Abstract
Breast cancer is a leading cause of death in women worldwide. Data suggests that hereditary factors only account for 5–10 % of breast cancer incidence, resulting in increased concern regarding the carcinogenicity involved with environmental and lifestyle-related factors. Among these, phthalates – ubiquitous endocrine disrupting chemicals founds in plastics, cosmetics, and food packaging – pose a growing concern. Human exposure to phthalates occurs through ingestion, inhalation, dermal contact, and critical windows such as intrauterine development. As an endocrine-responsive organ, the breast is particularly susceptible to disruption by these compounds. This review highlights emerging evidence linking phthalate exposure to the initiation, progression, and metastasis of breast cancer. This comprehensive overview of carcinogenesis-promoting mechanisms of phthalates, involving estrogen receptor signaling, oncogenic pathway activation, promotion of cancer stemness, and induction of therapy resistance, will provide crucial insights into phthalate-driven mechanisms in breast cancer that can inform future research directions, public health strategies, and regulatory efforts aimed at mitigating environmental cancer risks.
Keywords: breast cancer, endocrine-disrupting chemicals, phthalates, toxicology, environmental pollutants, carcinogenesis
1. Introduction
Breast cancer is a leading cause of mortality and morbidity among women worldwide (Sha et al., 2024). In the United States alone, the American Cancer Society projects approximately 316,950 new cases of invasive breast cancer in women in 2025, with an estimated 42,680 deaths (American Cancer Society, 2025). While mortality rates for female breast cancer in the U.S. peaked in 1989, decreasing by 42 % through 2021, the global death toll remained high, with 685,000 fatalities in 2020 (Siegel et al., 2024; Lei et al., 2021).
Observed declines in breast tumor-related deaths in the United States are largely attributed to earlier diagnoses through mammography screening, increased awareness, and advancements in treatment. Outside of screening, breast cancer diagnosis is generally prompted by symptoms, such as pain or a palpable mass. Screening of seemingly healthy women is associated with the detection of smaller tumors, lower likelihood of metastasis, and a reduced need for chemotherapy (McDonald et al., 2016). Apart from mammography, other modes of breast cancer screening recommended by the American Cancer Society include digital breast tomosynthesis, magnetic resonance imaging, and breast ultrasound based on risk of disease (DeSantis et al., 2019). Breast cancer is classified into three major subtypes based on molecular markers for estrogen receptors (ER), progesterone receptors (PR), and human epidermal growth factor receptor 2 (HER2/ERBB2): hormone receptor-positive (HR+)/HER2-negative (70 % of cases), HER2-positive (15–20 %), and triple-negative breast cancer (TNBC), which lacks all three markers (15 %) (Shien and Iwata, 2020) (Fig. 1). HR+ /HER2-cases are further divided into Luminal A and Luminal B subtypes, with the latter sometimes exhibiting HER2 expression. The HER2-enriched subtype refers to HR-negative, HER2-positive cases (Shien and Iwata, 2020). Data from the Surveillance, Epidemiology, and End Results (SEER) cancer registry was used to assess survival after breast cancer diagnosis in women from 2010 to 2013 and followed through December 2014. The best survival outcomes were observed among women with the HR+ /HER2− subtype, followed by HR+/HER+, HR−/HER2+, with the worst survival being the triple-negative subtype (Howlader et al., 2018).
Fig. 1.
