Abstract
Objectives
Knee osteoarthritis (KOA), a degenerative joint disease marked by chronic pain, is associated with systemic inflammation that may extend to neurocognitive dysfunction. While chronic low-grade inflammation in KOA has been implicated in mild cognitive impairment (MCI), a prodromal stage of dementia, the mediating role of inflammation in brain functional reorganization remains unclear.
Methods
This study integrated neuroimaging, inflammatory biomarkers, and machine learning to investigate inflammation-mediated brain functional alterations in 63 KOA patients with/without MCI. Serum levels of pro-inflammatory cytokines (IL-6, TNF-α) and resting-state fMRI data were analyzed using voxel-wise Regional Homogeneity (ReHo) and Amplitude of Low-Frequency Fluctuation (ALFF).
Results
Comparisons across healthy controls, KOA-MCI, and KOA-non-MCI groups identified MCI-linked functional alterations in the medial prefrontal cortex (mPFC), precuneus, and superior temporal gyrus. Mediation analysis revealed that mPFC ReHo significantly mediated the relationship between elevated IL-6 and cognitive decline. Machine learning models incorporating ReHo features from mPFC demonstrated robust classification of MCI status (AUC: 0.87), validated in an external dataset.
Conclusion
Our findings suggest that IL-6-driven mPFC dysfunction is a potential pathway linking KOA-related inflammation to MCI, while highlighting the combined utility of ReHo/ALFF metrics in mPFC, precuneus, and temporal regions as potential neuroimaging biomarkers. This multimodal approach advances understanding of neuroinflammatory mechanisms in osteoarthritis and provides a framework for early detection of cognitive vulnerability in KOA populations.
Keywords: Knee osteoarthritis, Mild cognitive impairment, Resting-State fMRI, Machine learning
Introduction
Knee osteoarthritis (KOA), an age-related disorder characterized by progressive cartilage degradation and chronic pain, affects over 30% of adults aged over 65 [1]. Beyond its musculoskeletal burden, KOA also demonstrates an epidemiological overlap with neurodegenerative diseases. Longitudinal studies have revealed an elevated risk of Alzheimer’s disease (AD) and dementia in KOA patients compared to age-matched healthy controls. Such comorbidity underscores potential shared pathophysiological mechanisms between KOA and these neurodegenerative diseases [2, 3]. Chronic low-grade inflammation, a hallmark of both aging (“inflammaging”) and KOA, emerges as a pivotal mediator [4]. Aging potentiates knee osteoarthritis (KOA) progression through multiple mechanisms, including cellular senescence, mitochondrial dysfunction, and oxidative stress. Simultaneously, aging fuels systemic inflammation through the senescence-associated secretory phenotype (SASP), which involves the sustained release of inflammatory mediators, such as IL-6, TNF-α [5, 6]. Notably, this inflammatory milieu mirrors the “inflammaging” observed in neurodegenerative disorders [7, 8], where elevated circulating IL-6 and C-reactive protein correlate with hippocampal atrophy and cortical thinning [9]. Such parallels suggest that KOA and cognitive decline may converge on shared inflammatory pathways that may contribute to neural degeneration in aging populations.
Notably, while IL-6 and TNF-α elevation occurs across chronic conditions like rheumatoid arthritis (RA) and obesity. KOA also exhibits similar synovial fluid cytokine patterns driven by joint-specific cellular senescence [10]. In KOA, synovial fluid IL-6 levels persistently increased [11], enabling cytokine infiltration into the brain via compromised blood-brain barrier integrity [12–14]. Preclinical evidence indicates IL-6 disrupts hippocampal-prefrontal synaptic plasticity [12], while TNF-α exacerbates amyloid-beta aggregation and tau pathology [15]. Neuroimaging studies further associate elevated IL-6 with functional abnormalities in the default mode network (DMN) [16], a network selectively vulnerable in early AD [17, 18]. Mild cognitive impairment (MCI), a transitional phase preceding dementia, may represent a critical window wherein KOA-driven inflammation exacerbates neural vulnerability. However, the neurobiological pathways linking KOA-associated inflammatory signaling to MCI-related brain alterations remain elusive, impeding targeted therapeutic strategies. Furthermore, whether KOA-specific inflammatory signatures preferentially target MCI-associated brain networks remains unexplored, representing a critical knowledge gap.
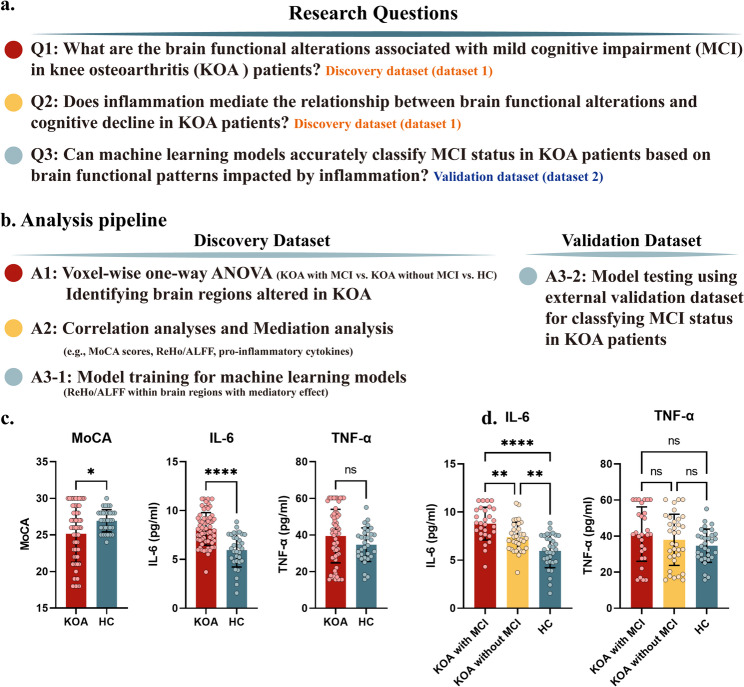
Resting-state fMRI (rs-fMRI) metrics such as Regional Homogeneity (ReHo) and Amplitude of Low-Frequency Fluctuation (ALFF) offer unique insights into early inflammation-driven neural reorganization preceding functional decline [19]. MCI patients exhibit ReHo reductions in the precuneus (a DMN hub) predictive of dementia progression [20] and ALFF decreases in language-related temporal regions [21], areas densely populated with IL-6 receptors and metabolically sensitive to systemic inflammation [22]. While chronic inflammatory conditions like rheumatoid arthritis demonstrate overlapping functional connectivity abnormalities, KOA’s unique triad of mechanical stress, chronic pain, and mobility restriction is likely to be associated with distinct neurofunctional adaptations [23]. Systematic characterization of these patterns could identify novel biomarkers for early intervention in comorbid cognitive decline. Therefore, in our current study, we investigated the regional brain functional alterations in KOA patients with/without MCI and investigated the association with brain functional alterations and serum levels of pro-inflammatory cytokines (IL-6, TNF-α). Machine learning models incorporating ReHo/ALFF features were also trained and validated on an external dataset for classifying MCI status. By conducting these analyses, we aimed to: (1) identify brain functional patterns associated with inflammation in KOA patients with MCI; (2) assess whether brain functional alterations influenced by inflammation can accurately predict MCI status in KOA patients using machine learning models (Fig. 1.a).
