Abstract
This study was conducted to investigate whether Accell gene gun coadministration of DNA encoding human interleukin-6 (IL-6) would enhance protective immune responses in mice to an equine influenza A virus hemagglutinin (HA) DNA vaccine. Mice that received HA DNA alone exhibited accelerated clearance of homologous challenge virus but were not protected from infection. In contrast, mice that received both HA and IL-6 DNA had no detectable virus in their lungs after challenge. These results strongly support the use of IL-6 as a cytokine adjuvant in DNA vaccination.
Influenza A viruses are important pathogens in a variety of mammalian and avian species (20). In humans, this virus is one of the most important respiratory pathogens, with infection leading to extensive morbidity and greater than 20,000 deaths in the United States during epidemic years (2). In horses, influenza A virus infection is also a medically and economically important disease throughout the world. In particular, it is one of the most common causes of viral respiratory disease among horses in North America (19, 36). As with humans, because of the high morbidity and economic losses associated with outbreaks, intense vaccination programs are employed for horses in an effort to control infection with influenza virus. Recovery from natural infection in horses results in complete immunity to reinfection for at least 6 months and partial immunity for over 1 year (10). However, the inactivated, whole-virus vaccines that are commercially available for horses offer only limited short-term protection (19). Therefore, new vaccines that elicit responses more like the responses to natural infection are needed.
We have evaluated Accell (PowderJect Vaccines Inc., Madison, Wis.) gene gun-mediated DNA vaccination as an alternative approach to influenza virus vaccination. DNA vaccination has been shown previously to elicit immune responses to a wide variety of viral, bacterial, and protozoal pathogens (7, 11, 31, 41). In particular, immune responses to avian influenza virus infection in chickens and human influenza virus infection in mice and ferrets have been demonstrated following DNA administration via intravenous, intramuscular, intranasal, and gene gun-mediated routes of delivery (8, 37, 38, 40). Cutaneous administration of DNA with the gene gun is, however, the most efficient approach, requiring 250 to 5,000-fold less DNA than parenteral injection techniques (9, 24). This technique also provides an added safety advantage over intramuscular injection since the administered DNA can be removed from the body through normal epidermal cell turnover (33).
Compared to administration of preformed protein antigen, DNA vaccination is particularly attractive for several reasons. Active synthesis of the immunogen de novo in transfected cells facilitates antigen expression in native form and expression by major histocompatibility complex class I as well as class II molecules (11, 40). In addition, DNA vaccination appears to be capable of generating long-term humoral and cellular immune responses (43) and may provide better heterologous protection against antigenic-drift variants of influenza virus (40). As such, this approach may be superior to the currently available killed equine influenza virus vaccines. We have demonstrated previously that Accell gene gun-mediated DNA vaccination with the hemagglutinin (HA) gene of A/Equine/Kentucky/1/81 (H3N8) (Eq/KY) virus induces virus-specific antibodies (Abs), including virus-neutralizing (VN) Abs, in mice (23). However, only partial protection from challenge infection was achieved with this HA DNA vaccine unless a very prolonged time period was allowed between doses of DNA. In contrast, recovery of mice from a previous infection with Eq/KY virus conferred complete immunity to homologous virus challenge 6 weeks later (23). We hypothesized that addition of a cytokine adjuvant could enhance the immune responses generated by our HA DNA vaccine and subsequent protection from infection, thus mimicking the host response to natural infection. Previous studies have investigated the use of a wide variety of cytokines (interleukin-1 [IL-1] to IL-8, IL-12, interferon, granulocyte-macrophage colony-stimulating factor, and tumor necrosis factor) as vaccine adjuvants (4, 12, 15, 16, 21, 25–27, 42), but our study is unique in its use of IL-6 DNA as a vaccine adjuvant administered by gene gun delivery. IL-6 is a critical factor in end-stage differentiation of B cells into immunoglobulin A (IgA)-secreting plasma cells (13, 17), and studies by Ramsay et al. of IL-6 knockout mice demonstrated that IL-6 is vital for maintenance of mucosal IgA responses (26). However, IL-6 also stimulates proliferation of T cells (39). Immunity to influenza is thought to be similarly dependent upon both local IgA responses for protection at the mucosal surfaces and cellular immune responses for clearance of virus from the body (20). Thus, we hypothesized that coadministration of IL-6 DNA would enhance the level of protection elicited by an influenza virus DNA vaccine.
Effect of HA DNA vaccination, with or without IL-6 DNA as a cytokine adjuvant, on protection from homologous virus challenge infection.
