Abstract
Background
Obesity and metabolic syndrome have been identified as risk factors for nephrolithiasis. Although dietary improvements and exercise effectively prevent cardiovascular disease, their impact on recurrent nephrolithiasis has not been well studied. We investigated the associations between changes in body shape and recurrent nephrolithiasis.
Methods
Patients who underwent computed tomography (CT) twice at intervals of more than 12 months after retrograde ureteroscopy to treat upper urinary stones of the calcium component from January 2014 to December 2021 at our hospital were retrospectively included. The changes in waist area (WA) according to CT, age, sex, previous stone episodes, number of stones at surgery, residual stones after surgery, and body mass index (BMI) at surgery were compared with those of patients who experienced recurrence and those who did not experience recurrence via univariate and multivariate analyses.
Results
A total of 96 patients were included in this study, with 48 patients in the recurrence group and 48 patients in the nonrecurrence group. The median age was younger in the recurrence group than in the nonrecurrence group (61.5 versus 70.5 years). The median BMI was 23 in both the recurrence group and the nonrecurrence group. The rate of recurrence in female patients with a decreased WA was greater than that in those with an increased WA (n = 9/5 versus 4/16) (p = 0.0137). Age under 70 years and a decreased WA were significant risk factors for the recurrence of renal stones according to multivariate analysis (p < 0.05).
Conclusion
A decreased WA on CT images is a risk factor for recurrent nephrolithiasis after ureteroscopy, especially in nonobese female patients.
Trial registration
Trial registration was not performed, and the number of clinical trials was not suitable because this was a retrospective observational study at a single institution. All participants were retrospectively registered, and the study was approved by the Institutional Review Board and Ethics Committee of the Faculty of Medicine, Saga Japan (number 2023-11-R-4) at the date of 2/02/2024.
Keywords: Nephrolithiasis, Recurrence, Waist area, Computed tomography, Ureteroscopy
Background
Recently, urolithiasis has been defined as a disease induced by metabolic syndrome. In particular, obesity has been reported to be a strong risk factor for renal stone recurrence [1]. A high rate of renal stone recurrence has been reported, with 19% symptomatic recurrence and 54% radiographic recurrence [2]. Recurrent urolithiasis and complications of repeat surgery [3, 4], such as acute pyelonephritis and ureteral stricture, can damage renal function [5], progress to chronic kidney disease and lead to the development of end-stage kidney disease [6, 7].
Lifestyle improvement, adequate fluid intake, a balanced diet, active exercise and reducing alcoholic fluid intake are important for the prevention of renal stones [8]. An abundance of visceral fat is a risk factor for not only renal stones but also cardiovascular diseases. The ability of cardiorespiratory fitness to improve adiposity effectively reduces the incidence of cardiovascular diseases in obese patients [9]. However, whether dietary weight- control can prevent the recurrence of nephrolithiasis has not been sufficiently studied [10].
In this study, we aimed to elucidate the association between changes in body shape and the recurrence of renal stones in patients who were treated with retrograde ureteroscopy.
Methods
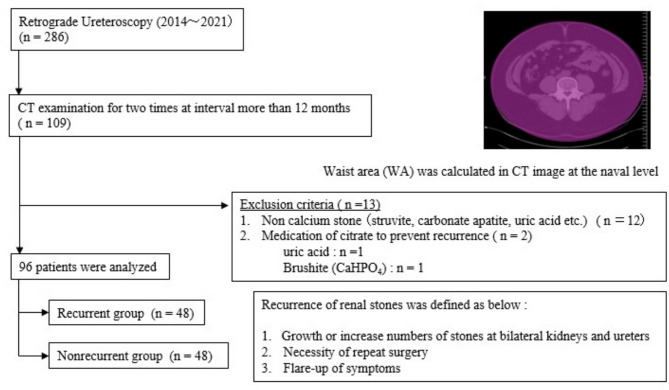
Adult patients older than 18 years who received treatment for upper urinary stones via retrograde ureteroscopy at our hospital between January 2014 and December 2021 (n = 286), and who underwent computed tomography (CT) examinations at intervals of more than 12 months, were retrospectively recruited (n = 109). Twelve patients who did not have a calcium stone composition (struvite; n = 4, carbonate apatite; n = 4, uric acid; n = 2 and others; n = 2) and 2 patients who received citrate medication for prevention of stone recurrence (uric acid; n = 1 and brushite (CaHPO4); n = 1) were excluded (n = 13).
