Abstract
During the unusually warm summer in Denmark in 1994, 11 clinical cases of Vibrio vulnificus infection were reported. These reports initiated an investigation of the occurrence of V. vulnificus biotypes in Danish marine environments. Samples of coastal water, sediment, shellfish, and wild fish were analyzed by preenrichment in alkaline peptone water amended with polymyxin B (2.0 × 104 U/liter) followed by streaking onto modified cellobiose-polymyxin B-colistin agar. V. vulnificus-like colonies were tested with a V. vulnificus-specific DNA probe. Low densities of V. vulnificus were detected in water (0.8 to 19 CFU/liter) from June until mid-September and in sediment (0.04 to >11 CFU/g) from July until mid-November. The presence of V. vulnificus was strongly correlated with water temperature. However, we isolated V. vulnificus from water from a mussel farm at a lower temperature than previously reported (7°C). In 1 of the 13 locations studied, V. vulnificus was found in mussels in 7 of 17 samples analyzed; this is the first report of V. vulnificus in European shellfish. V. vulnificus was also isolated from gills, intestinal contents, and mucus from wild fish. Although biotyping of 706 V. vulnificus strains isolated during our investigations revealed that the majority of the strains (99.6%) belonged to biotype 1, biotype 2 was detected in seawater at a low frequency (0.4%). Our findings provide further evidence that seawater can serve as a reservoir and might facilitate spread of V. vulnificus biotype 2 to eels, with subsequent spread to persons handling eels. In conclusion, our data demonstrate that V. vulnificus is ubiquitous in a temperate marine environment and that V. vulnificus biotype 2 is not strictly confined to eels.
Vibrio vulnificus is an estuarine bacterium capable of producing both septicemia and wound infections in humans (41). Primary septicemia occurs mainly in people who have eaten raw seafood, especially raw oysters, and in almost every case the patient has a chronic underlying disease. The fatality rate is high, up to 60% (35). In addition, V. vulnificus causes wound infections by entering preexisting skin lesions during exposure to seawater. The fatality rate for persons with wound infections may be up to 20%, but amputation or surgical debridement is often necessary (35).
V. vulnificus may be isolated from a wide variety of aquatic ecosystems, and the occurrence of the organism is favored by high temperatures and relatively low salinities. In the United States, V. vulnificus has been reported on the Atlantic, Pacific, and Gulf coasts (27, 28, 37). V. vulnificus occurs more rarely in temperate areas than in subtropical waters, and studies of the occurrence of this organism in temperate areas are few. However, V. vulnificus either has been isolated from water or has been implicated in human infections during the summer months in Denmark, Sweden, Germany, Holland, and Belgium (14, 25, 33, 34, 51).
Oysters, clams, mussels, and fish, as well as water, sediment, and plankton, have all been described as reservoirs for V. vulnificus (18, 38, 54). V. vulnificus has been isolated from waters with temperatures ranging from 8 to 31°C and salinities between 1 and 34‰ (27, 54). Despite an apparent tolerance for wide ranges of salinities and temperatures, V. vulnificus is observed at higher concentrations in water with temperatures above 20°C and salinities between 15 and 25‰ (26, 28).
In a comprehensive study of the distribution and ecology of V. vulnificus along the east coast of the United States, Oliver et al. (38) attempted to correlate the presence of V. vulnificus with the presence of fecal coliform bacteria. No correlation between the presence of lactose-fermenting vibrios (including V. vulnificus) and the presence of fecal coliform bacteria was found. Tamplin et al. (45) actually found a negative correlation between the presence of V. vulnificus and the presence of fecal coliform bacteria, and V. vulnificus was most often detected in waters with fecal coliform bacterial most probable numbers (MPN) of less than 3 organisms per 100 ml.
The procedures for isolation and identification of V. vulnificus have been improved during the last decade with the development of a selective and differential cellobiose-polymyxin B-colistin (CPC) agar (32) and a V. vulnificus-specific DNA probe directed against the cytolysin gene (55). CPC agar in combination with selective preenrichment in alkaline peptone water supplemented with polymyxin B (2.0 × 104 U/liter) has been used to recover V. vulnificus from Danish coastal water and sediment, as well as from imported seafood (13, 16). Also, we found that the DNA probe is superior to the API 20E system (bioMérieux, Marcy l’Etoile, France) for identification of V. vulnificus (13).
To date, phenotypic and genotypic characterizations of V. vulnificus have not identified particular traits that predict pathogenicity for humans. However, characterization of V. vulnificus strains has led to subdivision of the species into two biotypes which in the original description differed phenotypically, serologically, and in host range (48). Recent research suggests that biotyping should be based mainly on serological properties and host range since the biochemical traits have been found to vary (9, 11). Biotype 1 strains are pathogenic for humans, exhibit several immunologically distinct lipopolysaccharide (LPS) types, and are indole positive (11). Biotype 2 strains appear to be virulent for both humans and eels and express a common LPS type, and the majority of the biotype 2 strains are indole negative (2, 11). Little is known about the ecology of biotype 2 strains in the aquatic environment; until recently, these organisms had been isolated only from diseased eels and a single clinical case (3, 52). However, one V. vulnificus biotype 2 strain was recovered from a sample of Danish coastal water in 1994 (24), which indicates that both biotypes are present in the marine environment and that biotype 2 strains are not strictly confined to eels.
The recent focus on V. vulnificus in Denmark stems from 11 clinical cases of V. vulnificus infection which occurred in the unusually warm summer of 1994 (14). The route of infection in 10 of the 11 cases was a preexisting skin lesion, and the remaining case was an episode of near drowning. All of the patients had a history of exposure to seawater or handling of fish prior to infection, and none had consumed seafood (14). In addition, three wound infections associated with handling of fish were reported in the summer of 1995 (12). No clinical cases of V. vulnificus were reported in 1996. All of the clinical isolates were originally described as members of biotype 1 (14). However, it was shown by ribotyping that one isolate actually belonged to biotype 2, along with a strain isolated from a wound infection in Denmark in 1991 (6, 24). This provided further evidence that V. vulnificus biotype 2 should be regarded as an opportunistic human pathogen (2, 52).
