently presented as a cytosolic minigene, despite possessing Arg (the residue with the largest radius of gyration) as the penultimate NH2-terminal residue (67). These apparently contradictory results may suggest that the yeast and mammalian cells differ in their ability to remove initiating Met, that small peptides are processed by different proteases than proteins (proteins were used to establish the rules for yeast), or that factors intrinsic to the specific determinant peptide determine the extent to which a residual NH2 Met residue will interfere with accumulation of determinant-MHC class I molecules at the cell surface.
The antigenicity of the determinant V peptide expressed from rVV-V was enhanced by treating cells with the protease inhibitor cbz-LLL-CHO, a potent inhibitor of proteasomes that also inhibits other cellular proteases. The simplest explanation for the cbz-LLL-CHO-mediated rescue of determinant V is that cbz-LLL-CHO prevents its destruction by cellular proteases. For example, cbz-LLL-CHO-mediated inhibition of cellular proteolysis may allow extra time for removal of the NH2 Met extension. Alternatively, it is possible that the enhanced presentation of determinant V mediated by cbz-LLL-CHO is an indirect effect of reducing the amount of competing peptides and/or prolonging TAP interactions with peptide-receptive H-2Db molecules (68). Finally, if the relevant form of the protected determinant V peptide is the nonamer, our results could imply that the nonamer is not a cytosolic intermediate in the presentation of determinant V from T antigen (or the other constructs). This raises the specter of TAP-mediated transport of longer determinant V-containing peptides that are subsequently trimmed in the ER (27, 64, 67).
An intriguing finding is that the antigenicity and immunogenicity of the rVV-V construct were restored by addition of two Ala residues to the carboxy terminus of the determinant V sequence (rVV-V-A2 [Fig. 4 and Table 2]) to encode a primary translation product corresponding to MQGINNLDNLAA. The amino terminus of this (Ala)2-extended peptide is identical to the determinant V peptide encoded by the rVV-V minigene (MQGINNLDNL) which was only poorly antigenic and not immunogenic. Recognition of the Ala-extended peptide most likely does not result from enhanced import into the ER by TAP1/2 (Fig. 5) or improved MHC class I molecule binding (data not shown). Instead, these results suggest that longer peptides containing determinant V may be more resistant to cytosolic proteases which destroy a shorter determinant V peptide. This would be consistent with the enhancing effect of cbz-LLL-CHO on the presentation of MQGINNLDNL.
On the basis of the data at hand, we propose a simple model to unify the results of our analyses involving the recombinants which express cytosolic forms of determinant V. Inefficient removal of the amino-terminal initiating Met residue could limit the pool of determinant V peptide nonamer to levels insufficient to trigger CTL lysis. Since H-2Db complexes containing determinant V peptide are relatively unstable, the few which could be loaded from a limited pool of epitope V 9-mer may dissociate too rapidly for sufficient numbers to accumulate at the cell surface to trigger CTL lysis; alternately, if loaded, the Met-extended 10-mer determinant V peptide would be expected to form 10- to 100-fold-fewer complexes, and this level might also be below the threshold required to trigger CTL clone lysis. We hypothesize that stabilization of the core determinant V peptide (by treatment of rVV-V-infected targets with the protease inhibitor cbz-LLL-CHO or by extension of the minimal peptide) favors increasing the pool of available nonamer determinant V peptide.
In summary, we have shown the following. (i) The immunogenicity of an immunorecessive (or weak) CTL determinant was dramatically enhanced by ER targeting. Similar improvement was not observed for the dominant Tag CTL epitope I or II/III. (ii) The immunorecessive determinant V peptide was immunogenic without ER targeting, but only if short flanking residues were provided. (iii) Determinant V was antigenic and immunogenic when located within the context of an immunologically neutral (self-protein) context, namely, murine DHFR. (iv) The determinant V peptide forms relatively unstable complexes with H-2Db. These findings illustrate that appropriate immunization strategies can be used to effectively induce CTL specific for an immunorecessive epitope. We are currently using SV40 Tag transgenic mice to investigate the therapeutic potential of individual dominant or recessive Tag CTL epitopes in the rejection of SV40 Tag-induced tumors in vivo.
ACKNOWLEDGMENTS
This work was supported by grant CA25000 from the National Cancer Institute. T.D.S. is currently supported by a fellowship from the Cancer Research Institute of New York.
