Abstract
Background:
Nearly two-thirds of Alzheimer’s patients are women. Therapeutics for women are critical to lowering their elevated risk of developing this major cause of adult dementia. Moreover, targeting epigenetic processes such as histone acetylation that regulate multiple cellular pathways is advantageous given the multifactorial nature of Alzheimer’s. Histone acetylation is involved in memory consolidation, and its disruption is linked to Alzheimer’s.
Objective
Determine whether the investigational drug RG2833 has repurposing potential for Alzheimer’s. RG2833 is a histone deacetylase HDAC1/3 inhibitor that is orally bioavailable and permeates the blood-brain-barrier.
Methods:
RG2833 effects were determined on cognitive performance, gene expression, and Alzheimer’s-like pathology in 11-month TgF344-AD female and male rats. Treatment started early in the course of pathology when therapeutic intervention is predicted to be most effective.
Results
RG2833-treatment of 11-month TgF344-AD rats: (1) Significantly improved hippocampal-dependent spatial memory in females but not males. (2) Upregulated expression of immediate early genes, such as Arc, Egr1 and c-Fos, and other genes involved in synaptic plasticity and memory consolidation in females. Remarkably, out of 17,168 genes analyzed for each sex, no significant changes in gene expression were detected in males at P < 0.05, false discovery rate < 0.05, and fold-change equal or > 1.5. (3) Failed to ameliorate amyloid beta accumulation and microgliosis in female and male TgF344-AD rats.
Conclusion:
Our study highlights the potential of histone-modifying therapeutics such as RG2833 to improve cognitive behavior and drive the expression of immediate early, synaptic plasticity and memory consolidation genes, especially in female Alzheimer’s patients.
Keywords: Drug repurposing, HDAC1/3 inhibitor, epigenetic drug, TgF344-AD, spatial memory, neuroplasticity genes, Alzheimer’s disease
INTRODUCTION
Currently, there is no cure for Alzheimer’s. In the search for new, more reliable biomarkers and potential therapeutic targets, epigenetic modifications that affect how Alzheimer’s-related genes are expressed have emerged as important players in disease pathogenesis [1, 2]. Epigenetic dysregulation such as changes in DNA methylation[3–5], histone methylation [6] and acetylation [7, 8] occur in Alzheimer’s. Because these dysregulations can be reversed by targeting enzymes or factors that control them, epigenetic changes are important drug targets. One of the earliest modifications identified in histones is the acetylation of lysine residues by histone acetyltransferases (HAT), and removed by histone deacetylases (HDACs) [9]. Based on homology, the HDAC family is divided into I, II, III, and IV classes [10].
There is a wealth of evidence linking histone acetylation to Alzheimer’s and showing that the use of epigenetic modifying drugs can restore cognitive deficits previously lost in Alzheimer’s. In human postmortem brain tissue, there is evidence that epigenetic dysregulation of HDACs is implicated in cognitive decline. There is a significant increase in two of the class II enzymes HDAC1 and HDAC3 protein levels in mild cognitive impairment (MCI) and mild to moderate Alzheimer’s (mAD), followed by a decrease in severe Alzheimer’s (sAD) compared to non-cognitive impaired (NCI) controls [11]. A different study looking at microarray gene expression data, showed that the mRNA levels of HDAC1 and HDAC8 were significantly higher in the cortex of Alzheimer’s patients than in healthy controls, while those for HDAC2 and HDAC3 were significantly lower [12]. Together these findings suggest that although there is an overall decrease in HDAC levels in later stages of Alzheimer’s, there is an increase in the levels of HDACs in the early stages of the disease. A study on histone 3 acetylation (H3K9) and gene expression profiles of Alzheimer’s patients compared to healthy controls in different brain regions, identified hyperacetylation in Alzheimer’s cerebellum and hippocampus [13]. These findings also support a role for HDAC dysregulation in Alzheimer’s disease.
In the hippocampus of 6- and 9-month-old APPswe/PS1ΔE9 (APP/PS1) Alzheimer’s mouse model there is a significant increase in nuclear HDAC3 levels, while viral-mediated HDAC3 inhibition rescues spatial memory deficits observed in these transgenic mice [14]. Similarly, treatment of the triple transgenic Alzheimer’s mouse model (3xTg-AD) with the selective HDAC3 inhibitor RGFP966 increases histone H3 and H4 acetylation, reverses Alzheimer’s pathology and improves memory impairment [15]. These data support that HDACs could be a therapeutic target for Alzheimer’s. Some of the limitations of previous studies testing HDAC inhibitors for Alzheimer’s is the exclusion of female rats [15] and the short duration of drug administration [15, 16] along with small sample size [15, 17]. Overall, HDAC inhibitors rescue behavioral cognitive dysfunction in short-term studies with Alzheimer’s animal models. However, the functional consequences of such therapeutic intervention have not been described yet.
Our computational studies predicted that the investigational drug RG2833 (N-[6-(2-aminoanilino)-6-oxohexyl]-4-methylbenzamide) has repurposing potential for Alzheimer’s. RG2833 is a histone deacetylase HDAC1/3 inhibitor that is orally bioavailable and permeates the blood-brain-barrier. As far as we know, our studies are the first to investigate the long-term oral administration effects of RG2833 on cognitive deficits, pathology, and other molecular mechanisms relevant to Alzheimer’s. Remarkably, we found that RG2833-treatment initiated early in the course of pathology, has beneficial effects in female TgF344-AD rats but not in males. RG2833 reduced cognitive deficits, and altered the expression of immediate early, synaptic plasticity and memory consolidation genes in TgF344-AD females but not in males, corroborating the sex-dependent effect of this drug. Our results strongly support that RG2833-treatment is a potential therapeutic that affects multiple targets to mitigate Alzheimer’s pathology in females.
MATERIALS AND METHODS
TgF344-AD transgenic rat model of AD
Fisher transgenic F344-AD (TgF344-AD) rats exhibit age-dependent progressive Alzheimer’s-like pathology, including cognitive deficits, neuronal loss, Aβ plaque and neurofibrillary tangle burden, as well as gliosis [18, 19]. TgF344-AD rats express human APPswe and PS1ΔE9 mutations driven by the prion promoter [18]. As TgF344-AD rats successfully recapitulate hallmarks of Alzheimer’s that mimic its longitudinal progression, they are a suitable model for our studies.
The TgF344-AD rats and their wild-type (WT) littermates were purchased from the Rat Resource and Research Center (RRRC, Columbia, MO) at four weeks of age. The rats were housed in pairs upon arrival and maintained on a 12h light/dark cycle with food and water available ad libitum. The Institutional Animal Care and Use Committee (IACUC) at Hunter College approved all animal procedures that were in agreement with the standards outlined in the ARRIVE guidelines.
Drug choice
Currently, there are no available small molecule therapeutics that halt the progressive neurodegeneration in Alzheimer’s patients. Target-based screening is one of the popular approaches in drug discovery, where targets identified through publicly available Alzheimer’s ‘omic’ data are matched to drug databases to generate novel candidates for repurposing as Alzheimer’s therapeutics [20]. In our study, systems pharmacology integrating structure-based genome-scale off-target predictions and chemical-induced gene expression predictions, were used for screening FDA-approved or investigational drugs to target Alzheimer’s. Brain differential gene expression profiles from a group of Alzheimer’s patients vs healthy controls in the Accelerating Medicines Partnership: Alzheimer’s Disease (AMP-AD) data portal [21] was obtained, and considered as the molecular phenotypic signature for Alzheimer’s [22]. The genome-wide differential gene expression profile was considered as the molecular phenotypic signature for Alzheimer’s. Drug-induced gene expression profile of chemical compounds that can reverse the Alzheimer’s profiles were compared, meaning that downregulated and upregulated genes in Alzheimer’s would be upregulated and downregulated by the drug, respectively. Compounds that met these criteria were predicted to be repurposed as novel anti-Alzheimer’s therapeutics. This drug prediction method was previously described [23]. RG2833 was one of the HDAC inhibitor drugs selected for our studies because it was orally bioavailable and predicted to permeate the blood-brain-barrier (BBB).
Experimental design
Alzheimer’s pathological processes begin years before the appearance of disease hallmarks and cognitive deficits [24]. For a treatment to be effective, it is necessary that the drug be administered early in the “preclinical window” before the appearance of major pathologies [19]. For this reason, we started treatment at 5 months of age, prior to the development of pathology or cognitive deficits. Rats were treated chronically until 11 months of age, when pathology is known to be mild to advanced in TgF344-AD rats. Moreover, HDAC inhibitors induce widespread changes in gene expression at the cellular and organism levels. Therefore, we included two HDAC inhibitor-treatment groups, i.e. wild-type (WT) and TgF344-AD rats of both sexes. Our preventive approach and oral administration strategy may hold potential for delaying the onset of Alzheimer’s. Oral administration of drugs over a long period offers benefits in terms of convenience, personalized dosing and observation of the mechanisms that help slow the onset of Alzheimer’s.
A total of 89 rats for the combined female and male studies [WT n = 45 (17 females, 28 males), TgF344-AD n = 44 (24 females, 20 males)] across multiple cohorts were used. At 5 months of age TgF344-AD and WT rats began RG2833-treatment (cat #HY-16425, RG2833, MCE, Monmouth Junction, NJ) for 6 months, being sacrificed at 11 months of age. The drug was formulated into Purina 5001 Rodent Chow (Research Diets Inc. NJ) with 750 mg RG2833/kg diet. The rats consumed ~30 mg of RG2833/kg body weight/day/rat (Supplementary Fig. 1a and b).
We evaluated all rats at 9 and 11-months of age for hippocampal-dependent cognitive deficits estimated with the Active Place Avoidance Test (aPAT) described below [25]. Following behavioral testing, the rats were sacrificed at 11 months of age, and the brains were rapidly isolated and dissected into hemispheres and processed for the different assays as described below and in Fig. 1a.
Fig. 1.