The aims of our study were to investigate the occurrence of V. vulnificus in Danish coastal waters and especially to investigate the distribution of V. vulnificus biotype 2 strains in the aquatic environment. Also, we wanted to determine if there was any correlation between the occurrence of V. vulnificus and the occurrence of indicator bacteria routinely used to evaluate bathing water quality and presumptive Vibrio spp.
MATERIALS AND METHODS
Collection and treatment of samples.
During the 1996 bathing season from May to October, water was sampled weekly at the seven locations shown in Fig. 1. Two-liter samples of coastal water were collected by municipal food and environmental control units as part of the routine control of bathing water quality. The seawater temperature was measured in situ with a digital thermometer correct to 0.1°C, and salinity was measured in the laboratory with a refractometer (S/Mill, Atago, Japan). Water samples were mixed, filtered through 0.45-μm-pore-size membrane filters (Millipore, Bedford, Mass.), and analyzed by using a three-tube MPN method with alkaline peptone water preenrichment supplemented with polymyxin B (APWP) (1% NaCl, 2.0 × 104 U of polymyxin B per liter; pH 8.6) incubated at 37°C for 18 to 24 h, followed by streaking onto modified CPC (mCPC) agar (13, 32, 44). The total number of presumptive Vibrio spp. cells was estimated by streaking 0.1 ml of a water sample onto thiosulfate-citrate-bile-sucrose (Difco Laboratories, Detroit, Mich.) agar plates in duplicate and incubating the plates for 18 to 24 h at 37°C. Coliform bacterial, presumptive Escherichia coli, and fecal streptococcal organisms were enumerated by using standard procedures as previously described (46, 47). Sediment samples were collected weekly from locations 1 and 2 with a Van Veen sediment grab (Fig. 1). Samples of seawater and sediment were transported to the laboratory in insulated boxes containing cooling elements and were processed within 4 h of collection. Approximately 0.5 to 1 kg of top-layer sediment was transported in a sealed sterile plastic bag to the laboratory, where 300 g of sediment was homogenized with an equal volume of phosphate-buffered saline (pH 7.5) in a sterile stainless steel Waring commercial blender at high speed for 60 s, followed by inoculation into a three-tube MPN series with APWP. Samples of blue mussels (Mytilus edulis) and oysters (Oestra edulis and Crassostrea gigas) were obtained from July until December 1996 from a total of 13 sites (Fig. 1). Samples were collected by local fish inspectorates and were sent overnight in insulated boxes containing cooling elements to the laboratory, where they were processed. Two 250-ml water samples were collected along with each shellfish sample; one was a surface water sample, and one was a sample obtained from above the seabed at the shellfish sampling site. The shellfish were shucked aseptically with an autoclaved oyster knife, and the meat and liquor from 10 oysters or 25 mussels (approximately 100 to 200 g [pooled wet weight]) were homogenized for 60 s in a sterile Waring blender that contained an equal volume of phosphate-buffered saline. Homogenized samples (20, 2, and 0.2 g) were inoculated into APWP and incubated at 37°C for 18 to 24 h, after which samples showing turbidity were streaked onto mCPC agar. From July until October 1996, a total of 136 wild fish were analyzed; these fish included 29 flounders (Platichthys flesus), 14 eelpouts (Zoarches viviparus), and 93 eels (Anguilla anguilla) that were caught at various locations in Køge Bay and in the waters near location 2 (Fig. 1). The fish were caught with hook and line or in traps and were transported live at outdoor temperatures to the laboratory in plastic buckets. Approximately 1 g of mucus, gills, and intestinal contents was removed aseptically from each decapitated fish. Pooled samples of mucus, gills, and intestinal contents from five fish or samples from single fish were preenriched for 6 to 8 h in alkaline peptone water at 37°C and streaked onto mCPC agar. Samples from fish kidneys were streaked directly onto blood agar plates (Blood Agar base [Difco] supplemented with 5% citrated calf blood) and incubated at 20°C for 18 to 48 h to investigate if the fish were colonized with other fish-pathogenic bacteria. All mCPC plates were incubated at 40°C for 18 to 24 h.
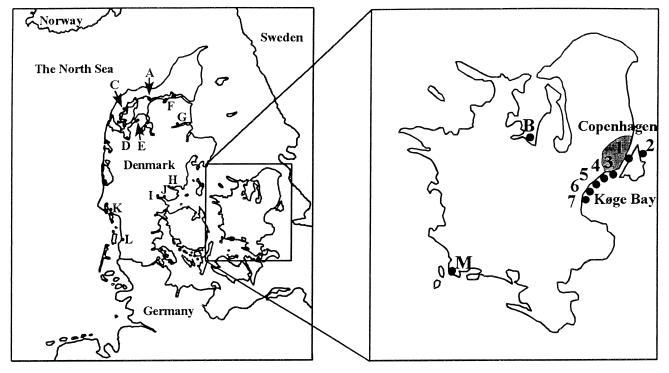
FIG. 1.
Geographical locations of sampling sites. Coastal water samples were collected at locations 1 to 7. Sediment samples were obtained at locations 1 and 2. Oysters were harvested in areas A and B, and blue mussels were harvested in areas C to M. Wild fish were caught in Køge Bay and at location 2.
Identification of V. vulnificus by colony hybridization.
Two V. vulnificus-like colonies were picked from each mCPC plate, cultured in veal infusion broth (Difco), and streaked onto blood agar plates to determine purity. Colony blots were prepared in triplicate from pure cultures grown on blood agar for 18 to 24 h at 37°C. Colonies were transferred to no. 541 filter paper (Whatman, Maidstone, United Kingdom) by overlaying the agar plates for 30 min. Cells were lysed by placing filters on no. 3 filter paper (Whatman) saturated with 10% sodium dodecyl sulfate for 10 min, followed by denaturation on filter paper saturated with 0.5 M NaOH for 20 min. Subsequently, the filters were placed three times (for 1 min each time) on filter paper saturated with 1 M Tris (pH 7.0) to obtain a neutral pH and then finally baked at 80°C for 2 h. Hybridization with an alkaline phosphatase-labeled DNA probe directed against the cytolysin gene and development of filters were done as previously described (55). The probe concentration was decreased from 10 to 1 nM compared with the method described by Wright et al. (55), and the probe was reused three or four times, with 0.5 nM fresh probe added for each hybridization. The alkaline phosphatase-labeled probe was purchased from DNA Technology, Aarhus, Denmark. Strains of V. vulnificus (ATCC 27562) and Vibrio cholerae O1 (V1075/25) (40) were included as positive and negative controls, respectively, on all filters. MPN tables were used to estimate the number of V. vulnificus cells originally present in a sample (49).
