Abstract
The adherence of Bifidobacterium strains isolated from infant feces and commercial fermented dairy products to enterocyte-like cells was correlated with the autoagglutination and hemagglutination properties of these organisms. These results allowed us to define two groups: (i) cell-adherent bacteria showing hemagglutination and autoagglutination and (ii) non-cell-adherent, nonhemagglutinating, nonautoagglutinating bacteria. Glass adherence was shown to be nonspecific and was discarded as a criterion for selection of adherent cells. Hydrophobicity appeared to be necessary for adhesion to enterocyte-like cells and autoagglutination. Adhesive strains were highly hydrophobic, and the degree of adherence was slightly dependent on the surface potential. Cells autoagglutinated more when the electrostatic negative charges on the cell surface were shielded by a decrease in the pH from 7 to 2. However, in some strains negative charges at the cell surface were adjuvant to adhesion, thus suggesting that specific chemical interactions occurred. The present results provide a method for preliminary selection of bacteria potentially adherent to epithelial cells by means of autoagglutination.
Bifidobacteria are nonmotile, gram-positive, anaerobic bacteria that are natural inhabitants of the guts of animals and humans (10, 14). These organisms have a number of beneficial effects on host health in both infants and adults. The most important of these include inhibition or displacement of undesirable microorganisms, elimination of procarcinogens, immunomodulation, and vitamin production (6, 9, 10, 12, 17). The effectiveness of these organisms is related to their ability to interact and adhere to the intestine wall. Adhesion of Bifidobacterium strains to the colon surface may occur by association of the bacteria with a secreted mucus gel or by adherence to the underlying epithelium (4).
Generally, adhesion can be ascribed to the interplay of attractive and repulsive forces between the approaching surfaces (5, 8, 13). Cell adhesion is a multistep process involving contact of the cell with the surface, and it is affected by the composition and structure of the cell membrane and interacting surfaces. Several workers have dealt with common features of the composition, structure, and forces of interaction related to bacterial adhesion. Mechanisms of cell-cell interactions follow the Derjaguin-Landau-Verwey-Overbeek theory of colloid stability, but specific macromolecular interactions have also been described. The molecular origin of each of these interactions in cells is a matter for discussion. As a way to understand cell-cell interactions at a molecular level, special procedures involving erythrocyte agglutination and adhesion to a variety of synthetic substrates without specific ligands have been examined (11, 15, 16, 18). On the other hand, the physical and chemical characteristics of the cell surface have been determined mainly by determining surface hydrophobicity and electrical mobility (2, 3, 5, 7). The hydrophobicity of the surface and zeta potentials account for the attractive and repulsive forces, respectively, that take place in autoaggregation and adhesion of bacteria to different surfaces.
Although adhesion to cultured human intestinal epithelial cells has been reported previously (1), no extensive inventory of the surface properties of Bifidobacterium strains has been made previously to correlate the properties of adherence of these strains to themselves, to other cells, and to synthetic surfaces. A determination of the properties of adherence to different materials (substrata) for a number of Bifidobacterium strains could provide in vitro insight into the effectiveness of these organisms for colonizing epithelia.
With this aim, the autoagglutination, hemagglutination, and adherence to enterocyte-like cells of Bifidobacterium strains of different origins were studied in order to establish their general behavior. In order to gain insight into the structural properties of the surfaces of the strains, the hydrophobicity and the electrokinetic mobility were determined for each strain.
MATERIALS AND METHODS
Bifidobacterium strains and culture conditions.
Strains CIDCA 532, CIDCA 533, CIDCA 536, CIDCA 537, CIDCA 538, CIDCA 539, CIDCA 5310, CIDCA 5311, CIDCA 5312, CIDCA 5313, CIDCA 5314, CIDCA 5315, CIDCA 5316, and CIDCA 5317 were isolated from infant feces in our laboratory. Strains MB-2, MB-9, MB-19, and MB-56 were obtained from Morinaga Milk Industry, Higashihara, Zama City, Japan. Strain CIDCA 531 was isolated from a commercial fermented milk product (Table 1). All of the strains were pleomorphic rods, gram positive, nonsporulating, strictly anaerobic, and positive for the fructose-6-phosphate phosphoketolase test (14). Further characterization was performed by analyzing sugar fermentation patterns and whole-cell proteins (unpublished data).