DNA vaccination was conducted with the Accell gene gun (11). Two doses of 5.0 μg of total DNA were administered into the epidermis of BALB/c mice, with a 3-week interval between vaccinations. The Eq/KY HA gene had been cloned previously (23) into a cytomegalovirus (CMV) promoter-based eukaryotic expression vector (pWRG7077; kindly provided by James Fuller, PowderJect) containing intron A from CMV, a poly(A) signal, and the kanamycin resistance gene. The resulting plasmid is hereafter designated pWRGHA. HA protein expression from pWRGHA was confirmed by immunofluorescent-antibody staining of transiently transfected Madin-Darby canine kidney (MDCK) cells (23). The human IL-6 (HuIL-6) gene was also expressed in a CMV promoter-based plasmid (32) and is hereafter referred to as pWRGIL-6. IL-6 expression from pWRGIL-6 was confirmed by testing the supernatants of transiently transfected MDCK cells with both a commercially available enzyme-linked immunosorbent assay (ELISA) kit (see below) and the B9-cell assay for IL-6 bioactivity (1).
In our first experiment, the mice were divided into three vaccination groups. One group of mice (n = 10) received only pWRG7077 DNA and served as controls. The second group of mice (n = 20) received 2.5 μg of pWRGHA DNA plus 2.5 μg of pWRG7077 control DNA, and the third group of mice (n = 20) received 2.5 μg of pWRGHA DNA plus 2.5 μg of pWRGIL-6 DNA. The reason for including control pWRG7077 DNA in the HA group was to equilibrate the total amount of DNA given and to account for any promoter competition in the mice receiving HA plus IL-6 DNA. However, for the sake of clarity, this HA-pWRG combination is hereafter referred to simply as HA DNA. To confirm our initial protection-from-challenge results and to obtain samples for assessment of mucosal immune responses, a second round of similar experiments was conducted (n = 10 mice per group), this time including a fourth group of mice that received pWRGIL-6 DNA plus pWRG7077 DNA. This group was included as an additional control to rule out any direct protective effect of IL-6 in the absence of HA antigen expression. Two weeks after the second vaccinations, all mice in the first experiment and half of the mice in each vaccination group in the second experiment were challenge infected with 6.65 × 104 50% egg infectious doses (EID50s) of Eq/KY virus by intranasal instillation under light methoxyflurane (Metofane; Pittman Moore, Mundelein, Ill.) sedation. (The Eq/KY virus was obtained from the Influenza Virus Repository at the University of Wisconsin—Madison and was propagated in embryonated chicken eggs [22].) The remaining mice in the second experiment were euthanatized 2 weeks after their second vaccination to obtain nasal wash samples for assessment of mucosal immune responses in the absence of a challenge infection. Challenged mice were euthanatized either 3 or 5 days after infection in experiment 1. (Half of the mice in each group were euthanatized on each day.) All challenged mice in experiment 2 were euthanatized 3 days after infection.
To assess protection from infection, the levels of virus in the lungs of challenged mice were calculated in EID50s/gram of lung tissue as previously described (23, 28). Virus titers for vaccination groups were compared statistically by one-way analysis of variance (ANOVA) with pairwise contrasts. The titers of virus in the lungs of the mice from the first experiment are shown in Fig. 1A. Compared to the control vaccinated mice, the mice that received HA DNA had reduced levels of virus in their lungs by day 3 after challenge (P = 0.001); they had also cleared their infections by 5 days after challenge. However, the mice vaccinated with HA plus IL-6 DNA were completely protected from pulmonary infection, as evidenced by a lack of detectable virus in their lungs after challenge. The difference in virus titers between the HA and HA plus IL-6 DNA-vaccinated mice 3 days after challenge is highly statistically significant (P < 0.0001). These results were confirmed in the second experiment (Fig. 1B). Once again, the mice that received both HA and IL-6 DNA were completely protected. The virus titers for the mice that received IL-6 DNA without HA DNA were comparable to those in the control DNA-vaccinated mice, thus confirming that the protection we observed cannot be attributed to an effect of IL-6 alone.
FIG. 1.