We are grateful for the pSC11 plasmid provided by B. Moss (National Institutes of Health) and T2/Db cells provided by P. Cresswell (Yale University). We thank Melanie Epler for excellent technical assistance.
REFERENCES
- 1.Anderson K, Cresswell P, Gammon M, Hermes J, Williamson A, Zweerink H. Endogenously synthesized peptide with an endoplasmic reticulum signal sequence sensitizes antigen processing mutant cells to class I-restricted cell-mediated lysis. J Exp Med. 1991;174:489–492. doi: 10.1084/jem.174.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson K S, Alexander J, Wei M, Cresswell P. Intracellular transport of class I MHC molecules in antigen processing mutant cell lines. J Immunol. 1993;151:3407–3419. [PubMed] [Google Scholar]
- 3.Anderson R W, Tevethia M J, Kalderon D, Smith A E, Tevethia S S. Fine mapping two distinct antigenic sites on simian virus 40 (SV40) T antigen reactive with SV40-specific cytotoxic T-cell clones by using SV40 deletion mutants. J Virol. 1988;62:285–296. doi: 10.1128/jvi.62.1.285-296.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Androlewicz M J, Anderson K S, Cresswell P. Evidence that transporters associated with antigen processing translocate a major histocompatibility complex class I-binding peptide into the endoplasmic reticulum in an ATP-dependent manner. Proc Natl Acad Sci USA. 1993;90:9130–9134. doi: 10.1073/pnas.90.19.9130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anton L C, Yewdell J W, Bennink J R. MHC class I-associated peptides produced from endogenous gene products with vastly different efficiencies. J Immunol. 1997;158:2535–2542. [PubMed] [Google Scholar]
- 6.Bacik I, Cox J H, Anderson R, Yewdell J W, Bennink J R. TAP (transporter associated with antigen processing)-independent presentation of endogenously synthesized peptides is enhanced by endoplasmic reticulum insertion sequences located at the amino- but not carboxyl-terminus of the peptide. J Immunol. 1994;152:381–387. [PubMed] [Google Scholar]
- 7.Bergmann C C, Tong L, Cua R, Sensintaffar J, Stohlman S. Differential effects of flanking residues on presentation of epitopes from chimeric peptides. J Virol. 1994;68:5306–5310. doi: 10.1128/jvi.68.8.5306-5310.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bergmann C C, Yao Q, Ho C-K, Buckwold S L. Flanking residues alter antigenicity and immunogenicity of multi-unit CTL epitopes. J Immunol. 1996;157:3242–3249. [PubMed] [Google Scholar]
- 9.Bergsagel D J, Finegold M J, Butel J S, Kupsky W J, Garcea R L. DNA sequences similar to those of simian virus 40 in ependymomas and choroid plexus tumors of childhood. N Engl J Med. 1992;326:988–993. doi: 10.1056/NEJM199204093261504. [DOI] [PubMed] [Google Scholar]
- 10.Brinster R L, Chen H Y, Messing A, van Dyke T, Levine A J, Palmiter R D. Transgenic mice harboring SV40 T-antigen genes develop characteristic brain tumors. Cell. 1984;37:367–379. doi: 10.1016/0092-8674(84)90367-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campbell A E, Foley F L, Tevethia S S. Demonstration of multiple antigenic sites of the SV40 transplantation rejection antigen by using cytotoxic T lymphocyte clones. J Immunol. 1983;130:490–492. [PubMed] [Google Scholar]
- 12.Cao W, Myers-Powell B A, Braciale T J. The weak CD8+ CTL response to an influenza hemagglutinin epitope reflects limited T cell availability. J Immunol. 1996;157:505–511. [PubMed] [Google Scholar]
- 13.Carbone M, Pass H I, Rizzo P, Marinetti M, Di Muzio M, Mew D J Y, Levine A S, Procopio A. Simian virus 40-like DNA sequences in human pleural mesothelioma. Oncogene. 1994;9:1781–1790. [PubMed] [Google Scholar]
