Abstract
Subgenomic expression plasmids for the so-called human foamy virus (HFV) structural gag, gag/pol, and env genes were constructed and used to analyze foamy virus particle formation by electron microscopy. Expression of an R-U5-gag-pol construct under control of the human cytomegalovirus immediate-early enhancer-promoter resulted in the formation of viral cores with a homogeneous size of approximately 50 nm located in the cytoplasm. Upon coexpression of an envelope construct, particles were observed budding into cytoplasmic vesicles and from the plasma membrane. Expression of the Gag protein precursor pr74 alone led to aberrantly formed viral particles of heterogeneous size and with open cores. Normal-shaped cores were seen after transfection of a construct expressing the p70gag cleavage product, indicating that p70gag is able to assemble into capsids. Coexpression of p70gag and Env resulted in budding virions, ruling out a requirement of the reverse transcriptase for capsid or virion formation. In sharp contrast to other retroviruses, the HFV cores did not spontaneously bud from cellular membranes. Radiochemical labeling followed by protein gel electrophoresis also revealed the intracellular retention of Env-deprived HFV capsids.
Foamy viruses (FVs) or Spumavirinae are a particular group of retroid viruses which have a way of replicating that is different from that of the other retroviruses and the hepadnaviruses (36, 39, 42). While the genome organization of the provirus is similar to that of the retroviruses, there are differences in the way the Pol protein is expressed (4, 10, 20, 27, 42), in the kind of functional nucleic acid in the virion (30, 42), in the structure and function of the Env protein (15, 16), and in the regulation of gene expression (7, 28). Some of these features appear to be at least partly analogous to the hepadnavirus replication strategy (35, 36).
The particle assembly of FVs also appears to differ from that of retroviruses. The primary sequence of the FV Gag protein lacks motifs, such as the major homology region in the capsid domain (CA) and the cysteine-histidine box in the nucleocapsid domain (NC), which are conserved in all retroviruses (29, 41). Instead, the FV region corresponding to NC harbors glycine-arginine boxes which are involved in nucleic acid binding and in the nuclear transport of the Gag precursor molecule (38, 43). However, the latter feature, although well conserved among primate FVs, was found not to be strictly required for the replication of the prototype human HFV (HFV) (43). In virus-producing cells, two forms of the primate FV Gag protein predominate, a pr74 and a p70 molecule (14, 18, 31). Both forms have also been found in approximately equimolar amounts in extracellular infectious virions (33). It has been shown that cleavage of the pr74gag precursor, which generates p70gag, is essential for virus replication (11). Furthermore, a protease-defective virus mutant, unable to perform the pr74-p70 cleavage, was found to lead to morphologically aberrant capsid structures (21).
The Gag protein-independent expression of the FV Pol protein from a spliced mRNA (4, 20, 42) raised the question of whether Pol is a structure-building component of FV particles or whether binding of Pol to the pregenomic RNA may be a prerequisite for particle assembly. To tackle this problem, cells were transiently transfected with subgenomic expression plasmids for the HFV gag, gag/pol, and env genes and analyzed by electron microscopy. In addition, we attempted to identify which of the two HFV Gag molecules is the major capsid-building element.