Biotyping.
All V. vulnificus isolates were tested for indole production in tryptone broth (1% tryptone [Difco], 0.5% NaCl; pH 7.5) supplemented with Kovács indole reagent after 48 h of incubation at 37°C (7). In addition, 127 strains isolated from seawater, sediment, and wild eels collected in Køge Bay during the summer of 1995 were included in the biotyping studies.
Statistical methods.
Statistical analysis was performed with the Statistical Analysis System (SAS Inc., Cary, N.C.) by using the General Linear Model procedure for evaluation of the correlation between salinity and temperature, the density of indicator bacteria, including presumptive Vibrio spp., and the occurrence of V. vulnificus. The data were log transformed and were assumed to be normally distributed. A linear relationship between log V. vulnificus MPN counts and the additive main effects of salinity, temperature, and location was assumed. The counts of presumptive Vibrio spp., coliform bacteria, presumptive E. coli, and fecal streptococci were regarded as covariants. The individual effect of each of the parameters and covariants was tested with additive tests against the full model.
RESULTS
Isolation of V. vulnificus.
Preenrichment of samples in APWP followed by subculturing on mCPC agar was useful for isolation of V. vulnificus. More than 95% of the presumptive isolates on mCPC agar were identified as V. vulnificus when they were tested with the cytolysin probe. Furthermore, it was possible to isolate low numbers of V. vulnificus from seawater, sediment, and shellfish by this method (Table 1 and Fig. 2). Our initial experiments proved that preenrichment in alkaline peptone water for 6 to 8 h was better than overnight preenrichment in APWP when samples from wild fish were analyzed. This finding may be explained by the lower level of background flora in samples from healthy fish than in samples of seawater, sediment, and shellfish, and therefore the addition of antibiotics may not be necessary.
TABLE 1.
Occurrence of V. vulnificus in water and mussels samples collected from area G in 1996
| Sample date (day/mo) | Water temp (°C) | Mussel sample depth(s) (m)a | Presence of V. vulnificus
|
|||
|---|---|---|---|---|---|---|
| Mussel samples
|
Water samplesb | |||||
| 10 g | 1 g | 0.1 g | ||||
| 24/9 | 14 | 1 | +c | + | + | −/−d |
| 1/10 | 13 | 1 | + | + | − | −/− |
| 22/10 | 11 | 1 | + | − | − | −/− |
| 29/10 | 10 | 1 | − | − | − | −/− |
| 29/10 | 10 | 6 | + | − | − | −/− |
| 5/11 | 10 | 1 | − | − | − | −/− |
| 5/11 | 10 | 1 | + | − | − | −/− |
| 5/11 | 10 | 6 | − | − | − | −/− |
| 5/11 | 10 | 6 | − | − | − | −/− |
| 11/11 | 8 | 1, 6 | + | + | − | +e |
| 11/11 | 8 | 1, 6 | + | − | − | + |
| 18/11 | 7 | 1, 6 | − | − | − | − |
| 18/11 | 7 | 1, 6 | − | − | − | + |
| 26/11 | 5 | 1, 6 | − | − | − | − |
| 26/11 | 5 | 1, 6 | − | − | − | − |
| 17/12 | 3 | 1, 3 | − | − | − | − |
| 17/12 | 3 | 1, 3 | − | − | − | − |
Mussels were cultivated on ropes, and various sampling depths were possible.
Water samples (250 ml) were collected at the surface and at a depth of 6 m.
+, V. vulnificus detected; −, V. vulnificus not detected.
Data for surface sample/data for sample collected at a depth of 6 m.
For samples that were collected on or after 11 November, the samples from the two depths were mixed before analysis.
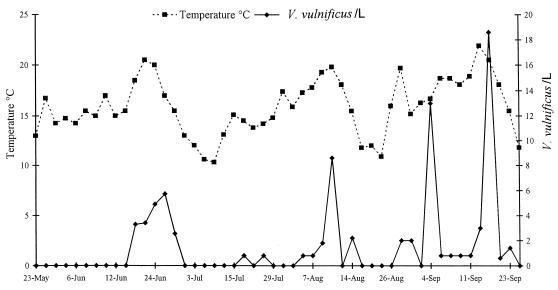
FIG. 2.
Mean densities of V. vulnificus in samples of coastal water and water temperatures from the seven sites studied.
Occurrence of V. vulnificus in water and sediment.
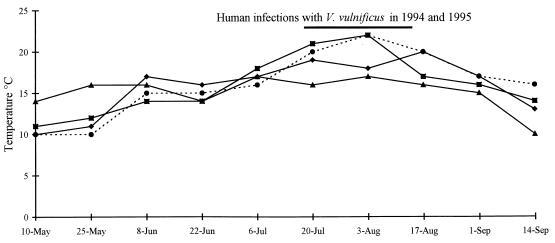
V. vulnificus was isolated from 72 (42%) of the 172 samples analyzed. Table 2 shows the occurrence by site and sample type and also shows the salinity and temperature ranges at sites that yielded samples which were positive and negative for V. vulnificus. The salinity and temperature ranges for water samples which contained V. vulnificus were 5 to 17‰ and 14 to 22°C, respectively, but varied considerably between sites. In particular, it should be noted that V. vulnificus was isolated at 9°C from the sediment at location 1 (Copenhagen Harbor), whereas the lowest recorded temperature for a water sample containing V. vulnificus was 14°C. All of the sediment samples from location 1 contained small blue mussels, and when these mussels were analyzed individually, 8 of 13 samples were found to contain V. vulnificus. The sediment samples remained positive for V. vulnificus when all mussels were removed before analysis. Statistical analysis revealed that there was a significant correlation (P < 0.0001) between densities of V. vulnificus and water temperatures, whereas the salinity did not influence the data. The seasonal relationship between the prevalence of V. vulnificus and water temperature is illustrated in Fig. 2. The seasonal relationship between V. vulnificus and water temperature is further emphasized in Fig. 3, which shows the mean water temperatures at three popular recreational beaches in the period from 1993 to 1996 and the approximate dates of clinical cases in 1994 and 1995 (12, 14). Figure 3 shows that the mean water temperatures were above 20°C in 1994 and 1995 when the clinical cases occurred. Statistical analysis further demonstrated that there was no correlation between the occurrence of presumptive Vibrio spp. and E. coli and the density of V. vulnificus. However, significant correlations between the occurrence of V. vulnificus and the occurrence of coliform bacteria (P < 0.0015) and between the occurrence of V. vulnificus and the occurrence of fecal streptococci (P < 0.0022) were found. Presumptive Vibrio spp. were present at concentrations of less than 10 cells per ml of water in almost every water sample analyzed (data not shown). The specific locations where samples were collected did not influence the results.
