Abstract
Background
Adiposity, insulin resistance, and lipid metabolism abnormalities are established risk factors for hyperuricemia; however, the impact of exercise on these factors remains unclear. Moreover, there is a lack of prospective data regarding the predictive value of these risk factors concerning the timing of hyperuricemia onset.
Methods
From 2017 to 2023, data were prospectively collected from annual health checkups and questionnaires. The primary outcome was the incidence of hyperuricemia, stratified into early-onset (age < 40 years) and late-onset (age > 60). Key predictive indicators included the systemic immune-inflammation index (SII), systemic inflammation response index (SIRI), non-high-density lipoprotein cholesterol/high-density lipoprotein cholesterol ratio (NHHR), triglyceride-glucose index (TyG), and body mass index (BMI). The effects of various exercise types on these indicators were analyzed using multivariate quantile regression models. Cox regression analyses were conducted to assess the hazard ratios (HRs) and their 95% confidence intervals (CIs) of these inflammation and metabolic indictors on hyperuricemia risk. Mediation analyses were performed to explore the roles of these indicators.
Results
Out of 23,534 participants free of hyperuricemia at baseline, 2,555 developed hyperuricemia. Regular exercise was linked to lower inflammation and metabolic indicators, particularly in their higher quantiles. SIRI, BMI, NHHR, and TyG were associated with increased hyperuricemia risk (log-rank p < 0.001), with stronger effect in the early-onset group. For early-onset hyperuricemia, HRs of the highest quantile (Q4) for NHHR, TyG, and BMI > 30 were 1.64 (95% CI, 1.27–2.12), 1.70 (1.32–2.29), and 1.84 (1.29–2.63), respectively. SIRI and SII at Q3 also indicated increased risk. NHHR mediated 5.63% of the overweight/obesity effect on overall hyperuricemia, increasing to 10.86% in early-onset cases, while TyG mediated 12.01%, which was similar to the early-onset group (11.85%).
Conclusions
Higher systemic inflammation, adiposity, and impaired lipid and glucose metabolism significantly increase hyperuricemia risk, particularly in early-onset cases. Exercise as interventions may reduce the levels of these risk factors, especially among individuals in higher quantiles.
Graphical Abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s12944-025-02713-4.
Keywords: Hyperuricemia, Inflammation, Metabolic risk, Insulin resistance, Risk factor, Cohort study
Background
Serum uric acid (SUA), the final product of purine metabolism, is generated exclusively via the oxidation of xanthine and hypoxanthine catalyzed by xanthine oxidoreductase (XOR) [1]. Hyperuricemia (HUA), a metabolic disorder characterized by abnormally elevated SUA levels in extracellular fluids and tissues, primarily arises from impaired uric acid excretion [2]– [3]. HUA is associated with multiple adverse health outcomes, and its prevalence has risen rapidly worldwide, particularly in developed countries [4]. Data from the National Health and Nutrition Examination Survey (NHANES) indicate a high prevalence of HUA, with an elevated co-occurrence of metabolic syndrome among affected individuals [5]. Furthermore, studies from the China National Health Survey and the National Survey of Thyroid Disorders and Diabetes demonstrate a notably high HUA prevalence among younger adults (age < 40), especially in males [6]– [7]. Due to prolonged cumulative exposure to elevated SUA, younger individuals face an increased lifetime risk of adverse health outcomes [8]. Consequently, identifying risk factors for early-onset HUA is essential for promoting long-term health and mitigating associated complications.
Although the association between hyperuricemia (HUA) and overweight/obesity is well-established, emerging evidence suggests that impaired atherogenic lipid profiles and glucose metabolism play a pivotal role in HUA development [9]– [10]. The non-high-density lipoprotein cholesterol (non-HDL-C) to high-density lipoprotein cholesterol (HDL-C) ratio (NHHR) has recently gained recognition as a novel composite marker of atherogenic lipids [11]. Notably, the UK’s National Institute for Health and Care Excellence (NICE) recommends non-HDL-C as a primary target for cardiovascular disease (CVD) risk reduction, highlighting its clinical relevance in assessing CVD susceptibility [12]. Furthermore, the triglyceride-glucose index (TyG) has been established as a reliable surrogate marker for insulin resistance (IR) and has been widely used in previous studies to assess its health implications [13].
In the pathogenesis of HUA and gout, systemic inflammation and immune dysregulation are considered key mechanisms modulating serum uric acid (SUA) levels [14]– [15]. However, most prior epidemiological studies investigating these biomarkers have employed cross-sectional designs, which inherently limit causal inference due to temporality constraints. Importantly, modifiable risk factors contribute significantly to HUA onset, underscoring the need for health promotion strategies targeting lifestyle modifications [16]. Among these, physical exercise and sports represent critical interventions for disease prevention and health enhancement.
Therefore, this study leverages data from the Diverse Life-Course Cohort (DLCC), a large-scale Chinese cohort, to achieve three objectives: First, to evaluate the effects of physical exercise and specific sports activities on key HUA risk factors, including body mass index (BMI), systemic immune-inflammation index (SII), systemic inflammation response index (SIRI), and metabolic markers (NHHR and TyG index); second, to investigate the predictive capacity of these biomarkers for HUA with different onset patterns; and third, to analyze potential mediating relationships among these risk factors.
Methods
Study population
This study analyzed data from the Tianjin-Beijing-Hebei Physical Examination multicenter cohort, a component of the Diverse Life-Course Cohort (DLCC) study. The cohort was established in 2017 with prospective follow-up continuing through July 2023. As previously described, the study employed a multi-stage stratified cluster sampling design to recruit participants from eight health examination centers across Beijing, Tianjin, and Hebei provinces [17]. Eligible participants met the following criteria: (1) Age ≥ 18 years; (2) Minimum one-year residence in the study area and (3) Voluntary participation in the study. Exclusion criteria included: (1) Pregnancy; (2) Severe physical or mental illness preventing survey completion; (3) Acute infections at baseline and (4) Follow-up duration < 6 months (to minimize latency bias). A complete participant flow diagram is presented in Supplementary Figure S1.
