Abstract
Background
Establishing an analytical method for the detection of related substances in ketoconazole cream is essential for improving product quality standards and ensuring the safety of this formulation.
Methods
In the 2024 National Drug Sampling Specification, we developed a novel high-sensitivity high-performance liquid chromatography (HPLC) method that effectively separates six known impurities, four preservatives, and one antioxidant present in ketoconazole creams. These creams were sourced from random market sampling and exhibited variations in prescription composition across 14 different manufacturers. Subsequently, we conducted the extraction, isolation, and characterization of common unknown impurities from these creams using mass spectrometry (MS), nuclear magnetic resonance spectroscopy (NMR), and infrared (IR) techniques. Furthermore, the structural analysis of unknown impurities within a specific enterprise was performed using high-resolution mass spectrometry and specialized software.
Results
The established method has been thoroughly validated, demonstrating its specificity, accuracy, sensitivity, and excellent repeatability, thereby confirming its suitability for determining related substances in ketoconazole creams from various manufacturers. This method allowed for both quantitative and qualitative evaluations of impurity content across different manufacturers, identifying sulfonated impurities in ketoconazole creams for the first time and analyzing their formation mechanisms. Furthermore, strategies to reduce these impurities were proposed through correlation analysis and process validation. Additionally, this study innovatively introduced a solvent smoothing agent to mitigate solvent effects, thereby preventing the degradation of the active pharmaceutical ingredient (API) during the heating and dissolution process of the cream matrix. This technique is applicable to the pretreatment of various cream-related substance determinations.
Conclusions
This method provides substantial reference value for establishing analytical methods for related substances in Ketoconazole cream and offers technical support for regulatory agencies in evaluating the quality of Ketoconazole cream.
Keywords: Ketoconazole cream, Related substances, National drug sampling inspection, Sulfonated impurities, Solvent effect
Introduction
Ketoconazole is an antifungal agent with the molecular formula C26H28Cl2N4O4. Although it is poorly soluble in water, it exhibits slight solubility in ethanol and dissolves readily in methanol and dichloromethane [1]. The molecular structure of ketoconazole contains amide and ether functional groups, which are susceptible to oxidation. Ketoconazole tablets were introduced for clinical use in 1978, however, due to severe hepatic toxicity reactions, the approval for oral ketoconazole preparations was withdrawn from the market in 2015. A 2% ketoconazole cream, developed by Janssen Pharmaceuticals, was first launched in Belgium and Germany in 1981, and it was registered for the first time in China in 1988. This formulation is effective in treating conditions such as tinea manus and pedis, although it may cause adverse reactions, including skin irritation [2–4].
In the production process of active pharmaceutical ingredients (APIs), various starting materials, intermediates, reagents, and by-products may ultimately enter the final product as impurities. Additionally, impurities may also be generated during the production and storage of formulations. These impurities can adversely affect the drug’s efficacy and potentially lead to adverse reactions in patients. Each identified impurity in the drug must undergo comprehensive qualitative and quantitative testing, as well as toxicological evaluation when necessary. Therefore, it is essential to develop effective techniques for the determination of pharmaceutical impurities [5–8].
The quality standards for ketoconazole cream are delineated in the 2025 Chinese Pharmacopoeia [1], the 2025 British Pharmacopoeia [9], and the 18th Japanese Pharmacopoeia [10]. Notably, only the British Pharmacopoeia specifies a standard for the analytical method regarding related substances [9], whereas the other pharmacopoeias do not include relevant inspections for the related substances of ketoconazole cream. However, the methods described in the British Pharmacopoeia are insufficient for identifying the related substances in ketoconazole creams produced by the 14 companies involved in the 2024 national drug sampling inspection in China. This inadequacy is particularly evident for formulations containing various bacteriostatic agents, such as hydroxyphenyl ethyl ester, propyl 4-hydroxybenzoate, benzyl alcohol, and thymol, as well as antioxidants like butylated hydroxytoluene.
In comparison, Rajndra A et al. [11] identified impurities in the raw material through the mandatory degradation of ketoconazole, Meanwhile, Roopali A et al. [12] employed automated screening alongside an orthogonal system and statistical tools to separate ketoconazole from its impurities using chromatographic techniques. Furthermore, Wenling Yang et al. [13] identified specific impurities, namely ketoconazole B and E, in the Compound Ketoconazole Ointment, which contains both ketoconazole and clobetasol propionate. However, the aforementioned methods have limitations, they can only separate related substances within the ketoconazole ingredients and do not effectively differentiate between various preservatives, antioxidants, and impurities present in ketoconazole cream. Consequently, these methods are inadequate for a comprehensive evaluation of the impurities in ketoconazole cream.
The impurities in ketoconazole can effectively activate human aryl hydrocarbon receptors, potentially inducing serious adverse side effects by altering various signaling and hormonal pathways [14]. Additionally, impurity D of ketoconazole has been linked to hepatic toxicity [15]. In accordance with the requirements of ICH Q3B [16], it is crucial to evaluate the risks posed by the impurities present in the formulation. During the 2024 national drug sampling research in China, the author optimized the chromatography conditions and verified the method for detecting impurities in ketoconazole creams from various manufacturers referring to the analytical method for ketoconazole cream related substances in the British Pharmacopoeia. The study involved the extraction and separation of unknown impurities identified across multiple enterprises, followed by structural analysis using mass spectrometry (MS) and nuclear magnetic resonance spectroscopy (NMR). Additionally, high-resolution mass spectrometers and compound software were employed to predict the structures of unknown impurities found in one company, facilitating a discussion on methods to minimize impurities.