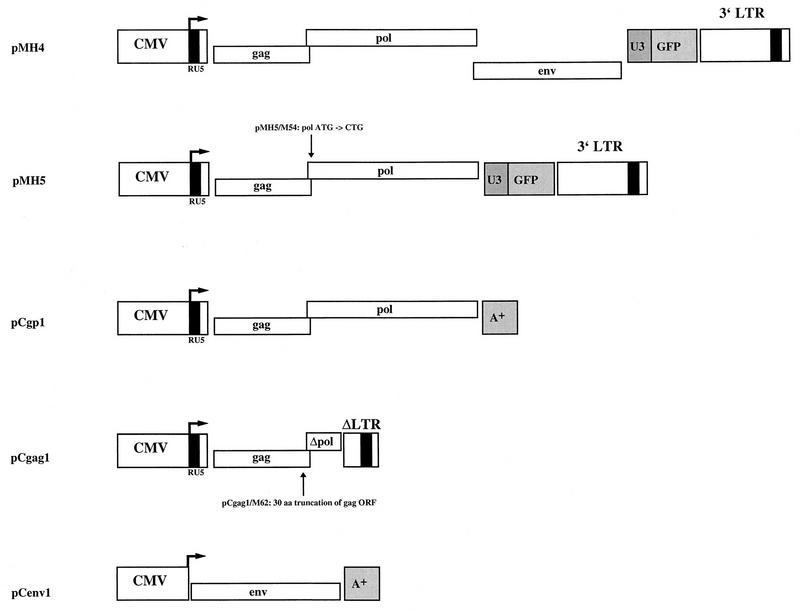
The expression plasmids shown in Fig. 1 were generated by conventional molecular cloning techniques (1, 37) in a pcDNA (Invitrogen) backbone. pMH4, pMH5, pMH5/M54, pCgp1, pCgag1, and pCgag1/M62 are human cytomegalovirus immediate-early promoter-enhancer-directed expression plasmids which use the transcriptional start of HFV as described previously for the pcHSRV2 plasmid (30). pMH4 and pMH5 harbor an internal cassette, made up of the U3 region of spleen focus-forming virus (2) driving the expression of the green fluorescent protein (26) in place of the HFV accessory reading frames (bel genes). This cassette, however, is not relevant to the topic under investigation in this study but simplifies the analysis of transfection efficiencies.
FIG. 1.
Plasmids used to analyze HFV particle formation. pMH4, pMH5, pMH5/M54, pCgp1, pCgag1, and pCgag1/M62 were derived from the infectious molecular clone pcHSRV2 (30). These plasmids use the human CMV enhancer-promoter and the transcriptional start of HFV. The pol ATG down-mutant M54 and the 30-amino-acid C-terminal gag truncation mutant M62 have been described previously (10, 11). The HFV genome in pCgag1 and pCgag1/M62 is deleted between the AflII sites in the pol gene and in the U3 region of the 3′ long terminal repeat (LTR). The internal cassette, made up of the U3 region of spleen focus-forming virus (2) directing the expression of green fluorescent protein (26), is irrelevant for this study. pCenv1 has been described earlier, where it was termed pCHFV wt (26). A+ indicates the bovine growth hormone gene poly(A) signal present in the pcDNA vector (Invitrogen). aa, amino acids; ORF, open reading frame.
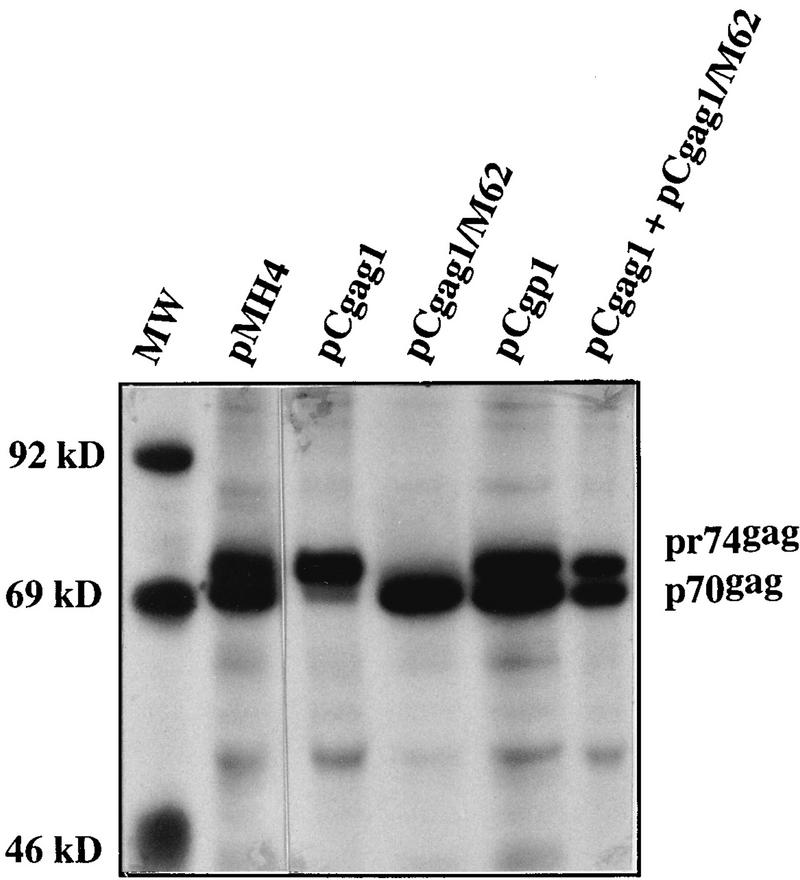
To demonstrate the correct expression of the proteins, the plasmids depicted in Fig. 1 were transiently transfected in 293T cells (8, 17, 25, 30). The cells were labeled with [35S]methionine-cysteine, cellular lysates were prepared, and after precipitation with HFV Gag-specific rabbit antiserum, the proteins were resolved by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) as described previously (11). As shown in Fig. 2, all plasmids gave rise to HFV Gag proteins of the expected sizes. The protease cleavage site in pr74gag, used to generate