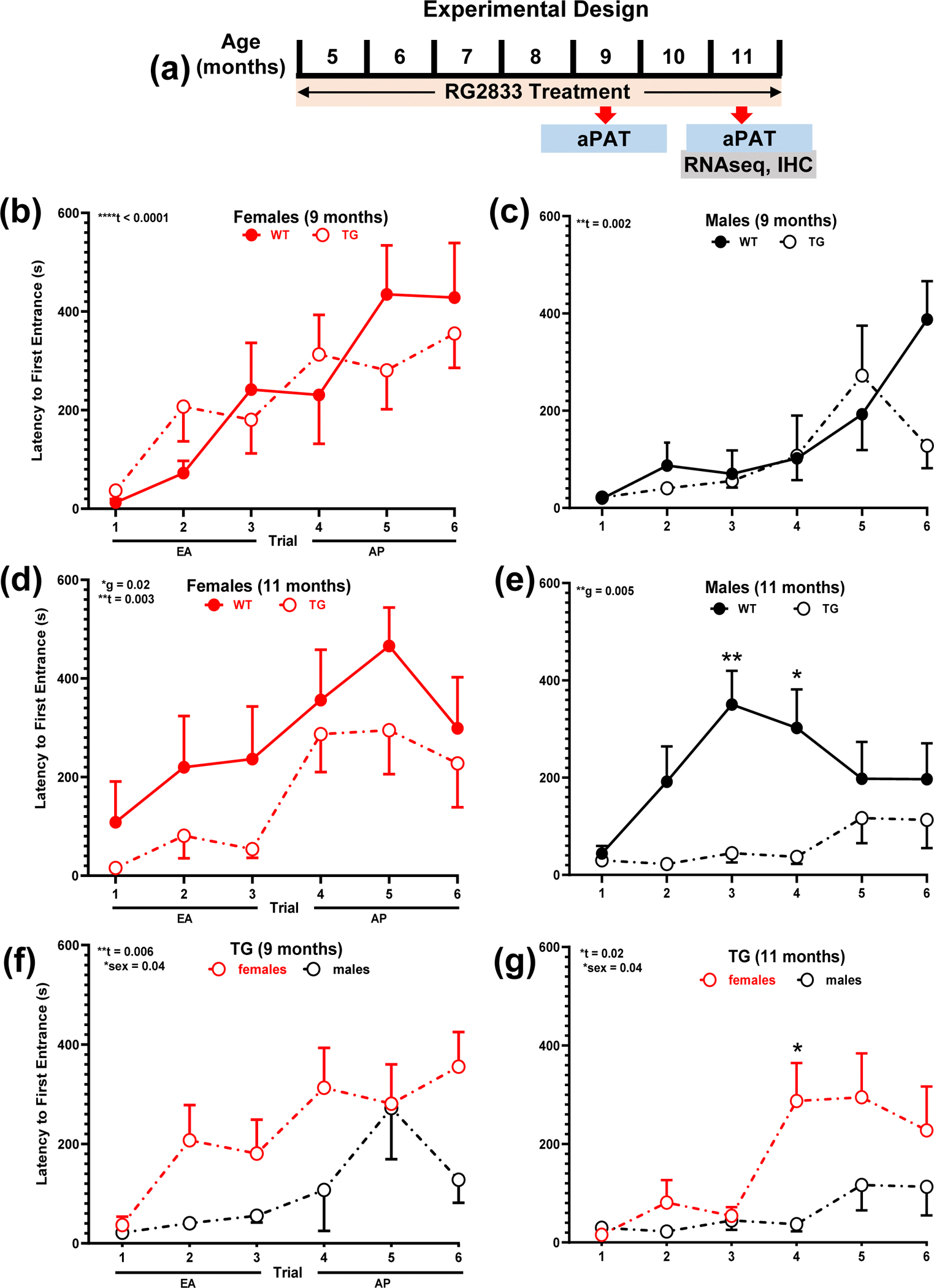
TgF344-AD rats develop spatial memory deficits from 9 to 11-months and females outperform males. a Experimental design. b-g Longitudinal spatial memory assessment with aPAT at 9- and 11-months of age for WT and TgF344-AD rats, represented by latency to first entrance in the shock zone during training. No differences were detected in early acquisition (EA) and asymptotic performance (AP) between WT and Tg344-AD b females or c males at 9-months. There is a trial effect for both sexes. Genotype differences are observed between WT and Tg344-AD d females as well as e males at 11-months. Besides a training effect for both sexes, there is also an overall sex effect for Tg344-AD rats at both ages, as females outperform males f,g. The TgF344-AD rat sex effect is not observed in WT rats. Unpaired t-test with Welch’s correction was used in this analysis, *P < 0.05, **P < 0.01, ****P < 0.0001. WT females (n = 7), males (n = 12), Tg344-AD females (n = 11), males (n = 7); t-trial effect, g-genotype effect, WT-wild-type, TG-transgenic, s-sex, female, male. For simplicity, error bars are shown in one direction only.
Spatial memory assessment with active place avoidance test (aPAT)
In addition to exhibiting Alzheimer-like pathology, TgF344-AD rats display memory deficits that resemble those in Alzheimer’s patients [18]. Alzheimer’s diagnosis is based on cognitive decline and a battery of tests that evaluate episodic memory, executive function, general orientation, and object recognition [26]. Spatial disorientation is one of the clinical tests that leads to Alzheimer’s diagnosis [27–29], with about 60% of Alzheimer’s patients having impaired spatial memory deficits and wandering behavior [30]. The hippocampus is involved in memory formation and consolidation and is a brain area affected in Alzheimer’s [31, 32]. In the early stages of the disease there is a loss of hippocampal neuronal number and volume [33, 34], which are associated with the functional disconnection with other parts of the brain and impaired memory and learning.
Based on these considerations, we assessed hippocampal-dependent cognitive functions including spatial learning and memory and the effects of treatment, with the spatial-dependent active place avoidance test (aPAT). This variant of aPAT is an active task that uses negative reinforcement (shock) to assess spatial learning [25]. We used the hippocampal-dependent aPAT as previously described, to assess short-term working memory performance at 9- followed by 11-month WT and TgF344-AD rats [35]. The latter exhibit alterations in the hippocampal and entorhinal neuronal processes that eventually disrupt cognitive maps, thus spatial memory deficits [36].
RNAseq analysis
Whole left hippocampal tissue prepared as previously described [35] was used for RNAseq analysis outsourced to the UCLA Technology Center for Genomics & Bioinformatics services. Samples from five untreated and five RG2833-treated transgenic male or female rats were compared. Gene expression data were normalized as reads per million (RPM) using the Trimmed Mean of M-values (TMM) method. Differentially expressed genes between untreated and RG2833-treated TgF344-AD rats for each sex were determined using the edgeR program. RPMs were analyzed for fold-change (FC), P values, and false discovery rate (FDR) for each gene. Additional analysis and visualization was performed with R-studio [37].
Tissue collection and preparation
At 11 months of age, the rats were anesthetized with an intraperitoneal injection containing ketamine (100 mg/kg body weight) and xylazine (10 mg/kg body weight), and then transcardially perfused with chilled RNAase free PBS. The brain left hemispheres were micro-dissected into different regions, snap frozen with a CoolRack over dry ice, and the hippocampal tissue used for RNAseq analyses. Whole right brain hemispheres were placed in a 4% paraformaldehyde/PBS solution for 48 hours at 4°C, followed by cryoprotection with a 30% sucrose/PBS solution to prevent water-freeze damage, and then flash frozen using 2-methylbutane, and stored at −80°C until sectioning for immunohistochemistry.
Immunohistochemistry (IHC)
IHC was restricted to dorsal hippocampal tissue within the following Bregma coordinates: −3.36 mm to −4.36 mm.[38] IHC and its analysis were performed as previously described [35].
We viewed the sections on a Zeiss Axio Imager M2 with AxioVision software to capture ZVI files of 10x and 20x mosaic images of the whole hippocampus, and then converted to TIF files. Optical Density (O.D.) was quantified using Image J as previously described [35]. Primary and secondary antibodies are listed in Supplementary Table 1. For quantification the following thresholds were used: Iba1: mean + 1.5*std, particles analyzed were in the range: 50–8,000, and circularity: 0–1.00; NeuN: mean + 1.5*std, particles analyzed were in the range: 10–10,000, and circularity: 0–1.00. Additionally, Iba1+ ramified, reactive, and amoeboid microglia phenotypes were analyzed for 0–1.00 circularity based on the ImageJ form factor FF = 4π x area/perimeter2 defined as ramified (FF < 0.50), reactive (FF: 0.50 to 0.70), and amoeboid (FF > 0.70). For quantification of plaque burden, the following thresholds were used: mean + 4*std, particles analyzed were in the range: 350-infinity, and circularity 0–1.00.
Statistics
Statistical analyses were performed with GraphPad Prism 9.5.1 (Boston, MA 02110). Data are represented as the mean ± standard error of the mean (SEM) with n representing the number of rats. We used the following analyzes: (1) Three-way ANOVAs to determine significant effects across independent variables for behavior and immunohistochemistry. (2) Two-way ANOVAs and post hoc Sidak’s-corrected t-test for behavior and immunohistochemistry assessments. (3) One-tail independent t-tests for Aβ plaque burden. The rolling ball algorithm was used for normalizing pixel intensity.[39] Macroscripts for image processing and quantification were added to GitHub (https://github.com/GiovanniOliveros33/Ibudilast-Manuscript). The alpha level was set at P < 0.05 with a 95% confidence interval for each effect. (3) Two-way ANOVA with Tukey’s post hoc for behavior across two age points (9 months and 11 months), two genotypes (WT and TgF344-AD, and treatments (RG2833-treatment vs. no treatment). (4) Multiple unpaired t-tests with Welch’s correction for differences in gene expression between TGNT and TGTR groups for each comparison. Significant differences were established at P < 0.05, FDR < 0.05, and fold change (FC) ≥ 1.5. determined by the two-stage step-up method.[40]
RESULTS
Significant spatial memory deficits develop from 9- to 11-months of age in TgF344-AD rats
We assessed short-term working memory performance with hippocampal-dependent aPAT, measuring latency to first entrance into the shock zone. A three-way ANOVA across six training trials, two ages (9- and 11-months), and two genotypes (WT and TG) showed a significant training effect for females [F(4.125, 132.0) = 11.75; P < 0.0001], but no effect of age [F(1, 32) = 0.09863; P = 0.755], genotype [F(1, 32) = 2.691; P = 0.111] or interaction [F(5, 160) = 0.4685; P = 0.799]. For males, there were significant effects of training [F(3.869, 131.6) = 4.998; P = 0.001] and genotype [F(1, 34) = 9.169; P < 0.005], but no age effect [F(1, 34) = 0.183; P = 0.672] or interaction [F(5, 170) = 2.122; P = 0.065].
A two-way ANOVA across training and genotype showed no genotype effect for 9-month females [Fig. 1b, F(1,16 ) = 0.015; P = 0.903] or 9-month males [Fig. 1c, F(1,17) = 0.912; P = 0.353]. In contrast, there were genotype effects for 11-month females [Fig. 1d, F(1,16) = 6.532; P = 0.021] and 11-month males [Fig. 1e, F(1,17) = 9.914; P = 0.005, Sidak’s post hoc t = 4.248, **P = 0.006 (trial 3); t = 3.292,*P = 0.039 (trial 4)]. Interestingly, these data establish that TG females and males show memory performance loss from 9- to 11-months of age.