The study protocol was approved by the ethics review board of Nankai University (NKUIRB2021063). All research was conducted in accordance with the Declarations of Helsinki and Istanbul. All participants signed a consent form before data collection.
Data collection and measurements
Data were collected through standardized questionnaire interviews, physical examinations, and laboratory analyses conducted by trained research staff. Face-to-face interviews captured demographic characteristics, lifestyle factors (including smoking status, alcohol consumption, and sedentary behaviors), physical activity patterns (frequency and types: swimming, ball games, cycling, jogging, or yoga), and medical history. Physical exercise was operationally defined as health-oriented activity performed consistently for ≥ 20 min per session over the preceding year, categorized as no exercise, occasional exercise (1–2 times/week), or regular exercise (≥ 3 times/week). Standardized physical examinations were conducted with participants wearing light clothing and no footwear. Height and weight were measured to 0.1 cm/kg precision using calibrated stadiometers (GL-310, Seoul, Korea), while blood pressure measurements were obtained from the right arm after 5 min of seated rest using automated sphygmomanometers (Omron HEM-907, Japan), with three sequential readings averaged for analysis. Fasting blood samples were collected in the morning for biochemical analysis. Morning fasting blood samples were collected for biochemical analysis, including lipid profile (total cholesterol, triglycerides [TG], HDL-C, LDL-C), glucose metabolism (fasting plasma glucose [FPG]), renal function markers (serum creatinine, blood urea nitrogen), inflammatory markers (complete blood count analyzed using SYSMEX XN-9000, Mindray BC-5380, and BACKMAN DXH800 analyzers), and serum uric acid (SUA) levels. All biochemical parameters were measured using an automated analyzer (Toshiba TBA-120FR) under strict quality control protocols. The estimated glomerular filtration rate (eGFR) was calculated using the Chinese-adapted Modification of Diet in Renal Disease (MDRD) equation for chronic kidney disease patients, with reduced renal function defined as eGFR < 90 mL/min/1.73 m² [18, 19].
BMI was calculated as weight (kg) divided by height squared (m²) and categorized according to World Health Organization criteria [20]. TyG was derived using the formula: TyG = ln[fasting plasma glucose (mg/dL) × triglycerides (mg/dL)/2] [21]. NHHR was calculated as (total cholesterol - HDL-C)/HDL-C [11]. SII was computed as (platelet count × neutrophil count)/lymphocyte count, while SIRI was determined as (neutrophil count × monocyte count)/lymphocyte count, with all cell counts expressed in 10⁹ cells/L [22].
Follow up and outcome assessment
This ongoing cohort study utilizes data collected through July 2023, with annual follow-up assessments conducted at examination centers using standardized physical and biochemical measurements consistent with baseline protocols. The primary outcome was incident HUA, defined as the first occurrence of SUA levels exceeding sex-specific thresholds: >360 µmol/L (≈ 6 mg/dL) in women and > 420 µmol/L (≈ 7 mg/dL) in men [19, 23].
Participants were followed from baseline until HUA diagnosis or their last available SUA measurement (whichever occurred first). Recognizing the cardiovascular implications of uric acid levels, we conducted sensitivity analyses using a more stringent cut-off (> 333 µmol/L [≈ 5.6 mg/dL] for both sexes) based on established associations between uric acid and metabolic syndrome risk [24]. Early-onset HUA is defined as the development of HUA in individuals younger than 40 years whereas late-onset HUA is defined as the development of HUA in individuals older than 60 years [9].
Statistical analysis
Participants with prevalent HUA at baseline or missing SUA measurements were excluded from analysis. Continuous variables with non-normal distributions were expressed as median (interquartile range), while categorical variables were reported as frequencies (percentages). Between-group comparisons were performed using χ² tests for categorical variables and Mann-Whitney U tests for continuous variables. We employed multivariate quantile regression to assess the association between physical activity patterns (including specific sports) and various quantiles of BMI, NHHR, SII, and SIRI, with adjustment for age, sex, smoking status, and alcohol consumption. Survival analyses included Kaplan-Meier estimation with log-rank testing to compare HUA incidence across BMI, NHHR, and inflammation index strata.
Cox proportional hazards models were constructed to evaluate the relationships between inflammatory/metabolic markers (SII, SIRI, NHHR, TyG) and HUA risk, with comprehensive adjustment for demographic, lifestyle, and clinical covariates (sex, age, smoking, alcohol use, physical activity, sedentary time, baseline hypertension, and renal function). These markers were analyzed as quartiles (Q1-Q4), with Q1 serving as the reference. The proportional hazards assumption was verified using likelihood ratio tests. Restricted cubic splines (3 knots) were implemented to examine potential nonlinear dose-response relationships.
Mediation analyses were performed to investigate potential intermediary roles of metabolic factors in the physical activity-HUA association, following established methodology from our prior work [25]. All analyses were conducted using SAS 9.4 (SAS Institute) and R 4.2.2, with statistical significance set at α = 0.05 (two-tailed).
Results
The baseline characteristics of the study population
The baseline characteristics of the 23,534 participants are presented in Table 1. Men accounted for 46.01% of the cohort. Significant sex differences were observed in lifestyle factors and clinical profiles. Compared to women, men had higher proportions of overweight/obesity and greater prevalence of chronic conditions including hypertension and diabetes, along with lower eGFR (all p < 0.001). Male participants also demonstrated significantly higher levels of neutrophils, monocytes, lymphocytes, SUA, FPG, and serum lipids (except HDL-C) than female participants (all p < 0.001).
Table 1.