p70gag, was mapped roughly in a previous study (11, 34). In the mutant pCgag1/M62, a premature stop codon was introduced into the gag reading frame, resulting in a 30-amino-acid truncation of Gag (11). As shown in Fig. 2, the protein expressed from pCgag1/M62 was found to have an apparent molecular weight similar to that of the wild-type p70gag protein. We did not observe significant cleavage of the pr74gag molecule upon transfection of pCgag1 (Fig. 2). Thus, a functional protease is not expressed from this plasmid under the conditions analyzed, although the 5′ part of the pol gene is present in this construct. This is in accordance with recent findings on the inability of isolated recombinant HFV protease to cleave the Gag precursor (34). The correct expression of the Env-encoding plasmid (pCenv1) and that of the pol gene ATG down-mutant M54 have been demonstrated previously (10, 26).
FIG. 2.
Protein analysis of Gag expression constructs. pMH4 (20 μg), pCgag1 (20 μg), pCgag1/M62 (20 μg), pCgp1 (20 μg), and pCgag1 (10 μg) together with pCgag1/M62 (10 μg) were transfected into 2 × 106 293T cells by using CaPO4 (17). At 36 h after transfection, the cells were labeled metabolically with [35S]methionine-cysteine (100 μCi/ml; PRO-MIX from Amersham) for 12 h and subsequently processed for immunoprecipitation with the HFV gag-2 rabbit antiserum (3) as described previously (10, 11). Precipitated proteins were resolved by SDS–7.5% PAGE, dried, and exposed to X-ray film. pMH5 transfection gave an identical result to pCgp1 transfection (data not shown). MW, molecular mass marker.
Transfected 293T cells were fixed and processed for electron microscopy. As shown in Fig. 3, transfection of pMH4 led to the accumulation of numerous cytoplasmically located capsids that had a homogeneous size of approximately 50 nm (Fig. 3A) (13). These capsids appeared to be embedded in a dark-staining matrix structure of unknown origin. In addition, particles budding into intracytoplasmic vesicles or from the cytoplasmic membrane were observed (Fig. 3B and data not shown). A similar result was obtained when pCgp1 or pMH5 was transfected alone, resulting in capsids located in the cytoplasm (Fig. 3C), or was transfected together with pCenv1, resulting in capsids and budding virions (Fig. 3D).
FIG. 3.