TgF344-AD females outperform TgF344-AD males in spatial memory
A three-way ANOVA across sex, age and training for TG rats, showed significant training [F(3.496, 111.9) = 8.351; P < 0.0001] and sex [F(1, 32) = 9.684; P = 0.004] effects, but no age effect [F(1, 32) = 2.436; P = 0.128] nor interaction [F(5, 160) = 0.959; P = 0.444]. Likewise, for WT rats, there were significant training [F(4.109, 139.7) = 8.931; P < 0.0001] and sex [F(1, 34) = 5.291; P = 0.028] effects, but no age effects [F(1, 34) = 2.697; P = 0.109] nor interaction (data not shown).
A two-way ANOVA for TG rats across sex and training showed that at 9-months there were significant sex [Fig. 1f, F(1,16) = 4.858; P = 0.043] and training effects [F(3.104, 49.66) = 4.592; P = 0.006]. At 11-months there were also significant sex [Fig. 1g, F(1,16) = 4.977; P = 0.040, Sidak’s post hoc t = 3.187; P = 0.052 (trial 4)] and training [F(2.695, 43.12) = 3.895; P = 0.02] effects. Notably, these results reveal that TG females are outperforming TG males on this spatial memory task. We address this finding in the discussion.
A similar analysis for WT rats showed a significant training effect [F(2.709, 46.06) = 10.35; P < 0.0001] but no sex-effect [F(1,17) = 3.811; P = 0.067] at 9-months. Likewise, at 11-months there was a significant training effect [F(5, 85) = 2.904; P = 0.018] but no sex effect [F(1,17) = 1.744; P = 0.204].
RG2833 improves spatial memory in female but not male TgF344-AD rats
A three-way ANOVA across training, drug-treatment and genotype for 11-month females shows significant training [F(5, 185) = 10.95; P < 0.0001] and drug effects [F(1, 37) = 4.304; P = 0.045], but no genotype effect [F(1, 37) = 2.459; P = 0.1254]. In contrast, a similar three-way ANOVA for 11-month males shows a significant training [F(4.628, 203.6) = 3.640; P = 0.0045], and genotype effects [F(1, 44) = 7.962; P = 0.0071], but no drug-treatment effect [F(1, 44) = 0.004431; P = 0.9472].
A two-way ANOVA for TG females (Fig. 2a) shows a significant effect of training [F(3.286, 72.29) = 7.191; P = 0.0002] and drug-treatment [F(1,22) = 9.029, P = 0.007] with post hoc differences at trial 2 (t = 3.004, P = 0.041) and trial 3 (t = 3.289, P = 0.034). There was no drug-treatment effect for WT females [Fig. 2b, F(1,15) = 0.026, P = 0.874], and TGTR females performed as well as WTTR females [F(1,21) = 0.035, P = 0.853, not shown].
Fig. 2.
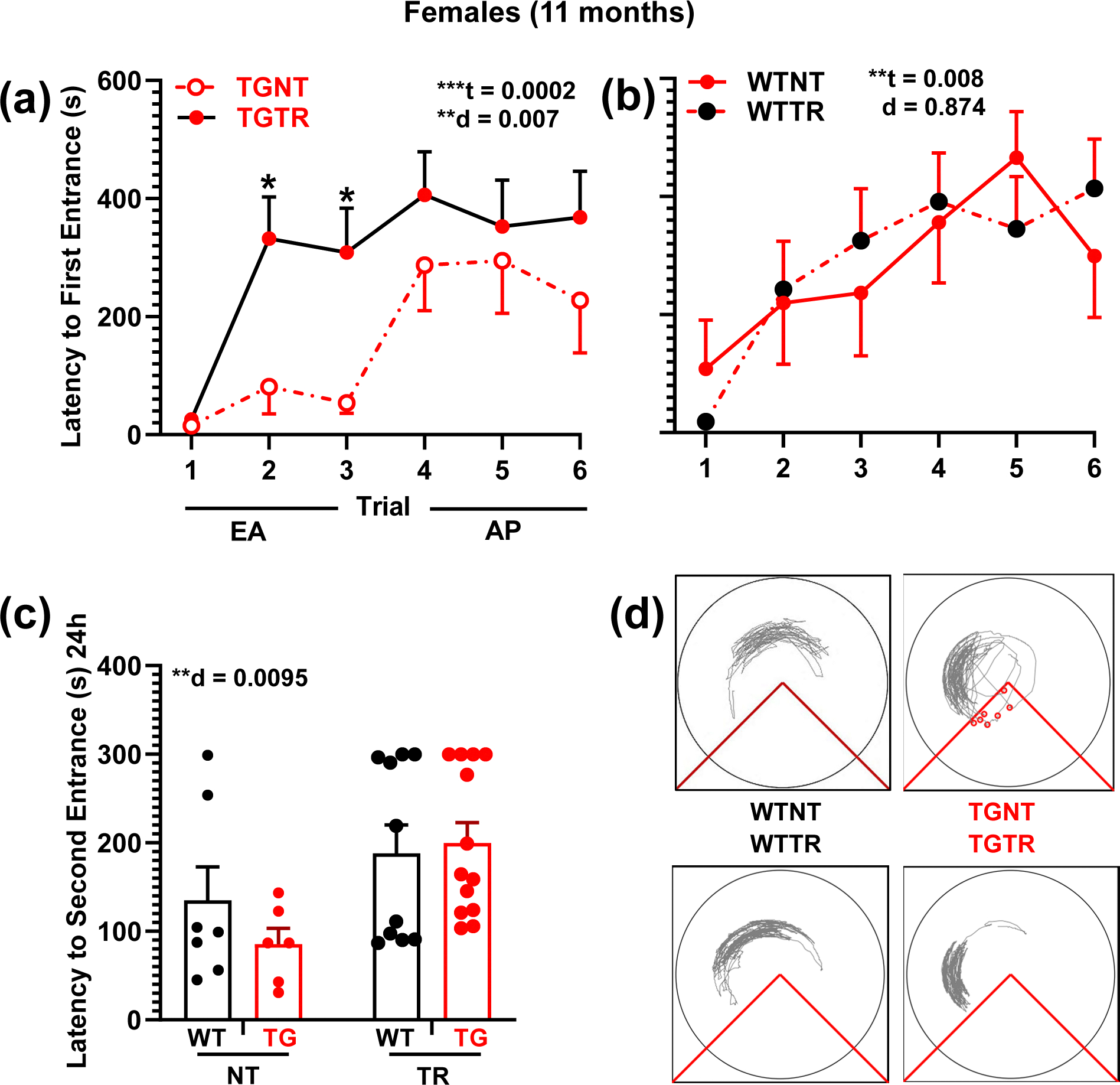
RG2833 improves spatial learning and memory in TgF344-AD females. a, b Latency to first entrance during training trials, c latency to second entrance during test trial. d Representative track tracing of individual female performance for trial 6 across treatment conditions. a TGTR females perform significantly better than TGNT females during early acquisition (EA). b WTNT vs WTTR females perform similarly. c Female test data show overall significant improvement in latency to second entrance following RG2833-treatment vs untreated, independent of genotype. No differences were observed in the asymptomatic performance (AP, trials 4–6) across all comparisons (not shown). Repeated measure two-way ANOVA with Sidak’s post hoc tests were used in 2a and 2b. Ordinary two-way ANOVA with Sidak’s post hoc analysis was used in 2c **P < 0.01, ***P < 0.001. n = 7 WTNT, n = 6 TGNT, n = 13 TGTR, n = 10 WTTR. EA - Early Acquisition; AP – Asymptotic Performance; WTNT – Wild-type not treated; TGNT – transgenic not treated; WTTR – Wild-type RG2833-treated; TGTR – transgenic RG2833-treated; t – trial effect, d – drug effect. For simplicity, error bars are shown in one direction only.
A two-way ANOVA for TG males (Fig. 3a) shows no effect of training [F(2.960, 53.28) = 1.777; P = 0.1632] or drug-treatment [F(1,18) = 1.189, P = 0.290]. Similarly, there was no drug-treatment effect for WT males [Fig. 3b, F(1,26) = 0.769, P = 0.388]. TGTR males were significantly different from WTTR males across training [F(4.139, 111.8) = 2.458; P = 0.0476], but not drug-treatment [F(1,27) = 1.271, P = 0.269; not shown].
Fig. 3.
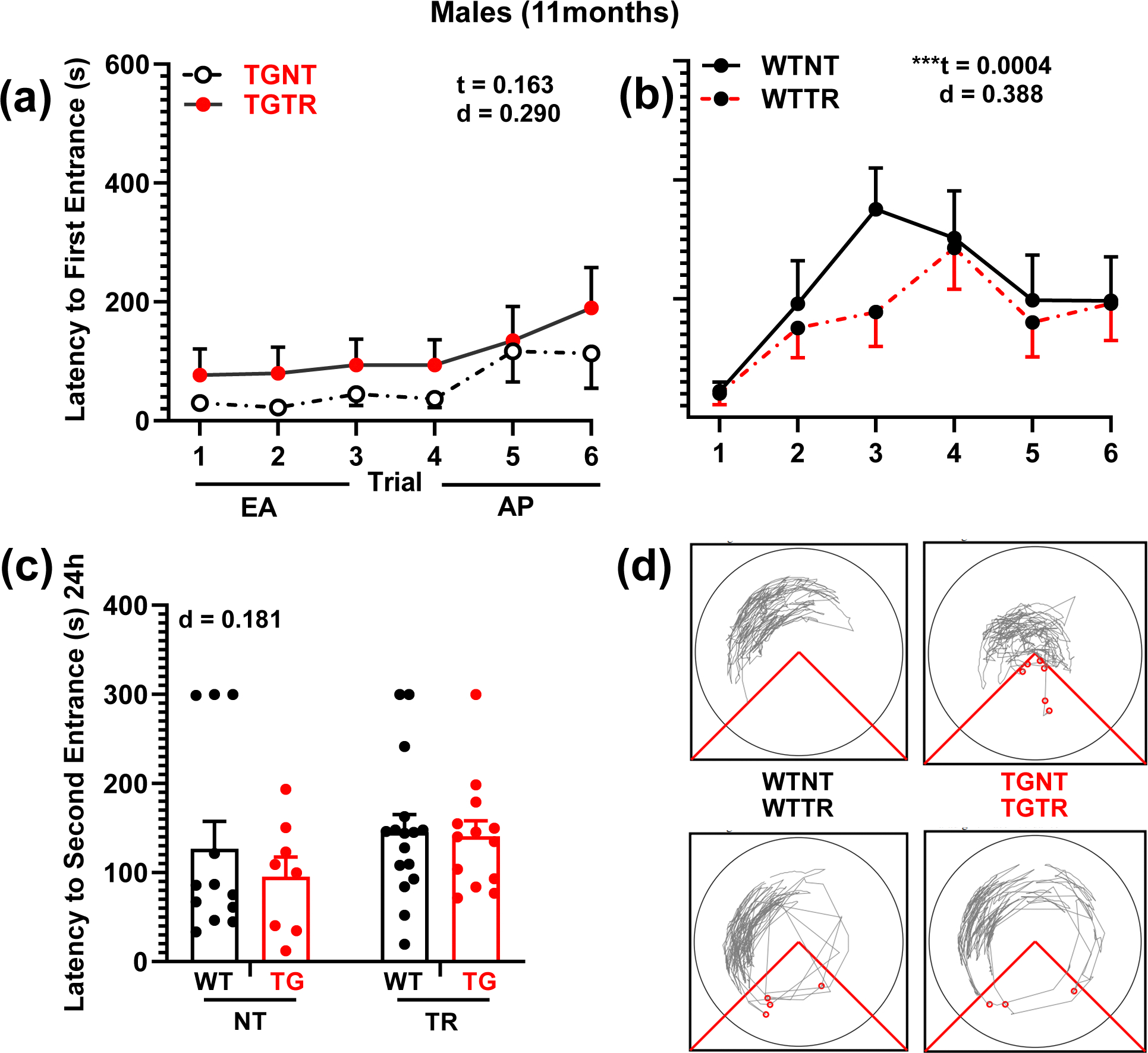
RG2833 failed to improve spatial learning and memory deficits in TgF344-AD males. a, b Latency to first entrance during training trials, c latency to second entrance during test trial. d Representative track tracing of individual male performance for trial 6 across treatment conditions. a TGTR males perform as poorly as TGNT males during early acquisition (EA). b WTNT vs WTTR males perform similarly. c Male test data show no significant improvement in latency to second entrance following RG2833-treatment vs no drug-treatment. No differences were observed in the asymptomatic performance (AP, trials 4–6) across all comparisons (not shown). Repeated measure two-way ANOVA with Sidak’s post hoc tests were used in 3a and 3b. Ordinary two-way ANOVA with Sidak’s post hoc analysis was used in 3c **P < 0.01. n = 12 WTNT, n = 8 TGNT, n = 13 TGTR, n = 16 WTTR. EA - Early Acquisition; AP – Asymptotic Performance; WTNT – Wild-type not treated; TGNT – transgenic not treated; WTTR – Wild-type RG2833-treated; TGTR – transgenic RG2833-treated; t – trial effect, d – drug effect. For simplicity, error bars are shown in one direction only.
