Abstract
Vaccinia virus has a wide host range and infects mammalian cells of many different species. This suggests that the cell surface receptors for vaccinia virus are ubiquitously expressed and highly conserved. Alternatively, different receptors are used for vaccinia virus infection of different cell types. Here we report that vaccinia virus binds to heparan sulfate, a glycosaminoglycan (GAG) side chain of cell surface proteoglycans, during virus infection. Soluble heparin specifically inhibits vaccinia virus binding to cells, whereas other GAGs such as condroitin sulfate or dermantan sulfate have no effect. Heparin also blocks infections by cowpox virus, rabbitpox virus, myxoma virus, and Shope fibroma virus, suggesting that cell surface heparan sulfate could be a general mediator of the entry of poxviruses. The biochemical nature of the heparin-blocking effect was investigated. Heparin analogs that have acetyl groups instead of sulfate groups also abolish the inhibitory effect, suggesting that the negative charges on GAGs are important for virus infection. Furthermore, BSC40 cells treated with sodium chlorate to produce undersulfated GAGs are more refractory to vaccinia virus infection. Taken together, the data support the notion that cell surface heparan sulfate is important for vaccinia virus infection. Using heparin-Sepharose beads, we showed that vaccinia virus virions bind to heparin in vitro. In addition, we demonstrated that the recombinant A27L gene product binds to the heparin beads in vitro. This recombinant protein was further shown to bind to cells, and such interaction could be specifically inhibited by soluble heparin. All the data together indicated that A27L protein could be an attachment protein that mediates vaccinia virus binding to cell surface heparan sulfate during viral infection.
Cell entry is the first step in the invasion of host cells by pathogens. Viruses have evolved strategies to recognize and discriminate their target host cells by binding to cell surface proteins, which may vary among tissue types. Identifying the receptors is essential for us to understand the biology of the virus. Receptor-mediated viral binding is shared by many viruses; however, the nature of the binding among different viruses appears diverse (16, 50). Some viruses require a single component for entry. For example, influenza virus binds to the terminal carbohydrate side chain sialic acids on cells, leading to endocytosis of virions (50). Poliovirus binds to an immunoglobulin-like protein, PVR, on the cell surface, resulting in rapid uncoating of virus particles inside cells (11, 22, 29, 35–37). Other viruses such as human immunodeficiency virus and herpesvirus require coreceptors to complete the cell entry process. HIV binds to CD4 on T cells, and subsequent interaction with a member of the chemokine receptor family, CCR-5 or CXCR-4, leads to membrane fusion (7, 10, 27, 40). Herpes simplex virus binds to cell surface glycosaminoglycan (GAGs) side chains (mainly heparan sulfate) of proteoglycans, and penetration requires a tumor necrosis factor receptor-like molecule, HVEM, to facilitate membrane fusion (2, 15, 31, 43, 45–47, 51).
Vaccinia virus belongs to the poxvirus family and infects many cells of different origins. Vaccinia virus produces two forms of infectious particles: intracellular mature virus (IMV) and extracellular enveloped virus (EEV). The membrane of IMV was thought to be derived from the intermediate compartment, whereas the EEV form of virions was derived from the trans-Golgi network (9, 44). Different structures of these particles suggested that they may infect cells by different routes; however, until now, no receptor for poxvirus has been demonstrated. We have previously isolated monoclonal antibody (MAb) B2, which recognizes a putative receptor on cells and blocks IMV virions of vaccinia virus binding to cells (4). B2 does not block EEV infection, indicating that different cellular receptors exist for IMV and EEV entry (48). B2 blocked vaccinia virus infection in all the susceptible cells we tested, but its efficiency varies among different cell lines and such partial inhibition suggested to us that other mediators for vaccinia virus may also exist.
GAGs are complex structures present on a variety of cells, with different carbohydrate moieties linked to core proteins through serine residues (17). Most cell types express GAGs such as condroitin sulfate, dermantan sulfate, or heparan sulfate to different extents. The chemical structures of GAGs have been well characterized with repeating disaccharides of a hexuronic acid and an N-acetylhexosamine sulfate modified with carboxylate and sulfate monoester groups (26). Due to these modifications on sugar side chains, cell surface GAGs are highly negatively charged. The biological roles of GAGs are quite diverse, ranging from cell attachment and migration, compressive resilience of cartilage, and control of fibrinogenesis to cell signaling (41). GAGs are also shown to be mediators of virus infections. Herpesviruses and the type O foot-and-mouth disease virus bind to cell surface heparan sulfate during infection (20, 43, 45, 46).
In this study, we explored the role of GAGs, particularly cell surface heparan sulfate and its related homolog heparin, in vaccinia virus infection. Our results suggested that vaccinia virus binds to heparan sulfate on cells. Not only vaccinia virus but also other poxviruses such as cowpox virus, rabbitpox virus, Shope fibroma virus and myxoma virus also bind to heparan sulfate as well. Furthermore, sulfate groups on GAGs contributed to the negative charges which are required for vaccinia virus infection.
We also investigated candidate virion membrane proteins on IMV that could mediate virus interaction with heparan sulfate. Our data suggested that A27L protein binds to cell surface heparan sulfate.
MATERIALS AND METHODS
Reagents and viruses.
Soluble heparin, chondroitin sulfate, and dermatan sulfate were purchased from Sigma Inc. A chemically modified heparin kit, containing completely desulfated N-acetylated heparin (CDSNAc-heparin, Na salt), completely desulfated N-sulfated heparin (CDSNS-heparin, Na salt), and N-desulfated N-acetylated heparin (NDSNAc-heparin, Na salt), was purchased from Seikagaku Corp. Heparin-Sepharose CL-6B beads and control CL-6B beads were purchased from Pharmacia. Wild-type vaccinia viruses (WR) were prepared as IMV stocks in BSC40 cells. A recombinant virus, vMJ360, expressing a lacZ gene from an early promoter was obtained from B. Moss (6).
Virus purification.
Vaccinia virus was purified as described previously (21). Briefly, BSC40 cells were infected with vaccinia virus at a multiplicity of infection (MOI) of 0.05 and the infection was allowed to proceed until a complete cytopathic effect was observed. The cells were scraped off the plates with a rubber policeman and centrifuged at 1,500 × g for 10 min. The cell pellets were resuspended in cold buffer containing 10 mM Tris (pH 7.5) and 5mM MgCl2 and homogenized with 20 strokes in a Dounce homogenizer. The large debris and nuclei were sedimented by centrifugation at 2,000 rpm for 10 min. The supernatant was sonicated and loaded on top of 36% sucrose, and the viruses were pelleted by centrifugation at 18,000 rpm for 80 min. The pelleted viruses were resuspended, sonicated and laid on top of a 25 to 40% sucrose gradient for centrifugation at 13,500 rpm for 40 min in a Beckman SW28 rotor. The band composed mainly of IMV was harvested and saved at −70°C as stocks. The stocks contained little contamination by extracellular enveloped viruses since plaque formation of these purified virions was readily neutralized (greater than 95%) by antibodies recognizing IMV-specific proteins.
Inhibition of soluble GAGs on vaccinia virus infection.
Heparin and other soluble GAGs were incubated with vaccinia virus at 4°C for 30 min, and the mixture was added to BSC40 cells at 37°C for 30 min. The inoculum was removed, the cells were washed and overlaid with agar, and plaque numbers were determined 3 days later. The numbers of plaques obtained in the absence of GAGs were used as 100%. For lacZ gene expression, BSC40 cells were infected at a MOI of 5 PFU per cell with vMJ360 (6). At 3 h postinfection (p.i.), the cells were fixed in 0.5% paraformaldehyde and analyzed for β-galactosidase (β-gal) activity by 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) staining.