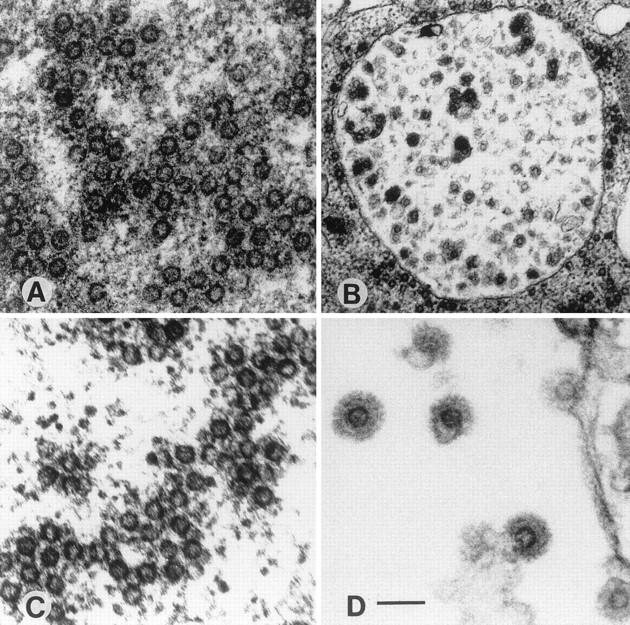
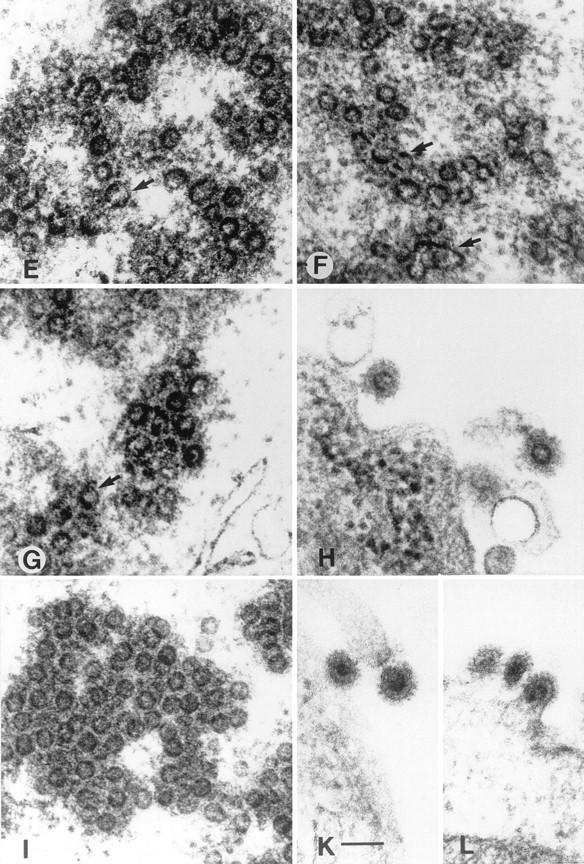
Electron micrographs of 293T cells transfected with the expression constructs pMH4 (A and B), pCgp1 (C), pCgp1 plus pCenv1 (D), pMH5/M54 (E), pCgag1 (F and G), pCgag1 plus pCenv1 (H), pCgag1/M62 (I), and pCgag1/M62 plus pCenv1 (K and L). The arrows indicate horseshoe-like structures often found in cells expressing only p74gag (E, F, and G) and a huge (120-nm-diameter) particulate structure (F). 293T cells were transfected as described in the legend to Fig. 2 with a total amount of 20 μg of DNA. Cotransfections with two plasmids were done with 10 μg of each DNA. At 48 h after transfection, the cells were washed with phosphate-buffered saline (PBS), fixed in cold 2.5% glutaraldehyde in PBS, and collected by low-speed centrifugation. After staining with 2% osmium tetroxide and 0.5% lead citrate, the cells were embedded in Epon 812 and further processed for ultrathin sectioning. pMH5 transfection gave an identical result to pCgp1 transfection, as shown in panel C. The micrographs were viewed under a Zeiss EM-109. Magnifications, ×33,000 (B) and ×100,000 (all other panels). Bars, 100 nm.
The transfection of pCgag1 resulted in morphologically altered structures (Fig. 3E and F). These particles were heterogeneous in size, ranging from 50 to 120 nm. Furthermore, the structures were often found to have a defect in circularization and presented as horseshoe-like open core structures. Similar abberantly formed particles were observed when the pol gene ATG down-mutant M54 was analyzed in the pMH5 background (Fig. 3G). Cotransfection of pCgag1 with pCenv1 led to budding particles with glycoprotein spike projections incorporated into the virus membrane (Fig. 3H). The transfection of pCgag1/M62 resulted in capsids with a wild-type morphology (Fig. 3I). Quantitation revealed that 19% (52 of 274) of the particulate structures in pCgp1-transfected cells showed an abnormal morphology, while 24% (90 of 324) and 92% (220 of 240) of the capsids in pCgag1/M62- and pCgag1-transfected cells, respectively, showed abnormal formations. The capsids in pCgag1/M62-transfected cells were located solely in the cytoplasm and were never observed to bud from any kind of cellular membrane. When pCgag1/M62 was cotransfected with pCenv1, enveloped particles with a regular spike morphology were observed (Fig. 3K and L).
