Abstract
Open reading frames within the unique short segment of alphaherpesvirus genomes participate in egress and cell-to-cell spread. The case of varicella-zoster virus (VZV) is of particular interest not only because the virus is highly cell associated but also because its most prominent cell surface protein, gE, bears semblance to the mammalian Fc receptor FcγRII. A previous study demonstrated that when expressed alone in cells, VZV gE was endocytosed from the cell surface through a tyrosine localization motif in its cytoplasmic tail (J. K. Olson and C. Grose, J. Virol. 71:4042–4054, 1997). Since VZV gE is normally found in association with gI in the infected cell, the present study was directed at defining the trafficking of the VZV gE:gI protein complex. First, VZV gI underwent endocytosis and recycling when it was expressed alone in cells, and interestingly, VZV gI contained a methionine-leucine internalization motif in its cytoplasmic tail. Second, VZV gI was found by confocal microscopy to colocalize with VZV gE during endocytosis and recycling in cells. Third, by a quantitative internalization assay, VZV gE:gI was shown to undergo endocytosis more efficiently (steady state, 55 to 60%) than either gE alone (steady state, ∼32%) or gI alone (steady state, ∼45%). Further, examination of endocytosis-deficient mutant proteins demonstrated that VZV gI exerted a more pronounced effect than gE on internalization of the complex. Most importantly, therefore, these studies suggest that VZV gI behaves as an accessory component by facilitating the endocytosis of the major constituent gE and thereby modulating the trafficking of the entire cell surface gE:gI Fc receptor complex.
Endocytosis is an important internalization process by which cells obtain extracellular molecules. Receptor-mediated endocytosis enables selective uptake of macromolecules by the cell. The process begins with receptors selectively concentrating in clathrin-coated pits on the cell membrane, after which they are internalized and delivered to endosomes. Some receptors continually cluster in coated pits and undergo rapid internalization such as the FcγRII, the transferrin receptor (TR), and the low-density-lipoprotein (LDL) receptor. Other receptors are concentrated in clathrin-coated pits only after binding their ligand, e.g., the epidermal growth factor receptor (42). The clustering in clathrin-coated pits and the internalization of receptors have been shown to be dependent on internalization signals in the cytoplasmic tail, which have been defined as tyrosine motifs or dileucine motifs (33). The internalization motifs form tight turns and interact with adaptor complexes associated with clathrin-coated pits (3, 31). Upon entering endosomes, the receptors are sorted into specific pathways, such as the recycling pathway or the lysosomal pathway. Receptors which enter the recycling pathway continually recycle from the cell surface to the endosomes and back to the cell surface again. These receptors include the TR and the LDL receptor, which have recycling efficiencies higher than 98% (19, 26, 40). In contrast, the epidermal growth factor receptor enters the lysosomal pathway along with its ligand and is subsequently degraded in the lysosomes (35).
Recent reports have demonstrated that viruses encode proteins which also undergo endocytosis from the cell membrane. First, both simian immunodeficiency virus and human immunodeficiency virus type 1 encode an envelope protein which undergoes endocytosis from the cell membrane. Retroviral envelope protein endocytosis was dependent on a tyrosine motif in the cytoplasmic tail region (9, 34, 36). Second, varicella-zoster virus (VZV) encodes a glycoprotein, gE, which has been demonstrated to undergo endocytosis from the cell membrane (1, 32). Likewise, VZV gE was shown to have a tyrosine-containing motif in its cytoplasmic tail which was important for internalization (32). Thus, virus-encoded proteins share similar trafficking sequence motifs with cellular receptors.
VZV is classified as one of the human alphaherpesviruses along with herpes simplex virus and pseudorabies virus. The VZV genome is the smallest of the human herpesviruses, including about 70 open reading frames (ORFs) (4). The gE glycoprotein (ORF 68; previously designated gpI or gp98) is the predominant glycosylated VZV cell surface antigen, where it is often localized along “viral highways” (15, 29, 45); it is also detected within the cytoplasmic vacuoles containing nascent virions (17, 28, 29). VZV gE has been designated a typical type I transmembrane glycoprotein; it is highly modified by both N-linked and O-linked glycosylation, sialylation, and sulfation, as well as serine/threonine and tyrosine phosphorylation (13, 33, 47). Further, VZV gE forms a protein complex with VZV gI during viral infection. VZV gI (ORF 67; previously designated gpIV) is another type I transmembrane glycoprotein which is N-linked and O-linked glycosylated and serine phosphorylated (13, 46). The gE:gI complex is located on the cell membrane of both virus-infected cultures and vesicular lesions in patients with chicken pox and herpes zoster (8, 23, 43, 45). Furthermore, the complex functions as a cell surface Fc receptor for nonimmune human immunoglobulin G (IgG) in both virus-infected cells and transfected cells (22, 23). Herpesviral Fc receptor activity has been proposed as a mechanism to protect virus-infected cells from lysis by the immune system (11).
The ORFs for gE and gI are located in the unique short (US) segment of the VZV genome. Based on genetic analyses of the evolution of herpesviruses, the US region is considered a recent addition to the herpesviral genome; it may function to provide a biological advantage for survival (25). The reason why an emergent VZV genome usurped an Fc receptor complex remains an interesting issue for investigation, particularly with regard to the role of alphaherpesviral gE:gI in neurotropic cell-to-cell spread (6, 7, 10, 20). The fact that a VZV gI null mutant spreads very poorly in cell culture reinforces the importance of the VZV gE:gI complex (24).
MATERIALS AND METHODS
Cells, plasmids, and antibodies.
HeLa cells (ATCC CCL2) were obtained from the American Type Culture Collection, Rockville, Md. HeLa cells were grown in Eagle complete medium supplemented with 10% fetal bovine serum. Descriptions of recombinant vaccinia virus (T7-vaccinia virus) and expression plasmid pTM1 have been published (30). Construction of plasmid pTM1-gE containing VZV ORF 68 and plasmid pTM1-gI containing VZV ORF 67 have been described previously (46, 47). Monoclonal antibody (MAb) 6B5 recognizes an epitope in the gI ectodomain (46), while MAb 3B3 binds to a defined epitope in the gE ectodomain (16).
Endocytosis assay with laser scanning confocal microscopy.
The endocytosis assays were performed as previously described (32). Briefly, HeLa cells were seeded in 35-mm-diameter culture dishes at a concentration of 6.2 × 105 cells per dish and incubated overnight at 37°C. The cells were infected with recombinant T7-vaccinia virus and then transfected with Lipofectin containing 4 μg of pTM1-gI construct. Six hours after transfection, fresh medium was added, and the cells were incubated overnight at 37°C; 16 h posttransfection, the cells were washed with cold phosphate-buffered saline (PBS; pH 7.4) and MAb 6B5 was added to each dish (1:1,500 dilution in PBS). After a 30-min incubation at 4°C, the cells were washed once with PBS and once with Eagle complete medium with 10% fetal bovine serum. Fresh medium was added, and the cells were incubated at 37°C for various time periods. Subsequently, the cells were fixed and permeabilized with 2% paraformaldehyde in 0.1 M Na2HPO4 with 0.05% Triton X-100. The cells were washed with PBS prior to incubation for 1 h with goat anti-mouse–fluorescein isothiocyanate (FITC) conjugate (1:1,000 dilution; Biosource). The cells were washed again and then viewed with a Bio-Rad 1024 laser scanning confocal microscope at the University of Iowa Central Microscopy Research Facility (33). Images were saved and analyzed as previously described (32).
Colocalization of gE and gI during endocytosis.
HeLa cells were transfected with both pTM1-gI and pTM1-gE as described above. The cells were incubated with MAb 6B5 (1:1,500 dilution) and rabbit monospecific polyclonal antiserum which recognizes VZV gE (1:1,000 dilution). The cells were incubated at 37°C with medium for 0, 15, 30, or 60 min. After each time period, the cells were fixed and permeabilized. The cells were then incubated for 1 h with secondary antibodies, goat anti-mouse–Texas red conjugate (1:1,000 dilution; Molecular Probes), and goat anti-rabbit–FITC conjugate (1:1,000 dilution; Biosource).
Endocytosis and recycling of VZV gE and gI after trypsin treatment.
HeLa cells were transfected with gI alone or with gE and gI as described above. The endocytosis and recycling assay closely resembles that previously described (32). Briefly, the cells were incubated with MAb 6B5 alone or both MAb 6B5 and polyclonal antiserum for gE at 4°C for 30 min. The cells were then incubated with medium at 37°C for 30 min. Next, the cells were treated with 1 mg of trypsin (Sigma) per ml at 0°C for 30 min. After trypsin treatment, the cells were washed and returned to 37°C with fresh medium containing 0.5 mg of trypsin inhibitor per ml for 30 min. After incubation at 37°C, the cells were fixed with 2% paraformaldehyde in 0.1 M Na2HPO4. The cells were then incubated with secondary antibody, goat anti-mouse–FITC conjugate alone, or both goat anti-mouse–Texas red and goat anti-rabbit–FITC conjugates for 1 h.
Quantitative internalization assay of VZV gE and gI.
