Abstract
The involvement of moesin in measles virus (MV) entry was investigated with moesin-positive and -negative mouse embryonic stem (ES) cells. MV infection of these cells was very ineffective and was independent of moesin expression. Furthermore, when these cells were transfected to express human CD46, a 100-fold increase in syncytium formation was observed with these cells and was independent of the expression of moesin. The only obvious difference between moesin-positive and -negative ES cells was the shape of the syncytia formed. Moesin-negative ES cells expressing or not expressing human CD46 formed separate pieces of fragmented syncytia which were torn apart during spreading, whereas ES cells expressing moesin exhibited typical syncytia. In addition, moesin was not detected on the surface of any murine cells or cell lines that we have tested by a flow cytometric assay with moesin-specific antibodies. These findings indicate that murine moesin is neither a receptor nor a CD46 coreceptor for MV entry into mouse ES cells. Moesin is involved in actin filament-plasma membrane interactions as a cross-linker, and it affects only the spreading and shape of MV-mediated syncytia.
CD46 (7, 10, 31, 33, 34) and moesin (11, 37) have been suggested to be implicated in measles virus (MV) entry. These two molecules are expressed on most human cells, consistent with the wide tissue tropism of MV. CHO cells, otherwise nonpermissive to MV, efficiently form syncytia on transfection with CD46 cDNA (10, 15). Rabbit anti-human CD46 antibody (Ab) and monoclonal Abs (MAbs) against human CD46 recognizing SCR2 block MV-mediated syncytium formation (16, 29, 40). Deglycosylation studies also support the importance of the sugars in SCR2 for MV infection (28). These results unequivocally indicate that CD46 serves as a receptor for MV. Since CD46 plays a primary role in the protection of host cells from homologous complement (20), it encompasses receptors for the complement system and viral infection.
Evidence supporting the role of moesin as a receptor for MV, on the other hand, seems to remain inconclusive. Moesin is a member of the ezrin-radixin-moesin family of proteins, which sustain cell surface molecules and the cytoskeleton (1, 2, 5, 24, 36, 44–46). Moesin is widely distributed as an essential intracellular element in cells of various species. It was reported that a MAb against a human astrocytoma cell line (U-251), named 119, inhibited MV infection and recognized a 75-kDa protein, which was identified as moesin (11). This result was confirmed with other MAbs against moesin and various cell lines of human, monkey, and murine origin (37). Indeed, murine cells with no detectable CD46 homolog were permissive to MV, although far less so than human cells (10, 12, 33, 48), and transfection of human CD46 conferred higher susceptibility to MV (10, 33, 48). These studies indicated that some murine cell lines that can be readily infected with MV must express receptor molecules other than CD46, and moesin is a candidate for such an alternative receptor molecule (6, 11, 12, 37). No structural homolog of CD46 has been found in these murine cell lines, and CRRY, a murine functional but not structural homolog of CD46 (14, 19, 25) in terms of complement regulation, is not involved in the entry of MV (12). Further supporting this issue is the fact that murine moesin is 98.3% identical to human moesin at the amino acid level (36), reasonably serving as a functional homolog (19, 25), while murine CRRY is <40% homologous to human CD46 (14, 25).
However, Ab blocking studies are sometimes difficult to interpret. In fact, Devaux and Gerlier (8) recently suggested that the cross-reactivity of antimoesin Abs with CD46 might explain the inhibitory effects of these Abs on MV entry. If this is the case, moesin, even though it forms a receptor complex with CD46 under the inner leaflet of membranes, may not be directly involved in MV binding.
To obtain conclusive evidence, MV infection studies were performed with moesin-positive and -negative embryonic stem (ES) cells expressing or not expressing human CD46.
MATERIALS AND METHODS
Cells and Abs.
CHO cells were obtained from the American Type Culture Collection. Vero cells and MV, a modified Nagahata strain (15, 16), which underwent four passages in hamster brain, were obtained from the Research Institute for Microbial Diseases, Osaka University. Anti-CD46 MAbs M160 and M177 (39) were prepared as described previously. The MAb against MV-H protein was a kind gift from S. Ueda. A rat anti-mouse moesin MAb, M22 (42), a rat anti-mouse ezrin MAb, M11 (42), and a mouse anti-chick gizzard radixin MAb, CR22 (36), which reacted strongly with mouse moesin, were prepared as described previously. Polyclonal Ab (PAb) TK89 was raised in rabbits against synthesized peptides corresponding to the mouse radixin sequence from amino acids 551 to 570, and it reacted with ezrin, radixin, and moesin.
ES cell line J1 (26) was used as the parent strain. Clones 145 and 199 (9), derived from ES cell line J1 in which the moesin gene was disrupted by homologous recombination, were used as moesin-negative cells. Both moesin-positive parent J1 cells and moesin-negative ES cell clones were cultured on gelatin-coated tissue culture dishes with high-glucose Dulbecco’s modified Eagle’s medium (DMEM; GIBCO BRL, Gaithersburg, Md.) supplemented with 20% fetal calf serum (FCS), 0.1 mM 2-mercaptoethanol (Sigma Chemical Co., St. Louis, Mo.), 1,000 U of leukemia inhibitory factor (Amrad Co., Kew, Victoria, Australia) per ml, 0.1 mM nonessential amino acids (ICN Biomedicals Inc., Costa Mesa, Calif.), 3 mM (each) adenosine, cytidine, guanosine, and uridine, and 1 mM thymidine (Sigma) in a humidified atmosphere of 5% CO2–95% air at 37°C.
Transfection of CD46 into ES cells.