The baseline characteristics of the cohort population
| Overall (n = 23534) | Male (n = 10829) | Female (n = 12705) | p | ||||
|---|---|---|---|---|---|---|---|
| n/median | %/IQR | n/median | %/IQR | n/median | %/IQR | ||
| Age | 46.25 | 14.42 | 48.09 | 14.70 | 44.68 | 13.98 | < 0.001 |
| Education | < 0.001 | ||||||
| Elementary school | 529 | 2.25 | 254 | 2.35 | 275 | 2.16 | |
| Junior high school | 1929 | 8.20 | 1179 | 10.89 | 750 | 5.90 | |
| Senior high school | 2990 | 12.71 | 1670 | 15.42 | 1320 | 10.39 | |
| College | 13,818 | 58.72 | 6113 | 56.45 | 7705 | 60.65 | |
| Graduate | 4268 | 18.14 | 1613 | 14.90 | 2655 | 20.90 | |
| Ever smoke | 4701 | 19.98 | 4449 | 41.08 | 252 | 1.98 | < 0.001 |
| Ever drink | 5154 | 21.90 | 4496 | 41.52 | 658 | 5.18 | < 0.001 |
| Exercise habits | < 0.001 | ||||||
| Rarely | 7907 | 33.60 | 3370 | 31.12 | 4537 | 35.71 | |
| Sometimes | 7659 | 32.54 | 3230 | 29.83 | 4429 | 34.86 | |
| Often | 7968 | 33.86 | 4229 | 39.05 | 3739 | 29.43 | |
| Exercise types | |||||||
| Jogging | 3501 | 14.88 | 1929 | 17.81 | 1572 | 12.37 | < 0.001 |
| Bicycling | 1579 | 6.71 | 774 | 7.15 | 805 | 6.34 | 0.0131 |
| Swimming | 1580 | 6.71 | 785 | 7.25 | 795 | 6.26 | 0.0024 |
| Ball game | 1901 | 8.08 | 1293 | 11.94 | 608 | 4.79 | < 0.001 |
| Yoga | 950 | 4.04 | 47 | 0.43 | 903 | 7.11 | < 0.001 |
| Taichi | 295 | 1.25 | 142 | 1.31 | 153 | 1.20 | 0.4619 |
| Other exercises | 387 | 1.64 | 183 | 1.69 | 204 | 1.61 | 0.6125 |
| Daily sitting hours | < 0.001 | ||||||
| < 2 | 2441 | 10.37 | 1290 | 11.91 | 1151 | 9.06 | |
| 2~ | 4487 | 19.07 | 2217 | 20.47 | 2270 | 17.87 | |
| 4~ | 6638 | 28.21 | 3099 | 28.62 | 3539 | 27.86 | |
| > 6 | 9968 | 42.36 | 4223 | 39.00 | 5745 | 45.22 | |
| Body mass index | 23.98 | 3.44 | 25.05 | 3.26 | 23.06 | 3.32 | < 0.001 |
| Neutrophil | 3.35 | 1.34 | 3.45 | 1.35 | 3.29 | 1.28 | < 0.001 |
| Monocyte | 0.33 | 0.15 | 0.36 | 0.16 | 0.30 | 0.14 | < 0.001 |
| Lymphocyte | 1.95 | 0.74 | 2.00 | 0.79 | 1.91 | 0.68 | < 0.001 |
| Platelets | 243 | 71 | 234 | 68 | 251 | 73 | < 0.001 |
| Serum uric acid | 297 | 93.4 | 342 | 76 | 263 | 70.6 | < 0.001 |
| eGFR | 115.12 | 33.68 | 109.38 | 29.13 | 120.81 | 36.7 | < 0.001 |
| Non-HDL-C | 3.35 | 1.19 | 3.44 | 1.13 | 3.26 | 1.22 | < 0.001 |
| HDL-C | 1.30 | 0.40 | 1.22 | 0.30 | 1.43 | 0.40 | < 0.001 |
| Fasting glucose plasma | 5.09 | 0.78 | 5.23 | 0.90 | 4.99 | 0.68 | < 0.001 |
| SBP | 123.18 | 18.39 | 128.53 | 17.42 | 118.60 | 17.95 | < 0.001 |
| DBP | 76.12 | 11.64 | 79.70 | 11.50 | 73.05 | 10.85 | < 0.001 |
| Hypertension | 5050 | 21.46 | 3157 | 29.15 | 1893 | 14.90 | < 0.001 |
| Diabetes | 2076 | 8.82 | 1375 | 12.70 | 701 | 5.52 | < 0.001 |
| Impaired kidney function | 3180 | 13.51 | 1704 | 15.74 | 1476 | 11.62 | < 0.001 |
Impaired kidney function was defined as an eGFR < 90 mL/min/1.73 m². The Chi-square test or Mann-Whitney U test was employed to compare baseline characteristics between sexes
IQR Interquartile range, eGFR Estimated glomerular filtration rate, HDL-C High-density lipoprotein cholesterol, SBP Systolic blood pressure, DBP Diastolic blood pressure
Effects of physical activity patterns on inflammatory and metabolic markers
Figure 1 presents the relationships between exercise frequency/type and levels of SII, SIRI, BMI, NHHR, and TyG. Compared to regular exercisers, participants with occasional or rare physical activity showed significantly higher values across all measured indicators. Certain sports modalities demonstrated particularly strong protective effects against elevated inflammatory markers, adiposity, and metabolic dysregulation. The magnitude of benefit varied by exercise type and across different quantiles of each outcome measure, suggesting differential effects depending on baseline risk profiles.
Fig. 1.
