Abstract
Studies conducted some 50 years ago showed that serial intracerebral passage of dengue viruses in mice selected for neurovirulent mutants that also exhibited significant attenuation for humans. We investigated the genetic basis of mouse neurovirulence of dengue virus because it might be directly or indirectly associated with attenuation for humans. Analysis of the sequence in the C-PreM-E-NS1 region of the parental dengue type 2 virus (DEN2) New Guinea C (NGC) strain and its mouse-adapted, neurovirulent mutant revealed that 10 nucleotide changes occurred during serial passage in mice. Seven of these changes resulted in amino acid substitutions, i.e., Leu55-Phe and Arg57-Lys in PreM, Glu71-Asp, Glu126-Lys, Phe402-Ile, and Thr454-Ile in E, and Arg105-Gln in NS1. The sequence of C was fully conserved between the parental and mutant DEN2. We constructed intertypic chimeric dengue viruses that contained the PreM-E genes or only the NS1 gene of neurovirulent DEN2 NGC substituting for the corresponding genes of DEN4. The DEN2 (PreM-E)/DEN4 chimera was neurovirulent for mice, whereas DEN2 (NS1)/DEN4 was not. The mutations present in the neurovirulent DEN2 PreM-E genes were then substituted singly or in combination into the sequence of the nonneurovirulent, parental DEN2. Intracerebral titration of the various mutant chimeras so produced identified two amino acid changes, namely, Glu71-Asp and Glu126-Lys, in DEN2 E as being responsible for mouse neurovirulence. The conservative amino acid change of Glu71-Asp probably had a minor effect, if any. The Glu126-Lys substitution in DEN2 E, representing a change from a negatively charged amino acid to a positively charged amino acid, most likely plays an important role in conferring mouse neurovirulence.
Among many members of the arthropod-borne flaviviruses, the four dengue virus serotypes (dengue virus types 1 to 4 [DEN1 to -4]) are most important in terms of human morbidity, which is estimated to involve millions of individuals every year (23). Dengue virus outbreaks and epidemics are a major public health problem in tropical and subtropical regions, where the Aedes aegypti and Aedes albopictus mosquito vectors are abundant. For this reason, the World Health Organization has assigned a high priority to the development of a dengue virus vaccine. Several groups of investigators working on different dengue virus serotypes or different strains of a serotype have reported that serial passage in primary dog kidney cells yielded dengue viruses that apparently exhibited attenuation or reduction of virulence in humans (1, 8). However, development of dengue virus vaccines by this approach still remains at the experimental stage. Shortly after DEN1 and DEN2 were first identified in human blood specimens more than 50 years ago, serial passage of these viruses in mouse brain was performed to recover virus in the laboratory and to adapt it to grow to a high titer in mice (16, 29). This process selected for dengue virus mutants that were neurovirulent for mice that also proved to be significantly attenuated for susceptible humans (27, 28, 34). This approach to dengue virus vaccine development was not pursued further because extensive virus purification would be required to remove contaminating mouse brain antigens from the vaccine preparation. Nevertheless, these mouse-adapted, neurovirulent dengue virus mutants could be used to identify mutations responsible for mouse neurovirulence that might also cause attenuation for humans.
The dengue virus-positive strand RNA genome codes for three virion structural proteins, i.e., the capsid protein (C), the precursor membrane protein (PreM), and the envelope protein (E), and a series of nonstructural proteins (designated NS1 to NS5) that are not present in the virion. There is evidence indicating that PreM and E form a heterodimer in which PreM serves to protect E present in the immature virions during intracellular transport through post-Golgi acidic vesicles (12). Subsequently, proteolytic cleavage of PreM takes place, generating the M protein of the mature virion prior to its exit from the infected cell (31, 32). E is the major virus surface antigen and is thought to be responsible for viral entry by binding to cell receptors, followed by fusion with the cell membranes and subsequent entry into the host cell (10, 11, 14). Both E and M (or its precursor, PreM) structural proteins are capable of mediating protective immune responses in the infected host (3, 6). Dengue virus nonstructural protein NS1 is also a protective antigen, presumably through its role in complement-dependent lysis of virus-infected cells (9, 30). In addition, NS1 is thought to play a role in viral RNA replication because it has been detected in association with the RNA replication complex and certain mutations in NS1 severely reduce the level of viral RNA replication (22, 24).
