Abstract
Mouse hepatitis virus strain A59 (MHV-A59) produces meningoencephalitis and severe hepatitis during acute infection. Infection of primary cells derived from the central nervous system (CNS) and liver was examined to analyze the interaction of virus with individual cell types derived from the two principal sites of viral replication in vivo. In glial cell cultures derived from C57BL/6 mice, MHV-A59 produces a productive but nonlytic infection, with no evidence of cell-to-cell fusion. In contrast, in continuously cultured cells, this virus produces a lytic infection with extensive formation of syncytia. The observation of few and delayed syncytia following MHV-A59 infection of hepatocytes more closely resembles infection of glial cells than that of continuously cultured cell lines. For MHV-A59, lack of syncytium formation correlates with lack of cleavage of the fusion glycoprotein, or spike (S) protein. The absence of cell-to-cell fusion following infection of both primary cell types prompted us to examine the cleavage of the spike protein. Cleavage of S protein was below the level of detection by Western blot analysis in MHV-A59-infected hepatocytes and glial cells. Furthermore, no cleavage of this protein was detected in liver homogenates from C57BL/6 mice infected with MHV-A59. Thus, cleavage of the spike protein does not seem to be essential for entry and spread of the virus in vivo, as well as for replication in vitro.
Mouse hepatitis virus strain A59 (MHV-A59) is a murine coronavirus which is both hepatotropic and neurotropic. In the central nervous system (CNS), this virus produces an acute meningoencephalitis, as well as chronic demyelinating disease in animals which survive acute infection (13, 14). To gain a better understanding of the tissue tropism of MHV-A59, we examined replication and fusogenicity of MHV-A59 in primary cell cultures. It is reasonable to presume that infection of primary cell cultures may more accurately reflect viral replication in vivo than infection of continuously cultured cells.
MHV-A59 produces a lytic infection, with extensive formation of syncytia, in continuously cultured cell lines. However, this virus produces a productive, nonlytic infection in primary glial cell cultures, with no evidence of a virus-mediated cytopathic effect (CPE) (15). Since the CNS and the liver represent the two primary sites of infection with MHV-A59, we were interested in determining what type of infection (lytic or nonlytic) would be established by this virus in primary hepatocytes.
Primary hepatocyte cultures were established by the technique of Seglin (21). Hepatocytes were isolated from 4- to 5-week-old C57BL/6 mice by in situ perfusion of the liver with collagenase. After removal of the liver, cells were dissociated and filtered on nylon mesh (100-μm pore diameter). Centrifugation of the cell suspension through Percoll resulted in the separation of single and viable hepatocytes (parenchymal cells) from dead, aggregated hepatocytes, debris, and nonparenchymal cells (mostly Kupffer and endothelial cells [12]). The viable hepatocytes were plated on collagen-coated dishes and grown in Hepatocyte-SFM medium (Gibco BRL). The purity of hepatocytes was assessed by the distinctive large size and morphology of the cells, and the cells appeared to be nearly 100% hepatocytes by this criterion.
Confluent monolayers of hepatocytes were infected with virus at a multiplicity of infection (MOI) of 5 PFU/cell. After 1 h of adsorption at 37°C, the cells were washed three times and incubated in medium. At various times postinfection, the supernatants were removed, and the cells were lysed by three cycles of freezing and thawing. Viral titers in both supernatant and cellular fractions were determined by plaque assay on L2 cells as described previously (9).