TABLE 1.
Bifidobacterial strains used in this study
| Strain | Species | Sourcea | Autoagglutinationb | Hemagglutinationc | Glass adherenced | Caco-2 cell adherencee |
|---|---|---|---|---|---|---|
| CIDCA 531 | Bifidobacterium pseudolongum | A | − | − | + | − |
| CIDCA 532 | Bifidobacterium breve | B | − | − | − | − |
| CIDCA 533 | Bifidobacterium bifidum | B | + | + | + | + |
| CIDCA 536 | Bifidobacterium bifidum | C | + | + | + | + |
| CIDCA 537 | Bifidobacterium bifidum | C | + | + | + | + |
| CIDCA 538 | Bifidobacterium infantis | D | − | − | − | − |
| CIDCA 539 | Bifidobacterium bifidum | E | + | + | + | + |
| CIDCA 5310 | Bifidobacterium bifidum | E | + | + | + | (+) |
| CIDCA 5311 | Bifidobacterium bifidum | E | + | − | − | + |
| CIDCA 5312 | Bifidobacterium breve | E | − | − | − | − |
| CIDCA 5313 | Bifidobacterium bifidum | E | + | + | + | + |
| CIDCA 5314 | Bifidobacterium breve | E | − | − | − | − |
| CIDCA 5315 | Bifidobacterium adolescentis | F | − | − | − | NDf |
| CIDCA 5316 | Bifidobacterium adolescentis | G | − | − | − | − |
| CIDCA 5317 | Bifidobacterium adolescentis | H | − | − | − | − |
| MB-2 | Bifidobacterium bifidum | I | − | − | − | ND |
| MB-9 | Bifidobacterium infantis | J | + | + | + | ND |
| MB-19 | Bifidobacterium breve | K | − | − | − | ND |
| MB-56 | Bifidobacterium longum | L | − | − | − | ND |
Strains with the same letter were isolated from the same individual or sample.
+, autoagglutinating strain; −, nonautoagglutinating strain.
+, hemagglutinating strain; −, nonhemagglutinating strain.
+, glass-adherent strain; −, non-glass-adherent strain.
+, strain adheres to Caco-2 cells; (+), strain slightly adheres to Caco-2 cells; −, strain does not adhere to Caco-2 cells.
ND, not determined.
Bacteria were cultured for 48 h at 37°C in TPY medium (14) containing (per liter) 10.0 g of tryptone, 5.0 g of soy peptone, 5.0 g of glucose, 2.5 g of yeast extract, 1.0 g of Tween 80, 0.5 g of cysteine hydrochloride, 2.0 g of K2HPO4, 5.0 g of MgCl2 · 6H2O, 0.25 g of ZnSO4 · 7H2O, and 0.15 g of CaCl2 and a trace of FeCl3; the pH was 7.0.
In all cases, bacteria were harvested in the stationary phase. Cells were collected by centrifugation at 14,000 × g for 1 min, washed twice in 50 mM K2HPO4 (pH 7.0), and finally resuspended in the same buffer. The cell concentration was adjusted to 0.2 mg (dry weight) per ml, and the A600 was measured.
Cell cultures.
Enterocyte-like Caco-2 cells were purchased from the American Type Culture Collection (Rockville, Md.). The cells were grown in Eagle’s minimum essential medium with Earle’s salts supplemented with l-glutamine, nonessential amino acids (Sigma Chemical Co., St. Louis, Mo.), and 20% inactivated fetal calf serum (GEN S.A., Buenos Aires, Argentina). Streptomycin and penicillin G were added to final concentrations of 100 mg/ml and 50 IU/ml, respectively.
Monolayers were prepared on glass coverslips which were placed in 24-well tissue plates (Corning Glass Works, Corning, N.Y.). Cells were seeded at a concentration of 2 × 105 cells/well and were incubated at 37°C in a 5% CO2 incubator. The culture medium was changed every 2 days, and adherence assays were performed after 15 days (late postconfluence). Cells were used after between 23 and 34 passages.
Agglutination index.
The agglutination index was defined as the ratio between the bacterial concentration (dry weight in milligrams per milliliter) and the A600 of a well-stirred sample. To determine the dry weight, 100 ml of a bacterial culture were centrifuged at 10,000 × g for 10 min, washed twice with distilled water, and allowed to dry at 100°C until the weight was constant.