These results demonstrate that the expression of the HFV p70gag molecule is necessary and sufficient for the formation of capsids. The Pol protein of HFV was found not to be required as a structural component for the initiation of capsid formation. The HFV Pol precursor protein is essential for capsid formation only because it cleaves pr74gag, which leads to the generation of the p70gag species. The p70gag molecule was also able to interact with the HFV Env protein, since budding virions with glycoprotein spikes were observed upon cotransfection of an Env-expressing plasmid. Furthermore, in contrast to all known exogenous retroviruses, where the capsids bud from the cytoplasmic membrane regardless of the presence of an Env protein (5, 9), in HFV, the Env protein appeared to be required for this process, at least in 293T cells.
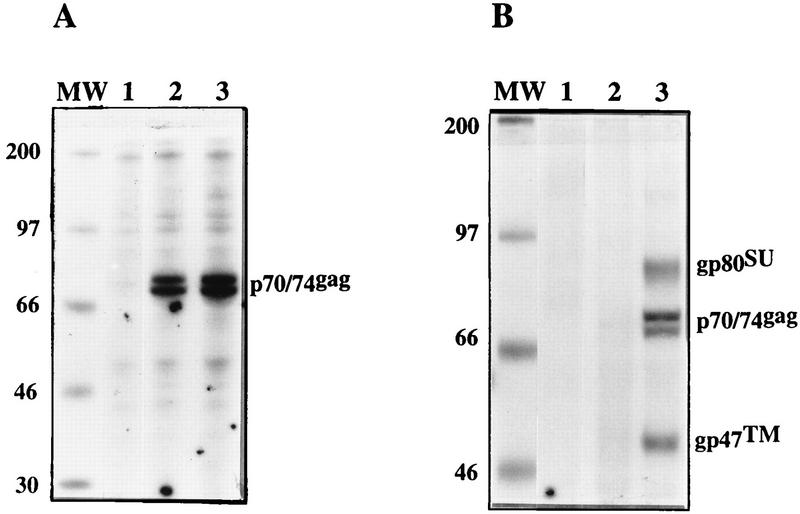
To corroborate the latter finding by a different experimental method, the supernatant of 293T cells transiently transfected with different constructs was analyzed for the presence of viral proteins. The cells were transfected with pCgp1 alone or with pCgp1 plus pCenv1. Intracellular proteins were precipitated with HFV Gag-specific serum (11), while the supernatant of the transfected cells was centrifuged through a sucrose cushion and the resulting virus pellet was analyzed directly by SDS-PAGE. As shown in Fig. 4, transfection of pCgp1 and of pCgp1 plus pCenv1 gave rise to similar amounts of intracellular Gag proteins. However, only in cells transfected with pCgp1 plus pCenv1 were viral particles detected in the supernatant, which is consistent with the result obtained by ultrastructural analysis.
FIG. 4.
SDS-PAGE of HFV proteins from 293T cells transfected with pCgp1 or with pCgp1 plus pCenv1. The cells were transfected with pcDNA (20 μg) (lanes 1), pCgp1 (10 μg) plus pcDNA (10 μg) (lanes 2), or pCgp1 (10 μg) plus pCenv1 (10 μg) (lanes 3). Lanes MW contain molecular mass markers (masses are shown in kilodaltons on the left). (A) Following metabolical labeling, a cellular lysate was prepared and the HFV Gag proteins were precipitated with Gag-specific antiserum (3, 11). (B) The supernatant of transfected cells (2 ml) was centrifuged through a 20% sucrose cushion (3 h at 25,000 rpm in an SW41 rotor at 4°C), and the pellet was resuspended in detergent buffer, boiled, and loaded directly onto the gel.