Fig. 1.
Panel (a) The research questions of current study. Panel (b) The analysis pipeline of current study. Panel (c) Pair-wise differences in MoCA scores, serum level of IL-6 and TNF-α between KOA patients and healthy controls. Panel (d) The one-way ANOVA for serum level of IL-6 and TNF-α among KOA patient with/without MCI and healthy controls. MoCA, Montreal Cognitive Assessment
Methods
Participants
The ethical approval of the current study was provided by the ethics committee of Xiangyang Central Hospital and Tianjin Baodi Hospital. For the discovery dataset, sixty-six KOA patients and thirty-two healthy controls (HC) were included in the current study from June 2022 to August 2024. Written informed consent was required for each participant before participating in the study. The inclusion criteria were as following: (1) Fulfilled the American College of Rheumatology (ACR) clinical criteria for knee osteoarthritis; (2) Radiographically confirmed Kellgren-Lawrence (KL) grade ≥ II in the affected knee; (3) Aged 50–65 years and right-handed; (4) No history of intra-articular corticosteroid injections within the preceding 3 months. Exclusion Criteria for All Participants: (1) Diagnosis of inflammatory arthritis (e.g., rheumatoid arthritis), traumatic knee injury, or secondary osteoarthritis; (2) Severe systemic comorbidities (e.g., uncontrolled diabetes, malignancy, cardiovascular events within 6 months); (3) Neurological disorders (e.g., stroke, Parkinson’s disease) or major psychiatric conditions (e.g., schizophrenia, bipolar disorder); (4) Contraindications to MRI (e.g., metal implants, claustrophobia); (5) Concurrent participation in other clinical trials involving anti-inflammatory or neuroactive medications. The inclusion criteria for HC include: (1) No history of knee pain, arthritis, or chronic pain syndromes; (2) Absence of structural knee abnormalities on radiography (KL grade 0); (3) Matched to KOA patients by age (± 5 years), sex, and education level (± 2 years). The analysis pipeline for the current study could be found in Fig. 1.b. For the testing dataset, the same inclusion and exclusion criteria were used, and thirty-eight KOA patients and thirty-five healthy controls were enrolled.
Cognitive evaluation
The Montreal Cognitive Assessment (MoCA) was chosen to evaluate the neurocognitive state of all participants, which evaluated several aspects of cognitive function, including visuospatial/executive, naming, language, attention, abstraction, memory (short-term immediate and deferred recall), and localization. The assessment time was approximately 10 min, with a maximum score of 30. Those subjects with scores of 26 or more were considered cognitively normal, and lower scores indicated poorer cognitive abilities. MoCA assessment was conducted for both the discovery and testing datasets.
Laboratory measurements
TNF-α and IL-6 levels were measured in the discovery dataset using ELISA. Peripheral blood was collected from patients with or without KOA, after which the serum was isolated via centrifugation at 3,000 x g at 4˚C for 15 min. Levels of TNF-α (cat. no. PT518; Beyotime Institute of Biotechnology) and IL-6 (cat. no. PI330; Beyotime Institute of Biotechnology) in serum were detected using ELISA kits according to the manufacturer’s protocol.
FMRI data acquisition
The brain functional MRI data were obtained in a 3T Siemens scanner with a 32-channel head coil at the Department of Radiology in Xiangyang Central Hospital. The participants were required to open their eyes, blink naturally concentrate on a dark screen during the entire fMRI scan, with a duration of 10 min. The gradient-echo echo-planar imaging sequence was applied to collect brain fMRI data with the following parameters: TR (repetition time) = 2000 ms, TE (echo time) = 30 ms, Slices = 44, Slice Thickness = 3.0 mm, in-plane resolution = 2.6 × 2.6 mm. A total of 300 volumes collected. After the functional imaging, a high-resolution T1-weighted structural image was acquired using an isotropic voxel MPRAGE sequence with a voxel size of 1 mm³ to ensure precise anatomical localization for subsequent analyses.
For testing dataset, brain fMRI data was collected in Tianjin Baodi Hospital for 6 min in 3T Siemens scanner with 64-channel head coil with following parameters: TR (repetition time) = 80 ms, TE (echo time) = 30 ms, field of view (FOV) = 222 mm ×222 mm, Slices = 44, Slice Thickness = 3.0 mm, in-plane resolution = 3 × 3 mm. A total of 450 volumes collected. After the functional imaging, a high-resolution T1-weighted structural image was also acquired using an isotropic voxel MPRAGE sequence with a voxel size of 1 mm³ to ensure precise anatomical localization for subsequent analyses.
Data preprocessing
The fMRI data were preprocessed using the DPARSFA toolbox, following a series of systematic steps to ensure data integrity and accuracy. The preprocessing included slice-timing correction to account for timing differences in image acquisition across slices, realignment to correct for head motion, and coregistration of functional images to the respective subjects’ structural images. Normalization was then performed to standardize the images to a common anatomical space, followed by smoothing using a 6 mm full-width at half maximum (FWHM) Gaussian kernel to enhance the signal-to-noise ratio. For the calculation of ReHo, the smoothing was conducted after the ReHo was calculated. Subsequently, segmentation of gray matter, white matter, and cerebrospinal fluid (CSF) was carried out to mitigate temporal confounding factors that could affect the analysis. To focus on relevant neural signals, band-pass filtering was applied within a frequency range of 0.01 to 0.1 Hz. To address potential correlations caused by head motion and artifacts, outlier time points in the motion parameters and global signal intensity were identified using the Artifact Detection Toolbox (ART, https://www.nitrc.org/projects/artifact_detect). Specifically, an image was flagged as an outlier if its composite displacement relative to the preceding image exceeded 0.5 mm, or if its global mean intensity deviated by more than 3 standard deviations from the mean image intensity. These identified outliers were subsequently incorporated as regressors into the first-order general linear model, together with motion parameters, to control for these confounding effects in subsequent analyses for calculating ReHo and ALFF.
Regional brain activity measurements
To measure the regional brain activity, Regional Homogeneity (ReHo) and Amplitude of Low Frequency Fluctuation (ALFF) were calculated in a voxel-wise manner. DPARSF software was also used for ALFF and ReHo analysis. To obtain the power spectrum, voxel time series were transformed into the frequency domain using the fast Fourier transform. The power spectrum was then square-rooted and averaged over the 0.01–0.10 Hz range for each voxel, defining the ALFF. The standardized ALFF values of each voxel were obtained by normalization to the global mean ALFF values. ReHo was calculated for each voxel using Kendall’s coefficient of concordance (KCC), assessing the temporal similarity with its 26 nearest voxels. The standardized ReHo was derived by normalizing each voxel’s KCC against the whole brain’s mean KCC, followed by smoothing with a 6 mm FWHM Gaussian kernel.