The waist area (WA) was measured with a CT image at the navel level, which was examined at the primary point and at the secondary point with ShadeQuest/ViewR-DG software (Fujifilm, Japan). The change in WA every 12 months was calculated on the basis of the interval between two CT examinations for each patient. The change in WA (± mm2) was calculated as [(WA at the second CT image)-(WA at the primary CT image)] and the change in WA per year (± mm2/year) was calculated as [(WA at the second CT image)།(WA at the primary CT image)]× 12/interval of two CT examinations (months). The electronic medical records of the patients were retrospectively reviewed. Age, sex, previous stone episodes before surgery, the number of stones at surgery, the presence of residual stones at the primary evaluation, and body mass index (BMI) at the time of primary surgery were collected as subvariables. The diameter of residual stones was defined as being longer than 2 mm on CT images.
The groups of patients who experienced recurrence of renal stones and those who did not were compared. The recurrence of renal stones was defined as follows. 1: There was growth or an increased number of stones in the bilateral kidneys and ureters in the CT image at the secondary point. 2: Secondary surgical intervention was needed 12 months after primary ureteroscopy. 3: Flare-up symptoms caused by upper urinary stones. A summary of how the patients were selected is shown in Fig. 1.
Fig. 1.
Patients who underwent computed tomography (CT) twice at intervals of more than 12 months after ureteroscopy were included in this study
Differences were evaluated via the chi-square test for categorical variables and the Mann-Whitney U test for continuous variables. Multivariate logistic regression analysis was performed to identify the risk factors for the recurrence of renal stones. All the statistical analyses were performed with EZR [11], which is a modified version of the R commander designed to add statistical functions frequently used in biostatistics.
Results
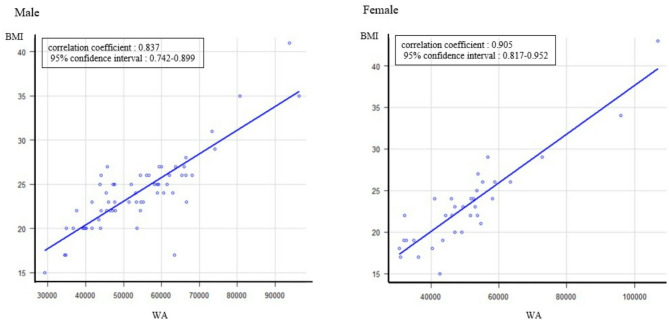
A total of 96 patients were included in this study. The median interval between CT examinations was 36 (range 12-108) months. Forty-eight patients experienced renal stone recurrence, and 48 patients experienced no recurrence. The median age of the recurrent group was younger than that of the nonrecurrent group (61.5 versus 70.5 years, p = 0.00243). There was no significant difference in sex (male/female, n = 35/13 versus 27/21, p = 0.135), previous stone episodes before surgery (n = 26 versus 20, p = 0.307), the number of stones at surgery (single/multiple, n = 17/31 versus 25/23,p = 0.149), residual fragment at the primary evaluation (n = 18 versus 16, p = 0.831), median BMI at surgery (23 versus 24, p = 0.808), median WA at the primary evaluation (50452 versus 53144 mm2, p = 0.47), secondary evaluation (50374 versus 56789.5 mm2, p = 0.481), median change in WA (+ 849.5 versus + 2013.5 mm2, p = 0.0858),or median change in WA per year (+ 278.5 versus + 784.5 mm2/year) (recurrent group versus nonrecurrent group). The recurrent group and the nonrecurrent group are compared in Table 1. The Pearson correlation coefficient between body mass index at surgery and WA at the primary point was 0.837 (95% confidence interval 0.742–0.899) for males and 0.905 (95% confidence interval 0.817–0.952) for females (Fig. 2).
Table 1.