Hemagglutination assays.
Bacterial cells collected by centrifugation at 14,000 × g for 1 min were washed twice with phosphate-buffered saline (pH 7) and resuspended to a final concentration of 1 mg/ml.
Human erythrocytes from type O+, A+, and B+ blood were obtained from healthy volunteers. Erythrocytes from different blood groups were assayed since they expressed different surface markers. In addition, it is known that surface antigens are also present in the enterocyte-like cells.
Hemagglutinating properties were measured in a 96-well U-shaped microliter plate (Nunc, Roskilde, Denmark) by using phosphate-buffered saline as the diluent. Twenty-five microliters of a twofold-diluted cell suspension was mixed with 25 μl of a 2% erythrocyte suspension and incubated for 1 h at room temperature. The hemagglutination titer was calculated as the reciprocal of the highest dilution showing hemagglutination. Macroscopic hemagglutination was confirmed microscopically by finding clusters of erythrocytes in which bacteria acted as a bridge.
Glass adherence.
Bacterial strains were grown in 10 ml of TPY medium at 37°C for 48 h in neutral glass tubes. The medium was carefully removed, and the A600 of the planktonic bacteria (Aplank) was measured.
The bacteria attached to the tube walls were resuspended by adding 10 ml of phosphate buffer (pH 7.0). To obtain a quantitative suspension, the bacteria on the surface were removed by scrapping the surface with a rubber policeman in order to avoid bacterial damage. The A600 of the resuspended bacteria (Aatt) was measured. The percentage of adherence (Adh%) was calculated with the formula Adh% = [Aatt/(Aatt + Aplank)] × 100.
Cell adherence.
Caco-2 monolayers prepared as described above were washed three times with GKN solution (which contained, per 100 ml of distilled water, 800 mg of NaCl, 40 mg of KCl, 69 mg of NaH2PO4 · H2O, and 157 mg of Na2HPO4; pH 7.2 to 7.4). Cultures of bifidobacteria in liquid TPY medium were washed with GKN solution, and 0.5 ml of a suspension containing 107 bacteria/ml was added to each well of the tissue culture plate. After incubation for 1 h at room temperature with agitation, the monolayers were washed with GKN solution, fixed for 5 min with methanol, Gram stained, and examined microscopically.
Hydrophobicity assays (microbial adhesion to hydrocarbons).
Two milliliters of bacterial suspension was put in contact with 0.4 ml of xylene by vortexing for 120 s. The phases were allowed to separate by decantation. The aqueous phase was carefully removed, and the A600 was measured.
The decrease in the absorbance of the aqueous phase was taken as a measure of the cell surface hydrophobicity (H%), which was calculated with the formula H% = [(Ao − A)/Ao] × 100, where Ao and A are the absorbance before and after extraction with xylene.
Electrophoretic mobility and zeta potential.
Electrophoretic mobilities of bacteria resuspended in 1 mM KCl were determined in a capillary H-cell with Ag/AgCl electrodes. The electrodes were connected to a continuous variable direct current source. The potential was fixed at 40 V, and measurements were obtained by alternatively changing the polarity of the electrodes to avoid polarization.
The electrophoretic mobility of bacteria (μ) was determined by measuring the rate of migration of the particles in the stationary layer when a constant electric field was applied. The effective electrical distance was calculated by using KCl solutions which had known conductivity at 25°C. The conditions were standardized by determining the zeta potential (ζ) of phosphatidylserine liposomes whose value was around −120 mV in 0.01 M NaCl at pH 7.4. The rate of migration was determined by microscopic observation of the displacement of individual cells with rectilinear and uniform movement along a reticular lattice (length, 1 mm). At least 10 determinations in each direction were made with different cells. The results reported below are the means and standard deviations. The temperature was maintained at 25°C in all cases. The zeta potential was calculated with the equation ζ = 4IIημ/ɛ, where η and ɛ are the viscosity and the dielectric constant of the solution, respectively.
Trypsinization procedure.
Bacterial suspensions were incubated with trypsin (final concentration, 1 g/liter) from Sigma Chemical Co. for 1 h at 37°C.
Statistical analysis.
The significance of differences was determined by a statistical analysis (analysis of variance) performed with Systat (Systat, Inc.).