Statistical analyses
To investigate the brain functional alterations among HC and KOA with/without MCI, voxel-wise one-way ANOVA was conducted for both ReHo and ALFF with age, gender, and years of education as covariates. Significant clusters were reported based on a voxel-wise threshold of P < 0.001 combined with an FDR-corrected cluster-wise threshold of P < 0.05 for multiple comparison correction. Subsequently, the average value within the resultant cluster obtained from one-way ANOVA for each participant was calculated. Post-hoc two-sample T test was conducted to reveal the pair-wise differences in ALFF and ReHo among HC and KOA with/without MCI group. Pearson correlation analyses were performed to investigate the association among MoCA scores, serum levels of IL-6 and TNF-α, and brain ReHo/ALFF value in the KOA and HC groups, respectively. Covariates (age, gender, education) were included in the whole-brain ANOVA model and carried forward into all subsequent post-hoc t-test comparisons of specific groups.
Drawing on the findings from one-way ANOVA and correlation analyses, mediation analyses were conducted to reveal the potential causal association between altered regional brain activity and clinical measures. Given the lack of empirical evidence for the directionality of the association among brain ALFF/ReHo, serum level of IL-6/TNF-α and MoCA scores, two models were tested in the current study: (1) brain ALFF/ReHo as the mediator, with IL-6/TNF-α as the independent variable and MoCA as the dependent variable; and (2) ALFF/ReHo as the mediator, with MoCA as the independent variable and IL-6/TNF-α as the dependent variable. The mediation effects were assessed using bootstrapped mediation analysis to evaluate the substantial difference between the total effect (path c) and the direct effect (path c’). The PROCESS macro (www.processmacro.org, version 2.16.3) in SPSS (IBM, version 23.0) was utilized, employing 5000 bootstrap samples to establish 95% confidence intervals for the model components. Mediation was deemed statistically significant if the 95% confidence intervals did not include zero.
The brain regions exhibited a significant mediatory effect were considered to be affected by inflammatory cytokines and impact cognitive ability in KOA patients. To further investigate their predictive utility for identifying MCI status in KOA patients, machine learning analyses incorporating ALFF/ReHo values as features to predict MCI status in a held-out testing set (detailed description could be found in supplementary materials). No feature selection or cross-validation was applied during model training. Detailly, five ML algorithms were employed, including linear discriminant analysis (LDA), gradient boosting (GB), adaptive boosting (AdaBoost), support vector machine (SVM), and Gaussian naïve Bayes (NB). Moreover, unlike logistic regression models, numerous ML algorithms do not provide probabilities. To address this, Platt scaling was utilized to transform the less interpretable output scores of the model into probabilities. The ML models were trained on the discovery dataset and tested on the held-out dataset. To evaluate model performance, multiple discrimination metrics were employed. The metrics included area under the receiver operating characteristic curve (ROC) or area under the curve (AUC), accuracy, sensitivity, brier score and specificity.
Results
Demographic data
The demographic data of the current study are shown in Table 1. No significant between-group differences in age, sex, and years of education were observed among KOA patients with/without MCI and healthy controls (HC).
Table 1.
Demographic and clinical data of current study. KOA, knee osteoarthritis; MCI, mild cognitive impairment; moca, Montreal cognitive assessment; HC, healthy controls
| KOA with MCI | KOA without MCI | HC | P value | |
|---|---|---|---|---|
| Age | 59.6 ± 16.3 | 58.7 ± 14.6 | 60.4 ± 13.2 | 0.49 |
| Sex (Female/Male) | 17/12 | 18/16 | 16/16 | |
| Years of education | 10.2 ± 9.2 | 10.1 ± 11.2 | 9.2 ± 10.4 | 0.81 |
| MoCA | 21.5 ± 2.4 | 28.2 ± 1.6 | 29.9 ± 1.5 | <0.001 |
| IL-6 | 8.8 ± 1.7 | 7.4 ± 1.5 | 5.9 ± 1.8 | <0.001 |
| TNF-α | 41.3 ± 15.1 | 37.9 ± 14.2 | 34.7 ± 9.2 | 0.16 |
Clinical assessment
KOA patients had lower MoCA scores than healthy controls, suggesting a link between peripheral joint disease and cognitive impairment. Furthermore, we found that, compared to HC, KOA patients exhibited higher levels of serum IL-6 and a trend of higher levels of serum TNF-α (Fig. 1.c). Notably, IL-6 levels were significantly higher in KOA patients with MCI compared to those without MCI and HC (Fig. 1.d), suggesting a potential link between inflammation and cognitive decline.
ReHo and ALFF analysis
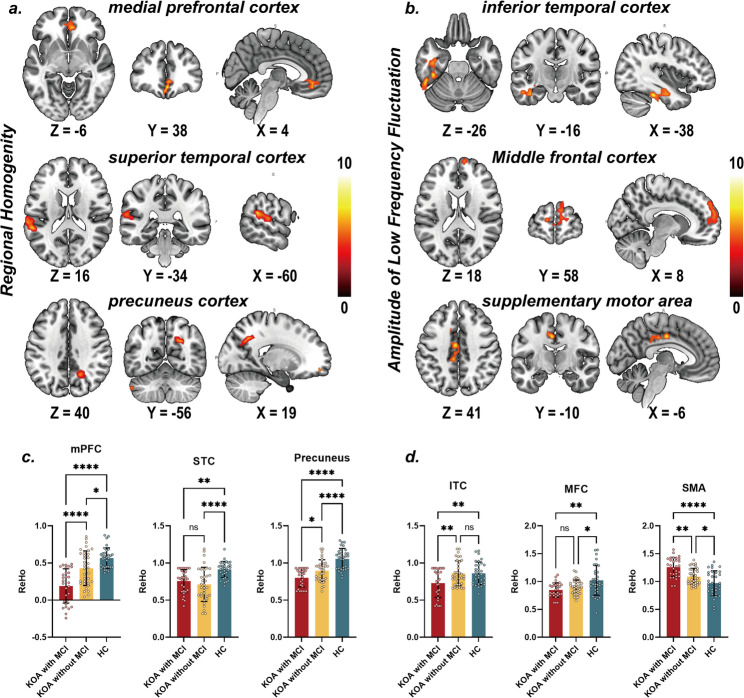
The brain imaging analysis identified significant alterations in ReHo and ALFF in KOA patients with and without MCI compared to HC (Table 2). Specifically, differences in ReHo in the medial prefrontal cortex (mPFC), superior temporal cortex (STC), and precuneus cortex were observed among KOA patients with MCI/without and HC (Fig. 2.a). Differences in ALFF were observed within the inferior temporal cortex, middle frontal cortex, and supplementary motor area in the same patient group (Fig. 2.b).