Experimental
Chemicals, reagents and samples
Acetonitrile of HPLC grade was procured from Honeywell International Inc. (New Jersey, USA) and used as the mobile phase. Glacial acetic acid, ammonium acetate, methanol, isopropanol, and other analytical grade reagents were sourced from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China). The sources and structures of ketoconazole and its impurities standards are shown in Table 1. The nomenclature of impurities aligns with the specifications outlined in the Ketoconazole and Ketoconazole Cream of the British Pharmacopoeia (BP) 2025 [9]. In this study, two impurities designated as B were collected, however, they exhibit different chromatographic behaviors. The impurity B sourced from Janssen Pharmaceuticals is referred to as BP impurity B, while the impurity B obtained from Chinese National Institutes for Food and Drug Control is simply referred to as impurity B.
Table 1.
The sources and structures of ketoconazole and its impurities standards
In the 2024 national drug sampling inspection conducted in China, all generic ketoconazole creams (2%) were manufactured by 14 Chinese companies, labeled from A to N, and referred to as tested samples. The original drug, produced by Johnson & Johnson GmbH, was sourced from the European market and is designated as Reference Listed Drug.
Instrument
HPLC
The primary method development utilized a high-performance liquid chromatography (HPLC) system, equipped with a diode-array detector (DAD) and operated using the Chromeleon 7 data acquisition system (ThermoFisher Scientific, Waltham, MA, USA). The liquid chromatography method for analyzing related substances in ketoconazole cream employs a CAPCELL PAK C18 MG II column (4.6 × 150 mm, 3 μm)(OSAKA SODA CO. LTD., Osaka, Japan ). The detection wavelength is set at 230 nm, and an Solvent-Smoother Solvs-X (Anavo, Benjing, China) is utilized to mitigate solvent effects. The column temperature is maintained at 40 °C, while the autosampler is kept at 20 °C. The mobile phase A consists of acetonitrile and 0.05 M ammonium acetate (pH adjusted to 6.0) in a 25:75 ratio, whereas mobile phase B comprises acetonitrile and 0.05 M ammonium acetate (pH adjusted to 6.0) in an 80:20 ratio. A two-phase gradient elution is employed in this study. The composition transitions as follows: from 0 to 17 min, it shifts from 100 to 77% A; from 17 to 30 min, it decreases from 77 to 60% A; from 30 to 55 min, it further decreases from 60 to 20% A; from 55 to 57 min, it reduces from 20 to 0% A; from 57 to 63 min, it remains at 0% A; from 63 to 65 min, it transitions from 0 to 100% A; and finally, from 65 to 70 min, it maintains at 100% A. The flow rate is set at 1.2 mL/min, with an injection volume of 20 µL.
MS
The structural characterization of unknown impurities was conducted using an Orbitrap Exploris 120 mass spectrometer (Thermo Fisher Scientific, Waltham, MA, USA), in conjunction with Compound Discoverer 3.3 software, under electrospray ionization (ESI) conditions. The parameters applied included a capillary voltage of ± 3.5 kV, sheath/auxiliary/sweep gas flow rates set at 35/10/1 arbitrary units (arb), a capillary temperature of 320 °C, and a vaporizer temperature of 350 °C. Full-scan acquisition was performed at a resolution of 12,000 (FWHM at m/z 200) over a mass-to-charge (m/z) range of 100–1000, utilizing simultaneous positive and negative ion detection, an intensity threshold of 2.0 × 10⁴counts. The full scan intensity dynamic (50–120,000 counts), and apex-triggered precursor exclusion with a duration of 3 s were emploied in this study. Data-dependent MS² analysis was executed using a 2 m/z isolation window and stepped normalized HCD collision energies of 20%, 40%, and 60%.
NMR
The molecular structure was elucidated using a JNM-ECZ400S/L1 nuclear magnetic resonance spectrometer (Jeol, Japan) operating at a frequency of 400 MHz. For the 1H-NMR measurements, the transmitter frequency was set to 100.5253 MHz, and the decoupling field frequency was set to 399.7822 MHz, utilizing CDCl3-D1 as the solvent. The spectrum was recorded over a range of δ 0–13 ppm. In the case of 13C-NMR measurements, the transmitter and decoupling field frequencies were consistent with those used for 1H NMR, employing DMSO-D6 as the solvent, with the spectrum recorded over a range of δ 0-210 ppm.
Preparation of solutions
The Balance XS205DU (METTLER TOLEDO, Zurich, Switzerland) was employed for weighing samples and standards. Weigh 2.5 g of ketoconazole cream and place it in a 50 mL conical centrifuge tube. Add 25 mL of isopropanol and vortex the mixture to ensure complete dispersion of the cream. Next, introduce 10 mL of methanol and employ ultrasonic oscillation to facilitate the dissolution of ketoconazole. Transfer the resulting solution into a 50 mL volumetric flask and adjust the volume to the 50 mL mark with methanol. Shake the flask thoroughly to ensure homogeneity, and then centrifuge at 0 °C for 30 min. Finally, collect the upper liquid obtained after filtration to serve as the test sample solution.
Take an appropriate amount of the test sample solution and dilute it 200 times with a mixture of isopropanol and methanol (1:1) to prepare a self-reference solution.
Prepare blank excipients by utilizing all excipients from each enterprise, excluding ketoconazole and the previously mentioned preservatives and antioxidants. Subsequently, formulate a blank excipient solution using the same method as that employed for the test sample solution. This procedure aims to investigate whether the presence of other excipients influences the determination of the substance under measurement.
The proposed solution comprises preservatives, antioxidants, and known impurities for each prescription, which are diluted with a 1:1 mixture of isopropanol and methanol to achieve the following concentrations: 0.185 mg of butylated hydroxytoluene, 0.4 mg of benzyl alcohol, 0.025 mg of propyl 4-hydroxybenzoate, 0.05 mg of hydroxyphenyl ethyl ester, 0.05 mg of thymol, 0.005 mg of impurity I, 0.005 mg of impurity II, 0.005 mg of impurity B, 0.005 mg of impurity D, 0.005 mg of impurity E, 0.005 mg of British Pharmacopoeia impurity B, and 1 mg of ketoconazole per mL. This solution serves as a system suitability solution for impurity analysis.