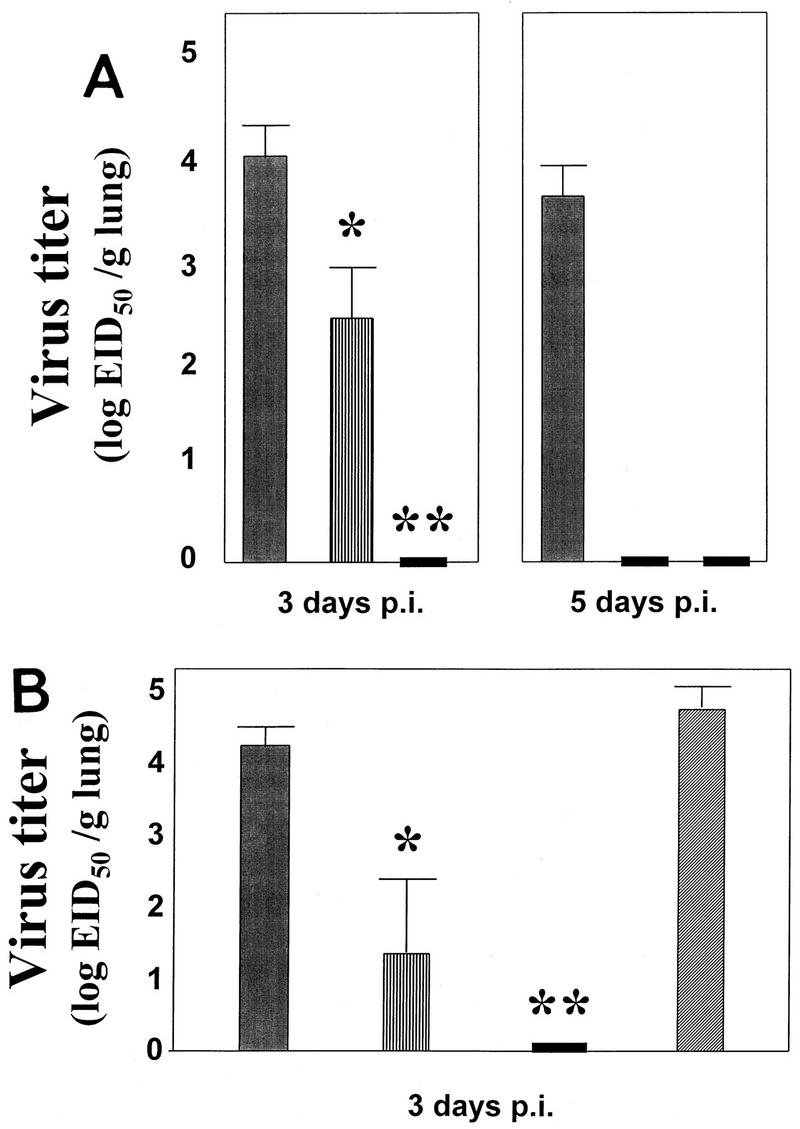
The mean titers, and standard errors of the means, of virus in the lungs of mice following vaccination with control pWRG7077 DNA (▪), pWRGHA plus pWRG7077 DNA (▥), pWRGHA plus pWRGIL-6 DNA (░⃞), or pWRGIL-6 plus pWRG7077 DNA (▨). Mice were challenged with 6.65 × 104 EID50s of Eq/KY virus by intranasal instillation under light methoxyflurane (Pittman Moore) sedation, 2 weeks after the second vaccinations. (A) Results from experiment 1. (B) Results from experiment 2. Virus was detected by inoculation (in triplicate) of serial dilutions of each of the mouse lungs into the allantoic cavities of 10-day-old embryonated chicken eggs (23). Mice were euthanatized for collection of lung samples either 3 or 5 days after challenge (p.i.). *, the reduction in titer achieved by administration of HA DNA compared to controls is statistically significant (P = 0.001) (one-way ANOVA and pairwise contrasts); **, the reduction in titer achieved by coadministration of HA plus IL-6 DNA compared to administration of HA DNA alone is highly statistically significant (P < 0.0001) (one-way ANOVA and pairwise contrasts).
Measurement of serum IL-6 levels after vaccination.
HuIL-6 is known to bind to murine IL-6 (MuIL-6) receptors and to be functional in the mouse (39). The use of HuIL-6 DNA in this study allowed us to distinguish between serum IL-6 activity expressed from our DNA construct and endogenous MuIL-6. Levels of both HuIL-6 and MuIL-6 in serum samples obtained 44 h after the first vaccinations were determined with commercially available ELISA kits (R&D Systems Inc., Minneapolis, Minn.). This time period after DNA administration was chosen based upon previous results of gene gun-mediated administration of IL-6 DNA in mice (32). Following vaccination, the level of HuIL-6 in serum was 40 pg/ml in the mice that received IL-6 DNA but was below the level of detection of the assay (<3 pg/ml) in the mice that received control or HA DNA alone. The MuIL-6 levels remained below the level of detection of the assay (<15.6 pg/ml) in all samples, suggesting that expression of HuIL-6 by DNA administration did not up-regulate endogenous MuIL-6 expression.
Virus-specific serum IgG, IgA, and VN-Ab production.
Virus-specific Abs were measured by isotype-specific ELISA and VN-Ab assay (23) with serum samples obtained immediately prior to the first vaccinations, immediately prior to the second vaccinations (3 weeks), immediately prior to challenge (5 weeks), and at the time of euthanasia. Due to volume restrictions, blood samples from all of the mice in each vaccination group were pooled at the time of collection. Administration of either HA or HA plus IL-6 DNA induced Eq/KY virus-specific serum IgG and VN Abs and primed the mice for production of virus-specific serum IgA following challenge. However, despite the dramatic enhancement of protection from challenge that we observed in the mice that received HA plus IL-6 DNA, we did not observe large differences in their immune responses compared to those in the mice that received HA DNA alone. Virus-specific serum IgG and IgA responses at the time of challenge were identical in both groups of mice (Fig. 2A and B), and only subtle enhancements in titer (1 serum dilution factor) were observed after challenge in the mice that received HA plus IL-6 DNA. The kinetics of the VN-Ab responses (Fig. 2C) paralleled those of the serum IgG responses. At the time of challenge, the VN-Ab titer for the mice that received HA plus IL-6 DNA was 50% higher than the titer for the mice that received HA DNA alone. However, this difference represents less than 1 serum dilution factor, and it is very unlikely that this small difference could account for their distinctly enhanced protection from pulmonary infection.