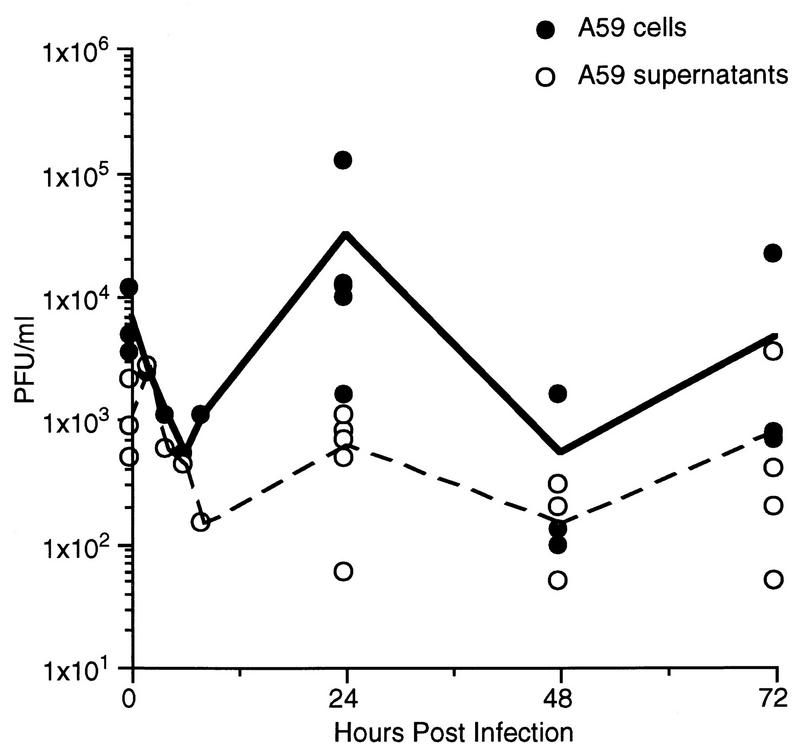
The titers of virus present in the supernatant and cellular fractions of primary cultures of hepatocytes infected with MHV-A59 are shown in Fig. 1. The data shown here are pooled from four independent experiments; each time point represents viral titers from an individual dish of hepatocytes. MHV-A59 was capable of limited replication in hepatocytes, but remained primarily cell associated. This differs from the pattern of replication seen in L2 cells or glial cells, where MHV-A59 replicates to a much greater extent and virus is released into the culture supernatant (7, 15).
FIG. 1.
Replication of MHV-A59 in primary hepatocyte cultures. Confluent monolayers of hepatocytes derived from C57BL/6 mice were infected with MHV-A59 at an MOI of 5 PFU/cell. Culture supernatants were removed, and cellular fractions were obtained by three consecutive rounds of freezing and thawing at various times postinfection. The viral titers of the cellular and supernatant fractions are shown. Each point represents the viral titer from a single 35-mm-diameter dish of MHV-A59-infected hepatocytes, while the lines are drawn between the means for each time point. Titers were determined by plaque assay on L2 cells.
Because the levels of infectious virus measured after infection of hepatocyte cultures were low (Fig. 1), we carried out immunofluorescent staining of infected cultures with an antiviral nucleocapsid protein monoclonal antibody (1.16.1; from J. Leibowitz) to determine the percentage of infected cells. At 24 h postinfection, we observed positive staining in a minority of the cells (approximately 5 to 10%) (data not shown). This suggests that the low level of infectious virus detected in hepatocyte cultures is due to virus replicating in a minority of the cells rather than virus replicating inefficiently in all of the cells. This was similar to what we have reported previously for MHV-A59-infected glial cells (15).
In continuously cultured cell lines, such as L2 or 17C1-1 cells, MHV-A59 produces syncytia within approximately 8 h postinoculation (at an MOI of 5), with extensive formation of syncytia observed by 12 h postinfection. In contrast, syncytia were not observed in MHV-A59-infected glial cell cultures, and only minimal syncytium formation was present in hepatocyte cultures and only at 48 h postinfection, after the peak of viral replication. The lack of syncytia observed in primary cells may be due to differences in the composition of the plasma membrane of these cells compared to continuously cultured cells or differences in synthesis or processing of the viral spike (S) protein, which is required for induction of cell fusion. Our observation that the processing and activity of S protein may differ for continuously cultured cells compared to primary cells is consistent with the report of Frana et al. (3a), who showed that the extent of syncytium formation varied even among continuously cultured cell lines.
MHV pathogenicity and tissue tropism are determined, at least in part, by the S protein. The S protein is an envelope glycoprotein responsible for viral attachment and virus-induced cell-to-cell fusion. For MHV, several studies have shown that viruses with mutations in the S protein are attenuated in vivo as well as in vitro, suggesting that this viral protein is an important mediator of pathogenicity (3–5). However, the importance of cell fusion in viral pathogenicity is unknown, since a defective fusion phenotype does not necessarily correlate with attenuation (9).
