Abstract
Infertility affects millions worldwide, and implantation failure remains a critical barrier in assisted reproduction. Endometrial receptivity, a fundamental prerequisite for successful implantation, involves precise regulation of extracellular matrix components, with hyaluronan (HA) being particularly significant due to its structural and signaling roles. This study evaluated the expression of genes involved in HA metabolism and regulation across different phases of the menstrual cycle, particularly emphasizing their role during the window of implantation in normal fertility and repeated in vitro fertilization failures. Analysis of publicly available transcriptomic datasets revealed distinct expression patterns of HA synthases (HAS2, HAS3), HA-degrading enzymes (HYAL2, CEMIP, CEMIP2), and HA receptors (CD44, RHAMM, layilin) in endometrium that dynamically change toward the receptive state. Notably, HAS enzymes and HA receptors were upregulated during the mid-secretory phase, whereas classical HA-degrading enzymes showed complex regulation, suggesting a balance between HA synthesis and degradation necessary for optimal endometrial function. In patients with repeated IVF failure, there was significant downregulation of key HA-related genes (HAS2, HAS3, CEMIP, CD44, versican, syndecans), implicating impaired HA metabolism in implantation failure. The results underscore the role of HA metabolism and HA receptors in establishing a receptive endometrial environment, highlighting HA and its associated pathways as potential therapeutic targets to enhance reproductive success rates. Further research is necessary to unravel the detailed molecular mechanisms of HA-mediated regulation and its translational implications for assisted reproduction technologies.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12958-025-01452-6.
Keywords: Hyaluronan, Endometrial receptivity, Extracellular matrix, Implantation, Infertility, Assisted reproduction technologies, CD44 receptor, Glycosaminoglycans
Introduction
Infertility, defined as the inability to conceive after a year of regular, unprotected sexual intercourse, is a global health problem affecting millions of couples worldwide, with significant psychological and social implications [1]. The World Health Organization estimates that 48 million couples and 186 million individuals live with infertility globally [2]. Approximately 20–30% of infertility cases are attributed to male factors, 20–35% to female factors, and 25–40% to combined causes. Female infertility is often associated with advanced age and is typically characterized by infrequent or absent menstrual periods. However, the cause remains unknown in 10–20% of infertility cases [3].
Assisted reproductive technologies have emerged as vital solutions to address this challenge, providing hope for those struggling to conceive. Among these technologies, in vitro fertilization (IVF) is the most prominent and widely used method and involves the fertilization of an egg outside the woman’s body, followed by the transfer of the resulting embryo into the uterus. Despite its widespread use and technological advancements, IVF has a success rate of approximately 30% per cycle [4]. This relatively low success rate can also be attributed to the failure of the embryo to implant in the uterus, a process heavily dependent on the receptivity of the endometrium [5].
The endometrium, the inner lining of the uterus, undergoes cyclical changes to become receptive to embryo implantation during a specific window of time. The remodeling of the endometrial architecture is fundamental for creating a suitable environment for the establishment of pregnancy [6]. Physiologically, successful embryo implantation can occur only within the window of implantation (WOI), typically during the mid-secretory phase of the menstrual cycle (6–10 days after ovulation), when the endometrium is in a receptive state. However, in many cases of IVF failure, the endometrium is nonreceptive or the WOI is shifted, leading to implantation failure [7]. To address this problem, various tests for endometrial receptivity have been developed, such as the Endometrial Receptivity Analysis (ERA test) or BeReady test, which aims to identify the optimal timing for embryo transfer [8, 9]. Despite these advancements, the precise mechanisms underlying endometrial receptivity remain largely unknown, and no definitive treatments exist for conditions of endometrial nonreceptivity. The complex interplay of hormonal, cellular, and molecular factors that govern endometrial receptivity continues to be a significant area of research, with the hope of improving IVF outcomes and providing better solutions for those affected by infertility.
The extracellular matrix (ECM), a three-dimensional scaffold composed of fibrous proteins, viscous proteoglycans (PGs) and glycosaminoglycans (GAGs) present on the surface of the endometrial epithelium, is the first point of contact between the embryo and the maternal tissue during implantation. The interactions between the embryo’s trophoectoderm and the luminal epithelium of the endometrium involve cell surface receptors, such as integrins and other adhesion molecules, that recognize complementary ligands present in the ECM of the opposing cells.
The composition and structure of the ECM are crucial for establishing a receptive environment, influencing processes such as cell adhesion, migration, and communication between the embryo and the endometrium [10, 11]. In general, the ECM functions as both connective tissue and a scaffold that provides structural support to cells while regulating their functions. During the menstrual cycle, the ECM undergoes significant remodeling to support the various stages of endometrial development and prepare for potential implantation [12, 13]. The composition of the endometrial ECM changes in response to varying levels of estrogen and progesterone [14]. During the proliferative phase, estrogen stimulates the synthesis and deposition of ECM components, leading to thickening of the endometrial lining. In the secretory phase, progesterone modifies the ECM such that it becomes more receptive to the implanting embryo. This phase is characterized by increased vascularization, glandular secretion, and the presence of decidual cells, all of which contribute to a supportive environment for implantation [6].
The key structural molecules of the ECM are GAGs, which are long polysaccharide chains that are typically covalently attached to a protein core to form PGs. Various types of PGs offer structural integrity to the ECM, forming three-dimensional structures that determine its mechanical properties. Owing to their negative charge, GAGs enable the ECM to retain large quantities of water. GAGs also interact with numerous growth factors, cytokines and chemokines, immobilizing them within the ECM structure (for a comprehensive overview, see Casale & Crane, 2024) [15]. The length of GAG chains can vary significantly, both among different GAG types and even within the same GAG type, leading to a broad range of molecular weights, from a few kDa to several thousand kDa. GAGs are either sulfated or nonsulfated, with hyaluronan (HA) being the only member. Among the various ECM components, HA stands out as a critical molecule because its unique physical and cell modulation properties contribute to tissue hydration, structural integrity, and cell signaling.
Functional and regulatory properties of HA
HA is composed of repeating units of N-acetylglucosamine (GlcNAc) and glucuronic acid (GlcA) that contribute to the viscoelastic properties of the ECM, providing a hydrated scaffold for cell migration and tissue remodeling. Its high molecular weight and water retention create a favorable microenvironment for cellular activities [16]. HA properties depend on its molecular weight, concentration, and interactions with receptors and proteins. Under physiological conditions, HA exists primarily as a high-molecular-weight polymer (> 10,000 kDa), providing structural support in the ECM but exhibiting rapid turnover to maintain tissue homeostasis [17]. HA concentration and molecular weight vary with tissue type, age, and pathological conditions and are influenced by the balance between its synthesis and degradation. In vertebrates, HA metabolism is regulated by three HA synthase isoforms (HASs) and several hyaluronidases (HYALs). In the human body, HASs 1, 2, and 3, which are membrane-bound proteins located on the plasma membrane of cells and are responsible for catalyzing the addition of repeating disaccharide units of GlcA and GlcNAc to form HA in the extracellular space, are present. Each HAS enzyme has unique properties that contribute to the complex regulation of HA in the body. Among the HYAL family members, HYAL1 and HYAL2 are widely expressed in mammalian tissues and are thought to be major contributors to HA catabolism. HYAL1 is the major lysosomal hyaluronidase, while HYAL2 works on the cell surface. More recently, cell migration-inducing protein (CEMIP), also known as KIAA1199, and CEMIP2, also known as transmembrane protein 2 (TMEM2), have been identified as novel molecules involved in extracellular HA degradation [18]. CEMIP is a secreted protein; however, its main localization was detected in intracellular compartments, whereas CEMIP2 can degrade HA bound in the ECM on the basis of cell–substratum contact [19]. However, the role of CEMIP and CEMIP2 in relation to endometrial receptivity has not yet been studied. In addition to the enzymatic degradation of HA, various free radicals and oxidants contribute to HA depolymerization, particularly during pathological processes such as inflammation.
HA functions not only as a structural molecule but also as an extracellular regulatory molecule that transmits signals and regulates a variety of cell behaviors, including cell adhesion, motility, and growth. This regulation relies on the physical-structural properties of HA as well as on cell signaling events triggered by HA-receptor interactions [20]. Notably, many different HA-binding or HA-interacting proteins are found in tissues. HA-mediated regulation is dependent on proteins known as hyaladherins, or HA-binding proteins, which include cell surface receptors, PGs anchored in the cell immediate extracellular surroundings and other various supporting proteins that interact with the HA-rich ECM. The specificity of HA interactions is determined by the structure of HA-binding partners, including the presence of structural domains, a link module and/or the BX7B motif [21]. From a localization and functional perspective, HA-interacting proteins can be divided into several groups. Cell receptors sequester HA on the cell surface to facilitate its internalization or to regulate cell adhesion, migration, and proliferation; other HA-binding proteins, including PGs from the lectican group, PGs indirectly interacting with HA-rich ECM and other secreted proteins, contribute to the crosslinking and stabilization of the HA-rich matrix. In addition, primary cell surface receptors can be cleaved from the membrane and bind HA in the extracellular space.
The primary HA receptors are CD44, Receptor for HA-Mediated Motility (RHAMM), and HA Receptor for Endocytosis (HARE)/Stabilin-2. CD44 is the most extensively studied HA receptor and functions in cell adhesion, migration, and signaling. CD44 is present in various segments of the reproductive tract in mice [22], cows [23], sheep [24], mars [25] and humans [26] under normal physiological conditions. RHAMM primarily mediates motility and cellular responses during wound healing and inflammation. HARE, which is expressed mainly in the liver and lymphatic system, facilitates HA endocytosis and clearance. Additional receptors include LYVE-1, which is involved in lymphatic vessel function, and Layilin [27–29].