Table 2.
Brain regions exhibited significant group differences in regional homogeneity (ReHo) and amplitude of low frequency fluctuation (ALFF) among KOA patients with/without MCI and healthy controls. KOA, knee osteoarthritis; MCI, mild cognitive impairment; mPFC, medial prefrontal cortex; STC, superior Temporal cortex; MFC, middle frontal cortex; SMA, supplementary motor area; ITC, inferior Temporal cortex
| Brain Region | Number of Voxels | F value | MNI coordinates (x, y,z) | ||
|---|---|---|---|---|---|
| ReHo | |||||
| mPFC | 119 | 8.9 | 3 | 39 | -15 |
| STC | 154 | 11.2 | -60 | -42 | 21 |
| Precuneus | 72 | 7.8 | 21 | -63 | 33 |
| ALFF | |||||
| MFG | 164 | 11.4 | -12 | 63 | 12 |
| SMA | 136 | 10.8 | -6 | -12 | 42 |
| ITC | 185 | 12.6 | -51 | -69 | -33 |
Fig. 2.
Panel a-b. Voxel-wise one-way ANOVA differences in ReHo and ALFF among KOA patient with/without MCI and healthy controls (FDR corrected, Voxel-wise P < 0.001, Cluster-level P < 0.05). Panel c-d. Post-hoc pair-wise comparison in ReHo and ALFF among KOA patient with/without MCI and healthy controls
Figure 2.c demonstrates regional ReHo differences. In the STC, ReHo was significantly higher in HC compared to the combined KOA groups (both with and without MCI). No significant difference was observed between KOA patients with MCI and those without MCI. In the mPFC and precuneus, ReHo was significantly higher in KOA patients with MCI than in both KOA patients without MCI and HCs. Furthermore, KOA patients without MCI also exhibited significantly higher ReHo than HC in these regions. Similarly, Fig. 2.d shows regional ALFF differences. In the supplementary motor area (SMA), ALFF was significantly higher in KOA patients with MCI compared to the other groups (both HC and KOA without MCI). Furthermore, KOA patients without MCI also exhibited significantly higher ALFF than HC in SMA. In ITC, ALFF was significantly lower in KOA patients with MCI compared to the other groups (both HC and KOA without MCI). No significant differences were observed between HC and KOA without MCI. ALFF in the middle frontal cortex was significantly higher in healthy controls than in both KOA patient groups (with and without MCI), with no significant difference observed between KOA patients with MCI and those without MCI. These findings suggest that specific brain regions exhibit altered regional functional connectivity and activity in KOA patients, particularly those with MCI, indicating potential neurobiological underpinnings of cognitive impairment in this patient population.
Mediation analyses
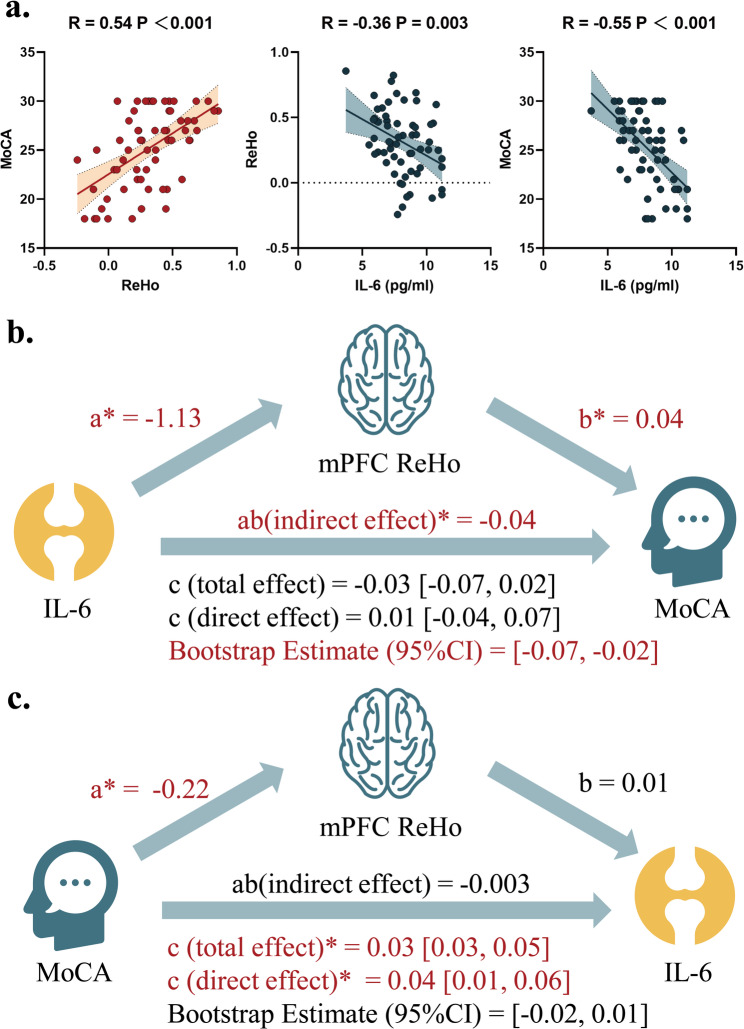
There was a strong positive correlation between mPFC ReHo and MoCA scores (R = 0.54, 95%CI = [0.35, 0.70], P < 0.001, Fig. 3.a), indicating that higher ReHo values are associated with better cognitive performance. Conversely, there is a negative correlation between IL-6 levels and MoCA scores (R = -0.55, 95%CI = [-0.70, -0.35], P < 0.001), suggesting that higher IL-6 levels are associated with worse cognitive performance. Additionally, IL-6 levels show a mild negative correlation with mPFC ReHo (R = -0.36, 95%CI = [-0.56, -0.12], P = 0.003), indicating that higher IL-6 levels are associated with lower ReHo values.
Fig. 3.
Panel (a) Scatter plots for pair-wise correlation among serum level of IL-6, mPFC ReHo and MoCA scores. Panel (b) Mediation analysis for the mediatory effect of mPFC for the association between IL-6 and MoCA scores. Panel (c) Mediation analysis for the mediatory effect of mPFC for the association between MoCA scores and IL-6. MoCA, Montreal Cognitive Assessment
To further identify the path association among IL-6, mPFC ReHo, and MoCA scores, mediation analysis was performed. We found that IL-6 levels have an indirect effect on MoCA scores through their influence on mPFC ReHo (Fig. 3.b). The indirect effect (ab) is -0.04, which is statistically significant, indicating that IL-6 levels negatively affect cognitive performance by reducing ReHo in the mPFC. Having said this, an alternative hypothesis is that IL-6 mediated the effect of MoCA scores on IL-6, and we also test this hypothesis by a different mediation pathway where MoCA scores have an indirect effect on IL-6 levels through their influence on mPFC ReHo. No significant indirect effect of this model was observed (Fig. 3.c). Overall, these results suggest that IL-6 levels and mPFC ReHo play a significant role in mediating the relationship between cognitive performance and inflammation in KOA patients.