CD46 cDNA (STc/CYT2 isoform) and its tail-less form (ΔCYT) were constructed as described previously (41). These cDNAs were ligated into the mammalian expression vector PCXN2 (41). Vector only-transfected cells were used as a control. ES cells (2 × 107) were electroporated in HEPES-buffered saline with vectors containing cDNA from CD46 or its tail-less form (20 μg) and 1 μg of pGKhph (carrying the hygromycin B phosphotransferase gene with the PGK promoter) at 0.25 kV and 960 μF by use of a Gene Pulser (Bio-Rad). The transfected ES cells were maintained for 24 h in the medium described above but containing 0.06% kanamycin, followed by selection with 120 μg of hygromycin B (Sigma) per ml. After 12 to 16 days, hygromycin B-resistant colonies were isolated with cloning cylinders and expanded on tissue culture plates. Expression of these gene products was confirmed by flow cytometry with M160 (not shown) and M177 as described below.
Flow cytometry.
Transfected cells (106) were incubated with 2 μg of murine anti-human CD46 MAb or nonimmune immunoglobulin G (IgG) for 45 min at 4°C and, after three washes with phosphate-buffered saline (PBS)–2% bovine serum albumin (BSA), stained with 2 μg of fluorescein isothiocyanate (FITC)-labeled goat anti-mouse IgG (Cappel, Westchester, Pa.) for 30 min at 4°C (39). The stained cells were analyzed with FACScan and/or Profile II flow cytometers to assess surface expression of CD46 and its mutants. J1 cells transfected with expression vector only were used as a control.
Reverse transcription (RT)-PCR and immunoblotting.
Total RNA was isolated from ES cells according to a standard procedure with guanidium-HCl and acid-phenol-chloroform, and cDNA was synthesized with SuperScriptII RNase H− reverse transcriptase (GIBCO BRL). Moesin primers (upstream, 5′-CTGGAGTTTGCCATTCAGCCC-3′; downstream, 5′-GAACAGGCGCTGGGTGATATC-3′) were designed to amplify a 261-bp segment. As a control for the presence of amplifiable RNA, hypoxanthine phosphoribosyltransferase (HPRT) primers were designed to amplify a 249-bp segment as previously described (18). Amplified PCR products were run on an agarose gel (2.0%) and stained with ethidium bromide.
Control ES cells (J1) and moesin knockout ES cell clones cultured on tissue culture dishes were washed with PBS and lysed in an equal volume of 2× SDS sample buffer (50 mM Tris-HCl [pH 6.8], 2% sodium dodecyl sulfate [SDS], 20% glycerol, 2% 2-mercaptoethanol, 0.01% bromophenol blue). Proteins were separated by SDS–10% polyacrylamide gel electrophoresis by the method of Laemmli (22). Proteins were electrophoretically transferred from gels to nitrocellulose membranes and incubated with PAb TK89 or a mixture of MAbs M22 and M11. For antibody detection, a blotting detection kit with biotinylated immunoglobulin and streptavidin-alkaline phosphatase conjugate (Amersham International Plc., Buckinghamshire, United Kingdom) was used.
MV binding assay.
Cells to be assayed for MV binding were detached from dishes at 80% confluency by the addition of 2 ml of PBS containing 5 mM EDTA. After two washes, aliquots containing 2 × 105 cells were incubated for 2 h at room temperature with 2 ml of concentrated MV (107 PFU/ml) in DMEM containing 5% FCS. After three washes with DMEM containing 5% FCS, cells were incubated at room temperature for 45 min with 5 μg of anti–MV-H protein MAb. After three washes with 10 ml of DMEM, cells were incubated with 5 μg of FITC-labeled goat anti-mouse IgG. The levels of MV-H protein in each ES cell clone were assessed by flow cytometry.
Determination of MV infectivity.
ES cell clones with or without CD46 or its tail-less mutants were cultured to 70% confluency in 24-well plates (Corning) for 15 h and infected with MV at 250 to 25,000 PFU/well. Simultaneously, plaque-forming assays were performed in some experiments, and the results were confirmed by the correlation between CHO cell syncytium formation and plaque formation (15). The syncytia formed were counted, and the cytopathic effects of the ES cell transfectants were observed 2 to 4 days postinfection. Cells were photographed under an Olympus microscope.
Virus production was determined as described previously (16). Briefly, ES cell clones were infected with MV (1,000 or 25,000 PFU/well) for 2 h, extensively washed, and cultured for 6 h. The supernatants were withdrawn, and the wells were washed again to remove free MV. Four days later, we removed the supernatants to determine the MV titers by a standard method with Vero cells.
Confocal analysis.
Cells cultured on chamber slides were fixed with 1.5% formaldehyde in PBS for 15 min, treated with 0.2% Triton X-100 in PBS for 10 min, and washed with PBS. After soaking in 1% BSA–PBS for 10 min, the samples were treated with the primary antibodies in 1% BSA–PBS at room temperature for 1 h. As the primary antibodies, rabbit anti-human CD46 Ab and M22 were used for double staining. The samples were washed with 1% BSA–PBS, followed by incubation with the secondary antibodies (rhodamine-conjugated goat anti-rabbit IgG or FITC-conjugated goat anti-rat IgG Abs [Cappel]) in 1% BSA–PBS for 30 min. The samples were washed with PBS and then examined with a confocal laser scanning microscope (Olympus Floview). Colocalized green (FITC) fluorescence and red (rhodamine) fluorescence appeared yellow in the merged images.
RESULTS
Moesin knockout ES cell clones.