Moreover, the HA-rich ECM matrix can be further stabilized by PGs and proteins interacting with HA either directly or indirectly. The hyalectan family members, also known as lecticans, which include aggrecan, versican, neurocan, and brevican, can directly interact with HA. These PGs share structural features, as an N-terminal domain that binds HA and a C-terminal domain that interacts with lectins (for a comprehensive overview, see Iozzo & Schaefer, 2015) [30]. The lecticans can further interact with various ECM proteins, such as tenascin-C, which is a large oligomeric glycoprotein that forms complex structures from HA and HA-binding lecticans, thus stabilizing the HA-rich matrix [31].
Additional stabilization of HA-rich ECM can be provided by certain cell surface–associated PGs, such as syndecans, glypicans or agrin. Syndecans are transmembrane heparan sulfate PGs that stabilize HA-rich ECM through their HS chains, which bind ECM proteins (such as tenascin-C or fibronectin). Like HA receptors on the cell surface, syndecan ectodomains can be shed after cleavage by proteolytic enzymes in response to injury and inflammation and can act as molecular reservoirs of cytokines and growth factors [32]. Similarly, glypicans (GPI-anchored heparan sulfate PGs tethered to the cell membrane or incorporated into the ECM) and soluble agrin can contribute to HA-rich ECM stabilization or influence HA-associated signaling via interactions with other matrix components or cell surface receptors [33].
Finally, further stabilization of the HA-rich matrix is provided by secreted proteins with the link domain that directly interacts with HA. Thus, another level of complexity (Fig. 1) is represented by the formation of complexes of HA with the heavy chains (HC) of the interα-trypsin inhibitor (IαI). These HA‒HC complexes are formed by the enzyme tumor necrosis factor-stimulated gene-6 (TSG-6), which is a key hyaladherin that crosslinks HA via the transfer of HCs from IαI [34]. The structural stability of the ECM provided by the formation of cross-linked HA multimolecular assemblages is essential for supporting cellular activities such as migration, adhesion, and proliferation. Furthermore, these complexes modulate the bioavailability of HA, regulating its interaction with hyaladherins, which are key mediators in cellular signaling pathways. Thus, alterations in the molecules responsible for HA crosslinking may affect ECM stability, which may lead to impaired endometrial receptivity. Interestingly, the most well-described role of these complexes is in cumulus oophorus matrix formation, where HA‒HC complexes increase the structural integrity of the ECM, facilitating successful ovulation and fertilization [34–36], and their role in endometrial receptivity remains the subject of future research.
Fig. 1.
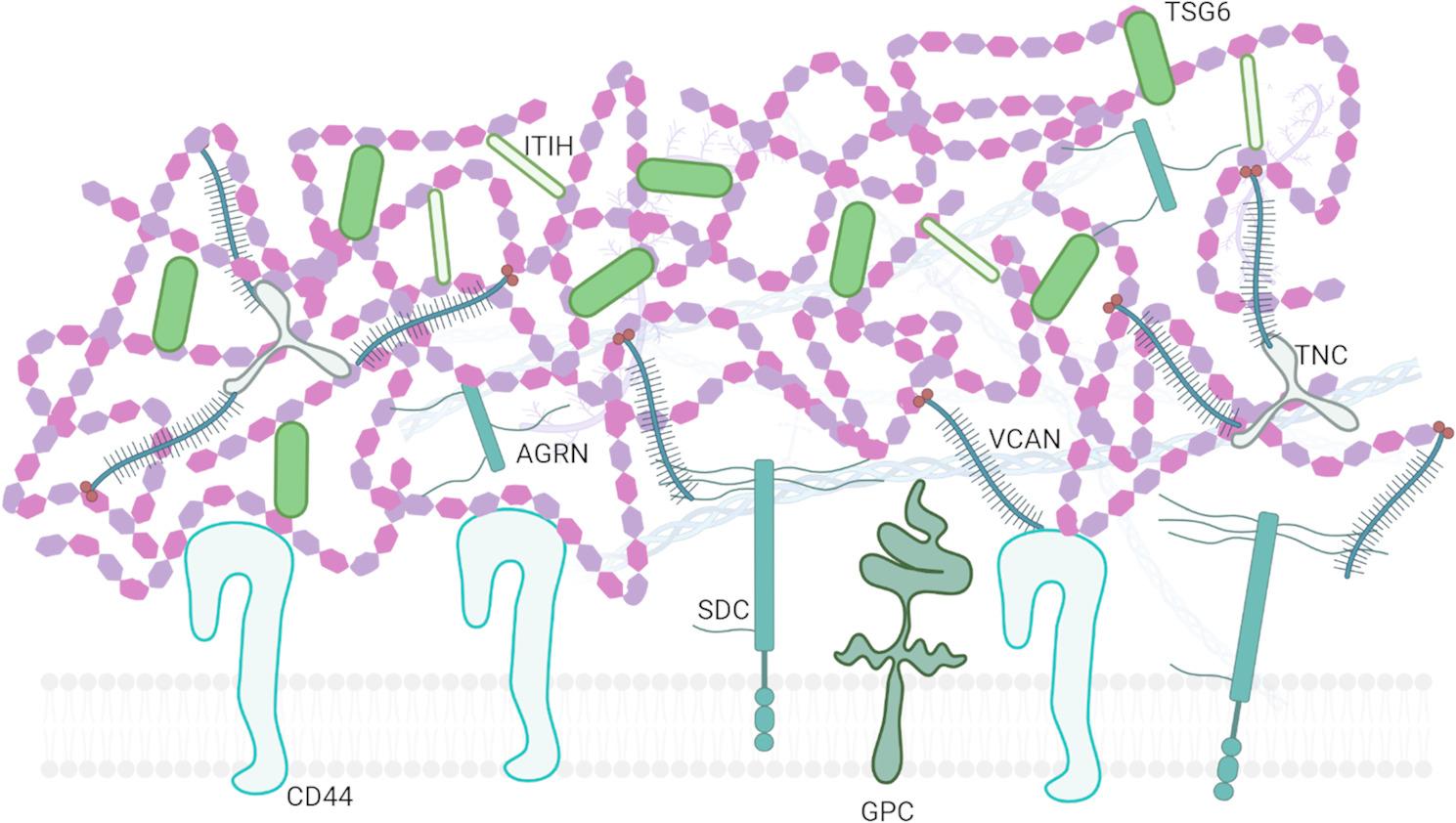
Scheme of complexity of HA-rich ECM that includes interactions with different type of cell surface receptors, PGs, and hyalectans. Abbreviations: AGRN– agrin, CD44– HA receptor CD44, GPC– glypican, HA– hyaluronan, ITIH– IαI heavy chains, SDC– syndecan, TNC– tenascin C, TSG-6– Tumor necrosis factor-Stimulated Factor 6, VCAN– versican
HA in complex processes of conception
The role of HA in reproductive biology and its clinical applications is gaining increasing recognition, as HA is suggested to play a significant role in various processes related to conception [37, 38]. In mammalian reproduction, HA is proposed to be involved in oocyte maturation [39], fertilization, implantation [40], and parturition [41]. The best documented examples are HA’s expansion of cumulus cells at ovulation (in mice) [42] and its induction of cervical ripening during parturition in rabbits and mice [41, 43]. HA is present in the oviduct, uterus and cervix in humans [44–46]. All cell types involved in folliculogenesis are capable of HA synthesis, such as oocytes, cumulus cells [47, 48], theca cells of primary and more advanced follicles [49] and granulosa cells [47–49], as shown in mice and rats. HA is also produced by cumulus and granulosa cells in the ovarian follicles of pigs, cows and sheep [39, 50, 51].
In addition to the effects of HA metabolism modulation on endometrial receptivity, extensive research has been conducted to evaluate the potential benefits of adding HA to embryo culture media. However, the results of these studies have been inconsistent. Some research suggests that HA supplementation may improve embryo quality and implantation rates [52–55], whereas other studies indicate no significant benefit [55–58]. The variability in outcomes suggests that the role of HA in embryo culture media is complex and may be influenced by additional factors, such as specific culture conditions and embryo characteristics. The variability in research findings on the addition of HA to embryo culture media and its inconsistent effects on implantation success further highlight the complexity of the role of HA in reproduction [55, 58–61].
Despite the well-established role of HA in overall female reproduction, less is known about its specific importance for endometrial receptivity during embryo implantation. The dynamic regulation of the synthesis and deposition of HA, in contrast to most other GAGs, during the menstrual cycle in both humans and animals suggests its critical involvement in preparing the endometrium for implantation. However, there is a lack of information about the expression of key enzymes and HA-interacting proteins involved in HA metabolism and deposition in the context of endometrial receptivity. To address this gap, we analyzed a dataset from the Gene Expression Omnibus (GEO) database and compared endometrial samples from different phases of the menstrual cycle from healthy women with a regular menstrual cycle, healthy women with a history of successful pregnancy and those with repeated IVF failure.
Methods
Three datasets available from the GEO database were analyzed. All of these data were generated via bulk RNA sequencing of endometrial biopsies. GEO dataset A, number GSE132711 from a study by Chi et al. [62]., contains transcriptomic data from the transcriptional profiling of the endometrium during the proliferative and secretory phases. Dataset A included six samples from donors in the proliferative phase (GSM3890612–GSM3890617) and five samples from donors in the secretory phase (GSM3890618–GSM3890622). These samples were originally collected from women aged 18–37 years with regular menstrual cycles (25–35 days), intercycle variability of no more than two days, a normal luteal phase without spotting, and a body mass index (BMI) between 19 and 28. The original study was conducted in the USA [62].
GEO datasets B and C include reanalyzed samples from the original GSE106602 dataset published by Suhorutshenko et al. [63]. This dataset comprises transcriptomic profiles of the endometrium during the early and mid-secretory phases, as well as samples from women with repeated IVF failure (Fig. 2).
Fig. 2.