Previously, we constructed intertypic dengue virus chimeras by substituting the C-PreM-E structural protein genes of the mouse-adapted, neurovirulent DEN2 mutant for the corresponding genes in full-length DEN4 DNA (4). When analyzed by intracerebral titration in mice, the DEN2 (C-PreM-E)/DEN4 chimera retained most of the mouse neurovirulence of the DEN2 mutant, as measured by 50% lethal dose (LD50) (4, 5). These observations suggest that major genetic determinants responsible for DEN2 mouse neurovirulence are located in the DEN2 C-PreM-E genes.
The present study was initiated to determine the amino acid changes in the mouse-adapted, neurovirulent mutant that are responsible for acquisition of mouse neurovirulence. For this purpose, we constructed intertypic chimeras of DEN4 that contained sequences for the following: (i) the virion structural proteins of a mouse neurovirulent DEN2 mutant or its non-mouse-adapted parental DEN2; or (ii) the structural proteins or the NS1 nonstructural protein of the parental nonneurovirulent DEN2 into which one or more of the mutant amino acids of the neurovirulent DEN2 mutant were substituted for the corresponding parental sequence. The neurovirulence of these chimeras was then analyzed by intracerebral inoculation in mice to identify the genetic determinants of mouse neurovirulence.
The parental DEN2 New Guinea C (NGC) strain (DEN2-P) at passage zero was recovered from mosquito C6/36 cells inoculated with the serum of a monkey that had been inoculated with the serum of the patient from whom the virus was originally recovered. This monkey serum was kindly provided by L. Rosen (University of Hawaii, Honolulu). A mouse brain preparation of a mouse neurovirulent DEN2 mutant, at mouse passage 38, was kindly provided by K. Eckels (Walter Reed Army Institute of Research, Washington, D.C.). This mouse neurovirulent mutant, designated DEN2-N, was passaged once in C6/36 cells before being used in the present study. Reverse transcription of virion RNA and PCR to produce cDNA corresponding to the C-PreM-E genes of DEN2-N was described earlier (4). The same procedure was performed to prepare the C-PreM-E cDNA of DEN2-P. Following cleavage with BglII and XhoI, the DEN2-P C-PreM-E DNA fragment was cloned into the plasmid p5-′2 (XhoI) vector. Similarly, a cDNA fragment which begins with the XhoI site near the end of the E gene and terminates at a PstI site introduced at the end of the NS1 gene was prepared by PCR by using appropriate primers. The cDNA fragment which contained the entire DEN2-N NS1 gene was also cloned in the p5′-2 (XhoI) vector or the p5′-2 chimeric DEN2 (C-PreM-E)/DEN4 intermediate vector replacing the DEN4 NS1 sequence to prepare the DEN2 (C-PreM-E-NS1)/DEN4 chimeric full-length construct. Construction of various chimeras containing the indicated DEN2 sequences and DEN4 strain 814669 cDNA was described earlier (20).
The nucleotide sequence of the C-PreM-E-NS1 genes of DEN2-P or DEN2-N was determined by using the Sequenase 2.0 DNA sequencing kit (Amersham Life Science, Cleveland, Ohio). The sequence of the DEN2-N C-PreM-E-NS1 genes was identical to the published sequence (17). Eight nucleotide changes in the C-PreM-E region occurred during passage of DEN2-P in mice, six of which resulted in amino acid substitutions (Table 1). In the neurovirulent mutant, there were two substitutions in PreM, i.e., Leu55-Phe and Arg57-Lys, and four substitutions in E, i.e., Glu71-Asp, Glu126-Lys, Phe402-Ile, and Thr454-Ile. These substitutions were designated sites 1 to 6, respectively, for ease of reference (Fig. 1). The amino acid sequence of the C protein was fully conserved between the parent and mutant strains. In the nonstructural NS1 gene region, two nucleotide changes were identified in the neurovirulent mutant. One of the changes produced an Arg-to-Gln mutation at amino acid position 105 in NS1, and the other was a silent mutation (Table 1).
TABLE 1.