Machine learning analyses
To further test whether inflammation impacted brain functional alterations could be used for classifying MCI status in KOA patients, we used the mPFC ReHo as features to train the machine learning models in the discovery set and tested on the external validation set. The results demonstrate that machine learning models, particularly Gradient Boosting (AUC = 0.77, 95%CI = [0.66,0.83]), Support Vector Machine (AUC = 0.82, 95%CI = [0.76, 0.94]), and AdaBoost (AUC = 0.86, 95%CI = [0.74,0.91]), can accurately classify MCI status in KOA patients based on brain functional patterns impacted by inflammation (Fig. 4; Table 3). The AdaBoost exhibited highest AUC compared with all other models (e.g., all P values < 0.05, DeLong test). AdaBoost also outperforms traditional methods like Linear Discriminant Analysis (AUC = 0.73, 95%CI = [0.64,0.88]) and Naive Bayes (AUC = 0.84, 95%CI = [0.68,0.88]), highlighting its potential utility in clinical settings for early detection and intervention.
Fig. 4.
Panel (a) The AUC values for each machine learning models respectively. Panel (b) The ROC curves for each ML models respectively, Panel (c) The DCA curve for each ML models respectively. LDA, Linear Discriminant Analysis (LDA); GB, Gradient Boosting; AdaBoost, Adaptive Boosting; SVM, Supported Vector Machine; NB, Gaussian Naïve Baye
Table 3.
Model performances for machine learning models respectively. LDA, linear discriminant analysis (LDA); GB, gradient boosting; adaboost, adaptive boosting; SVM, supported vector machine; NB, Gaussian Naïve Baye
| AUC | Accuracy | Sensitivity | Specificity | BrierScore | |
|---|---|---|---|---|---|
| LDA | 0.73 | 64.29 | 0.59 | 0.7 | 0.21 |
| GB | 0.77 | 73.81 | 0.73 | 0.75 | 0.2 |
| AdaBoost | 0.86 | 84.92 | 0.89 | 0.81 | 0.16 |
| SVM | 0.82 | 77.78 | 0.79 | 0.76 | 0.18 |
| NB | 0.84 | 80.16 | 0.79 | 0.81 | 0.17 |
Discussion
In this study, three main findings were observed: (1) KOA patients exhibited altered regional brain activity within mPFC, precuneus, and temporal cortices; (2) The altered regional brain activity measured by ALFF within mPFC mediated the effect of inflammation on MoCA scores in KOA patients; (3) Machine learning models incorporating ReHo/ALFF features from mPFC demonstrated robust classification of MCI status, validated in an external dataset.
Inflammation and regional brain alterations in KOA
The observed alterations in brain activity are consistent with existing literature that highlights the impact of inflammation on cognitive function. Chronic systemic inflammation, particularly elevated levels of IL-6 and TNF-α, has been implicated in neurodegenerative processes [24]. In KOA patients, peripheral IL-6 levels are significantly elevated, and this elevation is associated with greater pain and other clinical symptoms [12, 14]. Importantly, these pro-inflammatory cytokines can cross the blood-brain barrier and exert effects on the central nervous system [25]. This is particularly relevant in the context of aging, where chronic low-grade inflammation is common and contributes to a higher risk of cognitive decline [26].
Our study extends this knowledge by demonstrating that KOA patients exhibit specific altered regional brain activity, particularly in the medial prefrontal cortex (mPFC) and precuneus, areas crucial for executive functioning and self-referential thought processes [27, 28]. The mPFC, as part of the default mode network (DMN), is especially sensitive to inflammatory processes considering its role in integrating cognitive functions and emotional regulation [29]. This is further exacerbated in the aging population, where both KOA and cognitive decline are prevalent. In our analysis, the mPFC’s ReHo was significantly lower in KOA patients with MCI compared to HC and KOA patients without MCI. This finding suggests impaired local functional connectivity, which may contribute to the cognitive deficits observed in MCI. Furthermore, the precuneus, another critical hub of the DMN, showed similar alterations, reinforcing the idea that inflammatory states might disrupt normal brain network function [30]. Crucially, the directionality of these changes, observed decreases in ReHo within the DMN hubs in KOA MCI, requires careful interpretation. While such reductions often signify network dysfunction and correlate with cognitive impairment in prodromal Alzheimer’s disease and other neurodegenerative diseases [31–33]. The observed decreases in mPFC/precuneus ReHo in KOA MCI may reflect maladaptive processes where chronic inflammation directly impairs neuronal synchrony and network efficiency, rather than successful compensation. This pattern aligns with studies linking neuroinflammation to DMN hypoconnectivity and cognitive decline across various clinical cohorts, suggesting a common pathway towards dysfunction [34, 35].
The ability of peripheral IL-6 to cross the blood-brain barrier and influence brain function has been demonstrated in both animal models and human studies [25, 36]. Elevated IL-6 levels in the brain could disrupt synaptic plasticity and lead to functional alterations in regions such as the mPFC and precuneus [37]. This disruption may be a critical pathway linking peripheral inflammation in KOA to cognitive decline. The observed ReHo and ALFF alterations in these regions further support the hypothesis that inflammation-driven neural reorganization precedes functional changes and cognitive deficits.
Mediating effect of mPFC on the association between inflammation and cognitive decline
Our mediation analysis revealed that the ReHo of the mPFC significantly mediates the relationship between elevated IL-6 levels and cognitive decline in KOA patients. The lack of significant mediation or strong group differences for TNF-α, despite its established pro-inflammatory role, suggests a potentially more specific contribution of IL-6 to the KOA-MCI neuroinflammatory pathway within our cohort. This finding suggests a pathway through which peripheral inflammation affects central cognitive function, highlighting the mPFC as a critical region not only for cognitive processing but also as a potential target for therapeutic interventions. The mPFC is a key component of the DMN and is involved in various cognitive functions, including executive control, emotional regulation, and self-referential processing [38]. Elevated IL-6 levels, a hallmark of chronic systemic inflammation in KOA, might cross the blood-brain barrier and disrupt the functional integrity of the mPFC [39–42]. This disruption is evidenced by reduced ReHo values, indicating impaired local functional connectivity within the mPFC. The mediation effect observed in our study suggests that IL-6-induced inflammation specifically impacts the mPFC, leading to cognitive deficits characteristic of MCI. This association between peripheral inflammation and cognitive decline has been documented in previous research [43–45], with IL-6 identified as a key mediator that could affect neuroplasticity and synaptic function by modulating neurotransmitter systems and promoting neurodegeneration [46–48]. For instance, studies have shown that elevated IL-6 levels can impair synaptic plasticity in the hippocampus and prefrontal cortex, regions critical for memory and executive function [48]. Understanding the mechanisms through which IL-6 impacts the mPFC can provide valuable insights into potential therapeutic strategies aimed at reducing inflammation to preserve cognitive function in KOA patients. Our findings suggest that interventions targeting IL-6 and other inflammatory markers could be beneficial in mitigating cognitive decline in KOA patients. Anti-inflammatory medications, lifestyle modifications, and dietary interventions have all been shown to reduce systemic inflammation and improve cognitive outcomes in other inflammatory conditions [49]. For example, lifestyle changes such as regular low-impact physical activity, weight management, and an anti-inflammatory diet have been shown to reduce IL-6 levels and improve cognitive function [50]. Additionally, addressing chronic pain associated with KOA through pharmacological or psychological interventions may also help mitigate the cognitive repercussions of inflammation.