Schematic showing phases of menstrual cycle and time windows in which samples were taken in specific datasets. Dataset A (GSE132711 [62]) represent endometrial samples from fertile women with regular menstrual cycle in proliferative (P) and mid-secretory (MS) phases. Dataset B (GSE106602 [63]) contains transcriptomic data of profiling endometrial samples from women with regular menstrual cycle in early (ES) and mid-secretory phases. Dataset C (GSE106602 [63]) is combination of mid-secretory samples of dataset B which represent healthy fertile women and samples from women in mid-secretory phase with repeated IVF failure history
Dataset B contains paired endometrial biopsies collected from 15 women (GSM2593220–GSM2593252) during the early and mid-secretory phases. All participants had a history of successful pregnancy. Dataset C, derived from the same study (GSE106602), includes transcriptomic data from the mid-secretory endometria of 19 women who experienced IVF failure (GSM2593253–GSM2593271). These samples were compared with the mid-secretory phase samples from Dataset B.
All samples from Datasets B and C were collected in Spain from endometria of reproductive-aged women with a normal BMI and self-reported regular menstrual cycles. None of the participants had received hormonal treatment for at least three months prior to biopsy collection. The average age and BMI of the Spanish cohort were 29.1 ± 3.6 years and 23.2 ± 2.9 kg/m², respectively.
Relative gene expression of HA-related genes (HAS1–3, HYAL1–2, CEMIP, CEMIP2, CD44, HMMR, LAYN, TSG6, VCAN, BCAN, SDC1–4, GPC1–6, AGRN, ITIH1–5, and TNSC) was analyzed via GEO2R, an interactive web tool for comparing two or more groups of samples within a GEO series to identify differentially expressed genes. GEO2R utilizes the DESeq2 package for differential expression analysis on the basis of NCBI-computed raw count matrices. Statistical comparisons within each dataset were conducted via an unpaired t test. The results are presented as the means ± SEMs.
Next, relative gene expression data were analyzed to assess potential relationships between gene expression levels. Pearson correlation coefficients were calculated to evaluate the strength and direction of linear associations between pairs of genes. The corresponding p values were determined to assess the statistical significance of each correlation. All analyses were performed via GraphPad Prism software (version 8), with significance thresholds set at p < 0.01.
Results
Relative gene expression of genes encoding HA synthases
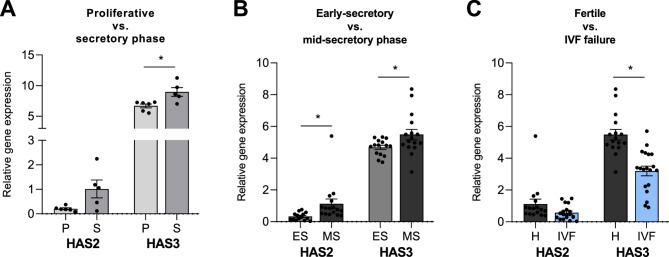
The most abundant HAS in the endometrium is HAS3 (Fig. 3), while HAS1 expression is mostly not detectable (data not shown). We observed the upregulation of HAS2 and HAS3 expression in the endometrium during the secretory phase of the menstrual cycle, as demonstrated by our reanalysis of GEO dataset A (Fig. 3 A). This trend was further confirmed by the reanalysis of another GEO dataset B. Notably, the HAS2 and HAS3 expression levels were greater in the mid-secretory phase than in the early secretory phase (Fig. 3B). During the whole menstrual cycle, HAS3 was significantly upregulated (Fig. 3 A and B). In contrast, in the comparison of the fertile and IVF failure samples, the expression of HASs decreased, whereas that of HAS3 decreased significantly (Fig. 3 C).
Fig. 3.
Comparison of HAS2 and HAS3 gene expression in the proliferative and secretory phases (A), in the early secretory and mid-secretory phases of the menstrual cycle in endometrial samples from healthy women (B) and in the mid-secretory phase of endometrial samples from women with a history of successful pregnancy and women with IVF failure (C) based on reanalysis of GEO datasets A: GSE132711, B: GSE106602, C: GSE106602
Relative gene expression of HA degradation enzymes
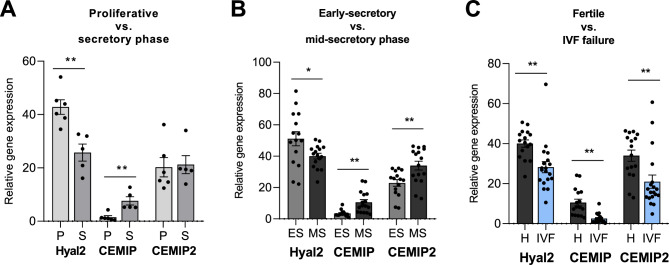
Compared with HYAL2 and CEMIP2, CEMIP was expressed at lower levels in the endometrium (Fig. 4). Negligible expression of HYAL1 in the endometrium independent of the phase of the menstrual cycle was found in both reanalyzed GEO datasets (data not shown). Significant downregulation of HYAL2 expression was present in the secretory phase (GEO dataset A) (Fig. 4 A). However, the gene expression of another HA-degrading protein, CEMIP, was upregulated in the secretory phase (Fig. 4 A). Notably, these trends of decreased HYAL2 expression and increased CEMIP expression were detected in the middle-secretory phase compared with the early secretory phase (GEO dataset B). Additionally, CEMIP2 is also upregulated in the mid-secretory phase (Fig. 4B).
Fig. 4.
Comparison of HYAL2, CEMIP and CEMIP2 gene expression in the proliferative and secretory phases (A), in the early secretory and mid-secretory phases of the menstrual cycle in endometrial samples from healthy women (B) and in the mid-secretory phase of endometrial samples from women with a history of successful pregnancy and women with IVF failure (C) based on reanalysis of GEO datasets A: GSE132711, B: GSE106602, C: GSE106602
Interestingly, the data revealed a comparison of patients who experienced IVF failure with patients who were fertile. All genes encoding HA degradation enzymes with detectable expression of HYALl2, CEMIP, and CEMIP2 were significantly lower in patients with IVF failure than in fertile patients (GEO dataset C) (Fig. 4 C).
Relative gene expression of cell surface HA receptors
The most highly expressed gene for the cell surface HA receptor was the gene encoding CD44 (Fig. 5). CD44 expression was increased during the secretory phase compared with the proliferative phase (GEO dataset A) (Fig. 5 A). This trend was confirmed by the increased expression of CD44 during the mid-secretory phase compared with the early secretory phase (Fig. 5B; GEO dataset B). However, an opposite trend of RHAMM to CD44 expression was observed after reanalysis of both datasets (Fig. 5A-B; GEO datasets A and B). Similar to RHAMM, the HA receptor layilin was expressed to a greater extent during the proliferative phase than during the secretory phase and during the early phase than during the mid-secretory phase (Fig. 5A-B; GEO datasets A and B). The expression of the other cell surface HA receptors Stabilin 2 and LYVE-1 was very low at both the secretory and proliferative phases (data not shown). In contrast, the expression of CD44 was lower in the endometrium during the WOI of women with repeated IVF failure than in that of fertile women according to the analysis of GEO dataset C (Fig. 5 C).
Fig. 5.
Comparison of CD44, HMMR (RHAMM) and LAYN (Layilin) gene expression in the proliferative and secretory phases (A), in the early secretory and mid-secretory phases of the menstrual cycle in endometrial samples from healthy women (B) and in the mid-secretory phase of endometrial samples from women with a history of successful pregnancy and women with IVF failure (C) based on reanalysis of GEO datasets A: GSE132711, B: GSE106602, C: GSE106602
Relative gene expression of HA-binding PGs
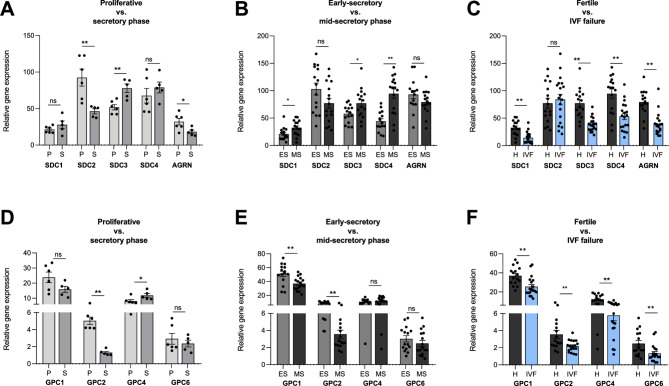
Syndecans 1–4 are expressed in the endometrium with increasing expression of syndecan 1, 3 and 4 from the proliferative phase to the mid-secretory phase (Fig. 6A-B; GEO datasets A and B). In contrast, syndecan 2 showed the opposite trend (Fig. 6A-B). In the case of glypicans, all isoforms 1–6 presented the highest expression of glypican 1 (Fig. 6D-E; GEO datasets A and B) and the lowest expression of glypican 3 and 5 (data not shown). The expression of glypicans During the menstrual cycle was stable, except for significantly lower expression of glypican 2 during the secretory phase, especially the mid-secretory phase, than during the proliferative phase (Fig. 6D-E; GEO datasets A and B). The expression of other cell surface-attached PG agrin tended to decrease among the analyzed phases of the menstruation cycle (Fig. 6A-B; GEO datasets A and B).
Fig. 6.
Comparison of SDC1-4 (syndecan), AGRN (agrin) and GPC1, 2, 4 and 6 (glypican) gene expression in the proliferative and secretory phases (A), in the early secretory and mid-secretory phases of the menstrual cycle in endometrial samples from healthy women (B) and in the mid-secretory phase of endometrial samples from women with a history of successful pregnancy and women with IVF failure (C) based on reanalysis of GEO datasets A: GSE132711, B: GSE106602, C: GSE106602
Analysis of GEO dataset C revealed significantly lower expression of genes for syndecans 1, 3 and 4 in the endometria of women with IVF failure than in those of fertile women (Fig. 6 C). Similarly, the expression of agrin (Fig. 6 C) and glypicans 1, 4 and 6 (Fig. 6 F) was decreased in IVF failure samples (GEO dataset C).