Accurately weigh appropriate amounts of each impurity reference standard and dissolve them in methanol. Subsequently, dilute to prepare impurity reference stock solutions with the following concentrations: 0.2734 mg/ml for impurity I, 0.4648 mg/ml for impurity II, 0.4671 mg/ml for impurity D, 0.4613 mg/ml for BP impurity B, 0.4788 mg/ml for impurity B, and 0.4781 mg/ml for impurity E. Next, Precisely measure 1 mL of each impurity reference stock solution and transfer them into a 10 mL volumetric flask. Then, dilute to the mark with methanol to prepare the mixed impurity reference stock solution. For the preparation of linear solutions, accurately measure 0.1 mL, 0.2 mL, 0.4 mL, 1 mL, and 2 mL aliquots of the mixed impurity reference stock solution into separate 20 mL volumetric flasks. Finally, dilute each to the mark with a methanol-isopropanol mixture (1:1, v/v) and mix thoroughly to obtain the desired series of linear solutions for each impurity.
Accurately weigh the appropriate amount of the ketoconazole reference standard, dissolve it in methanol into a stock solution with a concentration of 4.782 mg/ml, then measure 3 ml, 2 ml, 1 ml, 1 ml, 0.1 ml, and 0.05 ml respectively into 10 ml, 10 ml, 10 ml, 100 ml, 100 ml, and 1000 ml volumetric flasks with a methanol-isopropanol mixture (1:1) diluent to prepare the ketoconazole linear solutions.
According to the preparation method of the test solution, nine samples of Ketoconazole Cream from enterprise G were placed in 50 ml pointed-bottom centrifuge tubes. To each tube, 1 ml, 2.5 ml, and 5 ml of a mixed impurity reference stock solution were accurately added, with three samples allocated for each volume. The recovery solution was then prepared in accordance with the established preparation method for the test solution.
Results and discussion
Validation of the HPLC method
The developed method for qualitative and quantitative analysis of impurity for ketoconazole cream was validated by considering the current regulatory guidelines of ICH Q2R2 [17]and USP general chapter < 1225>[18].
The system suitability and specificity
The chromatogram of the system suitability solution is presented in Fig. 1. Each known impurity is effectively separated from the active pharmaceutical ingredient (API), preservatives, and antioxidants. The blank excipient does not interfere with the determination of known impurities, preservatives, and antioxidants, as illustrated in Fig. 2. This blank excipient comprises 40 auxiliary materials sourced from 14 companies, including paraffin, glycerol, monoglycerides, and laurocapram with a retention time of about 61.5 min among others, excluding the API and the aforementioned preservatives and antioxidants, thereby demonstrating the specificity of the employed method.
Fig. 1.
The chromatogram of the system suitability solution. (1.Benzyl alcohol; 2.Hydroxyphenyl ester;3.Impurity II; 4.Impurity I; 5.Propyl 4-hydroxybenzoate; 6.Impurity D; 7. Thymol; 8. Impurity B from Janssen Pharmaceuticals; 9.Ketoconazole ; 10. Impurity B; 11.Impurity E; 12.Dibutylhydroxytoluene)
Fig. 2.
The chromatogram of blank excipients
A batch of samples from Manufacturer G, which contained an impurity with a relative retention time (RRT) of 0.49 that exceeded the acceptable limit, was subjected to forced degradation testing. The results of the forced degradation chromatogram are illustrated in Fig. 3., while the mass balance under various degradation conditions is summarized in Table 2. Analysis of Fig. 3.and Table 2.indicates that ketoconazole demonstrates relative stability in the formulation when exposed to alkaline conditions and light. Although the ketoconazole drug substance can generate impurity D under alkaline degradation, it remains stable within the formulation under such conditions. Under thermal degradation conditions, a 0.25% impurity with an RRT of 1.16 is produced, however, this impurity was not detected in formulations from various manufacturers, and was not subjected to further investigation. In the case of acid degradation, small quantities of impurity Ⅰ, impurity Ⅱ, and 3.35% of impurity D are generated. Conversely, oxidative degradation primarily results in the formation of impurity Ⅰ, with the impurity with an RRT of 0.49 showing a tendency to convert into impurity Ⅰ.
Fig. 3.
The chromatogram of degradation(a: Original; b:Thermal; c:Oxidation; d:Base Hydrolysis; e:Acid Hydrolysis; f:Photolysis) Impurity, Ⅱ, 2-ImpurityⅠ, 3-Unknown impurity༈RRT0.49༉,4-ImpurityD, 5-BP ImpurityB, 6-ketoconazole, 7-Unknown impurity(RRT1.16) )
Table 2.
Mass balance analysis results of forced degradation
| Original | Acid Hydrolysis | Base Hydrolysis | Oxidation | Photolysis | Thermal | |
|---|---|---|---|---|---|---|
| ImpurityⅡ(%) | / | 0.07 | / | / | / | / |
| ImpurityⅠ(%) | / | 0.07 | / | 1.09 | / | / |
| Unknown impurity(RRT0.49)༈%༉ | 2.03 | 1.98 | 2.09 | 1.73 | 2.07 | 2.12 |
| ImpurityD(%) | / | 3.35 | / | 0.08 | / | / |
| Impurity B from Janssen Pharmaceuticals(%) | 0.08 | 0.07 | 0.07 | 0.09 | 0.08 | 0.08 |
| ketoconazole(%) | 97.76 | 94.38 | 97.76 | 96.84 | 97.71 | 97.47 |
| Unknown impurity(RRT1.16)༈%༉ | 0.05 | / | / | 0.09 | 0.05 | 0.25 |
| ImpurityE(%) | 0.08 | 0.08 | 0.08 | 0.08 | 0.09 | 0.08 |
Linearity and correction factor
The linear regression equations for the impurities were established as follows:
For Impurity I, the equation was Y = 507.42X − 0.0273 (R = 0.9994), demonstrating a strong linear relationship across the concentration range of 0.1367 to 2.734 µg/mL. Similarly, Impurity IIexhibited a robust linear correlation, with the equation Y = 368.94X + 0.0039 (R = 1), covering the range of 0.2324 to 4.648 µg/mL.Moving on to Impurity D, the regression equation was Y = 537.9X-0.0014 (R = 1), confirming a good linear relationship within 0.4671 to 4.671 µg/mL. Meanwhile, Impurity Bshowed excellent linearity, as described by Y = 560.21X + 0.0011 (R = 0.9999), spanning 0.2307 to 4.613 µg/mL. In contrast, BP Impurity B followed the equation Y = 599.13X − 0.0274 (R = 0.9996), maintaining a strong linear correlation over 0.2394 to 4.788 µg/mL. Additionally, Impurity E displayed a highly linear trend, with Y = 622.09X + 0.0089 (R = 0.9999), across 0.2391 to 4.781 µg/mL. Finally, for ketoconazole, the regression equation was Y = 555.68X + 0.4268 (R = 1), indicating excellent linearity over a broad concentration range of 0.2361 to 1416.8 µg/mL. Overall, all regression analyses demonstrated strong linear correlations, as evidenced by their high correlation coefficients (R ≥ 0.9994), ensuring reliable quantification across the specified concentration ranges. The linear relationships of various known impurities are robust. The slope of the standard curve is utilized to calculate correction factor for each impurity. Except for impurity II, which has a correction factor of 1.5, the correction factors for other known impurities range from 0.9 to 1.1. This indicates that, aside from impurity II, major component self-comparison without calibration factor can be employed for the quantitative assessment of the known impurities.