Comparison between the recurrence group and the nonrecurrence group
| Recurrent group (n = 48) | Nonrecurrent group (n = 48) | p value | |
|---|---|---|---|
| Median Age (range) | 61.5 (19ཞ79) | 70.5 (40ཞ90) | 0.00243 |
| Sex : Male/Female (n) | 35/13 | 27/21 | 0.135 |
| Previous stone episodes before surgery (n) | 26 | 20 | 0.307 |
| The number of stones at surgery: Single/Multiple (n) | 17/31 | 25/23 | 0.149 |
| Residual fragment * at the primary evaluation (n) | 18 | 16 | 0.831 |
| Median BMI at surgery | 23 (14ཞ41) | 23 (15ཞ43) | 0.808 |
| Median WA at the primary evaluation (range, mm2) | 50,452 (31999ཞ96356) | 53,144 (29225ཞ106925) | 0.47 |
| Median WA at the secondary evaluation (range, mm2) | 50,374 (29311ཞ106329) | 56789.5 (23427ཞ110072) | 0.481 |
| Median change of WA (range, ±mm2) | + 849.5 (−34113ཞ+13098) | + 2013.5 (−12625ཞ+17719) | 0.0858 |
| Median change of WA per year (range, ±mm2/year) | + 278.5 (−13645ཞ+4793) | + 784.5 (−6313ཞ+13207) | 0.0743 |
WA Waist area, BMI Body Mass Index
*Diameter longer than 2 mm on CT images
Fig. 2.
Pearson correlation coefficient between body mass index at ureteroscopy and waist area at the time of primary computed tomography
Additionally, we compared the recurrent group and the nonrecurrent group of males and females (Tables 2 and 3). There was no significant difference in the change in WA in males (median + 2583 versus + 1580 mm2, p = 0.481), but there was a significant difference in females in that the change in WA in the recurrent group was less than that in the nonrecurrent group (median − 2175 versus + 3019 mm2, p = 0.0368).
Table 2.
Comparison between the recurrence group and the nonrecurrence group in male patients
| Recurrent group (n = 35) | Nonrecurrent group (n = 27) | p value | |
|---|---|---|---|
| Median Age (range) | 63 (19ཞ78) | 70 (40ཞ86) | 0.0112 |
| Previous stone episodes before surgery (n) | 20 | 12 | 0.443 |
| The number of stones at surgery: Single/Multiple (n) | 11/24 | 15/12 | 0.0722 |
| Residual fragment * at the primary evaluation (n) | 15 | 10 | 0.795 |
| Median BMI at surgery | 24 (17ཞ41) | 23 (15ཞ29) | 0.206 |
| Median WA at the primary evaluation (range, mm2) | 54,484 (36651ཞ96356) | 53,211 (29225ཞ74076) | 0.103 |
| Median WA at the secondary evaluation (range, mm2) | 52,170 (29311ཞ106329) | 56,520 (23427ཞ76227) | 0.612 |
| Median change of WA (range, ±mm2) | + 2583 (−34113ཞ+12499) | + 1580 (−5798ཞ+17497) | 0.481 |
| Median change of WA per year (range, ±mm2/year) | + 545 (−13645ཞ+4166) | + 861 (−2525ཞ+11665) | 0.397 |
WA Waist area, BMI Body Mass Index
*Diameter longer than 2 mm on CT images
Table 3.
Comparison between the recurrence group and the nonrecurrence group in female patients
| Recurrent group (n = 13) | Nonrecurrent group (n = 21) | p value | |
|---|---|---|---|
| Median Age (range) | 57 (35ཞ79) | 74 (43ཞ90) | 0.123 |
| Previous stone episodes before surgery (n) | 6 | 8 | 0.728 |
| The number of stones at surgery: Single/Multiple (n) | 6/7 | 10/11 | 1 |
| Residual fragment * at the primary evaluation (n) | 3 | 6 | 1 |
| Median BMI at surgery | 22 (15ཞ29) | 24 (17ཞ43) | 0.0978 |
| Median WA at the primary evaluation (range, mm2) | 46,221 (31999ཞ56746) | 53,077 (30754ཞ106925) | 0.137 |
| Median WA at the secondary evaluation (range, mm2) | 45,097 (33575ཞ56562) | 57,664 (30729ཞ110072) | 0.0503 |
| Median change of WA (range, ±mm2) | −2175 (−8054ཞ+13098) | + 3019 (−12625ཞ+17719) | 0.0386 |
| Median change of WA per year (range, ±mm2/year) | −363(−6041ཞ+4793) | + 708 (−6313ཞ+13207) | 0.0503 |
WA Waist area, BMI Body Mass Index
*Diameter longer than 2 mm on CT images
The groups with increased WAs and decreased WAs were subsequently compared in each sex cohort (Table 4). There was no significant difference in the male cohort (n = 22/18 versus 13/9, p = 0.795), but there was a significantly greater rate of recurrence in the female cohort (9 recurrent patients out of 14 with decreased WA vs. 4 recurrent patients out of 20 with increased WA, p = 0.0137).