The effect of physical exercise and different sport patterns on SIRI, SII, NHHR, TyG and BMI. SIRI: systemic inflammation response index; SII: systemic immune‑inflammation index; NHHR: non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio; TyG: triglyceride-glucose index; BMI: body mass index
HUA incidence and associated inflammatory/metabolic risk factors
Over the 6-year follow-up (median duration: 1.1 years), 2,555 participants (10.86%) developed HUA. Sex-stratified distributions of SII, SIRI, BMI, NHHR, and TyG between HUA and non-HUA groups are shown in Supplementary Figure S2. Kaplan-Meier analysis (Fig. 2) revealed significantly higher HUA incidence among participants with elevated BMI, NHHR, SIRI, and TyG levels (all p < 0.001), though no significant association was observed for SII (p = 0.21).
Fig. 2.
The Kaplan-Meier cumulative risk curves of HUA risk in the study population, stratified by sex, BMI, NHHR, TyG, SII and SIRI groups. The groups are based on first to forth quantiles for SII, SIRI, NHHR and TyG. For BMI groups, 1–4 represents underweight, normal weight, overweight, and obesity, respectively. Sex = 1 represents Male and 2 represents females. P values were estimated using the log-rank tests. SIRI: systemic inflammation response index; SII: systemic immuneinflammation index; NHHR: non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio; TyG: triglyceride-glucose index; BMI: body mass index; HUA: hyperuricemia
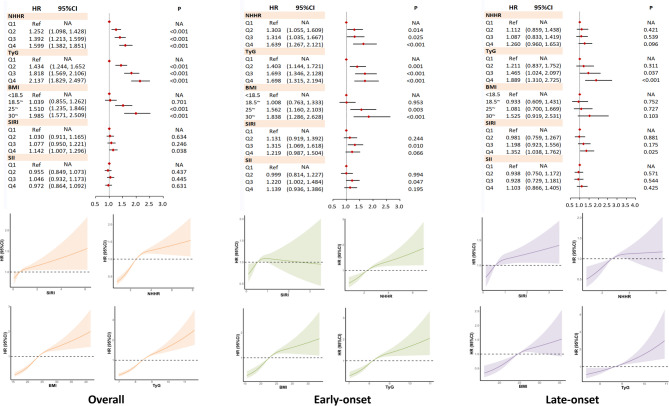
As shown in Fig. 3, increased NHHR, TyG, BMI, and SIRI levels were positively associated with HUA risk, with effect sizes varying by onset timing. Compared to the lowest quartile, the highest quartile groups showed significantly elevated HUA risk: NHHR (HR = 1.60, 95%CI:1.38–1.85), TyG (HR = 2.14, 95%CI:1.83–2.50), BMI (HR = 1.96, 95%CI:1.57–2.51), and SIRI (HR = 1.15, 95%CI:1.01–1.30). These associations remained significant in early-onset HUA (< 60 years) but were generally attenuated in late-onset cases (Supplementary Table S1).
Fig. 3.
The effect of inflammation indexes, NHHR, TyG and BMI on the risk of HUA, early-onset HUA (onset age < 40) and late-onset HUA (onset age > 60). The regression models were restricted to participants younger than 40 years old (for the early-onset HUA) at the end of follow-up or over 60 years old (for the late-onset HUA). The covariates adjusted were age, sex, ever-drink, ever-smoke, exercise habits, daily sedentary behavior, baseline hypertension, baseline impaired eGFR (eGFR < 90). SIRI: systemic inflammation response index; SII: systemic immuneinflammation index; NHHR: non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio; TyG: triglyceride-glucose index; BMI: body mass index; HUA: hyperuricemia; eGFR: estimated glomerular filtration rate
Sex-specific associations between metabolic/inflammatory markers and HUA risk
Sex-stratified analyses revealed differential associations between risk indicators and HUA development (Supplementary Figures S3-S4, Table S1). In males, elevated NHHR, TyG, BMI, and SIRI levels showed consistent positive associations with HUA risk, mirroring population-level trends. For early-onset HUA, all four markers (NHHR, TyG, SIRI, and SII) demonstrated significant risk associations, though only TyG maintained significance after full adjustment.
Among females, increased NHHR, TyG, and BMI levels were similarly associated with higher HUA risk, particularly for early-onset cases. The TyG Q4 group showed marginal association with late-onset HUA (HR = 1.81, 95%CI: 1.10–2.99). Notably, SII Q2 in females demonstrated an inverse relationship with HUA risk (HR = 0.82, 95%CI:0.68–0.98), suggesting a potential U-shaped association.
Mediation effects of metabolic markers on HUA risk
Given the nonsignificant associations observed for late-onset HUA, mediation analyses focused on overall and early-onset cases (Fig. 4). NHHR mediated 5.63% (95%CI:3.32–7.95%) of the total effect of overweight/obesity on HUA risk in the overall population, increasing to 10.86% (95%CI:4.39–17.34%) for early-onset cases. TyG demonstrated stronger mediation effects, accounting for 12.01% (95%CI:8.14–15.88%) of the total effect.
Fig. 4.
The mediated and interacted role of NHHR and TyG in the associations between BMI and HUA/early-onset HUA. TE: total effect; NDE: natural direct effect; NIE: natural indirect effect. HUA: hyperuricemia. NHHR: non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio; TyG: triglyceride-glucose index; BMI: body mass index
Sensitivity analysis
Sensitivity analyses employing a lower HUA threshold (333 µmol/L; Supplementary Tables S2-S3) yielded findings consistent with our primary analysis. Using this alternative cut-off, 4,481 participants (19.04%) were identified with HUA. The regression results maintained robustness when applying either conventional or cardiovascular-optimized HUA diagnostic criteria.
Discussion
To our knowledge, this represents the first investigation examining the predictive utility of novel inflammatory and metabolic markers for hyperuricemia. Our results demonstrate that elevated inflammatory markers, NHHR, and TyG levels significantly increase HUA risk, with particularly strong associations observed for early-onset cases. Additionally, our study suggests that regular physical exercise and specific activity types may effectively mitigate these adverse metabolic and inflammatory profiles. These findings highlight the potential value of targeted interventions addressing modifiable HUA risk factors.