Relative gene expression of HA-binding proteins that form a three-dimensional HA matrix in the extracellular space
Compared with that in the proliferative phase, the expression of lectican versican was significantly greater during the secretory phase, especially during the mid-secretory phase (Fig. 7A-B; GEO datasets A and B). Other lecticans, such as aggrecan, neurocan, (data not shown) and brevican, are expressed at lower levels than versican; however, brevican expression tends to decrease during the menstrual cycle (Fig. 7A-B; GEO datasets A and B). Compared with that in the proliferative phase, tenascin-C expression was found to be extensive during the proliferative phase, with a significant decrease during the secretory phase, especially the mid-secretory phase (Fig. 7A-B; GEO datasets A and B). In the endometrium, HA binding protein 4 (IHABP-4) is expressed at similar levels during both the secretory and proliferative phases (data not shown).
Fig. 7.
Comparison of VCAN (versican), BCAN (brevican) and TNC (tenascin C) gene expression in the proliferative and secretory phases (A), in the early secretory and mid-secretory phases of the menstrual cycle in endometrial samples from healthy women (B) and in the mid-secretory phase of endometrial samples from women with a history of successful pregnancy and women with IVF failure (C) based on reanalysis of GEO datasets A: GSE132711, B: GSE106602, C: GSE106602
The analysis of datasets from the GEO database revealed significant differences in the expression of TSG-6 in the endometrium during the proliferative and secretory phases (Fig. 8 A; GEO dataset A), whereas minimal differences were detected between the early and mid-secretory phases (Fig. 8B; GEO dataset B). Decreased expression of TSG-6 was observed in endometrial samples from patients with IVF failure (Fig. 8 C; GEO dataset C). Among the genes encoding HCs, ITIH1-5, ITIH4 and ITIH5 were expressed at the highest levels in the endometrium (Fig. 8A-B; GEO datasets A and B). ITIH2 and ITIH3 were expressed at minimal levels, and ITH1 was almost undetectable (data not shown). A decreasing trend in the expression of ITH4 and ITIH5 occurred from the proliferative phase to the mid-secretory phase (Fig. 8A-B; GEO datasets A and B). IVF failure samples presented decreasing trends in the expression of both ITIH4 and ITH5 (Fig. 8 C, GEO dataset C).
Fig. 8.
Comparison of ITIH4-5 (IαI heavy chains) and TSG-6 gene expression in the proliferative and secretory phases (A), in the early secretory and mid-secretory phases of the menstrual cycle in endometrial samples from healthy women (B) and in the mid-secretory phase of endometrial samples from women with a history of successful pregnancy and women with IVF failure (C) based on reanalysis of GEO datasets A: GSE132711, B: GSE106602, C: GSE106602
Relationships among HA-related genes
The mutual relationships among genes with significant differences were evaluated and are depicted in detail in Supplementary Figs. 1, 2, 3 and summarized in Fig. 9. Among all the datasets, HAS3, the most highly expressed HA synthase (Fig. 9), correlated positively with versican, a molecule that binds HA in the extracellular space (Fig. 7), since it tended to be expressed at increased levels during the menstrual cycle (GEO datasets A and B). However, their expression in IVF failure samples (GEO dataset C) was downregulated (Fig. 9).
Fig. 9.
Heatmaps representing gene expression profiles of HA-associated genes across three independent GEO datasets (Dataset A, Dataset B, and Dataset C). Red indicates gene upregulation, while blue denotes downregulation. Genes are categorized based on their functional roles in HA metabolism: HA synthesis, HA degradation, and HA-binding/receptors. Sample groups within each dataset are indicated: P– proliferative phase, S– secretory phase, ES– early secretory, MS– mid-secretory, F– fertile, RIF– recurrent implantation failure. Abbreviations: HAS– hyaluronan synthase, CEMIP– cell migration-inducing and HA-binding protein, HMMR– HA-mediated motility receptor, LAYN– layilin, TSG6 - tumor necrosis factor-inducible gene 6, VCAN– versican, BCAN– brevican, SDC– syndecan, GPC– glypican, AGRN– agrin, ITIH– Interalpha-trypsin inhibitor heavy chain, TNC– tenascin C
With respect to degradation enzymes (Fig. 4), HYAL2 was positively correlated with the HA-interacting molecules glypican 1 and agrin, which all presented decreasing trends (Fig. 9) in the menstrual cycle (GEO datasets A and B). Interestingly, the expression of these genes was also lower in IVF failure samples than in fertile control samples (GEO dataset C). CEMIP1 expression was negatively correlated with decreasing expression of the receptor RHAMM (Fig. 9) during the menstrual cycle (GEO datasets A and B) but not in IVF failure samples (GEO dataset C), in which the expression of both genes had decreasing trends (Fig. 9). CEMIP2 was positively correlated with syndecan 1, syndecan 4 and glypican 4 in all analyzed datasets (GEO datasets A, B and C), and its expression tended to increase during the menstrual cycle but was downregulated in IVF failure samples (Fig. 9).
An increasing trend of HA surface receptor CD44 gene expression (Fig. 5) was present during the menstrual cycle (Fig. 9), and its expression correlated positively with that of other cell surface HA receptors, including syndecan 1, syndecan 3 and the ECM receptor versican, but negatively with that of the HA receptor glypican 2 and the ECM receptor tenascin C throughout the menstrual cycle (GEO datasets A and B; Fig. 9).
Interestingly, CD44 gene expression did not correlate with HA-interacting protein-encoding gene expression in IVF failure samples (GEO dataset C). In contrast, the expression of other HA-binding proteins and receptors was positively correlated across all datasets (GEO datasets A, B and C), particularly Layilin and syndecan 2, agrin and glypican 1, and Agrin and ITIH4, whose expression decreased during the menstrual cycle (Fig. 9), and their expression was also lower in IVF failure samples than in fertile control samples (Fig. 9).
General gene expression patterns in samples from women with repeated IVF failure compared with those from fertile controls
Considering the genes with the most significant changes in expression and a uniform pattern, it can generally be concluded that the expression of genes related to HA metabolism or genes encoding proteins that interact with HA is mostly downregulated in endometrial samples from women with unsuccessful IVF compared with fertile samples (Fig. 9). The most important HA-related genes in the context of IVF failure are HAS2, HAS3, CEMIP1, CEMIP2, CD44, versican, syndecan 1, syndecan 3 and syndecan 4, as their expression is upregulated in the mid-secretory phase of the menstrual cycle and downregulated in IVF failure samples (Fig. 9). Interestingly, syndecan 2 was the only gene among the analyzed genes whose expression was upregulated in the IVF failure samples (Fig. 9). However, their role in the mechanism of endometrial receptivity remains a subject of future research.
Discussion
This study provides comprehensive insights into the critical role of HA metabolism in endometrial receptivity and its potential implications for reproductive success. By systematically analyzing publicly available RNA sequencing datasets from endometrial biopsies, we identified significant dynamic changes in the expression profiles of genes associated with HA synthesis, degradation, and interactions throughout the menstrual cycle. During the mid-secretory phase, coinciding with WOI, genes encoding HA synthases (HAS2 and HAS3) were distinctly upregulated, emphasizing the importance of enhanced HA synthesis for receptivity. Concurrently, increased expression of nonclassical HA-degrading enzymes (CEMIP and CEMIP2) was observed, suggesting a controlled degradation process essential for generating bioactive HA fragments. Disruptions in this delicate equilibrium, as observed in women experiencing repeated IVF failures, were characterized by notable downregulation of HAS enzymes, HA-degrading enzymes, and primary HA receptors, particularly CD44. This suggests that impaired HA metabolism may compromise endometrial receptivity, ultimately leading to implantation failure.
HA metabolism
The importance of HA synthesis for endometrial receptivity is supported by increased expression of HAS2 and HAS3 during the menstrual cycle, with a peak in the mid-secretory phase when the WOI is present. Each HAS enzyme has a unique expression profile and properties that contribute to the complex regulation of HA in the body. In general, compared with the other two enzymes, HAS1 has lower activity and functions optimally at high substrate concentrations, making it less significant in many tissues. This is supported by almost no detectable expression of HAS1 in the analyzed samples of the endometrium. HAS2 is the most critical HAS in many tissues and has the highest level of activity under normal physiological conditions. However, in the human endometrium, the most abundantly expressed HAS independent of the phase of the cycle is HAS3, according to the data from GEO datasets A and B and the Human Protein Atlas.
Increased HAS expression in the endometrium during the receptive state is correlated with increased HA deposition reported in the literature, which is reported to be initially faint and patchy during the proliferative phase, gradually increases during the secretory phase and peaks during the mid-secretory phase [44, 64]. Throughout the menstrual cycle, HA is localized mainly in the endometrial stroma. However, during the late secretory phase, in addition to the abundant HA predecidualized, HA may also be present adjacent to the endometrial surface [44]. HA synthesis increases dramatically around the time of implantation [44, 65, 66]. Ovarian steroid hormones are thought to have differential effects on the expression of specific HASs [44, 45, 67, 68]. In mice, the deposition of HA in the uterus increases after progesterone treatment [69] and before embryo implantation [70]. The expression profiles of different HAS isoforms should be considered in terms of their ability to synthetize HA of different molecular weights with different biological properties, which is known for the production of low-molecular-weight HA [20, 68, 71]. HAS3, while having high activity, synthesizes shorter HA chains than HAS2 does, which influences its specific role in cellular signaling and tissue remodeling. Low-molecular-weight HA is supposed to have mostly cell-activating properties, including the ability to modulate immune responses and promote cellular interactions; thus, it can be crucial for implantation. The importance of HA synthesis for a receptive endometrium is supported by the observed significant decrease in HAS2 and HAS3 expression in the endometrium of patients with repeated IVF failure compared with that in the endometrium of fertile women on the basis of a reanalysis of GEO dataset C. However, despite the suggested importance of HA during the WOI, other studies have shown that the loss of HA from the endometrial surface caused by pharmacological inhibition of uterine HA synthesis in sheep leads to an increase in embryo attachment [72] and enhances the interaction between the trophectoderm and the endometrial epithelium in isolated human endometrial cells in vitro [73]. This finding suggests that the potentiated HA synthesis during the endometrial change toward the WOI should be counterbalanced by HA degradation. The expression of the nonclassical hyaluronidases CEMIP and CEMIP2 is upregulated in the endometrium during the menstrual cycle, with a peak in the mid-secretory phase, when the WOI occurs. However, at the same time, the expression of classical HYAL2 was significantly downregulated in the secretory phase, as demonstrated by our reanalysis of GEO datasets A and B. Interestingly, the expression of HYAL2, CEMIP and CEMIP2 decreased in repeated IVF failure samples, suggesting the importance of HA degradation before embryo implantation. Similarly, enzymatic degradation of HA from the surface of a monolayer of the endometrial cell line Ishikawa helps establish stable attachment [74]. Conversely, infusion of HA into the uterine lumen inhibits implantation [72]. While classical hyaluronidases to which HYAL2 belongs typically mediate HA breakdown through lysosomal or surface-associated enzymatic activity, CEMIP1 and CEMIP2 represent alternative, mostly nonlysosomal mechanisms of HA catabolism. CEMIP1 is often induced by inflammatory stimuli, and the observed upregulation of these enzymes may reflect a cellular response to inflammatory or stress signals, often associated with tissue remodeling or altered ECM dynamics typical of changes in the endometrium during endometrial changes toward the WOI. Moreover, CEMIP-mediated pathways may preferentially degrade high-molecular-weight HA, generating bioactive HA fragments that further influence cell behavior, promoting cell proliferation and migration.