As mentioned above, the replication strategy of FVs differs from the classical retroviral replication strategy in several ways. The results reported here demonstrate an additional difference in the method of virus particle generation. In exogenous retroviruses, the matrix (MA) domains of the Gag proteins harbor sequences which mediate the interaction of the precursor proteins with the cytoplasmic membrane and promote the release of viral particles in the absence of Env (9, 22). Env-independent particle release of exogenous retroviruses has been found by using many different expression systems and cell types (5). The mechanism does not require N-terminal myristoylation of MA and is also independent of the type of ultrastructural particle morphology (22). In the case of intracisternal A-type particles, (IAPs), the situation is somewhat different. IAPs lack a functional Env and assemble on membranes of the endoplasmic reticulum (ER) and bud into ER-derived cisternae (23, 40). Again, the interaction of IAP capsids with the ER membrane is mediated by a motif located in the MA domain (23, 40). FVs are also known to bud into ER-derived vesicles. An ER retention signal, located at the C terminus of the Env precursor, has been identified to be at least partly responsible for this (15, 16). Nonetheless, one might assume that Env-deprived FV capsids behave like IAPs with preassembled cores. However, upon an extensive search of 293T cells transfected with pCgag1/M62 or pCgp1, we did not observe any HFV particles that budded spontaneously into the ER or from any other cellular membrane.
The inability of FVs to release particles without the coexpression of Env protein is another feature that distinguishes this retrovirus subgroup from the other retroviruses. It again points to a similarity with hepadnaviruses (6, 12, 19, 24, 32); further investigations on the Gag-Env interaction will be required to elucidate more precisely the FV replication strategy.
Acknowledgments
We are indebted to Ian Johnston for critically reading the manuscript.
This work was supported by grants from the DFG (SFB165), BMBF, EU, and Bayerische Forschungsstiftung. D.L. is supported by the Virology fellowship program of the BMBF.
REFERENCES
- 1.Ausubel F M, Brent R, Kingston R E, Moore D, Seidman J G, Smith J A, Struhl K. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1987. [Google Scholar]
- 2.Baum C, Hegewisch-Becker S, Eckert H-G, Stocking C, Ostertag W. Novel retroviral vectors for efficient expression of the multidrug resistance (mdr-1) gene in early hematopoietic cells. J Virol. 1995;69:7541–7547. doi: 10.1128/jvi.69.12.7541-7547.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baunach G, Maurer B, Hahn H, Kranz M, Rethwilm A. Functional analysis of human foamy virus accessory reading frames. J Virol. 1993;67:5411–5418. doi: 10.1128/jvi.67.9.5411-5418.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bodem J, Löchelt M, Winkler I, Flower R P, Delius H, Flügel R M. Characterization of the spliced pol transcript of feline foamy virus: the splice acceptor site of the pol transcript is located in gag of foamy viruses. J Virol. 1996;70:9024–9027. doi: 10.1128/jvi.70.12.9024-9027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boulanger P, Jones I. Use of heterologous expression systems to study retroviral morphogenesis. Curr Top Microbiol Immunol. 1996;214:237–260. doi: 10.1007/978-3-642-80145-7_8. [DOI] [PubMed] [Google Scholar]
- 6.Bruss V, Ganem D. The role of envelope proteins in hepatitis B virus assembly. Proc Natl Acad Sci USA. 1991;88:1059–1063. doi: 10.1073/pnas.88.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campbell M, Eng C, Luciw P A. The simian foamy virus type 1 transcriptional transactivator (Tas) binds and activates an enhancer element in the gag gene. J Virol. 1996;70:6847–6855. doi: 10.1128/jvi.70.10.6847-6855.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DuBridge R B, Tang P, Hsia H C, Leong P-M, Miller J H, Calos M P. Analysis of mutation in human cells by using an Epstein-Barr virus shuttle system. Mol Cell Biol. 1987;7:379–387. doi: 10.1128/mcb.7.1.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Einfeld D. Maturation and assembly of retroviral glycoproteins. Curr Top Microbiol Immunol. 1996;214:133–176. doi: 10.1007/978-3-642-80145-7_5. [DOI] [PubMed] [Google Scholar]