Serum lipid disorders, insulin resistance, and obesity are pathophysiologically interrelated, sharing common risk factors and underlying mechanisms including oxidative stress and chronic inflammation [26]. Supporting this connection, Fortuna et al. demonstrated reduced functional capacity in HUA patients using the 6-Minute Walking Test [27]. As a practical non-pharmacological intervention, physical exercise exhibits well-established anti-inflammatory properties, particularly beneficial for chronic inflammatory rheumatic and musculoskeletal conditions [28]. Exercise also modulates body composition and Lipid metabolism. Meta-analytic evidence indicates that weekly 30-minute aerobic exercise yields modest but significant improvements in body weight, waist circumference, and adiposity measures in overweight/obese adults [29]. Recent clinical trial data further reveal that aerobic exercise induces favorable changes in lipid metabolites associated with decreased hepatic fat accumulation [30].
Aerobic and resistance training demonstrate comprehensive metabolic advantages, including enhanced insulin sensitivity, improved lipid profiles, better circulation, and optimized skeletal muscle function [31]. Our investigation extends these findings by examining the differential effects of exercise modalities across metabolic profile quantiles. The analysis reveals that aerobic activities (swimming, ball sports, jogging) and resistance exercises (yoga) predominantly affect upper quantiles of metabolic indicators rather than median levels. Quantile regression analysis provides distinct advantages over conventional approaches by: (1) enabling precise modeling of any outcome distribution percentile, (2) eliminating the homogeneity assumption of traditional regression, and (3) resolving boundary disparity issues. This methodology offers particular value for preventive medicine by identifying intervention effects on high-risk individuals in upper quantiles of HUA-related markers.
Our results demonstrate that elevated baseline levels of SIRI, BMI, NHHR, and TyG were significantly associated with increased risk of HUA development, with distinct patterns observed between early- and late-onset cases. These findings align with previous reports suggesting different pathophysiological mechanisms may underlie HUA development at different life stages [32]. Specifically, early-onset HUA showed stronger associations with metabolic disturbances including adiposity, systemic inflammation, and impaired lipid/glucose metabolism, whereas late-onset cases appeared more closely related to age-related declines in renal function (as indicated by our Cox regression analyses, not presented in the results). These differential risk profiles underscore the importance of age-specific prevention strategies, with younger individuals potentially benefiting more from interventions targeting inflammatory pathways and metabolic health through physical activity. The observed variations in risk factor profiles between age groups may reflect distinct etiological pathways in HUA pathogenesis, suggesting that early-onset cases could represent a more metabolically-driven phenotype compared to the predominantly renal-mediated late-onset form. These findings have important clinical implications for developing targeted prevention approaches based on age-specific risk factor profiles.
Current evidence regarding the association between systemic inflammatory indices and hyperuricemia remains limited. While previous case-control and cross-sectional studies have established SIRI as a reliable marker for gouty arthritis prevalence and disease activity, our longitudinal cohort study reveals important sex-specific differences [22, 33, 34]. We observed a significant positive association between SIRI and HUA incidence in male participants that was not evident in females - a finding that contrasts with prior reports [33]. This discrepancy may stem from methodological differences in study design (particularly regarding temporal relationships), population characteristics, or potential sex-specific biological mechanisms. Our prospective design provides stronger evidence for causality by establishing clear temporality between exposure and outcome. The pathophysiological basis for the observed onset-time variations in SIRI effects warrants further investigation. We hypothesize that these differences may reflect age-related variations in lifestyle factors, particularly among younger individuals who tend to exhibit higher rates of pro-inflammatory behaviors including consumption of high-fat diets and other modifiable risk factors known to promote obesity, metabolic dysregulation (including diabetes mellitus), and cardiovascular diseases [35]. These findings highlight the complex interplay between inflammatory pathways and HUA pathogenesis, suggesting that the relative contribution of systemic inflammation may vary by both sex and age at onset.
HUA represents a significant metabolic disorder that frequently coexists with chronic kidney disease, diabetes mellitus, and dyslipidemia, reflecting systematic metabolic disturbances commonly observed in these patients [36]. While previous cross-sectional studies, including NHANES analyses, have demonstrated associations between NHHR and HUA prevalence, our prospective cohort study provides stronger evidence that elevated NHHR - an integrated measure of pro- and anti-atherogenic lipid particles - independently predicts HUA incidence [37, 38]. These findings emphasize the clinical importance of comprehensive metabolic monitoring incorporating both SUA and lipid profile assessments for HUA and gout prevention. Notably, Mendelian randomization studies have established a causal relationship between IR and both HUA and gout, suggesting that IR reduction may effectively lower SUA levels and gout risk [10]. The underlying pathophysiology involves hyperinsulinemia-induced downregulation of renal urate transporters, impairing uric acid excretion [39]. While existing literature has primarily employed cross-sectional designs to examine TyG-HUA associations, our longitudinal data identify TyG as an independent predictor of HUA development across all onset periods. Importantly, our mediation analyses reveal that both TyG and NHHR not only directly influence HUA risk but also significantly mediate the adiposity-HUA relationship. This is particularly relevant given the well-documented progression from obesity to IR and subsequent glucose metabolism dysregulation, which collectively contribute to hyperuricemia pathogenesis.
The predictive capacity of NHHR and TyG was particularly pronounced for early-onset HUA compared to late-onset cases. Despite the established high prevalence of HUA among young males, lipid and glucose management in this demographic remains frequently overlooked. Our findings emphasize the critical importance of implementing community-based glycemic and lipid control measures as preventive strategies against HUA in younger populations. The mediating roles of NHHR and TyG in the adiposity-HUA association provide novel insights into the complex interplay of metabolic risk factors in HUA pathogenesis, while highlighting the dual benefits of weight management for both HUA prevention and metabolic health improvement.