- 14.Chakrabarti S, Brechling K, Moss B. Vaccinia virus expression vector: coexpression of beta-galactosidase provides visual screening of recombinant virus plaques. Mol Cell Biol. 1985;5:3403–3409. doi: 10.1128/mcb.5.12.3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen W, Khilko S, Fecondo J, Margulies D, McCluskey J. Determinant selection of major histocompatibility complex class I-restricted antigenic peptides is explained by class I-peptide affinity and is strongly influenced by nondominant anchor residues. J Exp Med. 1994;180:1471–1483. doi: 10.1084/jem.180.4.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Christinck E R, Luscher M A, Barber B H, Williams D B. Peptide binding to class I MHC on living cells and quantitation of complexes required for CTL lysis. Nature (London) 1991;352:67–70. doi: 10.1038/352067a0. [DOI] [PubMed] [Google Scholar]
- 17.Cox J H, Bennink J R, Yewdell J W. Retention of adenovirus E19 glycoprotein in the endoplasmic reticulum is essential to its ability to block antigen presentation. J Exp Med. 1991;174:1629–1637. doi: 10.1084/jem.174.6.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Daly K, Nguyen P, Woodland D L, Blackman M A. Immunodominance of major histocompatibility complex class I-restricted influenza virus epitopes can be influenced by the T-cell receptor repertoire. J Virol. 1995;69:7416–7422. doi: 10.1128/jvi.69.12.7416-7422.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deckhut A M, Lippolis J D, Tevethia S S. Comparative analysis of core amino acid residues of H-2Db-restricted cytotoxic T-lymphocyte recognition epitopes in simian virus 40 T antigen. J Virol. 1992;66:440–447. doi: 10.1128/jvi.66.1.440-447.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Del Val M, Schlicht H J, Ruppert T, Reddehase M J, Koszinowski U H. Efficient processing of an antigenic sequence for presentation by MHC class I molecules depends on its neighboring residues in the protein. Cell. 1991;66:1145–1153. doi: 10.1016/0092-8674(91)90037-y. [DOI] [PubMed] [Google Scholar]
- 21.Deng Y, Yewdell J W, Eisenlohr L C, Bennink J R. MHC affinity, peptide liberation, T cell repertiore, and immunodominance all contribute to the paucity of MHC class I-restricted peptides recognized by anti-viral cytotoxic T lymphocytes. J Immunol. 1997;158:1507–1515. [PubMed] [Google Scholar]
- 22.Dick L R, Aldrich C, Jameson S C, Moomaw C R, Pramanik B C, Doyle C K, DeMartino G N, Bevan M J, Forman J M, Slaughter C A. Proteolytic processing of ovalbumin and β-galactosidase by the proteasome to yield antigenic peptides. J Immunol. 1994;152:3884–3894. [PMC free article] [PubMed] [Google Scholar]
- 23.Earl P L, Hügin A W, Moss B. Removal of cryptic poxvirus transcription termination signals from the human immunodeficiency virus type 1 envelope gene enhances expression and immunogenicity of a recombinant vaccinia virus. J Virol. 1990;64:2448–2451. doi: 10.1128/jvi.64.5.2448-2451.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Earl, P. L., and B. Moss. Generation of recombinant vaccinia viruses, p. 16.17.1–16.17.16. In F. M. Ausubel, R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.), Current protocols in molecular biology. John Wiley & Sons, Inc., New York, N.Y.
- 25.Eisenlohr L C, Bacik I, Bennink J R, Bernstein K, Yewdell J W. Expression of a membrane protease enhances presentation of endogenous antigens to MHC class I-restricted T lymphocytes. Cell. 1992;71:963–972. doi: 10.1016/0092-8674(92)90392-p. [DOI] [PubMed] [Google Scholar]
- 26.Eisenlohr L C, Yewdell J W, Bennink J R. Flanking sequences influence the presentation of an endogenously synthesized peptide to cytotoxic T lymphocytes. J Exp Med. 1992;175:481–487. doi: 10.1084/jem.175.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elliott T, Willis A, Cerundolo V, Townsend A. Processing of major histocompatibility class I-restricted antigens in the endoplasmic reticulum. J Exp Med. 1995;181:1481–1491. doi: 10.1084/jem.181.4.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fanning E. Structure and function of simian virus 40 large tumor antigen. Annu Rev Biochem. 1992;61:55–85. doi: 10.1146/annurev.bi.61.070192.000415. [DOI] [PubMed] [Google Scholar]