RESULTS
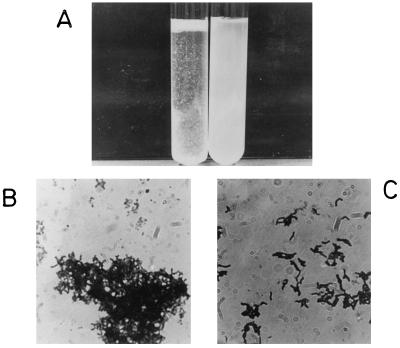
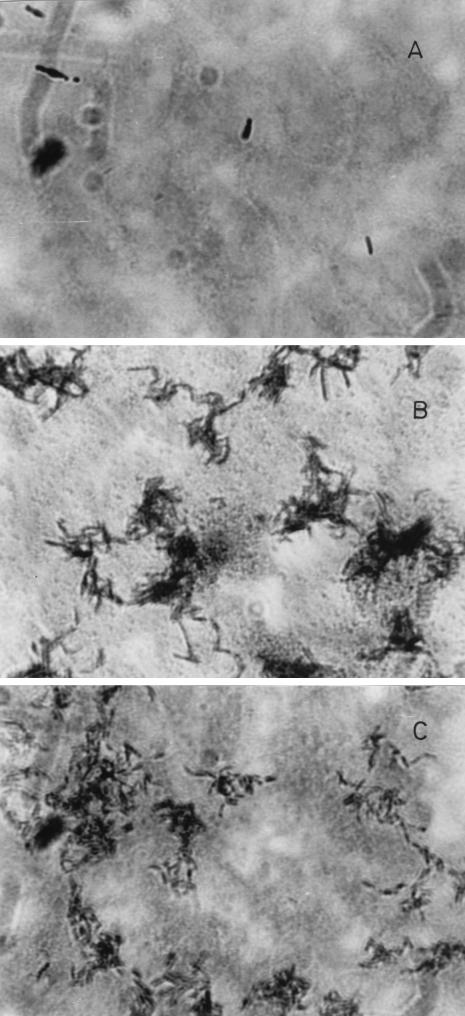
Bifidobacterium strains grown in aqueous media were classified into two groups on the basis of the visual appearance of their suspensions, as shown in Fig. 1A. One group, designated the autoagglutinating cells, formed a precipitate, resulting in a clear solution. The second group, designated the nonagglutinating cells, produced constant turbidity for long periods of time. These two macroscopic behaviors can be explained by the formation of large clusters in the case of the agglutinating bacteria (Fig. 1B) and the lack of clusters in the case of the nonagglutinating bacteria (Fig. 1C).
FIG. 1.
Macroscopic and microscopic views of autoagglutinating and nonautoagglutinating Bifidobacterium cells. (A) Autoagglutinating cells gave a clear solution after the floccules precipitated. Nonagglutinating cells gave a turbid solution after more than 24 h. (B) Microscopic image of agglutinated strain CIDCA 5310 cells. Magnification, ×910. (C) Microscopic image of nonagglutinated strain CIDCA 5317 cells. Magnification, ×910.
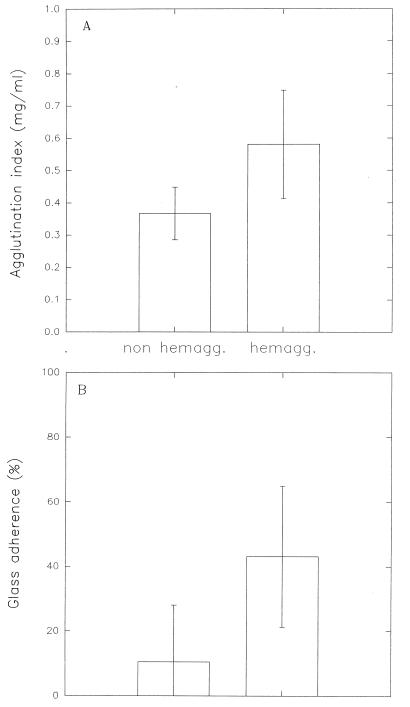
By using this procedure, Bifidobacterium strains having different origins were classified as autoagglutinating or nonautoagglutinating (Table 1). The agglutination index, obtained from turbidity measurements as described in above, is a function of the hemagglutinating properties of each strain. Bacteria positive for hemagglutination had a higher autoagglutination index (Fig. 2A) and a greater adherence to glass than nonagglutinating strains (Fig. 2B).