TABLE 2.
Occurrence of V. vulnificus in water and sediment samples from the sites studied
| Location | Sample type | No. of positive samples/total no. of samples | Positive samples
|
Negative samples
|
||
|---|---|---|---|---|---|---|
| Temp (°C) | Salinities (‰) | Temp (°C) | Salinities (‰) | |||
| 1 | Water | 13/19 | 15–22 | 7–17 | 9–16 | 9–14 |
| 1 | Sediment | 19/25 | 9–22a | —b | 6–16 | — |
| 2 | Water | 7/19 | 14–20 | 7–17 | 10–18 | 8–18 |
| 2 | Sediment | 6/19 | 16–20 | — | 10–18 | — |
| 3 | Water | 4/17 | 17–19 | 5–17 | 12–18 | 5–15 |
| 4 | Water | 2/9 | 14–20 | 9 | 12–20 | 8–11 |
| 5 | Water | 5/17 | 16–21 | 6–11 | 13–17 | 5–12 |
| 6 | Water | 5/17 | 15–20 | 5–9 | 13–17 | 5–15 |
| 7 | Water | 3/17 | 17–20 | 7–9 | 12–20 | 5–12 |
Temperature of the water column 1 m above where sediment was collected.
—, salinity was not determined for sediment samples.
FIG. 3.
Mean water temperatures at three popular recreational beaches in Denmark from 1993 to 1996. The horizontal bar shows the time period when human infections occurred in 1994 and 1995. Symbols: ▴, 1993; ▪, 1994; •, 1995; ⧫, 1996.
Occurrence of V. vulnificus in shellfish.
Sampling of blue mussels was initiated in September 1996, and samples were received from areas C to M (Fig. 1). The salinities and surface water temperatures in these areas were studied in September and ranged from 18 to 35‰ and from 12 to 14°C, respectively. Only the sample from area G was found to be positive for V. vulnificus, which led to extended monitoring of this area until December 1996. In area G, mussels were cultivated on suspended ropes attached to floating rafts, whereas at the other sampling areas mussels were cultivated on the seabed at depths varying from 2 to 10 m. Table 1 shows the results obtained with water and mussel samples from area G. V. vulnificus was found in mussels at water temperatures between 8 and 14°C and at a salinity of approximately 17‰ throughout the sampling period. V. vulnificus was detected in water when the water temperatures were 7 to 8°C, but not at higher temperatures. Sampling of oysters at areas A and B was carried out in August when the water temperature was approximately 20°C; the water salinities at these two areas were 35 and 20‰, respectively. V. vulnificus was not detected in any oyster sample or in water samples from the oyster beds.
Occurrence of V. vulnificus in wild fish.
Table 3 shows the distribution of V. vulnificus in samples of gills, mucus, and intestinal contents from eels, eelpouts, and flounders. The majority of the positive samples were obtained in late August and early September when the water temperatures were high (Fig. 2). No V. vulnificus or other fish-pathogenic bacteria were detected in kidney samples. The highest prevalence of V. vulnificus was found in the gills from eels; 19 (26%) of 73 samples were positive. The prevalence of V. vulnificus in the mucus and intestinal contents of eels was lower; 3 of 20 (15%) and 4 of 73 (5%) of the samples were positive, respectively. We detected V. vulnificus in 4 of 75 samples from eelpouts and flounders. However, none of the mucus samples from these two fish species contained V. vulnificus.
TABLE 3.
Occurrence of V. vulnificus in samples of gills, mucus, and intestinal contents from different fish species
| Fish species | No. of positive samples/total no. of samples
|
||
|---|---|---|---|
| Gills | Mucus | Intestinal contents | |
| Eels | 19/73 | 3/20 | 4/73 |
| Eelpouts | 2/27 | 0/6 | 0/27 |
| Flounders | 1/5 | 0/5 | 1/5 |
| Total | 22/105 | 3/31 | 5/105 |
Biotyping.
A total of 706 V. vulnificus strains from seawater (325 strains), sediment (245 strains), wild fish (110 strains), and shellfish (26 strains) were tested for indole production. Two strains from seawater and one strain from sediment were indole negative as determined by repeated tests and were assumed to belong to biotype 2. The remaining strains were all indole positive.
DISCUSSION
Until recently, V. vulnificus biotype 2 had never been isolated from seawater, suggesting that this organism was transmitted from one eel to another by direct contact and that it did not survive well in seawater. In the present study, we isolated biotype 2 strains from sediment and seawater samples, although the frequency of isolation was very low. The low probability of finding a V. vulnificus biotype 2 strain in an environmental sample may explain why the occurrence of this organism in seawater has not been reported previously. The isolation procedure may also influence which biotype is detected since a few biotype 2 cells present in a sample might be overgrown by biotype 1 cells or other bacteria. Even though virulence determinants, phenotypic properties, and physiological characteristics of the two biotypes have been reported to be similar in many respects (4, 8, 10, 11), our study revealed that in the Danish marine environment the ecology of biotype 1 and the ecology of biotype 2 appear to be different. Biotype 1 can be isolated from various environmental sources, whereas biotype 2 is rarely found in the environment. Our findings indicate that the marine environment should be regarded as a reservoir and possible vehicle of transmission for V. vulnificus biotype 2. The biotype 2 strains isolated from water and sediment, along with biotype 2 strains isolated from wound infections and diseased eels in Denmark, are currently being characterized to determine their genotypes, serotypes, and virulence for eels to further investigate the ecology of V. vulnificus biotype 2 in marine environments.