HeLa cells were transfected with gE, gI, or gE and gI as described above. This assay was performed similarly to that previously described (32). Six hours posttransfection, 250 μCi of [35S]methionine-cysteine (specific activity, 7.15 mCi/ml; PRO-MIX [Amersham]) per ml of medium was added to each dish, and the cells were incubated for 10 h at 37°C. The cells were washed and incubated with MAb 6B5 (1:1,500 dilution) or with MAb 3B3 (1:2,000 dilution) for 30 min at 4°C. The cells were washed and incubated at 37°C for different time periods. At the given times, the cells were treated with 1 mg of trypsin per ml for 30 min at 0°C to remove surface proteins. A previous experiment demonstrated that all surface protein is removed by trypsin treatment (33). The cells were then lysed in radioimmunoassay buffer containing 0.5 mg of soybean trypsin inhibitor (Sigma) per ml on ice for 30 min. The lysates which contain antigen-antibody complexes were incubated with protein A-Sepharose CL-4B beads (Pharmacia) and precipitated as previously described (32). The proteins were eluted from the protein A beads in reducing buffer (125 mM Tris [pH 6.8], 6% glycerol, 10% 2-mercaptoethanol). The immunoprecipitated proteins were analyzed on 10 to 18% gradient polyacrylamide gels containing 0.1% sodium dodecyl sulfate. The gels were analyzed with a Packard Instantimager and exposed to radiographic film.
Construction of endocytosis mutant gI by recombination PCR.
To mutate methionine residue at position 328 and leucine residue at position 329 in the gI cytoplasmic tail into alanine residues, site-directed mutagenesis was performed by recombination PCR (48). Four oligonucleotide primers were prepared to generate two linear fragments containing homologous ends. One pair of mutating primers and one pair of nonmutating primers were designed. The nonmutating primers were prepared as previously described (33, 48). The mutating primers were prepared by Genosys through the University of Iowa DNA Core facility. Sequences of the mutating primers were 5′ TCCGATGTGGCTGCAGAGGCCGCCATTGCAC 3′ and 5′ GTGCAATGGCGGCCTCTGCAGCCACATCGGA 3′ with a 31-bp overlap. Plasmid pTM1-gI was first linearized with restriction endonuclease SacI or SpeI. One mutating and one nonmutating primer were used in pairs to generate linear fragments. Amplification of the DNA fragments from the linearized plasmid template was performed by PCR methods previously described (33, 48). The two linear DNA products were combined and transformed into Max competent Escherichia coli DH5α cells (BRL, Life Technologies). Recombination between the homologous fragments produced a plasmid, gI-AA, containing the designated mutation. The mutation was verified by partial sequencing at the University of Iowa DNA Core facility.
RESULTS
Endocytosis of VZV gI.
Recently, VZV gE was shown to undergo endocytosis from the cell membrane in a manner similar to that of the TR (32). In the infected cells, VZV gE is usually associated with a second viral protein, gI, to form the gE:gI complex (13, 44). When gE and gI are coexpressed in a dual transient transfection system, they also form a gE:gI complex on the cell surface (22, 46, 47). Since the gE:gI complex may be a more accurate representation of the situation in the infected cell, we investigated the trafficking of the gI molecule alone and together with gE. To this end, VZV gI was expressed in HeLa cells, and a confocal-microscopy endocytosis assay was conducted. HeLa cells were transfected with plasmid pTM1-gI, which contains the entire wild-type gI gene. Sixteen hours posttransfection, the cells were incubated with MAb 6B5 at 4°C for 30 min. Incubation of cells at 4°C inhibits endocytosis of proteins from the cell membrane, thus allowing the antibody to bind to surface proteins. Murine MAb does not induce endocytosis of the protein (32). The cells were then incubated at 37°C for 0, 15, 30, 45, or 60 min to allow internalization of gI. After the timed incubations at 37°C, the cells were fixed, permeabilized, and probed with secondary antibody conjugated with FITC to determine the localization of the antibody-bound gI proteins within the cell. The cells were examined by confocal microscopy with laser sectioning in 1-μm increments (zeta series). Multiple images were analyzed for each time point, and the central sections from each cell were compared for gI localization.
As shown in Fig. 1A, when VZV gI-transfected cells were incubated with MAb 6B5 and not returned to 37°C, the antibody-bound gI was detectable on the surface of the cell but was not observed within the cell. When the cells were incubated with MAb 6B5 and then incubated at 37°C for 15 min (Fig. 1B), some gI was still observed on the surface while some was also internalized within the cell. After 30, 45, or 60 min at 37°C (Fig. 1C to E), larger amounts of gI were localized within the cell, especially in small vesicle-shaped clusters. Since these cells were optically sectioned to examine intracellular VZV proteins, the gI visible in Fig. 1C to E cannot be located only on the cell surface. As a control, mock-transfected HeLa cells were not bound by MAb 6B5 during the 30-min incubation (Fig. 1F). The results of this experiment indicate that when VZV gI was expressed alone in transfected cells, it underwent endocytosis from the cell membrane in a manner similar to that of VZV gE.
FIG. 1.
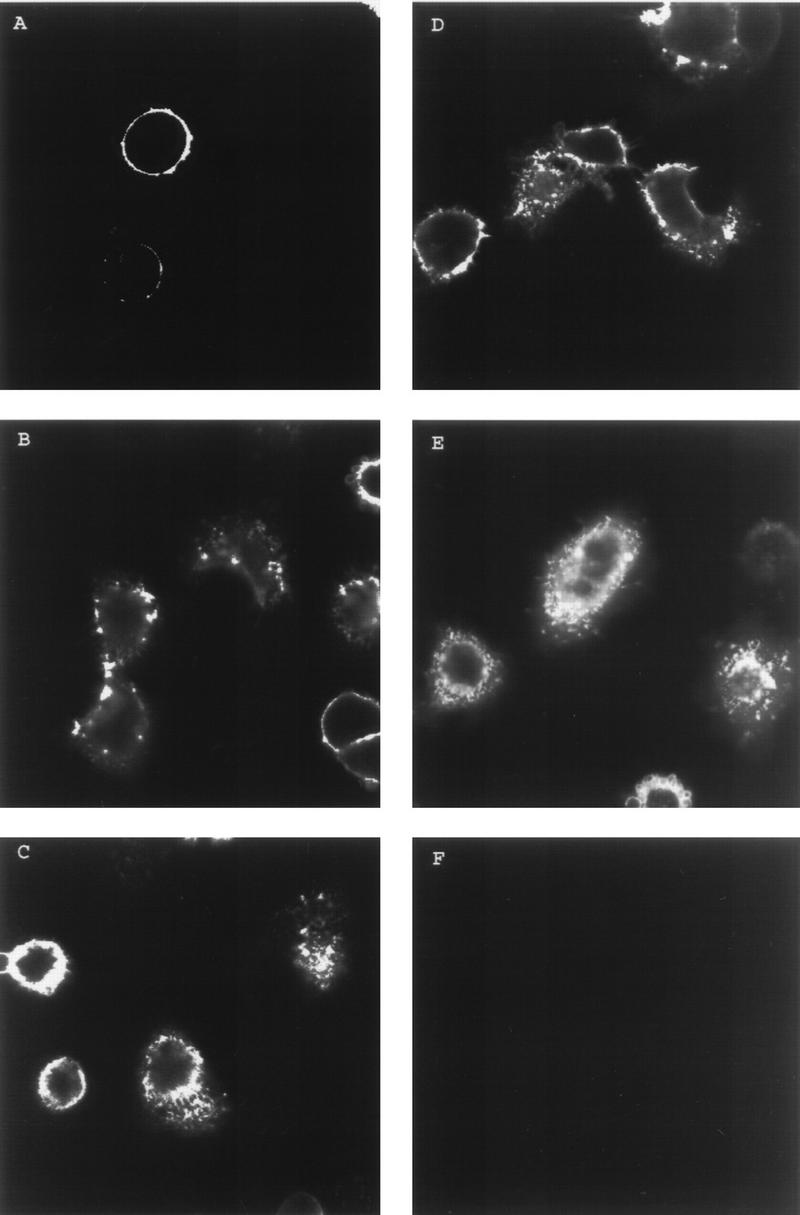
Endocytosis of VZV gI. HeLa cells were transfected with the gI gene. Sixteen hours posttransfection, the cells were incubated with anti-gI MAb 6B5 at 4°C for 30 min. The cells were returned to 37°C with fresh medium for 0 (A), 15 (B), 30 (C), 45 (D), or 60 (E) min. The cells were fixed and permeabilized and then incubated with secondary antibody for 1 h. The images shown represent the central section from the cells analyzed by laser scanning confocal microscopy. Panel F shows HeLa cells mock transfected and stained as a negative control.
Colocalization of VZV gE and gI during endocytosis.