Table 4.
Comparison between the increased WA group and the decreased WA group
| Increased WA group (n = 60) | Decreased WA group (n = 36) | p value | |
|---|---|---|---|
| Male (n) | 40 | 22 | |
| Recurrence/No recurrence (n) | 22/18 | 13/9 | 0.795 |
| Female (n) | 20 | 14 | |
| Recurrence/No recurrence (n) | 4/16 | 9/5 | 0.0137 |
The risk of recurrence of renal stones was subsequently evaluated via logistic regression analysis (Table 5). Age (≦ 70 years) and decreased WA were significant risk factors (odds ratios: 6.520 and 2.750, 95% confidence intervals 2.280-18.700 and 1.040།7.290, p = 0.000477 and 0.041400). Sex (female) and BMI at surgery (≧ 25) were not significantly related (odds ratios: 0.523 and 0.757; 95% confidence intervals: 0.198།1.380 and 0.293།1.950; p = 0.190000 and 0.564000, respectively).
Table 5.
Multivariate analysis of the risk of recurrent renal stones
| Odds ratio | 95% confidential intervals | p value | |
|---|---|---|---|
| Age (≦ 70 years old) | 6.520 | 2.280 - 18.700 | 0.000477 |
| Sex (female) | 0.523 | 0.198 - 1.380 | 0.190000 |
| BMI at surgery (≧ 25) | 0.757 | 0.293 - 1.950 | 0.564000 |
| Decreased WA | 2.750 | 1.040 - 7.290 | 0.041400 |
| Logistic regression analysis with EZR | |||
Discussion
Recently, urolithiasis has become symptom associated with metabolic syndrome. Obesity and unhealthy metabolic status have been reported to be risk factors for the development and growth of renal stones [1]. It is expected that dietary habits and improvements in obesity reduce the recurrence of renal stones, but these findings are difficult to prove. In a rat model, a weight-loss intervention was shown to reduce the risk of renal stones [12]. However, clinical evidence for the potential benefits of dietary weight loss strategies for urolithiasis is limited [10]. The results of our study were contrary to expectations, especially for female patients, whose decreased WA was a risk factor for the recurrence of renal stones.
However, whether WA is correlated with weight and obesity is unknown. Obesity-related indices such as BMI, waist circumference, waist-to-height ratio, waist-to-hip ratio, abdominal volume index, body roundness index, conicity index, and triglyceride glucose index were associated with a greater incidence of renal stones in a longitudinal cohort study [13]. It was assumed that WA was correlated with changes in weight and body shape.
WAs are mainly composed of the bowel intestine, visceral fat, subcutaneous fat, psoas muscle, abdominal wall muscle and paravertebral muscle. The visceral adiposity index can accurately identify and assess visceral adiposity function [14]. It has been reported that recurrent stone-formers have a greater visceral fat area ratio on computed tomography than first-time stone-formers do [15].
Compared with large and small WAs, subcutaneous and visceral fat strongly affect patients. However, when a timely change in WA is focused on each patient, the amount of muscle may be affected more strongly than the amount of adipose tissue. Physical activity may reduce waist gain and the risk of type 2 diabetes and potentially have a beneficial effect on the risk of nephrolithiasis. Low -to-moderate levels of physical activity have the potential to prevent kidney stones, but further studies are needed. The geographic risk factor for BMI and nephrolithiasis was also reported to be more strongly associated in European and American studies than in Asian studies [16]. It has been reported that physical inactivity with loss of muscle is associated with the incidence of renal stones. The link between physical activity and kidney stones has long been controversial. The protective effect of vigorous recreational activity on kidney stones has been reported. Physical activity might promote bone calcium deposition and reduce urinary calcium excretion, which is related to the formation of calcium-containing stones [17].