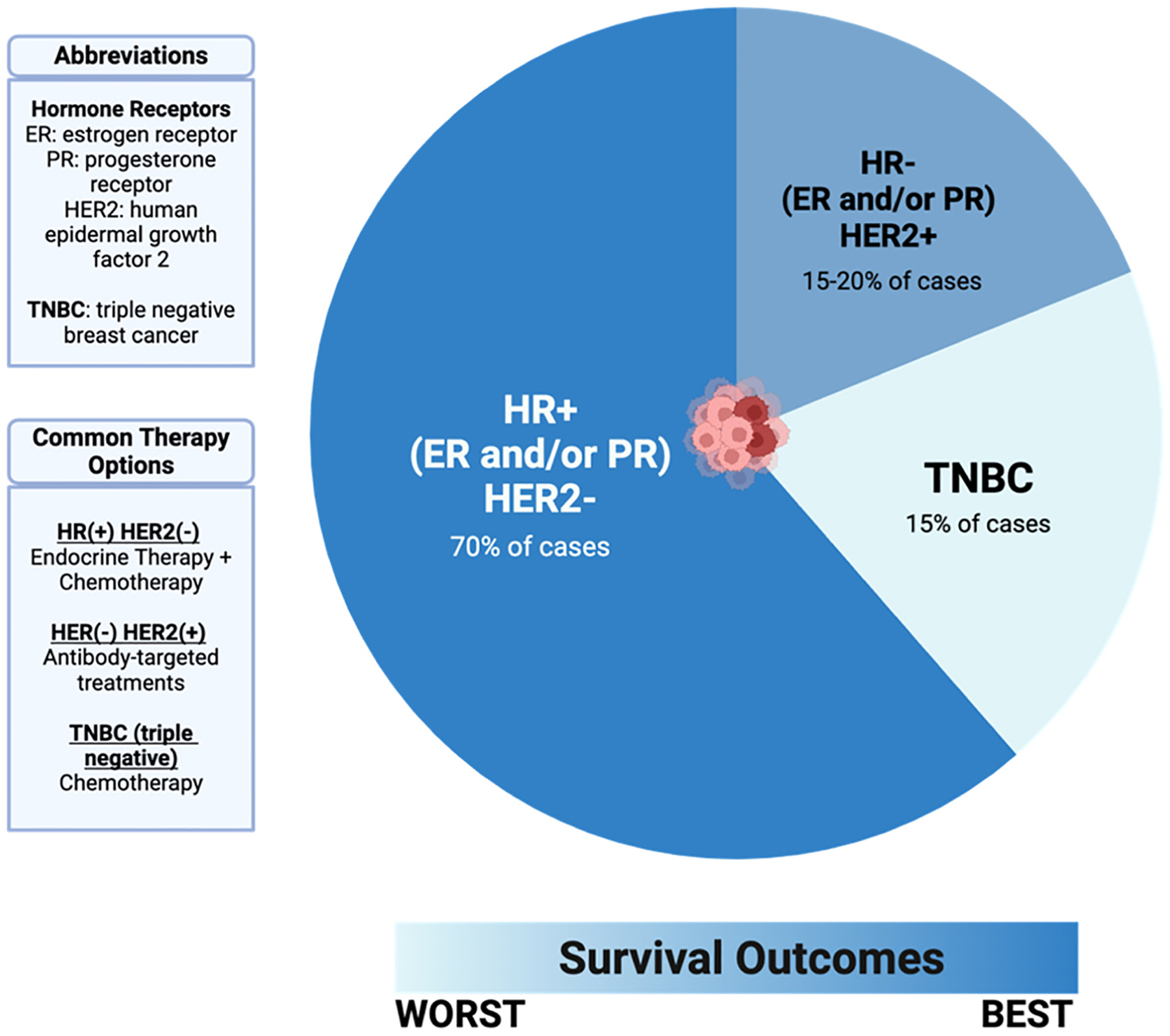
Breast cancers are categorized into 3 subtypes based on molecular profiles. These subtypes determine possible routes for therapeutic interventions and provide insights on prognosis and health outcomes.