To determine if gI follows a similar pattern of endocytosis and recycling when expressed with gE, colocalization experiments were performed with confocal microscopy. Colocalization of VZV gE and gI was examined in cells cotransfected with the genes for gE and gI and double labeled with antibody for gE and gI during the endocytosis assay. To this end, HeLa cells were transfected with plasmids pTM1-gE and pTM1-gI. Sixteen hours posttransfection, the cells were incubated with murine MAb 6B5 and rabbit polyclonal antiserum for gE at 4°C for 30 min. The cells were returned to 37°C for various times, after which they were fixed and permeabilized. The cells were then incubated with goat anti-mouse–Texas red conjugate and goat anti-rabbit–FITC conjugate to identify the localization of each protein within the cell. When the cells were incubated with primary antibody but not returned to 37°C, gE was localized on the cell membrane as previously documented (Fig. 2A), and gI was also localized on the cell membrane in the same manner as that shown in Fig. 1A (Fig. 2B). When the two images were merged, the two proteins colocalized to the cell surface, as shown by the yellow color (Fig. 2C). When the cells were incubated with primary antibodies and returned to 37°C for 15 min, gE was internalized, as shown by staining within the cell (Fig. 2D), and gI was internalized in the same manner as that shown in Fig. 1B (Fig. 2E). When the two images were merged, the two proteins were internalized to the same areas of the cell (Fig. 2F). After 30 min at 37°C, both gE and gI were still being internalized (Fig. 2G and H). The two proteins were colocalizing within the cell, and small vesicles within the cell contained both proteins (Fig. 2I). After incubation at 37°C for 60 min, gE and gI were both internalized (Fig. 2J and K), and both proteins colocalized within the cell (Fig. 2L). Therefore, these results showed that gI was internalized along the same pathway as that of gE during endocytosis from the cell membrane.
FIG. 2.
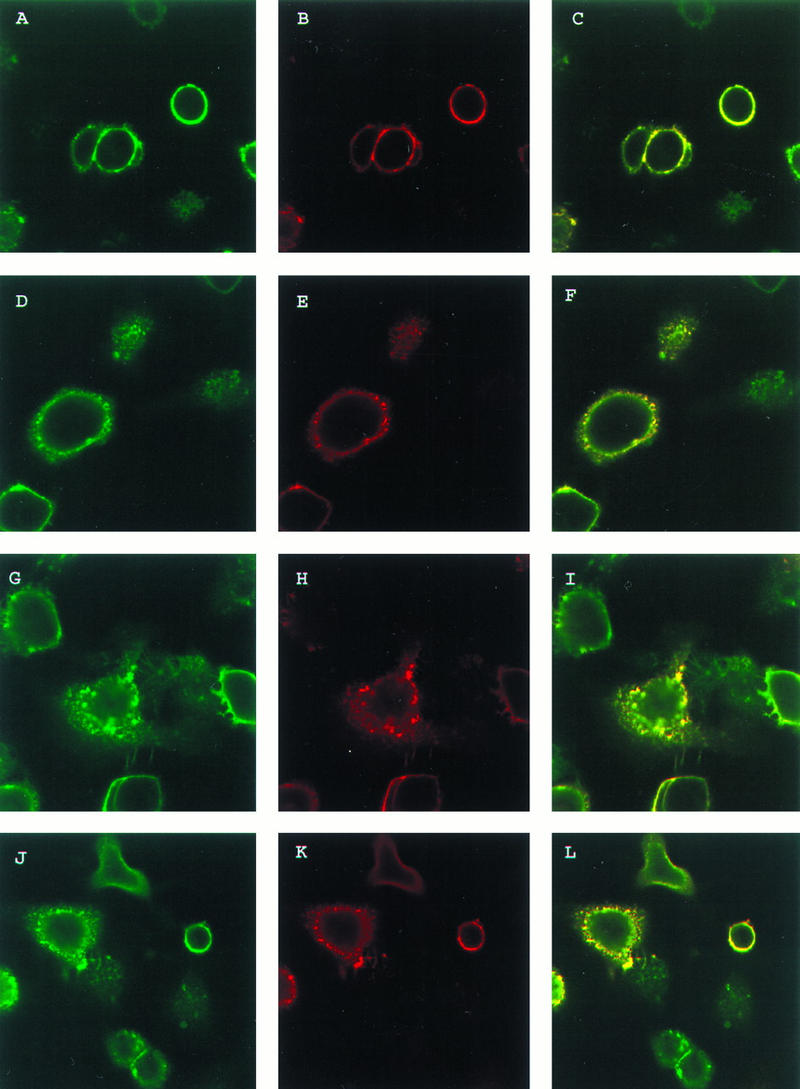
Colocalization of VZV gE and gI during endocytosis. HeLa cells were transfected with the gE gene and the gI gene. The cells were incubated with MAb 6B5 and polyclonal antiserum for gE at 4°C for 30 min. The cells were then returned to 37°C for 0 (A, B, and C), 15 (D, E, and F), 30 (G, H, and I), or 60 (J, K, and L) min. The cells were fixed and permeabilized and then incubated with goat anti-mouse–Texas red conjugate and goat anti-rabbit–FITC conjugate for 1 h. The cells were analyzed by laser scanning confocal microscopy with gE (green stain) (A, D, G, and J) and gI (red stain) (B, E, H, and K); the merged images (yellow stain) are also shown (C, F, I, and L).
Recycling of VZV gI.
Since gE has been previously shown to be recycled to the cell surface after internalization, the colocalization of gI with gE during endocytosis suggested that gI may also be recycling. To analyze recycling, HeLa cells were transfected with the gI gene alone or cotransfected with both the gE and gI genes. Sixteen hours posttransfection, the cells were incubated with primary antibody MAb 6B5 alone or MAb 6B5 and polyclonal antiserum for gE at 4°C for 30 min. The cells were then returned to 37°C for 30 min to allow internalization of the antibody-bound proteins. Some of the cells were then incubated with trypsin at 0°C for 30 min to remove surface proteins before being returned to 37°C for 30 min. Treating the cells with trypsin removes surface proteins without affecting internalized proteins, while returning the cells to 37°C allows the internalized proteins to return to the cell surface (32). At various time points, the cells were fixed and then stained with anti-mouse–FITC conjugate or both anti-mouse–Texas red conjugate and anti-rabbit–FITC conjugate. Since the cells were not permeabilized, the secondary antibody was able to bind only surface antibody-bound proteins. The recycling of gI expressed alone in HeLa cells was analyzed first. In the cells which were not trypsin treated after 30 min of incubation at 37°C, some gI remained on the surface (Fig. 3A). When the cells were treated with trypsin but not returned to 37°C after treatment, all gI was removed from the surface (Fig. 3B). When the cells were returned to 37°C for 30 min following trypsin treatment, gI was again observed on the surface of the cells (Fig. 3C). Thus, the gI which was initially found on the surface of the cell (where it bound antibody) was internalized during the first incubation at 37°C and subsequently returned to the cell surface.
FIG. 3.
Colocalization of VZV gE and gI during recycling. HeLa cell monolayers were singly transfected with the gI gene (A, B, and C) or dually transfected with the gE and gI genes (D to I). Subsequently, monolayers were incubated with MAb 6B5 (A to C) or with both MAb 6B5 and polyclonal antiserum for gE (D to I) while on ice. Some monolayers were incubated at 37°C for 30 min to allow internalization and then treated with trypsin to remove surface proteins. The cells were returned to 37°C with fresh medium containing trypsin inhibitor for 0 (B, E, and H) or 30 (C, F, and I) min. Transfected cells that were neither trypsin treated nor returned to 37°C represented positive controls (A, D, and G). At the given time points, the cells were fixed and stained with goat anti-mouse–FITC conjugate (A to C) or both goat anti-mouse–Texas red conjugate and goat anti-rabbit–FITC conjugate (D to I). Singly transfected monolayers were analyzed by confocal microscopy for gI in panels A to C; cotransfected monolayers were probed for gI in panels D to F and for gE in panels G to I.
The recycling of gI when expressed with gE was then analyzed by double staining for gE and gI. When cotransfected cells were incubated with both MAb 6B5 and anti-gE antiserum, the recycling pattern of gI and gE together was similar to that observed in cells expressing either gI or gE alone. Initially, gI was present on the surface of cells which were not treated with trypsin after the first incubation at 37°C (Fig. 3D). Similarly, gI was removed from the surface of the cells by trypsin treatment (Fig. 3E). Finally, gI coexpressed with gE recycled back to the cell surface, as indicated by the presence of gI on the cell surface after incubation at 37°C following trypsin treatment (Fig. 3F). An identical pattern was seen in the three gE staining profiles (Fig. 3G to I). These results documented that gI underwent internalization and recycled back to the cell surface whether expressed alone or together with gE.
Endocytosis of gE and gI as a protein complex.
By confocal microscopy, gE and gI were both shown to undergo endocytosis and recycle when they were expressed alone as well as when they were expressed together. Further, the proteins colocalized during endocytosis and recycling when expressed together in HeLa cells. To verify that the gE:gI complex itself was endocytosed and recycled, gE and gI were analyzed by a quantitative internalization protein assay (Table 1).
TABLE 1.