FIG. 2.
Correlation of the agglutination index (A) and glass adherence (B) with hemagglutination. For hemagglutinating (hemagg.) strains the agglutination index was 0.58 ± 0.17 (n = 27) and the percentage of adherence was 43.0 ± 21.8% (n = 33). For nonhemagglutinating (non hemagg.) strains the agglutination index was 0.37 ± 0.08 (n = 33) and the percentage of adherence was 10.4 ± 17.7% (n = 48). In all cases, significant differences were observed (P < 0.001).
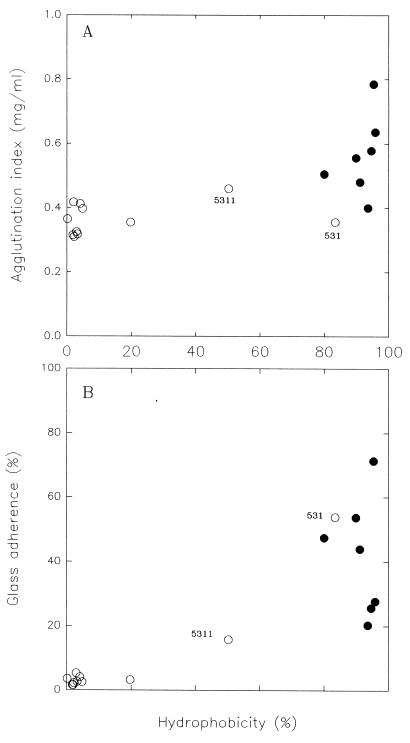
High agglutination index and glass adherence values corresponded to hydrophobicities of 80% or more (Fig. 3). In contrast, the autoagglutination and glass adherence values were low and nearly constant at hydrophobicity values below 80%. All of the strains with high hydrophobicities autoagglutinated and adhered. However, strains with almost the same hydrophobicity value had wide ranges of adherence and hemagglutination values (Fig. 3). This implies that other forces may modulate the adhesion driven by hydrophobicity.
FIG. 3.
Agglutination index (A) and glass adherence (B) as a function of surface hydrophobicity for hemagglutinating (•) and nonhemagglutinating (○) strains. Values are the averages from at least two experiments.
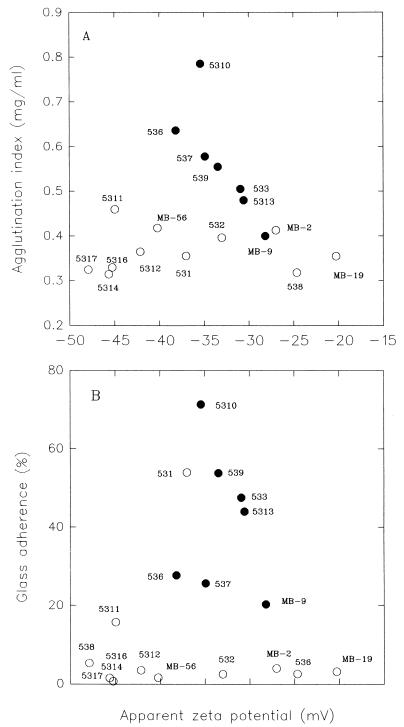
The autoagglutination index is plotted against the electrokinetic potential in Fig. 4A. With hemagglutinating hydrophobic strains there was an inverse correlation between agglutination index and zeta potential. On the other hand, nonhemagglutinating nonhydrophobic strains had agglutination indexes around 0.35 mg/ml over the entire range of electrokinetic potential. Figure 4B shows that hemagglutinating hydrophobic strains had glass adherence values that ranged from 20 to 70% and zeta values between −28 and −39 mV. The nonhemagglutinating nonhydrophobic strains had low glass adherence values and a wide range of electrokinetic potentials. On the base of these results we defined two groups, one containing hemagglutinating hydrophobic strains (strains that showed autoagglutination) and one containing nonhemagglutinating nonhydrophobic strains (strains that did not show autoagglutination). Strains CIDCA 531 and CIDCA 5311 are not in either of these groups. Strain CIDCA 531 is nonhemagglutinating and showed relatively high adherence to glass but had a low agglutination index (Table 1) and a high hydrophobicity value (85%). In addition, strain CIDCA 5311, which is also nonhemagglutinating, autoagglutinates but shows low glass adherence.