To study the role of moesin in MV entry, we used parent ES (J1) cells and two independent moesin knockout ES cell clones, 145 and 199, in which the moesin gene was disrupted by homologous recombination (9). As the murine moesin gene is located on the X chromosome (47), it could be disrupted by mutation of a single gene. We confirmed that these clones lacked moesin mRNA and moesin protein expression by RT-PCR (Fig. 1a) and immunoblotting (Fig. 1b), respectively. These clones were morphologically indistinguishable from parent ES cells and showed similar growth rates. The results obtained with these clones were comparable, so differences, if any, were due to moesin loss rather than to clonal variation. Details of the analysis of moesin knockout mice will be presented elsewhere.
FIG. 1.

Lack of moesin expression in targeted ES cells. (a) RT-PCR analysis of mRNAs from parent [J1 (+/Y)] and moesin knockout [145 (−/Y) and 199 (−/Y)] ES cells. Moesin primers directed the amplification of a 261-bp fragment from parent cell-derived cDNA. RT-PCR amplification was also performed with HPRT primers as a control. (b) Immunoblot analysis of moesin expression in cell lysates from parent and moesin knockout ES cells (designations as in panel a) with three distinct antibodies. Each sample was loaded on an SDS–10% polyacrylamide gel with the same amount of total protein and then transferred to a nitrocellulose membrane. The blots were probed with PAb TK89 or a mixture of MAbs M22 and M11, followed by antibody detection with a blotting detection kit. In the targeted ES cells (145 and 199), moesin was not detected. Ezrin, 85 kDa; radixin, 82 kDa; moesin, 75 kDa.
MV infection assay.
Moesin-positive and -negative ES cells were infected with MV strain Nagahata. Both were highly resistant to MV (Table 1). Syncytia were formed in these clones to a similar extent regardless of moesin expression at high titers of MV. Accordingly, the MV-H protein was poorly amplified, again independently of the presence of moesin (data not shown). Similar results were obtained with a variety of strains of MV which use CD46 as an entry receptor (35) (Table 2).
TABLE 1.
Numbers of syncytia formed in the MV infection assay
| Cells | h after infection | No. of syncytia formed with the following MV titer (102 PFU/well)a:
|
|||||
|---|---|---|---|---|---|---|---|
| 250 | 125 | 75 | 25 | 2.5 | 0 | ||
| CD46 negative | |||||||
| J1 | 72 | 1 | 0 | 0 | 0 | 0 | 0 |
| 96 | 5 | 2 | 0 | 0 | 0 | 0 | |
| 120 | 18 | 5 | 2 | 0 | 0 | 0 | |
| 145 | 72 | 0 | 0 | 0 | 0 | 0 | 0 |
| 96 | 3 | 0 | 0 | 0 | 0 | 0 | |
| 120 | 15 | 4 | 3 | 0 | 0 | 0 | |
| 199 | 72 | 0 | 0 | 0 | 0 | 0 | 0 |
| 96 | 5 | 0 | 0 | 0 | 0 | 0 | |
| 120 | 8 | 2 | 1 | 0 | 0 | 0 | |
| CD46 positive | |||||||
| J1 | 72 | TM | 50 | 31 | 28 | 3 | 0 |
| 96 | TM | TM | TM | 50 | 12 | 0 | |
| 120 | TM | TM | TM | TM | 20 | 0 | |
| 145 | 72 | TM | 51 | 32 | 21 | 1 | 0 |
| 96 | TM | TM | TM | 50 | 2 | 0 | |
| 120 | TM | TM | TM | TM | 5 | 0 | |
| 199 | 72 | TM | 50 | 38 | 15 | 3 | 0 |
| 96 | TM | TM | TM | 50 | 4 | 0 | |
| 120 | TM | TM | TM | TM | 10 | 0 | |
TM, too many to count.
TABLE 2.
MV strains amplified in the ES cell clones
| MV strain | Virus production (102 PFU/ml) in the following cellsa:
|
|||
|---|---|---|---|---|
| CD46 negativeb
|
CD46 positivec
|
|||
| J1 | 199 | J1 | 199 | |
| Edmonston | 1.2 | 1.2 | 2.6 | 3.0 |
| Toyoshima | 0.5 | 0.3 | 1.1 | 0.8 |
| Nagahata | 2.3 | 2.1 | 4.5 | 4.6 |
Determined with Vero cells as described in Materials and Methods.
Two doses of MV were used. ES cell clones were infected with MV at 1,000 or 25,000 PFU/well. Plaque-forming activities were not detected in the supernatants of 1,000 PFU-treated samples (data not shown) but were detected in the supernatants of 25,000 PFU-treated samples, as indicated.
The values are for the supernatants of 1,000 PFU-treated samples.
The only obvious difference between moesin-positive and -negative cells was in the properties of the syncytia (Fig. 2a) (clone 199 is not shown). In moesin-negative cells, MV induced the formation of uniquely shaped syncytia in which the balloonlike small fragments were torn apart during spreading. Under the same experimental conditions, no balloonlike shape was observed in cells expressing moesin (Fig. 2a).
FIG. 2.