To investigate the inhibition of GAGs on virus binding, BSC40 cells were infected with vaccinia virus at a MOI of 10 PFU per cell with or without GAGs (50 μg/ml) at 4°C for 30 min. The cells were washed thoroughly with cold phosphate-buffered saline (PBS), lysed with sodium dodecyl sulfate (SDS)-containing sample buffer, and loaded on an SDS-polyacrylamide gel electrophoresis (PAGE) gel (10% polyacrylamide) for Western blot analysis with a serum against vaccinia virus virions as described previously (4).
Inhibition of sulfation of GAGs by sodium chlorate.
All chlorate experiments were done as described previously with minor modifications (47). In brief, BSC40 cells were seeded in sulfate-free Joklik modified minimal essential medium (Gibco-Bethesda Research Laboratories) supplemented with 1.8 mM CaCl2, 10 mM sodium chlorate, and 8% fetal bovine serum previously dialyzed against PBS. In control experiments 0.8 mM sodium sulfate was also added to the above chlorate-containing medium (sulfate-reconstituted medium) to reverse the in vivo sulfation block in cells. After 2 days, the cells were infected with a recombinant vMJ360 at a MOI of 5 PFU per cell and fixed at 3 h p.i. for β-gal activity determination with X-Gal. Alternatively, chlorate-treated cells were infected with vaccinia virus at 4°C for 30 min, washed in PBS, and lysed in SDS-containing sample buffer for Western blot analysis.
Heparin binding assays.
Heparin-Sepharose CL-6B beads or control Sepharose beads were swollen in PBS–0.5% Nonidet P-40–0.5% sodium deoxycholate at 4°C overnight and washed in PBS as described previously (19). The washed beads were blocked for 1 h at 4°C with PBS containing 1% bovine serum albumin, and vaccinia virions (4,000 PFU) were added. The mixture was incubated at 4°C for 1 h, and the unbound virions were collected from the supernatant after a brief centrifugation. The virus titers in the supernatant were determined by plaque assays in BSC40 cells.
For determination of protein binding to heparin beads, 10 μg of purified recombinant A27L or A4L proteins was incubated in PBS–Nonidet P-40–sodium deoxycholate buffer with 200 μl of heparin beads (50% vol/vol) at 4°C for 1 h and the supernatant was collected after a brief centrifugation as described previously (19). The pellets were then washed with the same buffer three times. Both the supernatant and the pellet were resuspended in protein sample buffer containing 5% 2-mercaptoethanol, and the same proportion of each (1/15) was loaded onto a SDS-PAGE gel (15% polyacrylamide) and transferred for Western blot analysis with a MAb against T7 tag sequences (1:5,000) (Novagen), as suggested by the manufacturer.
Protein expression and purification.
For expression of the soluble recombinant A27L protein truncated before the transmembrane region, two primers were made for PCR amplification. The 5′ primer (5′-AAAGGATCCTCTACAAAGGCTGCTAAA) and the 3′ primer (5′-AAAAAGCTTATTTTCCAACCTAAATAG) were used with viral DNA template in a PCR amplification with a program of 94°C for 1 min, 42°C for 1.5 min, and 72°C for 1.5 min for 25 cycles. The amplified DNA fragment contained sequences corresponding to A27L amino acids 21 to 84. The DNA fragment was digested with BamHI and HindIII and cloned into pET21a (Novagen). The resulting plasmid expressed the A27L ectodomain with a T7 tag peptide at the N terminus for easy identification and hexahistidine sequences at the C terminus for purification with a nickel column.
For A4L, the 5′ primer (5′-TATGAATTCATGGACTTCTTTAACAAG) and the 3′ primer (5′-TATAAGCTTCTTTTGGAATCGTTCAAA) were used with vaccinia virus DNA template, and the amplified DNA fragment contained sequences encoding the entire amino acids (1 to 841) of the A4L open reading frame. The DNA fragment was digested with EcoRI and HindIII and cloned into pET21a (Novagen).
For protein expression, the bacterial cultures were transformed with individual plasmids as described above and the cultures were induced with 0.2 mM isopropyl-β-d-thiogalactopyranoside (IPTG) for 1 h at 37°C and harvested. The bacterial pellets were sonicated, and the supernatant recovered after centrifugation was loaded onto a nickel column as suggested in the pET system manual (Novagen). The column was washed, and the bound protein was eluted with 0.3M imidazole and dialyzed against PBS at 4°C overnight before use.
Biotinylation of A27L protein and cell binding assays.
Biotinylation of A27L protein was performed with an ECL biotinylation system purchased from Amersham Life Science, Inc. In brief, 2 mg of purified A27L protein was mixed with 40 μl of the biotinylation reagent N-hydroxysuccinamide ester in 40 mM bicarbonate buffer (pH 8.6) at room temperature for 1 h as suggested by the manufacturer. The biotinylated mixture was then loaded on a 9-ml Sephadex G-25 column previously equilibrated with PBS. Biotinylated A27L protein was collected in fractions 4 to 6, and the extent of biotinylation was confirmed by spectrophotometry and by Western blot analysis with horseradish peroxidase-conjugated streptavidin.
For cell binding assays, BSC40 cells (2 × 104 per well) were incubated with biotinylated A27L protein (0.2 μg/μl) alone or with GAGs (50 μg/ml) in a volume of 50 μl in a 96-well plate at 4°C for 30 min. The cells were subsequently washed with cold PBS, and phycoerythrin-conjugated streptavidin (1:100) was added for another 30 min at 4°C. The cells were washed three more times with cold PBS, and images were photographed under a Nikon fluorescence microscope with a Nikon G-2A filter (wavelength, 510 to 560 nm) connected to a Kodak photoMicroGraphy Digitize-Integrate system (MGDS system). All images were analyzed by the FreePlus Editing program or Adobe Photoshop.
RESULTS
Vaccinia virus binds to cell surface heparan sulfate during viral infection.
The three most abundant GAGs on plasma membrane proteoglycans of mammalian cells are heparan sulfate, chondroitin sulfate, and dermantan sulfate (24). Heparin, on the other hand, is found only in secretary granules of mast cells (26). Technically, soluble heparin is often used as an effective competitor for heparan sulfate on cells. To investigate the effects of cell surface GAGs on vaccinia virus binding, we incubated various soluble GAGs with viruses during infection and determined the inhibitory effects of each GAG in vaccinia virus plaque formation (Fig. 1A). At 1 μg/ml, heparin blocks 35% of the vaccinia virus plaque formation. Heparin blocked vaccinia virus better at higher concentrations (2 to 5 μg/ml) and reached a maximum of 60% inhibition at 5 to 10 μg/ml. Other GAGs such as condroitin sulfate and dermatan sulfate did not show any significant inhibition.
FIG. 1.