Comparison of the nucleotide and amino acid sequences of the structural protein and nonstructural NS1 protein gene region of the parental DEN2 NGC strain and its mouse-adapted neurovirulent mutant
| Position | Nucleotide
|
Position in protein | Amino acid
|
||
|---|---|---|---|---|---|
| Parent | Mutant | Parent | Mutant | ||
| 601 | CTT | TTT | PreM55 | Leu | Phe |
| 608 | AGG | AAG | PreM57 | Arg | Lys |
| 1149 | GAA | GAT | E71 | Glu | Asp |
| 1176 | CCT | CCC | E80 | Pro | Pro |
| 1255 | TTA | CTA | E107 | Leu | Leu |
| 1312 | GAA | AAA | E126 | Glu | Lys |
| 2140 | TTT | ATT | E402 | Phe | Ile |
| 2297 | ACT | ATT | E454 | Thr | Ile |
| 2735 | CGG | CAG | NS1105 | Arg | Gln |
| 3130 | CTA | TTA | NS1277 | Leu | Leu |
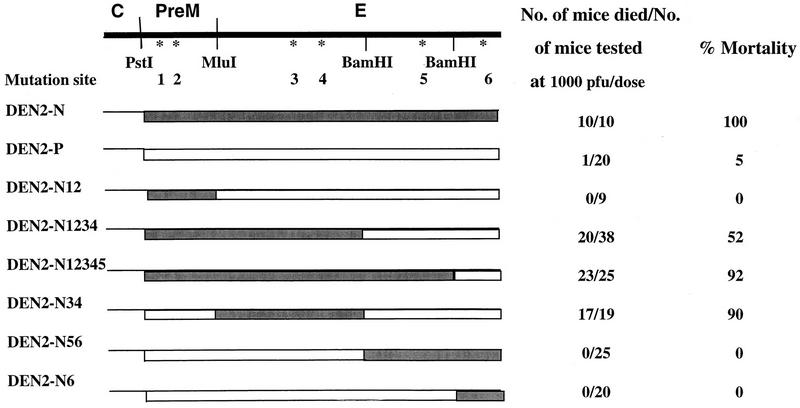
FIG. 1.
Plasmid constructs of chimeric DEN2 (PreM-E)/DEN4 viruses and analysis of neurovirulence in suckling mice. Chimeric cDNAs that contained the PreM-E genes of DEN2-P or DEN2-N, or their derived sequences, were constructed by using the PstI site previously introduced at the end of the DEN4 C gene for joining with the DEN2 DNA fragment cleaved at the PstI site (nucleotide [nt] 400) and the XhoI site near the end of the DEN2 E sequence. A new MluI site was introduced at nt 888, generating a conservative Ala266-Val substitution at the hydrophobic N terminus of E. In addition, two BamHI sites at nt 1696 and 2203 were also chosen to generate DEN2-N PreM-E DNA subfragments to substitute for the corresponding DEN2-P DNA sequences in the series of chimeras containing amino acid substitutions in the PreM-E polyprotein. The procedure to construct chimeric cDNAs from p5′-2 (XhoI) that contained the cloned DEN2 PreM-E genes and p3′-A that contained most of the remaining DEN4 DNA sequences was essentially as described earlier (4). Transcription of Asp718 linearized chimeric cDNA and transfection of mosquito C6/36 cells with the RNA transcripts were done as described previously (20). One week after transfection, the cells were transferred to a T75 flask and also to a chamber slide. An indirect immunofluorescence assay was performed with cells in the chamber slide to monitor the fraction of cells infected with progeny virus. Virus was harvested from the medium fluid, and titers were determined on monolayers of C6/36 cells, when 80% or more of cells showed positive fluorescence. Chimeras containing the PreM-E of DEN2-N or DEN2-P or derived mutants were recovered readily, reaching 106 PFU/ml or more 10 to 12 days after transfection. The initial harvest of the chimera containing DEN2 NS1 was obtained under the same condition. This chimera was subsequently amplified once in C6/36 cells to give a titer of 2 × 107 PFU/ml. The series of intertypic dengue chimeric viruses, the parental nonneurovirulent DEN2, and the mouse-adapted neurovirulent DEN2 mutant were analyzed for neurovirulence in mice by intracerebral inoculation (4, 18).
Since the sequence of C was fully conserved between DEN2-P and DEN2-N, the site of mouse neurovirulence mutations would be expected to reside within PreM and/or E and/or NS1. Thus, only intertypic chimeras involving the PreM-E or NS1 gene were constructed. Viruses with the chimeric PreM-E gene constellation were recovered from simian LLC-MK2 cells transfected with the RNA transcripts of full-length cDNA. To further map the mutation site in the PreM-E region of DEN2-N, a series of chimeric mutants was constructed; they contained a DNA subfragment of DEN2-N substituted into the DEN2-P sequence in the DEN2-P (PreM-E)/ DEN4 chimera. When constructed in this manner, the chimeras contained either single or multiple amino acid substitutions representing the mutations in the DEN2-N PreM-E genes. Specifically, chimera DEN2-N12/DEN4 contained substitutions Leu55-Phe (site 1) and Arg57-Lys (site 2) present in the PreM of DEN2-N. Chimera DEN2-N34/DEN4 contained the amino acid substitutions Glu71-Asp at site 3 and Glu126-Lys at site 4 in E. Chimera DEN2-N56/DEN4 contained the substitutions Phe402-Ile (site 5) and Thr454-Ile (site 6) in E of DEN2-N, whereas chimera DEN2-N6/DEN4 contained a single amino acid substitution at site 6. Chimera DEN2-N1234/DEN4 contained both substitutions in PreM plus substitutions at sites 3 and 4 in E of DEN2-N. Likewise, chimera DEN2-N12345/DEN4 contained five amino acid substitutions in PreM and E at the indicated sites (Fig. 1). Figure 1 shows the site of the amino acid substitutions in the chimeric DEN2-P (PreM-E)/DEN4 cDNA constructs. Prior to transcription of RNA, each of the full-length cDNA constructs was sequenced to verify the presence of the mutation site(s). Progeny of each of the chimeric constructs was recovered from the medium of the RNA-transfected LLC-MK2 cells, and the titer of each was determined on the mosquito C6/36 cells.