Studies of the seven coastal sites examined showed that the occurrence of V. vulnificus in water was strongly correlated to water temperature, as reported by other researchers (28, 39, 54). Most water samples containing V. vulnificus had temperatures above 15°C, similar to temperatures reported previously (28, 45). However, V. vulnificus was detected in coastal water obtained at a mussel farm at a temperature of 7°C, which is lower than the temperature of any previously reported positive sample (54). V. vulnificus has only occasionally been isolated from the Gulf of Mexico when the water temperature is below 20°C (28), but it has been isolated from waters with temperatures of 11 and 8°C in the Great Bay Estuary of New Hampshire and Maine and in the Chesapeake Bay (39, 54). Detection of V. vulnificus at low temperatures, as seen in the present study, may be a function of the sensitivity of the isolation method and/or of adaptation of V. vulnificus to colder temperatures. The inability to isolate V. vulnificus when the temperature drops below 7°C may be due to entry of the organism into a viable but nonculturable state, a survival response by V. vulnificus to low-temperature stress which has been well described (36).
The highest recorded mean density of V. vulnificus in seawater was 1.9 CFU/100 ml, which was reached 1 week after the mean water temperature peaked at 22°C. In comparison, the highest densities of V. vulnificus recorded in water in the Great Bay Estuary and the Chesapeake Bay were 104 and 102 CFU/100 ml at water temperatures of 23 and 27°C, respectively (39, 54). However, both of these areas have water temperatures above 20°C for several months, and this could explain the higher levels of V. vulnificus compared to the levels in Danish coastal areas.
In the statistical linear model, V. vulnificus counts in water samples did not correlate with salinity. All of the sampling sites included in the present study had salinities ranging from 5 to 17‰, which in a previous study were reported to be optimal for isolation of V. vulnificus (28). Therefore, the lack of statistical correlation between salinity and occurrence of V. vulnificus may be explained by the fact that no sampling site had a salinity that limited the growth of V. vulnificus.
The control of Danish coastal bathing water is based on the use of presumptive E. coli and coliform bacteria as indicators of water quality. No correlation between the occurrence of presumptive E. coli and the occurrence of V. vulnificus was seen, which is in agreement with studies reported previously (15, 37, 39, 45). The reason for the apparent positive correlation between the occurrence of coliform bacteria and fecal streptococci and the occurrence of V. vulnificus is not known.
It has been suggested that Vibrio anguillarum and Vibrio alginolyticus are better indicators than presumptive E. coli for assessing the risk of contracting human bacterial infections in the marine environment (31). In the present study there was no correlation between the occurrence of presumptive Vibrio spp., defined as colonies growing on thiosulfate-citrate-bile-sucrose agar at 37°C, and densities of V. vulnificus. The incubation temperature (37°C) was chosen to give an estimate of vibrios pathogenic to humans. The sensitivity of the method was 10 bacteria per ml of water, which means that low numbers of vibrios were not detected. A possible correlation may have been revealed by a more sensitive method, such as membrane filtration, and/or a preenrichment step. Lower incubation temperatures (20 to 25°C) may also have yielded higher levels of vibrios since some Vibrio spp., including V. anguillarum, do not grow well at 37°C.
No obvious correlation was found between V. vulnificus counts in synoptic sediment and water samples. However, V. vulnificus was frequently found in sediment from Copenhagen Harbor (location 1), which was found to be a natural habitat for mussels. The persistence of V. vulnificus in the tissue of filter-feeding shellfish has been described previously, and at high water temperatures V. vulnificus can replicate in oyster tissue and be released at rates as high as 100,000 cells per oyster per h (21, 43). It has been suggested that V. vulnificus overwinters in a floc zone at the water-sediment interface and is resuspended in the spring following changes in temperature (50). However, the distribution of V. vulnificus in sediment is not clear-cut. Plankton and seawater samples collected at sites where sediment samples were collected have been reported to contain V. vulnificus when no V. vulnificus was found in the sediment (27, 54). Nevertheless, sediment in and around oyster beds has been reported to be more likely to contain V. vulnificus than areas not associated with oysters (54). Our data also indicate that the presence of mussels may favor the survival of V. vulnificus in sediment by providing an environment suitable for persistence and replication.
Samples from Danish shellfish cultivation areas were analyzed to determine if V. vulnificus was part of the natural microbial flora in Danish oysters and mussels. V. vulnificus was found in both water and mussel samples from 1 of the 13 blue mussel cultivation areas tested. We found no obvious correlation between the occurrence of V. vulnificus in water and the occurrence of V. vulnificus in mussel samples. V. vulnificus was isolated from water samples at low temperatures but not at higher temperatures when the highest concentrations of V. vulnificus were obtained from the mussels. Temperature and salinity values do not explain why V. vulnificus was detected only in this area since other areas had similar values. The concentrations of V. vulnificus in blue mussels were very low, and the low probability of finding a viable V. vulnificus cell may explain why this organism was not isolated from other areas. However, area G differed in several ways from the other areas investigated: (i) mussels are cultivated in area G on suspended ropes, a cultivation method in which the mussels are raised above the seabed and exploitation of food at all depths is more efficient than exploitation of food when mussels are cultivated on the seabed; (ii) cormorants (Phalacrocorax carbo) are present in high numbers at the area G mussel farm; and (iii) area G is among the most nutrient-rich water areas in Denmark. The location of mussels in surface waters provides favorable temperatures for growth of V. vulnificus during the summer, and the high levels of nutrients may provide conditions favorable for growth of V. vulnificus, even at low temperatures. The presence of high numbers of defecating cormorants above the mussel farm may be of importance for the prevalence of V. vulnificus in this particular mussel farm. One could hypothesize that when cormorants eat mussels containing V. vulnificus, the organisms may survive or even replicate in the intestines of the birds and be released back into the environment in the feces. The body temperature of birds is 41 to 42°C, a temperature which allows growth of V. vulnificus (19). Furthermore, V. vulnificus and V. cholerae have been isolated from the intestinal contents of a variety of seabirds from the Gulf of Mexico area (17). Birds have also been reported to be a potential vector for the fish-pathogenic bacterium Yersinia ruckeri, which has been isolated from the intestines of several fish-eating birds (20, 53). The role of birds in the ecology of V. vulnificus is currently being investigated in our laboratory.