This regulation underscores the complexity of HA turnover and suggests that the potentiation of both HA synthesis and HA degradation occurs in the endometrium toward the WOI. HA degradation is associated with the formation of biologically active molecules that promote changes in endometrial morphology and the cell phenotype. Thus, we can hypothesize that endometrial receptivity can be regulated by small fragments of HA produced by increased turnover in the endometrium provided by increased HA synthesis counterbalanced by potentiated degradation of the produced HA. Overall, the reported HA deposition and modulation of the expression of enzymes responsible for HA synthesis and degradation suggest that balanced HA metabolism within the endometrium, involving both synthesis and timely degradation, is crucial for successful embryo implantation and that disruptions in this balance may contribute to implantation failure.
Cell surface HA receptors
The response of cells to HA is dependent on its recognition by different types of HA receptors, which also play essential roles in the attachment of HA to the cell surface and the formation of complex three-dimensional HA-rich ECMs. In general, increased expression of HA receptors during the secretory phase has been reported [68], which may be connected to maintaining HA on the cell surface. However, importantly, these receptors also facilitate interactions between the endometrium and the HA-rich ECM of the trophectoderm. Reciprocally, HA present on the endometrial surface serves as a ligand for HA-binding proteins and cell receptors.
The major HA receptor CD44 is the most highly expressed among the cell surface HA receptors in the endometrium, and its expression is increased during both the early and mid-secretory phases compared with the proliferative phase. In contrast, other HA-binding receptors, RHAMM and laylin, were significantly downregulated in the endometrium during the menstrual cycle, with decreased expression in the receptive state during the WOI. However, the expression levels of RHAMM and laylin in the endometrium are markedly lower than those of CD44. Increased expression of CD44 by the endometrium can contribute to increased deposition of HA during the WOI reported in the literature [44, 64] or, on the other hand, can promote adhesion to HA present in the ECM of developing embryo.
In agreement with the hypothesis that CD44 is important for endometrial receptivity, the expression of CD44 in the endometria of infertile women during the WOI was lower than that in the endometria of fertile women. There were no significant changes in the expression of RHAMM or laylin in endometrial samples from women with RIF compared with those from fertile controls. Similarly, increased expression of CD44, specifically CD44v3, was reported in endometrial stromal cells during the mid-secretory phase and was notably downregulated in IVF failure samples [75, 76]. Thus, CD44 may be a key HA receptor during WOI. The functional importance of CD44 at the endometrial interface during embryo implantation was highlighted in the study of Illera and coworkers (2004), where intrauterine infusion of anti-CD44 antibodies hindered embryo implantation in rabbits [77]. However, in mice, CD44-null mice are fertile and viable with no morphological defects [74, 78, 79], suggesting either an alternative role of HA in embryo implantation in mice or, more likely, compensatory mechanisms involving alternative HA receptor development during CD44-null mouse development. However, direct implantation-focused knockout experiments appear to be lacking.
In previous studies, CD44 in the endometrium was shown to colocalize in the stroma and immune cells [80, 81]; however, they did not study the luminal epithelium, which is in first contact with embryo during implantation. Furthermore, the analyzed gene expression of CD44 does not necessarily mirror the presence of the CD44 receptor on the surface of the endometrial epithelium. In addition, a possible regulatory mechanism of the interaction between CD44 and HA is CD44 glycosylation [82, 83]. Furthermore, CD44 cleavage of the soluble form of CD44 from the cell surface is a well-described phenomenon. Accordingly, the level of CD44 released in the uterine lavage fluid of infertile women in the proliferative phase is reportedly lower than that in the uterine lavage fluid of fertile women according to proteomic analysis [84], which may be related to the decreased expression of CD44 in the endometria of infertile women.
A possible role in endometrial receptivity was also suggested for another HA receptor, RHAMM, on the basis of spatiotemporal expression in the mouse endometrium during the estrous cycle and the peri-implantation period [85]. However, these observations may also be specific to mice since reanalysis of human samples revealed downregulation of the gene expression of these receptors during the menstrual cycle and no significant difference between the fertile and repeated IVF failure samples. Similar gene expression trends to those of RHAMM were also observed for laylin. The absence of significant changes in expression in RIF samples suggests that RHAMM and laylin are not involved in the induction of endometrial receptivity.
Proteins forming an HA three-dimensional matrix in the extracellular space
Several HA-binding or HA-associated proteins, both directly and indirectly interacting with HA, exhibit dynamic expression changes throughout the menstrual cycle. The major skeleton for HA without direct contact with the cell surface is formed by lecticans, with the most highly expressed lectican in the endometrium being versican, whose important role in endometrial receptivity is supported by its upregulation during the menstrual cycle and decreased expression in repeated IVF failure samples. Similarly, the upregulation of versican was reported in the receptive endometrium of goats but not in the prereceptive endometrium during the menstrual cycle [86]. Moreover, the importance of versican for embryo implantation was shown by Miyazaki et al. [87], who proposed the hypothesis that versican improves embryo implantation through interaction with CD44 and integrin β1 [88] that are both expressed by the luminal epithelium and preimplantation human embryos [89]. Another detectable lectican in the endometrium is brevican, which has a similar pattern during the menstrual cycle; however, no difference was detected between the RIF samples and the fertile controls. Interestingly, the expression of tenascin-C, which interacts with lecticans, was decreased during the menstrual cycle, with no significant difference between the fertile and IVF failure samples.
Other proteins that are key for stabilization and the three-dimensional organization of HA-rich ECM are PGs syndecans, glypicans, and agrin. The expression of syndecans 1, 3, and 4 was found to be increased in the endometrium in the mid-secretory phase than in the proliferative and early-secretory phases of the menstrual cycle, which is in agreement with the current literature [32]. In addition, these syndecans were decreased in the IVF failure samples. Similarly, altered syndecan 1 expression has been reported in other reproductive pathologies, such as premature delivery, fetal growth restriction or preeclampsia, and is used for chronic endometritis diagnosis in humans [32]. Interestingly, syndecan 2, which is expressed primarily by vascular cells and fibroblasts [90], presented opposite expression trends to those of syndecan 1, 3 and 4. Syndecan 2 is connected to shedding of E-cadherin in epithelial cells, as shown in colorectal carcinoma [91]. As E-cadherin is known to be important for endometrial receptivity, it can be hypothesized that downregulation of syndecan 2 During the WOI is necessary for maintaining E-cadherin in epithelial cells. Compared with that in fertile endometrial samples, the expression of syndecan 2 in IVF failure endometrium samples was not significantly changed. Thus, the different expression patterns of syndecans can be explained by their different functions other than stabilizing the HA-rich matrix. The most highly expressed glypican in the endometrium is glypican 1, together with other members of this subfamily [92], glypicans 2, 4 and 6. In contrast, the second subfamily of glypicans, glypican 3 and 5 [92], showed almost no expression in the endometrium. The downregulation of glypican 1 and 2 during the menstrual cycle toward the WOI could suggest the importance of loosening the complexity of the HA-rich ECM for endometrial receptivity. Interestingly, all of these genes were downregulated in the IVF failure samples compared with the fertile controls. Although the decrease in the expression of several PGs stabilizing the HA-rich matrix during the menstrual cycle suggests the importance of loosening the complexity of this matrix, which is consistent with the HA-disappearance hypothesis, their even lower expression in IVF failure samples suggests that their presence may still be required to stabilize the HA-rich matrix on the trophoblast and support adhesion to endometrium during implantation.
Regarding HCs, a group of secreted proteins that form complexes with HA, the detectable expression of these genes in the endometrium is only observed for ITIH4 and ITIH5. In general, these genes are downregulated during the menstrual cycle, and even greater decreases in expression were detected in the IVF failure samples than in the fertile control samples. Analysis revealed increased expression of TSG-6, a secreted protein providing HA-HC complexes formation, during the secretory phase compared with the proliferative phase. The change in TSG-6 expression during the secretory phase (early- to mid-secretory) is variable, which is in agreement with the literature reporting that TSG-6 mRNA is expressed at similar levels in endometrial tissues (and isolated cell populations) collected during the late-proliferative and middle-secretory phases [93]. This finding suggests that TSG-6 is not subject to endocrine regulation; however, the expression of the TSG-6 gene is elevated in endometrial stromal cells following exposure to trophoblasts or placenta-conditioned media [35]. Capp et al. reported reduced expression of TSG-6 in the endometria of women with unexplained infertility compared with fertile controls at the time of implantation [93]. Similarly, decreased expression of TSG-6 was observed in endometrial samples from patients with repeated IVF failure, suggesting that TSG-6 is important for endometrial receptivity. Interestingly, TSG-6−/− female mice are able to ovulate, but the sterility of TSG-6−/− females is the result of the inability of their oocytes to fertilize in vivo [35]. Nevertheless, the role of TSG-6 in endometrial receptivity remains unknown.