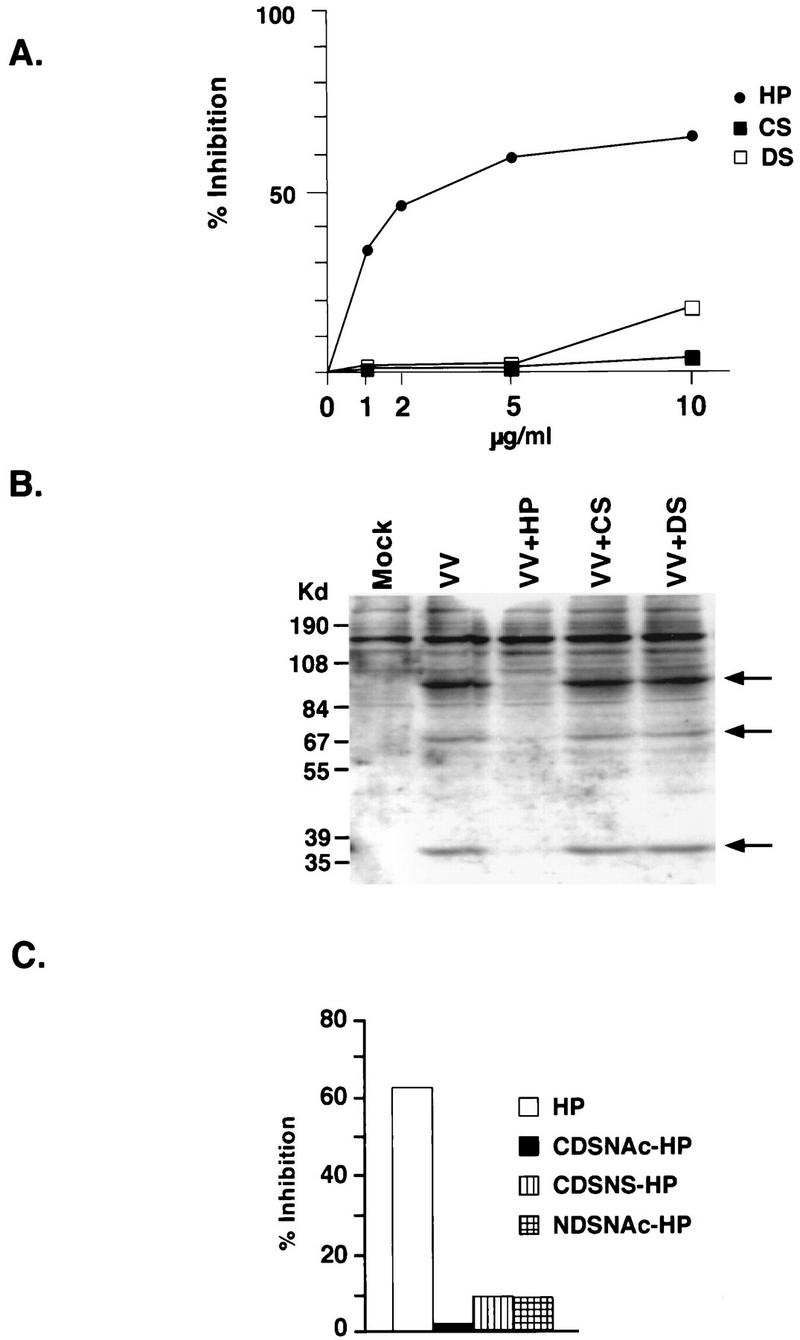
(A) Soluble heparin blocks vaccinia virus infection of cells. BSC40 cells were infected with vaccinia virus in the presence of different GAGs (heparin [HP], chondroitin sulfate [CS], and dermatan sulfate [DS]) at various concentrations (0, 1, 2, 5, and 10 μg/ml) for 60 min at 37°C. The cells were then washed with PBS and overlaid with agar for plaque assays. The numbers of plaques obtained from infection without GAGs, within the range of 100 to 150 plaques, were used as 100%. The data were obtained from an average of four plates. (B). Heparin blocks vaccinia virus binding to cells. BSC40 cells were infected with vaccinia virus at a MOI of 10 PFU per cell in the presence of different GAGs (50 μg/ml), as shown above the lanes, at 4°C for 30 min, washed with PBS, and lysed in gel sample buffer containing 5% 2-mercaptoethanol. The samples were separated by SDS-PAGE (10% polyacrylamide) and transferred for Western blot analysis with an antiserum (1:100) previously raised against purified virions. The arrow indicates the position of a viral 35-kDa protein encoded by the D8L gene. (C). Heparin analogs fail to block vaccinia virus infection. BSC40 cells were infected with vaccinia virus in the presence of heparin or its analogs CDSNAc-heparin, CDSNS-heparin, or NDSNAc-heparin as described in Materials and Methods. The cells were washed with PBS and overlaid with agar, and plaque numbers were determined after 3 days. The number of plaques obtained from infection without GAGs was used as 100%.
We then determined the mechanism of heparin inhibition of vaccinia virus infection. BSC40 cells were infected with vaccinia virus at 4°C for 30 min, and cell-associated virions were determined by Western blot analysis (Fig. 1B). With a serum against purified virions, several viral proteins were specifically detected in virus-infected cells. The intensity of these bands was greatly reduced when heparin was incubated with cells during infection. Again, condroitin sulfate and dermatan sulfate had little effect on virus binding to cells. The data indicated that heparin specifically blocked vaccinia virus binding to mammalian cells during infection.
Negative charges contributed by sulfation of heparan sulfate on cells are required for vaccinia virus infection.
Heparan sulfate and heparin contain a number of N-sulfate groups in place of N-acetyl groups and contain l-iduronic acid in place of some glucuronic acid residues with some of the former sulfated in the 2 position. Generally heparin has a higher proportion of N-sulfate and l-iduronic acid than does heparan sulfate (26). To address the chemical structure responsible for heparin competition, we obtained different heparin analogs that contain reduced sulfate contents by chemical modifications. NDSNAc-heparin and CDSNS-heparin contains less sulfate (4.5 to 8% sulfate), and CDSNAc-heparin is completely desulfated (less than 1.5% sulfate). Removing any sulfate from heparin dramatically reduces its inhibitory effect on vaccinia virus infection (Fig. 1C). This therefore suggested that the charge density contributed by sulfate is important for virus interaction with cell surface heparan sulfate. This result is similar to what has been reported for herpes simplex virus and pseudorabies virus (18).
We then sought to deplete sulfate from cell surface GAGs with sodium chlorate to determine if sulfate moieties are indeed required for vaccinia virus infection. BSC40 cells plated in sulfate-free medium continued to grow with a normal morphology indistinguishable from the controlled cells in sulfate-reconstituted medium (Fig. 2A and D). This is expected since, without exogenous sulfate source, metabolism of methionine and cysteine could still be used as the endogenous source of sulfation in vivo (32). In addition, these cells grown in sulfate-free medium could be infected by vaccinia virus to similar extents to the controlled cells when lacZ expression from a viral early promoter was monitored (Fig. 2B and E). The addition of 10 mM sodium chlorate sulfate-free medium blocked the activities of cellular ATP-sulfurylase and sulfate adenyltransferase and reduced sulfate incorporation into GAGs by up to 96% (1, 14, 23). At the same time, chlorate treatment significantly inhibited virus infection (Fig. 2C). It therefore provided direct evidence that sulfation of cell surface GAGs is required for vaccinia virus infection. We could still detect 5 to 10% blue cells, suggesting either that chlorate blockage is leaky or that vaccinia virus could use alternative means of cell entry in the absence of sulfation. Nevertheless, desulfation of GAGs by chlorate treatment had a profound effect on vaccinia virus infection. Such chlorate inhibition could be reversed by the addition of exogenous sodium sulfate to chlorate-containing medium (47). Consistently, reconstitution of the normal sulfation state in vivo also rendered cells susceptible to virus infection again (Fig. 2F). These data, taken together, demonstrated that the sulfate moieties on heparan sulfate are involved in vaccinia virus entry.
FIG. 2.