Precision
The method’s precision was demonstrated by adding a known impurity reference solution to samples from G Enterprise and conducting six determinations of impurity content. Two analysts prepared six samples each for the intermediate precision experiment, which were subsequently injected into the HPLC system. The relative standard deviation (RSD%) values for method precision were recorded as follows: Impurity II (0.2), Impurity I (0.3), 4-Impurity D (0.3), BP Impurity B (0.7), Impurity B (0.6), and Impurity E (0.8). The RSD% values for intermediate precision were noted as 0.5, 0.8, 1.0, 1.2, 1.5, and 1.0, respectively. This method exhibits excellent reproducibility.
Accuracy
The recovery rates of known impurities were assessed by introducing three different concentrations of reference solutions containing known impurities into the test samples. The accuracy data are presented in Table 3. The recovery rates for all impurities fell within the range of 90–110%, with relative standard deviations (RSDs) not exceeding 3.0%. The established method exhibited strong accuracy and is suitable for the quantitative determination of impurities in Ketoconazole Cream.
Table 3.
Recovery rate of known impurities
| ImpurityⅡ | ImpurityⅠ | ImpurityD | BP Impurity B | Impurity B | ImpurityE | |
|---|---|---|---|---|---|---|
| 0.10% Mean ± SD | 104.5 ± 2.3 | 102.3 ± 2.8 | 105.6 ± 3.5 | 100.2 ± 0.8 | 100.1 ± 0.6 | 100.5 ± 1.2 |
| 0.25% Mean ± SD | 98.5 ± 1.0 | 99.1 ± 1.6 | 103.8 ± 3.0 | 98.5 ± 1.0 | 98.5 ± 0.7 | 99.7 ± 1.1 |
| 0.50% Mean ± SD | 102.1 ± 0.6 | 101.3 ± 0.8 | 1002.5 ± 2.8 | 99.5 ± 0.5 | 98.6 ± 0.6 | 98.5 ± 1.0 |
Stability
The test solution of Enterprise G was precisely sampled at 20 µL at 0, 2, 4, 8, 12, and 24-hour intervals and injected into the liquid chromatograph for analysis under the established chromatographic conditions. The results showed no changes in the content of any impurities in the test solution, indicating good method stability and confirming the stability of the test solution within 24 h.
Limit of detection (LOD) and limit of quantitation (LOQ)
Accurately measure 20 µL of the impurity reference standard and the linear first-point solution of ketoconazole for analysis to achieve LOD with a signal-to-noise ratio (S/N) of approximately 3 and a LOQ with a signal-to-noise ratio (S/N) of approximately 10. LOD for ketoconazole, impurity I, impurity B, impurity D, impurity E, and British Pharmacopoeia impurity B are 0.06 µg/ml, 0.07 µg/ml, 0.14 µg/ml, 0.12 µg/ml, 0.23 µg/ml, and 0.12 µg/ml, respectively. Correspondingly, LOQ for these substances are 0.12 µg/ml, 0.14 µg/ml, 0.23 µg/ml, 0.24 µg/ml, 0.46 µg/ml, and 0.24 µg/ml, respectively.
Based on the analysis above, this method exhibits high specificity, accuracy, and precision, along with excellent detection sensitivity. It is suitable for both qualitative and quantitative analyses of impurities in ketoconazole cream produced by various manufacturers.
Sample determination results
In accordance with the limit standards for ketoconazole cream as outlined in the British Pharmacopoeia, the limit standards for related substances in the 2024 national drug sampling have been established. Specifically, according to major component self-comparison without calibration factor, the content of impurity D must not exceed 0.4%, while unknown impurities should not surpass 0.2%. Additionally, the total impurity content is required to remain below 1.0%. Notably, impurities E and B, as defined by the British Pharmacopoeia, are classified as unknown impurities. Among the 94 batches analyzed, 20 were found to be unqualified, resulting in a failure rate of 21.3%. The box chart illustrating the total impurity measurement results for each enterprise is presented in Fig. 4.
Fig. 4.