- 10.Enssle J, Jordan I, Mauer B, Rethwilm A. Foamy virus reverse transcriptase is expressed independently from the gag protein. Proc Natl Acad Sci USA. 1996;93:4137–4141. doi: 10.1073/pnas.93.9.4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Enssle J, Fischer N, Moebes A, Mauer B, Smola U, Rethwilm A. Carboxy-terminal cleavage of the human foamy virus Gag precursor molecule is an essential step in the viral life cycle. J Virol. 1997;71:7312–7317. doi: 10.1128/jvi.71.10.7312-7317.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ganem D. Assembly of hepadnaviral virions and subviral particles. Curr Top Microbiol Immunol. 1991;168:62–83. doi: 10.1007/978-3-642-76015-0_4. [DOI] [PubMed] [Google Scholar]
- 13.Gelderblom H, Frank H. Spumavirinae. In: Nermut M V, Steven A C, editors. Animal virus structure. Amsterdam, The Netherlands: Elsevier; 1987. pp. 305–311. [Google Scholar]
- 14.Giron M-L, Colas S, Wybier J, Rozain F, Emanoil-Ravier R. Expression and maturation of human foamy virus Gag precursor polypeptides. J Virol. 1997;71:1635–1639. doi: 10.1128/jvi.71.2.1635-1639.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goepfert P A, Wang G, Mulligan M J. Identification of an ER retrieval signal in a retroviral glycoprotein. Cell. 1995;82:543–544. doi: 10.1016/0092-8674(95)90026-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goepfert P A, Shaw K L, Ritter G D J, Mulligan M J. A sorting motif localizes the foamy virus glycoprotein to the endoplasmic reticulum. J Virol. 1997;71:778–784. doi: 10.1128/jvi.71.1.778-784.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Graham F, van der Eb A. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973;52:456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- 18.Hahn H, Baunach G, Bräutigam S, Mergia A, Neumann-Haefelin D, Daniel M D, McClure M O, Rethwilm A. Reactivity of primate sera to foamy virus gag and bet proteins. J Gen Virol. 1994;75:2635–2644. doi: 10.1099/0022-1317-75-10-2635. [DOI] [PubMed] [Google Scholar]
- 19.Hirsch R, Lavine J E, Chang L, Varmus H E, Ganem D. Polymerase gene products of hepatitis B viruses are required for genomic RNA packaging as well as for reverse transcription. Nature. 1990;344:552–555. doi: 10.1038/344552a0. [DOI] [PubMed] [Google Scholar]
- 20.Jordan I, Enssle J, Güttler E, Mauer B, Rethwilm A. Expression of human foamy virus reverse transcriptase involves a spliced pol mRNA. Virology. 1996;224:314–319. doi: 10.1006/viro.1996.0534. [DOI] [PubMed] [Google Scholar]
- 21.Konvalinka J, Löchelt M, Zentgraf H, Flügel R M, Kräusslich H-G. Active foamy virus proteinase is essential for virus infectivity but not for formation of a Pol polyprotein. J Virol. 1995;69:7264–7268. doi: 10.1128/jvi.69.11.7264-7268.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kräusslich H-G, Welker R. Intracellular transport of retroviral capsid proteins. Curr Top Microbiol Immunol. 1996;214:25–63. doi: 10.1007/978-3-642-80145-7_2. [DOI] [PubMed] [Google Scholar]
- 23.Kuff E L, Lueders K K. The intracisternal A particle gene family: structure and functional aspects. Adv Cancer Res. 1988;51:183–276. doi: 10.1016/s0065-230x(08)60223-7. [DOI] [PubMed] [Google Scholar]
- 24.Kuroki K, Russnak R, Ganem D. Novel N-terminal amino acid sequence required for retention of a hepatitis B virus glycoprotein in the endoplasmic reticulum. Mol Cell Biol. 1989;9:4459–4466. doi: 10.1128/mcb.9.10.4459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lindemann, D., and A. Rethwilm. Characterization of a human foamy virus 170 kD Env-Bet fusion protein generated by alternative splicing. Submitted for publication. [DOI] [PMC free article] [PubMed]