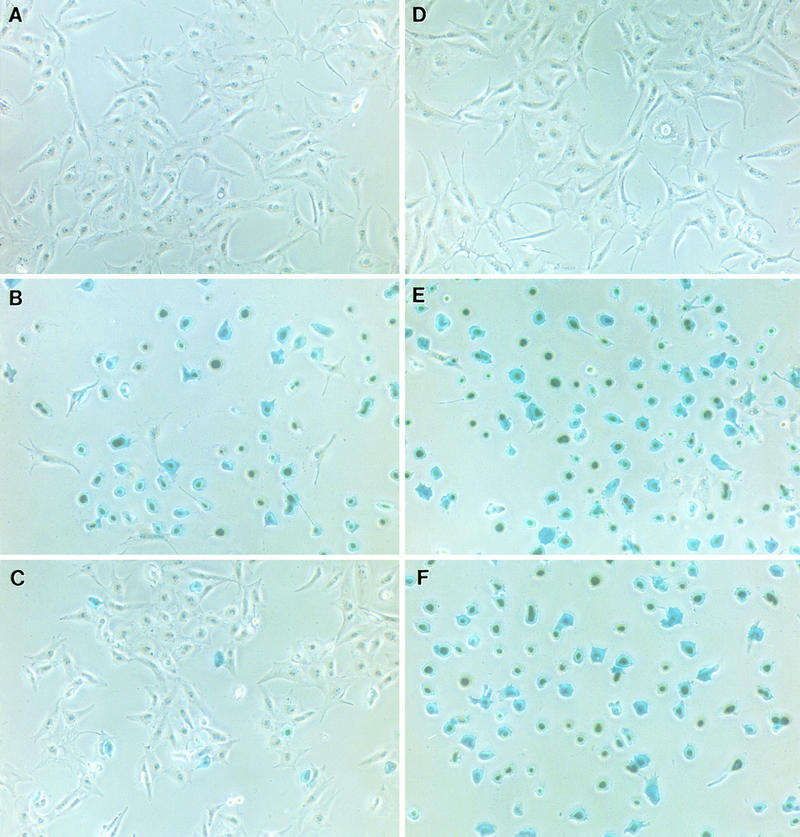
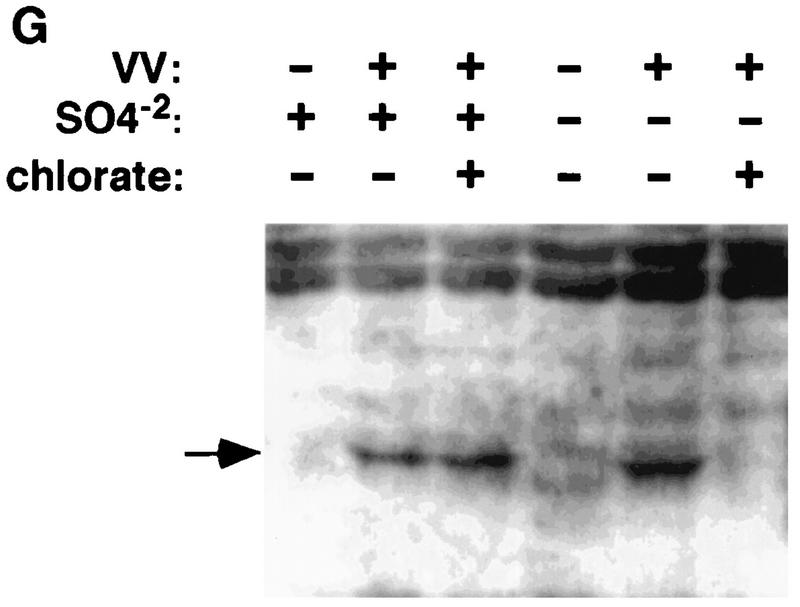
Undersulfation of GAGs by chlorate treatment blocks vaccinia virus binding. BSC40 cells were seeded in sulfate-free medium (A to C) or sulfate-reconstituted medium (D to F). In addition, 10 mM sodium chlorate was added to BSC cells in panels C and F to block sulfation in vivo. After 2 days, BSC cells were infected with vMJ360 at a MOI of 5 PFU per cell and fixed at 3 h p.i. for β-gal activity with X-Gal. (G) BSC40 cells were prepared as described above, infected with vaccinia virus at 4°C for 30 min, washed, and lysed immediately for Western blot analysis with an antiserum against a viral D8L protein, as marked by an arrow.
To demonstrate the mechanism of chlorate treatment on virus entry, we pretreated cells with chlorate and infected them with vaccinia virus at 4°C for 30 min. These cells were washed and lysed for Western blot analysis with an antiserum against a viral D8L protein. As shown in Fig. 2G, the amount of cell-associated viruses was greatly reduced when chlorate was added to sulfate-free medium. When exogenous sulfate was added, virus binding to cells was increased. The data correlated with β-gal staining in Fig. 2A to F and therefore provided direct evidence that undersulfation of cell surface GAGs by chlorate treatment resulted in reduction of vaccinia virus binding to cells.
Other poxviruses also bind to cell surface GAGs during infection.
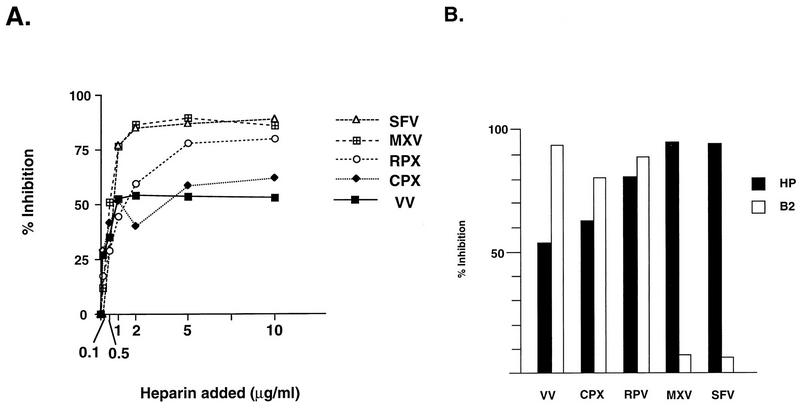
We then investigated if other poxviruses also adopt a similar strategy for cell entry process. BSC40 cells were infected with cowpox virus, rabbitpox virus, Shope fibroma virus, or myxoma virus in the presence of heparin, as described in the legend to Fig. 1. Plaque numbers for each virus were determined (Fig. 3A). Heparin blocked vaccinia virus and cowpox virus to a similar extent, around 55 to 60%. It inhibited rabbitpox virus better, at 78%. Furthermore, it blocked most plaque formation by Shope fibroma virus and myxoma virus, reaching more than 80% inhibition. Therefore, the data indicated that heparin could act as a general inhibitor of poxvirus infections.
FIG. 3.
(A) Heparin blocks infections by other poxviruses. BSC40 cells were infected with Shope fibroma virus (SFV), myxoma virus (MXV), rabbitpox virus (RPV), cowpox virus (CPX), and vaccinia virus (VV) in the presence of heparin at various concentrations as shown at the bottom of the figure, and plaque numbers were determined as described in the legend to Fig. 1A. (B) MAb B2 blocked various poxvirus infections differently from the blocking effect of heparin. BSC40 cells were infected with different poxviruses in the presence of heparin (10 μg/ml) or hybridoma culture supernatant containing B2, and the plaque numbers were determined as described in the legend to Fig. 1A.
Previously, we isolated MAb B2, which binds to cells and blocks vaccinia virus infection (4). Similar to heparin, B2 also interferes with vaccinia virus binding to cells. However, we have shown that cells treated with trypsin or pronase lost their reactivity to B2, suggesting that B2 recognizes a protein on the cell surface (4). Since heparin also blocked vaccinia virus binding to cells, we were curious to test if B2 cross-reacts with GAGs structures on cells. One simple experiment was to test whether the blocking effect of B2 on different poxviruses was identical to the blocking effect of heparin. We performed parallel experiments to compare MAb B2 with heparin for their inhibitory effects on these four poxviruses. Both inhibitors were present in excess to ensure that maximum effect would be achieved. As shown in Fig. 3B, the sensitivities of these poxviruses to MAb B2 could be divided into two categories. Vaccinia virus, cowpox virus, and rabbitpox virus were sensitive to MAb B2 treatment, resulting in 95, 80, and 88% inhibition, respectively. On the other hand, infections of Shope fibroma virus and myxoma virus were resistant to B2, with less than 10% inhibition. At the same time, heparin blocked all five poxviruses. The spectrum of virus sensitivity to heparin did not correlate with B2, suggesting that heparin and B2 compete for two different constituents on cells for virus binding during infection and that not all poxviruses infect cells through identical routes.
Recombinant A27L protein binds to heparin beads in vitro.