The series of progeny chimeras were analyzed for mouse neurovirulence by intracerebral inoculation in 3-day-old outbred Swiss mice (Fig. 1). As predicted, chimera DEN2-P/DEN4, which contained the DEN2-P PreM-E genes, was not neurovirulent for mice: 19 of 20 inoculated mice remained healthy following inoculation with 1,000 PFU of the chimera. Chimera DEN2-N/DEN4, containing the PreM-E genes of DEN2-N, proved to be neurovirulent, and the mortality rate was 100%. Mutant DEN2-N12/DEN4, which contained the two substitutions in PreM, was not neurovirulent, suggesting that neither mutation in the PreM gene played a significant role in neurovirulence. Chimeric mutant DEN2-N34/DEN4, which contained the substitutions Glu71-Asp and Glu126-Lys in E, was essentially as neurovirulent as DEN2-N/DEN4. Mutants DEN2-N1234/DEN4 and DEN2-N12345/DEN4, each of which contained two mutations in PreM plus two or three substitutions in E, exhibited mouse neurovirulence. Chimeras DEN2-N56/DEN4 and DEN2-N6/DEN4, which contained, respectively, substitutions 5 and 6 or substitution 6 alone, were not mouse neurovirulent. It was noted that the mortality rate of mice inoculated with mutant DEN2-N1234/DEN4 was slightly lower than that of mice inoculated with mutant DEN2-N34/DEN4 or mutant DEN2-N12345/DEN4. Nevertheless, mutant DEN2-N1234/DEN4 was definitely neurovirulent for mice. Analysis of the LD50 of each of these viruses in mice should further clarify whether the reduced mortality rate of mice inoculated with DEN2-N1234/DEN4 was biologically significant. At any rate, this analysis indicated that the Glu71-Asp and/or Glu126-Lys substitution in E represented the major determinant of mouse neurovirulence. It is possible that the conservative amino acid substitution Glu71-Asp probably had only a minor effect, but the Glu126-Lys mutation represents a change from a negatively charged amino acid to a positively charged amino acid, suggesting an important role in acquisition of DEN2 mouse neurovirulence. As indicated later, other independent data support this interpretation.
Studies performed earlier indicated that there was a 3- to 5-day delay in death of mice inoculated with the chimera DEN2-N (C-PreM-E)/DEN4 compared to that of mice inoculated with the same dose of neurovirulent DEN2-N from which the C-PreM-E genes were derived (4). Subsequently, the LD50 of DEN2 (C-PreM-E)/DEN4 was shown to be 23 PFU, whereas the LD50 of DEN2-N was in the range of 0.1 to 1 PFU for animals of the same age (5). Thus, the intertypic dengue virus chimera exhibited some diminution of mouse neurovirulence compared to the high level of neurovirulence of DEN2-N. One interpretation for the observation is that during serial passage of DEN2 in mice, neurovirulence mutations also developed in the nonstructural protein region of the viral genome. Sequence analysis of DEN2-N NS1 indicated that there were two nucleotide substitutions, one of which resulted in a Gln105-Arg substitution in NS1. We addressed the question of whether the NS1 mutation might also contribute to mouse neurovirulence by constructing two chimeric DEN4 cDNAs that contained (i) the DEN2-N C-PreM-E-NS1 genes or (ii) only the DEN2-N NS1 gene as a replacement for the corresponding DEN4 sequence. The RNA transcripts from each of the two chimeric DNA constructs were used to transfect cultured C6/36 cells. Interestingly, a viable virus was recovered from the chimeric DNA construct that contained only the DEN2 NS1 gene. A viable chimeric construct containing the DEN2 C-PreM-E-NS1 genes was not recovered, even though repeated transfections were performed.