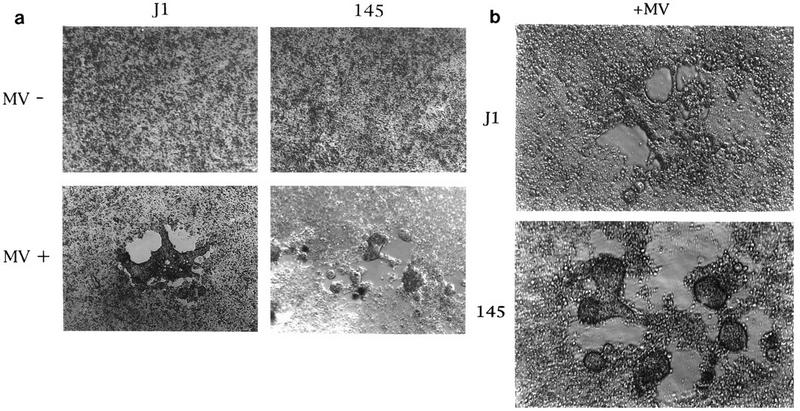
Shapes of MV-mediated syncytia in moesin-positive and -negative ES cells. (a) Parent ES cells J1 and clone 145, which lacks moesin, were incubated with or without MV (2.5 × 105 PFU) for 72 h at 37°C and viewed by phase-contrast microscopy (Olympus). Magnification, ×51. The experiments were performed three times, and representative syncytia are shown in the lower panel. Results similar to those shown in the right panel were obtained with clone 199. (b) J1 and 145 which had been transfected with human CD46 were incubated with MV (2.5 × 103 PFU) for 72 h at 37°C, and representative syncytia were photographed under phase-contrast microscopy. Magnification, ×100.
Transfection of CD46.
CD46 is a receptor for most laboratory-adapted MV strains. We examined whether moesin serves as a coreceptor for CD46, the complex of which confers high susceptibility to MV on cells. CD46 was transfected into ES cells with or without moesin (Fig. 3a). Cells expressing similar levels of CD46 were selected by flow cytometry (Fig. 3a) and cloned by limiting dilution. We first examined MV binding to these cells. An MV-H-mediated fluorescence shift was detected with CD46-positive cells (Fig. 3b), suggesting specific binding of MV to CD46. An MV infection assay was next performed with the clones. An approximately 100-fold-higher sensitivity to MV was observed in the CD46-positive clones than in the CD46-negative clones (Table 1). The degrees of MV sensitivity were almost identical in the CD46-positive clones regardless of moesin expression. The same syncytium shape was observed in CD46 transfectants (Fig. 2b) as in CD46-negative ES cells (Fig. 2a). MV-H was synthesized and appeared to be colocalized with CD46 in the center of the syncytia regardless of moesin expression (data not shown). Furthermore, with several MV strains in addition to Nagahata, effective virus production could be detected (Table 2). Viral amplification therefore proceeded in moesin-negative cells with CD46. These results reinforce the finding that moesin is not a receptor for MV and support the inference that it does not affect the MV receptor function of CD46.
FIG. 3.

Flow cytometric analysis of the levels of CD46 and MV binding in the established ES cell clones. (a) Levels of human CD46 in ES cell transfectants. Cells were established as described in Materials and Methods. Cells were sequentially incubated with M177 and FITC-labeled goat anti-mouse IgG. After being fixed with paraformaldehyde, cells were analyzed by flow cytometry within 3 days. (b) Binding of MV to CD46 transfectants. The established ES cell clones with or without CD46 were detached from dishes and incubated with MV. After extensive washes, cells were incubated with anti–MV-H protein MAb. After being washed again, cells were incubated with FITC-labeled goat anti-mouse IgG. The levels of the MV-H protein in each ES cell clone were assessed by flow cytometry. Here, we show representative results for clone 145, although similar results were obtained for clone 199. The mean fluorescence shift values are shown in each panel.
Transfection of a tail-less form of CD46.
Moesin has been reported to be partially expressed on the cell surface (11, 23) and to form a complex with CD46 in the same cell membrane (37). However, we could not detect moesin on the surface of CHO cells or the murine or human cell lines tested by flow cytometry with MAbs M22 and CR22 (data not shown). Thus, CD46 may contact moesin through its cytoplasmic domains inside the cells. Indeed, CD46 has an RRKKK sequence beneath the transmembrane domain which may sustain moesin binding, like CD44 and ICAM-2 (50). To rule out the possibility that the association between the cytoplasmic tail of CD46 and moesin plays a role in syncytium formation, particularly the shape of the syncytia, we transfected cDNA encoding a tail-less form of CD46 into moesin-positive cells and examined the syncytia formed. Even the tail-less form of CD46 allowed the formation of normal, nonfragmented syncytia in moesin-positive cells (data not shown), consistent with previous reports (13, 49). These results indicate that even if the cytoplasmic tail of CD46 is associated with moesin, this association does not affect syncytium formation.
Localization of moesin and CD46 in ES cells.
It has been reported that CD46, like moesin, is localized in microvilli in some cell lines (37), which may facilitate MV infection. Localization profiles for CD46 were compared with those for moesin by use of ES and CHO cells expressing CD46 and moesin. CD46 was distributed in areas of cell-to-cell contact in both ES cells (Fig. 4) and CHO cells (39). By confocal analysis, moesin was also seen to be predominantly distributed in a pattern similar to that of CD46 (Fig. 4). Villi were not stained with anti-CD46 Ab but were faintly stained with antimoesin Ab (Fig. 4). The same was true for CD46-transfected CHO cells (39). We interpret these results as indicating that moesin is colocalized with CD46 in areas of cell-to-cell contact, although their direct association remains to be conclusively demonstrated. Moesin clustered in microvilli is free from CD46. This distribution profile was observed even with cells expressing the tail-less form of CD46 (data not shown). We initially anticipated that the absence of a cytoplasmic domain which may associate with moesin would cause disordering of the CD46 distribution. CD46, however, retained its predominantly lateral expression pattern even in such mutant transfectants.