These results suggest that the release of complex HA mesh in the ECM of the endometrium and the formation of biologically active low-molecular-weight fragments of HA is probably necessary for endometrial receptivity. This is due to more intensive HA synthesis together with the active degradation of HA chains into smaller fragments. The release of HA-rich mesh is further supported by the decreased presence of stabilizing PGs such as tenascin-C or cross-linking proteins such as ITIH5 or TSG6. Other HA-interacting partners, such as the CD44 receptor and versican or syndecans, are upregulated, and HA-rich matrix release enables them to support the adhesive interaction between the endometrium and HA-rich ECM of embryo.
Given the importance of HA in the endometrial environment, there is ongoing interest in therapeutic applications that utilize exogenous HA or modulate endogenous HA metabolism and deposition during implantation and decidualization. Research has shown that HA degradation and pharmacological inhibition of HA synthesis improve embryo implantation in sheep [72]. Similarly, the clearance of HA from endometrial epithelial cells improved mouse embryo attachment in vitro [74]. Conversely, the infusion of HA into the uterine lumen inhibits embryo implantation in sheep [72].
Limitations
Following limitations must be considered while interpreting the presented findings. The study is based on the reanalysis of publicly available RNA sequencing data from endometrial biopsies. Although all datasets provide high-quality transcriptomic profiles, they originate from different studies with notable methodological differences. Dataset A used TRIzol-based RNA extraction and sequencing in a single U.S. facility, whereas Datasets B and C used QIAzol extraction with miRNA depletion and were sequenced at multiple European sites. Additionally, the datasets differ in geographic origin (USA vs. Spain) and have unequal sample sizes across groups, which may affect comparability and statistical power.
Conclusion
This study emphasizes the critical role of HA metabolism and associated ECM components in establishing endometrial receptivity. Our analysis demonstrated dynamic regulation of HA synthesis enzymes, HA-degrading enzymes, and key HA receptors during the menstrual cycle, with marked upregulation during the mid-secretory phase, corresponding to WOI. These results underscore the finely tuned balance of HA synthesis and degradation required to achieve optimal conditions for embryo attachment and implantation. Notably, dysregulated expression patterns of HA-related genes were observed in women experiencing repeated IVF failure. Reduced expression of HAS enzymes, HA-degrading enzymes, and critical HA receptors in these patients suggests disrupted HA metabolism and altered ECM composition as potential underlying mechanisms for impaired endometrial receptivity.
The findings presented herein suggest the significant contribution of HA and associated molecules to endometrial receptivity and also can indicate specific molecular targets potentially contributing to IVF failure. This provides a foundation for future research aimed at clarifying the detailed mechanistic pathways through which HA modulates endometrial receptivity. Importantly, prospective studies should explore the functional implications of altered HA metabolism, including direct analyses of HA fragment generation, receptor interactions, and ECM structural remodeling in both physiological and pathological contexts. Clinical validation of HA-related biomarkers as predictive indicators of endometrial receptivity may further refine embryo transfer strategies, ultimately enhancing implantation success rates.
Furthermore, therapeutic manipulation of HA pathways can present a promising avenue for improving outcomes in assisted reproductive technologies. Future research should investigate pharmacological interventions or targeted molecular therapies designed to restore HA metabolic balance in the endometrium of patients with recurrent IVF failures.
In conclusion, our study highlights HA metabolism as a crucial regulator of endometrial receptivity, identifies disturbances in these pathways as potential contributors to IVF failure, and lays the groundwork for future translational research. Addressing the gaps in our understanding of HA’s precise roles in implantation will likely open novel therapeutic and diagnostic opportunities, providing tangible benefits for patients facing infertility.
Supplementary Information
Abbreviations
- AGRN
Agrin
- BCAN
Brevican
- CEMIP
Cell migration-inducing and hyaluronan-binding protein (also known as KIAA1199)
- CEMIP2
Transmembrane protein 2 (also known as TMEM2)
- ECM
Extracellular matrix
- ES
Early secretory phase (of the menstrual cycle)
- F
Fertile (or fertile samples in dataset comparisons)
- GAG
Glycosaminoglycan
- GEO
Gene expression omnibus
- GlcA
Glucuronic acid
- GlcNAc
N-acetylglucosamine
- GPC (GPC1, GPC2, etc.)
Glypican
- HAS (HAS1, HAS2, HAS3)
Hyaluronan synthase
- HA
Hyaluronan (also called hyaluronic acid)
- HC
Heavy chain (in reference to interα-trypsin inhibitor heavy chains)
- HMMR
Hyaluronan-mediated motility receptor (also called RHAMM)
- HYAL (HYAL1, HYAL2, etc.)
Hyaluronidase
- IαI
Interα-trypsin inhibitor
- ITIH (ITIH1, ITIH2, etc.)
Interα-trypsin inhibitor heavy chain
- IVF
In vitro fertilization
- LAYN
Layilin
- LYVE-1
Lymphatic vessel endothelial hyaluronan receptor 1
- MS
Mid-secretory phase (of the menstrual cycle)
- P
Proliferative phase (of the menstrual cycle)
- PG
Proteoglycan
- RHAMM
Receptor for hyaluronic acid-mediated motility (alternative name for HMMR)
- S
Secretory phase (of the menstrual cycle)
- SDC (SDC1, SDC2, etc.)
Syndecan
- TNC
Tenascin C
- TSG-6
Tumor necrosis factor-stimulated gene 6
- WOI
Window of implantation
Authors’ contributions
KE: Formal analysis, software, data curation, and writing; MiM: Writing– original draft; FN: Formal analysis and writing; MaM: Writing; TS: Writing; KL: Formal analysis, writing, and funding acquisition; RM: Writing and funding acquisition.
Funding
This work was supported by the VEGA grant scheme of the Ministry of Education, Science, Research, and Sport of the Slovak Republic [Grant No. 1/0747/24] and by the institutional support of the Institute of Biophysics of the Czech Academy of Sciences (68081707).
Data availability
No datasets were generated during the current study.
Declarations
Ethics approval and consent to participate
This study did not require ethical approval or patient consent, as it was based solely on reanalysis of publicly available data from previously published studies in the GEO database (GSE106602 and GSE132711). All analyses followed the methods reported in the original publications.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Silvia Toporcerova, Email: silvia.toporcerova@upjs.sk.
Lukas Kubala, Email: kubalal@ibp.cz.
References
- 1.Mascarenhas MN, Flaxman SR, Boerma T, Vanderpoel S, Stevens GA, National. Regional, and Global Trends in Infertility Prevalence Since 1990: A Systematic Analysis of 277 Health Surveys. PLoS Med. 2012;9:e1001356. [DOI] [PMC free article] [PubMed]
- 2.WHO. Infertility prevalence estimates, 1990–2021 [Internet] Geneva: World Health Organization; 2023 [cited 2025 Feb 24]. Available from: https://www.who.int/publications/i/item/978920068315.
- 3.Walker MH, Tobler KJ. Female infertility [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 [cited 2025 Feb 24]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK556039/. [PubMed]
- 4.Society for Assisted Reproductive Technology. IVF success rates [Internet]. Washington (DC): SART; 2022 [cited 2025 Feb 24]. Available from https://www.sartcorsonline.com/EmbryoOutcome/PublicSARTOutcomeTables?reportingYear=2022&ClinicPKID=.