FIG. 4.
Correlation of agglutination index (A) and glass adherence (B) with the electrokinetic potentials of the different strains. The plots show the results for two groups of strains. Group I, strains with hydrophobic indexes greater than 80% (•) (hemagglutinating strains). Group II, strains with hydrophobic indexes less than 80% (○) (nonhemagglutinating strains).
Typical results for adherence of Bifidobacterium strains to Caco-2 epithelial cells are shown in Fig. 5. Autoagglutinating and hemagglutinating strain CIDCA 536 showed a high degree of adherence to the cell monolayer. In contrast, nonautoagglutinating strains did not show this property. For example, strain CIDCA 531 did not show adherence to the cell monolayer in spite of its hydrophobicity and glass adherence. Table 1 shows that all of the autoagglutinating strains tested except strain CIDCA 5310 were able to adhere to the cell monolayer. On the basis of these results it is possible to conclude that autoagglutinating and hemagglutinating strains have the ability to adhere to enterocyte-like cells. In contrast, glass adherence did not correlate to cell adherence. Strains CIDCA 5310 and CIDCA 5317 were subjected to different treatments in order to characterize the surfaces of representative examples of each of the groups defined above. Table 2 shows that the hydrophobic index of strain CIDCA 5310 was slightly decreased at pH 2 and that there was a strong change in the electrokinetic potential. This induced an increase in autoagglutination. At pH 2 nonagglutinating strain CIDCA 5317 showed an increase in the hydrophobicity value (it increased to 64%) and a change in the surface potential similar to that found in the autoagglutinating strain, which resulted in a slight increase in autoagglutination.
FIG. 5.
Adherence of different Bifidobacterium strains to Caco-2 cells. (A) CIDCA 531. (B) CIDCA 536. (C) CIDCA 5311.
TABLE 2.
Effects of different treatments on surface properties of bifidobacterial strainsa
| Strain | Treat- ment | Zeta potential (mV) | Hydropho- bicity (%) | Autoagglu- tinationb |
|---|---|---|---|---|
| CIDCA 5310 | pH 7 | −39.5 ± 8.7 | 95.3 ± 0.9 | + |
| pH 2 | −7.03 ± 3.2 | 76.9 ± 3.3 | ++ | |
| Trypsin | −51.2 ± 18.0 | −0.1 ± 2.6 | − | |
| CIDCA 5317 | pH 7 | −42.0 ± 5.1 | 3.0 ± 0.6 | − |
| pH 2 | −15.9 ± 2.7 | 63.7 ± 6.4 | (+) | |
| Trypsin | −57.5 ± 10.6 | −8.9 ± 8.5 | − |
The zeta potential values are means from 7 to 14 determinations. The hydrophobicity values are means from two or three determinations.
+, autoagglutinating bacteria; −, nonautoagglutinating bacteria; ++, highly autoagglutinating bacteria; (+), slightly autoagglutinating bacteria.
Trypsinization had a dramatic effect on the hydrophobicity of strain CIDCA 5310, eliminating its autoagglutination (Table 2). However, trypsinization at pH 7 had no effect on the hydrophobicity of nonagglutinating strain CIDCA 5317. In this case, the nonagglutinating characteristic was maintained. Trypsinization had no significant effect on the zeta potential of either type of strains. These results indicate that the autoagglutinating behavior of strain CIDCA 5310 is due to a hydrophobic protein and negative surface charges.
DISCUSSION
Figures 2 and 5 and Table 1 show that autoagglutination and hemagglutination are direct indications that Bifidobacterium strains will adhere to enterocyte-like cells. When autoagglutination and hemagglutination are not present, adhesion to Caco-2 cells is not observed in spite of the high hydrophobicity values of the bacterial strains. Adherence to glass is not indicative of a bacterial ability to interact with enterocyte-like cells. Strain CIDCA 5311 is an example of an autoagglutinating strain that shows adherence to cells but not to glass. In contrast, strain CIDCA 531, a highly hydrophobic and nonautoagglutinating strain, does not adhere to cells in spite of its adherence to glass. Multiple reasons may account for adherence to glass, and it was not the purpose of the present work to analyze these reasons since this property is an ambiguous criterion for selecting cell-adherent bacteria.