It has been reported that cleavage of the MHV S protein into its S1 and S2 subunits results in more efficient induction of syncytia than that induced by uncleaved S protein (1, 6, 23, 24). The relationship between cleavage of S protein and cell-to-cell fusion and the lack of syncytium formation observed in MHV-A59-infected hepatocyte or glial cell cultures suggested that the S protein may not be cleaved by these primary cells.
We postulated that lack of virus-induced CPE correlates with lack of cleavage of the S protein. To test this hypothesis, we examined the extent of cleavage of S protein by various cell types, including a continuously cultured cell line (L2 cells) and primary cultures (glial cells and hepatocytes). Cleavage of S protein was assessed by Western blot analysis of virions present in culture supernatants or lysates of infected cells. S proteins were visualized by an enhanced chemiluminescence detection system with a polyclonal goat anti-S protein antibody probe (obtained from K. Holmes). For comparison, the cleavage properties of the S protein of MHV-3, a second hepatotropic strain of MHV, were examined.
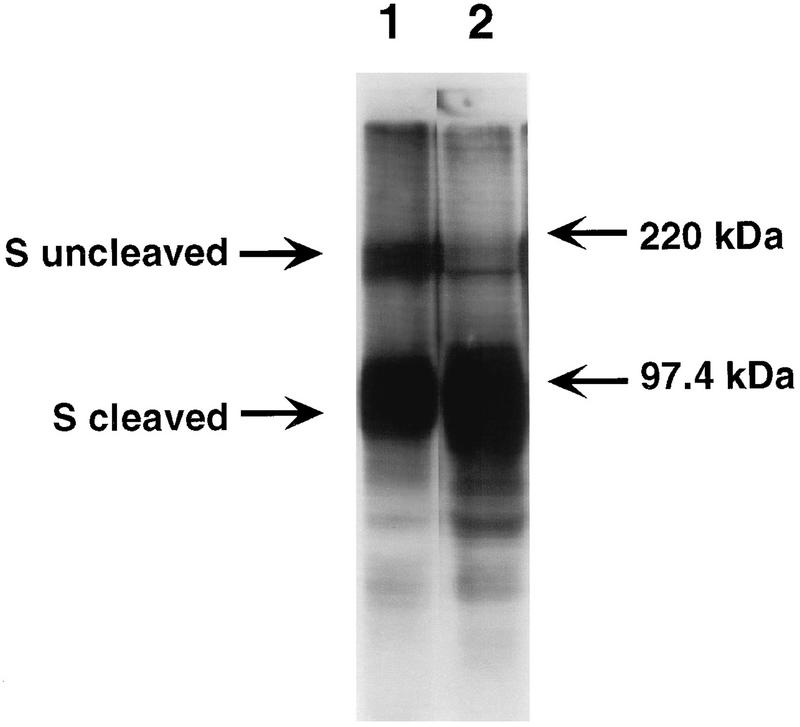
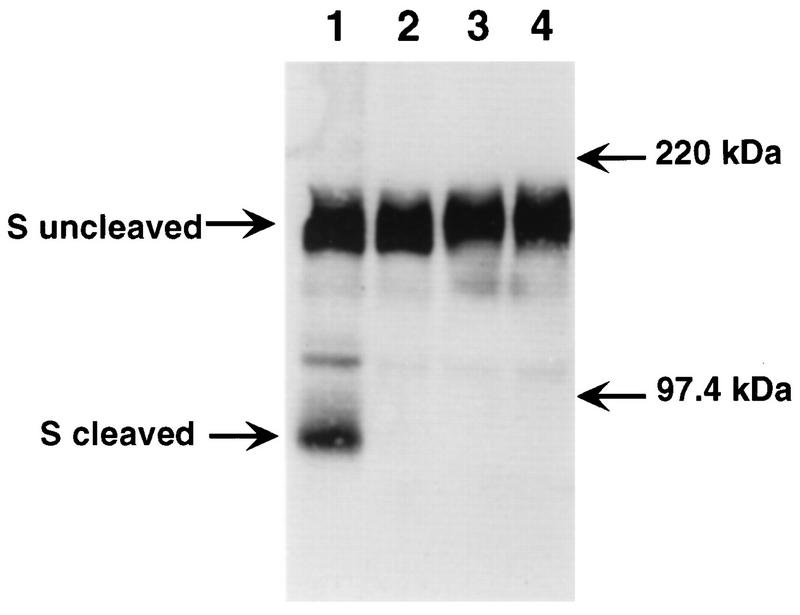
As shown in Fig. 2, the S proteins of MHV-A59 and MHV-3 were cleaved upon replication in continuously cultured L2 cells. In contrast, the MHV-A59 S protein showed no cleavage following viral replication in both hepatocyte and glial cell cultures (Fig. 3 and 4). Interestingly, the MHV-3 S protein was cleaved by primary hepatocytes, but not by glial cells.