Machine learning classification of MCI
The performance of our machine learning models, which achieved an AUC of 0.87 in classifying MCI status based on ReHo and ALFF features from the mPFC, underscores the potential of neuroimaging biomarkers in predicting cognitive outcomes. The robustness of these models, validated with an external dataset, highlights their utility in clinical settings for early identification of cognitive vulnerability among KOA patients. Machine learning approaches have gained momentum in neurological research, providing the ability to analyze complex, high-dimensional data sets to identify subtle patterns that may not be apparent through traditional statistical methods [51–54]. Importantly, this model could be adapted for use in other populations experiencing neuroinflammation, thereby broadening its application and relevance. Our findings have significant implications for the clinical management of KOA patients, particularly as it relates to cognitive health. The identification of inflammation as a mediating factor in cognitive decline suggests that routine assessments of inflammatory markers could be beneficial in predicting cognitive outcomes. Furthermore, the early identification of cognitive impairment through neuroimaging biomarkers can prompt timely interventions, potentially altering the disease trajectory for these patients. Additionally, given the aging demographic and the increasing prevalence of KOA, integrating cognitive assessments and inflammation screening into standard care protocols for KOA patients is warranted. This holistic approach can lead to comprehensive management strategies that address both physical and cognitive health, ultimately enhancing patients’ overall quality of life.
Limitations
This study, while providing important insights, is not without limitations. The cross-sectional design restricts our ability to draw causal inferences regarding the relationship between inflammation and cognitive decline. Longitudinal studies are essential to clarify the temporal dynamics between inflammatory markers, brain alterations, and cognitive outcomes. Moreover, while we focused on IL-6 and TNF-α, exploring additional cytokines and inflammatory pathways relevant to KOA and cognitive decline could yield further insights. Future research should also consider the potential impact of other confounding variables, such as lifestyle factors, comorbidities, and genetic predispositions, that may influence cognitive health in KOA patients. Furthermore, expanding the sample size and diversity of participants would strengthen the generalizability of our findings, making the results more applicable to the broader population of older adults with KOA. Given the established role of chronic pain in modulating mPFC connectivity and its potential interaction with neuroinflammation, future longitudinal studies should incorporate detailed pain phenotyping and specifically account for pain-related neural reorganization to fully elucidate the distinct and shared contributions of pain and inflammation to cognitive decline in KOA. Future research should prioritize longitudinal designs in KOA cohorts. Such studies are essential to rigorously determine whether sustained peripheral inflammation precedes and predicts progressive mPFC functional alterations, which subsequently mediate cognitive decline.
Conclusion
In conclusion, our study highlights the intricate relationship between inflammation, regional brain alterations, and cognitive decline in KOA patients. The mediating role of mPFC dysfunction driven by elevated IL-6 levels presents a critical pathway for understanding cognitive impairment in this population. The successful application of machine learning models demonstrates the promise of neuroimaging biomarkers in predicting MCI status, paving the way for innovative approaches to early diagnosis and intervention. By addressing the dual burden of KOA and cognitive decline, we can ultimately improve the quality of life for affected individuals and contribute to better health outcomes in an aging population.
Abbreviations
- ACR
American college of rheumatology
- AD
Alzheimer’s disease
- ALFF
Amplitude of low-frequency fluctuation
- AUC
Area under the curve
- DMN
Default mode network
- ELISA
Enzyme-linked immunosorbent assay
- fMRI
functional magnetic resonance imaging
- HC
Healthy controls
- IL
6-Interleukin-6
- ITC
Inferior temporal cortex
- KL
Kellgren-lawrence (grade)
- KOA
Knee osteoarthritis
- MCI
Mild cognitive impairment
- MFC
Middle frontal cortex
- MoCA
Montreal cognitive assessment
- mPFC
medial prefrontal cortex
- ReHo
Regional homogeneity
- rs-fMRI
Resting-state functional magnetic resonance imaging
- SMA
Supplementary motor area
- STC
Superior temporal cortex
- TNF-α
Tumor necrosis factor-alpha
Author contributions
CL (a) ZL (*) conceptualized and designed the study, supervised the project, and contributed to the interpretation of the results.CH (a) and KZ (a) jointly contributed to the acquisition of clinical data and laboratory measurements, and participated in the initial drafting of the manuscript.PZ (*) and QS (*) analyzed the neuroimaging data, conducted statistical analyses, and contributed to the interpretation of the results. They also participated in the revision of the manuscript.ZL (*) interpreted the results and drafted the final version of the manuscript.All authors read and approved the final manuscript.Note: CL, CH, and KZ are co-first authors (a).PZ, QS, and ZL are co-corresponding authors (*).
Funding
Not applicable.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethic approval
Ethics approval was given by the Ethic committee of Xiangyang Central Hospital, Affiliated Hospital of Hubei University and Ethic committee of Baodi Hospital Affiliated Hospital of Tianjin Medical University.
Consent to participate
Informed consent was provided by each participant before any procedures.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Chao Li, Congqiang Hao and Kechong Zhou contributed equally to this work.
Contributor Information
Peng Zhang, Email: 1016600575@qq.com.
Quan Sun, Email: 562022663@qq.com.
Zhengxi Li, Email: lizx2025@163.com.