Impurity measurement results of each enterprise
Impurity B is a by-product of the synthesis of raw materials, whereas impurity E serves as an intermediate in the same process. With the exception of reference preparations and companies J and I, all other companies detected both impurity B from Janssen Pharmaceuticals and impurity E; however, none detected impurity B from Chinese National Institutes for Food and Drug Control in any samples. The levels of impurity B across various enterprises ranged from 0.02 to 0.16%, while the levels of impurity E varied from 0.02 to 0.20%. Stability studies indicated that neither impurity B nor impurity E increased over time, suggesting that the concentrations of both impurities can be effectively managed by optimizing the purification process of the raw materials. The impurity B reference materials from different sources have been confirmed by NMR and HPLC to exhibit configurational differences. In actual sample testing, no impurity with a retention time consistent with the impurity B reference material issued by Chinese National Institutes for Food and Drug Control was detected; however, an impurity with a retention time consistent with the impurity B reference material provided by the enterprise was identified. Given that the ketoconazole structure contains two chiral carbon atoms, leading to multiple possible configurations, it is imperative for the issuing institutions of the reference material to recheck the configuration of impurity B to ensure the effectiveness of ketoconazole quality control.
In a batch of samples from Enterprise G, the impurity content with a relative retention time (RRT) of 0.49 was measured at 2.1%, which exceeds the specified limit (see Fig. 4.). In comparison, the impurity content at the same relative retention time from Enterprises C and B also surpassed the limit requirement of 0.2%. The total impurity level in Company C’s products is significantly higher than that of other manufacturers. Specifically, the impurity with a relative retention time of 0.49 exceeds the permissible limit by 0.2%. Across various batches, the content of impurity ranges from 0.22 to 1.22%. Although the levels of impurity D in this company’s samples comply with the British Pharmacopoeia’s limit of 0.4%, they are notably higher than those found in other manufacturers, where the batch-to-batch content of impurity D ranges from 0.09 to 0.26%. Furthermore, two batches of samples from Enterprise I and three batches from Enterprise D failed to meet the requirements due to the presence of other unidentified impurities that exceeded the permissible limits.
3.3 Structural Prediction and Source Analysis of unknown impurities with a relative retention time of 0.49.
The unknown impurity was extracted and separated from a sample provided by Company G, which exhibited an impurity content of 2.1% and a relative retention time of 0.49. Structural analysis was conducted using MS (Fig. 5), nuclear magnetic resonance (NMR) spectroscopy (Figs. 6 and 7), and infrared spectroscopy (Fig. 8). This compound was identified as ketoconazole with an additional sulfonic acid group.
Fig. 5.
mass spectrum of sulfonated impurities
Fig. 6.
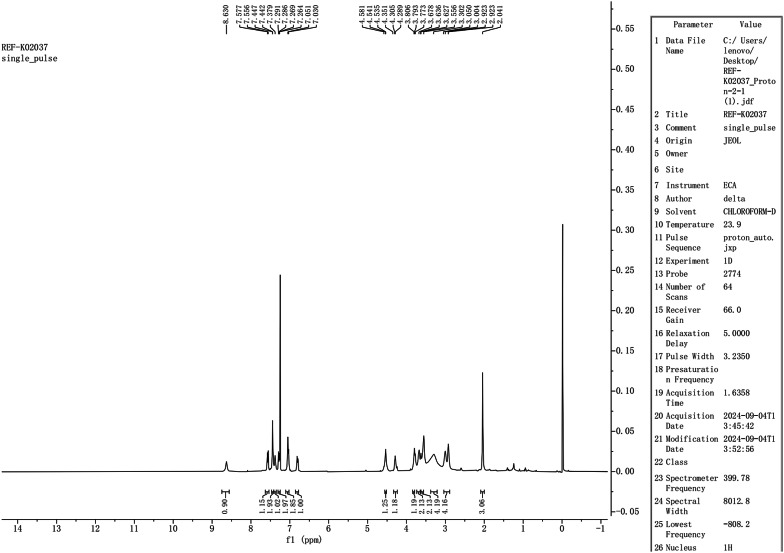
Hydrogen spectrum of sulfonated impurities
Fig. 7.
Carbon spectrum of sulfonated impurities
Fig. 8.
Infrared spectrum of sulfonated impurities
Low-resolution Electrospray Ionization Mass Spectrometry (ESI-MS) analysis identified a peak for [M + H]+ at 611.48, while High-Resolution Mass Spectrometry (HRMS) confirmed the precise molecular weight (MW) of [M + H]+ as 611.11334. The molecular formula was determined to be C26H28Cl2N4O6S (calculated value: 610.11Da). Compared to ketoconazole (C26H28Cl2N4O4, MW 531.43), the mass increased by 80 Da, indicating the presence of a sulfonic acid modification (SO3 group).
Proton NMR (1 H-NMR) analysis identified a proton signal for the sulfonic acid group (-SO3H) at 8.630 ppm (s, 1 H), which is characteristic of acidic hydrogen. The chemical shift range of 7.577–6.795 ppm, corresponding to the aromatic region, reflects the aromatic protons of the ketoconazole core (including the benzene and imidazole rings). The characteristic peaks of the benzene ring are observed as doublets at δ7.051 and δ7.029, corresponding to two hydrogen atoms, as well as at δ6.816 and δ6.795, corresponding to one hydrogen atom. This observation indicates the attachment of the sulfonate group to the benzene ring.Notably, there were no significant absences or additions of peaks, indicating that sulfonation did not disrupt the aromatic structure. Additionally, the aliphatic region (3.004–1.972 ppm) includes a signal at 3.347–3.254 ppm (m, 4 H), which corresponds to the methylene groups on the side chain of ketoconazole (C23,24-H), consistent with the core structure. The peak at 1.972 ppm (s, 3 H) represents the acetyl group (-COCH3), aligning with the core structure.
Carbon spectrum(13 C-NMR) analysis revealed a signal at 168.742 ppm corresponding to the acetyl carbonyl (C25), consistent with the parent nucleus, while signals within the range of 130.736-116.012 ppm were attributed to aromatic carbons (benzene and imidazole rings), with no abnormal shifts detected. The absence of additional carbon signals ruled out any carbon skeleton breakage or rearrangement, thereby supporting the notion of sulfonic acid group substitution on the existing framework.
The IR spectroscopy confirmed the presence of a sulfonic acid O-H stretching vibration at 3435 cm− 1, an amide C = O stretching vibration at 1619 cm− 1 (consistent with ketoconazole), and aromatic ring C = C skeleton vibrations in the range of 1400–1600 cm− 1, with no anomalous peaks detected.