Bottom-feeding fish have been reported to play a role in the ecology of V. vulnificus in Alabama and Gulf of Mexico waters (18). High densities (108 CFU/g) of V. vulnificus were found in the intestinal contents of fish that consumed mollusks and crustaceans, and it was suggested that fish may play a role in the growth and transport of V. vulnificus (18). V. vulnificus was rarely (5% of samples) found to be a part of the microbial flora in the intestines of eels, eelpouts, and flounders. The greatest prevalence of V. vulnificus (21% of samples) was found in gills, especially the gills of eels (26% of samples). This is to the best of our knowledge the first report of isolation of V. vulnificus from flounder and eelpout. Previous laboratory studies have reported that V. vulnificus biotypes 1 and 2 both adhere to mammalian cells but that only biotype 2 strains adhere to fish cells, suggesting that specialized adhesins are produced (5, 8). Specific adhesion was not investigated in the present study, but the data indicate that V. vulnificus biotype 1 strains can colonize fish. Fish mucus has been reported to contain proteins and glycoproteins that react with environmental antigens and serve as defense barriers to bacterial colonization (1, 22). The low number of mucus samples found to contain V. vulnificus may be explained by a possible bactericidal effect toward V. vulnificus of fish mucus. Detection of V. vulnificus in samples from Danish wild fish provides further evidence that estuarine fish may be important in understanding the ecology of V. vulnificus (18). Although our data do not support the hypothesis that V. vulnificus overwinters in intestines of wild fish, migrating fish colonized by V. vulnificus may facilitate the spread of this organism to new areas where the bacterium can survive.
Shellfish are often implicated in the transmission of V. vulnificus infections in the United States, especially in states bordering the Gulf of Mexico (23, 29, 30). Concentrations of V. vulnificus in raw oysters from this region are reported to be as high as 105 to 106 organisms per g of oyster during the summer, when more than 90% of raw oyster-associated V. vulnificus infections, mainly septicemia, occur (23, 29, 42). Wound infections due to occupational activities around seawater have been reported to show a similar seasonal pattern, with the highest number of cases occurring from April to October (23). In Denmark, infections due to V. vulnificus, mainly wound infections, occurred only in warm summers (12, 14). To date, no V. vulnificus infections have been associated with consumption of raw shellfish in Denmark or elsewhere in Europe. No epidemiological reports have established that there is a risk associated with consumption of raw shellfish containing V. vulnificus in low numbers, as reported in this study.
The detection of V. vulnificus in samples from Danish wild fish during the summer may have possible health implications for fishermen. Fishermen are often exposed to the marine environment on a daily basis and often have abrasions on their hands because of their work. In 1994, 4 of 11 patients contracted V. vulnificus wound infections when fishing (14), and in 1995, three wound infections associated with fishing were recorded. Fishing or handling of eels was involved in four of these cases (12, 14). Fishermen are therefore at risk of V. vulnificus wound infections during the summer.
No human V. vulnificus infections were recorded during the summer of 1996 when low concentrations (<2 CFU/100 ml) of V. vulnificus in coastal water were detected. This may indicate that the levels of V. vulnificus were too low to cause infection, even in susceptible individuals. Epidemiological data from 1994 and 1995 suggest that the risk of contracting a V. vulnificus infection following exposure to seawater is correlated to water temperatures. Thus, medical doctors and authorities responsible for controlling bathing water quality should pay particular attention to potential V. vulnificus wound infections when water temperatures exceed 20°C for several weeks.
ACKNOWLEDGMENTS
This work received financial support from The Fisheries Directorates, Ministry of Agriculture and Fisheries, and The Danish Environmental Protection Agency, Ministry of Environment and Energy. Lise Høi was supported by a fellowship from The Royal Veterinary and Agricultural University.
The technical assistance of Anita Forslund, Mahawash Houssain, and Jane Pedersen is greatly appreciated. Also, we thank Vibeke From Jeppesen, Municipal Food Control Unit, Køge, Denmark, and Jan Rasmussen, Environmental Protection Agency, Copenhagen, Denmark, for their help in collecting water samples and providing data for levels of indicator bacteria. Birgitte Christensen and Thyra Bjergskov, The Danish Veterinary and Food Administration, are thanked for their assistance in coordinating the sampling of shellfish. Brita Bruun, Statens Serum Institut, is thanked for providing information about the clinical cases.
REFERENCES
- 1.Alexander J B, Ingram G A. Noncellular nonspecific defense mechanisms of fish. Annu Rev Fish Dis. 1992;2:249–279. [Google Scholar]
- 2.Amaro C, Biosca E G. Vibrio vulnificus biotype 2, pathogenic for eels, is also an opportunistic pathogen for humans. Appl Environ Microbiol. 1996;62:1454–1457. doi: 10.1128/aem.62.4.1454-1457.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amaro C, Biosca E G, Fouz B, Alcaide E, Esteve C. Evidence that water transmits Vibrio vulnificus biotype 2 infections to eels. Appl Environ Microbiol. 1995;61:1133–1137. doi: 10.1128/aem.61.3.1133-1137.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amaro C, Biosca E G, Fouz B, Toranzo A E, Garay E. Role of iron, capsule, and toxins in the pathogenicity of Vibrio vulnificus biotype 2 for mice. Infect Immun. 1994;62:759–763. doi: 10.1128/iai.62.2.759-763.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amaro C, Biosca E H, Esteve C, Fouz B, Toranzo A E. Comparative study of phenotypic and virulence properties in Vibrio vulnificus biotypes 1 and 2 obtained from an European eel farm experiencing mortalities. Dis Aquat Org. 1992;13:29–35. [Google Scholar]
- 6.Andersen H K. Vibrio vulnificus. J Dan Med Assoc. 1991;153:2361–2362. [PubMed] [Google Scholar]
- 7.Barrow G I, Feltham R K A, editors. Cowan and Steel’s manual for the identification of medical bacteria. 3rd ed. Cambridge, United Kingdom: Cambridge University Press; 1993. [Google Scholar]
- 8.Biosca E G, Amaro C. Toxic and enzymatic activities of Vibrio vulnificus biotype 2 with respect to host specificity. Appl Environ Microbiol. 1996;62:2331–2337. doi: 10.1128/aem.62.7.2331-2337.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Biosca E G, Amaro C, Larsen J L, Pedersen K. Phenotypic and genotypic characterization of Vibrio vulnificus: proposal for the substitution of the subspecific taxon biotype for serovar. Appl Environ Microbiol. 1997;63:1460–1466. doi: 10.1128/aem.63.4.1460-1466.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Biosca E G, Amaro C, Marco-Noales E, Oliver J D. Effect of low temperature on starvation-survival of the eel pathogen Vibrio vulnificus biotype 2. Appl Environ Microbiol. 1996;62:450–455. doi: 10.1128/aem.62.2.450-455.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Biosca E G, Oliver J D, Amaro C. Phenotypic characterization of Vibrio vulnificus biotype 2, a lipopolysaccharide-based homogeneous O serogroup within Vibrio vulnificus. Appl Environ Microbiol. 1996;62:918–927. doi: 10.1128/aem.62.3.918-927.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bruun, B. Personal communication.