FIG. 4.
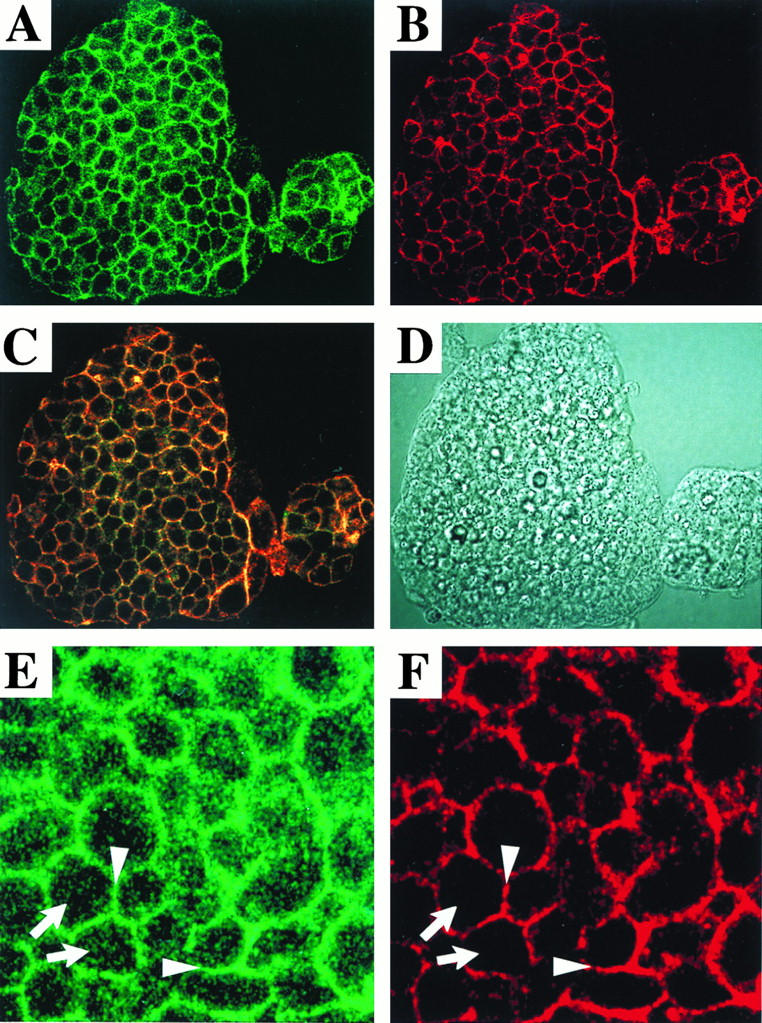
Confocal analysis of CD46 and moesin in ES cell clones. The localization of moesin and CD46 was examined with CD46-expressing J1 cells. Moesin (A) and CD46 (B) were stained with green (FITC) fluorescence and red (rhodamine) fluorescence, respectively, as described in Materials and Methods. (C) Colocalization of green fluorescence and red fluorescence appears yellow. (D) Phase-contrast micrograph. Areas from panels A and B are enlarged in panels E and F, respectively. Moesin and CD46 were colocalized in the lateral portion (arrowheads), but only moesin was localized in microvilli (arrows).
DISCUSSION
The following conclusions were drawn from the results of the present study. Murine moesin per se is not a receptor for MV entry. It is localized in areas of cell-to-cell attachment with a distribution profile similar to that of CD46. Moesin appears to affect only the spreading and shape of MV-mediated syncytia.
As direct evidence for these conclusions, high doses of MV induced syncytia in both moesin-positive and -negative ES cells, and the sensitivities to MV of these ES clones were indistinguishable. The sensitivities to MV were increased 100-fold when these ES clones were transfected with CD46. Again, the differences in the MV sensitivities of these transfectants were minimal. In addition, no moesin epitopes were detected on any cells or cell lines by a flow cytometric assay with Abs, consistent with a previous report (8). Thus, CD46 but not moesin contributed to MV susceptibility in our system. However, we cannot exclude the possibility that human moesin serves as an entry receptor for MV. Whether purified moesin can actually bind to MV, as reported previously (11), should be reexamined.
In a previous report, moesin and CD46 were shown to be MV receptors acting in an additive manner and colocalized in microvilli (37), a situation that is advantageous for MV attachment and fusion to cells. Our results did not support the localization of these two molecules in microvilli; only moesin was present in villi, and it probably assembled with other adhesion molecules as described previously (44, 45, 50). The predominantly lateral distribution of CD46 and moesin was confirmed with nonpolarized CHO cells by confocal analysis (data not shown), a result which differs from previous results obtained with polarized cells (4). Passage of MV strains in nonpolarized cells may have influenced the patterns of entry and release of virus. The lateral distribution of CD46 in ES cells may facilitate the basolateral infectious pathway of MV through the cell membrane.
Which molecule is responsible for the reported MV sensitivity of murine cells is a relevant question. In Vero cells, with a high sensitivity to MV, CD46 is likely to be colocalized extracellularly with CD9 (a tetraspan transmembrane protein which renders cells susceptible to canine distemper virus [27]), α3β1 integrin (30, 32), and heparin-binding epidermal growth factor (HB-EGF) (32) and intracellularly with moesin (37). Thus, the clustering of these molecules in areas of cell-to-cell attachment may indicate that they participate in MV entry in conjunction with CD46. Yet, there is no evidence that CD9, α3β1 integrin, or HB-EGF is responsible for the susceptibility of murine cells to MV. Moreover, moesin is a cytoplasmic protein with no receptor function. Hence, at least in murine ES cells, it is possible that unknown molecules, but not moesin, accumulating with these hetero-oligomeric complexes allow for weak MV susceptibility.