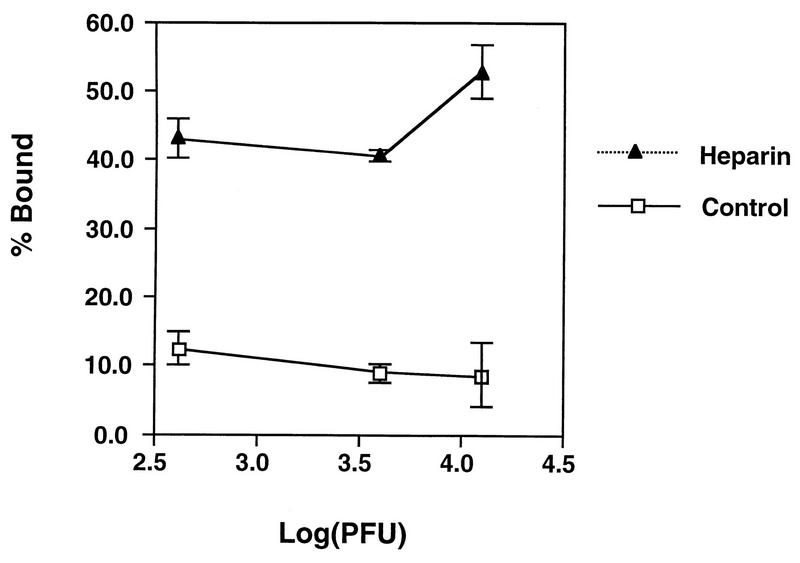
To investigate the biochemical nature of vaccinia virus binding to GAGs, we used heparin-Sepharose beads to determine if virions indeed bind to heparin. Purified vaccinia virus virions were incubated with heparin-conjugated beads at 4°C for 1 h, the unbound viruses were collected, and the virus titers were determined. As shown in Figure 4, about 10% of the input virions bound to the control Sepharose beads whereas an average of 50% of the input virions bound to the heparin-Sepharose beads.
FIG. 4.
Vaccinia virus virions bind to the heparin-Sepharose beads in vitro. Different amounts of purified vaccinia virus virions, as indicated at the bottom of the figure, were incubated with heparin-Sepharose beads at 4°C for 60 min as described in Materials and Methods. The unbound virions were collected in the supernatant, and the titers were determined. The data shown on the y axis were obtained by the following formula % Bound = [1-(PFUsupernatant/PFUinput)] × 100.
To search for a candidate viral protein responsible for GAGs interaction, we overexpressed vaccinia virus candidate proteins in a prokaryotic system to obtain large quantities. For example, both recombinant A27L and A4L proteins were tagged with 6-histidine at the C termini and purified with a nickel column. Both recombinant proteins were stained with Coomassie blue to check for their purity (Fig. 5A, lanes 1 and 2). Also, Western blot analysis was performed to confirm the presence of T7-tagged peptide sequences at the N termini of these recombinant proteins (lanes 3 and 4). Subsequently, these purified proteins were incubated with control Sepharose beads or heparin-Sepharose beads at 4°C for 30 min, and after the beads were washed the amount of proteins retained in the beads was determined by Western blot analysis. Neither protein bound to the control beads (Fig. 5B, lanes 4 and 9), and the proteins were collected in the supernatant (lanes 5 and 10). At the same time, A27L protein specifically bound to the heparin-Sepharose beads (lane 2) whereas A4L did not (lane 7).
FIG. 5.
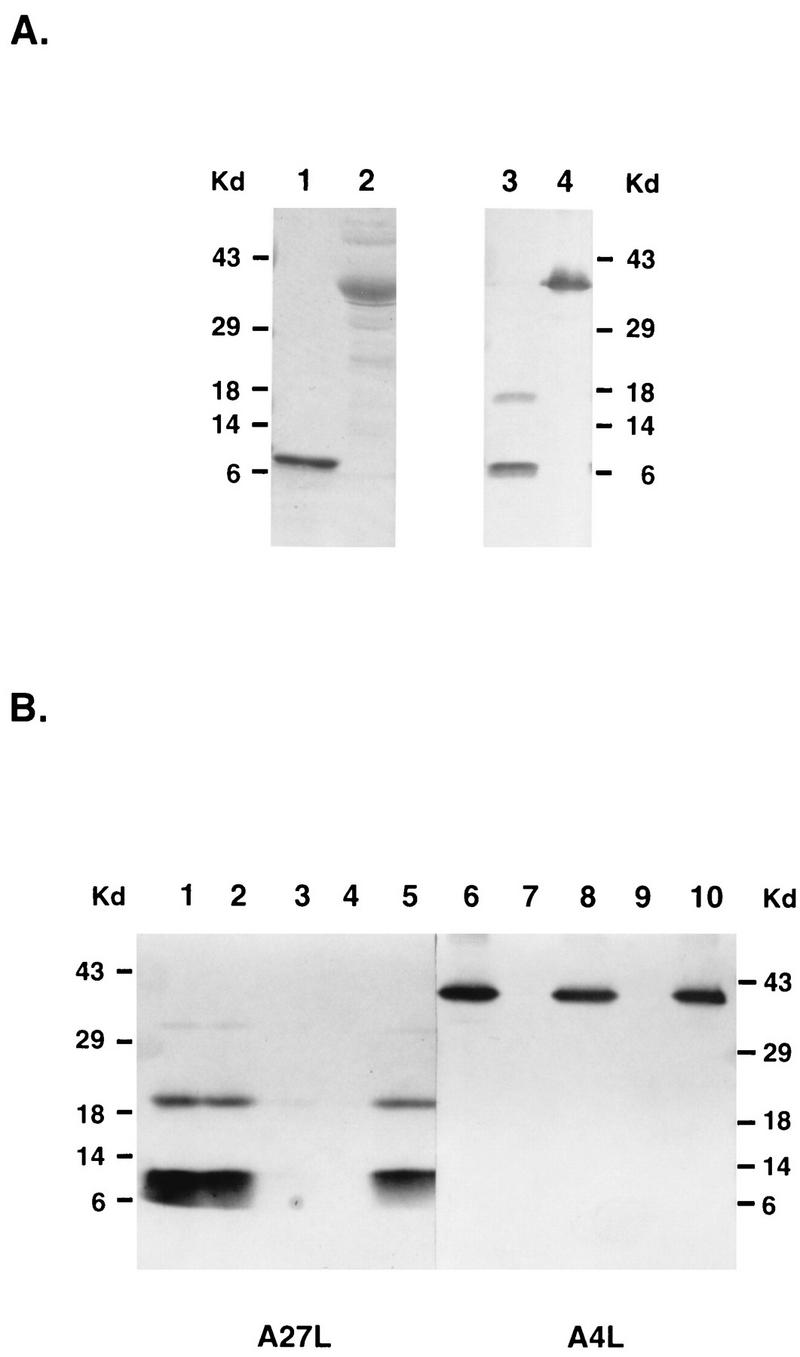
(A) Purification of the recombinant A27L and A4L proteins. Both A27L (lanes 1 and 3) and A4L (lanes 2 and 4) recombinant proteins were expressed in BL21(DE3) as described in Materials and Methods and purified through a nickel column. The left panel is an SDS-PAGE gel stained with Coomassie brilliant blue, and the right panel is a Western blot probed with a MAb against a T7 tag (1:5,000) at the N terminus of these recombinant proteins. (B) Recombinant A27L protein binds to a heparin beads in vitro. Purified A27L (lanes 1 to 5) or A4L (lanes 6 to 10) protein was incubated with heparin-Sepharose beads (lanes 2, 3, 7, and 8) or control Sepharose beads (lanes 4, 5, 9, and 10) at 4°C for 60 min, and the supernatant was collected. The beads were washed three times, and the volumes of the bound (lanes 2, 4, 7, and 9) or supernatant (lanes 3, 5, 8, and 10) samples were adjusted so that equal proportions of each sample were loaded on a 15% polyacrylamide gel for SDS-PAGE. Purified A27L and A4L proteins are shown as controls in lane 1 and 6, respectively. The gel was transferred for Western blot analysis with a MAb against a T7 tag (1:5,000) at the N terminus of these recombinant proteins.