Evidence that the viable DEN2-N (NS1)/DEN4 chimera produced the predicted DEN2 NS1 protein and other proteins from DEN4 in infected C6/36 cells is presented in Fig. 2. Radioimmunoprecipitation was performed with a DEN2 NS1-specific monoclonal antibody, 34-23 (13), kindly provided by E. Henchal (Walter Reed Army Institute of Research), and DEN2- or DEN4-specific hyperimmune mouse ascitic fluid. As a control, parental DEN4 and DEN2-N were studied in parallel. Monoclonal antibody 34-23 precipitated a labeled band with the molecular size predicted for DEN2 NS1 from DEN2-N-infected cells and from chimeric virus-infected cells. DEN2 NS1 from DEN2-N or from chimeric virus-infected cells migrated slightly slower than DEN4 NS1 immunoprecipitated from DEN4-infected cell lysates. On the other hand, the chimera produced DEN4 E that was distinct from DEN2 E, which migrated slightly faster on the gel.
FIG. 2.
Analysis of dengue virus proteins by radioimmunoprecipitation. Confluent mosquito C6/36 cells in a T25 flask were infected with the chimera (DEN2 (NS1)/DEN4 [(D2 NS1)/D4] or with DEN2-N or the DEN4 control at a multiplicity of 1.0 for 6 days. Infected cells were then labeled with [35S]methionine (specific activity, 3,000 Ci/mmol; 100 μCi/flask in 2 ml of methionine-free minimal essential medium) for 6 h. The cells were then lysed with RIPA buffer (1% sodium deoxycholate, 1% Nonidet P-40, 0.1% sodium dodecyl sulfate [SDS], 0.01 M Tris-HCl [pH 7.5], 0.15 M NaCl), and the radiolabeled lysates were analyzed by immunoprecipitation with DEN2 (lanes 2)- or DEN4 (lanes 4)-specific hyperimmune mouse ascitic fluid or with DEN2 NS1-specific monoclonal antibody 34-23 (lanes M). The immunoprecipitates were analyzed by SDS-polyacrylamide gel electrophoresis. The radiolabeled protein bands were visualized by autoradiography of the dried gel.
The chimera DEN2 (NS1)/DEN4 was analyzed for neurovirulence by intracerebral inoculation of 3-day-old Swiss mice with 1,000 PFU. Mice in the control groups were inoculated with parental DEN4, DEN2-N, or its derived chimera, DEN2 (C-PreM-E)/DEN4, at the same dose (Fig. 3). Mice developed encephalitis and died within 9 days after inoculation with DEN2-N or 11 days after inoculation with the chimera DEN2-N (C-PreM-E)/DEN4. On the other hand, all mice which were inoculated with the chimera DEN2(NS1)/DEN4, similar to animals inoculated with DEN4, failed to develop symptoms of encephalitis or die. Thus, a role in mouse neurovirulence could not be demonstrated for the Gln105-Arg substitution identified in DEN2-N NS1.
FIG. 3.
Mouse neurovirulence of DEN2 (NS1)/DEN4. Three-day-old Swiss outbred mice in groups of 10 were inoculated intracerebrally with the chimera DEN2 (NS1)/DEN4 at 1,000 PFU. Neurovirulent DEN2-N, nonneurovirulent DEN4, and the derived DEN2 (C-PreM-E)/DEN4 chimera at the same dose were included as controls. Inoculated mice were observed daily for signs of encephalitis, and deaths were recorded for a 24-day period postinoculation.
The present study suggests that Glu71-Asp and Glu126-Lys substitutions in E played a major role in acquisition of neurovirulence during serial passage of the parental DEN2 NGC strain in mice. The conservative amino acid substitution of Glu71-Asp probably had only a minor effect, if any, on neurovirulence. On the other hand, the Glu126-Lys substitution represents a change from a negatively charged amino acid to a positively charged amino acid. As demonstrated earlier, introduction of a single Glu126-Lys substitution in the corresponding position of E from a nonneurovirulent DEN3 in an intertypic chimera produced a neurovirulent virus with DEN3 antigenicity (7). This suggests that the latter mutation alone could be responsible for the acquisition of DEN2 mouse neurovirulence. Also, these observations indicated that in one situation, a genetic change responsible for the mouse neurovirulence phenotype could be transferred from one dengue virus serotype to another. Glu at amino acid position 126 of DEN2 E is conserved in the sequence of DEN1 E and DEN3 E but not in the sequence of DEN4 E. According to the folded structure of E deduced for tick-borne encephalitis virus (26), this amino acid residue is located in the d-e loop that is occupied by several positively charged amino acids within the dimerization domain (domain II). The Glu126-Lys substitution in the mutant DEN2 E results in a loss of the only negatively charged amino acid in the loop region. The loop’s outward location on the dimeric E surface suggests the possibility that a change of charged amino acids could alter virus-cell interactions.