We tested rats for avoidance of the shock zone (latency to second entrance) in the absence of shock 24h after the last training trial. A two-way ANOVA across genotype and drug-treatment shows a significant effect of drug-treatment in females [Fig. 2c, F(1,32) = 7.616, P = 0.0095] and no effect on genotype [F(1, 32) = 0.3850; P = 0.539]. In contrast, two-way ANOVA for males shows no significant drug-treatment [Fig. 3c, F(1,45) = 1.843, P = 0.181] or genotype effect [F(1, 45) = 0.5768; P = 0.451].
Overall, our data demonstrate that the benefits of RG2833-treatment on spatial memory are sex-dependent, enhancing cognitive performance only in TGNT females but not TGNT males. Tracking for individual rats during trial 6 across the four treatment conditions is shown in Fig. 2d for females and Fig. 3d for males. Furthermore, we show that RG2833 is well tolerated by both WT females and males.
Spatial memory improvement by RG2833 is age-dependent in TgF344-AD females
A three-way ANOVA across sex, drug-treatment, and age on early acquisition training (trials 1–3) showed that for TG rats, there are sex [F(1, 40) = 12.75; P = 0.0009] and drug-treatment [F(1, 40) = 5.486; P = 0.024] effects and interaction [F(1, 40) = 5.354; P = 0.026], but no age effect [F(1, 40) = 0.017; P = 0.896]. For WT rats there are significant sex [F(1, 41) = 6.568; P = 0.014] and age [F(1, 41) = 10.79; P = 0.002] effects, but no drug-treatment effect [F(1, 41) = 0.08091; P = 0.777].
A two-way ANOVA for TG females (Fig. 4a) shows a significant effect of drug-treatment [F(3,32) = 3.477; P = 0.027] but no age effect [F(12,37) = 0.938; P = 0.523]. RG2833-treatment improved spatial memory in 11-month but not 9-month TG females. This effect is likely due to TGTR females maintaining their memory performance from 9 to 11 months, which is not the case for TGNT females. TG males (Fig. 4b) show no significant overall effect of drug-treatment [F(3,24) = 0.788; P = 0.512] or age [F(12,24) = 0.960; P = 0.509].
Fig. 4.

Age and sex-dependent effects of RG2833 on WT and TgF344-AD rats. a - d Latency to first entrance during early acquisition (EA, trials 1 – 3). At 9-months of age, there is no significant drug effect on WT and TG rats independently of sex a - d. At 11-months of age, there is a drug positive effect on TG females a, and a negative effect on WT males d. WT males perform significantly better at 11-months than at 9-months of age d, but TG males do not b. No differences were observed in the asymptomatic performance (AP, trials 4–6) across all comparisons (not shown). Two-way ANOVA with Tukey’s post-hoc was used across two age points (9 and 11 months) and two drug-treatments (RG2833-treatment vs. no treatment). *P < 0.05, **P < 0.01. Females n = 7 WTNT, n = 11 TGNT, n = 13 TGTR, n = 10 WTTR. Males n = 12 WTNT, n = 7 TGNT, n = 13 TGTR, n = 14 WTTR. EA - Early Acquisition; AP – Asymptotic Performance; WTNT – Wild-type not treated; TGNT – transgenic not treated; WTTR – Wild-type RG2833-treated; TGTR – transgenic RG2833-treated; age – age effect, d – drug effect. For simplicity, error bars are shown in one direction only.
A two-way ANOVA for WT females (Fig. 4c) shows no significant effects of drug-treatment [F(3,21) = 0.596; P = 0.624] or age [F(9, 21) = 0.633; P = 0.757]. WT males (Fig. 4d) show significant age [F(15,37) = 2.298; P = 0.020] and drug-treatment effects [F(3,37) = 8.505; P = 0.0002]. The spatial memory of WT males improved from 9 to 11 months of age most likely because they remembered the previous training.
RG2833 alters gene expression and pathway enrichments in TgF344-AD females
To understand the potential molecular consequences of chronic RG2833-treatment, we assessed differential gene expression profiles and enriched pathways in 11-month TgF344-AD rats. The gene expression analysis generated output files containing an initial 17,168 genes. After removing those genes considered not differentially expressed between the two groups (TGNT vs TGTR), we were left with 358 genes for further analysis in females. This set contained 126 upregulated and 232 downregulated genes, significant at P < 0.05, FDR < 0.05, and fold change (FC) ≥ 1.5. The male gene analysis yielded no genes that were differentially expressed with these criteria.
Principal component analysis shows a clear separation of the five biological replicates for the female TGNT vs TGTR groups (Fig. 5a). There was no clear separation of the replicates for the male group (Fig. 5b). The volcano plots revealed significantly more differentially expressed (combined up and downregulated) genes in females compared to males at P < 0.05 and −2< FC >+2 (Figs. 5c and 5d). We subsequently focused on the genes that were significantly up or downregulated by RG2833-treatment and performed gene set enrichment analysis (GSEA) to find pathways that were significantly enriched or suppressed (Figs. 5e and 5f).
Fig. 5.

RNA sequencing analyses comparing RG2833-treated and untreated TgF344-AD females and males. Principal Component Analysis (PCA) plots for RNA sequencing (RNAseq) data comparing female a and male b treated (blue) and untreated (red) TgF344-AD rats. The five biological replicates corresponding to each treatment (TGNT vs. TGTR) show consistent clustering for females (on the left) but not for males (on the right). Volcano plots of differentially expressed genes comparing female c and male d treated (blue) and untreated (red) TgF344-AD rats. Gene Set Enrichment Analysis (GSEA) evaluating activated (blue) and suppressed (red) enriched pathways based on transcriptional data from female e and male f TgF344-AD rats. The plots show the relationship between the top 4 most significantly enriched (activated and supressed) pathways (padj.), by grouping genes that are in similar pathways. The color represents the P-values relative to the other displayed terms (brighter red is more significant) and the size of the terms represents the number of genes that are significant from our list.
Genes upregulated in females were significantly enriched in four top pathways involved in “chondroitin sulfotransferase activity”, “gpi linked ephrin receptor”, “regulation of AMPA receptor activity”, and “regulation of postsynaptic neurotransmitter receptor activity”. Genes downregulated in females were significantly enriched in four top pathways that are involved in “mitochondrial protein-containing complex”, “ribosome”, “innate immune response” and “regulation of hormone levels” (Fig. 5e).
Furthermore, the female top upregulated genes are related to HDAC inhibition, upregulation in memory, regulation by estrogen and downregulation in Alzheimer’s (Fig. 6, Venn diagram). Three of these genes, Arsi, Hunk, and Eya2, are associated with the estrogen pathway (green elypse). Five other genes, Fos, Cacrig5, Egr1, Arc, and Egr4 are related to the four pathways, i.e., HDAC inhibition, upregulation in memory, regulation by estrogen and downregulation in Alzheimer’s.
Fig. 6.

Venn diagram of differentially expressed genes (DEGs) in TgF344-AD females. The diagram depicts the literature annotation of the number of genes related to distinct pathways of interest in response to RG2833. The overlapping ellipses in the Venn diagram depict the relationships among the four sets of categories: downregulation in Alzheimer’s (yellow), related to estrogen (green), related to HDAC (pink) and upregulated in memory (lavender), highlighting how the categories are similar and different.
Genes upregulated in males were significantly enriched in four top pathways involved in “Regulation of cortisol secretion”, “threonine metabolic process”, “translation at synapse”, and “Aryl sulfotransferase activity”. Genes downregulated in males were significantly enriched in four top pathways involved in “cilium”, “embryonic morphogenesis”, “epithelial cell differentiation”, and “sensory organ development” (Fig. 5f). We will address the relevance of these findings in the discussion.
RG2833 does not alter hippocampal Aβ plaque burden
Fig. 7 shows IHC for Aβ plaque load and DAPI in TgF344 untreated (A) and RG2833-treated (B) females, and untreated (G) and RG2833-treated (H) males. Aβ plaque load was analyzed as a three-way ANOVA across brain region, drug-treatment, and sex for TG animals. Our results show a significant brain region effect [F(2.066, 74.38) = 31.12; P < 0.0001], but no sex [F(1, 36) = 0.2739; P = 0.6040] or drug-treatment [F(1, 36) = 0.01249; P = 0.9116] effects. Subsequent analysis of hippocampal regions as independent t-tests showed no significant reduction in Aβ plaque burden for TG females (Fig. 7c–f and Supplementary Table 2A) or TG males (Fig. 7i–l and Supplementary Table 2b).