FIG. 2.
Western blot analysis of lysates of infected fibroblasts. Proteins in lysates from MHV-A59-infected L2 cells (lane 1) and MHV-3-infected L2 cells (lane 2) were separated by electrophoresis in sodium dodecyl sulfate–8% polyacrylamide gel electrophoresis gel, transferred to nitrocellulose, and visualized by chemiluminescence (Amersham) with a polyclonal goat anti-S protein antibody (obtained from K. Holmes) as the primary antibody to detect cleaved and uncleaved forms of S protein. The migration of the molecular mass markers is indicated.
FIG. 3.
Comparison of the cleavage of S protein in fibroblasts and in glial cells. Culture supernatants of MHV-A59- and C12-infected L2 cells (lanes 1 and 2, respectively) and MHV-A59- and MHV-3-infected glial cells (lanes 3 and 4, respectively) were analyzed by sodium dodecyl sulfate–8% polyacrylamide gel electrophoresis and Western blotting as described in the legend to Fig. 2. C12 is a variant of MHV-A59 with an uncleaved S protein (6) and is included here as a negative control for cleavage. The migration of the molecular mass markers is indicated.
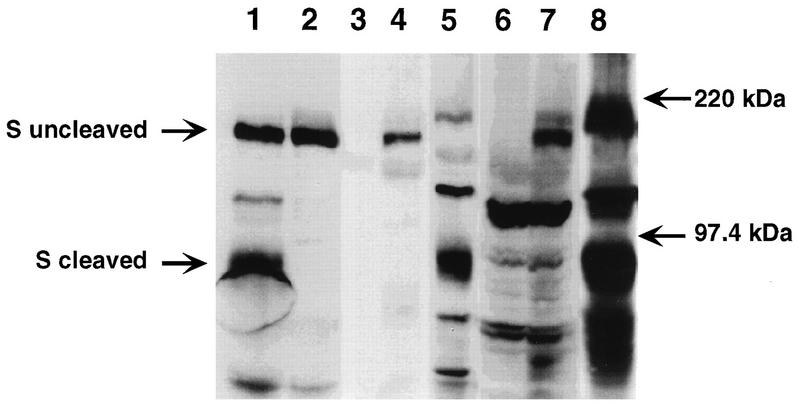
FIG. 4.
Western blot analysis of S protein present in hepatocytes and liver lysates. Culture supernatants of MHV-A59- and C12-infected L2 cells (lanes 1 and 2, respectively), cell lysates of uninfected, MHV-A59-infected, and MHV-3-infected hepatocytes (lanes 3, 4, and 5, respectively), and liver homogenates of uninfected, MHV-A59-infected, and MHV-3-infected mice (lanes 6, 7, and 8, respectively) were analyzed by sodium dodecyl sulfate–8% polyacrylamide gel electrophoresis and Western blotting as described in the legend to Fig. 2. Lanes 1 and 2 were included as positive and negative controls for cleavage, respectively. The migration of the molecular mass markers is indicated.