Based on the structure of ketoconazole, the sulfonic acid group may be attached to either the 19th or the 20th carbon in Fig. 9., but further two-dimensional NMR is required to precisely determine the exact position.
Fig. 9.
Chemical structure of sulfonated impurities (a or b)
The compatibility tests of raw materials and excipients indicate that no sulfonated impurities of ketoconazole are present under various mixed and destructive testing conditions. The concentration of sulfonated impurities from each enterprise increases with extended placement time. In this study, we observed that when leakage occurred at the tail of the aluminum tube, the difference in sodium sulfite antioxidant content between the head and tail of the paste increased, while the sodium sulfite antioxidant content at the tail decreased significantly. This phenomenon was accompanied by a decrease in pH and an increase in the content of sulfonated impurities, which gradually formed through oxidation in the presence of air. Furthermore, the ketoconazole cream packaged in aluminum tubes exhibited a higher susceptibility to leakage due to the thinning of the tube walls and a reduced number of tail folding layers. The relevant analytical results are presented in Fig. 10. Based on the above analysis, the mechanism of impurity formation is proposed as shown in Fig. 11. Due to the presence of bisulfite ions in the cream, which are oxidized to sulfate ions, a Friedel-Crafts reaction, specifically an electrophilic substitution reaction, occurs with ketoconazole under acidic conditions. This reaction proceeds slowly in the presence of air.To mitigate the formation of impurities, it is essential to ensure the integrity of the packaging seal, adjust the pH to an appropriate range, and maintain the concentration of sodium sulfite as antioxidants.
Fig. 10.
Heatmap of correlation between safety indicators of ketoconazole cream
Fig. 11.
The mechanism of sulfonic acid impurity formation
The ketoconazole cream from each manufacturer gradually turns pink after prolonged exposure to air. Simulating the conditions for each manufacturer without the addition of antioxidants, it was observed that heating above 80 °C also causes the ketoconazole cream to turn pink. However, the sulfonated impurities obtained through separation and the properties of each impurity provided by the manufacturer remain white, and the solutions of these compounds are colorless. This indicates that the discoloration is not caused by known impurities. Based on the structure of ketoconazole, it is speculated that the reddening of the cream results from oxidation.
Phenols may be produced following the cleavage of the ether bond, which can subsequently oxidize into a compound containing a quinone structure that exhibits color. Given that the discoloration impurities are trace substances, this study has not provided effective information regarding their nature, and further investigation will be conducted in the future. Due to its strong correlation with oxidation, it is advisable to focus on the comprehensiveness of antioxidant content and the integrity of packaging seals.
Structure speculation on unknown impurities exclusive to a certain enterprise
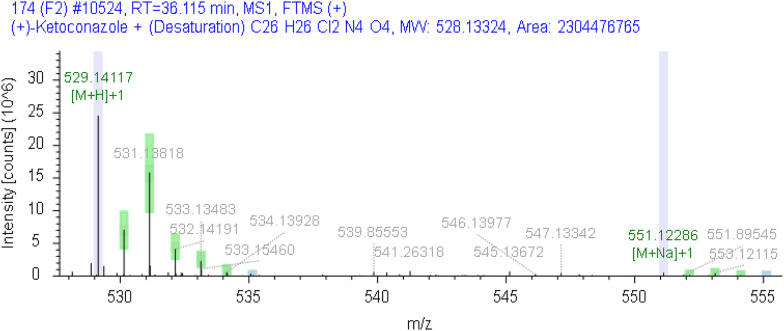
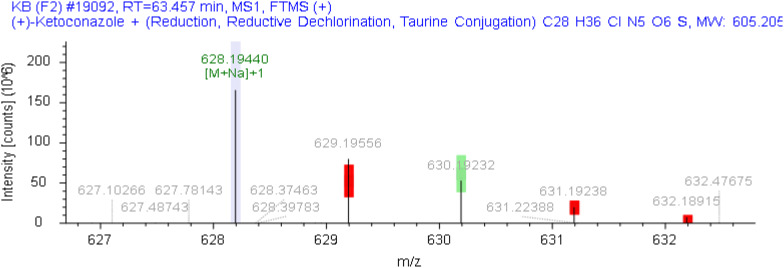
The unknown impurities in the samples from each enterprise were analyzed and structurally elucidated using the Orbitrap Exploris 120 mass spectrometer (Compound Discoverer 3.3). In the sample from Company I, an unknown impurity with a relative retention time of 0.83 was identified as a product of ketoconazole following the loss of C2H4, while another unknown impurity with a relative retention time of 1.19 was identified as a product of ketoconazole after the loss of H2. The chromatogram is presented in Fig. 12, and the corresponding mass spectra are illustrated in Figs. 13 and 14, respectively. Furthermore, the molecular formula of the unknown impurity with a relative retention time of 2.2 from Company E is estimated to be C28H36ClN5O6S, as depicted in the chromatogram in Fig. 15 and the mass spectrum in Fig. 16. This compound is formed as a result of the reduction and dechlorination of ketoconazole, followed by a reaction with sulfonic acid substances present in the excipients.
Fig. 12.
The chromatogram of related substances in enterprise I (1: unknown impurity with relative retention time of 0.83 ;2༚ketoconazole༛3༚unknown impurity with relative retention time of 1.19 )
Fig. 13.
The mass spectrum of impurities with relative retention time of 0.83 from enterprise I
Fig. 14.
The mass spectrum of Impurities with relative retention time of 1.19 from enterprise I
Fig. 15.
The chromatogram of related substances in enterprise E
Fig. 16.
The mass spectrum of impurities with relative retention time of 2.2 from enterprise E
(1: ketoconazole;2༚unknown impurity with relative retention time of 2.2)
According to the production process of I Enterprise, heating at 115 °C for 4 h results in the formation of unknown impurities with relative retention times of 0.83 and 1.19. Conversely, when the production temperature is lowered to 80 °C, these two unknown impurities are not produced. Furthermore, these impurities exhibited a gradual increase during stability testing. It is recommended that enterprises revise their preparation processes to minimize the formation of these unknown impurities.