Quantitative internalization assay results for VZV gE and gI
| Incubation time (min)a | gI aloneb
|
gI with gEc
|
gE aloned
|
gE with gIe
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Radioactivity
|
Internalization
|
Radioactivity
|
Internalization
|
Radioactivity
|
Internalization
|
Radioactivity
|
Internalization
|
|||||||||
| cpmf | Corrected cpmg | %h | % (mean ± SD)i | cpm | Corrected cpm | % | % (mean ± SD) | cpm | Corrected cpm | % | % (mean ± SD) | cpm | Corrected cpm | % | % (mean ± SD) | |
| 0 (without trypsin) | 35,235 | 33,272 | 100 | 100 | 34,936 | 33,655 | 100 | 100 | 34,942 | 33,607 | 100 | 100 | 27,026 | 25,912 | 100 | 100 |
| 0 (with trypsin) | 1,963 | 0 | 0 | 0 | 1,281 | 0 | 0 | 0 | 1,335 | 0 | 0 | 0 | 1,114 | 0 | 0 | 0 |
| 15 | 16,206 | 14,243 | 42.8 | 43.3 ± 0.8 | 20,874 | 19,593 | 58.2 | 58.4 ± 0.9 | 11,686 | 10,351 | 30.8 | 30.7 ± 0.5 | 15,236 | 14,122 | 54.5 | 55.2 ± 0.9 |
| 30 | 17,344 | 15,381 | 46.2 | 46.3 ± 0.7 | 21,656 | 20,375 | 60.5 | 60.5 ± 0.5 | 11,961 | 10,626 | 31.6 | 31.9 ± 1 | 15,806 | 14,692 | 56.7 | 56.8 ± 0.6 |
| 45 | 17,580 | 15,617 | 46.9 | 47.3 ± 0.7 | 22,019 | 20,738 | 61.6 | 61.8 ± 0.9 | 12,197 | 10,862 | 32.3 | 32.5 ± 0.5 | 16,013 | 14,899 | 57.5 | 57.3 ± 0.8 |
| 60 | 17,644 | 15,681 | 47.1 | 47.4 ± 0.6 | 21,786 | 20,505 | 60.9 | 61.1 ± 0.8 | 11,780 | 10,445 | 32.1 | 32.2 ± 0.5 | 15,858 | 14,744 | 56.9 | 56.9 ± 0.9 |
The cells were incubated for the time indicated at 37°C following incubation with antibody during the endocytosis assay. The cells were either left untreated or were treated with trypsin, which removes all the surface proteins, before (0 min) or after the incubation times indicated at 37°C. The proteins which were left after trypsin treatment represented the internalized protein.
The VZV gI gene was transfected into HeLa cells, and the gI protein was precipitated with anti-gI MAb 6B5.
The VZV gI gene and the VZV gE gene were transfected into HeLa cells, and the gE:gI complex was precipitated with MAb 6B5.
The VZV gE gene was transfected into HeLa cells, and the gE protein was precipitated with anti-gE MAb 3B3.
The VZV gE gene and the VZV gI gene were transfected into HeLa cells, and the gE:gI complex was precipitated with MAb 3B3.
The radioactivity of each protein in counts per minute was determined with an Instantimager.
The radioactivity of each protein was corrected by subtracting the background radioactivity measured in the control sample (incubation time, 0 min; with trypsin).
The percentage of protein internalized was determined by dividing the corrected counts per minute at each time point by the corrected counts per minute of the total protein present on the surface at the beginning of the assay (time, 0 min; without trypsin), and multiplying this value by 100.
These values represent data collected for this experiment and three additional independent experiments conducted in a similar manner, as described in Materials and Methods.
The amount of VZV gE protein internalized at each time point is graphically represented in Fig. 4A. The curves of these graphs are indicative of proteins which are continually internalized and recycled since the total protein present did not vary throughout the experiment (Table 1). Interestingly, when gE was expressed alone, 30 to 32% of the protein was internalized at a steady state; when gE was coprecipitated with gI during endocytosis, 55 to 57% of the protein was internalized at any given time point. A similar result was observed with gI internalization (Fig. 4B). When gI was expressed alone and incubated with MAb 6B5 during the endocytosis assay, 43 to 47% of gI was internalized at a steady state. When gI was coexpressed with gE and coprecipitated with MAb 3B3 during endocytosis, gI was internalized at a rate of 58 to 62% at any given time point. These results clearly demonstrated that a greater amount of the gE:gI complex underwent internalization than either protein expressed alone.
FIG. 4.
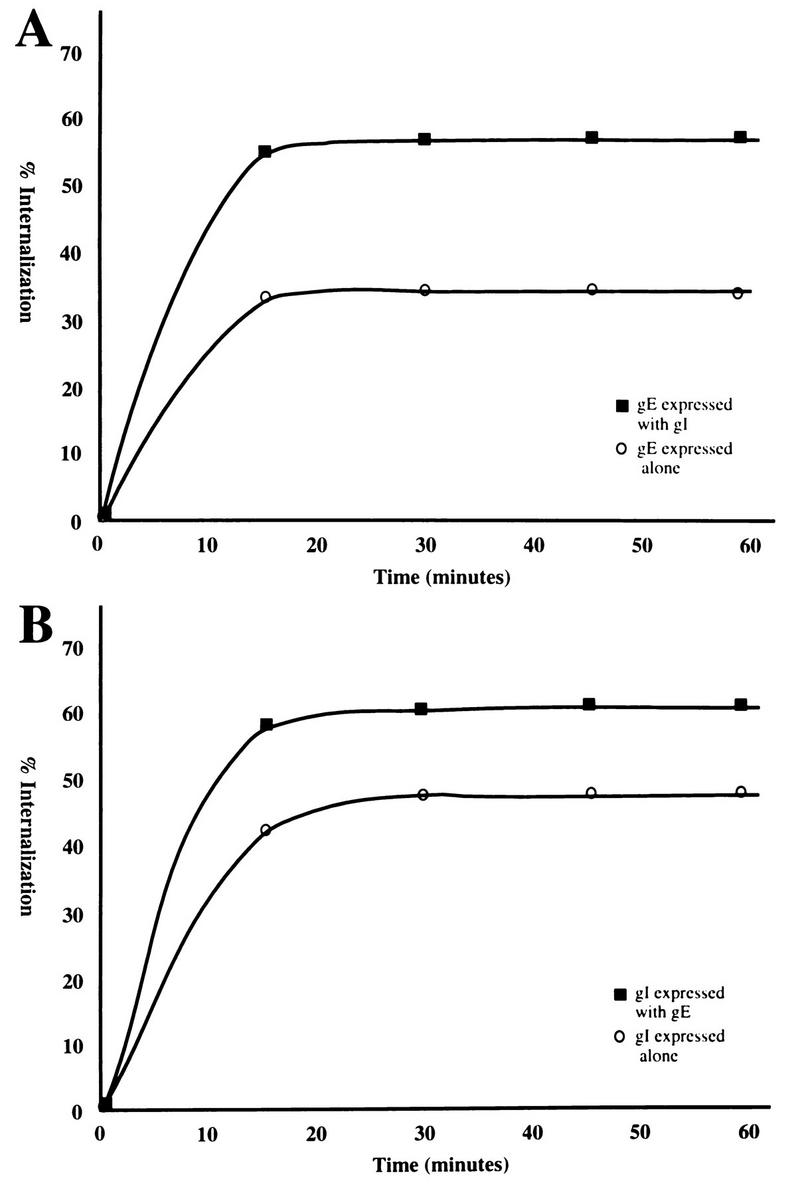
Analysis of internalized VZV gE (A) and gI (B). (A) The graph of internalized gE was derived from gE either expressed alone or with gI. This graph is representative of four separate experiments (Table 1). (B) The graph of internalized gI was derived from gI either expressed alone or with gE. This graph is representative of four separate experiments (Table 1).
Endocytosis of gI and endocytosis mutant gE as a complex.