Earlier, we analyzed the genetic loci responsible for DEN4 mouse neurovirulence, and these studies identified two mutations in DEN4 E as responsible for mouse neurovirulence (18). One is a substitution of Pro for Ser at position 155 which ablates a glycosylation site within the central domain (domain I) of the E structure, and the other is a substitution of Leu for Phe at position 402 located within the postulated stem-anchor region adjacent to the putative receptor-binding domain (domain III) of the E protein structure (33). Amino acid substitutions in the stem-anchor region of E were also identified in a DEN3 strain following passage in mouse brain, although it was not established whether such substitutions alone were responsible for DEN3 mouse neurovirulence (21). Analysis of neurovirulence mutations in DEN2 and DEN4 identified a shared substitution of Phe that was located at position 402 in the sequence of E. Our findings indicated that the Phe402-Leu substitution in DEN4 E alone confers the DEN4 mouse neurovirulence phenotype (18). On the other hand, acquisition of mouse neurovirulence could not be demonstrated for the mutant containing an analogous Phe402-Ile substitution at the corresponding position in DEN2 E. Thus, not all neurovirulence mutations of dengue viruses can be transferred from one serotype to another.
Our analysis indicated that mouse-adapted DEN2 and DEN4 mutants contained multiple amino acid substitutions in the structural proteins and that one or more such changes were responsible for dengue mouse neurovirulence, depending on dengue virus serotype. Although the genetic loci determining mouse neurovirulence selected by serial intracerebral passage in mice appear to be different among dengue virus serotypes, it is possible to confer neurovirulence upon a nonneurovirulent dengue virus by introducing a mutation in E that is responsible for neurovirulence of a mouse-adapted neurovirulent mutant of another serotype. A central issue of such studies remains whether the dengue virus mutations determining mouse neurovirulence and the potential mutations responsible for human attenuation are the same, distinct, or overlapping. Recently, molecular characterization of dengue virus attenuation was attempted by comparison of sequences of the parental virus and its derived vaccine strain prepared by serial passage in primary dog kidney cells (2, 19, 25). In these studies, multiple nucleotide changes in various regions of the dengue virus genome, some of which result in amino acid changes, have been found in the vaccine strains. It remains to be sorted out which mutations are responsible for attenuation and which are not. To provide an answer to the series of questions, a clinical trial would be required to assess attenuation of these mutants in humans. For such clinical trials to become feasible, identification of other viral characteristics in vitro is needed. We recently observed that certain mouse-adapted DEN1 or -4 mutants replicated less efficiently than the wild-type virus in simian cell culture (15). For example, a mouse-adapted DEN1 MD-1 strain that was tested earlier as a candidate vaccine grew to a titer of 104 PFU/ml in LLC-MK2 cells, whereas its parental DEN1 strain yielded 106 PFU/ml under the same condition (15). In contrast, certain mouse-adapted variants of DEN3 were reported to replicate as efficiently as the parental virus in Vero cells (21). Presumably, successful selection of altered growth phenotype variants may require a high level of mouse brain passage. At least, experience with several well-studied live vaccines directed against other viruses indicates that growth restriction in nonhuman primate cells is usually associated with attenuation in humans. For this reason, mutations that affect viral growth probably play a more important role in conferring attenuation in humans than mutations that affect only neurovirulence, because dengue viruses are inherently not neurotropic.
Acknowledgments
We are grateful to L. Rosen and K. Eckels for providing viruses; E. Henchal for providing monoclonal antibodies; H. Kawano, M. Tadano, and K. Hiramatsu for helpful discussions; and especially R. Chanock for continuing support and encouragement of the flavivirus research program.