This study possesses several notable strengths. To our knowledge, it represents the first comprehensive evaluation of BMI, NHHR, and TyG effects across different HUA onset timings. The prospective design with a large sample size established clear temporal relationships, strengthening causal inference. Furthermore, our application of quantile regression enabled examination of exercise effects across the entire distribution of risk factors, overcoming limitations of conventional regression approaches. However, several limitations warrant consideration. However, several limitations warrant consideration. Second, the information on exercise habits were lack of intensity, and together with inflammatory markers, and metabolic indicators were assessed at baseline, necessitating future research to explore how baseline exercise influences these indicators over time using longitudinal data. Third, we did not collect specific data on antihypertensive (especially diuretic medication) and antidiabetic medication use as these factors could potentially influence the observed outcomes, neither the data of heart rate, which has been shown to be an influencing factor on the association between HUA and CVDs [40]. Finally, reduced statistical power in age-stratified subgroups may have limited detection of significant interaction effects.
Conclusions
Elevated levels of systemic inflammation, NHHR, TyG, and adiposity are independently linked to an increased risk of HUA, particularly in individuals with early-onset HUA. Moreover, NHHR and TyG mediate the relationship between overweight/obesity and HUA risk, highlighting the importance of monitoring lipid profiles and insulin resistance in young individuals as a preventive measure against HUA. Additionally, our findings indicate that regular exercise is associated with lower levels of these risk factors, especially in the higher quantiles of their distribution.
Supplementary Information
Supplementary Material 1. Table S1. The effect of inflammation indexes, NHHR, TyG and BMI on the risk of HUA among people who developed HUA between 40~60 years old. Table S2. The effect of inflammation indexes, NHHR, TyG and BMI on the risk of HUA, according to a lower cut-off threshold of HUA (serum uric acid ≥5.6 mg/dL). Table S3. The effect of inflammation indexes, NHHR, TyG and BMI on the risk of HUA with different onset timing, according to a lower cut-off threshold of HUA (serum uric acid ≥5.6 mg/dL). Figure S1. The flow chart of the study. DLCC: the diverse life course cohort study. Figure S2. Violin diagrams of the distribution of BMI, SII, SIRI, NHHR and TyG in HUA and non-HUA groups. SIRI: Systemic inflammation response index; SII: Systemic immune‑inflammation index; NHHR: Non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio; TyG: Triglyceride-glucose index; BMI: Body mass index; HUA: hyperuricemia. Figure S3. The effect of inflammation indexes, NHHR, TyG and BMI on the risk of HUA in men. Adjusted covariates included age, ever-drink, ever-smoke, exercise habits, daily sedentary behavior, baseline hypertension and impaired eGFR (eGFR<90). SIRI: Systemic inflammation response index; SII: Systemic immune‑inflammation index; NHHR: Non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio; TyG: Triglyceride-glucose index; BMI: Body mass index; HUA: hyperuricemia. Figure S4. The effect of inflammation indexes, NHHR, TyG and BMI on the risk of HUA in women. Adjusted covariates included age, ever-drink, exercise habits, daily sedentary behavior, baseline hypertension and impaired eGFR (eGFR<90). SIRI: Systemic inflammation response index; SII: Systemic immune‑inflammation index; NHHR: Non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio; TyG: Triglyceride-glucose index; BMI: Body mass index; HUA: hyperuricemia.
Acknowledgements
We thank all the participants and collaborators in the cohort and appreciate the help from Dr. Wei Han for methodological support.
Abbreviations
- BMI
Body mass index
- CI
Confidence interval
- CKD
Chronic kidney disease
- CVD
Cardiovascular disease
- DLCC
Diverse Life-Course Cohort
- eGFR
Estimated glomerular filtration rate
- FPG
Fasting glucose plasma
- HDL-C
High-density lipoprotein cholesterol
- HR
Hazard ratio
- HUA
Hyperuricemia
- IQR
Interquartile range
- IR
Insulin resistance
- LDL-C
Low density lipoprotein cholesterol
- NDE
Natural direct effect
- NHHR
Non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio
- NIE
Natural indirect effect
- SII
Systemic immune‑inflammation index
- SIRI
Systemic inflammation response index
- SUA
Serum uric acid
- TG
Triglycerides
- TE
Total effect
- TyG
Triglyceride-glucose index
- XOR
Oxidoreductase
Authors’ contributions
He Huijing: Conceptualization, Methodology, Software, Writing- Original draft preparation. Li Chunjun: Investigation, Supervision. Zhang Li: Data curation, Project administration and Resources. Guo Fenghua: Project administration and Investigation. Zhang Mianzhi: Supervision and Resources. Guo Congfang: Investigation. Cheng Qiaolu: Visualization and Software. Guo Yirui: Investigation. Zhang Minying: Project administration, Supervision, Resources, Writing- Reviewing and Editing.