The data in Fig. 1 suggest that hepatocytes are not a major site of replication for MHV-A59; thus, it is likely that MHV-A59-induced hepatitis correlates with infection of nonparenchymal cells. Consistent with this are studies comparing MHV-3 and a nonhepatotropic strain of MHV (MHV-4), which demonstrated that replication in Kupffer cells and/or hepatic endothelial cells, but not hepatocytes, correlated with hepatotropism (11, 17, 18). It is possible, therefore, that cleavage of the S protein does occur in the liver in vivo as a result of infection of nonparenchymal cells. To address this question, we examined the migration of the S protein present in liver homogenates from infected animals. The results with liver homogenates were similar to those from primary hepatocyte cultures; as seen in Fig. 4, only the MHV-3 S protein was cleaved in liver homogenates from infected mice. Thus, the MHV-3 S protein is more readily cleaved by cellular proteases in livers of C57BL/6 mice than that of MHV-A59.
To maximize our ability to detect cleaved spike protein, we would have preferred to examine the state of cleavage of spike protein from virions secreted from hepatocytes rather than from cellular lysates as in Fig. 4. However, because of the low levels of virus replication (Fig. 1), this was not possible. Nevertheless, we feel that if cleaved MHV-A59 spike protein were present in virions from hepatocyte lysates or liver homogenates, we would have observed it in hepatocyte lysates, because we easily observed cleaved MHV-3 spike protein from similar samples (Fig. 4). Although we cannot completely rule out the possibility that the lack of cleavage observed in lysates from MHV-A59-infected hepatocytes is a consequence of examination of cellular proteins rather than secreted virus, we also observed no evidence of cleaved spike protein in the virion-associated spike protein from MHV-A59-infected glial cells (Fig. 2).
For many coronaviruses, the ability to induce cell-to-cell fusion correlates with cleavage of the spike protein; however, there are coronaviruses (feline infectious peritonitis virus and bovine coronavirus) that fuse cells without cleavage of S protein (2). Furthermore, the relationship between cleavage and fusion differs among various MHV strains. Syncytium formation and cleavage of the S protein both occur when continuously cultured cell lines are infected with either MHV-A59 or MHV-3. For MHV-A59, while cleavage is not necessary for fusion of continuously cultured cell lines, the kinetics of fusion of infected cells are greatly enchanced by cleavage of S protein (1, 6, 23, 24). In contrast, the S protein of the MHV-2 strain, which is not cleaved, does not induce cell fusion, even with reduced kinetics, in continuously cultured cell lines. When MHV-2-infected cells are treated with trypsin, however, some fusion of cells is observed (unpublished observations). Thus, cleavage seems to be necessary for the induction of fusion by MHV-2. The data for MHV-3 suggest that cleavage of S protein is not sufficient to allow fusion in some cell types, such as hepatocytes. Syncytium formation was not observed following MHV-3 infection of either primary cell type, despite the observation that cleavage of the S protein was observed in virions derived from infected hepatocytes and in liver homogenates from infected animals.
If cleavage of MHV S protein occurs, the spike protein is cleaved into an amino-terminal S1 subunit and a carboxy-terminal S2 subunit following a series of basic residues having a b-b-x-b-b motif, where b represents a basic amino acid (22). This cleavage motif is consistent with the substrate requirement for furin-like proteases, cellular proteases which have been shown to cleave other viral fusion glycoproteins (8, 19) and which similarly may be responsible for cleaving coronaviral spike proteins. The cleavage signal for MHV-A59 is RRAHR (16). We sequenced the entire S gene of MHV-3 and determined the cleavage signal to be RRARR (data not shown), identical to that of the JHM strain of MHV (20). The observation that the S protein of MHV-3, but not that of MHV-A59, is cleaved by hepatocytes suggests that RRARR is perhaps a better substrate than RRAHR for the cellular proteases present in the livers of C57BL/6 mice. Furthermore, cleavage of the spike protein of MHV-3 appears to be more complete than that of A59, even in fibroblast cell lines (Fig. 2). Thus, the spike protein of MHV-3 appears to be generally more susceptible to cleavage by cellular furin-like enzymes. This is consistent with the observation that mutagenesis of the MHV-A59 spike cleavage site to RRARR results in more complete cleavage (1). The lack of cleavage of the MHV-3 S protein by glial cells suggests that the cellular proteases which cleave S protein in the liver may differ from those in the CNS.