Fig. 7.
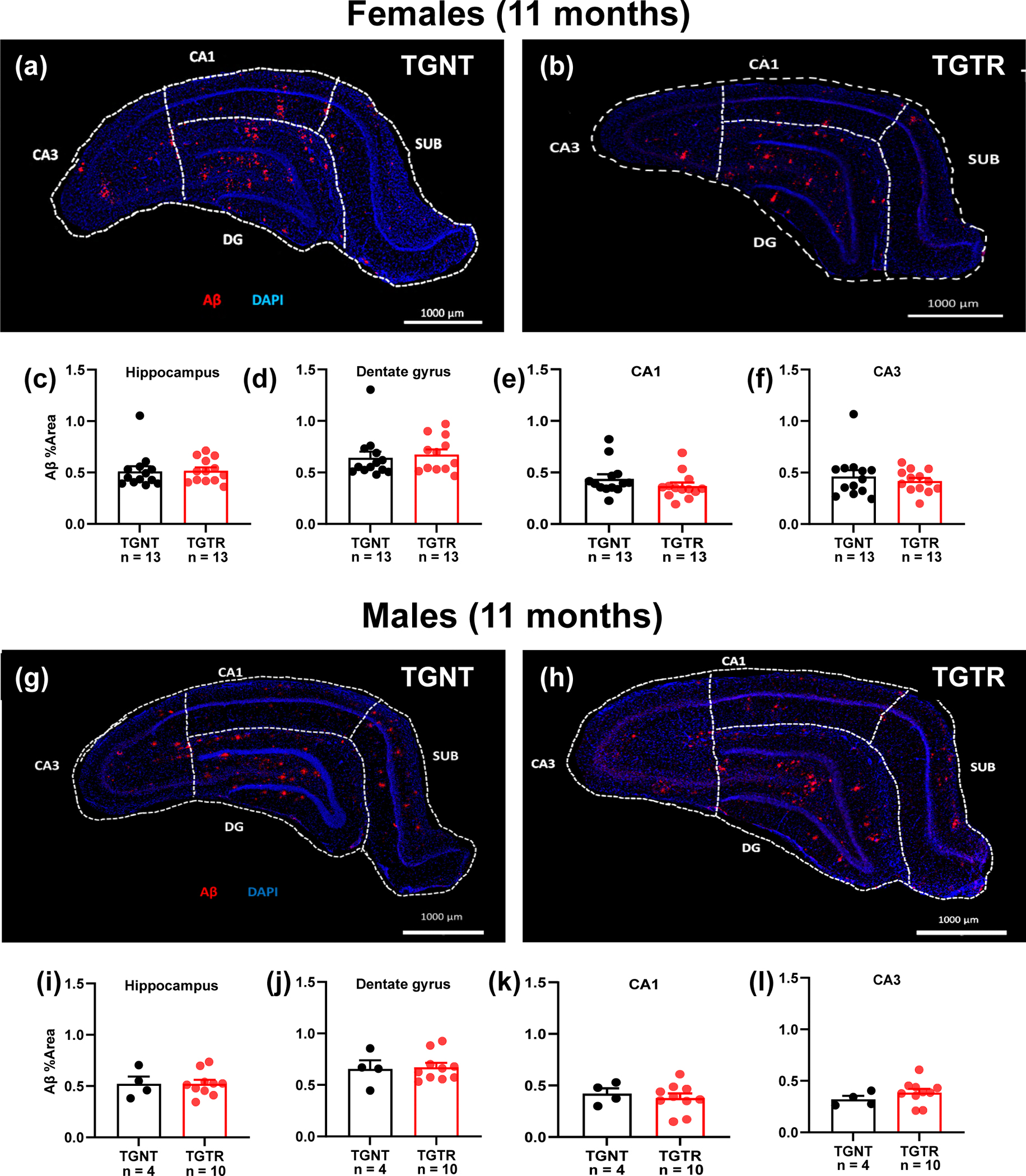
RG2833 does not reduce Aβ plaque load in the hippocampus of TgF344-AD females and males. Immunohistochemical analyses for Aβ plaque load and DAPI in TgF344 untreated a and RG2833-treated b females, and untreated g and RG2833-treated h males. Plaque load was not reduced in the hippocampus of both females c, and males i, or in the DG (d, j), CA1 (e, k), and CA3 (f, l). Unpaired one-tail t-tests with Welch’s corrections were used for quantification. n = 13 female TGNT, n = 13 female TGTR, n = 4 male TGNT, n = 10 male TGTR. CA – Cornu Ammonis; DG – Dentate Gyrus; TGNT – transgenic not treated; TGTR – transgenic RG2833-treated. Scale bar = 1000μm for panels (a, b, g, h). For simplicity, error bars are shown in one direction only.
RG2833 does not prevent hippocampal microgliosis or neuronal loss
Microglia exhibit a variety of morphologies associated with their functions. Based on shape (Fig. 8a) and function, we considered three microglia groups defined as follows: Ramified, actively engaged in neuronal maintenance and providing neurotrophic factors; Reactive, responsive to CNS injury; Amoeboid, with amorphous cell bodies with pseudopodia that remove cell debris [41].
Fig. 8.

RG2833 does not reduce microgliosis in the hippocampus of TgF344-AD females and males. Microglia immunohistochemical analyses with the Iba1 antibody identified three different types of microglia morphology expressed by specific form factor ranges for circularity: ramified, reactive and amoeboid a. Our analyses focused on hippocampal amoeboid/ramified microglial ratios. In females, amoeboid/ramified microglia ratio is significantly increased in TGNT compared to WTNT in the hippocampus and its regions DG and CA1, as well as in TGTR compared to WTTR in the hippocampus and DG (b – e). In males, there was no significant increase in amoeboid/ramified microglia ratio of TGNT vs WTNT in the hippocampus as well as in the DG, CA1 and CA3 regions (f – i). There was a significant increase in amoeboid/ramified microglia ratio of TGTR vs WTTR males in the hippocampus as well as in the DG, CA1 and CA3 regions (f – i). There was also a significant increase in the amoeboid/ramified microglia ratio of the hippocampal CA3 region of TGTR vs TGNT males. Ordinary two-way ANOVA with Sidak’s post hoc tests were used in 8B through 8I. *P < 0.05, **P < 0.01, ***P < 0.001. Females: n = 6 WTNT, n = 13 TGNT, n = 10 WTTR, n = 13 TGTR. Males: n = 4 WTNT, n = 4 TGNT, n = 6 WTTR, n = 10 TGTR, CA – Cornu Ammonis, DG – Dentate Gyrus, WTNT – Wild-Type not treated, TGNT – transgenic not treated, WTTR – Wild-type RG2833-treated, TGTR – transgenic RG2833 -treated. For simplicity, error bars are shown in one direction only.
Microglia in a postnatal brain are mostly amoeboid (active phagocytosis). As the brain develops, they gradually undergo a transition to a surveillant non-phagocytic state characterized by a highly branched (ramified) morphology. In neurodegenerative conditions, ramification is reversed during the process of microglia activation. Ramified (surveillant microglia) can undergo a transformation to amoeboid morphology and become phagocytic in response to disease [42]. We preformed microglia analyses in rat hippocampus using anti-Iba1 staining separately for females and males. We quantified the ratio of amoeboid over ramified microglia, expressed as a percentage.
Female microglia analyzed as a three-way ANOVA showed a significant effect across brain regions [F(1.773, 67.39) = 24.20; P < 0.0001], drug-treatment [F(1, 38) = 7.341; P = 0.010] and genotype [F(1, 38) = 49.65; P < 0.0001]. Similarly, males showed a significant effect of brain regions [F(1.379, 27.58) = 6.951; P = 0.008], drug-treatment [F(1, 20) = 5.473; P = 0.029] and genotype [F(1, 20) = 8.470; P = 0.009].
Individual brain regions [hippocampus, DG, CA1 and CA3] were analysed for amoeboid/ramified ratios in females as a two-way ANOVA across drug-treatment and genotype (Fig. 8b–e, Supplementary Table 3A). Fig. 8b–e show a significant effect of genotype [Fig. 8b, whole hippocampus F(1, 39) = 22.87, P < 0.0001; Fig. 8c, DG F(1, 37) = 16.71, P = 0.0002; Fig. 8d, CA1 F(1, 38) = 16.24; P = 0.0003; Fig. 8e, CA3 F(1, 38) = 9.700; P = 0.0035]. There is no drug-treatment effect [Fig. 8b, whole hippocampus F(1, 39) = 3.526, P = 0.0679; Fig. 8c, DG F(1, 37) = 3.881; P = 0.0564; Fig. 8d, CA1 F(1, 38) = 0.4703; P = 0.497; Fig. 8e, CA3 F(1, 38) = 1.792; P = 0.1886].
A similar two-way ANOVA analysis in males showed a significant genotype effect in the hippocampus and its DG and CA3 regions, but not in CA1 (Fig. 8f–i, Supplementary Table 3b). There was no significant drug-treatment effect in the hippocampus and its DG and CA1 regions but was observed in CA3 (Fig. 8f–i, Supplementary Table 3b).
Overall, we compared the data for the hippocampus and its regions with a two-way ANOVA and post hoc Sidak’s-corrected t-test. The results indicate that (1) RG2833-treatment does not improve microgliosis assessed as amoeboid/ramified ratios in TgF344-AD rats of both sexes, (2) non-treated TgF344-AD females exhibit significantly higher amoeboid/ramified ratios in the hippocampus and its regions than untreated WT females, but males do not, (3) treated TgF344-AD males exhibit significantly higher amoeboid/ramified ratios in the hippocampus and its regions than treated WT males, but females show the trend only in the hippocampus and DG region.
Neuronal loss (NeuN IHC) was analyzed as a three-way ANOVA across brain region, drug-treatment and genotype for each sex. For females, there was a significant effect of brain region [F(2.383, 85.79) = 8.932; P = 0.0001], and drug-treatment [F(1, 36) = 4.440; P = 0.042], but no genotype effect [F(1, 36) = 0.03886; P = 0.845]. For males, there was a significant brain region effect [F(1.643, 37.78) = 22.45; P < 0.0001], but no drug-treatment [F(1, 23) = 0.1521; P = 0.7001] nor genotype effects [F(1, 23) = 2.179; P = 0.153]. Subsequent two-way ANOVA analyses of individual brain regions [hippocampus, DG, CA1 and CA3] across drug-treatment or genotype for neuronal loss in males and females are shown in Supplementary Table 4A–B. There was no significant effect of drug-treatment across any of the brain regions. Other significant effects of genotype are shown in Supplementary Table 4A–B.