Funding
This study is supported by National Key R&D Program of China (Grant No. 2016YFC0900604) and the National Natural Science Foundation of China (Grant No. 82373669).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
The study has been carried out in accordance with the Declaration of Helsinki. Ethical approval was obtained from the Ethics Review Boards of Nankai University (NKUIRB2021063), Tianjin First Central Hospital (2017N052KY), Tianjin Union medical center (2018C02), Hebei Medical University (2016021), and Ethics Committee of Beijing Health Examination Center (2023-005). All participants provided written informed consent before the survey.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Crawley WT, Jungels CG, Stenmark KR, Fini MA. U-shaped association of uric acid to overall-cause mortality and its impact on clinical management of hyperuricemia. Redox Biol. 2022;51: 102271. 10.1016/j.redox.2022.102271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang T, Liu W, Gao S. Exercise and hyperuricemia: an opinion article. Ann Med. 2024;56(1): 2396075. 10.1080/07853890.2024.2396075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li L, Zhang Y, Zeng C. Update on the epidemiology, genetics, and therapeutic options of hyperuricemia. Am J Transl Res. 2020;12(7):3167–81. [PMC free article] [PubMed] [Google Scholar]
- 4.Du L, Zong Y, Li H, et al. Hyperuricemia and its related diseases: mechanisms and advances in therapy. Signal Transduct Target Ther. 2024;9(1):212. 10.1038/s41392-024-01916-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim YJ, Kim S, Seo JH, Cho SK. Prevalence and associations between metabolic syndrome components and hyperuricemia by race: findings from US population, 2011–2020. Arthritis Care Res. 2024;76(8):1195–202. 10.1002/acr.25338. [DOI] [PubMed] [Google Scholar]
- 6.He H, Pan L, Ren X, et al. The effect of body weight and alcohol consumption on hyperuricemia and their population attributable fractions: a national health survey in China. Obes Facts. 2022;15(2):216–27. 10.1159/000521163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Song J, Jin C, Shan Z, et al. Prevalence and risk factors of hyperuricemia and gout: a cross-sectional survey from 31 provinces in Mainland China. J Transl Int Med. 2022;10(2):134–45. 10.2478/jtim-2022-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang X, Tian X, Chen S, et al. Early onset of hyperuricemia is associated with the risk of nonalcoholic fatty liver disease across life course. Nutr Metab Cardiovasc Dis. 2024;34(12):2740–8. 10.1016/j.numecd.2024.07.022. [DOI] [PubMed] [Google Scholar]
- 9.Kvasnička A, Friedecký D, Brumarová R, et al. Alterations in lipidome profiles distinguish early-onset hyperuricemia, gout, and the effect of urate-lowering treatment. Arthritis Res Ther. 2023;25(1): 234. 10.1186/s13075-023-03204-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCormick N, O’Connor MJ, Yokose C, et al. Assessing the causal relationships between insulin resistance and hyperuricemia and gout using bidirectional Mendelian randomization. Arthritis Rheumatol. 2021;73(11):2096–104. 10.1002/art.41779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sheng G, Liu D, Kuang M, et al. Utility of non-High-Density lipoprotein cholesterol to High-Density lipoprotein cholesterol ratio in evaluating incident diabetes risk. Diabetes Metab Syndr Obes. 2022;15:1677–86. 10.2147/DMSO.S355980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu B, Li M, Yu Z, et al. The non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio (NHHR) as a predictor of all-cause and cardiovascular mortality in US adults with diabetes or prediabetes: NHANES 1999–2018. BMC Med. 2024;22(1): 317. 10.1186/s12916-024-03536-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lopez-Jaramillo P, Gomez-Arbelaez D, Martinez-Bello D, et al. Association of the triglyceride glucose index as a measure of insulin resistance with mortality and cardiovascular disease in populations from five continents (PURE study): a prospective cohort study. Lancet Healthy Longev. 2023;4(1):e23–33. 10.1016/S2666-7568(22)00247-1. [DOI] [PubMed] [Google Scholar]
- 14.Niu Z, Chen T, Duan Z, et al. Associations of exposure to phthalate with serum uric acid and hyperuricemia risk, and the mediating role of systemic immune inflammation. Ecotoxicol Environ Saf. 2024;287: 117269. 10.1016/j.ecoenv.2024.117269. [DOI] [PubMed] [Google Scholar]
- 15.Xie F, Wu Z, Feng J, et al. Association between systemic immune-inflammation index and serum uric acid in U.S. adolescents: a population-based study. Nutr Metab Cardiovasc Dis. 2024;34(1):206–13. 10.1016/j.numecd.2023.10.008. [DOI] [PubMed] [Google Scholar]
- 16.Choi HK, McCormick N, Lu N, et al. Population impact attributable to modifiable risk factors for hyperuricemia. Arthritis Rheumatol. 2020;72(1):157–65. 10.1002/art.41067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He H, Pan L, Hu Y, et al. The diverse life-course cohort (DLCC): protocol of a large-scale prospective study in China. Eur J Epidemiol. 2022;37(8):871–80. 10.1007/s10654-022-00894-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma YC, Zuo L, Chen JH, et al. Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol. 2006;17(10):2937–44. 10.1681/ASN.2006040368. [DOI] [PubMed] [Google Scholar]
- 19.He H, Pan L, Wang D, et al. Fat-to-muscle ratio is independently associated with hyperuricemia and a reduced estimated glomerular filtration rate in Chinese adults: the China National health survey. Nutrients. 2022;14(19): 4193. 10.3390/nu14194193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Health Organization. Obesity. (Accessed at https://www.who.int/health-topics/obesity#tab=tab_1.).