Recombinant A27L protein binds to heparan sulfate on cells.
The binding of A27L protein to heparin in vitro indicated that A27L protein may play an important role in vaccinia virus entry. We examined the interaction of soluble recombinant A27L protein with live BSC40 cells to determine the specificity of its binding to cells. Monolayers of BSC40 cells were incubated with biotinylated A27L protein at 4°C, and the bound A27L protein was detected by phycoerythrin-conjugated streptavidin by immunofluorescence. BSC40 cells incubated with streptavidin alone showed very low background staining (Fig. 6A). Binding of A27L protein to cells was seen as intense homogeneous staining on the cell surface (Fig. 6B). Binding of A27L recombinant protein to cells was greatly diminished in the presence of soluble heparin, except that some small speckles were observed (Fig. 6C). In contrast, A27L protein that had been incubated with condrointin sulfate (Fig. 6D) or dermatan sulfate (Fig. 6E) bound to cells in a pattern indistinguishable from that observed with A27L protein alone.
FIG. 6.
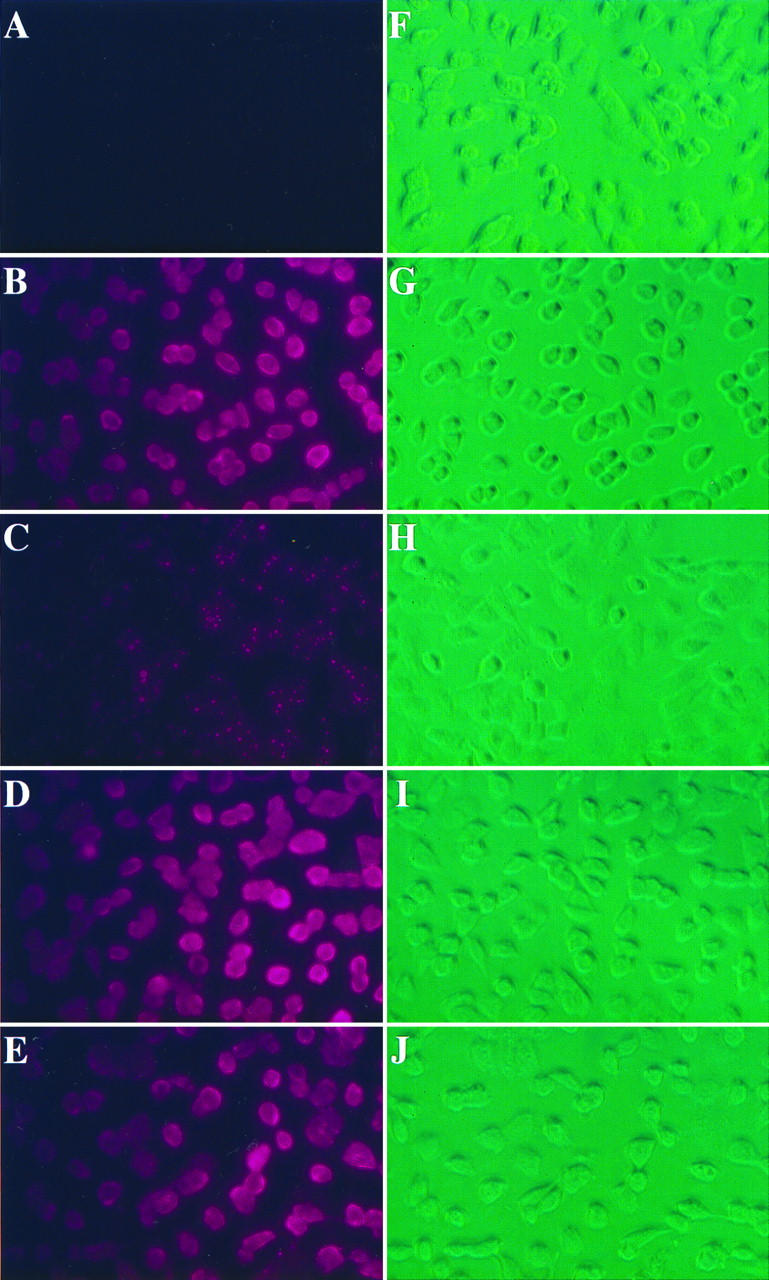
Recombinant A27L protein binds to the cell surface. Live BSC40 cells were incubated at 4°C for 30 min with PBS (A and F) or biotinylated A27L protein alone (B and G) or A27L protein previously mixed with heparin (C and H), condrointin sulfate (D and I), or dermatan sulfate (E and J) as described in Materials and Methods. The cells were washed three times and phycoerythrin-conjugated streptavidin was added for another 30-min incubation. After being washed, the cells were observed with a fluorescence microscope (A to E) or reverse-phase light microscope (F to J). All the images were analyzed as described in Materials and Methods.
DISCUSSION
Poxviruses are known to infect a variety of different cell types both in vitro and in vivo (34). Vaccinia virus, as the prototype orthopoxvirus, has been shown to infect multiple organs in the hosts. The nature of such a wide host range is not understood, and no receptor for vaccinia virus has been reported. In this study, we show that vaccinia virus binds to cell surface heparan sulfate during infection. The evidence came from several experiments. First, soluble heparin specifically competed with cells for virus binding. In addition, undersulfation of cell surface heparan sulfate by chlorate treatment reduced cell susceptibility to virus infection. Furthermore, purified vaccinia virus virions bound directly to the heparin-Sepharose column. Since heparan sulfate proteoglycans are found in the extracellular matrix and on the surface of most adherent mammalian cells, it would be advantageous for viruses to adopt such an interaction during infections (24). Indeed, results from four other poxviruses we tested also suggested a similar role of heparan sulfate for poxvirus entry. The nature of the virus-heparan sulfate interaction was governed mainly by the charge density contributed by different extents of sulfation on sugar moieties. However, undersulfated heparin sulfate produced in the presence of chlorate was reported to contain a reduced level of iduronic acids (23). Therefore, sugar structures of heparan sulfate may also specify vaccinia virus-cell interactions. We did notice that the dependence of vaccinia virus on heparan sulfate may not be as tight as that of herpesvirus, since the extent of inhibition by soluble heparin was never complete. Besides BSC40 cells, heparin could also block vaccinia virus infection of RK13 and HeLa cells to similar extents. We cannot completely rule out an involvement of other GAGs which may play a role for vaccinia virus but have escaped detection.
Cell surface heparan sulfate has been reported to play a role in the invasion of several pathogens into mammalian cells; these pathogens include bacteria such as Chlamydia trachomatis and Neisseria gonorrhoeae, the intracellular parasite Leishmania, and viruses such as herpesviruses and type O foot-and-mouth disease virus (20, 28, 46, 49, 52). The abundance and ubiquitous distribution of proteoglycans on various cells may justify a role as common attachment sites for different pathogens through a relatively nonspecific charge interaction. Since the entry process often has multiple stages, subsequent steps after such initial interactions may have been diversified according to the unique structure of individual pathogens. For example, herpesvirus binding to heparan sulfate is followed by membrane fusion between viruses and cells. Such a fusion factor, HVEM, was recently isolated and shown to be specific for herpes simplex virus type 1 (31). Vaccinia virus was also shown to enter cells via plasma membrane fusion (8). Thus, in principle, a different fusion factor may exist for vaccinia virus. Since we found that MAb B2 blocked vaccinia virus infection differently from soluble heparin, it is possible that B2 reacts with a surface molecule which plays some roles in the fusion event. Experiments to address these issues are in progress.