FIG. 2.
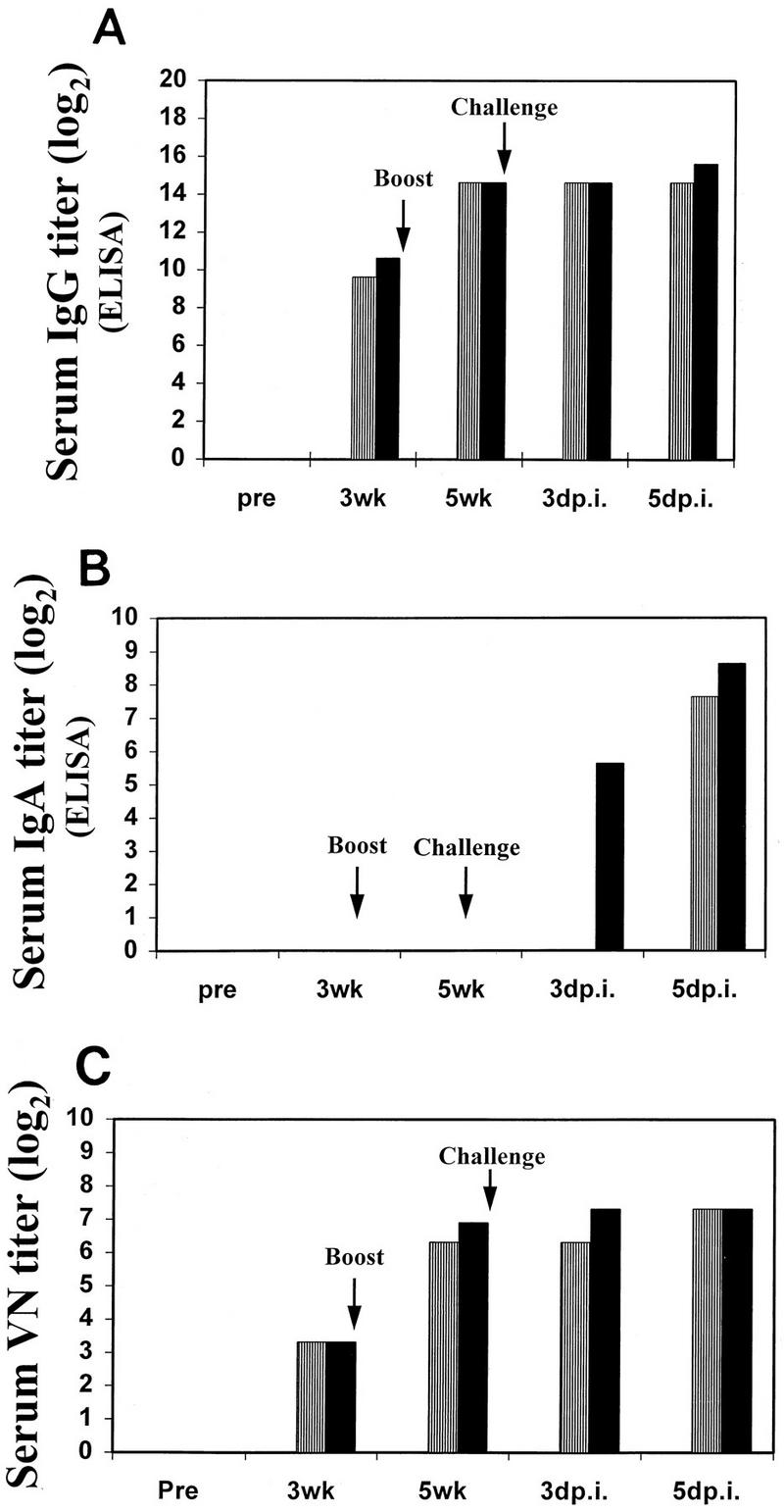
(A and B) Virus-specific serum IgG (A) and IgA (B) titers, as measured by ELISA, in mice vaccinated with control pWRG7077 DNA, pWRGHA plus pWRG7077 DNA (▥), or pWRGHA plus pWRGIL-6 DNA (▪). The ELISAs were performed as previously described (23). ELISA titers are defined as the reciprocal of the highest dilution of serum for which the optical density was a least twice as great as the optical density of the negative control sample on that plate. (C) VN-Ab titers in mice vaccinated with control pWRG7077 DNA, pWRGHA plus pWRG7077 DNA (▥), or pWRGHA plus pWRGIL-6 DNA (▪). VN Abs were measured by inoculation (in triplicate) of MDCK cells with serial dilutions of serum incubated with 50% tissue culture infective doses (50 doses) of Eq/KY virus (23). The VN-Ab titers are defined as the reciprocal of the highest dilution of serum that completely inhibited the Eq/KY virus-induced cytopathic effect. In each panel, the times of sampling are shown. pre, immediately prior to the first vaccination; 3wk, immediately prior to the second vaccination; 5wk, immediately prior to challenge; and 3dp.i. or 5dp.i., 3 or 5 days after challenge infection.