Obesity and metabolic syndrome are known risk factors for nephrolithiasis. In the present study, there was no significant difference in the median BMI or initial WA between the recurrent and nonrecurrent groups, and BMI was not a significant risk factor according to multivariate analysis. Interestingly, a decreased WA in female patients was associated with a greater risk of recurrent nephrolithiasis than was a decreased WA in male patients in this study. The associations of urolithiasis with hypercalciuria and low bone mineral density, particularly in postmenopausal women, have been recognized. Bone metabolism in patients with urolithiasis is associated not only with a high risk of recurrence but also with a potential risk of fracture. Low bone mineral density is associated with hypercalciuria, and it could be an independent risk factor for development of urolithiasis. Postmenopausal women have a relatively high incidence of osteoporosis, and physical inactivity might promote bone resorption and increased calcium excretion in the urine. Additionally, weight- loss might cause metabolic acidosis and hypocitraturia, which promote the development of urolithiasis [18]. Nephrolithiasis is associated with low bone mineral density. Estrogen in premenopausal women has a particular influence on cortical bone and contribute to bone quality. Further studies are warranted to confirm whether sex-related differences are due to the beneficial effects of estrogen on cortical bone among premenopausal women with nephrolithiasis [19].
Interestingly, “age under 70 years” was also a significant risk factor for recurrent nephrolithiasis. The peak age of incidence for upper urinary stones was reported to be 50 years in males and 60 years in females in a national cross-sectional survey in Japan [20]. These ages might present a potential metabolic and hormonal risk for recurrent nephrolithiasis.
There are several Limitations in this study because of its retrospective design. First, the rate of recurrence was greater in these patients than in patients who received a consultation at the public urological office. In this study, the patients were Limited to those who were examined twice via CT at a single regional referral hospital. Therefore, the selection bias for these patients could be caused by their comorbidities and increased risk of recurrence. Second, almost all patients were nonobese and had a normal weight. The median BMI at surgery was 23 in both groups. Caution should be taken because this study was not performed in obese patients. The opposite findings could be obtained if the materials used were limited to obese patients. Data on dietary intake, physical activity, menopausal status, muscle mass, fat distribution, and bone mineral density were lacking because of the retrospective study-design. The sample size was too small to generalize, and the statistical power was limited.
Even after these limitations are overcome, the results of this study reflect the features of high-risk patients who experience renal stone recurrence after surgery. In the future, prospective studies with larger sample sizes should investigate whether the recurrence of urolithiasis is related to changes in muscle mass, fat volume, bone mineral density, physical activity, menopausal status and hormone levels.
Conclusions
Several limitations were included in this retrospective single-center study, whereas a decreased WA on CT images was a significant risk factor for the recurrence of renal stones after ureteroscopy in nonobese Japanese female patients. The relationships among age, sex, muscle mass, fat distribution, bone mineral density, dietary intake, physical activity, menopausal status and recurrent nephrolithiasis should be further studied.
Acknowledgements
Not applicable.
Clinical trial number
Not applicable.
Abbreviations
- CT
Computed tomography
- WA
Waist area
- BM
Body mass index
Authors’ contributions
Conceptualization, formal analysis and investigation, writing-original draft preparation: Hiroaki Kakinoki. Methodology: Yuka Kakinoki. Writing-review and editing: Kazuma Udo, Shohei Tobu. Supervision: Mitsuru Noguchi.
Data availability
The datasets and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
This study adhered to the Declaration of Helsinki and was conducted in accordance with relevant guidelines and regulations. Ethical approval of the manuscript was obtained from the Institutional Review Board and Ethics Committee of the Faculty of Medicine, Saga Japan (number 2023-11-R-4) at the date of 2/02/2024. Owing to the study’s retrospective nature, the requirement for informed consent was waived; however, participants were given the option to opt out via the websites of our institution.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zhenyang Ye C, Wu Y, Bai, et al. Obesity, metabolic dysfunction, and risk of kidney stone disease: a National cross-sectional study. Aging Male. 2023;26(1):2195932. 10.1080/13685538.2023.2195932. [DOI] [PubMed] [Google Scholar]