- 5.Koot YEM, Teklenburg G, Salker MS, Brosens JJ, Macklon NS. Molecular aspects of implantation failure. Biochim Biophys Acta. 2012;1822:1943–50. [DOI] [PubMed] [Google Scholar]
- 6.Rossi F, Luppi S, Fejza A, Giolo E, Ricci G, Andreuzzi E. Extracellular matrix and pregnancy: functions and opportunities caught in the net. Reprod Biol Endocrinol. 2025;23:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aghajanova L, Hamilton AE, Giudice LC. Uterine receptivity to human embryonic implantation: histology, biomarkers, and transcriptomics. Semin Cell Dev Biol. 2008;19:204–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruiz-Alonso M, Blesa D, Díaz-Gimeno P, Gómez E, Fernández-Sánchez M, Carranza F, et al. The endometrial receptivity array for diagnosis and personalized embryo transfer as a treatment for patients with repeated implantation failure. Fertil Steril. 2013;100:818–24. [DOI] [PubMed] [Google Scholar]
- 9.Ruiz-Alonso M, Valbuena D, Gomez C, Cuzzi J, Simon C. Endometrial receptivity analysis (ERA): data versus opinions. Hum Reprod Open. 2021;2021(2):hoab011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aplin J. Embryo implantation: the molecular mechanism remains elusive. Reprod Biomed Online. 2006;13:833–9. [DOI] [PubMed] [Google Scholar]
- 11.Cakmak H, Taylor HS. Implantation failure: molecular mechanisms and clinical treatment. Hum Reprod Update. 2011;17:242–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zambuto SG, Jain I, Clancy KBH, Underhill GH, Harley BAC. Role of extracellular matrix biomolecules on endometrial epithelial cell attachment and cytokeratin 18 expression on gelatin hydrogels. ACS Biomater Sci Eng. 2022;8:3819–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sillem M. Extracellular matrix remodelling in the endometrium and its possible relevance to the pathogenesis of endometriosis. Hum Reprod Update. 1998;4:730–5. [DOI] [PubMed] [Google Scholar]
- 14.O’Connor BB, Pope BD, Peters MM, Ris-Stalpers C, Parker KK. The role of extracellular matrix in normal and pathological pregnancy: future applications of microphysiological systems in reproductive medicine. Exp Biol Med (Maywood). 2020;245:1163–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Casale J, Crane JS, Biochemistry. Glycosaminoglycans.Glycosaminoglycans [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 [cited 2025 Feb 24]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK544295/. [PubMed]
- 16.Jiang D, Liang J, Noble PW. Hyaluronan as an immune regulator in human diseases. Physiol Rev. 2011;91:221–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Triggs-Raine B. Biology of hyaluronan: insights from genetic disorders of hyaluronan metabolism. WJBC. 2015;6:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kobayashi T, Chanmee T, Itano N. Hyaluronan. Metabolism and Function. Biomolecules. 2020;10:1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamamoto H, Tobisawa Y, Inubushi T, Irie F, Ohyama C, Yamaguchi Y. A mammalian homolog of the zebrafish transmembrane protein 2 (TMEM2) is the long-sought-after cell-surface hyaluronidase. J Biol Chem. 2017;292:7304–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Misra S, Hascall VC, Markwald RR, Ghatak S. Interactions between hyaluronan and its receptors (CD44, RHAMM) regulate the activities of inflammation and cancer. Front Immunol. 2015;6:201. [DOI] [PMC free article] [PubMed]
- 21.Day AJ. The structure and regulation of hyaluronan-binding proteins. Biochem Soc Trans. 1999;27(2):115–21. [DOI] [PubMed]
- 22.Kennel SJ, Lankford TK, Foote LJ, Shinpock SG, Stringer C. CD44 expression on murine tissues. J Cell Sci. 1993;104(Pt 2):373–82. [DOI] [PubMed] [Google Scholar]
- 23.Bergqist A-S, Yokoo M, Bage R, Sato E, Rodriguez-Martinez H. Detection of the hyaluronan receptor CD44 in the bovine oviductal epithelium. J Reprod Dev. 2005;51:445–53. [DOI] [PubMed] [Google Scholar]
- 24.Perry K, Haresign W, Wathes DC, Khalid M. Hyaluronan (HA) content, the ratio of HA fragments and the expression of CD44 in the ovine cervix vary with the stage of the oestrous cycle. Reproduction. 2010;140:133–41. [DOI] [PubMed] [Google Scholar]
- 25.Rodriguez Hurtado I, Stewart AJ, Wolfe DF, Caldwell FJ, Harrie M, Whitley EM. Immunolocalization of the hyaluronan receptor CD44 in the reproductive tract of the mare. Theriogenology. 2011;75:276–86. [DOI] [PubMed] [Google Scholar]
- 26.López J, Valdez-Morales FJ, Benítez-Bribiesca L, Cerbón M, Carrancá AG. Normal and cancer stem cells of the human female reproductive system. Reproductive Biology Endocrinol. 2013;11:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harris EN, Baker E. Role of the hyaluronan receptor, Stabilin-2/HARE, in health and disease. Int J Mol Sci. 2020;21:3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Du Y, Liu H, He Y, Liu Y, Yang C, Zhou M, et al. The Interaction between LYVE-1 with Hyaluronan on the Cell Surface May Play a Role in the Diversity of Adhesion to Cancer Cells. PLoS ONE. 2013;8:63463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shendi D, Marzi J, Linthicum W, Rickards AJ, Dolivo DM, Keller S, et al. Hyaluronic acid as a macromolecular crowding agent for production of cell-derived matrices. Acta Biomater. 2019;100:292–305. [DOI] [PubMed] [Google Scholar]
- 30.Iozzo RV, Schaefer L. Proteoglycan form and function: A comprehensive nomenclature of proteoglycans. Matrix Biol. 2015;42:11–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lundell A, Olin AI, Mörgelin M, al-Karadaghi S, Aspberg A, Logan DT. Structural basis for interactions between tenascins and Lectican C-Type lectin domains: evidence for a crosslinking role for tenascins. Structure. 2004;12:1495–506. [DOI] [PubMed] [Google Scholar]
- 32.Xu Y, Mei J, Diao L, Li Y, Ding L. Chronic endometritis and reproductive failure: role of syndecan-1. Am J Rep Immunol. 2020;84:e13255. [DOI] [PubMed] [Google Scholar]
- 33.Sarrazin S, Lamanna WC, Esko JD. Heparan sulfate proteoglycans. Cold Spring Harb Perspect Biol. 2011;3:a004952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Day AJ, Milner CM. TSG-6: A multifunctional protein with anti-inflammatory and tissue-protective properties. Matrix Biol. 2019;78–79:60–83. [DOI] [PubMed] [Google Scholar]
- 35.Fülöp C, Szántó S, Mukhopadhyay D, Bárdos T, Kamath RV, Rugg MS, et al. Impaired cumulus mucification and female sterility in tumor necrosis factor-induced protein-6 deficient mice. Development. 2003;130:2253–61. [DOI] [PubMed] [Google Scholar]
- 36.Wisniewski H-G, Vilček J. Cytokine-induced gene expression at the crossroads of innate immunity, inflammation and fertility: TSG-6 and PTX3/TSG-14. Cytokine Growth Factor Rev. 2004;15:129–46. [DOI] [PubMed] [Google Scholar]
- 37.Suchanek E, Simunic V, Juretic D, Grizelj V. Follicular fluid contents of hyaluronic acid, follicle-stimulating hormone and steroids relative to the success of in vitro fertilization of human oocytes. Fertil Steril. 1994;62:347–52. [DOI] [PubMed] [Google Scholar]
- 38.Lee CN, Ax RL. Concentrations and composition of glycosaminoglycans in the female bovine reproductive tract. J Dairy Sci. 1984;67:2006–9. [DOI] [PubMed] [Google Scholar]
- 39.Schoenfelder M, Einspanier R. Expression of hyaluronan synthases and corresponding hyaluronan receptors is differentially regulated during oocyte maturation in cattle. Biol Reprod. 2003;69:269–77. [DOI] [PubMed] [Google Scholar]
- 40.Babayan A, Neuer A, Dieterle S, Bongiovanni AM, Witkin SS. Hyaluronan in follicular fluid and embryo implantation following in vitro fertilization and embryo transfer. J Assist Reprod Genet. 2008;25:473–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Straach KJ, Shelton JM, Richardson JA, Hascall VC, Mahendroo MS. Regulation of hyaluronan expression during cervical ripening. Glycobiology. 2005;15:55–65. [DOI] [PubMed] [Google Scholar]
- 42.Salustri A, Yanagishita M, Hascall VC. Synthesis and accumulation of hyaluronic acid and proteoglycans in the mouse cumulus cell-oocyte complex during follicle-stimulating hormone-induced mucification. J Biol Chem. 1989;264:13840–7. [PubMed] [Google Scholar]
- 43.Maradny EE, Kanayama N, Kobayashi H, Hossain B, Khatun S, Liping S, et al. The role of hyaluronic acid as a mediator and regulator of cervical ripening. Hum Reprod. 1997;12:1080–8. [DOI] [PubMed] [Google Scholar]
- 44.Afify A, Craig S, Paulino MD. Temporal variation in the distribution of hyaluronic acid, CD44s, and CD44v6 in the human endometrium across the menstrual cycle. Appl Immunohistochem Mol Morphology. 2006;14:328–33. [DOI] [PubMed] [Google Scholar]
- 45.Raheem KA, Marei WF, Mifsud K, Khalid M, Wathes DC, Fouladi-Nashta AA. Regulation of the hyaluronan system in ovine endometrium by ovarian steroids. Reproduction. 2013;145:491–504. [DOI] [PubMed] [Google Scholar]
- 46.Perry K, Haresign W, Wathes DC, Pitsillides AA, Khalid M. Cervical expression of hyaluronan synthases varies with the stage of the estrous cycle in the Ewe. Theriogenology. 2012;77:1100–10. [DOI] [PubMed] [Google Scholar]
- 47.Salustri A, Yanagishita M, Underhill CB, Laurent TC, Hascall VC. Localization and synthesis of hyaluronic acid in the cumulus cells and mural granulosa cells of the preovulatory follicle. Dev Biol. 1992;151:541–51. [DOI] [PubMed] [Google Scholar]
- 48.Ueno S, Yoshida N, Niimura S. Amount of hyaluronan produced by mouse oocytes and role of hyaluronan in enlargement of the perivitelline space. J Reprod Dev. 2009;55:496–501. [DOI] [PubMed] [Google Scholar]
- 49.Takahashi N, Tarumi W, Ishizuka B. Involvement of hyaluronan synthesis in ovarian follicle growth in rats. Reproduction. 2014;147:189–97. [DOI] [PubMed] [Google Scholar]
- 50.Kimura N, Konno Y, Miyoshi K, Matsumoto H, Sato E. Expression of hyaluronan synthases and CD44 messenger RNAs in Porcine Cumulus-Oocyte complexes during in vitro maturation. Biol Reprod. 2002;66:707–17. [DOI] [PubMed] [Google Scholar]