References
- 1.Weng Q, Chen Q, Jiang T, Zhang Y, Zhang W, Doherty M, Xie J, Liu K, Li J, Yang T, et al. Global burden of early-onset osteoarthritis, 1990–2019: results from the global burden of disease study 2019. Ann Rheum Dis. 2024;83(7):915–25. [DOI] [PubMed] [Google Scholar]
- 2.Endo Y, Kobayashi H, Watanabe K, Otani K, Otoshi K, Numazaki H, Sekiguchi M, Sato M, Nikaido T, Ono R et al. Radiographic Knee Osteoarthritis Is a Risk Factor for the Development of Dementia: Locomotive Syndrome and Health Outcomes in the Aizu Cohort Study. J Clin Med 2024, 13(16). [DOI] [PMC free article] [PubMed]
- 3.Weber A, Mak SH, Berenbaum F, Sellam J, Zheng YP, Han Y, Wen C. Association between osteoarthritis and increased risk of dementia: A systemic review and meta-analysis. Med (Baltim). 2019;98(10):e14355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greene MA, Loeser RF. Aging-related inflammation in osteoarthritis. Osteoarthritis Cartilage. 2015;23(11):1966–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ansari MY, Ahmad N, Haqqi TM. Oxidative stress and inflammation in osteoarthritis pathogenesis: role of polyphenols. Biomed Pharmacother. 2020;129:110452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou J, Huang J, Li Z, Song Q, Yang Z, Wang L, Meng Q. Identification of aging-related biomarkers and immune infiltration characteristics in osteoarthritis based on bioinformatics analysis and machine learning. Front Immunol. 2023;14:1168780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Irwin MR, Vitiello MV. Implications of sleep disturbance and inflammation for alzheimer’s disease dementia. Lancet Neurol. 2019;18(3):296–306. [DOI] [PubMed] [Google Scholar]
- 8.Wang XX, Zhang B, Xia R, Jia QY. Inflammation, apoptosis and autophagy as critical players in vascular dementia. Eur Rev Med Pharmacol Sci. 2020;24(18):9601–14. [DOI] [PubMed] [Google Scholar]
- 9.Tang Y, Wang Z, Cao J, Tu Y. Bone-brain crosstalk in osteoarthritis: pathophysiology and interventions. Trends Mol Med. 2024;31(3):281–295. 10.1016/j.molmed.2024.09.006 [DOI] [PubMed]
- 10.Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier JP, Fahmi H. Role of Proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 2011;7(1):33–42. [DOI] [PubMed] [Google Scholar]
- 11.Xu L, Ma J, Zhou C, Shen Z, Zhu K, Wu X, Chen Y, Chen T, Lin X. Identification of key hub genes in knee osteoarthritis through integrated bioinformatics analysis. Sci Rep. 2024;14(1):22437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kerkis I, da Silva ÁP, Araldi RP. The impact of interleukin-6 (IL-6) and mesenchymal stem cell-derived IL-6 on neurological conditions. Front Immunol. 2024;15:1400533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin Z, Liu T, Hu Z, Que W, Qiu H, Chen L. Effects of different running intensity on serum levels of IL-6 and TNF-α in patients with early knee osteoarthritis. J Coll Physicians Surg Pak. 2022;32(7):899–903. [DOI] [PubMed] [Google Scholar]
- 14.Wang P. Diagnostic value of combined serum IL-6, TNF-α, and leptin levels in patients with Post-Traumatic osteoarthritis. Clin Lab 2020;66(10). [DOI] [PubMed]
- 15.Gupta DP, Lee YS, Choe Y, Kim KT, Song GJ, Hwang SC. Knee osteoarthritis accelerates amyloid beta deposition and neurodegeneration in a mouse model of alzheimer’s disease. Mol Brain. 2023;16(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmitz CN, Sammer G, Neumann E, Blecker C, Gründer G, Adolphi H, Lamadé EK, Pedraz-Petrozzi B. Functional resting state connectivity is differentially associated with IL-6 and TNF-α in depression and in healthy controls. Sci Rep. 2025;15(1):1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giorgio J, Adams JN, Maass A, Jagust WJ, Breakspear M. Amyloid induced hyperexcitability in default mode network drives medial Temporal hyperactivity and early Tau accumulation. Neuron. 2024;112(4):676–e686674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ibrahim B, Suppiah S, Ibrahim N, Mohamad M, Hassan HA, Nasser NS, Saripan MI. Diagnostic power of resting-state fMRI for detection of network connectivity in alzheimer’s disease and mild cognitive impairment: A systematic review. Hum Brain Mapp. 2021;42(9):2941–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sezer I, Pizzagalli DA, Sacchet MD. Resting-state fMRI functional connectivity and mindfulness in clinical and non-clinical contexts: A review and synthesis. Neurosci Biobehav Rev. 2022;135:104583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yuan X, Han Y, Wei Y, Xia M, Sheng C, Jia J, He Y. Regional homogeneity changes in amnestic mild cognitive impairment patients. Neurosci Lett. 2016;629:1–8. [DOI] [PubMed] [Google Scholar]
- 21.Yuan Q, Qi W, Xue C, Ge H, Hu G, Chen S, Xu W, Song Y, Zhang X, Xiao C, et al. Convergent functional changes of default mode network in mild cognitive impairment using activation likelihood Estimation. Front Aging Neurosci. 2021;13:708687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zheng C, Zhou X-W, Wang J-Z. The dual roles of cytokines in alzheimer’s disease: update on interleukins, TNF-α, TGF-β and IFN-γ. Translational Neurodegeneration. 2016;5(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abe N, Fujieda Y, Tha KK, Narita H, Aso K, Karino K, Kanda M, Kono M, Kato M, Amengual O, et al. Aberrant functional connectivity between anterior cingulate cortex and left Insula in association with therapeutic response to biologics in inflammatory arthritis. Semin Arthritis Rheum. 2022;55:151994. [DOI] [PubMed] [Google Scholar]
- 24.Shan C, Zhang C, Zhang C. The role of IL-6 in neurodegenerative disorders. Neurochem Res. 2024;49(4):834–46. [DOI] [PubMed] [Google Scholar]
- 25.Liang N, Nho K, Newman JW, Arnold M, Huynh K, Meikle PJ, Borkowski K, Kaddurah-Daouk R, Kueider-Paisley A, Doraiswamy PM, et al. Peripheral inflammation is associated with brain atrophy and cognitive decline linked to mild cognitive impairment and alzheimer’s disease. Sci Rep. 2024;14(1):17423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mekhora C, Lamport DJ, Spencer JPE. An overview of the relationship between inflammation and cognitive function in humans, molecular pathways and the impact of nutraceuticals. Neurochem Int. 2024;181:105900. [DOI] [PubMed] [Google Scholar]
- 27.Garin CM, Hori Y, Everling S, Whitlow CT, Calabro FJ, Luna B, Froesel M, Gacoin M, Ben Hamed S, Dhenain M, et al. An evolutionary gap in primate default mode network organization. Cell Rep. 2022;39(2):110669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Müller NCJ, Dresler M, Janzen G, Beckmann CF, Fernández G, Kohn N. Medial prefrontal decoupling from the default mode network benefits memory. NeuroImage. 2020;210:116543. [DOI] [PubMed] [Google Scholar]