- 26.Lindemann D, Bock M, Schweizer M, Rethwilm A. Efficient pseudotyping of murine leukemia virus particles with chimeric human foamy virus envelope proteins. J Virol. 1997;71:4815–4820. doi: 10.1128/jvi.71.6.4815-4820.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Löchelt M, Flügel R M. The human foamy virus pol gene is expressed as a Pro-Pol polyprotein and not as a Gag-Pol fusion protein. J Virol. 1996;70:1033–1040. doi: 10.1128/jvi.70.2.1033-1040.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Löchelt M, Muranyi W, Flügel R M. Human foamy virus possesses an internal, bel-1-dependent and functional promoter. Proc Natl Acad Sci USA. 1993;90:7317–7321. doi: 10.1073/pnas.90.15.7317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maurer B, Bannert H, Darai G, Flügel R M. Analysis of the primary structure of the long terminal repeat and the gag and pol genes of the human spumaretrovirus. J Virol. 1988;62:1590–1597. doi: 10.1128/jvi.62.5.1590-1597.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moebes A, Enssle J, Bieniasz P D, Heinkelein M, Lindemann D, Bock M, McClure M O, Rethwilm A. Human foamy virus reverse transcription that occurs late in the viral replication cycle. J Virol. 1997;71:7305–7311. doi: 10.1128/jvi.71.10.7305-7311.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morozov V A, Copeland T D, Nagashima K, Gonda M A, Oroszlan S. Protein composition and morphology of human foamy virus intracellular cores and extracellular particles. Virology. 1997;228:307–317. doi: 10.1006/viro.1996.8379. [DOI] [PubMed] [Google Scholar]
- 32.Nassal M, Schaller H. Hepatitis B virus replication. Trends Microbiol. 1993;1:221–228. doi: 10.1016/0966-842x(93)90136-f. [DOI] [PubMed] [Google Scholar]
- 33.Netzer K-O, Rethwilm A, Maurer B, ter Meulen V. Identification of the major immunogenic structural proteins of human foamy virus. J Gen Virol. 1990;71:1237–1241. doi: 10.1099/0022-1317-71-5-1237. [DOI] [PubMed] [Google Scholar]
- 34.Pfrepper K-I, Löchelt M, Schnölzer M, Flügel R M. Expression and molecular characterization of an enzymatically active recombinant human spumaretrovirus protease. Biochem Biophys Res Commun. 1997;237:548–553. doi: 10.1006/bbrc.1997.7187. [DOI] [PubMed] [Google Scholar]
- 35.Rethwilm A. Regulation of foamy virus gene expression. Curr Top Microbiol Immunol. 1995;193:1–24. doi: 10.1007/978-3-642-78929-8_1. [DOI] [PubMed] [Google Scholar]
- 36.Rethwilm, A. 1996. Unexpected replication pathways of foamy viruses. J. Acquired Immune Defic. Syndr. Hum. Retrovirol. 13(Suppl. 1):S248–S253. [DOI] [PubMed]
- 37.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 38.Schliephake A W, Rethwilm A. Nuclear localization of foamy virus Gag precursor protein. J Virol. 1994;68:4946–4954. doi: 10.1128/jvi.68.8.4946-4954.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weiss R A. Foamy viruses bubble on. Nature. 1996;380:201. doi: 10.1038/380201a0. [DOI] [PubMed] [Google Scholar]
- 40.Welker R, Janetzko A, Kräusslich H-G. Plasma membrane targeting of chimeric intracisternal A-type particle polyproteins leads to particle release and specific activation of the viral proteinase. J Virol. 1997;71:5209–5217. doi: 10.1128/jvi.71.7.5209-5217.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wills J W, Craven R C. Form, function, and use of retroviral gag proteins. AIDS. 1991;5:639–654. doi: 10.1097/00002030-199106000-00002. [DOI] [PubMed] [Google Scholar]
- 42.Yu S F, Baldwin D N, Gwynn S R, Yendapalli S, Linial M L. Human foamy virus replication: a pathway distinct from that of retroviruses and hepadnaviruses. Science. 1996;271:1579–1582. doi: 10.1126/science.271.5255.1579. [DOI] [PubMed] [Google Scholar]
- 43.Yu S F, Edelmann K, Strong R K, Moebes A, Rethwilm A, Linial M L. The carboxyl terminus of the human foamy virus Gag protein contains separable nucleic acid binding and nuclear transport domains. J Virol. 1996;70:8255–8262. doi: 10.1128/jvi.70.12.8255-8262.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]