Virus-specific mucosal IgG and IgA responses.
In the second experiment, nasal wash samples were collected for assessment of Ab responses in the nasal passages. These samples were obtained 2 weeks after the second vaccination from mice that were not challenged and 3 days after infection from the challenged mice. Nasal washes were performed after euthanasia by inserting a 22-gauge intravenous catheter retrograde from the tracheal bifurcation toward the head, positioning the end of the catheter at the caudal area of the nasal turbinates. One milliliter of sterile phosphate-buffered saline plus 1% bovine serum albumin was flushed through the catheter and collected as it drained from the nares into a sterile petri dish. The flush was repeated three times with the same volume of fluid. As an additional measure of mucosal immune responses, virus-specific Abs in fecal pellets that were collected immediately prior to challenge and at the time of euthanasia were measured. One fecal pellet was collected per mouse and homogenized to a concentration of 1 mg/ml (wt/vol) in phosphate-buffered saline containing 5% fetal bovine serum and 0.01% Tween 20.
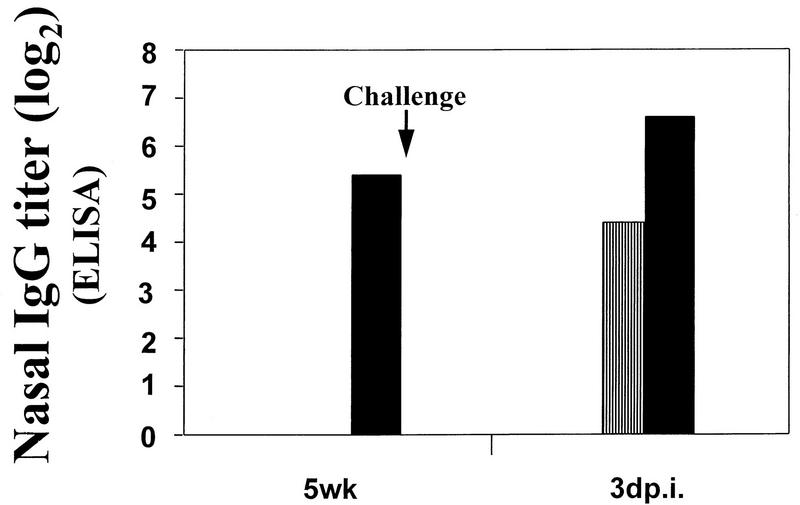
No virus-specific IgA was detectable in any of the nasal washes or fecal pellets tested. This was not simply a technical problem with the assay, because we could detect virus-specific IgA when we spiked a portion of these samples with immune sera (data not shown). Virus-specific IgG was detected in the nasal washes. In fact, nasal IgG was the only immunologic parameter that differed substantially at the time of challenge for the mice that received HA plus IL-6 DNA and those that received HA DNA alone. This may be an important correlate to protection against influenza virus infection in our model system. Virus-specific IgG was present in the nasal washes prior to challenge in the mice vaccinated with HA plus IL-6 DNA, but it was not detectable until after challenge infection in the mice that received HA DNA alone. In addition, by 3 days after challenge, the virus-specific IgG titer was fourfold higher in the mice vaccinated with HA plus IL-6 DNA (Fig. 3). The procedure for obtaining nasal wash samples is very nontraumatic. Therefore, we are confident that this IgG does not simply represent blood contamination at the time of sampling.
FIG. 3.
Virus-specific IgG titers, as measured by ELISA, in nasal wash specimens from mice vaccinated with control pWRG7077 DNA, pWRGHA plus pWRG7077 DNA (▥), pWRGHA plus pWRGIL-6 DNA (▪), or pWRGIL-6 plus pWRG7077 DNA. Titers are defined as the reciprocal of the highest dilution of sample for which the optical density was a least twice the optical density of the negative control samples on that plate. The times of sampling are shown. 5wk, immediately prior to challenge; 3dp.i., 3 days after challenge infection.
IgA is the predominant isotype in mucosal secretions because it is selectively transported across mucosal surfaces by the polymeric immunoglobulin receptor transport system (18). In contrast, IgG cannot utilize this transport system and, therefore, most likely enters mucosal secretions either via transudation from systemic sources or following local production. Results of numerous studies indicate that protection from initial infection with influenza virus in the upper airways of mice (3, 14, 29, 30, 34, 35) and humans (5, 20) correlates with local IgA responses. However, this was not the case in our study, and it is important to note that there is a precedent for protection from influenza virus lethal challenge in mice in the absence of a mucosal IgA response (6). Furthermore, preliminary results of DNA vaccination of horses indicate that protective immune responses correlate with nasal IgG responses in the absence of IgA prior to challenge (16a). To more completely understand the immunologic basis for the IL-6 adjuvant effect that we have observed in mice, future studies will be needed to determine whether virus-specific IgG-secreting cells are present in the upper airways of vaccinated mice and whether their frequency correlates with protection from initial infection in the nasal passages, as well as in the lungs. In addition, it will be very important to assess the effect of IL-6 DNA administration on cytotoxic-T-lymphocyte responses in our system, since cytotoxic T lymphocytes may be very important in clearance of influenza virus from the body (20).