- 13.Dalsgaard A, Dalsgaard I, Høi L, Larsen J L. Comparison of a commercial biochemical kit and an oligonucleotide probe for identification of environmental isolates of Vibrio vulnificus. Lett Appl Microbiol. 1996;22:184–188. doi: 10.1111/j.1472-765x.1996.tb01138.x. [DOI] [PubMed] [Google Scholar]
- 14.Dalsgaard A, Frimodt-Møller N, Bruun B, Høi L, Larsen J L. Clinical manifestations and epidemiology of Vibrio vulnificus infections in Denmark. Eur J Clin Microbiol Infect Dis. 1996;15:227–231. doi: 10.1007/BF01591359. [DOI] [PubMed] [Google Scholar]
- 15.Dalsgaard A, Huss H H, Kittikun A H-, Larsen J L. Prevalence of Vibrio cholerae and Salmonella in a major shrimp production area in Thailand. Int J Food Microbiol. 1995;28:101–113. doi: 10.1016/0168-1605(94)00165-3. [DOI] [PubMed] [Google Scholar]
- 16.Dalsgaard A, Høi L. Prevalence and characterization of Vibrio vulnificus isolated from shrimp products imported into Denmark. J Food Prot. 1997;60:1132–1135. doi: 10.4315/0362-028X-60.9.1132. [DOI] [PubMed] [Google Scholar]
- 17.DePaola, A. Personal communication.
- 18.DePaola A, Capers G M, Alexander D. Densities of Vibrio vulnificus in the intestines of fish from the U.S. Gulf Coast Appl Environ Microbiol. 1994;60:984–988. doi: 10.1128/aem.60.3.984-988.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Freeman B M. Body temperature and thermoregulation. In: Freeman B M, editor. Physiology and biochemistry of the domestic fowl. London, United Kingdom: Academic Press; 1983. pp. 365–377. [Google Scholar]
- 20.Furones M D, Rodgers C J, Munn C B. Yersinia ruckeri, the causal agent of enteric redmouth disease (ERM) in fish. Annu Rev Fish Dis. 1993;3:105–125. [Google Scholar]
- 21.Harris-Young L, Tamplin M L, Fisher W S, Mason J W. Effects of physicochemical factors and bacterial colony morphotype on association of Vibrio vulnificus with hemocytes of Crassostrea virginica. Appl Environ Microbiol. 1993;59:1012–1017. doi: 10.1128/aem.59.4.1012-1017.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hjelmeland K, Christie M, Raa J. Skin mucus protease from rainbow trout, Salmo gairdneri Richardson, and its biological significance. J Fish Biol. 1983;23:13–22. [Google Scholar]
- 23.Hlady W G, Klontz K C. The epidemiology of Vibrio infections in Florida, 1981–1993. J Infect Dis. 1996;173:1176–1183. doi: 10.1093/infdis/173.5.1176. [DOI] [PubMed] [Google Scholar]
- 24.Høi L, Dalsgaard A, Larsen J L, Warner J M, Oliver J D. Comparison of ribotyping and randomly amplified polymorphic DNA polymerase chain reaction (RAPD-PCR) for characterization of Vibrio vulnificus. Appl Environ Microbiol. 1997;63:1674–1678. doi: 10.1128/aem.63.5.1674-1678.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoyer J, Engelmann E, Liehr R-M, Distler A, Hahn H, Shimada T. Septic shock due to Vibrio vulnificus serogroup O4 wound infection acquired from the Baltic Sea. Eur J Clin Microbiol Infect Dis. 1995;14:1016–1018. doi: 10.1007/BF01691388. [DOI] [PubMed] [Google Scholar]
- 26.Kaspar C W, Tamplin M L. Effects of temperature and salinity on the survival of Vibrio vulnificus in seawater and shellfish. Appl Environ Microbiol. 1993;59:2425–2429. doi: 10.1128/aem.59.8.2425-2429.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaysner C A, Abeyta C J, Wekell M M, DePaola A, Stott R F, Leitch J M. Virulent strains of Vibrio vulnificus isolated from estuaries of the United States west coast. Appl Environ Microbiol. 1987;53:1349–1351. doi: 10.1128/aem.53.6.1349-1351.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kelly M T. Effect of temperature and salinity on Vibrio (Beneckea) vulnificus occurrence in a Gulf Coast environment. Appl Environ Microbiol. 1982;44:820–824. doi: 10.1128/aem.44.4.820-824.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klontz K C, Lieb S, Schreiber M, Janowski H T, Baldy L M, Gunn R A. Syndromes of Vibrio vulnificus infections. Clinical and epidemiologic features in Florida cases, 1981–1987. Ann Intern Med. 1988;109:318–323. doi: 10.7326/0003-4819-109-4-318. [DOI] [PubMed] [Google Scholar]
- 30.Klontz K C, Williams L, Baldy L M, Campos M. Raw oyster-associated Vibrio infections: linking epidemiologic data with laboratory testing of oysters obtained from a retail outlet. J Food Prot. 1994;56:977–979. doi: 10.4315/0362-028X-56.11.977. [DOI] [PubMed] [Google Scholar]
- 31.Larsen J L, Willeberg P. The impact of terrestrial and estuarial factors on the density of environmental bacteria (Vibrionaceae) and faecal coliforms in coastal water. Zentralbl Bakteriol Parasitenkd Infektionskr Hyg Abt 1 Orig. 1984;179:308–323. [PubMed] [Google Scholar]
- 32.Massad G, Oliver J D. New selective and differential medium for Vibrio cholerae and Vibrio vulnificus. Appl Environ Microbiol. 1987;53:2262–2264. doi: 10.1128/aem.53.9.2262-2264.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Melhus Å, Homldahl T, Tjernderg I. First documented case of bacteremia with Vibrio vulnificus in Sweden. Scand J Infect Dis. 1995;27:81–82. doi: 10.3109/00365549509018980. [DOI] [PubMed] [Google Scholar]
- 34.Mertens A, Nagler J, Hansen W, Gepts-Friedenreich E. Halophilic, lactose-positive Vibrio in a case of fatal septicemia. J Clin Microbiol. 1979;9:233–235. doi: 10.1128/jcm.9.2.233-235.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oliver J D. Vibrio vulnificus. In: Doyle M P, editor. Foodborne bacterial pathogens. New York, N.Y: Marcel Dekker, Inc.; 1989. pp. 569–600. [Google Scholar]