DISCUSSION
Our studies investigate the effects of a long-term oral administration of the HDAC1/3 inhibitor RG2833 on cognitive performance, gene expression, and Alzheimer’s-like pathology in female and male TgF344-AD rats.
Age- and sex-dependent spatial learning and memory deficits in TgF344-AD rats
We assessed hippocampal-dependent spatial learning and memory, which are significantly impacted in Alzheimer’s [43]. We show that female and male TgF344-AD rats exhibit poorer performance compared to their WT littermates at 11- but not 9-months of age. There is also a sex-dependent difference in this cognitive behavior, as females perform better than males of similar ages.
Cognitive performance encompasses many processes like learning and memory, attention, and decision-making. The spatial learning and memory assessed with the aPAT task that we used in the current study, is allothetic or allocentric meaning that the rat has to use visual cues/landmarks to orient itself relative to the room in order to avoid a fixed shock zone within the rotating arena [44]. This type of memory is heavily dependent on the use of the hippocampus according to previous studies [44, 45]. It is not a stress response because the foot shock does not significantly raise the cortisone levels compared to exploration without the shock [25]. Our results are highly relevant to Alzheimer’s, as in these patients the hippocampal function is compromised as a result of the neuropathology. Previous research showed that Alzheimer’s patients show a higher cognitive deficit in allocentric tasks than healthy controls [46, 47]. Other studies using different animal models support our findings, as they show that there is allocentric spatial memory impairment in later ages above the 9-month time point. Significant neurocognitive decline was detected using two different allocentric spatial navigation tasks. One study showed a difference between TgF344-AD and WT rats at 10–11 months using the Morris water maze (MWM) [48]. Others found a similar pattern at 12 months of age using the radial-arm maze [49], and at 15 months of age using the Barnes maze test [50]. These studies together with ours, which show that TgF344-AD rats exhibit cognitive deficits at 11- but not 9-months of age, strongly support that the TgF344-AD rat model incorporates aging as a risk factor for Alzheimer’s. This is highly important as aging is a major risk factor for Alzheimer’s. Further investigations focusing on mechanisms responsible for this age-dependent susceptibility to Alzheimer’s could lead to the discovery of new therapeutic targeting in the early stages of the disease.
We also observed that TgF344-AD females outperform males at 9- and 11-months of age. We previously reported a similar 9-month sex-dependent trend using the same rat model [51]. In the latter study we found that 9-month TgF344-AD females had higher Aβ plaque burden and GluA2 subunit levels than males. GluA2 is an AMPA receptor subunit that is important for spatial memory [52, 53], and could provide a neuroprotective mechanism for females independently of Aβ plaque burden.
Females could also find a better navigational strategy to avoid the shock zone compared to males. We used standard performance measures for the aPAT, comparing number of shocks, entrances to the shock zone, etc. Using more rigorous analytical techniques, like machine learning methods to classify animal navigational paths into behavioral patterns as described previously for MWM [54] and active allothetic PAT [55], could clarify the strategies used by females to better avoid the shock zone in aPAT than males. Moreover, looking at the molecular profile changes occurring between TgF344-AD females and males at 9- and 11-months of age, could provide a better understanding of the sex effects observed at these ages when full pathology is not yet manifested. Some studies including the original one by Cohen and co-workers [18], do not report a sex-dependent effect on cognition. This discrepancy could be attributed to different tests or/and smaller sample size used for the analyses.
RG2833 reduces spatial memory deficits specifically in TgF344-AD females
We showed that chronic oral administration of RG2833 improves spatial learning and memory only in TgF344-AD females. This observation was unexpected, because a previous study showed that in the 3xTg-AD mouse model, males that received daily intraperitoneal (i.p.) injections of RGFP966, a potent and specific HDAC3 inhibitor, for 12 weeks performed better in spatial and recognition memory compared to untreated mice [15]. A different study using the APPswe/PS1ΔE9 mouse model of Alzheimer’s, showed that chronic i.p. injections of HDAC1 inhibitors for 2–3 weeks, restored contextual memory in males and females [56]. However, due to the low sample size, no analysis was performed to investigate any sex differences [56].
A variation in HDAC inhibitor type, drug metabolism, and/or the amount of drug reaching the brain could explain the sex difference attributed to RG2833-treatment. Regardless of the cause, it is clear in our study that RG2833 has sex-specific effects on TgF344-AD rats that affect spatial memory and gene expression, the latter discussed below.
RG2833 changes gene expression and pathway enrichments specifically in TgF344-AD females
Our analyses of Aβ plaque pathology, microgliosis, and neuronal loss did not show any differences between TgF344-AD untreated and RG2833-treated rats. This could indicate that the RG2833 effect on Alzheimer’s pathology is not the main mechanism mediating the drug beneficial effect on spatial learning and memory. In contrast, other studies show that infusion of an HDAC3 inhibitor decreases Aβ1–42 oligomer levels, and reverses tau pathology in transgenic Alzheimer’s mice [14, 15]. Experimental and species differences could explain the contrasting results of these studies.
To understand the mechanism of improved cognitive behavior observed in RG2833-treated TgF344-AD females, we analyzed hippocampal gene expression profiles in both sexes, using bulk RNA-sequencing. The results showed that at the criteria P < 0.05, FDR < 0.05, and fold change (FC) ≥ 1.5, only females showed differentially expressed genes, while males did not.
For females, we included the RG2833-effect on Gene Set Enriched Analysis (GSEA) that organizes the differentially expressed genes into functional groups. The female upregulated genes are involved in neurotransmitter and synaptic functions, and the downregulated genes in the immune system and hormone regulation. Gene ontology (GO) evaluation showed that most genes affected by RG2833 are involved in learning and neuronal synaptic plasticity, with some involved in the positive regulation of transcription or translation. Most of these proteins are located in the nucleus, and the ones on the plasma membrane function as synaptic proteins.
Our results with RG2833 show a consistency between the improved cognition and RNAseq data, such as upregulation of neuronal signaling pathways leading to improved learning and hippocampal-dependent memory consolidation. A previous study reported that there is HDAC enrichment at the promoter regions of neuroplasticity genes (Arc, Egr1, BDNF),[57–59] and HDAC3 negatively regulates spatial and long-term memory [14, 60]. Also inhibiting the activity of HDACs, therefore promoting histone acetylation, results in gene expression required for memory consolidation [17, 61, 62]. For example, histone post-translational modifications including H3 acetylation, are associated with the expression of the immediate early gene Egr1 (zif268), a transcription factor that favors memory consolidation [63].
Other genes that are significantly upregulated in RG2833-treated TgF344-AD females are Arc, Fos, Egr1, Egr4, and Cacng5. These genes are induced by estrogen and associated with memory [64, 65]. 17β-Estradiol, a steroid hormone produced by the ovaries, enhances cognition and spatial memory by a similar mechanism as HDAC inhibitors. Both types of chemicals promote histone acetylation in the dorsal hippocampus, while also reducing levels of histone deacetylases (HDAC2 and HDAC3) in the brain [66]. The rise in histone acetylation promotes increased transcription of brain-derived neurotrophic factor (BDNF), a crucial protein for synaptic plasticity and memory formation [67].
Genes downregulated by the RG2833-treatment in TgF344-AD females belong to two important groups, one involved in drug efflux transport (Abcg2) and the other in metabolism (Ugt1a3, Gsta4, Gstm2, Gstt1). Downregulation of Abcg2 could mediate intracellular accumulation of RG2833, therefore allowing it to function longer.
Glutathione transferase (GST) is a superfamily of phase II detoxifying enzymes that catalyze the reaction of toxic compounds or drugs with reduced glutathione (GSH). This could explain why RG2833 affects females only, as some of these enzymes are strongly regulated in females [68–70]. The UDP glucuronosyltransferase family 1 member A3 (Ugt1a3) catalyzes the glucuronidation reaction, which helps to detoxify and remove unwanted as well as endogenous substances (eg. drugs, estrogen). The Ugt1A family is also known to clear various HDAC inhibitors such as Vorinostst, Belinostat and Panobinostat [71–73], and estrogen [74]. Downregulation of the UDP glucuronosyltransferase family 1 and the glutathione transferase family could lead to accumulation of RG2833 and other drugs.
Conclusion
Overall, our results show that the HDAC1/3 inhibitor RG2833 reduces cognitive deficits and modulates the expression of immediate early, neuroprotective and synaptic plasticity genes in female but not male TgF344-AD rats. The RG2833 action showed results when administered in a chronic manner over a long period, but before any known pathology developed. These findings have a significant implication for clinical trials testing HDAC inhibitors, which are currently used to treat a broad range of human diseases. Repurposing HDAC inhibitors for Alzheimer’s has to take into account the stage of the disease as well as the role biological sex plays in the effects of the drug.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to thank Sebastian Yumiseba for helping with the feeding, weighing, and behavior analysis of some of the rats.
FUNDING
This work was supported in part by NIH/NIA R01AG057555 to LX, NIH training grants R25GM060665 to support KN, and the City University of New York (Ph.D. program CUNY Neuroscience Collaborative, Graduate Center).
Footnotes
CONFLICT OF INTEREST
The authors declare that they have no conflict of interests.
DATA AVAILABILITY
The datasets supporting the conclusions of this article are available in the NIH GEO repository, ACCESSION NUMBERS:
Male TGNT: GSM7884145 (M1), GSM7884146 (M2), GSM7884147 (M3), GSM7884148 (M4), GSM7884149 (M5).
Male TGTR: GSM7884150 (MRG1),GSM7884151(MRG2),GSM7884152(MRG3), GSM7884153 (MRG4), GSM7884154 (MRG5)
Female TGNT: GSM7884155 (F1), GSM7884156 (F2), GSM7884157 (F3), GSM7884158 (F4), GSM7884159 (F5)
Female TGTR: GSM7884160 (FRG1), GSM7884161 (FRG2), GSM7884162 (FRG3), GSM7884163 (FRG4), GSM7884164 (FRG5)
To review GEO accession GSE247142:
Go to https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE247142
Enter token mfobggwmhrspnur into the box.