It is generally accepted that mice harbor CRRY, which is a functional substitute for CD46, as a complement regulatory protein (14, 25) and that mice harbor no CD46. However, we recently cloned a murine structural analog of CD46 which was predominantly expressed in the testis, although its message was found in almost all tissues to a lesser extent (43). Although whether the murine CD46 homolog serves as a receptor for MV in murine cells remains to be tested, it would be an alternative candidate as a murine MV receptor.
It is notable that the fragmented syncytial debris was observed in moesin knockout ES cells regardless of the expression of human CD46. There are two points to be made about syncytial debris. First, it can be a vehicle carrying MV and can be spread on cultured monolayers. There are some organs in which the amounts of moesin are relatively low (3, 36). So, it would be interesting to test whether this infectious pathway exists in vivo. Second, moesin is thought to function as a general cross-linker between the plasma membrane and actin filaments (1, 2, 5, 36, 45, 46). Thus, in cases of cytoskeletal changes such as syncytium formation, moesin loss may affect syncytium morphology. Further studies on the molecular mechanisms, including signaling, fusion, and cellular responses (17, 21, 38), whereby these unique syncytia are formed are needed to lead to a better understanding of this virus-derived phenotype.
ACKNOWLEDGMENTS
We are grateful to A. Ueda (Research Institute for Microbial Diseases, Osaka University), H. Sakata (National Institute of Health Japan), and K. Toyoshima (Osaka Medical Center) for their generous gifts of reagents and valuable discussions. Thanks are also due to K. Shida and T. Hara for technical assistance.
REFERENCES
- 1.Amieva M R, Furthmayr H. Subcellular localization of moesin in dynamic filopodia, retraction fibers, and other structures involved in substrate exploration, attachment, and cell-cell contacts. Exp Cell Res. 1995;219:180–196. doi: 10.1006/excr.1995.1218. [DOI] [PubMed] [Google Scholar]
- 2.Arpin M, Algrain M, Louvard D. Membrane-actin microfilament connections: an increasing diversity of players related to band 4.1. Curr Opin Cell Biol. 1994;6:136–141. doi: 10.1016/0955-0674(94)90127-9. [DOI] [PubMed] [Google Scholar]
- 3.Berryman M, Franck Z, Bretscher A. Ezrin is concentrated in the apical microvilli of a wide variety of epithelial cells whereas moesin is found primarily in endothelial cells. J Cell Sci. 1993;105:1025–1043. doi: 10.1242/jcs.105.4.1025. [DOI] [PubMed] [Google Scholar]
- 4.Blau D M, Compans R W. Adaptation of measles virus to polarized epithelial cells: alterations in virus entry and release. Virology. 1997;231:281–289. doi: 10.1006/viro.1997.8520. [DOI] [PubMed] [Google Scholar]
- 5.Bretscher A. Microfilament structure and function in the cortical cytoskeleton. Annu Rev Cell Biol. 1991;7:337–374. doi: 10.1146/annurev.cb.07.110191.002005. [DOI] [PubMed] [Google Scholar]
- 6.Buckland R, Wild T F. Is CD46 the cellular receptor for measles virus? Virus Res. 1997;48:1–9. doi: 10.1016/s0168-1702(96)01421-9. [DOI] [PubMed] [Google Scholar]
- 7.Devaux P, Loveland B, Christiansen D, Milland J, Gerlier D. Interactions between the ectodomains of haemagglutinin and CD46 as a primary step in measles virus entry. J Gen Virol. 1996;77:1477–1481. doi: 10.1099/0022-1317-77-7-1477. [DOI] [PubMed] [Google Scholar]
- 8.Devaux P, Gerlier D. Antibody cross-reactivity with CD46 and lack of cell surface expression suggest that moesin might not mediate measles virus binding. J Virol. 1997;71:1679–1682. doi: 10.1128/jvi.71.2.1679-1682.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doi, Y., H. Takano, S. Yonemura, M. Itoh, S. Tsukita, S. Tsukita, and T. Noda. Unpublished data.