REFERENCES
- 1.Bhamarapravati N, Yoksan S, Chayaniyayothin T, Angsubphakorn S, Bunyaratvej A. Immunization with a live attenuated dengue-2 virus candidate vaccine (16681-PDK53): clinical, immunological and biological responses in adult volunteers. Bull W H O. 1987;65:189–195. [PMC free article] [PubMed] [Google Scholar]
- 2.Blok J, McWilliam M, Butler H C, Gibbs A J, Weiller G, Herring B L, Hemsley A G, Aaskov J G, Yoksan S, Bhamarapravati N. Comparison of a dengue-2 virus and its candidate vaccine derivative: sequence relationships with the flaviviruses and other viruses. Virology. 1992;187:573–590. doi: 10.1016/0042-6822(92)90460-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bray M, Lai C J. Dengue virus pre-membrane and membrane proteins elicit a protective immune response. Virology. 1991;185:505–508. doi: 10.1016/0042-6822(91)90809-p. [DOI] [PubMed] [Google Scholar]
- 4.Bray M, Lai C J. Construction of intertypic chimeric dengue viruses by substitution of structural protein genes. Proc Natl Acad Sci USA. 1991;88:10342–10346. doi: 10.1073/pnas.88.22.10342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bray M, Lai C J. Construction and characterization of chimeric dengue viruses. In: Brown F, Chanock R M, Ginsberg H S, Lerner R A, editors. Vaccine 92. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1992. pp. 271–276. [Google Scholar]
- 6.Bray M, Zhao B, Markoff L, Eckels K H, Chanock R M, Lai C J. Mice immunized with recombinant vaccinia virus expressing dengue 4 virus structural proteins with or without nonstructural protein NS1 are protected against fatal dengue virus encephalitis. J Virol. 1989;63:2853–2856. doi: 10.1128/jvi.63.6.2853-2856.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen W, Kawano H, Men R, Clark D, Lai C J. Construction of intertypic chimeric dengue viruses exhibiting type 3 antigenicity and neurovirulence for mice. J Virol. 1995;69:5186–5190. doi: 10.1128/jvi.69.8.5186-5190.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edelman R, Tacket C O, Wasserman S S, Vaughn D W, Eckels K H, Dubois D R, Summers P L, Hoke C H. A live attenuated dengue-1 vaccine candidate (45AZ5) passaged in primary dog kidney cell culture is attenuated and immunogenic for humans. J Infect Dis. 1994;170:1448–1455. doi: 10.1093/infdis/170.6.1448. [DOI] [PubMed] [Google Scholar]
- 9.Falgout B, Bray M, Schlesinger J J, Lai C J. Immunization of mice with recombinant vaccinia virus expressing authentic dengue virus nonstructural protein NS1 protects against lethal dengue virus encephalitis. J Virol. 1990;64:4356–4363. doi: 10.1128/jvi.64.9.4356-4363.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guirakhoo F, Heinz F X, Mandl C W, Holzmann H, Kunz C. Fusion activity of flaviviruses: comparison of mature and immature (prM-containing) tick-borne encephalitis virions. J Gen Virol. 1991;72:1323–1329. doi: 10.1099/0022-1317-72-6-1323. [DOI] [PubMed] [Google Scholar]
- 11.Heinz F X. Epitope mapping of flavivirus glycoproteins. Adv Virus Res. 1986;31:103–168. doi: 10.1016/s0065-3527(08)60263-8. [DOI] [PubMed] [Google Scholar]
- 12.Heinz F X, Stiasny K, Pueschner-Auer G, Holzmann H, Allison S, Mandl C W, Kunz C. Structural change and functional control of the tick-borne encephalitis virus glycoprotein E by the heterodimeric association with protein prM. Virology. 1994;198:109–117. doi: 10.1006/viro.1994.1013. [DOI] [PubMed] [Google Scholar]
- 13.Henchal E A, Henchal L S, Thaisomboonsuk B K. Topological mapping of unique epitopes on the dengue-2 virus NS1 protein using monoclonal antibodies. J Gen Virol. 1987;68:845–851. doi: 10.1099/0022-1317-68-3-845. [DOI] [PubMed] [Google Scholar]
- 14.Henchal E A, Putnak J R. The dengue viruses. Clin Microbiol Rev. 1990;3:376–396. doi: 10.1128/cmr.3.4.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hiramatsu, K., R. Men, and C. J. Lai. 1997. Unpublished observations.