REFERENCES
- [1].Nikolac Perkovic M, Videtic Paska A, Konjevod M, Kouter K, Svob Strac D, Nedic Erjavec G, Pivac N, Epigenetics of Alzheimer’s Disease, Biomolecules 11(2) (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Graff J, Tsai LH, The potential of HDAC inhibitors as cognitive enhancers, Annu Rev Pharmacol Toxicol 53 (2013) 311–30. [DOI] [PubMed] [Google Scholar]
- [3].Pellegrini C, Pirazzini C, Sala C, Sambati L, Yusipov I, Kalyakulina A, Ravaioli F, Kwiatkowska KM, Durso DF, Ivanchenko M, Monti D, Lodi R, Franceschi C, Cortelli P, Garagnani P, Bacalini MG, A Meta-Analysis of Brain DNA Methylation Across Sex, Age, and Alzheimer’s Disease Points for Accelerated Epigenetic Aging in Neurodegeneration, Front Aging Neurosci 13 (2021) 639428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Altuna M, Urdánoz-Casado A, Sánchez-Ruiz de Gordoa J, Zelaya MV, Labarga A, Lepesant JMJ, Roldán M, Blanco-Luquin I, Perdones Á, Larumbe R, Jericó I, Echavarri C, Méndez-López I, Di Stefano L, Mendioroz M, DNA methylation signature of human hippocampus in Alzheimer’s disease is linked to neurogenesis, Clin Epigenetics 11(1) (2019) 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Wei X, Zhang L, Zeng Y, DNA methylation in Alzheimer’s disease: In brain and peripheral blood, Mech Ageing Dev 191 (2020) 111319. [DOI] [PubMed] [Google Scholar]
- [6].Smith AR, Smith RG, Macdonald R, Marzi SJ, Burrage J, Troakes C, Al-Sarraj S, Mill J, Lunnon K, The histone modification H3K4me3 is altered at the ANK1 locus in Alzheimer’s disease brain, Future Sci OA 7(4) (2021) Fso665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Marzi SJ, Leung SK, Ribarska T, Hannon E, Smith AR, Pishva E, Poschmann J, Moore K, Troakes C, Al-Sarraj S, Beck S, Newman S, Lunnon K, Schalkwyk LC, Mill J, A histone acetylome-wide association study of Alzheimer’s disease identifies disease-associated H3K27ac differences in the entorhinal cortex, Nat Neurosci 21(11) (2018) 1618–1627. [DOI] [PubMed] [Google Scholar]
- [8].Schueller E, Paiva I, Blanc F, Wang XL, Cassel JC, Boutillier AL, Bousiges O, Dysregulation of histone acetylation pathways in hippocampus and frontal cortex of Alzheimer’s disease patients, Eur Neuropsychopharmacol 33 (2020) 101–116. [DOI] [PubMed] [Google Scholar]
- [9].Wood IC, The Contribution and Therapeutic Potential of Epigenetic Modifications in Alzheimer’s Disease, Front Neurosci 12 (2018) 649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Haggarty SJ, Tsai LH, Probing the role of HDACs and mechanisms of chromatin-mediated neuroplasticity, Neurobiol Learn Mem 96(1) (2011) 41–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Mahady L, Nadeem M, Malek-Ahmadi M, Chen K, Perez SE, Mufson EJ, Frontal Cortex Epigenetic Dysregulation During the Progression of Alzheimer’s Disease, J Alzheimers Dis 62(1) (2018) 115–131. [DOI] [PubMed] [Google Scholar]
- [12].Geng F, Zhao N, Chen X, Liu X, Zhu M, Jiang Y, Ren Q, Transcriptome analysis identifies the role of Class I histone deacetylase in Alzheimer’s disease, Heliyon 9(7) (2023) e18008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Santana DA, Bedrat A, Puga RD, Turecki G, Mechawar N, Faria TC, Gigek CO, Payão SL, Smith MA, Lemos B, Chen ES, The role of H3K9 acetylation and gene expression in different brain regions of Alzheimer’s disease patients, Epigenomics 14(11) (2022) 651–670. [DOI] [PubMed] [Google Scholar]
- [14].Zhu X, Wang S, Yu L, Jin J, Ye X, Liu Y, Xu Y, HDAC3 negatively regulates spatial memory in a mouse model of Alzheimer’s disease, Aging Cell 16(5) (2017) 1073–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Janczura KJ, Volmar CH, Sartor GC, Rao SJ, Ricciardi NR, Lambert G, Brothers SP, Wahlestedt C, Inhibition of HDAC3 reverses Alzheimer’s disease-related pathologies in vitro and in the 3xTg-AD mouse model, Proc Natl Acad Sci U S A 115(47) (2018) E11148–e11157. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [16].Benito E, Urbanke H, Ramachandran B, Barth J, Halder R, Awasthi A, Jain G, Capece V, Burkhardt S, Navarro-Sala M, Nagarajan S, Schütz AL, Johnsen SA, Bonn S, Lührmann R, Dean C, Fischer A, HDAC inhibitor-dependent transcriptome and memory reinstatement in cognitive decline models, J Clin Invest 125(9) (2015) 3572–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Rumbaugh G, Sillivan SE, Ozkan ED, Rojas CS, Hubbs CR, Aceti M, Kilgore M, Kudugunti S, Puthanveettil SV, Sweatt JD, Rusche J, Miller CA, Pharmacological Selectivity Within Class I Histone Deacetylases Predicts Effects on Synaptic Function and Memory Rescue, Neuropsychopharmacology 40(10) (2015) 2307–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Cohen RM, Rezai-Zadeh K, Weitz TM, Rentsendorj A, Gate D, Spivak I, Bholat Y, Vasilevko V, Glabe CG, Breunig JJ, Rakic P, Davtyan H, Agadjanyan MG, Kepe V, Barrio JR, Bannykh S, Szekely CA, Pechnick RN, Town T, A transgenic Alzheimer rat with plaques, tau pathology, behavioral impairment, oligomeric abeta, and frank neuronal loss, J. Neurosci 33(15) (2013) 6245–6256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Saré RM, Cooke SK, Krych L, Zerfas PM, Cohen RM, Smith CB, Behavioral Phenotype in the TgF344-AD Rat Model of Alzheimer’s Disease, Front Neurosci 14 (2020) 601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Rodriguez S, Hug C, Todorov P, Moret N, Boswell SA, Evans K, Zhou G, Johnson NT, Hyman BT, Sorger PK, Albers MW, Sokolov A, Machine learning identifies candidates for drug repurposing in Alzheimer’s disease, Nat Commun 12(1) (2021) 1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hodes RJ, Buckholtz N, Accelerating Medicines Partnership: Alzheimer’s Disease (AMP-AD) Knowledge Portal Aids Alzheimer’s Drug Discovery through Open Data Sharing, Expert Opin Ther Targets 20(4) (2016) 389–91. [DOI] [PubMed] [Google Scholar]
- [22].Hodes RJ, Buckholtz N, Accelerating Medicines Partnership: Alzheimer’s Disease (AMP-AD) Knowledge Portal Aids Alzheimer’s Drug Discovery through Open Data Sharing, 10.1517/14728222.2016.1135132 (2016). [DOI] [PubMed] [Google Scholar]
- [23].Lim H, Poleksic A, Yao Y, Tong H, He D, Zhuang L, Meng P, Xie L, Large-Scale Off-Target Identification Using Fast and Accurate Dual Regularized One-Class Collaborative Filtering and Its Application to Drug Repurposing, PLOS Computational Biology 12(10) (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Sperling R, Mormino E, Johnson K, The evolution of preclinical Alzheimer’s disease: implications for prevention trials, Neuron 84(3) (2014) 608–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Lesburguères E, Sparks FT, O’Reilly KC, Fenton AA, Active place avoidance is no more stressful than unreinforced exploration of a familiar environment, Hippocampus 26(12) (2016) 1481–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Rajan KB, Wilson RS, Weuve J, Barnes LL, Evans DA, Cognitive impairment 18 years before clinical diagnosis of Alzheimer disease dementia, Neurology 85(10) (2015) 898–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Piccini C, Pecori D, Campani D, Falcini M, Piccininni M, Manfredi G, Amaducci L, Bracco L, Alzheimer’s disease: patterns of cognitive impairment at different levels of disease severity, J Neurol Sci 156(1) (1998) 59–64. [DOI] [PubMed] [Google Scholar]
- [28].Cronin-Golomb A, Corkin S, Rizzo JF, Cohen J, Growdon JH, Banks KS, Visual dysfunction in Alzheimer’s disease: relation to normal aging, Ann Neurol 29(1) (1991) 41–52. [DOI] [PubMed] [Google Scholar]
- [29].Schmidtke K, Olbrich S, The Clock Reading Test: validation of an instrument for the diagnosis of dementia and disorders of visuo-spatial cognition, Int Psychogeriatr 19(2) (2007) 307–21. [DOI] [PubMed] [Google Scholar]
- [30].Hope T, Keene J, McShane RH, Fairburn CG, Gedling K, Jacoby R, Wandering in dementia: a longitudinal study, Int Psychogeriatr 13(2) (2001) 137–47. [DOI] [PubMed] [Google Scholar]
- [31].Rao YL, Ganaraja B, Murlimanju BV, Joy T, Krishnamurthy A, Agrawal A, Hippocampus and its involvement in Alzheimer’s disease: a review, 3 Biotech 12(2) (2022) 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Allen G, Barnard H, McColl R, Hester AL, Fields JA, Weiner MF, Ringe WK, Lipton AM, Brooker M, McDonald E, Rubin CD, Cullum CM, Reduced hippocampal functional connectivity in Alzheimer disease, Arch Neurol 64(10) (2007) 1482–7. [DOI] [PubMed] [Google Scholar]
- [33].Simić G, Kostović I, Winblad B, Bogdanović N, Volume and number of neurons of the human hippocampal formation in normal aging and Alzheimer’s disease, J Comp Neurol 379(4) (1997) 482–94. [DOI] [PubMed] [Google Scholar]
- [34].Kril JJ, Hodges J, Halliday G, Relationship between hippocampal volume and CA1 neuron loss in brains of humans with and without Alzheimer’s disease, Neurosci Lett 361(1–3) (2004) 9–12. [DOI] [PubMed] [Google Scholar]
- [35].Wallace CH, Oliveros G, Serrano PA, Rockwell P, Xie L, Figueiredo-Pereira M, Timapiprant, a prostaglandin D2 receptor antagonist, ameliorates pathology in a rat Alzheimer’s model, Life Sci Alliance 5(12) (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Silva A, Martínez MC, Spatial memory deficits in Alzheimer’s disease and their connection to cognitive maps’ formation by place cells and grid cells, Front Behav Neurosci 16 (2022) 1082158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].R.C. Team, R: A language and environment for statistical computing. R Foundation for Statistical Computing, 2021. https://www.R-project.org/. [Google Scholar]
- [38].Paxinos G, Watson C, The rat brain in stereotaxic coordinates, 7th ed., Academic Press; 2013. [DOI] [PubMed] [Google Scholar]
- [39].Stemberg S, Biomedical Image Processing, Computer 16(1) (1983) 22–34. [Google Scholar]