- 10.Dorig R E, Marcil A, Chopra A, Richardson C D. The human CD46 molecule is a receptor for measles virus (Edmonston strain) Cell. 1993;75:295–305. doi: 10.1016/0092-8674(93)80071-l. [DOI] [PubMed] [Google Scholar]
- 11.Dunster L M, Schneider-Schaulies J, Loffler S, Lankes W, Schwartz-Albiez R, Lottspeich F, ter-Meulen V. Moesin: a cell membrane protein linked with susceptibility to measles virus infection. Virology. 1994;198:265–274. doi: 10.1006/viro.1994.1029. [DOI] [PubMed] [Google Scholar]
- 12.Dunster L M, Schneider-Schaulies J, Dehoff M H, Holers V M, Schwartz-Albiez R, ter-Meulen V. Moesin, and not the murine functional homologue (Crry/p65) of human membrane cofactor protein (CD46), is involved in the entry of measles virus (strain Edmonston) into susceptible murine cell lines. J Gen Virol. 1995;76:2085–2089. doi: 10.1099/0022-1317-76-8-2085. [DOI] [PubMed] [Google Scholar]
- 13.Hirano A, Yant S, Iwata K, Korte-Sarfaty J, Seya T, Nagasawa S, Wong T C. Human cell receptor CD46 is down regulated through recognition of a membrane-proximal region of the cytoplasmic domain in persistent measles virus infection. J Virol. 1996;70:6929–6936. doi: 10.1128/jvi.70.10.6929-6936.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holers V M, Kinoshita T, Morina H. The evolution of mouse and human complement C3-binding proteins: divergence of form but conservation of function. Immunol Today. 1992;13:231–236. doi: 10.1016/0167-5699(92)90160-9. [DOI] [PubMed] [Google Scholar]
- 15.Iwata K, Seya T, Ariga H, Ueda S, Nagasawa S. Modulation of complement regulatory function and measles virus receptor function by the serine-threonine-rich domains of membrane cofactor protein (CD46) Biochem J. 1994;304:169–175. doi: 10.1042/bj3040169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iwata K, Seya T, Yanagi Y, Pesando J M, Johnson P M, Okabe M, Ueda S, Ariga H, Nagasawa S. Diversity of sites for measles virus binding and for inactivation of complement C3b and C4b on membrane cofactor protein CD46. J Biol Chem. 1995;270:15148–15152. doi: 10.1074/jbc.270.25.15148. [DOI] [PubMed] [Google Scholar]
- 17.Karp C L, Wysocka M, Wahl L M, Ahearn J M, Cuomo P J, Sherry B, Trinchieri G, Griffin D E. Mechanism of suppression of cell-mediated immunity by measles virus. Science. 1996;273:228–231. doi: 10.1126/science.273.5272.228. [DOI] [PubMed] [Google Scholar]
- 18.Keller G, Kennedy M, Papayannopoulou T, Wiles M V. Hematopoietic commitment during embryonic stem cell differentiation in culture. Mol Cell Biol. 1993;13:473–786. doi: 10.1128/mcb.13.1.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim Y U, Kinoshita T, Molina H, Hourcade D, Seya T, Wagner L M, Holers V M. Mouse complement regulatory protein CRRY/P65 uses the specific mechanisms of both human decay-accelerating factor and membrane cofactor protein. J Exp Med. 1995;181:151–159. doi: 10.1084/jem.181.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kinoshita T, Seya T. Complement regulatory proteins on nucleated cells. In: Erdei A, editor. New aspects of complement structure and function. R. G. Dallas, Tex: Landes Co.; 1995. pp. 35–58. [Google Scholar]
- 21.Krantic S, Gimenez C, Rabourdin-Combe C. Cell-to-cell contact via measles virus haemagglutinin-CD46 interaction triggers CD46 downregulation. J Gen Virol. 1995;76:2793–2800. doi: 10.1099/0022-1317-76-11-2793. [DOI] [PubMed] [Google Scholar]
- 22.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 23.Lankes W T, Griesmacher A, Grunwald J, Schwartz-Albiez R, Keller R. A heparin-binding protein involved in inhibition of smooth muscle cell proliferation. Biochem J. 1988;251:831–842. doi: 10.1042/bj2510831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lankes W T, Furthmayr H. Moesin: a member of the protein 4.1-talin-ezrin family of proteins. Proc Natl Acad Sci USA. 1991;88:8297–8301. doi: 10.1073/pnas.88.19.8297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li B, Sallee C, Dehoff M, Foley S, Molina H, Holers V M. Mouse Crry/p65: characterization of monoclonal antibodies and the tissue distribution of a functional homologue of human MCP and DAF. J Immunol. 1993;151:4295–4305. [PubMed] [Google Scholar]
- 26.Li E, Bestor T H, Jaenisch R. Targeted mutation of the DNA methyltransferase gene results in embryonic lethality. Cell. 1992;69:915–926. doi: 10.1016/0092-8674(92)90611-f. [DOI] [PubMed] [Google Scholar]
- 27.Loffler S, Lottspeich F, Lanza F, Azorsa D O, ter-Meulen V, Schneider-Schaulies J. CD9, a tetraspan transmembrane protein, renders cells susceptible to canine distemper virus. J Virol. 1997;71:42–49. doi: 10.1128/jvi.71.1.42-49.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maisner A, Alvarez J, Liszewski M K, Atkinson D J, Atkinson J P, Herrler G. The N-glycan of the SCR 2 region is essential for membrane cofactor protein (CD46) to function as a measles virus receptor. J Virol. 1996;70:4973–4977. doi: 10.1128/jvi.70.8.4973-4977.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Manchester M, Valsamakis A, Kaufman R, Liszewski M K, Alvarez J, Atkinson J P, Lublin D M, Oldstone M B. Measles virus and C3 binding sites are distinct on membrane cofactor protein (CD46) Proc Natl Acad Sci USA. 1995;92:2303–2307. doi: 10.1073/pnas.92.6.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mori K, Heuler M E. A possible molecular complex involving CD46. Proc Jpn Immunol Soc. 1997;27:110. . (Abstract.) [Google Scholar]