In summary, Accell gene gun administration of DNA encoding IL-6 induced a very significant adjuvant effect on the protection from challenge infection elicited by an equine influenza virus HA DNA vaccine in mice. We have initiated similar studies of horses and pigs, in both of which influenza virus is an important pathogen. Immune responses against influenza virus have been elicited previously by gene gun-mediated DNA vaccination in both of these species (16a, 16b), but as in our mouse model system, HA DNA vaccination alone has not been sufficient to induce complete protection from challenge. Therefore, we have cloned the porcine (kindly provided by Michael Murtaugh, University of Minnesota) and equine (kindly provided by David Horohov, Louisiana State University) IL-6 genes into the pWRG7077 expression vector, demonstrated that they are functionally expressed in vitro in bioactive forms (13a, 31a), and are currently assessing the adjuvant effect of IL-6 DNA on influenza virus DNA vaccination in these outbred species.
Acknowledgments
We thank Virginia Hinshaw (University of Wisconsin) for providing the Eq/KY virus isolate, Brian Aldridge (University of Wisconsin) for performing the statistical analyses, and Dennis McCabe (Agracetus) and Michael Macklin (PowderJect) for technical assistance with the gene gun. We also thank Jackie Katz (Centers for Disease Control and Prevention) for advice on the nasal lavage procedure.
This work was supported in part by grants from the Companion Animal Fund of the University of Wisconsin (C.W.O.), the Agricultural Experiment Station of the USDA (C.W.O.), and the Grayson-Jockey Club Research Foundation (D.P.L.).
REFERENCES
- 1.Aarden L A, De Groot E R, Schapp O L, Lansdorp P M. Production of hybridoma growth factor by monocytes. Eur J Immunol. 1987;17:1411–1416. doi: 10.1002/eji.1830171004. [DOI] [PubMed] [Google Scholar]
- 2.Arden N H, Cox N J, Schonberger L B. Prevention and control of influenza. Morbid Mortal Weekly Rep. 1996;45:1–24. [Google Scholar]
- 3.Bender B S, Rowe C A, Taylor S F, Wyatt L S, Moss B, Small P A., Jr Oral immunization with a replication-deficient recombinant vaccinia virus protects mice against influenza. J Virol. 1996;70:6418–6424. doi: 10.1128/jvi.70.9.6418-6424.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chow Y-H, Huang W-L, Chi W-K, Chu Y-D, Tao M-H. Improvement of hepatitis B virus DNA vaccines by plasmids coexpressing hepatitis B surface antigen and interleukin-2. J Virol. 1997;71:169–178. doi: 10.1128/jvi.71.1.169-178.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clements M L, O’Donnell S, Levine M M, Chanock R M, Murphy B R. Dose response of A/Alaska/6/77 (H3N2) cold-adapted reassortant vaccine virus in adult volunteers: role of local antibody in resistance to infection with vaccine virus. Infect Immun. 1983;40:1044–1051. doi: 10.1128/iai.40.3.1044-1051.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deck R R, DeWitt C M, Donnelly J J, Liu M A, Ulmer J B. Characterization of humoral immune responses induced by an influenza hemagglutinin DNA vaccine. Vaccine. 1997;15:71–78. doi: 10.1016/s0264-410x(96)00101-6. [DOI] [PubMed] [Google Scholar]
- 7.Donnelly J J, Ulmer J B, Liu M A. Immunization with DNA. J Immunol Methods. 1994;176:145–152. doi: 10.1016/0022-1759(94)90308-5. [DOI] [PubMed] [Google Scholar]
- 8.Fynan E F, Webster R G, Fuller D H, Haynes J R, Santoro J C, Robinson H L. DNA vaccines: protective immunizations by parenteral, mucosal, and gene-gun inoculations. Proc Natl Acad Sci USA. 1993;90:11478–11482. doi: 10.1073/pnas.90.24.11478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fynan E F, Webster R G, Fuller D H, Haynes J R, Santoro J C, Robinson H L. DNA vaccines: a novel approach to immunization. Int J Immunopharmacol. 1995;17:79–83. doi: 10.1016/0192-0561(94)00090-b. [DOI] [PubMed] [Google Scholar]
- 10.Hannant D, Mumford J A, Jessett D M. Duration of circulating antibody and immunity following infection with equine influenza virus. Vet Rec. 1988;122:125–128. doi: 10.1136/vr.122.6.125. [DOI] [PubMed] [Google Scholar]
- 11.Haynes J R, McCabe D E, Swain W F, Widera G, Fuller J T. Particle-mediated nucleic acid immunization. J Biotechnol. 1996;44:37–42. doi: 10.1016/0168-1656(96)80298-7. [DOI] [PubMed] [Google Scholar]
- 12.Hengge U R, Chan E F, Foster R A, Walker P S, Vogel J C. Cytokine gene expression in epidermis with biological effects following injection of naked DNA. Nat Genet. 1996;10:161–166. doi: 10.1038/ng0695-161. [DOI] [PubMed] [Google Scholar]
- 13.Holmgren J, Czerkinsky C, Lycke N, Svennerholm A-M. Mucosal immunity: implications for vaccine development. Immunobiology. 1992;184:157–179. doi: 10.1016/S0171-2985(11)80473-0. [DOI] [PubMed] [Google Scholar]
- 13a.Larsen, D. L., et al. Unpublished results.