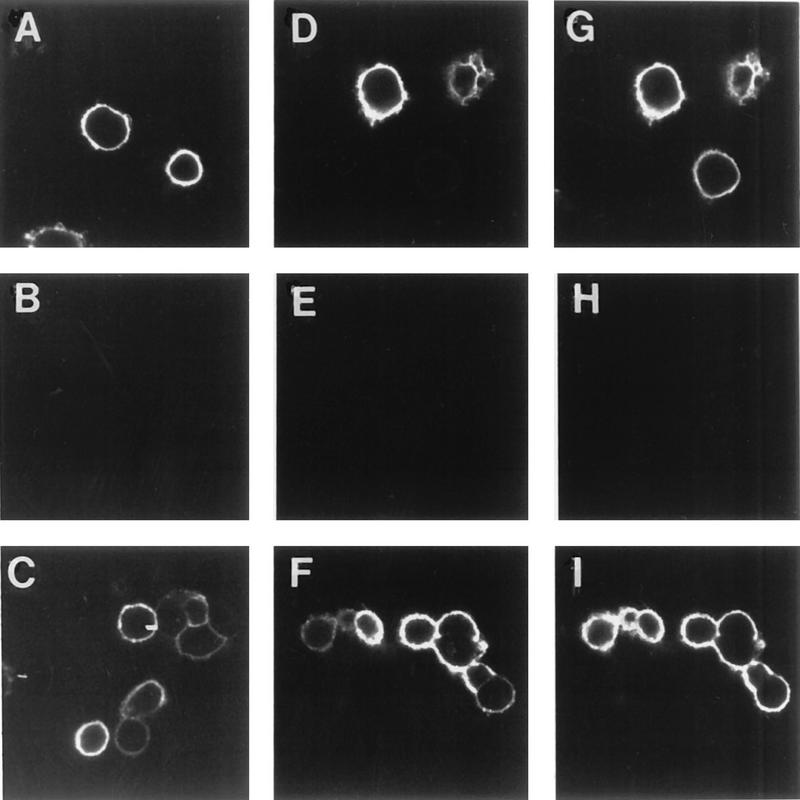
The results described above suggest an important role in endocytosis for the formation of the VZV protein complex. To determine the relative roles of each protein within the complex, we selected a previously described endocytosis mutant gE which contains a tyrosine mutation at residue 582 in the cytoplasmic tail (32). The effect of this gE mutation on the endocytosis of the gE:gI complex was examined. HeLa cells were transfected with the gE-Y582G gene or with both the gI gene and the gE-Y582G gene. When gE-Y582G was expressed alone in cells, the protein was present on the cell surface after incubation with MAb 3B3 and without returning the cells to 37°C (Fig. 5A). After incubation at 37°C for 30 (Fig. 5B) or 60 (Fig. 5C) min, gE-Y582G remained on the surface of the cells and was not internalized, similar to a previous observation (32). When gE-Y582G was coexpressed with gI and incubated with MAb 3B3, gE-Y582G was present on the surface prior to incubation at 37°C (Fig. 5D). However, after incubation at 37°C for 30 min (Fig. 5E), gE-Y582G was localized not only on the surface but also within the cell in a characteristic multivesicular endocytosis pattern (compare with Fig. 1). After a 60-min incubation at 37°C (Fig. 5F), gE-Y582G was almost completely internalized within the cell, indicating that the gE mutant was being endocytosed when it was coexpressed with gI. Further experiments with cells cotransfected with gE-Y582G and gI and then incubated with polyclonal antiserum for gE and MAb 6B5 demonstrated colocalization of gE-Y582G and gI during endocytosis. When the cells were not incubated at 37°C following incubation with the primary antibodies (Fig. 5G), both proteins were localized to the cell membrane, as indicated by the yellow color. After incubation at 37°C for 30 min (Fig. 5H), both gE-Y582G and gI were colocalized both within the cell and on the cell surface. Further, after incubation at 37°C for 60 min (Fig. 5I), both proteins were colocalized in the cell during endocytosis. These results documented that the tyrosine mutant gE was able to undergo endocytosis when expressed with wild-type gI.
FIG. 5.
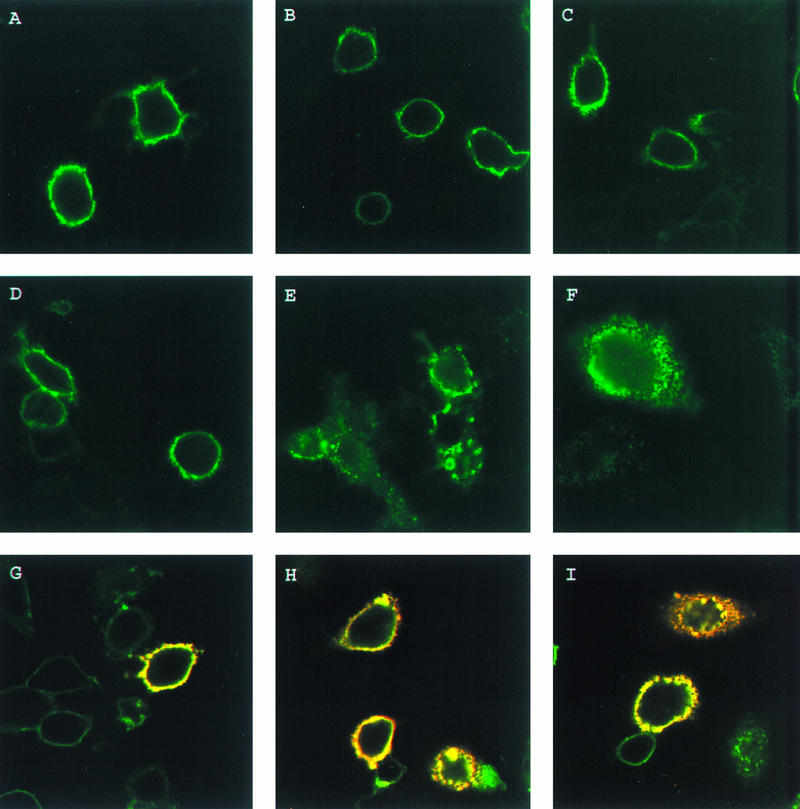
Endocytosis of VZV gI and endocytosis mutant gE. HeLa cells were transfected with the gE-Y582G gene (A, B, and C) or with both the gE-Y582G gene and the wild-type gI gene (D to I). The cells were incubated with MAb 3B3 (A to F) or with MAb 6B5 and polyclonal antiserum for gE (G, H, and I) at 4°C for 30 min. The cells were returned to 37°C for 0 (A, D, and G), 30 (B, E, and H), or 60 (C, F, and I) min. After the incubations, the cells were fixed and permeabilized prior to incubation with goat anti-mouse–FITC conjugate (A to F) or both goat anti-mouse–Texas red conjugate and goat anti-rabbit–FITC conjugate (G, H, and I) for 1 h. The cells were analyzed by confocal microscopy, with gE-Y582G staining green in all panels and the merged images with gI (red) staining yellow in panels G, H, and I.
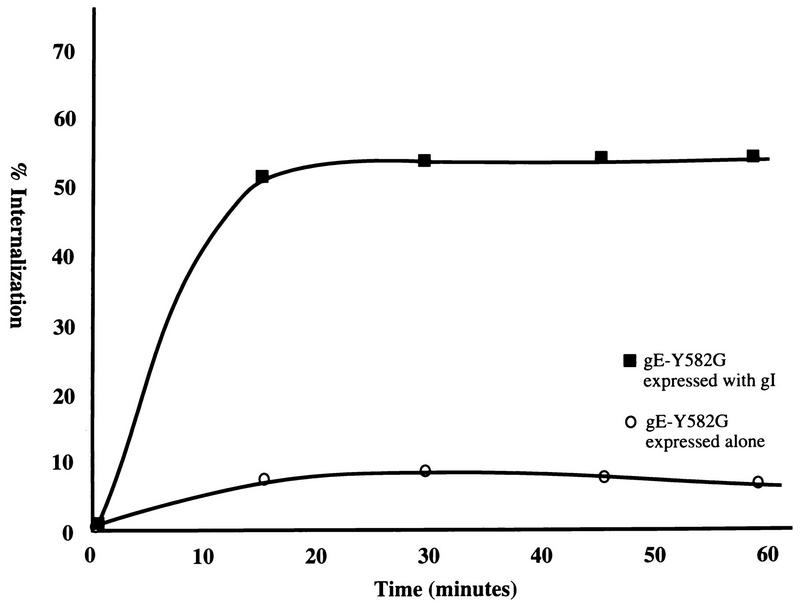
To determine whether gI directly complexed with gE-Y582G enabled endocytosis of the tyrosine mutant gE, a quantitative coprecipitation endocytosis assay was performed. HeLa cells were transfected with gE-Y582G or gE-Y582G and gI and radiolabeled as described above. Cells expressing gE-Y582G alone were incubated with MAb 3B3, and cells expressing gE-Y582G and gI were incubated with MAb 6B5 during the assay. When gE-Y582G was expressed alone, the protein was not efficiently internalized, as shown in Fig. 6. In contrast, when gE-Y582G was coprecipitated with gI during endocytosis, gE-Y582G was internalized very efficiently (Fig. 6). Since internalization of the endocytosis mutant gE:gI complex returned to near that of wild-type gE alone, gI was able to overcome the endocytosis-deficient function of the gE mutant and allow efficient endocytosis of the gE-Y582G:gI protein complex.
FIG. 6.
Analysis of VZV gI with endocytosis mutant gE. HeLa cells were transfected with the gE-Y582G gene or with both the gE-Y582G and gI genes and radiolabeled with [35S]methionine-cysteine. After 16 h, the cells were incubated with MAb 3B3 or MAb 6B5, respectively, at 4°C for 30 min. A quantitative internalization assay was performed. The percentage of protein internalized was calculated for gE-Y582G when expressed alone or with gI. This graph is representative of four experiments.
Endocytosis sequence in the gI cytoplasmic tail.