- 16.Hotta S. Experimental studies on dengue. I. Isolation, identification, and modification of the virus. J Infect Dis. 1952;90:1–9. doi: 10.1093/infdis/90.1.1. [DOI] [PubMed] [Google Scholar]
- 17.Irie K, Mochan P M, Sagaguri Y, Putnak R, Padmanabhan R. Sequence analysis of cloned dengue virus type 2 genome (New Guinea C strain) Gene. 1989;75:197–211. doi: 10.1016/0378-1119(89)90266-7. [DOI] [PubMed] [Google Scholar]
- 18.Kawano H, Rostapshov V, Rosen L, Lai C J. Genetic determinants of dengue type 4 virus neurovirulence for mice. J Virol. 1993;67:6567–6575. doi: 10.1128/jvi.67.11.6567-6575.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kinney R M, Butrapet S, Chang G J, Tsuchiya K R, Roehrig J T, Bhamarapravati N, Gubler D J. Construction of infectious cDNA clones for dengue 2 virus: strain 16681 and its attenuated vaccine derivative, strain PDK-53. Virology. 1997;230:300–308. doi: 10.1006/viro.1997.8500. [DOI] [PubMed] [Google Scholar]
- 20.Lai C J, Zhao B, Hori H, Bray M. Infectious RNA transcribed from stably cloned full-length cDNA of dengue type 4 virus. Proc Natl Acad Sci USA. 1991;88:5139–5143. doi: 10.1073/pnas.88.12.5139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee E, Weir R C, Dalgarno L. Changes in the dengue virus major envelope protein on passaging and their localization on the three-dimensional structure of the protein. Virology. 1997;232:281–290. doi: 10.1006/viro.1997.8570. [DOI] [PubMed] [Google Scholar]
- 22.Mackenzie J M, Jones M J, Young P R. Immunolocalization of the dengue virus nonstructural glycoprotein NS1 suggests a role in viral RNA replication. Virology. 1996;220:232–240. doi: 10.1006/viro.1996.0307. [DOI] [PubMed] [Google Scholar]
- 23.Monath T. Dengue: the risk to developed and developing countries. Proc Natl Acad Sci USA. 1994;91:2395–2400. doi: 10.1073/pnas.91.7.2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muylaert I R, Galler R, Rice C. Mutagenesis of the N-glycosylation sites of the yellow fever virus NS1 protein: effects on RNA replication and mouse neurovirulence. Virology. 1996;222:159–168. doi: 10.1006/viro.1996.0406. [DOI] [PubMed] [Google Scholar]
- 25.Puri B, Nelson W M, Henchal E A, Hoke C H, Eckels K H, Dubois D R, Porter K R, Hayes C G. Molecular analysis of dengue virus attenuation after serial passage in primary dog kidney cells. J Gen Virol. 1997;78:2287–2291. doi: 10.1099/0022-1317-78-9-2287. [DOI] [PubMed] [Google Scholar]
- 26.Rey F A, Heinz F X, Mandl C, Kunz C, Harrison S C. The envelope glycoprotein from tick-borne encephalitis virus at 2 Å resolution. Nature. 1995;375:291–298. doi: 10.1038/375291a0. [DOI] [PubMed] [Google Scholar]
- 27.Sabin A B, Schlesinger R W. Production of immunity to dengue with virus modified by propagation in mice. Science. 1945;101:640–642. doi: 10.1126/science.101.2634.640. [DOI] [PubMed] [Google Scholar]
- 28.Sabin A B. Research on dengue during World War II. Am J Trop Med Hyg. 1952;1:30–50. doi: 10.4269/ajtmh.1952.1.30. [DOI] [PubMed] [Google Scholar]
- 29.Sabin A B. Recent advances in our knowledge of dengue and sandfly fever. Am J Trop Med Hyg. 1955;4:198–207. doi: 10.4269/ajtmh.1955.4.198. [DOI] [PubMed] [Google Scholar]
- 30.Schlesinger J J, Brandriss M W, Walsh E E. Protection against 17D yellow fever encephalitis in mice by passive transfer of monoclonal antibodies to the nonstructural glycoprotein gp48 and by active immunization with gp48. J Immunol. 1985;135:2805–2809. [PubMed] [Google Scholar]
- 31.Shapiro D, Brandt W E, Russell P K. Change involving a viral membrane glycoprotein during morphogenesis of group B arboviruses. Virology. 1972;50:906–911. doi: 10.1016/0042-6822(72)90445-x. [DOI] [PubMed] [Google Scholar]
- 32.Stadler K, Allison S L, Schalich J, Heinz F X. Proteolytic activation of tick-borne encephalitis virus by furin. J Virol. 1997;71:8475–8481. doi: 10.1128/jvi.71.11.8475-8481.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stiasny K, Allison S L, Marchler-Bauer A, Kunz C, Heinz F X. Structural requirements for low-pH-induced rearrangements in the envelope glycoprotein of tick-borne encephalitis virus. J Virol. 1996;70:8142–8147. doi: 10.1128/jvi.70.11.8142-8147.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wisseman C L, Jr, Sweet B H, Rosenzweig E C, Eylar O R. Attenuated living type 1 dengue vaccines. Am J Trop Med Hyg. 1963;12:620–623. [Google Scholar]