- 14.Liew F Y, Russell S M, Appleyard G, Brand C M, Beale J. Cross-protection in mice infected with influenza A virus by the respiratory route is correlated with local IgA antibody rather than serum antibody or cytotoxic T cell reactivity. Eur J Immunol. 1984;14:350–356. doi: 10.1002/eji.1830140414. [DOI] [PubMed] [Google Scholar]
- 15.Lin R, Tarr P E, Jones T C. Present status of the use of cytokines as adjuvants with vaccines to protect against infectious diseases. Clin Infect Dis. 1995;21:1439–1449. doi: 10.1093/clinids/21.6.1439. [DOI] [PubMed] [Google Scholar]
- 16.Lofthouse S A, Andrews A E, Nash A D, Bowles V M. Humoral and cellular responses induced by intradermally administered cytokine and conventional adjuvants. Vaccine. 1995;13:1131–1137. doi: 10.1016/0264-410x(94)00053-p. [DOI] [PubMed] [Google Scholar]
- 16a.Lunn, D. P., et al. Unpublished results.
- 16b.Macklin M D, McCabe D, McGregor M W, Neumann V, Meyer T, Callan R, Hinshaw V S, Swain W F. Immunization of pigs with a particle-mediated DNA vaccine to influenza A virus protects against challenge with homologous virus. J Virol. 1998;72:1491–1496. doi: 10.1128/jvi.72.2.1491-1496.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McGhee J R, Kiyono H. Mucosal immunity to vaccines: current concepts for vaccine development and immune response analysis. In: Ciardi J E, editor. Genetically engineered vaccines. New York, N.Y: Plenum Press; 1992. pp. 3–12. [DOI] [PubMed] [Google Scholar]
- 18.Mostov K E. Transepithelial transport of immunoglobulins. Annu Rev Immunol. 1994;12:63–84. doi: 10.1146/annurev.iy.12.040194.000431. [DOI] [PubMed] [Google Scholar]
- 19.Mumford J. Progress in the control of equine influenza. In: Plowright W, Rossdale P D, Wade J F, editors. Equine infectious diseases VI. Proceedings of the Sixth International Conference. Newmarket: R and W Publications; 1992. pp. 207–217. , •••. [Google Scholar]
- 20.Murphy B R, Webster R G. Orthomyxoviruses. In: Fields B N, Knipe D M, Howley P M, Chanock R M, Melnick J L, Monath T P, Roizman B, Straus S E, editors. Field’s virology. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 1397–1445. [Google Scholar]
- 21.Noll A, Autenrieth I B. Immunity against Yersinia enterocolitica by vaccination with Yersinia HSP60 immunostimulating complexes or YersiniaHSP60 plus interleukin-12. Infect Immun. 1996;64:2955–2961. doi: 10.1128/iai.64.8.2955-2961.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olsen C W, McGregor M W, Cooley A J, Schantz B, Hotze B, Hinshaw V S. Antigenic and genetic analysis of a recently isolated H1N1 swine influenza virus. Am J Vet Res. 1993;54:1630–1636. [PubMed] [Google Scholar]
- 23.Olsen C W, McGregor M W, Dybdahl-Sissoko N, Schram B R, Nelson K M, Lunn D P, Macklin M D, Swain W F, Hinshaw V S. Immunogenicity and efficacy of baculovirus-expressed and DNA-based equine influenza virus hemagglutinin vaccines in mice. Vaccine. 1997;15:1149–1156. doi: 10.1016/s0264-410x(96)00309-x. [DOI] [PubMed] [Google Scholar]
- 24.Pertmer T M, Eisenbraun M D, McCabe D, Prayaga S K, Fuller D F, Haynes J R. Gene gun-based nucleic acid immunization: elicitation of humoral and cytotoxic T lymphocyte responses following epidermal delivery of nanogram quantities of DNA. Vaccine. 1995;13:1427–1430. doi: 10.1016/0264-410x(95)00069-d. [DOI] [PubMed] [Google Scholar]
- 25.Pockley A G, Montgomery P C. In vivo adjuvant effect of interleukins 5 and 6 on rat tear IgA antibody responses. Immunology. 1991;73:19–23. [PMC free article] [PubMed] [Google Scholar]
- 26.Ramsay A J, Husband A J, Ramshaw I A, Bao S, Matthaei K I, Koehler G, Kopf M. The role of interleukin-6 in mucosal IgA antibody responses in vivo. Science. 1994;264:561–563. doi: 10.1126/science.8160012. [DOI] [PubMed] [Google Scholar]
- 27.Ramsay A J, Kohonsen-Corish M. Interleukin-5 expressed by a recombinant virus vector enhances specific mucosal IgA responses in vivo. Eur J Immunol. 1993;23:3141–3145. doi: 10.1002/eji.1830231215. [DOI] [PubMed] [Google Scholar]
- 28.Reed L J, Muench H. Simple method of estimating 50 percent endpoints. Am J Hyg. 1938;27:493–497. [Google Scholar]
- 29.Renegar K B, Small P A., Jr Passive transfer of local immunity to influenza virus infection by IgA antibody. J Immunol. 1991;146:1972–1978. [PubMed] [Google Scholar]
- 30.Renegar K B, Small P A., Jr Immunoglobulin A mediation of murine nasal anti-influenza virus immunity. J Virol. 1991;65:2146–2148. doi: 10.1128/jvi.65.4.2146-2148.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sakaguchi M, Nakamura H, Sonoda K, Hamada F, Hirai K. Protection of chickens from Newcastle disease by vaccination with a linear plasmid DNA expressing the F protein of Newcastle disease virus. Vaccine. 1996;14:747–752. doi: 10.1016/0264-410x(95)00254-x. [DOI] [PubMed] [Google Scholar]
- 31a.Soboll, G., et al. Unpublished results.