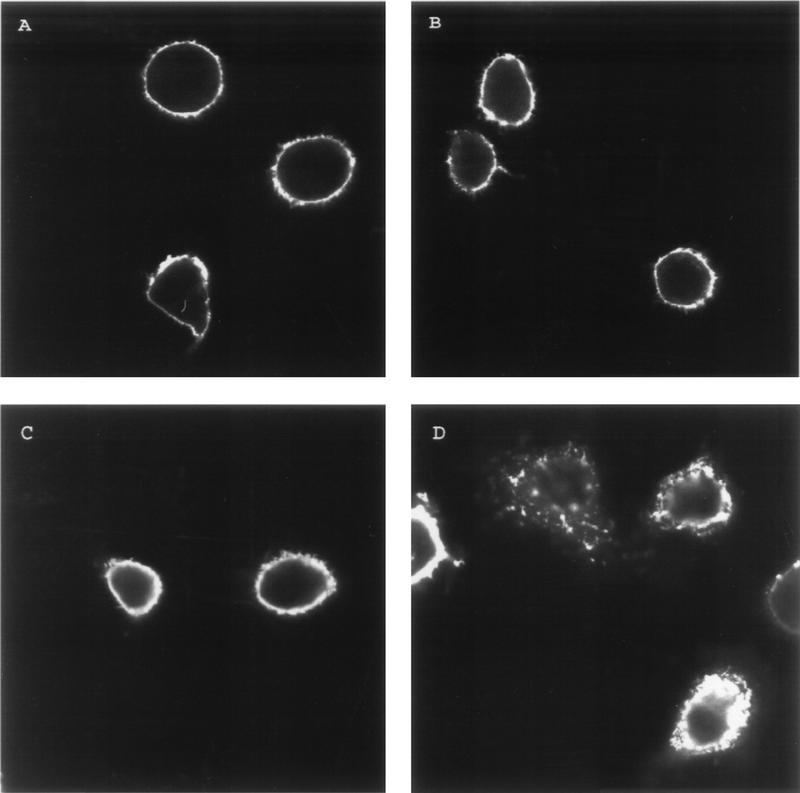
Endocytosis of cell surface receptors is known to be dependent on specific amino acid sequence within the cytoplasmic tails. As described above, the gE cytoplasmic tail contains a YXXL internalization motif similar to those in other cellular receptors such as the FcγRII (12, 27, 32). Upon examination of the gI cytoplasmic tail sequence, no tyrosine-containing endocytosis sequence was identified (4). However, the gI cytoplasmic tail did contain a potential dileucine-type endocytosis motif: ML residues 328 and 329 (2, 4, 35). To determine whether this motif was important for endocytosis of VZV gI, the ML sequence was changed to an AA sequence by site-directed mutagenesis. HeLa cells were transfected with the mutant gI gene, designated gI-AA, or the wild-type gI gene. When the gI-AA gene was expressed in cells and incubated with MAb 6B5 (Fig. 7A), gI-AA was present on the cell surface as shown by surface localization. After 30 min at 37°C (Fig. 7B), gI-AA was still localized to the cell membrane. In contrast, wild-type gI was internalized within the cells after 30 min at 37°C (Fig. 7D). Further timed incubations with mutant and wild-type gI showed similar results. Thus, the ML motif was clearly important for efficient internalization of gI in the transfected cell.
FIG. 7.
Diminished endocytosis of VZV gI with a mutated internalization motif. HeLa cells were transfected with the mutant gI-AA gene (A and B) or the wild-type gI gene (C and D). The cells were incubated with MAb 6B5 at 4°C for 30 min. The cells were returned to 37°C with fresh medium for 0 (A and C) or 30 (B and D) min. After the incubation at 37°C, the cells were fixed and permeabilized prior to incubation with goat anti-mouse–FITC conjugate for 1 h. The cells were analyzed by laser scanning confocal microscopy.
Endocytosis of gE and endocytosis mutant gI as a complex.
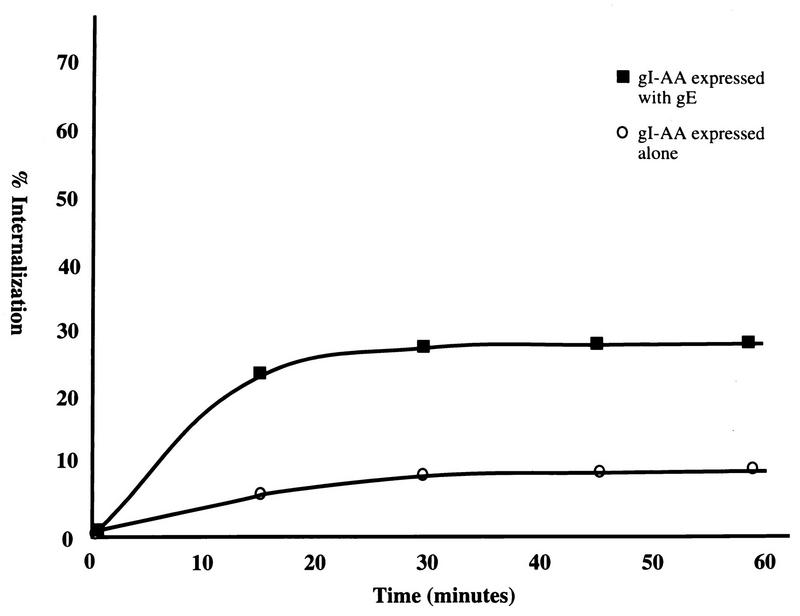
To examine in more detail the effect of the endocytosis mutant gI on the endocytosis of the gE:gI complex, gI-AA was coexpressed with gE in HeLa cells and analyzed by a quantitative coprecipitation endocytosis assay. As shown in Fig. 8, gI-AA expressed alone demonstrated only 7% internalization. A similarly low rate of internalization was determined for a tailless gI mutant (data not shown). Internalization of endocytosis mutant gI-AA when complexed with wild-type gE was then examined through coprecipitation of the proteins. Interestingly, internalization of gI-AA increased to only 28% when it was complexed with wild-type gE (Fig. 8). This rate of internalization was substantially lower than that for wild-type gI complexed with wild-type gE or wild-type gI complexed with endocytosis mutant gE (both between 50 and 60%).
FIG. 8.
Analysis of VZV gE with endocytosis-mutant gI. HeLa cells were transfected with the gI-AA gene or with both the gI-AA gene and the wild-type gE gene and then radiolabeled with [35S]methionine-cysteine. The cells were incubated with MAb 6B5 or MAb 3B3, respectively, at 4°C for 30 min. A quantitative internalization assay was performed. The percentage of protein internalized was calculated for gI-AA when expressed alone or with gI. This graph is representative of four experiments.
DISCUSSION
VZV gE is the predominant cell surface glycoprotein in the infected cell, both in cell culture and in the vesicular lesions seen in humans with chicken pox (13, 45). The fact that viral glycoprotein gE resembles mammalian cell surface receptors, such as the LDL receptor and the FcγRII, has increased interest in defining whether it retains related trafficking patterns. To that end, Alconada et al. (1) reported that VZV gE undergoes internalization from the cell membrane and trafficks to the trans-Golgi network (TGN), where it localizes in the transiently transfected cell. Olson and Grose (32) documented that gE endocytosis from the cell surface occurs in clathrin-coated vesicles; subsequently, gE recycles through the endosomes and trafficks back to the cell membrane. Colocalization studies clearly demonstrate that both gE and TR follow a similar, if not identical, recycling pathway from the plasma membrane (32). Based on the observations described above, Olson and Grose (32) showed by mutagenesis studies that a YAGL sequence in the gE cytoplasmic tail was indeed the internalization motif. They also confirmed within a transfection system an earlier report by Litwin et al. (23) that VZV gE binds the Fc portion of human (or rabbit) IgG with a distinctive punctate pattern on the cell surface; however, much larger amounts of Fc fragments than those provided by the diluted rabbit antiserum used in the current study are required. In short, VZV gE displays a combination of structural features commonly observed in cell surface receptors (18, 33, 37, 38). Since the VZV Fc receptor does not bind the Fc portion of murine IgG (23), attachment of murine MAb 3B3 to VZV gE or MAb 6B5 to VZV gI by their Fab domains should not induce the endocytosis events described in this report.
The present study took note of the repeated observation that gE in VZV-infected cells is usually found together with VZV gI in a gE:gI complex (13, 18). Likewise, in cells cotransfected with gE and gI genes, the two molecules form a complex which is easily detected on the cell surface (18, 22). Relevant studies of the major histocompatibility complex class II and the T-cell receptor focus on cell surface proteins which strictly rely on complex formation for proper cellular localization and function. The T-cell receptor contains several protein chains, all of which are required for the receptor to be assembled and transported to the cell surface. Two different internalization motifs, one dileucine and one YXXL located on one component, are responsible for cellular localization of the T-cell receptor (21). Another complex, major histocompatibility complex class II, requires association of invariant chain with the complex for localization to the cell membrane; the invariant chain contains dileucine-type internalization signals to mediate rapid internalization of the complex (2). Likewise, the ML endocytosis signal in the gI cytoplasmic tail enables the more efficient endocytosis of gE in the gE:gI complex. Further, mutation to the tyrosine-based endocytosis signal in gE which results in diminished endocytosis was overcome by association with gI. However, the reverse situation was not true; the endocytosis mutant gI was not efficiently endocytosed even when associated with wild-type gE. Thus, VZV gI appears to be important to the virus in facilitating the cellular localization of gE when gE and gI associate in virus-infected cells.