Heparan sulfate-virus interaction could induce conformational rearrangements that may enhance or optimize subsequent fusion events. In herpes simplex virus, such process is mediated by multiple viral proteins on virions. Herpes simplex virus virion binding to heparan sulfate is mediated by two viral proteins, gC and gB. Overexpression of gB in cells results in cell fusion after low-pH treatment (3, 42). Other viral membrane proteins such as gD, gH, and gL are also known to be required for cell fusion, although they do not directly bind to heparan sulfate (42).
For vaccinia virus, our results suggested that one viral membrane protein, A27L, could mediate virus attachment to heparan sulfate moieties on cells. A27L protein was previously reported to bind to cells, but the nature of the binding was not explored (25). We showed that recombinant A27L protein bound to heparin in vitro. In addition, purified A27L protein bound to cells, and this interaction could be specifically blocked by soluble heparin. These data revealed a molecular basis to explain the functions of A27L protein in the virus-host interaction. Similar to gB in HSV, A27L protein was previously reported to be an envelope protein on the surface of IMV and was required for cell fusion triggered by low-pH treatment (38, 39). Deletion of the N-terminal region of A27L protein eliminated its fusion activity, and many neutralizing MAbs recognize an epitope located near the N-terminus of A27L protein (13, 30). It is interesting that a cluster of positively charged amino acids are also located within the N-terminal region. These arginine and lysine residues, in principle, could be the GAG-binding domain of A27L protein. In future, it will be worthwhile to generate site-specific mutations of A27L proteins and to determine the relationship between the heparin binding activity of A27L protein and its fusion activity.
Natural mutations of A27L gene in vaccinia virus were found in persistently infected cells (5, 33). Mutations of A27L protein resulted in viable mutant viruses with a small-plaque phenotype (5, 12). It could mean that A27L mutant viruses enter cells via surface molecules distinct from heparan sulfate. Alternatively, other viral membrane proteins may substitute for the heparin-binding functions of A27L protein in these A27L mutant viruses. These two possibilities are not mutually exclusive and will be worth studying in the future.
Vaccinia virus has two forms of infectious particles, IMV and EEV. These viruses are composed of distinct membrane structures, and hence the entry of individual forms must be different from others. Our present study focuses on IMV entry, and any new information may help us understand its roles in virus spread within hosts. Studying the molecular mechanisms of virus entry may also help explain why multiple forms of poxviruses have evolved. Our study of GAG-vaccinia virus interaction is only the beginning of an attempt to dissect vaccinia virus entry pathways. More experiments are needed to further understand the molecular events that facilitate vaccinia virus entry processes.
ACKNOWLEDGMENTS
We thank Chi-Long Lin for computer assistance. We also thank Douglas Platt for carefully reviewing the manuscript.
This work is supported by research grants from the National Science Council (grant NSC 86-2316-B-001-016) and from Academia Sinica.
REFERENCES
- 1.Baeuerle P A, Huttner W B. Chlorate–a potent inhibitor of protein sulfation in intact cells. Biochem Biophys Res Commun. 1986;141:870–877. doi: 10.1016/s0006-291x(86)80253-4. [DOI] [PubMed] [Google Scholar]
- 2.Banfield B W, Leduc Y, Esford L, Schubert K, Tufaro F. Sequential isolation of proteoglycan synthesis mutants by using herpes simplex virus as a selective agent: evidence for a proteoglycan-independent virus entry pathway. J Virol. 1995;69:3290–8. doi: 10.1128/jvi.69.6.3290-3298.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Butcher M, Raviprakash K, Ghosh H P. Acid pH induced fusion of cells by herpes simplex virus glycoproteins gB and gD. J Biol Chem. 1990;265:5862–5868. [PubMed] [Google Scholar]
- 4.Chang W, Hsiao J-C, Chung C-S, Bair C-H. Isolation of a monoclonal antibody which blocks vaccinia virus infection. J Virol. 1995;69:517–522. doi: 10.1128/jvi.69.1.517-522.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dallo S, Rodriguez J F, Esteban M. A 14K envelope protein of vaccinia virus with an important role in virus-host cell interactions is altered during virus persistence and determines the plaque size phenotype of the virus. Virology. 1987;159:423–32. doi: 10.1016/0042-6822(87)90481-8. [DOI] [PubMed] [Google Scholar]
- 6.Davison A J, Moss B. Structure of vaccinia virus early promoters. J Mol Biol. 1989;210:749–769. doi: 10.1016/0022-2836(89)90107-1. [DOI] [PubMed] [Google Scholar]
- 7.Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton R E, Hill C M, Davis C B, Peiper S C, Schall T J, Littman D R, Landau N R. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 8.Doms R W, Blumenthal R, Moss B. Fusion of intra- and extracellular forms of vaccinia virus with the cell membrane. J Virol. 1990;64:4884–4892. doi: 10.1128/jvi.64.10.4884-4892.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dubochet J, Adrian M, Richter K, Garces J, Wittek R. Structure of intracellular mature vaccinia virus observed by cryoelectron microscopy. J Virol. 1994;68:1935–1941. doi: 10.1128/jvi.68.3.1935-1941.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feng Y, Broder C C, Kennedy P E, Berger E A. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272:872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 11.Gomez Yafal A, Kaplan G, Racaniello V R, Hogle J M. Characterization of poliovirus conformational alteration mediated by soluble cell receptors. Virology. 1993;197:501–505. doi: 10.1006/viro.1993.1621. [DOI] [PubMed] [Google Scholar]
- 12.Gong S C, Lai C F, Dallo S, Esteban M. A single point mutation of Ala-25 to Asp in the 14,000-Mr envelope protein of vaccinia virus induces a size change that leads to the small plaque size phenotype of the virus. J Virol. 1989;63:4507–4514. doi: 10.1128/jvi.63.11.4507-4514.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gong S C, Lai C F, Esteban M. Vaccinia virus induces cell fusion at acid pH and this activity is mediated by the N-terminus of the 14-kDa virus envelope protein. Virology. 1990;178:81–91. doi: 10.1016/0042-6822(90)90381-z. [DOI] [PubMed] [Google Scholar]
- 14.Greve H, Cully Z, Blumberg P, Kresse H. Influence of chlorate on proteoglycan biosynthesis by cultured human fibroblasts. J Biol Chem. 1988;263:12886–12892. [PubMed] [Google Scholar]
- 15.Gruenheid S, Gatzke L, Meadows H, Tufaro F. Herpes simplex virus infection and propagation in a mouse L cell mutant lacking heparan sulfate proteoglycans. J Virol. 1993;67:93–100. doi: 10.1128/jvi.67.1.93-100.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haywood A M. Virus receptors: binding, adhesion strengthening, and changes in viral structure. J Virol. 1994;68:1–5. doi: 10.1128/jvi.68.1.1-5.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heinegard D, Franzen A, Hedbom E, Sommarian Y. Common structures of the core proteins of interstitial proteoglycans. Chichester, United Kingdom: John Wiley & Sons; 1986. [DOI] [PubMed] [Google Scholar]
- 18.Herold B C, Gerber S I, Polonsky T, Belval B J, Shaklee P N, Holme K. Identification of structural features of heparin required for inhibition of herpes simplex virus type 1 binding. Virology. 1995;206:1108–1116. doi: 10.1006/viro.1995.1034. [DOI] [PubMed] [Google Scholar]