Our data suggest that cell-to-cell fusion is not an important aspect of MHV-A59 pathogenesis in vivo. The lack of syncytia observed in MHV-A59-infected hepatocyte or glial cell cultures correlated with the lack of cleavage of the S protein. The lack of cleavage of MHV-A59 S protein in virions present in liver homogenates of infected C57BL/6 mice suggests that in the livers of MHV-A59-infected animals, cell-to-cell fusion may not occur to any great extent. This observation implies that efficient cell fusion may not be required for viral virulence in vivo. Consistent with this, persistent MHV-A59 infection of glial cells selects for viruses that have a mutation in the RRAHR signal for cleavage of S protein (6). Such mutants express an uncleaved S protein and induce cell fusion inefficiently, yet still cause acute encephalitis and, in some cases, acute hepatitis (9, 10). We are currently addressing the role of cell-to-cell fusion in pathogenesis by examining the virulence of recombinant viruses containing a mutation in S protein which renders them poorly fusogenic.
Acknowledgments
This work was supported by Public Health Service grants NS-21954 and NS-30606 (S.R.W.).
REFERENCES
- 1.Bos E C W, Heijnen L, Luytjes W, Spaan W J M. Mutational analysis of the murine coronavirus spike protein: effect on cell to cell fusion. Virology. 1996;214:453–463. doi: 10.1006/viro.1995.0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cavanagh D. The coronavirus surface glycoprotein. In: Siddell S G, editor. The Coronaviridae. New York, N.Y: Plenum Press; 1995. pp. 73–113. [Google Scholar]
- 3.Fleming J O, Trousdale M D, El-Zaatari F A K, Stohlman S A, Weiner L P. Pathogenicity of antigenic variants of murine coronavirus JHM selected with monoclonal antibodies. J Virol. 1986;58:869–875. doi: 10.1128/jvi.58.3.869-875.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3a.Frana M F, Behnke J N, Sturman L S, Holmes K V. Proteolytic cleavage of the E2 glycoprotein of murine coronavirus: host-dependent differences in proteolytic cleavage and cell fusion. J Virol. 1985;56:912–920. doi: 10.1128/jvi.56.3.912-920.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gallagher T M, Escarmis C, Buchmeier M J. Alteration of pH dependence of coronavirus-induced cell fusion: effect of mutations in the spike glycoprotein. J Virol. 1991;65:1916–1928. doi: 10.1128/jvi.65.4.1916-1928.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gallagher T M, Parker S E, Buchmeier M J. Neutralization-resistant variants of a neurotropic coronavirus are generated by deletions within the amino-terminal half of the spike glycoprotein. J Virol. 1990;64:731–741. doi: 10.1128/jvi.64.2.731-741.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gombold J L, Hingley S T, Weiss S R. Fusion-defective mutants of mouse hepatitis virus A59 contain a mutation in the spike protein cleavage signal. J Virol. 1993;67:4504–4512. doi: 10.1128/jvi.67.8.4504-4512.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gombold J L, Weiss S R. Mouse hepatitis virus A59 increases steady state levels of MHC mRNAs in primary glial cell cultures and in the murine central nervous system. Microb Pathog. 1992;13:493–505. doi: 10.1016/0882-4010(92)90015-G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gotoh B, Ohnishi Y, Inocencio N M, Esaki E, Nakayama K, Barr P J, Thomas G, Nagai Y. Mammalian subtilisin-related proteinases in cleavage activation of the paramyxovirus fusion glycoprotein: superiority of furin/PACE to PC2 or PC1/PC3. J Virol. 1992;66:6391–6397. doi: 10.1128/jvi.66.11.6391-6397.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hingley S T, Gombold J L, Lavi E, Weiss S R. MHV-A59 fusion mutants are attenuated and display altered hepatotropism. Virology. 1994;200:1–10. doi: 10.1006/viro.1994.1156. [DOI] [PubMed] [Google Scholar]