- 29.Fu T-M, Bonneau R H, Epler M, Tevethia M J, Alam S, Verner K, Tevethia S S. Induction and persistence of a cytotoxic T lymphocyte (CTL) response against a herpes simplex virus-specific CTL epitope expressed in a cellular protein. Virology. 1996;222:269–274. doi: 10.1006/viro.1996.0419. [DOI] [PubMed] [Google Scholar]
- 30.Fu T-M, Friedman A, Ulmer J B, Liu M A, Donnelly J J. Protective cellular immunity: cytotoxic T-lymphocyte responses against dominant and recessive epitopes of influenza virus nucleoprotein induced by DNA immunization. J Virol. 1997;71:2715–2721. doi: 10.1128/jvi.71.4.2715-2721.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gairin J E, Oldstone M B A. Design of high-affinity major histocompatibility complex-specific antagonist peptides that inhibit cytotoxic T-lymphocyte activity: implications for control of viral disease. J Virol. 1992;66:6755–6762. doi: 10.1128/jvi.66.11.6755-6762.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Germain R N, Margulies D H. The biochemistry and cell biology of antigen processing and presentation. Annu Rev Immunol. 1993;11:403–450. doi: 10.1146/annurev.iy.11.040193.002155. [DOI] [PubMed] [Google Scholar]
- 33.Hahn Y S, Yang B, Braciale T J. Regulation of antigen processing and presentation to class I MHC restricted CD8+ T lymphocytes. Immunol Rev. 1996;151:31–49. doi: 10.1111/j.1600-065x.1996.tb00702.x. [DOI] [PubMed] [Google Scholar]
- 34.Hanahan D. Heritable formation of pancreatic β-cell tumors in transgenic mice expressing recombinant insulin/simian virus 40 oncogenes. Nature (London) 1985;315:115–122. doi: 10.1038/315115a0. [DOI] [PubMed] [Google Scholar]
- 35.Jennings S R, Fresa K L, Lippe P A, Milici J E, Tevethia S S. Frequency analysis of simian virus 40-specific cytotoxic T lymphocyte precursors in the high responder C57BL/6 mouse strain. J Gen Virol. 1988;69:2493–2503. doi: 10.1099/0022-1317-69-10-2493. [DOI] [PubMed] [Google Scholar]
- 36.Kärre K, Ljunggren H G, Piontek G, Kiessling R. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature (London) 1986;319:675–678. doi: 10.1038/319675a0. [DOI] [PubMed] [Google Scholar]
- 37.Knowles B B, McCarrick J, Fox N, Solter D, Damjanov I. Osteosarcomas in transgenic mice expressing an α-amylase-SV40 T-antigen hybrid gene. Am J Pathol. 1990;137:259–262. [PMC free article] [PubMed] [Google Scholar]
- 38.Kozak M. The scanning model for translation: an update. J Cell Biol. 1989;108:229–240. doi: 10.1083/jcb.108.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lanford R E. Expression of simian virus 40 T antigen in insect cells using a baculovirus expression vector. Virology. 1988;167:72–81. doi: 10.1016/0042-6822(88)90055-4. [DOI] [PubMed] [Google Scholar]
- 40.Lawson C M, Bennink J R, Restifo N P, Yewdell J W, Murphy B R. Primary pulmonary cytotoxic T lymphocytes induced by immunization with a vaccinia virus recombinant expressing influenza A virus nucleoprotein peptide do not protect mice against challenge. J Virol. 1994;68:3505–3511. doi: 10.1128/jvi.68.6.3505-3511.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lednicky J A, Garcea R L, Bergsagel D J, Butel J S. Natural simian virus 40 strains are present in human choroid plexus and ependymoma tumors. Virology. 1995;212:710–717. doi: 10.1006/viro.1995.1529. [DOI] [PubMed] [Google Scholar]