- 32.Sun W H, Burkholder J K, Sun J, Culp J, Turner J, Lu X G, Pugh T D, Ershler W B, Yang N-S. In vivo cytokine gene transfer by gene gun reduces tumor growth in mice. Proc Natl Acad Sci USA. 1995;92:2889–2893. doi: 10.1073/pnas.92.7.2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Swain W F, Macklin M D, Neumann V, McCabe D E, Drape R, Fuller J T, Widera G, McGregor M W, Callan R J, Hinshaw V. Manipulation of immune responses via particle-mediated polynucleotide vaccines. Behring Inst Res Commun. 1997;98:73–78. [PubMed] [Google Scholar]
- 34.Takase H, Murakami Y, Endo A, Ikeuchi T. Antibody responses and protection in mice immunized orally against influenza virus. Vaccine. 1996;14:1651–1656. doi: 10.1016/s0264-410x(96)00128-4. [DOI] [PubMed] [Google Scholar]
- 35.Tamura S, Funato H, Hirabayashi Y, Suzuki Y, Nagamine T, Aizawa C, Kurata T. Cross-protection against influenza A virus infection by passively transferred respiratory tract IgA antibodies to different hemagglutinin molecules. Eur J Immunol. 1991;21:1337–1344. doi: 10.1002/eji.1830210602. [DOI] [PubMed] [Google Scholar]
- 36.Traub-Dargatz J L, Salman M D, Voss J L. Medical problems of adult horses, as ranked by equine practitioners. J Am Vet Med Assoc. 1991;198:1745–1747. [PubMed] [Google Scholar]
- 37.Ulmer J B, Deck R R, DeWitt C M, Friedman A, Donnelly J J, Liu M A. Protective immunity by intramuscular injection of low doses of influenza virus DNA vaccines. Vaccine. 1994;12:1541–1544. doi: 10.1016/0264-410x(94)90081-7. [DOI] [PubMed] [Google Scholar]
- 38.Ulmer J B, Donnelly J J, Parker S E, Rhodes G H, Felgner P L, Dwarki V J, Gromkowski S H, Deck R R, DeWitt C M, Friedman A, Hawe L A, Leander K R, Martinez D, Perry H C, Shiver J W, Montgomery D L, Liu M A. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science. 1993;259:1745–1749. doi: 10.1126/science.8456302. [DOI] [PubMed] [Google Scholar]
- 39.Van Snick J. Interleukin-6: an overview. Annu Rev Immunol. 1990;8:253–278. doi: 10.1146/annurev.iy.08.040190.001345. [DOI] [PubMed] [Google Scholar]
- 40.Webster R G, Fynan E F, Santoro J C, Robinson H. Protection of ferrets against influenza challenge with a DNA vaccine to the haemagglutinin. Vaccine. 1994;12:1495–1498. doi: 10.1016/0264-410x(94)90071-x. [DOI] [PubMed] [Google Scholar]
- 41.Whalen R G. DNA vaccines for emerging infectious diseases: what if? Emerg Infect Dis. 1996;2:168–175. doi: 10.3201/eid0203.960302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xiang Z, Ertl H C. Manipulation of the immune response to a plasmid-encoded viral antigen by coinoculation with plasmids expressing cytokines. Immunity. 1995;2:129–135. doi: 10.1016/s1074-7613(95)80001-8. [DOI] [PubMed] [Google Scholar]
- 43.Yankauckas M A, Morrow J E, Parker S E, Abai A, Rhodes G H, Dwarki V J, Gromkowski S H. Long-term anti-nucleoprotein cellular and humoral immunity is induced by intramuscular injection of plasmid DNA containing the NP gene. DNA Cell Biol. 1993;12:771–776. doi: 10.1089/dna.1993.12.771. [DOI] [PubMed] [Google Scholar]