E Company exhibits an unidentified impurity peak at a relative retention time of 2.2, with the impurity content significantly increasing during accelerated, long-term, and freeze-thaw stability tests. The pH of the sample from this company is measured at 5.0. Following the adjustment of the sample to a pH of 7.0, the subsequent increase in the peak area of the unidentified impurity is notably smaller compared to the increase observed under acidic conditions during stability testing. This observation suggests that acidic conditions promote the formation of the unidentified impurity. In light of the impurity’s growth, it is recommended that the company optimize the pH of the formulation and control the content of the unidentified impurity.
Optimization of sample pretreatment methods and the mobile phase
The cream formulation comprises various pharmaceutical excipients, including paraffin, vaseline, glycerin, and other oil-phase agents. Notably, the ketoconazole present in the matrix poses challenges in dissolving within the solvent. The solvents most frequently referenced in the literature are methanol and acetonitrile. However, a significant issue arises as elevated temperatures of 60–80 °C often lead to the degradation of active pharmaceutical ingredients (APIs), resulting in the formation of impurities [19–22]. Accurately representing the actual impurities present in the cream is a challenge. To address this issue, the current study employs a method in which isopropanol is added first, followed by vortex mixing to ensure uniform dispersion. Subsequently, methanol is introduced to dissolve the ketoconazole. Consequently, the solvent used for the dissolved sample is a methanol-isopropanol mixture in a 1:1 ratio.
The differing solvents used for the dissolved sample and mobile phase may produce solvent effects that result in forked chromatographic peaks, ultimately hindering the completion of the test. To address this issue, this work presents an innovative solvent elimination device. The principle behind this device is to minimize the disparity between the sample solvent and the mobile phase, achieved through a specially designed cavity that reduces the solvent effect. Specifically, the device features a unique structural cavity located at the front end of the chromatographic column. During the system’s equilibrium phase, this cavity is filled with the mobile phase. As the sample solution passes through the cavity, it generates slight turbulence in the small air pocket, effectively mixing the sample solution with the mobile phase. This hybridization optimizes the surrounding environment of the sample molecules, ensuring compatibility with the mobile phase prior to entering the chromatographic column. Consequently, this approach eliminates the solvent effect and facilitates the successful completion of the test.
In this study, the chromatographic conditions employed a pre-mixed mobile phase consisting of mobile phase A (acetonitrile/0.05 M ammonium acetate, pH 6.0, 25:75) and mobile phase B (acetonitrile/0.05 M ammonium acetate, pH 6.0, 80:20), as opposed to the conventional mobile phases where solvent A is 100% 0.05 M ammonium acetate (pH 6.0) and solvent B is 100% acetonitrile. This choice was informed by the mobile phase specifications for related substances of ketoconazole cream outlined in BP 2025, with further optimization of the gradient elution program conducted accordingly. The rationale for using pre-mixed mobile phases stems from the fact that employing separate pumps for acetonitrile and ammonium acetate can induce an instantaneous salting-out effect due to their interaction, potentially resulting in clogging of the one-way valve in the liquid chromatograph. Consequently, pre-mixed mobile phases are utilized for both Phase A and Phase B.
In the 2024 national drug sampling inspection, ketoconazole cream preparations from 14 participating companies were found to contain various preservatives and antioxidants with ultraviolet absorption properties, including methylparaben, propylparaben, benzyl alcohol, and thymol. The 28-minute gradient specified in the British Pharmacopoeia was inadequate for separating unknown impurities from known impurities 1 and 2, and it failed to distinguish the bacteriostatic agent thymol, British Pharmacopoeia impurity B, and ketoconazole. Additionally, it did not elute the antioxidant butylated BHT. Consequently, this study revised the original 28-minute elution procedure by increasing the proportion of the aqueous phase during the first 30 min and extending the elution time from 30 to 65 min to facilitate the elution of BHT. The method developed in this study not only quantifies the impurity content but also simultaneously determines the concentrations of various preservatives and antioxidants, thereby effectively evaluating the quality of ketoconazole cream.
Conclusion
This study establishes qualitative and quantitative analytical methods for the related substances in ketoconazole cream. The methods demonstrated high specificity and sensitivity, effectively analyzing impurities, preservatives, and antioxidants in ketoconazole creams from 14 companies participating in the 2024 National Drug Sampling Inspection in China. During the method development process, the sample dissolution and analysis technique, which utilized a solvent-smoother, provided significant references for analyzing various creams. Through MS and NMR the common impurity identified during stability tests conducted by various companies was characterized as sulfonated products. Correlation analysis indicated relationships among factors such as the presence of impurities, pH value, packaging integrity, and antioxidant content. Furthermore, an examination of the unknown impurities present in each enterprise revealed that their formation was influenced by the formulation’s pH value and processing temperature. It is recommended to enhance the packaging and sealing processes to ensure the stability of the antioxidant sodium sulfite throughout the product’s shelf life, thereby effectively controlling impurities. This study provides technical support for the establishment of comprehensive quality standards and the enhancement of the quality of ketoconazole cream.
Author contributions
CX, QW, PW and DD: data curation, validation, investigation, resources, writing—original draft. YX and FL: validation, formal analysis, investigation, data curation, supervi‑sion, writing—review and editing, visualization. YZ and FW: validation, formal analysis, investigation, data curation, supervision, writing—review and editing, visualization. LL: conceptualization, methodology, validation, formal analysis, investigation, data curation, supervision, review and editing, visualization. LZ and LY: conceptualization, methodology, validation, formal analysis, investigation, data curation, supervision, review and editing, visualization. All authors read and approved the final manuscript.