- 42.Lill N L, Tevethia M J, Hendrickson W G, Tevethia S S. Cytotoxic T lymphocytes (CTL) against a transforming gene product select for transformed cells with point mutations within sequences encoding CTL recognition epitopes. J Exp Med. 1992;176:449–457. doi: 10.1084/jem.176.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lippolis J D, Mylin L M, Simmons D T, Tevethia S S. Functional analysis of amino acid residues encompassing and surrounding two neighboring H-2Db-restricted cytotoxic T-lymphocyte epitopes in simian virus 40 tumor antigen. J Virol. 1995;69:3134–3146. doi: 10.1128/jvi.69.5.3134-3146.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ljunggren H G, Kärre K. Host resistance directed selectively against H-2-deficient lymphoma variants. Analysis of the mechanism. J Exp Med. 1985;162:1745–1759. doi: 10.1084/jem.162.6.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mackett M, Smith G L, Moss B. General method for production and selection of infectious vaccinia virus recombinants expressing foreign genes. J Virol. 1984;49:857–864. doi: 10.1128/jvi.49.3.857-864.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martini F, Iaccheri L, Lazzarin L, Carinci P, Corallini A, Gerosa M, Iuzzolino P, Barbanti-Brodano G, Tognon M. SV40 early region and large T antigen in human brain tumors, peripheral blood cells, and sperm fluids from healthy individuals. Cancer Res. 1996;56:4820–4825. [PubMed] [Google Scholar]
- 47.Michalek M T, Grant E P, Gramm C, Goldberg A L, Rock K L. A role for the ubiquitin-dependent proteolytic pathway in MHC class I-restricted antigen presentation. Nature (London) 1992;363:552–554. doi: 10.1038/363552a0. [DOI] [PubMed] [Google Scholar]
- 48.Moerschell R P, Hosokawa Y, Tsunasawa S, Sherman F. The specificities of yeast methionine aminopeptidase and acetylation of amino-terminal methionine in vivo. J Biol Chem. 1992;265:19638–19643. [PubMed] [Google Scholar]
- 49.Monaco J J. A molecular model of MHC class-I-restricted antigen processing. Immunol Today. 1992;13:173–179. doi: 10.1016/0167-5699(92)90122-N. [DOI] [PubMed] [Google Scholar]
- 50.Mylin L M, Bonneau R H, Lippolis J D, Tevethia S S. Hierarchy among multiple H-2b-restricted cytotoxic T-lymphocyte epitopes within simian virus 40 T antigen. J Virol. 1995;69:6665–6677. doi: 10.1128/jvi.69.11.6665-6677.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mylin L M, Deckhut A M, Bonneau R H, Kierstead T D, Tevethia M J, Simmons D T, Tevethia S S. Cytotoxic T lymphocyte escape variants, induced mutations and synthetic peptides define a dominant H-2Kd-restricted determinant in simian virus 40 tumor antigen. Virology. 1995;208:159–172. doi: 10.1006/viro.1995.1139. [DOI] [PubMed] [Google Scholar]
- 51a.Mylin, L. M., and S. S. Tevethia. Unpublished results.
- 52.Nanda N K, Sercarz E. A truncated T cell receptor repertoire reveals underlying immunogenicity of an antigenic determinant. J Exp Med. 1996;184:1037–1043. doi: 10.1084/jem.184.3.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Neisig A, Roelse J, Sijts A J, Ossendorp F, Feltkamp M C, Kast W M, Melief C J M, Neefjes J J. Major differences in transporter associated with antigen processing (TAP)-dependent translocation of MHC class I-presentable peptides and the effect of flanking sequences. J Immunol. 1995;154:1273–1279. [PubMed] [Google Scholar]
- 54.Niedermann G, Butz S, Ihlenfeldt H G, Grimm R, Lucchiari M, Hoschützky H, Jung G, Maier B, Eichmann K. Contribution of proteosome-mediated proteolysis to the hierarchy of epitopes presented by major histocompatibility complex class I molecules. Immunity. 1995;2:289–299. doi: 10.1016/1074-7613(95)90053-5. [DOI] [PubMed] [Google Scholar]
- 55.Nuchtern J G, Bonifacino J S, Biddison W E, Klausner R D. Brefeldin A implicates egress from endoplasmic reticulum in class I restricted antigen presentation. Nature (London) 1989;339:223–226. doi: 10.1038/339223a0. [DOI] [PubMed] [Google Scholar]
- 56.Oldstone M B A, Lewicki H, Borrow P, Hudrisier D, Gairin J E. Discriminated selection among viral peptides with the appropriate anchor residues: implications for the size of the cytotoxic T-lymphocyte repertoire and control of viral infection. J Virol. 1995;69:7423–7429. doi: 10.1128/jvi.69.12.7423-7429.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ossendorp F, Eggers M, Neisig A, Ruppert T, Groettrup M, Sijts A, Mengedé E, Kloetzel P-M, Neefjes J, Koszinowski U, Melief C. A single residue exchange within a viral CTL epitope alters proteasome-mediated degradation resulting in lack of antigen presentation. Immunity. 1996;5:115–124. doi: 10.1016/s1074-7613(00)80488-4. [DOI] [PubMed] [Google Scholar]