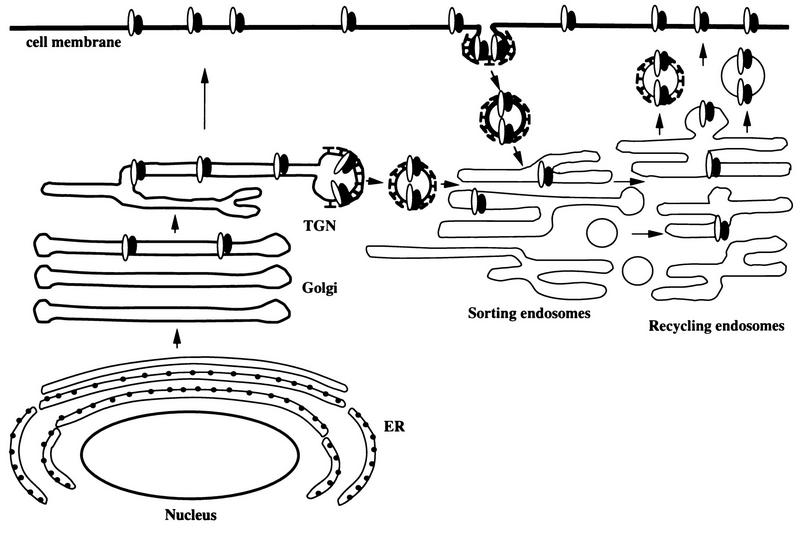
Conclusions from previous studies in conjunction with the results described above are illustrated in a model of VZV gE:gI cellular trafficking (Fig. 9). Cellular trafficking and localization are dependent on sorting signals located in the cytoplasmic tail of the proteins (42, 44). VZV gE and gI are first translocated into the endoplasmic reticulum via a signal sequence which is subsequently cleaved from the protein (47). VZV gE and gI are then transported through the endoplasmic reticulum and into the Golgi apparatus, where the proteins obtain their mature forms (47). At this point, gE (or the gE:gI complex) may be shuttled to the TGN via an AYRV motif localized in the gE cytoplasmic tail near the transmembrane domain (49, 50). Alternatively, the VZV gE:gI complex may traffick to the cell membrane directly, like many other cell membrane proteins. After the VZV gE:gI complex is incorporated into the cell membrane, the complex is internalized through use of the respective internalization signals, YAGL and ML. Serine and tyrosine phosphorylation/dephosphorylation within the cytoplasmic tails of gE and gI may further modulate internalization (31, 33, 46, 47). The clathrin-coated vesicles which contain VZV gE:gI are transported to the early endosomes. The vesicles lose their clathrin coating prior to fusion with the sorting endosome, which is one of the early endosomes (14). The sorting endosome separates proteins into either the recycling pathway or the lysosomal pathway (41). After gE:gI has entered the sorting endosome either directly from the TGN or by endocytosis from the cell membrane, the gE:gI complex is sorted for transport to the recycling endosome. Since (i) VZV gE colocalizes with the TR during endocytosis and recycling and (ii) the TR does not recycle to the TGN, gE presumably does not recycle to the TGN but rather recycles to the cell membrane (39). Finally, concepts in Fig. 9 may be relevant to neuronal VZV infection because endocytosis is a recognized trafficking mechanism for neuronal synaptic vesicles (5).
FIG. 9.
Schema for trafficking of the VZV gE:gI complex. The VZV gE:gI complex is represented by adjacent black and white ovals. The different trafficking patterns are described in detail in the Discussion. Besides the results in this report, this schema is based on data collated from references 1, 13, 17, 28, 32, 49 and 50. ER, endoplasmic reticulum.
ACKNOWLEDGMENTS
We thank the staff, especially Katherine Walters, at the Central Microscopy Facility at the University of Iowa for technical assistance.
This research was supported by U.S. Public Health Service grant AI 22795.
REFERENCES
- 1.Alconada A, Bauer U, Hoflack B. A tyrosine-based motif and a casein kinase II phosphorylation site regulate the intracellular trafficking of the varicella-zoster virus glycoprotein I, a protein localized in the trans-Golgi network. EMBO J. 1996;15:6096–6110. [PMC free article] [PubMed] [Google Scholar]
- 2.Bremnes B, Madsen T, Gedde-Dahl M, Bakke O. An LI and ML motif in the cytoplasmic tail of the MHC-associated invariant chain mediates rapid internalization. J Cell Sci. 1994;107:2021–2032. doi: 10.1242/jcs.107.7.2021. [DOI] [PubMed] [Google Scholar]
- 3.Collawn J F, Stangel M, Kuhn L A, Esekogwu V, Jing S, Trowbridge I S, Trainer J A. Transferrin receptor internalization sequence YXRF implicates a tight turn as the structural recognition motif for endocytosis. Cell. 1990;63:1061–1072. doi: 10.1016/0092-8674(90)90509-d. [DOI] [PubMed] [Google Scholar]
- 4.Davison A J, Scott J E. The complete DNA sequence of varicella-zoster virus. J Gen Virol. 1986;67:1759–1816. doi: 10.1099/0022-1317-67-9-1759. [DOI] [PubMed] [Google Scholar]
- 5.De Camilli P. The eighth Datta lecture. Molecular mechanisms in synaptic vesicle recycling. FEBS Lett. 1995;369:3–12. doi: 10.1016/0014-5793(95)00739-v. [DOI] [PubMed] [Google Scholar]
- 6.Dingwell K S, Doering L C, Johnson D C. Glycoproteins E and I facilitate neuron-to-neuron spread of herpes simplex virus. J Virol. 1995;69:7087–7098. doi: 10.1128/jvi.69.11.7087-7098.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dingwell K S, Brunetti C R, Hendricks R L, Tang Q, Tang M, Rainbow A J, Johnson D C. Herpes simplex virus glycoproteins E and I facilitate cell-to-cell spread in vivo and across junctions of cultured cells. J Virol. 1994;68:834–845. doi: 10.1128/jvi.68.2.834-845.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edson C M, Hosler B A, Poodry C A, Scooley R T, Waters D J, Thorley-Lawson D A. Varicella-zoster virus envelope glycoproteins: biochemical characterization and identification in clinical material. Virology. 1985;145:62–71. doi: 10.1016/0042-6822(85)90201-6. [DOI] [PubMed] [Google Scholar]
- 9.Egan M A, Carruth L M, Rowell J F, Yu X, Siliciano R F. Human immunodeficiency virus type 1 envelope protein endocytosis mediated by a highly conserved intrinsic internalization signal in the cytoplasmic domain of gp41 is suppressed in the presence of the Pr55gag precursor protein. J Virol. 1996;70:6547–6556. doi: 10.1128/jvi.70.10.6547-6556.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Enquist L W. Infection of the mammalian nervous system by pseudorabies virus (PRV) Semin Virol. 1994;5:221–231. [Google Scholar]
- 11.Frank I, Friedman H M. A novel function of the herpes simplex virus type I Fc receptor: participation in bipolar bridging of antiviral immunoglobulin G. J Virol. 1989;63:4479–4488. doi: 10.1128/jvi.63.11.4479-4488.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghazizadeh S, Fleit H B. Tyrosine phosphorylation provides an obligatory early signal for FcγRII-mediated endocytosis in the monocytic cell line THP-1. J Immunol. 1994;152:30–41. [PubMed] [Google Scholar]
- 13.Grose C. Glycoproteins encoded by varicella-zoster virus: biosynthesis, phosphorylation, and intracellular trafficking. Annu Rev Microbiol. 1990;44:59–80. doi: 10.1146/annurev.mi.44.100190.000423. [DOI] [PubMed] [Google Scholar]
- 14.Gruenberg J, Howell K E. Membrane traffic in endocytosis: insights from cell-free assays. Annu Rev Cell Biol. 1989;5:453–481. doi: 10.1146/annurev.cb.05.110189.002321. [DOI] [PubMed] [Google Scholar]
- 15.Harson R, Grose C. Egress of varicella-zoster virus from the melanoma cell: a tropism for the melanocyte. J Virol. 1995;69:4994–5010. doi: 10.1128/jvi.69.8.4994-5010.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hatfield C, Duus K M, Jones D H, Grose C. Epitope mapping and tagging by recombination PCR mutagenesis. BioTechniques. 1997;22:332–337. doi: 10.2144/97222rr02. [DOI] [PubMed] [Google Scholar]
- 17.Jones F, Grose C. Role of cytoplasmic vacuoles in varicella-zoster virus glycoprotein trafficking and virion envelopment. J Virol. 1988;62:2701–2711. doi: 10.1128/jvi.62.8.2701-2711.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kimura H, Straus S E, Williams R K. Varicella-zoster virus glycoproteins E and I expressed in insect cells form a heterodimer that requires the N-terminal domain of glycoprotein I. Virology. 1997;233:382–391. doi: 10.1006/viro.1997.8625. [DOI] [PubMed] [Google Scholar]
- 19.Kishimoto A, Brown M S, Slaughter C A, Goldstein J L. Phosphorylation of serine 833 in cytoplasmic domain of low density lipoprotein receptor by a high molecular weight enzyme resembling casein kinase II. J Biol Chem. 1987;262:1344–1351. [PubMed] [Google Scholar]