- 31.Mumenthaler C, Schneider U, Buchholz C J, Koller D, Braun W, Cattaneo R. A 3D model for the measles virus receptor CD46 based on homology modeling, Monte Carlo simulations, and hemagglutinin binding studies. Protein Sci. 1997;6:588–597. doi: 10.1002/pro.5560060308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakamura K, Iwamoto R, Mekada E. Membrane-anchored heparin-binding EGF-like growth factor (HB-EGF) and diphtheria toxin receptor-associated protein (DRAP27)/CD9 form a complex with integrin alpha 3 beta 1 at cell-cell contact sites. J Cell Biol. 1995;129:1691–1705. doi: 10.1083/jcb.129.6.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Naniche D, Varior-Krishnan G, Cervoni F, Wild T F, Rossi B, Rabourdin-Combe C, Gerlier D. Human membrane cofactor protein (CD46) acts as a cellular receptor for measles virus. J Virol. 1993;67:6025–6032. doi: 10.1128/jvi.67.10.6025-6032.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nussbaum O, Broder C C, Moss B, Stern L B, Rozenblatt S, Berger E A. Functional and structural interactions between measles virus hemagglutinin and CD46. J Virol. 1995;69:3341–3349. doi: 10.1128/jvi.69.6.3341-3349.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sakata, H., M. Kurita, Y. Murakami, S. Nagasawa, N. Watanabe, S. Ueda, M. Matsumoto, and T. Seya. Two modes of CD46 down-regulation induced by Japanese wild measles virus strains. Submitted for publication. [DOI] [PubMed]
- 36.Sato N, Funayama N, Nagafuchi A, Yonemura S, Tsukita S, Tsukita S. A gene family consisting of ezrin, radixin, and moesin. Its specific localization at actin filament/plasma membrane association sites. J Cell Sci. 1992;103:131–143. doi: 10.1242/jcs.103.1.131. [DOI] [PubMed] [Google Scholar]
- 37.Schneider-Schaulies J, Dunster L M, Schwartz-Albiez R, Krohne G, ter-Meulen V. Physical association of moesin and CD46 as a receptor complex for measles virus. J Virol. 1995;69:2248–2256. doi: 10.1128/jvi.69.4.2248-2256.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schnorr J J, Xanthakos S, Keikavoussi P, Kampge E, ter-Meulen V, Schneider-Schaulies S. Induction of maturation of human blood dendritic cell precursors by measles virus is associated with immunosuppression. Proc Natl Acad Sci USA. 1997;94:5326–5331. doi: 10.1073/pnas.94.10.5326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seya T, Hara T, Matsumoto M, Akedo H. Quantitative analysis of membrane cofactor protein (MCP) of complement: high expression of MCP on human leukemia cell lines, which is down-regulated during cell-differentiation. J Immunol. 1990;145:238–245. [PubMed] [Google Scholar]
- 40.Seya T, Kurita M, Hara T, Iwata K, Semba T, Hatanaka M, Matsumoto M, Yanagi Y, Ueda S, Nagasawa S. Blocking measles virus infection with a recombinant soluble form of, or monoclonal antibodies against, membrane cofactor protein of complement (CD46) Immunology. 1995;84:619–625. [PMC free article] [PubMed] [Google Scholar]
- 41.Seya T, Kurita M, Iwata K, Yanagi Y, Tanaka K, Shida K, Hatanaka M, Matsumoto M, Jung S, Hirano A, Ueda S, Nagasawa S. The CD46 transmembrane domain is required for efficient formation of measles-virus-mediated syncytium. Biochem J. 1997;322:135–144. doi: 10.1042/bj3220135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takeuchi K, Sato N, Kasahara H, Funayama N, Nagafuchi A, Yonemura S, Tsukita S, Tsukita S. Perturbation of cell adhesion and microvilli formation by antisense oligonucleotide to ERM family members. J Cell Biol. 1994;125:1371–1384. doi: 10.1083/jcb.125.6.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tsujimura, A., K. Shida, K. Kitamura, J. Takeda, K. Matsumiya, Y. Tanaka, M. Matsumoto, Y. Nishimune, M. Okabe, and T. Seya. Molecular cloning of murine homologue of membrane cofactor protein (MCP, CD46). Biochem. J., in press. [DOI] [PMC free article] [PubMed]
- 44.Tsukita S, Oishi K, Sato N, Sagara J, Kawai A, Tsukita S. ERM family members as molecular linkers between the cell surface glycoprotein CD44 and actin-based cytoskeletons. J Cell Biol. 1994;126:391–401. doi: 10.1083/jcb.126.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tsukita S, Yonemura S, Tsukita S. ERM proteins: head-to-tail regulation of actin-plasma membrane interaction. Trends Biochem Sci. 1997;22:53–58. doi: 10.1016/s0968-0004(96)10071-2. [DOI] [PubMed] [Google Scholar]
- 46.Tsukita S, Yonemura S, Tsukita S. ERM (ezrin/radixin/moesin) family: from cytoskeleton to signal transduction. Curr Opin Cell Biol. 1997;9:70–75. doi: 10.1016/s0955-0674(97)80154-8. [DOI] [PubMed] [Google Scholar]
- 47.Wilgenbus K K, Hsieh C L, Lankes W T, Milatovich A, Francke U, Furthmayr H. Structure and localization on the X chromosome of the gene coding for the human filopodial protein moesin. Genomics. 1994;15:326–333. doi: 10.1006/geno.1994.1065. [DOI] [PubMed] [Google Scholar]
- 48.Yanagi Y, Hu H L, Seya T, Yoshikura H. Measles virus infects mouse fibroblast cell lines, but its multiplication is severely restricted in the absence of CD46. Arch Virol. 1994;138:39–53. doi: 10.1007/BF01310037. [DOI] [PubMed] [Google Scholar]
- 49.Yant S, Hirano A, Wong T C. Identification of a cytoplasmic Tyr-X-X-Leu motif essential for down regulation of the human cell receptor CD46 in persistent measles virus infection. J Virol. 1997;71:766–770. doi: 10.1128/jvi.71.1.766-770.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yonemura, S., M. Hirao, Y. Doi, N. Takahashi, T. Kondo, S. Tsukita, and S. Tsukita. Submitted for publication.