- 21.Tao LC, Xu JN, Wang TT, et al. Triglyceride-glucose index as a marker in cardiovascular diseases: landscape and limitations. Cardiovasc Diabetol. 2022;21(1): 68. 10.1186/s12933-022-01511-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang Y, Tu X, Liao X, et al. New inflammatory marker associated with disease activity in gouty arthritis: the systemic inflammatory response index. J Inflamm Res. 2023;16:5565–73. 10.2147/JIR.S432898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson RJ, Bakris GL, Borghi C, et al. Hyperuricemia, acute and chronic kidney disease, hypertension, and cardiovascular disease: report of a scientific workshop organized by the National Kidney Foundation. Am J Kidney Dis. 2018;71(6):851–65. 10.1053/j.ajkd.2017.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maloberti A, Tognola C, Garofani, et al. Uric acid and metabolic syndrome: importance of hyperuricemia cut-off. Int J Cardiol. 2024;417:132527. 10.1016/j.ijcard.2024.132527. [DOI] [PubMed] [Google Scholar]
- 25.He H, Pan L, Liu F, et al. The mediation effect of body composition on the association between menopause and hyperuricemia: evidence from China National health survey. Front Endocrinol. 2022;13: 879384. 10.3389/fendo.2022.879384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Varra FN, Varras M, Varra VK, et al. Molecular and pathophysiological relationship between obesity and chronic inflammation in the manifestation of metabolic dysfunctions and their inflammationmediating treatment options (review). Mol Med Rep. 2024;29(6): 95. 10.3892/mmr.2024.13219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fortuna M, Tognola C, Algeri M, et al. Hyperuricemia in cardiac rehabilitation patients: prevalence and association with functional improvement and left ventricular ejection fraction. High Blood Press Cardiovasc Prev. 2024;31(5):461–71. 10.1007/s40292-024-00665-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Metsios GS, Moe RH, Kitas GD. Exercise and inflammation. Best Pract Res Clin Rheumatol. 2020;34(2): 101504. 10.1016/j.berh.2020.101504. [DOI] [PubMed] [Google Scholar]
- 29.Jayedi A, Soltani S, Emadi A, et al. Aerobic exercise and weight loss in adults: a systematic review and dose-response meta-analysis. JAMA Netw Open. 2024;7(12):e2452185. 10.1001/jamanetworkopen.2024.52185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qi Z, Le S, Cheng R, et al. Responses of the serum lipid profile to exercise and diet interventions in nonalcoholic fatty liver disease. Med Sci Sports Exerc. 2024;56(6):1036–45. 10.1249/MSS.0000000000003388. [DOI] [PubMed] [Google Scholar]
- 31.Zhang J, Tam W, Hounsri K, et al. Effectiveness of combined aerobic and resistance exercise on cognition, metabolic health, physical function, and health-related quality of life in middle-aged and older adults with type 2 diabetes mellitus: a systematic review and meta-analysis. Arch Phys Med Rehabil. 2024;105(8):1585–99. 10.1016/j.apmr.2023.10.005. [DOI] [PubMed] [Google Scholar]
- 32.Pascart T, Norberciak L, Ea HK, et al. Patients with early-onset gout and development of earlier severe joint involvement and metabolic comorbid conditions: results from a cross-sectional epidemiologic survey. Arthritis Care Res. 2019;71(7):986–92. 10.1002/acr.23706. [DOI] [PubMed] [Google Scholar]
- 33.Chen MQ, Wang HY, Shi WR, et al. Estimate of prevalent hyperuricemia by systemic inflammation response index: results from a rural Chinese population. Postgrad Med. 2021;133(2):242–9. 10.1080/00325481.2020.1809870. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Y, Han S, Duan Z, et al. Associations of systemic inflammation and systemic immune inflammation with serum uric acid concentration and hyperuricemia risk: the mediating effect of body mass index. Front Endocrinol. 2024;15: 1469637. 10.3389/fendo.2024.1469637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Evans AK, Saw NL, Woods CE, et al. Impact of high-fat diet on cognitive behavior and central and systemic inflammation with aging and sex differences in mice. Brain Behav Immun. 2024;118:334–54. 10.1016/j.bbi.2024.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shen X, Wang C, Liang N, et al. Serum metabolomics identifies dysregulated pathways and potential metabolic biomarkers for hyperuricemia and gout. Arthritis Rheumatol. 2021;73(9):1738–48. 10.1002/art.41733. [DOI] [PubMed] [Google Scholar]
- 37.Jiang Z, Zhu X, Zhao D, et al. Associations between non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio and hyperuricemia: a cross-sectional study. Lipids Health Dis. 2024;23(1): 280. 10.1186/s12944-024-02269-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang Z, Wu M, Du R, et al. The relationship between non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio (NHHR) and hyperuricaemia. Lipids Health Dis. 2024;23(1): 187. 10.1186/s12944-024-02171-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vadakedath S, Kandi V. Probable potential role of urate transporter genes in the development of metabolic disorders. Cureus. 2018;10(3):e2382. 10.7759/cureus.2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maloberti A, Mengozzi A, Russo E, et al. The results of the URRAH (Uric acid right for heart Health) project: a focus on hyperuricemia in relation to cardiovascular and kidney disease and its role in metabolic dysregulation. High Blood Press Cardiovasc Prev. 2023;30(5):411–25. 10.1007/s40292-023-00602-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 1. Table S1. The effect of inflammation indexes, NHHR, TyG and BMI on the risk of HUA among people who developed HUA between 40~60 years old. Table S2. The effect of inflammation indexes, NHHR, TyG and BMI on the risk of HUA, according to a lower cut-off threshold of HUA (serum uric acid ≥5.6 mg/dL). Table S3. The effect of inflammation indexes, NHHR, TyG and BMI on the risk of HUA with different onset timing, according to a lower cut-off threshold of HUA (serum uric acid ≥5.6 mg/dL). Figure S1. The flow chart of the study. DLCC: the diverse life course cohort study. Figure S2. Violin diagrams of the distribution of BMI, SII, SIRI, NHHR and TyG in HUA and non-HUA groups. SIRI: Systemic inflammation response index; SII: Systemic immune‑inflammation index; NHHR: Non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio; TyG: Triglyceride-glucose index; BMI: Body mass index; HUA: hyperuricemia. Figure S3. The effect of inflammation indexes, NHHR, TyG and BMI on the risk of HUA in men. Adjusted covariates included age, ever-drink, ever-smoke, exercise habits, daily sedentary behavior, baseline hypertension and impaired eGFR (eGFR<90). SIRI: Systemic inflammation response index; SII: Systemic immune‑inflammation index; NHHR: Non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio; TyG: Triglyceride-glucose index; BMI: Body mass index; HUA: hyperuricemia. Figure S4. The effect of inflammation indexes, NHHR, TyG and BMI on the risk of HUA in women. Adjusted covariates included age, ever-drink, exercise habits, daily sedentary behavior, baseline hypertension and impaired eGFR (eGFR<90). SIRI: Systemic inflammation response index; SII: Systemic immune‑inflammation index; NHHR: Non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio; TyG: Triglyceride-glucose index; BMI: Body mass index; HUA: hyperuricemia.
Data Availability Statement
No datasets were generated or analysed during the current study.