- 19.Herold B C, WuDunn D, Soltys N, Spear P G. Glycoprotein C of herpes simplex virus type 1 plays a principal role in the adsorption of virus to cells and in infectivity. J Virol. 1991;65:1090–1098. doi: 10.1128/jvi.65.3.1090-1098.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jackson T, Ellard F M, Ghazaleh R A, Brookes S M, Blakemore W E, Corteyn A H, Stuart D I, Newman W I, King A Q. Efficient infection of cells in culture by type O foot-and-mouth disease virus requires binding to cell surface haparan sulfate. J Virol. 1996;70:5282–5287. doi: 10.1128/jvi.70.8.5282-5287.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joklik W K. The purification of four strains of poxvirus. Virology. 1962;18:9–18. doi: 10.1016/0042-6822(62)90172-1. [DOI] [PubMed] [Google Scholar]
- 22.Kaplan G, Freistadt M S, Racaniello V R. Neutralization of poliovirus by cell receptors expressed in insect cells. J Virol. 1990;64:4697–4702. doi: 10.1128/jvi.64.10.4697-4702.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keller K M, Brauer P B, Keller J M. Modulation of cell surface heparan sulfate structure by growth of cells in the presence of chlorate. Biochemistry. 1989;28:8100–8107. doi: 10.1021/bi00446a021. [DOI] [PubMed] [Google Scholar]
- 24.Kjellen L, Lindahl U. Proteoglycans: structures and interactions. Annu Rev Biochem. 1991;60:443–475. doi: 10.1146/annurev.bi.60.070191.002303. [DOI] [PubMed] [Google Scholar]
- 25.Lai C, Gong S, Esteban M. Structural and functional properties of the 14-kDa envelope protein of vaccinia virus synthesized in Escherichia coli. J Biol Chem. 1990;265:22174–22180. [PubMed] [Google Scholar]
- 26.Lindahl U. Heparin: chemical and biological properties, clinical applications. London, United Kingdom: Edward Arnold; 1989. [Google Scholar]
- 27.Liu R, Paxton W A, Choe S, Ceradini D, Martin S R, Horuk R, MacDonald M E, Stuhlmann H, Koup R A, Landau N R. Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell. 1996;86:367–77. doi: 10.1016/s0092-8674(00)80110-5. [DOI] [PubMed] [Google Scholar]
- 28.Love D C, Esko J D, Mosser D M. A heparin-binding activity on Leishmania amastigotes which mediates adhesion to cellular proteoglycans. J Cell Biol. 1993;123:759–766. doi: 10.1083/jcb.123.3.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mendelsohn C L, Wimmer E, Racaniello V R. Cellular receptor for poliovirus: molecular cloning, nucleotide sequence, and expression of a new member of the immunoglobulin superfamily. Cell. 1989;56:855–865. doi: 10.1016/0092-8674(89)90690-9. [DOI] [PubMed] [Google Scholar]
- 30.Meyer H, Osterrieder N, Czerny C-P. Identification of binding sites for neutralizing monoclonal antibodies on the 14-kDa fusion protein of orthopox viruses. Virology. 1994;200:778–783. doi: 10.1006/viro.1994.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Montgomery R I, Warner M S, Lum B J, Spear P G. Herpes simplex virus-1 entry into cells mediated by a novel member of the TNF/NGF receptor family. Cell. 1996;87:427–36. doi: 10.1016/s0092-8674(00)81363-x. [DOI] [PubMed] [Google Scholar]
- 32.Mulder G I. Sulfation of drugs and related compounds. Boca Raton, Fla: CRC Press; 1981. [Google Scholar]
- 33.Paez E, Dallo S, Esteban M. Virus attenuation and identification of structural proteins of vaccinia virus that are selectively modified during virus persistence. J Virol. 1987;61:2642–2647. doi: 10.1128/jvi.61.8.2642-2647.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pogo B G, Dales S. Biology of poxviruses. New York, N.Y: Springer-Verlag; 1981. [DOI] [PubMed] [Google Scholar]
- 35.Racaniello V R. Early events in poliovirus infection: virus-receptor interactions. Proc Natl Acad Sci USA. 1996;93:11378–11381. doi: 10.1073/pnas.93.21.11378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Racaniello V R. Virus-receptor interaction in poliovirus entry and pathogenesis. Harvey Lect. 1991;87:1–16. [PubMed] [Google Scholar]
- 37.Racaniello V R, Ren R. Transgenic mice and the pathogenesis of poliomyelitis. Arch Virol Suppl. 1994;9:79–86. doi: 10.1007/978-3-7091-9326-6_9. [DOI] [PubMed] [Google Scholar]
- 38.Rodriguez J F, Esteban M. Mapping and nucleotide sequence of the vaccinia virus gene that encodes a 14-kilodalton fusion protein. J Virol. 1987;61:3550–3554. doi: 10.1128/jvi.61.11.3550-3554.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rodriguez J F, Paez E, Esteban M. A 14,000-Mr enveloped protein of vaccinia virus is involved in cell fusion and forms covalently linked trimers. J Virol. 1987;61:395–404. doi: 10.1128/jvi.61.2.395-404.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rucker J, Samson M, Doranz B J, Libert F, Berson J F, Yi Y, Smyth R J, Collman R G, Broder C C, Vassart G, Doms R W, Parmentier M. Regions in beta-chemokine receptors CCR5 and CCR2b that determine HIV-1 cofactor specificity. Cell. 1996;87:437–46. doi: 10.1016/s0092-8674(00)81364-1. [DOI] [PubMed] [Google Scholar]
- 41.Ruoslahti E. Proteoglycans in cell regulation. J Biol Chem. 1989;264:13369–13372. [PubMed] [Google Scholar]
- 42.Shieh M-T, Spear P G. Herpesvirus-induced cell fusion that is dependent on cell surface heparan sulfate or soluble heparin. J Virol. 1994;68:1224–1228. doi: 10.1128/jvi.68.2.1224-1228.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shieh M T, WuDunn D, Montgomery R I, Esko J D, Spear P G. Cell surface receptors for herpes simplex virus are heparan sulfate proteoglycans. J Cell Biol. 1992;116:1273–1281. doi: 10.1083/jcb.116.5.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sodeik B, Cudmore S, Ericsson M, Esteban M, Niles E G, Griffiths G. Assembly of vaccinia virus: incorporation of p14 and p32 into the membrane of the intracellular mature virus. J Virol. 1995;69:3560–3574. doi: 10.1128/jvi.69.6.3560-3574.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Spear P G. Entry of alphaherpesviruses into cells. Semin Virol. 1993;4:167–180. [Google Scholar]
- 46.Spear P G, Shieh M T, Herold B C, WuDunn D, Koshy T I. Heparan sulfate glycosaminoglycans as primary cell surface receptors for herpes simplex virus. Adv Exp Med Biol. 1992;313:341–353. doi: 10.1007/978-1-4899-2444-5_33. [DOI] [PubMed] [Google Scholar]
- 47.Subramanian G, McClain D S, Perez A, Fuller A O. Swine testis cells contain functional heparan sulfate but are defective in entry of herpes simplex virus. J Virol. 1994;68:5667–5676. doi: 10.1128/jvi.68.9.5667-5676.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vanderplasschen A, Smith G L. A novel virus binding assay using confocal microscopy: demonstration that the intracellular and extracellular vaccinia virions bind to different cellular receptors. J Virol. 1997;71:4032–4041. doi: 10.1128/jvi.71.5.4032-4041.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van Putten J P M, Paul S M. Binding of syndecan-like cell surface proteoglycan receptor is required for Neisseria gonorrhoeae entry into human mucosal cells. EMBO J. 1995;14:2144–2154. doi: 10.1002/j.1460-2075.1995.tb07208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wimmer E. Cellular receptors for animal viruses. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1994. [Google Scholar]
- 51.WuDunn D, Spear P G. Initial interaction of herpes simplex virus with cells is binding to heparan sulfate. J Virol. 1989;63:52–8. doi: 10.1128/jvi.63.1.52-58.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zaretzky F R, Pearce-Pratt R, Phillips D M. Sulfated polyanions block Chlamydia trachomatis infection of cervix-derived human epithelia. Infect Immun. 1995;63:3520–3526. doi: 10.1128/iai.63.9.3520-3526.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]