- [40].Chen S, Wang C, Eberly LE, Caffo BS, Schwartz BS, Adaptive control of the false discovery rate in voxel-based morphometry, Hum Brain Mapp 30(7) (2009) 2304–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Karperien A, Ahammer H, Jelinek HF, Quantitating the subtleties of microglial morphology with fractal analysis, Front Cell Neurosci 7 (2013) 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Zusso M, Methot L, Lo R, Greenhalgh AD, David S, Stifani S, Regulation of postnatal forebrain amoeboid microglial cell proliferation and development by the transcription factor Runx1, J Neurosci 32(33) (2012) 11285–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Joo IL, Lai AY, Bazzigaluppi P, Koletar MM, Dorr A, Brown ME, Thomason LAM, Sled JG, McLaurin J, Stefanovic B, Early neurovascular dysfunction in a transgenic rat model of Alzheimer’s disease, Sci Rep 7 (2017) 46427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Cimadevilla JM, Fenton AA, Bures J, New spatial cognition tests for mice: passive place avoidance on stable and active place avoidance on rotating arenas, Brain Res Bull 54(5) (2001) 559–63. [DOI] [PubMed] [Google Scholar]
- [45].Duda W, Węsierska M, Spatial working memory in rats: Crucial role of the hippocampus in the allothetic place avoidance alternation task demanding stimuli segregation, Behav Brain Res 412 (2021) 113414. [DOI] [PubMed] [Google Scholar]
- [46].Kalová E, Vlcek K, Jarolímová E, Bures J, Allothetic orientation and sequential ordering of places is impaired in early stages of Alzheimer’s disease: corresponding results in real space tests and computer tests, Behav Brain Res 159(2) (2005) 175–86. [DOI] [PubMed] [Google Scholar]
- [47].Nedelska Z, Andel R, Laczó J, Vlcek K, Horinek D, Lisy J, Sheardova K, Bures J, Hort J, Spatial navigation impairment is proportional to right hippocampal volume, Proc Natl Acad Sci U S A 109(7) (2012) 2590–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Berkowitz LE, Harvey RE, Drake E, Thompson SM, Clark BJ, Progressive impairment of directional and spatially precise trajectories by TgF344-Alzheimer’s disease rats in the Morris Water Task, Sci Rep 8(1) (2018) 16153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Bernaud VE, Bulen HL, Peña VL, Koebele SV, Northup-Smith SN, Manzo AA, Valenzuela Sanchez M, Opachich Z, Ruhland AM, Bimonte-Nelson HA, Task-dependent learning and memory deficits in the TgF344-AD rat model of Alzheimer’s disease: three key timepoints through middle-age in females, Scientific Reports 12(1) (2022) 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Cohen RM, Rezai-Zadeh K, Weitz TM, Rentsendorj A, Gate D, Spivak I, Bholat Y, Vasilevko V, Glabe CG, Breunig JJ, Rakic P, Davtyan H, Agadjanyan MG, Kepe V, Barrio JR, Bannykh S, Szekely CA, Pechnick RN, Town T, A transgenic Alzheimer rat with plaques, tau pathology, behavioral impairment, oligomeric abeta, and frank neuronal loss, J Neurosci 33(15) (2013) 6245–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Chaudry O, Ndukwe K, Xie L, Figueiredo-Pereira M, Serrano P, Rockwell P, Females exhibit higher GluA2 levels and outperform males in active place avoidance despite increased amyloid plaques in TgF344-Alzheimer’s rats, Scientific Reports 12(1) (2022) 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Sebastian V, Vergel T, Baig R, Schrott LM, Serrano PA, PKMzeta differentially utilized between sexes for remote long-term spatial memory, PLoS One 8(11) (2013) e81121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Migues PV, Hardt O, Wu DC, Gamache K, Sacktor TC, Wang YT, Nader K, PKMzeta maintains memories by regulating GluR2-dependent AMPA receptor trafficking, Nat Neurosci 13(5) (2010) 630–4. [DOI] [PubMed] [Google Scholar]
- [54].Vouros A, Gehring TV, Szydlowska K, Janusz A, Tu Z, Croucher M, Lukasiuk K, Konopka W, Sandi C, Vasilaki E, A generalised framework for detailed classification of swimming paths inside the Morris Water Maze, Sci Rep 8(1) (2018) 15089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Vouros A, Gehring TV, Jura B, Węsierska MJ, Wójcik DK, Vasilaki E, Strategies discovery in the active allothetic place avoidance task, Sci Rep 12(1) (2022) 12675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Kilgore M, Miller CA, Fass DM, Hennig KM, Haggarty SJ, Sweatt JD, Rumbaugh G, Inhibitors of class 1 histone deacetylases reverse contextual memory deficits in a mouse model of Alzheimer’s disease, Neuropsychopharmacology 35(4) (2010) 870–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Shivakumar M, Subbanna S, Joshi V, Basavarajappa BS, Postnatal Ethanol Exposure Activates HDAC-Mediated Histone Deacetylation, Impairs Synaptic Plasticity Gene Expression and Behavior in Mice, Int J Neuropsychopharmacol 23(5) (2020) 324–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Guan JS, Haggarty SJ, Giacometti E, Dannenberg JH, Joseph N, Gao J, Nieland TJ, Zhou Y, Wang X, Mazitschek R, Bradner JE, DePinho RA, Jaenisch R, Tsai LH, HDAC2 negatively regulates memory formation and synaptic plasticity, Nature 459(7243) (2009) 55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Bredy TW, Wu H, Crego C, Zellhoefer J, Sun YE, Barad M, Histone modifications around individual BDNF gene promoters in prefrontal cortex are associated with extinction of conditioned fear, Learn Mem 14(4) (2007) 268–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].McQuown SC, Barrett RM, Matheos DP, Post RJ, Rogge GA, Alenghat T, Mullican SE, Jones S, Rusche JR, Lazar MA, Wood MA, HDAC3 Is a Critical Negative Regulator of Long-Term Memory Formation, J Neurosci 31(2) (2011) 764–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Intlekofer KA, Berchtold NC, Malvaez M, Carlos AJ, McQuown SC, Cunningham MJ, Wood MA, Cotman CW, Exercise and sodium butyrate transform a subthreshold learning event into long-term memory via a brain-derived neurotrophic factor-dependent mechanism, Neuropsychopharmacology 38(10) (2013) 2027–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Singh P, Konar A, Kumar A, Srivas S, Thakur MK, Hippocampal chromatin-modifying enzymes are pivotal for scopolamine-induced synaptic plasticity gene expression changes and memory impairment, J Neurochem 134(4) (2015) 642–51. [DOI] [PubMed] [Google Scholar]
- [63].Gräff J, Woldemichael BT, Berchtold D, Dewarrat G, Mansuy IM, Dynamic histone marks in the hippocampus and cortex facilitate memory consolidation, Nature Communications 3(1) (2012) 1–8. [DOI] [PubMed] [Google Scholar]
- [64].Gallo FT, Katche C, Morici JF, Medina JH, Weisstaub NV, Immediate Early Genes, Memory and Psychiatric Disorders: Focus on c-Fos, Egr1 and Arc, Front Behav Neurosci 12 (2018) 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Yagi S, Drewczynski D, Wainwright SR, Barha CK, Hershorn O, Galea LAM, Sex and estrous cycle differences in immediate early gene activation in the hippocampus and the dorsal striatum after the cue competition task, Horm Behav 87 (2017) 69–79. [DOI] [PubMed] [Google Scholar]
- [66].Fortress AM, Kim J, Poole RL, Gould TJ, Frick KM, 17β-Estradiol regulates histone alterations associated with memory consolidation and increases Bdnf promoter acetylation in middle-aged female mice, Learn Mem 21(9) (2014) 457–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Heldt SA, Stanek L, Chhatwal JP, Ressler KJ, Hippocampus-specific deletion of BDNF in adult mice impairs spatial memory and extinction of aversive memories, Mol Psychiatry 12(7) (2007) 656–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Park HJ, Kim MJ, Rothenberger C, Kumar A, Sampson EM, Ding D, Han C, White K, Boyd K, Manohar S, Kim YH, Ticsa MS, Gomez AS, Caicedo I, Bose U, Linser PJ, Miyakawa T, Tanokura M, Foster TC, Salvi R, Someya S, GSTA4 mediates reduction of cisplatin ototoxicity in female mice, Nat Commun 10(1) (2019) 4150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Lopes-Ramos CM, Kuijjer ML, Ogino S, Fuchs CS, DeMeo DL, Glass K, Quackenbush J, Gene Regulatory Network Analysis Identifies Sex-Linked Differences in Colon Cancer Drug Metabolism, Cancer Res 78(19) (2018) 5538–5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Knight TR, Choudhuri S, Klaassen CD, Constitutive mRNA expression of various glutathione S-transferase isoforms in different tissues of mice, Toxicol Sci 100(2) (2007) 513–24. [DOI] [PubMed] [Google Scholar]
- [71].Wang LZ, Ramírez J, Yeo W, Chan MY, Thuya WL, Lau JY, Wan SC, Wong AL, Zee YK, Lim R, Lee SC, Ho PC, Lee HS, Chan A, Ansher S, Ratain MJ, Goh BC, Glucuronidation by UGT1A1 is the dominant pathway of the metabolic disposition of belinostat in liver cancer patients, PLoS ONE 8(1) (2013) e54522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Balliet RM, Chen G, Gallagher CJ, Dellinger RW, Sun D, Lazarus P, Characterization of UGTs active against SAHA and association between SAHA glucuronidation activity phenotype with UGT genotype, Cancer Res 69(7) (2009) 2981–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Dong D, Zhang T, Lu D, Liu J, Wu B, In vitro characterization of belinostat glucuronidation: demonstration of both UGT1A1 and UGT2B7 as the main contributing isozymes, Xenobiotica 47(4) (2017) 277–283. [DOI] [PubMed] [Google Scholar]
- [74].Raftogianis R, Creveling C, Weinshilboum R, Weisz J, Estrogen metabolism by conjugation, J Natl Cancer Inst Monogr (27) (2000) 113–24. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting the conclusions of this article are available in the NIH GEO repository, ACCESSION NUMBERS:
Male TGNT: GSM7884145 (M1), GSM7884146 (M2), GSM7884147 (M3), GSM7884148 (M4), GSM7884149 (M5).
Male TGTR: GSM7884150 (MRG1),GSM7884151(MRG2),GSM7884152(MRG3), GSM7884153 (MRG4), GSM7884154 (MRG5)
Female TGNT: GSM7884155 (F1), GSM7884156 (F2), GSM7884157 (F3), GSM7884158 (F4), GSM7884159 (F5)
Female TGTR: GSM7884160 (FRG1), GSM7884161 (FRG2), GSM7884162 (FRG3), GSM7884163 (FRG4), GSM7884164 (FRG5)
To review GEO accession GSE247142:
Go to https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE247142
Enter token mfobggwmhrspnur into the box.