The correlation of autoagglutination and hemagglutination with cell adherence provides a simple method for preliminary selection of strains with cell-adhesive features by means of simple visual inspection of liquid cultures (Fig. 1). Autoagglutination and cell adherence (Fig. 3 through 5) result from a complex interplay between hydrophobic (attractive) and electrostatic (repulsive) interactions. Hydrophobicity appears to be necessary for adhesion to epithelial cells and autoagglutination. Cell-cell interaction occurs when the hydrophobic indexes are greater than 80%. However, the zeta potentials of autoagglutinating strains vary between −29 and −40 mV.
Figure 4 shows that autoagglutination increases with a decrease in zeta potential, indicating that negative charges affect adhesion. The decrease in electrostatic repulsion could result in greater adhesion by allowing the approach of the hydrophobic surfaces. Assays performed at pH 2 with strain CIDCA 5310 (Table 2) showed that when the zeta potential changes from −39 mV (pH 7) to −7 mV (pH 2), autoagglutination of the cells increases. This could be explained by the shielding by protons of the negative charges laying on the cell surface, leading to a decrease in cell repulsion.
In general, adhesion is expected to increase at high attractive hydrophobic forces and low surface potentials. In autoagglutinating bacteria the hydrophobicity remains high (around 65%) with a decrease in pH (Table 2). As a consequence of the reduction of the electrostatic repulsion, autoagglutination increases. The same reduction in surface potential makes a nonagglutinating strain slightly autoagglutinating, and there is a parallel increase in hydrophobicity. Hydrophobicity is eliminated by trypsin, with no effect on the zeta potential. In this case, autoagglutination disappears. Thus, negative surface charges and hydrophobic moieties of the surface protein could be two independent structural features modulating the autoagglutination of these bacteria.
These results show that Bifidobacterium strains of human origin have a wide range of surface properties. However, it is interesting that strains with such differences can colonize the intestinal tract. Moreover, one individual can harbor strains with different surface features (Table 1). Probably, this could produce complementary responses in the interactions with other intestinal bacteria and the gut wall.
It is important to note that all autoagglutinating strains belong to the species Bifidobacterium bifidum, but not all B. bifidum strains are autoagglutinating and cell adherent. The question of whether Bifidobacterium species other than B. bifidum have these properties is raised by this comparison.
In summary, the present results show that in most cases hemagglutination is related to the simultaneous presence of cell adherence and autoagglutination. Both of these properties are related to a high hydrophobic index, and the electrostatic (negative) potential has a minor influence.
The use and interrelationship between these properties could provide a tool for characterizing intestinal bacteria without sophisticated and time-consuming physical methods. The simple observation of liquid cultures should allow workers to select, in a quick orientative assay, strains with the ability to adhere to intestinal epithelial cells.
ACKNOWLEDGMENTS
The technical assistance of Lucía Brandi and Sergio de la Lama (Consejo de Investigaciones Científicas y Técnicas de la República Argentina) is gratefully acknowledged. G.L.D.A. is a member of the Research Career of the Comisión de Investigaciones Científicas de la Provincia de Buenos Aires. P.F.P. and E.A.D. are members of the Research Career of the Consejo de Investigaciones Científicas y Técnicas de la República Argentina.
This work was supported in part by funds from the Secretaría de Ciencia y Técnica (Universidad de Buenos Aires).