- 2.Jaime Uribarri. Chronic kidney disease and kidney stones. Curr Opin Nephrol Hypertens. 2020;29(2):237–42. 10.1097/MNH.0000000000000582. [DOI] [PubMed] [Google Scholar]
- 3.Riccardo Lombardo A, Guidotti, Cosimo DE, Nunzio, et al. Trends and incidence of reported events associated with ureteral stents: an analysis of the food and drug administration’s manufacturer and user facility device experience (MAUDE) datebase. World J Urol. 2024;42(1):524. 10.1007/S00345-024-05225-5. [DOI] [PubMed] [Google Scholar]
- 4.Antonio Cicione J, Stira C, De Nunzio, et al. Ureteral stent encrustation: evaluation of available scores as predictors of a complex surgery. Minerva Urol Nephrol. 2023;75(3):359–65. 10.23736/S2724-6051.22.04999-0. Epub 2022 Oct 26. [DOI] [PubMed] [Google Scholar]
- 5.Nunzio CD, Ghahhari J, Tubaro A, et al. Development of a nomogram predicting the probability of stone free rate in patients with ureteral stone free rate in patients with ureteral stones eligible for semi-rigid primary laser uretero-lithotripsy. World J Urol. 2021;39(11):4267–74. 10.1007/s00345-021-03768-5. Epub 2021 Jun 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matthew R, D’Costa WE, Haley, Kristin C, Mara, et al. Symptomatic and radiographic manifestations of kidney stone recurrence and their prediction by risk factors: A prospective cohort study. J Am Soc Nephrol. 2019;30(7):1251–60. 10.1681/ASN.20181241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Antonio Franco S, Riolo C, De Nunzio, et al. Renal function preservation in purely Off-Clamp sutureless robotic partial nephrectomy: initial experience and technique. Diagnostics (Basel). 2024;14(15):1579. 10.3390/diagnostics14151579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Slolarikos A, Somani B, Gambaro G, et al. Metabolic evaluation and recurrence prevention for urinary stone patients: an EAU guidelines update. Eur Urol. 2024;86(4):343–63. 10.1016/j.eururo.2024.05.029. [DOI] [PubMed] [Google Scholar]
- 9.Haider A. Tamara horwich. Obesity, cardiorespiratory fitness, and cardiovascular disease. Curr Cardiol Rep. 2023;25(11):1565–71. 10.1007/s11886-023-01975-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roswitha Siener C, Metzner. Dietary weight loss strategies for kidney stone patients. World J Urol. 2023;41(5):1221–8. 10.1007/s00345-022-04268-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transpl. 2013;48:452–8. 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sasaki Y, Kohjimoto Y, Iba A, et al. Weight loss intervention reduces the risk of kidney stone formation in a rat model of metabolic syndrome. Int J Urol. 2015;22(4):404–9. 10.1111/iju.12691. [DOI] [PubMed] [Google Scholar]
- 13.Lee M-R, Ke H-L, Geng J-H, et al. Obesity-related indices and its association with kidney stone disease: a cross-sectional and longitudinal cohort study. Urolithiasis. 2022;50(1):55–63. 10.1007/s00240-021-01288-w. [DOI] [PubMed] [Google Scholar]
- 14.Liang D, Liu C, Yang M, et al. The association of visceral adiposity index with the risk of kidney stone and kidney stone recurrence. BMC Nephrol. 2023;12(1):368. 10.1186/s12882-023-03421-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shimpei Yamashita T, Iguchi I, Hara, et al. Recurrent stone-forming patients have high visceral fat ratio based on computed tomography images compared to first-time stone-forming patients. Int J Urol. 2018;25(6):569–73. 10.1111/iju.13564. [DOI] [PubMed] [Google Scholar]
- 16.Dagfinn Aune Y, Mahamat-Saleh E, Riboli, et al. Body fatness, diabetes, physical activity and risk of kidney stones: a systematic review and meta-analysis of cohort studies. Eur J Epidemiol. 2018;33:1033–47. 10.1007/s10654-018-0426-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Y, Di X, Liao B, et al. Association between daily sitting time and kidney stones based on the National health and nutrition examination survey (NHANES) 2007–2016: a cross-sectional study. Int J Surg. 2024;110(8):4624–32. 10.1097/JS9.0000000000001560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kazumi Taguchi S, Hamamoto T, Yasui, et al. Low bone mineral density is a potential risk factor for symptom onset and related with hypocitraturia in urolithiasis patients: a single center retrospective cohort study. BMC Urol. 2020;20(1):174. 10.1186/s12894-020-00749-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Priscila Ligeiro Goncalves Esper, Rodrigues FG, Heilberg IP, et al. Bone density, microarchitecture and estimated strength in stone formers: a cross-sectional HR-pQCT study. Nephrol Dial Transpl. 2023;38(2):425–34. 10.1093/ndt/gfac128. [DOI] [PubMed] [Google Scholar]
- 20.Shinichi Sakamoto K, Miyazawa T, Ichikawa, et al. Chronological changes in the epidemiological characteristics of upper urinary tract urolithiasis in Japan. Int J Urol. 2018;25(4):373–8. 10.1111/iju.13552 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets and/or analyzed during the current study are available from the corresponding author upon reasonable request.