Funding
This research was funded by Drug Sampling Inspection (Central Subsidy to Local) Funding Project by National Medical Products Administration(NMPA).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Pharmacopoeia Commission of the People’s Republic of China. Pharmacopoeia of the people’s Republic of China. Beijing: China Medical Science; 2025. [Google Scholar]
- 2.Proksch E, FLster-Holst R, Jensen JM. Skin barrier function, epidermal proliferation and differentiation in eczema. J Dermatol Sci. 2006;43(3):159–69. 10.1016/j.jdermsci.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 3.Heeres J, Backx LJJ, Mostmans JH. ,et al.antimycotic imidazoles. Part 4. Synthesis and antifungal activity of ketoconazole, a new potent orally active broad-spectrum antifungal agent. J Med Chem. 1979;22(8):1003–5. 10.1021/jm00194a023. [DOI] [PubMed] [Google Scholar]
- 4.Nimura K, Niwano Y, Ishiduka S, et al. Comparison of in vitro antifungal activities of topical antimycotics launched in 1990s in Japan. Int J Antimicrob Agents. 2001;18(2):173–8. 10.1016/S0924-8579(01)00365-X. [DOI] [PubMed] [Google Scholar]
- 5.Bolt HM, Foth H, Hengstler JG, Degen GH. Carcinogenicity categorization of chemicals-new aspects to be considered in a European perspective. Toxicol Lett. 2004;151(1):29–41. 10.1016/S0378-4274(04)00210-3. [DOI] [PubMed] [Google Scholar]
- 6.De Oliveira Melo SR, Homem-de-Mello M, Silveira D, Simeoni LA. Advice on degradation products in pharmaceuticals: a toxicological evaluation. PDA J Pharm Sci Technol. 2014;68(3):221–38. 10.5731/pdajpst.2014.00974. [DOI] [PubMed] [Google Scholar]
- 7.Abdel Rahman MA, Elghobashy MR, Zaazaa HE, et al. Validated HPLC–PDA methodology utilized for simultaneous determination of Etoricoxib and Paracetamol in the presence of Paracetamol toxic impurities. BMC Chem. 2022;16(1):1–11. 10.1186/s13065-022-00904-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Görög S, Babjak M, Balogh G, Brlik J, Csehi A, Dravecz F, et al. Drug impurity profiling strategies. Talanta. 1997;44(9):1517–26. 10.1016/S0039-9140(96)02179-0. [PubMed] [Google Scholar]
- 9.British Pharmacopoeia Commission. British Pharmacopoeia. Ketoconazole cream. London: The StationeryOffice; 2025. [Google Scholar]
- 10.Pharmaceutical and Medical Device Regulatory Science Society of Japan Japanese Pharmacopoeia 18th[M]. Tokyo: Japanese Ministry of Health, Labour and Welfare. 2021.
- 11.Rajendra A, Mhaske, et al. Identification of major degradation products of ketoconazole. Sci Pharm. 201210.3797/scipharm.1107-18.. [DOI] [PMC free article] [PubMed]
- 12.Roopali A, Sateesh B, Chandrakant B. A new strategy for the Software-Assisted LC separations of ketoconazole and its impurities. J Chromatogr Sci. 2023;61(5):418–27. 10.1093/chromsci/bmac049. [DOI] [PubMed] [Google Scholar]
- 13.Yang W, Yang X, Shi F, et al. Qualitative and quantitative assessment of related substances in the compound ketoconazole and Clobetasol propionate cream by HPLC-TOF-MS and HPLC. J Pharm Anal. 2019;9(3):7. 10.1016/j.jpha.2018.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aneta G, Aneta D, Zdeněk D. Impurities contained in antifungal drug ketoconazole are potent activators of human Aryl hydrocarbon receptor. Toxicol Lett. 2015;239(2):67–72. [DOI] [PubMed] [Google Scholar]
- 15.Souza PDC, Wilhems RZ, Queiroz LHK, et al. Kinetic study and structural Elucidation of the main ketoconazole metabolite. J Mol Struct. 2020;1220:128737. [Google Scholar]
- 16.International Council for Harmonisation. ICH: assessment and control of DNA reactive (mutagenic) impurities in pharmaceuticals to limit potential carcinogenic risk M7(R1). EB/OL. 2017-06-22;2025–06–21. https://database.ich.org/sites/default/files/M7_R1_Guideline.pdf.
- 17.International Council for Harmonisation (ICH). 2022. Validation of Analytical Procedures: Q2(R2).[EB/OL]. (2023-12-28) [2025-08-18]. https://www.ich.org/page/quality-guidelines
- 18.FDA. USP-NF < 1225>. Validation of compendial procedures. Rockville, MD: United States Pharmacopeial Convention; 2025. [Google Scholar]
- 19.Siva KM, Kumar N, Kumar K. Unique green chromatographic method for the qualitative and quantitative analysis of ketoconazole, its impurities and preservatives from ketoconazole cream formulation: identification of degradants by Q-ToF LCMS and robustness bydesign of experimentsr. Sustainable Chem Pharm. 2023;36. 10.1016/j.scp.2023.101247.
- 20.Elumalai S, Thenmozhi K, Senthilkumar S, et al. Identification and characterization of unknown degradation impurities in beclomethasone dipropionate cream formulation using HPLC, ESI, MSand NMR. J Pharm Biomed Anal. 2019;167. 10.1016/j.jpba.2019.02.013. [DOI] [PubMed]
- 21.Jian W, Shuxian Z, Yu X, et al. Development of a novel HPLC method for thedetermination of the impurities in Desonide cream and characterization of its impurities by 2D LC-IT-TOF MS. J Pharm Biomed Anal. 2018;161:399–406. 10.1016/j.jpba.2018.08.055. [DOI] [PubMed] [Google Scholar]
- 22.Elumalai S, Thenmozhi K, Senthilkumar S, et al. Identification and characterization of unknown degradation impurities in beclomethasone dipropionate cream formulation using HPLC, ESI-MS and NMR. J Pharm Biomed Anal. 2019;167:123–31. 10.1016/j.jpba.2019.02.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.