- 58.Palombella V J, Rando O J, Goldberg A L, Maniatis T. The ubiquitin-proteasome pathway is required for processing the NF-κB1 precursor protein and the activation of NF-κB. Cell. 1994;78:773–785. doi: 10.1016/s0092-8674(94)90482-0. [DOI] [PubMed] [Google Scholar]
- 59.Paltimer R D, Chen H Y, Messing A, Brinster R L. SV40 enhancer and large T antigen are instrumental in development of choroid plexus tumors in transgenic mice. Nature (London) 1985;316:457–460. doi: 10.1038/316457a0. [DOI] [PubMed] [Google Scholar]
- 60.Potter T A, Boyer C, Verhuslt A, Goldstein P, Rajan T V. Expression of H-2Db on the cell surface in the absence of detectable β2 microglobulin. J Exp Med. 1984;160:317–322. doi: 10.1084/jem.160.1.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Restifo N P, Bacik I, Irvine K R, Yewdell J W, McCabe B J, Anderson R W, Eisenlohr L C, Rosenberg S A, Bennink J R. Antigen processing in vivo and the elicitation of primary CTL responses. J Immunol. 1995;154:4414–4422. [PMC free article] [PubMed] [Google Scholar]
- 62.Rhim J S, Cho H Y, Huebner R J. Non-producer human cells induced by murine sarcoma virus. Int J Cancer. 1975;15:23–29. doi: 10.1002/ijc.2910150104. [DOI] [PubMed] [Google Scholar]
- 63.Rock K L, Gramm G, Rothstein L, Clark K, Stein R, Dick L, Hwang D, Goldberg A L. Inhibitors of the proteasome block the degradation of most cell proteins and the generation of peptides presented on MHC class I molecules. Cell. 1994;78:761–771. doi: 10.1016/s0092-8674(94)90462-6. [DOI] [PubMed] [Google Scholar]
- 64.Roelse J, Grommé M, Momberg F, Hämmerling G, Neefjes J. Trimming of TAP-translocated peptides in the endoplasmic reticulum and in the cytosol during recycling. J Exp Med. 1994;180:1591–1597. doi: 10.1084/jem.180.5.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schirmbeck R, Böhm W, Reimann J. DNA vaccination primes MHC class I-restricted, simian virus 40 large tumor antigen-specific CTL in H-2d mice that reject syngeneic tumors. J Immunol. 1996;157:3550–3558. [PubMed] [Google Scholar]
- 66.Schumacher T N M, Kantesaria D V, Heemels M-T, Ashton-Rickardt P G, Shepherd J C, Fruh K, Yang Y, Peterson P A, Tonegawa S, Ploegh H L. Peptide length and sequence specificity of the mouse TAP1/TAP2 translocator. J Exp Med. 1994;179:533–540. doi: 10.1084/jem.179.2.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Snyder H L, Yewdell J W, Bennink J R. Trimming of antigenic peptides in an early secretory compartment. J Exp Med. 1994;180:2389–2394. doi: 10.1084/jem.180.6.2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Suh W-K, Mitchell E K, Yang Y, Peterson P A, Waneck G L, Williams D B. MHC class I molecules form ternary complexes with calnexin and TAP and undergo peptide-regulated interaction with TAP via their extracellular domains. J Exp Med. 1996;184:337–348. doi: 10.1084/jem.184.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tanaka Y, Anderson R W, Maloy W L, Tevethia S S. Localization of an immunorecessive epitope on SV40 T antigen by H-2Db-restricted cytotoxic T-lymphocyte clones and a synthetic peptide. Virology. 1989;171:205–213. doi: 10.1016/0042-6822(89)90527-8. [DOI] [PubMed] [Google Scholar]
- 70.Tanaka Y, Tevethia M J, Kalderon D, Smith A E, Tevethia S S. Clustering of antigenic sites recognized by cytotoxic T lymphocyte clones in the amino terminal half of SV40 T antigen. Virology. 1988;162:427–436. doi: 10.1016/0042-6822(88)90483-7. [DOI] [PubMed] [Google Scholar]
- 71.Tanaka Y, Tevethia S S. In vitro selection of SV40 T antigen epitope loss variants by site-specific cytotoxic T lymphocyte clones. J Immunol. 1988;140:4348–4354. [PubMed] [Google Scholar]