- 10.Hingley S T, Gombold J L, Lavi E, Weiss S R. Hepatitis mutants of mouse hepatitis virus strain A59. Adv Exp Med Biol. 1995;380:577–582. doi: 10.1007/978-1-4615-1899-0_92. [DOI] [PubMed] [Google Scholar]
- 11.Joseph J, Kim R, Siebert K, Lublin F D, Offenbach C, Knobler R L. Organ specific endothelial cell heterogeneity influences differential replication and cytopathogenicity of MHV-3 and MHV-4. Adv Exp Med Biol. 1995;380:43–50. doi: 10.1007/978-1-4615-1899-0_6. [DOI] [PubMed] [Google Scholar]
- 12.Kreamer B L, Staecker J L, Sawada G L, Sattler G L, Hsia M T S, Pitot H C. Use of low speed, iso-density percoll centrifugation method to increase the viability of isolated rat hepatocyte preparations. In Vitro Cell Dev Biol. 1986;22:201–211. doi: 10.1007/BF02623304. [DOI] [PubMed] [Google Scholar]
- 13.Lavi E, Fishman P S, Highkin M K, Weiss S R. Limbic encephalitis after inhalation of a murine coronavirus. Lab Invest. 1988;58:31–36. [PubMed] [Google Scholar]
- 14.Lavi E, Murray E M, Makino S, Stohlman S A, Lai M M C, Weiss S R. Determinants of coronavirus MHV pathogenesis are localized to 3′ portions of the genome as determined by ribonucleic acid-ribonucleic acid recombination. Lab Invest. 1990;62:570–578. [PubMed] [Google Scholar]
- 15.Lavi E, Suzumura A, Hirayama M, Highkin M K, Dambach D M, Silberberg D H, Weiss S R. Coronavirus mouse hepatitis virus (MHV)-A59 causes a persistent, productive infection in primary glial cell cultures. Microb Pathog. 1987;3:79–86. doi: 10.1016/0882-4010(87)90066-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luytjes W, Sturman L, Bredenbeck P J, Charite J, van der Zeijst B A M, Horzinek M C, Spaan W J M. Primary structure of the glycoprotein E2 of coronavirus MHV-A59 and identification of the trypsin cleavage site. Virology. 1987;161:479–487. doi: 10.1016/0042-6822(87)90142-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin J P, Chen W, Koehren F, Pereira C A. The virulence of mouse hepatitis virus 3, as evidenced by permissivity of cultured hepatic cells toward escape mutants. Res Virol. 1994;145:297–302. doi: 10.1016/S0923-2516(07)80034-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martin J P, Chen W, Obert G, Koehren F. Characterization of attenuated mutants of MHV3: importance of the E2 protein in organ tropism and infection of isolated liver cells. Adv Exp Med Biol. 1990;276:403–410. doi: 10.1007/978-1-4684-5823-7_55. [DOI] [PubMed] [Google Scholar]
- 19.Ohnishi Y, Shioda T, Nakayama K, Iwata S, Gotoh B, Hamaguchi M, Nagai Y. A furin-defective cell line is able to process correctly the gp160 of human immunodeficiency virus type I. J Virol. 1994;68:4075–4079. doi: 10.1128/jvi.68.6.4075-4079.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmidt I, Skinner M, Siddell S. Nucleotide sequence of the gene encoding the surface projection glycoprotein of coronavirus MHV-JHM. J Gen Virol. 1987;68:47–56. doi: 10.1099/0022-1317-68-1-47. [DOI] [PubMed] [Google Scholar]
- 21.Seglin P O. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- 22.Spaan W J M, Cavanagh D, Horzinek M C. Coronaviruses. Structure and genome expression. J Gen Virol. 1988;69:2939–2952. doi: 10.1099/0022-1317-69-12-2939. [DOI] [PubMed] [Google Scholar]
- 23.Stauber R, Pfleiderer M, Siddell S G. Proteolytic cleavage of the murine coronavirus surface glycoprotein is not required for infectivity. J Gen Virol. 1993;74:183–191. doi: 10.1099/0022-1317-74-2-183. [DOI] [PubMed] [Google Scholar]
- 24.Taguchi F, Ikeda T, Shida H. Molecular cloning and expression of a spike protein of neurovirulent murine coronavirus JHMV variant cl-2. J Gen Virol. 1992;73:1065–1072. doi: 10.1099/0022-1317-73-5-1065. [DOI] [PubMed] [Google Scholar]