- 51.Chavoshinejad R, Marei WFA, Hartshorne GM, Fouladi-Nashta AA. Localisation and endocrine control of hyaluronan synthase (HAS) 2, HAS3 and CD44 expression in sheep granulosa cells. Reprod Fertil Dev. 2014;28:765–75. [DOI] [PubMed] [Google Scholar]
- 52.Gardner DK, Rodriegez-Martinez H, Lane M. Fetal development after transfer is increased by replacing protein with the glycosaminoglycan hyaluronan for mouse embryo culture and transfer. Hum Reprod. 1999;14:2575–80. [DOI] [PubMed] [Google Scholar]
- 53.Friedler S, Schachter M, Strassburger D, Esther K, Ron El R, Raziel A. A randomized clinical trial comparing Recombinant hyaluronan/recombinant albumin versus human tubal fluid for cleavage stage embryo transfer in patients with multiple IVF-embryo transfer failure. Hum Reprod. 2007;22:2444–8. [DOI] [PubMed] [Google Scholar]
- 54.Hambiliki F, Ljunger E, Karlström P-O, Stavreus-Evers A. Hyaluronan-enriched transfer medium in cleavage-stage frozen-thawed embryo transfers increases implantation rate without improvement of delivery rate. Fertil Steril. 2010;94:1669–73. [DOI] [PubMed] [Google Scholar]
- 55.Heymann D, Vidal L, Shoham Z, Kostova E, Showell M, Or Y. The effect of hyaluronic acid in embryo transfer media in donor oocyte cycles and autologous oocyte cycles: a systematic review and meta-analysis. Hum Reprod. 2022;37:1451–69. [DOI] [PubMed] [Google Scholar]
- 56.Loutradi KE, Prassas I, Bili E, Sanopoulou T, Bontis I, Tarlatzis BC. Evaluation of a transfer medium containing high concentration of hyaluronan in human in vitro fertilization. Fertil Steril. 2007;87:48–52. [DOI] [PubMed] [Google Scholar]
- 57.Fancsovits P, Lehner A, Murber A, Kaszas Z, Rigo J, Urbancsek J. Effect of hyaluronan-enriched embryo transfer medium on IVF outcome: a prospective randomized clinical trial. Arch Gynecol Obstet. 2015;291:1173–9. [DOI] [PubMed] [Google Scholar]
- 58.Horiuchi Y, Yanaihara A, Hatakeyama S, Taga Y, Kondou T, Kuramoto G et al. Efficacy of Hyaluronan-Enriched Transfer Medium for Low-Grade Frozen Embryo Transfer. Cureus. 2023;15(5):e39210. [DOI] [PMC free article] [PubMed]
- 59.Hazlett WD, Meyer LR, Nasta TE, Mangan PA, Karande VC. Impact of embryoglue as the embryo transfer medium. Fertil Steril. 2008;90:214–6. [DOI] [PubMed] [Google Scholar]
- 60.Loutradi KE, Tarlatzi TB, Kolibianakis EM, Tarlatzis BC. Does hyaluronan improve embryo implantation? Curr Opin Obstet Gynecol. 2008;20:305–7. [DOI] [PubMed]
- 61.Heymann D, Vidal L, Or Y, Shoham Z. Hyaluronic acid in embryo transfer media for assisted reproductive technologies. Cochrane Database Syst Rev. 2020;9(9):CD007421. [DOI] [PMC free article] [PubMed]
- 62.Chi R-PA, Wang T, Adams N, Wu S-P, Young SL, Spencer TE, et al. Human endometrial transcriptome and progesterone receptor cistrome reveal important pathways and epithelial regulators. J Clin Endocrinol Metab. 2020;105:e1419–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Suhorutshenko M, Kukushkina V, Velthut-Meikas A, Altmäe S, Peters M, Mägi R, et al. Endometrial receptivity revisited: endometrial transcriptome adjusted for tissue cellular heterogeneity. Hum Reprod. 2018;33:2074–86. [DOI] [PubMed] [Google Scholar]
- 64.Afify A, Craig S, Stern R, Paulino P. Expression of hyaluronic acid and its receptors, CD44s and CD44v6, in normal, hyperplastic, and neoplastic endometrium. Annals Diagn Pathol. 2005;9:312–8. [DOI] [PubMed] [Google Scholar]
- 65.Zorn TM, Pinhal MA, Nader HB, Carvalho JJ, Abrahamsohn PA, Dietrich CP. Biosynthesis of glycosaminoglycans in the endometrium during the initial stages of pregnancy of the mouse. Cell mol biol. 1995;41:97–106. [PubMed] [Google Scholar]
- 66.Salamonsen L, Shuster S, Stern R. Distribution of hyaluronan in human endometrium across the menstrual cycle. Cell Tissue Res. 2001;306:335–40. [DOI] [PubMed] [Google Scholar]
- 67.Teixeira Gomes RC, Verna C, Nader H, dos Santos Simoes R, Dreyfuss J, Martins J, et al. Concentration and distribution of hyaluronic acid in mouse uterus throughout the estrous cycle. Fertil Steril. 2009;92:785–92. [DOI] [PubMed] [Google Scholar]
- 68.Fouladi-Nashta AA, Raheem KA, Marei WF, Ghafari F, Hartshorne GM. Regulation and roles of the hyaluronan system in mammalian reproduction. Reproduction. 2017;153:R43–58. [DOI] [PubMed] [Google Scholar]
- 69.Maioral G, Gomes R, Verna C, Simões M, Nader H, Simões R, et al. Concentration of glycosaminoglycan in ovariectomized mice uterus after treatment with ovarian steroids. Gynecol Endocrinol. 2016;21:1–5. [DOI] [PubMed] [Google Scholar]
- 70.San Martin S, Soto-Suazo M, Zorn TMT. Distribution of versican and hyaluronan in the mouse uterus during decidualization. Braz J Med Biol Res. 2003;36:1067–71. [DOI] [PubMed] [Google Scholar]
- 71.Itano N, Sawai T, Yoshida M, Lenas P, Yamada Y, Imagawa M, et al. Three isoforms of mammalian hyaluronan synthases have distinct enzymatic properties. J Biol Chem. 1999;274:25085–92. [DOI] [PubMed] [Google Scholar]
- 72.Marei WF, Wathes DC, Raheem KA, Mohey-Elsaeed O, Ghafari F, Fouladi-Nashta AA. Influence of hyaluronan on endometrial receptivity and embryo attachment in sheep. Reprod Fertil Dev. 2017;29:1763. [DOI] [PubMed] [Google Scholar]
- 73.McLaughlin JE, Santos MT, Binkley PA, Sultana M, Tekmal RR, Schenken RS, et al. Inhibition of hyaluronic acid synthesis decreases endometrial cell attachment, migration, and invasion. Reprod Sci. 2020;27:1058–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Berneau SC, Ruane PT, Brison DR, Kimber SJ, Westwood M, Aplin JD. Investigating the role of CD44 and hyaluronate in embryo-epithelial interaction using an in vitro model. Mol Hum Reprod. 2019;25:265–73. [DOI] [PubMed] [Google Scholar]
- 75.Canan S, İnan MA, Erdem A, Demirdağ E, Gündüz Mİ, Erdem Ö, et al. Evaluation of endometrial receptivity in recurrent pregnancy loss and recurrent implantation failure. Turk J Obstet Gynecol. 2024;21:22–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhou X, Cao Y, Zhou M, Han M, Liu M, Hu Y, et al. Decreased CD44v3 expression impairs endometrial stromal cell proliferation and decidualization in women with recurrent implantation failure. Reprod Biol Endocrinol. 2022;20:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Illera M, Bermejo P, Hernandez J, Gonzalez A, Illera J. The effect of ant-CD44 on embryo implantation in rabbits. Reprod Fertility Dev. 2004;16:190. [Google Scholar]
- 78.Schmits R, Filmus J, Gerwin N, Senaldi G, Kiefer F, Kundig T, et al. CD44 regulates hematopoietic progenitor distribution, granuloma formation, and tumorigenicity. Blood. 1997;90:2217–33. [PubMed] [Google Scholar]
- 79.Protin U, Schweighoffer T, Jochum W, Hilberg F. CD44-deficient mice develop normally with changes in subpopulations and recirculation of lymphocyte subsets. J Immunol. 1999;163:4917–23. [PubMed] [Google Scholar]
- 80.Koo YH, Na YJ, Ahn MY, Jeon HN, Yeom JI, Lee KS. Expression of CD44 in endometrial stromal cells from women with and without endometriosis and its effect on the adherence to peritoneal mesothelial cells. Obstet Gynecol Sci. 2013;56:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kong C-S, Ordoñez AA, Turner S, Tremaine T, Muter J, Lucas ES et al. Involvement of uterine natural killer cells in the natural selection of human embryos at implantationn [Preprint]. bioRxiv. 2020.
- 82.Bartolazzi A, Nocks A, Aruffo A, Spring F, Stamenkovic I. Glycosylation of CD44 is implicated in CD44-mediated cell adhesion to hyaluronan. J Cell Biol. 1996;132:1199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Katoh S, Kincade PW. Glycosylation of CD44 negatively regulates its recognition of hyaluronan. J Exp Med. 1995;182:419–29. [DOI] [PMC free article] [PubMed]
- 84.Fitzgerald HC, Evans J, Johnson N, Infusini G, Webb A, Rombauts LJR, et al. Idiopathic infertility in women is associated with distinct changes in proliferative phase uterine fluid proteins†. Biol Reprod. 2018;98:752–64. [DOI] [PubMed] [Google Scholar]
- 85.Ozbilgin K, Boz B. RHAMM expression in the rat endometrium during the estrous cycle and following implantation. 2012;13:131–7. [PMC free article] [PubMed]
- 86.Zhang L, An X-P, Liu X-R, Fu M-Z, Han P, Peng J-Y, et al. Characterization of the transcriptional complexity of the receptive and Pre-receptive endometria of dairy goats. Sci Rep. 2015;5:14244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Miyazaki Y, Horie A, Tani H, Ueda M, Okunomiya A, Suginami K et al. Versican V1 in human endometrial epithelial cells promotes bewo spheroid adhesion in vitro. Reproduction. 2019;53–64. [DOI] [PubMed]
- 88.Wu YJ, Pierre DPL, Wu J, Yee AJ, Yang BB. The interaction of versican with its binding partners. Cell Res. 2005;15:483–94. [DOI] [PubMed] [Google Scholar]
- 89.Kimber SJ. Molecular interactions at the Maternal-Embryonic interface during the early phase of implantation. Semin Reprod Med. 2000;18:237–54. [DOI] [PubMed] [Google Scholar]
- 90.Czarnowski D. Syndecans in cancer: A review of function, expression, prognostic value, and therapeutic significance. Cancer Treat Res Commun. 2021;27:100312. [DOI] [PubMed] [Google Scholar]
- 91.Jang B, Jung H, Chung H, Moon B-I, Oh E-S. Syndecan-2 enhances E-cadherin shedding and fibroblast-like morphological changes by inducing MMP-7 expression in colon cancer cells. Biochem Biophys Res Commun. 2016;477:47–53. [DOI] [PubMed] [Google Scholar]
- 92.Pan J, Ho M. Role of glypican-1 in regulating multiple cellular signaling pathways. Am J Physiology-Cell Physiol. 2021;321:C846–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Capp E, Milner CM, Williams J, Hauck L, Jauckus J, Strowitzki T, et al. Modulation of tumor necrosis factor-stimulated gene-6 (TSG-6) expression in human endometrium. Arch Gynecol Obstet. 2014;289:893–901. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated during the current study.