- 72.Tanaka Y, Tevethia S S. Loss of immunorecessive cytotoxic T lymphocyte determinant V on SV40 T antigen following cocultivation with site-specific cytotoxic T lymphocyte clone Y-5. Intervirology. 1990;31:197–202. doi: 10.1159/000150154. [DOI] [PubMed] [Google Scholar]
- 73.Tevethia M J. Immortalization of primary mouse embryo fibroblasts with SV40 virions, viral DNA, and a subgenomic DNA fragment in a quantitative assay. Virology. 1984;137:414–421. doi: 10.1016/0042-6822(84)90234-4. [DOI] [PubMed] [Google Scholar]
- 74.Tevethia M J, Ripper L W. Biology of SV40 transplantation antigen (TrAg). II. Isolation and characterization of additional temperature sensitive mutants of SV40. Virology. 1977;81:192–211. doi: 10.1016/0042-6822(77)90137-4. [DOI] [PubMed] [Google Scholar]
- 75.Tevethia M J, Ripper L W, Tevethia S S. A simple qualitative spot complementation test for temperature sensitive mutants of SV40. Intervirology. 1974;3:245–255. doi: 10.1159/000149761. [DOI] [PubMed] [Google Scholar]
- 76.Tevethia S S. Immunology of simian virus 40. In: Klein G, editor. Viral oncology. New York, N.Y: Raven Press; 1980. pp. 581–601. [Google Scholar]
- 77.Tevethia S S. Recognition of simian virus 40 T antigen by cytotoxic T lymphocytes. Mol Biol Med. 1990;7:83–96. [PubMed] [Google Scholar]
- 78.Tevethia S S, Greenfield R S, Flyer D C, Tevethia M J. SV40 transplantation antigen: relationship to SV40-specific proteins. Cold Spring Harbor Symp Quant Biol. 1980;1:235–242. doi: 10.1101/sqb.1980.044.01.027. [DOI] [PubMed] [Google Scholar]
- 79.Tooze J. DNA tumor viruses. In: Tooze J, editor. Molecular biology of tumor viruses. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1980. pp. 799–829. [Google Scholar]
- 80.Townsend A, Bastin J, Gould K, Brownlee G, Andrew M, Coupar B, Boyle D, Chan S, Smith G. Defective presentation to class I-restricted cytotoxic T lymphocytes in vaccinia-infected cells is overcome by enhanced degradation of antigen. J Exp Med. 1988;168:1211–1224. doi: 10.1084/jem.168.4.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Townsend A, Ohlen C, Bastin J, Ljunggren H G, Foster L, Karre K. Association of class I major histocompatibility heavy and light chains induced by viral peptides. Nature (London) 1989;340:443–448. doi: 10.1038/340443a0. [DOI] [PubMed] [Google Scholar]
- 82.van der Burg S H, Visseren M J W, Brandt R M P, Kast W M, Melief C J M. Immunogenicity of peptides bound to MHC class I molecules depends on the MHC-peptide complex stability. J Immunol. 1996;156:3308–3314. [PubMed] [Google Scholar]
- 83.Van Kaer L, Ashton-Rickardt P G, Ploegh H L, Tonegawa S. TAP1 mutant mice are deficient in antigen presentation, surface class I molecules, and CD4−8+ T cells. Cell. 1992;71:1205–1214. doi: 10.1016/s0092-8674(05)80068-6. [DOI] [PubMed] [Google Scholar]
- 84.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- 85.Yewdell J W, Bennink J R. Brefeldin A specifically inhibits presentation of protein antigens to cytotoxic T lymphocytes. Science. 1989;244:1072–1075. doi: 10.1126/science.2471266. [DOI] [PubMed] [Google Scholar]
- 86.Yewdell J W, Bennink J R. Cell biology of antigen processing and presentation to major histocompatibility complex class I molecule-restricted T lymphocytes. Adv Immunol. 1992;52:1–123. doi: 10.1016/s0065-2776(08)60875-5. [DOI] [PubMed] [Google Scholar]
- 87.Yewdell J W, Esquivel F, Arnold D, Spies T, Eisenlohr L C, Bennink J R. Presentation of numerous viral peptides to mouse major histocompatibility complex (MHC) class I-restricted T lymphocytes is mediated by the human MHC-encoded transporter or by a hybrid mouse-human transporter. J Exp Med. 1993;177:1785–1790. doi: 10.1084/jem.177.6.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.York I A, Rock K L. Antigen processing and presentation by the class I major histocompatibility complex. Annu Rev Immunol. 1996;14:369–396. doi: 10.1146/annurev.immunol.14.1.369. [DOI] [PubMed] [Google Scholar]