- 29.Joyce MKP, Uchendu S, Arnsten AFT. Stress and inflammation target dorsolateral prefrontal cortex function: neural mechanisms underlying weakened cognitive control. Biol Psychiatry. 2025;97(4):359–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bennet L, Dhillon S, Lear CA, van den Heuij L, King V, Dean JM, Wassink G, Davidson JO, Gunn AJ. Chronic inflammation and impaired development of the preterm brain. J Reprod Immunol. 2018;125:45–55. [DOI] [PubMed] [Google Scholar]
- 31.Guo W, Jin W, Li N, Gao J, Wang J, Chang Y, Yin K, Chen Y, Zhang S, Wang T. Brain activity alterations in patients with parkinson’s disease with cognitive impairment based on resting-state functional MRI. Neurosci Lett. 2021;747:135672. [DOI] [PubMed] [Google Scholar]
- 32.Liang X, Wen J, Ni L, Zhong J, Qi R, Zhang LJ, Lu GM. Altered pattern of spontaneous brain activity in the patients with end-stage renal disease: a resting-state functional MRI study with regional homogeneity analysis. PLoS ONE. 2013;8(8):e71507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu J, Qin W, Wang H, Zhang J, Xue R, Zhang X, Yu C. Altered spontaneous activity in the default-mode network and cognitive decline in chronic subcortical stroke. J Neurol Sci. 2014;347(1–2):193–8. [DOI] [PubMed] [Google Scholar]
- 34.Ma X, Zheng W, Li C, Li Z, Tang J, Yuan L, Ouyang L, Jin K, He Y, Chen X. Decreased regional homogeneity and increased functional connectivity of default network correlated with neurocognitive deficits in subjects with genetic high-risk for schizophrenia: A resting-state fMRI study. Psychiatry Res. 2019;281:112603. [DOI] [PubMed] [Google Scholar]
- 35.Zhu J, Zhao YP, Zhang YQ. The rs-fMRI study of effects of fornix and hippocampus-related brain function after the transcallosal interforniceal approach. Brain Res Bull. 2019;150:207–15. [DOI] [PubMed] [Google Scholar]
- 36.Conole ELS. Chronic inflammation and brain health: the case for early monitoring. Neurology. 2024;103(2):e209613. [DOI] [PubMed] [Google Scholar]
- 37.Serra D, Almeida LM, Dinis TCP. The impact of chronic intestinal inflammation on brain disorders: the Microbiota-Gut-Brain axis. Mol Neurobiol. 2019;56(10):6941–51. [DOI] [PubMed] [Google Scholar]
- 38.Sambuco N. Cognition, emotion, and the default mode network. Brain Cogn. 2024;182:106229. [DOI] [PubMed] [Google Scholar]
- 39.Nikolopoulos D, Manolakou T, Polissidis A, Filia A, Bertsias G, Koutmani Y, Boumpas DT. Microglia activation in the presence of intact blood-brain barrier and disruption of hippocampal neurogenesis via IL-6 and IL-18 mediate early diffuse neuropsychiatric lupus. Ann Rheum Dis. 2023;82(5):646–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rummel C, Matsumura K, Luheshi GN. Circulating IL-6 contributes to peripheral LPS-induced mPGES-1 expression in the rat brain. Brain Res Bull. 2011;86(5–6):319–25. [DOI] [PubMed] [Google Scholar]
- 41.Ting EY, Yang AC, Tsai SJ. Role of Interleukin-6 in depressive disorder. Int J Mol Sci 2020;21(6). [DOI] [PMC free article] [PubMed]
- 42.Zhao Z, Zhang J, Wu Y, Xie M, Tao S, Lv Q, Wang Q. Plasma IL-6 levels and their association with brain health and dementia risk: A population-based cohort study. Brain Behav Immun. 2024;120:430–8. [DOI] [PubMed] [Google Scholar]
- 43.Feng X, Valdearcos M, Uchida Y, Lutrin D, Maze M, Koliwad SK. Microglia mediate postoperative hippocampal inflammation and cognitive decline in mice. JCI Insight. 2017;2(7):e91229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gulen MF, Samson N, Keller A, Schwabenland M, Liu C, Glück S, Thacker VV, Favre L, Mangeat B, Kroese LJ, et al. cGAS-STING drives ageing-related inflammation and neurodegeneration. Nature. 2023;620(7973):374–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang RP, Ho YS, Leung WK, Goto T, Chang RC. Systemic inflammation linking chronic periodontitis to cognitive decline. Brain Behav Immun. 2019;81:63–73. [DOI] [PubMed] [Google Scholar]
- 46.Koutentaki E, Basta M, Antypa D, Zaganas I, Panagiotakis S, Simos P, Vgontzas AN. IL-6 Enhances the Negative Impact of Cortisol on Cognition among Community-Dwelling Older People without Dementia. Healthcare (Basel) 2023, 11(7). [DOI] [PMC free article] [PubMed]
- 47.Zhu X, Shen J, Feng S, Huang C, Wang H, Huo F, Liu H. Akkermansia muciniphila, which is enriched in the gut microbiota by metformin, improves cognitive function in aged mice by reducing the Proinflammatory cytokine interleukin-6. Microbiome. 2023;11(1):120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kummer KK, Zeidler M, Kalpachidou T, Kress M. Role of IL-6 in the regulation of neuronal development, survival and function. Cytokine. 2021;144:155582. [DOI] [PubMed] [Google Scholar]
- 49.Cheng HM, Koutsidis G, Lodge JK, Ashor A, Siervo M, Lara J. Tomato and lycopene supplementation and cardiovascular risk factors: A systematic review and meta-analysis. Atherosclerosis. 2017;257:100–8. [DOI] [PubMed] [Google Scholar]
- 50.Alme KN, Askim T, Assmus J, Mollnes TE, Naik M, Næss H, Saltvedt I, Ueland PM, Ulvik A, Knapskog AB. Investigating novel biomarkers of immune activation and modulation in the context of sedentary behaviour: a multicentre prospective ischemic stroke cohort study. BMC Neurol. 2021;21(1):318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Song J, Li J, Zhao R, Chu X. Developing predictive models for surgical outcomes in patients with degenerative cervical myelopathy: a comparison of statistical and machine learning approaches. Spine J. 2024;24(1):57–67. 10.1016/j.spinee.2023.07.021 [DOI] [PubMed]
- 52.Su Q, Li J, Chu X, Zhao R. Preoperative pain hypersensitivity is associated with axial pain after posterior cervical spinal surgeries in degenerative cervical myelopathy patients: a preliminary resting-state fMRI study. Insights Imaging. 2023;14(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhao R, Chu X, Ge Y, Guo X, Xue Y. Brain connectivity markers in degenerative cervical myelopathy patients with depression for predicting the prognosis following decompression surgery. Front Neurol. 2022;13:1003578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhao R, Guo X, Wang Y, Song Y, Su Q, Sun H, Liang M, Xue Y. Functional MRI evidence for primary motor cortex plasticity contributes to the disease’s severity and prognosis of cervical spondylotic myelopathy patients. Eur Radiol. 2022;32(6):3693–704. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.