- 36.Oliver J D. The viable but non-culturable state in the human pathogen Vibrio vulnificus. FEMS Microbiol Lett. 1995;133:203–208. doi: 10.1111/j.1574-6968.1995.tb07885.x. [DOI] [PubMed] [Google Scholar]
- 37.Oliver J D, Warner R A, Cleland D R. Distribution and ecology of Vibrio vulnificus and other lactose-fermenting marine vibrios in coastal waters of the southeastern United States. Appl Environ Microbiol. 1982;44:1404–1414. doi: 10.1128/aem.44.6.1404-1414.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oliver J D, Warner R A, Cleland D R. Distribution of Vibrio vulnificus and other lactose-fermenting vibrios in the marine environment. Appl Environ Microbiol. 1983;45:985–998. doi: 10.1128/aem.45.3.985-998.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O’Neill K R, Jones S H, Grimes D J. Seasonal incidence of Vibrio vulnificus in the Great Bay estuary of New Hampshire and Maine. Appl Environ Microbiol. 1992;58:3257–3262. doi: 10.1128/aem.58.10.3257-3262.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tabtieng R, Wattanasri S, Echeverria P, Seriwatana J, Bodhidatta L, Chatkaeomorakot A, Rowe B. An epidemic of V. cholerae El Tor Inaba resistant to several antibiotics with a conjugative group C plasmid coding for type II dihydrofolate reductase in Thailand. Am J Trop Med Hyg. 1989;41:680–686. doi: 10.4269/ajtmh.1989.41.680. [DOI] [PubMed] [Google Scholar]
- 41.Tacket C O, Brenner F, Blake P A. Clinical features and an epidemiological study of Vibrio vulnificus infections. J Infect Dis. 1984;149:558–561. doi: 10.1093/infdis/149.4.558. [DOI] [PubMed] [Google Scholar]
- 42.Tamplin M L. Proceedings of the 1994 Vibrio vulnificus Workshop. U.S. Washington, D.C: Food and Drug Administration; 1995. The ecology of Vibrio vulnificus; pp. 75–86. [Google Scholar]
- 43.Tamplin M L, Capers G M. Persistence of Vibrio vulnificus in tissues of Gulf Coast oysters, Crassostrea virginica, exposed to seawater disinfected with UV light. Appl Environ Microbiol. 1992;58:1506–1510. doi: 10.1128/aem.58.5.1506-1510.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tamplin M L, Martin A L, Ruple A D, Cook D W, Kaspar C W. Enzyme immunoassay for identification of Vibrio vulnificus in seawater, sediment, and oysters. Appl Environ Microbiol. 1991;57:1235–1240. doi: 10.1128/aem.57.4.1235-1240.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tamplin M L, Rodrick G E, Blake N J, Cuba T. Isolation and characterization of Vibrio vulnificus from two Florida estuaries. Appl Environ Microbiol. 1982;44:1466–1470. doi: 10.1128/aem.44.6.1466-1470.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.The International Organization for Standardization. Water quality—detection and enumeration of fecal enterococci. Part 2. Membrane filtration method. Publication ISO 7899/1. Geneva, Switzerland: The International Organization for Standardization; 1984. [Google Scholar]
- 47.The International Organization for Standardization. Water quality—detection and enumeration of coliform organisms, thermotolerant coliform organisms, and presumptive Escherichia coli. Part 1. Membrane filtration method. Publication ISO 9308/1. Geneva, Switzerland: The International Organization for Standardization; 1990. [Google Scholar]
- 48.Tison D L, Nishibuchi M, Greenwood J D, Seidler R J. Vibrio vulnificus biogroup 2; new biogroup pathogenic for eels. Appl Environ Microbiol. 1982;44:640–646. doi: 10.1128/aem.44.3.640-646.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.U.S. Food and Drug Administration. Bacteriological analytical manual. 8th ed. Arlington, Va: Association of Official Analytical Chemists; 1984. [Google Scholar]
- 50.Vanoy R W, Tamplin M L, Schwartz J R. Ecology of Vibrio vulnificus in Galveston Bay oysters, suspended particulate matter, sediment and seawater: detection by monoclonal antibody-immunoassay-most probable number procedures. J Ind Microbiol. 1992;9:219–223. [Google Scholar]
- 51.Veenstra J, Rietra P J, Coster J M, Slaats E, Dirks Go S. Seasonal variations in the occurrence of Vibrio vulnificus along the Dutch coast. Epidemiol Infect. 1994;112:285–290. doi: 10.1017/s0950268800057691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Veenstra J, Rietra P J, Stoutenbeek C P, Coster J M, de Gier H H, Dirks-Go S. Infection by an indole-negative variant of Vibrio vulnificus transmitted by eels. J Infect Dis. 1992;166:209–210. doi: 10.1093/infdis/166.1.209. [DOI] [PubMed] [Google Scholar]
- 53.Willumsen B. Birds and wild fish as potential vectors of Yersinia ruckeri. J Fish Dis. 1989;12:275–277. [Google Scholar]
- 54.Wright A C, Hill R T, Johnson J A, Roghman M-C, Colwell R R, Morris J G. Distribution of Vibrio vulnificus in Chesapeake Bay. Appl Environ Microbiol. 1996;62:717–724. doi: 10.1128/aem.62.2.717-724.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wright A C, Miceli G A, Landry W L, Christy J B, Watkins W D, Morris J G. Rapid identification of Vibrio vulnificus on nonselective media with an alkaline phosphatase-labeled oligonucleotide probe. Appl Environ Microbiol. 1993;59:541–546. doi: 10.1128/aem.59.2.541-546.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]