REFERENCES
- 1.Bernet M F, Brassart D, Neeser J R, Servin A L. Adhesion of human bifidobacterial strains to cultured human epithelial cells and inhibition of enteropathogen-cell interactions. Appl Environ Microbiol. 1993;59:4121–4128. doi: 10.1128/aem.59.12.4121-4128.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Busscher H J, Geertsema G I, van der Mei H C. On mechanisms of oral microbial adhesion. J Appl Bacteriol Symp Suppl. 1993;74:136S–146S. doi: 10.1111/j.1365-2672.1993.tb04350.x. [DOI] [PubMed] [Google Scholar]
- 3.Crow V L, Gopal P K, Wicken A J. Cell surface differences of lactococcal strains. Int Dairy J. 1995;5:45–68. [Google Scholar]
- 4.Fontaine I F, Aissi E A, Bouquelet S J L. In vitro binding of Bifidobacterium bifidum DSM 20082 to mucosal glycoproteins and hemagglutinating activity. Curr Microbiol. 1994;28:325–330. [Google Scholar]
- 5.Geertsema-Doornbusch G I, van der Mei H C, Busscher H J. Microbial cell surface hydrophobicity. The involvement of electrostatic interactions in microbial adhesion to hydrocarbons (MATH) J Microbiol Methods. 1993;18:61–68. [Google Scholar]
- 6.Gibson G R, Wang X. Regulatory effects of bifidobacteria on the growth of other colonic bacteria. J Appl Bacteriol. 1994;77:412–420. doi: 10.1111/j.1365-2672.1994.tb03443.x. [DOI] [PubMed] [Google Scholar]
- 7.Gilbert P, Evans D J, Brown M R W. Formation and dispersal of bacterial biofilms “in vivo” and “in situ.”. J Appl Bacteriol Symp Suppl. 1993;74:67S–78S. doi: 10.1111/j.1365-2672.1993.tb04343.x. [DOI] [PubMed] [Google Scholar]
- 8.Greene J D, Klaenhammer T R. Factors involved in adherence of lactobacilli to human Caco-2 cells. Appl Environ Microbiol. 1994;60:4487–4494. doi: 10.1128/aem.60.12.4487-4494.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kado-Oka Y, Fujiwara S, Hirota T. Effects of bifidobacteria cells on mitogenic response of splenocytes and several functions of phagocytes. Milchwissenschaft. 1991;46:626–630. [Google Scholar]
- 10.Modler H W, McKellar R C, Yaguchi M. Bifidobacteria and bifidogenic factors. Can Inst Food Sci Technol J. 1990;23:29–41. [Google Scholar]
- 11.Nakasone N, Iwanaga M. Cell-associated hemagglutinin of classical Vibrio cholerae O1 with reference to intestinal adhesion. FEMS Microbiol Lett. 1993;113:67–70. doi: 10.1111/j.1574-6968.1993.tb06489.x. [DOI] [PubMed] [Google Scholar]
- 12.Noda H, Akasaka N, Ohsugi M. Biotin production by bifidobacteria. J Nutr Sci Vitaminol. 1994;40:181–188. doi: 10.3177/jnsv.40.181. [DOI] [PubMed] [Google Scholar]
- 13.Ronner U, Husmark V, Henriksson A. Adhesion of Bacillus spores in relation to hydrophobicity. J Appl Bacteriol. 1990;69:550–556. doi: 10.1111/j.1365-2672.1990.tb01547.x. [DOI] [PubMed] [Google Scholar]
- 14.Scardovi V. Genus Bifidobacterium. In: Sneath P H A, Mair N S, Sharpe M E, Holt J G, editors. Bergey’s manual of systematic bacteriology. Vol. 2. Baltimore, Md: Williams & Wilkins; 1986. pp. 1418–1434. [Google Scholar]
- 15.Sengupta T K, Sengupta P K, Ghose A C. A 20-kDa pilus protein with hemagglutination and intestinal adherence properties expressed by a clinical isolate of non-O1 Vibrio cholerae. FEMS Microbiol Lett. 1993;112:237–242. doi: 10.1111/j.1574-6968.1993.tb06454.x. [DOI] [PubMed] [Google Scholar]
- 16.Wai S N, Takade A, Amako K. The hydrophobic surface protein layer of enteroaggregative Escherichia coli strains. FEMS Microbiol Lett. 1996;135:17–22. doi: 10.1111/j.1574-6968.1996.tb07960.x. [DOI] [PubMed] [Google Scholar]
- 17.Wilhelm H P, Lee D T, Rosenblatt J E. Bacterial interference by anaerobic species isolated from human feces. Eur J Clin Microbiol. 1987;6:266–270. doi: 10.1007/BF02017610. [DOI] [PubMed] [Google Scholar]
- 18.Yamada M, Saito T, Toba T, Kitazawa H, Vemura J, Itoh T. Hemagglutination activity of Lactobacillus acidophilus group lactic acid bacteria. Biosci Biotechnol Biochem. 1994;58:910–915. doi: 10.1271/bbb.58.910. [DOI] [PubMed] [Google Scholar]