- 20.Knapp A C, Husak P J, Enquist L W. The gE and gI homologs from two alphaherpesviruses have conserved and divergent neuroinvasive properties. J Virol. 1997;71:5820–5827. doi: 10.1128/jvi.71.8.5820-5827.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Letourneur F, Klausner R D. A novel di-leucine motif and a tyrosine-based motif independently mediate lysosomal targeting and endocytosis of CD3 chains. Cell. 1992;69:1143–1157. doi: 10.1016/0092-8674(92)90636-q. [DOI] [PubMed] [Google Scholar]
- 22.Litwin V, Jackson W, Grose C. Receptor properties of two varicella-zoster virus glycoproteins, gpI and gpIV, homologous to herpes simplex virus gE and gI. J Virol. 1992;66:3643–3651. doi: 10.1128/jvi.66.6.3643-3651.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Litwin V, Sandor M, Grose C. Cell surface expression of varicella-zoster virus glycoproteins and Fc receptor. Virology. 1990;178:263–272. doi: 10.1016/0042-6822(90)90402-d. [DOI] [PubMed] [Google Scholar]
- 24.Mallory S, Sommer M, Arvin A M. Mutational analysis of the role of glycoprotein I in varicella-zoster virus replication and its effects on glycoprotein E conformation and trafficking. J Virology. 1997;71:8279–8288. doi: 10.1128/jvi.71.11.8279-8288.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McGeoch D J, Cook S, Dolan A, Jamieson F E, Telford E A R. Molecular phylogeny and evolutionary timescale for the family of mammalian herpesviruses. J Mol Biol. 1995;247:443–458. doi: 10.1006/jmbi.1995.0152. [DOI] [PubMed] [Google Scholar]
- 26.McGraw T E, Maxfield F R. Internalization and sorting of macromolecules: endocytosis. In: Juliano R L, editor. Targeted drug delivery. Berlin, Germany: Springer-Verlag; 1991. pp. 11–41. [Google Scholar]
- 27.Miettinen H M, Rose J K, Mellman I. Fc receptor isoforms exhibit distinct abilities for coated pit localization as a result of cytoplasmic domain heterogeneity. Cell. 1989;58:317–327. doi: 10.1016/0092-8674(89)90846-5. [DOI] [PubMed] [Google Scholar]
- 28.Montalvo E A, Parmley R T, Grose C. Varicella-zoster viral glycoprotein envelopment: ultrastructural cytochemical localization. J Histochem Cytochem. 1986;34:281–284. doi: 10.1177/34.2.3003184. [DOI] [PubMed] [Google Scholar]
- 29.Montalvo E A, Parmley R T, Grose C. Structural analysis of the varicella-zoster virus gp98-gp62 complex: posttranslational addition of N-linked and O-linked oligosaccharide moieties. J Virol. 1985;53:761–770. doi: 10.1128/jvi.53.3.761-770.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moss B, Elroy-Stein O, Mijukani T, Alexander W A, Fuerst T R. New mammalian expression vectors. Nature (London) 1990;348:91–92. doi: 10.1038/348091a0. [DOI] [PubMed] [Google Scholar]
- 31.Ohno H, Stewart J, Fournier M-C, Bosshart H, Rhee I, Miyatake S, Saito T, Gallusser A, Kirchhausen T, Bonifacino J S. Interaction of tyrosine based sorting signals with clathrin-associated proteins. Science. 1995;269:1872–1875. doi: 10.1126/science.7569928. [DOI] [PubMed] [Google Scholar]
- 32.Olson J K, Grose C. Endocytosis and recycling of varicella-zoster virus Fc receptor glycoprotein gE: internalization mediated by a YXXL motif in the cytoplasmic tail. J Virol. 1997;71:4042–4054. doi: 10.1128/jvi.71.5.4042-4054.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Olson J K, Bishop G A, Grose C. Varicella-zoster virus Fc receptor gE glycoprotein: serine/threonine and tyrosine phosphorylation of monomeric and dimeric forms. J Virol. 1997;71:110–119. doi: 10.1128/jvi.71.1.110-119.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rowell J F, Stanhope P E, Siliciano R F. Endocytosis of endogenously synthesized HIV-1 envelope protein. J Immunol. 1995;155:473–488. [PubMed] [Google Scholar]
- 35.Sandoval I V, Bakke O. Targeting of membrane proteins to endosomes and lysosomes. Trends Cell Biol. 1994;4:292–297. doi: 10.1016/0962-8924(94)90220-8. [DOI] [PubMed] [Google Scholar]
- 36.Sauter M M, Pelchen-Mathews A, Bron R, Marsh M, LaBranche C C, Vance P J, Romano T, Haggarty B S, Hart T K, Lee W M F, Hoxie J A. An internalization signal in the simian immunodeficiency virus transmembrane protein cytoplasmic domain modulates expression of envelope glycoprotein on the cell surface. J Cell Biol. 1996;132:795–811. doi: 10.1083/jcb.132.5.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scholl P R, Ahern D, Geha R S. Protein tyrosine phosphorylation induced via the IgG receptors FcγRI and FcγRII in the human monocytic cell line THP-1. J Immunol. 1992;149:1751–1757. [PubMed] [Google Scholar]
- 38.Smith C A, Davis T, Anderson D, Solam L, Bechman M P, Jerzey R, Dower S K, Cosman D, Goodwin R G. A receptor for tumor necrosis factor defines an unusual family of cellular and viral proteins. Science. 1990;248:1019–1023. doi: 10.1126/science.2160731. [DOI] [PubMed] [Google Scholar]
- 39.Stoorvogel W, Oorschot V, Geuze H J. A novel class of clathrin-coated vesicles budding from the endosomes. J Cell Biol. 1996;132:21–33. doi: 10.1083/jcb.132.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stoorvogel W, Geuze H J, Stous G J. Sorting of endocytosed transferrin and asialoglycoprotein occurs immediately after internalization in HepG2 cells. J Cell Biol. 1987;104:1261–1268. doi: 10.1083/jcb.104.5.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Trowbridge I S, Collawn J F, Hopkins C R. Signal-dependent trafficking in the endocytic pathway. Annu Rev Cell Biol. 1993;9:129–161. doi: 10.1146/annurev.cb.09.110193.001021. [DOI] [PubMed] [Google Scholar]
- 42.Trowbridge I S. Endocytosis and signals for internalization. Curr Opin Cell Biol. 1991;3:634–641. doi: 10.1016/0955-0674(91)90034-v. [DOI] [PubMed] [Google Scholar]
- 43.Vafai A, Wroblewska Z, Mahalingam R, Cabirac G, Wellish M, Cisco M, Gilden D. Recognition of similar epitopes on varicella-zoster virus gpI and gpIV by monoclonal antibodies. J Virol. 1988;62:2544–2551. doi: 10.1128/jvi.62.8.2544-2551.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Watts C, Marsh M. Endocytosis: what goes in and how? J Cell Biol. 1992;103:1–8. doi: 10.1242/jcs.103.1.1a. [DOI] [PubMed] [Google Scholar]
- 45.Weigle K A, Grose C. Common expression of varicella-zoster viral glycoprotein antigens in vitro and in chickenpox and zoster vesicles. J Infect Dis. 1983;148:630–638. doi: 10.1093/infdis/148.4.630. [DOI] [PubMed] [Google Scholar]
- 46.Yao Z, Grose C. Unusual phosphorylation sequence in the gpIV (gI) component of the varicella-zoster virus gpI-gpIV glycoprotein complex (VZV gE-gI) J Virol. 1994;68:4202–4211. doi: 10.1128/jvi.68.7.4204-4211.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yao Z, Jackson W, Grose C. Identification of the phosphorylation sequence in the cytoplasmic tail of the varicella-zoster virus Fc receptor glycoprotein gpI. J Virol. 1993;67:4464–4473. doi: 10.1128/jvi.67.8.4464-4473.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yao Z, Jones D H, Grose C. Site-directed mutagenesis of herpesvirus glycoprotein phosphorylation sites by recombination polymerase chain reaction. PCR Methods Appl. 1992;1:205–207. doi: 10.1101/gr.1.3.205. [DOI] [PubMed] [Google Scholar]
- 49.Zhu Z, Gershon M D, Hao Y, Ambron R T, Gabel C A, Gershon A A. Envelopment of varicella-zoster virus: targeting of viral glycoproteins to the trans-Golgi network. J Virol. 1995;69:7951–7959. doi: 10.1128/jvi.69.12.7951-7959.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhu Z, Hao Y, Gershon M D, Ambron R T, Gershon A A. Targeting of glycoprotein I (gE) of varicella-zoster virus to the trans-Golgi network by an AYRV sequence and an acidic amino acid-rich patch in the cytosolic domain of the molecule. J Virol. 1996;70:6563–6575. doi: 10.1128/jvi.70.10.6563-6575.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]