The initiation of cancer is an enigmatic process that overlaps potent external influences as well as transformative biomechanisms. A cell must undergo several aberrant changes before becoming malignant, with genetic alterations being necessary but insufficient for the initiation of cancer (de Visser and Joyce, 2023). These alterations can encompass epigenetic, cellular, functional, and microenvironmental changes, including proto-oncogene activation, as well as the amplification, mutation, or loss of function of tumor suppressors (Mughees et al., 2022). Several other factors also impact one’s susceptibility to cancer, such as genetic predispositions, lifestyle and environmental exposures (Kentsis, 2020). Hereditary factors account for just 5–10 % of breast cancer incidence, suggesting that nongenetic factors are the main culprit for the increase in breast cancer cases over the last several decades. These may include reproductive factors such as early age at menarche, later age at menopause, nulliparity or late age at first birth (Guo et al., 2020).
2. Chemistry of phthalates
Endocrine-disrupting chemicals (EDCs) are substances that mimic, block, or interfere with any aspect of normal hormonal function, including the synthesis, release, and transport of natural hormones. Examples of commonly found EDCs are dioxins, polyfluoroalkyl substances (PFAS), polychlorinated biphenyls (PCBs), bisphenol A (BPA), and phthalates. These chemicals become widely disseminated throughout the environment in processes that involve soil, water, and air due to their varying resistance to environmental degradation (González, 2022). EDCs can indirectly impede proper endocrine systems, thereby imposing carcinogenic effects. Subsequent effects include, but are not limited to, chemoresistance, amplified cell proliferation, angiogenesis, increased expression of oncogenes, and alterations in apoptosis regulator genes (Mughees et al., 2022; Del Pup et al., 2016).
Phthalates, also known as phthalic esters, are pervasive substances with an annual global consumption exceeding three million metric tons. They are found in a wide variety of industrial products and consumer goods, including plastics, paints, children’s toys, pharmaceuticals, polyvinyl chloride (PVC) pipes, and food packaging (Holahan and Smith, 2015; National Institute of Environmental Health Sciences, 2025) (Fig. 2). Phthalates are commonly added to plastics as plasticizers. Low molecular weight phthalates such as dimethyl phthalate, diethyl phthalate, butyl benzyl phthalate (BBP), di-iso-butyl phthalate, and di-n-butyl phthalate (DBP) are often used in finishing materials like tiles, varnishes, sealants, welds, and coatings. Di-(2-ethylhexyl) phthalate (DEHP), di-iso-nonyl phthalate, and di-iso-decyl phthalate, which are high molecular weight phthalates, are more frequently used in building materials, toys, and food packaging (Zhang et al., 2021). Phthalate plasticizers are not chemically bound to the polymer backbones of plastics; therefore, they easily leach into the environment during plastic degradation, allowing them to migrate and evaporate into indoor air, food, and other materials. Phthalate compounds can make their way to the aqueous systems via wastewater treatment plants, sewage, urban land surfaces, agricultural runoff, and septic systems (Pironti et al., 2021). Hence, humans are exposed to contamination through ingestion, inhalation, and dermal contact throughout their lifetime, including during intrauterine development (Heudorf et al., 2007). Food is the major source of exposure to phthalates in humans as they are present in water, alcohol, oils, dairy products, meat, and edible plants (Giuliani et al., 2020).
Fig. 2.

Phthalates are ubiquitous chemicals found in everyday items. Their presence in personal care items, food packing, and medical devices create opportunities for daily exposure via several routes including oral ingestion, inhalation and more. Chronic phthalate exposure is linked to a variety of diseases, including breast cancer.
Phthalate pollution is a worldwide issue as phthalates are present in air, soil, and water in concentrations ranging from ng/L to μg/L (Giuliani et al., 2020; Kumari and Pulimi, 2023). Phthalates are listed on the ATSDR 2022 Substance Priority List. Specifically, DBP is number #59 on the list; DEHP, number #77; butyl methyl phthalate, number #98; and BBP, number #207. Concerns about the health risks of phthalate exposure arose around 1970, when a report was published examining the presence of DEHP in human blood stored in PVC bags (Jaeger and Rubin, 1972). Subsequently, the same authors published a report revealing the presence of DEHP in tissue samples of the lung, liver, and spleen from patients who received blood transfusions before death (Jaeger and Rubin, 1970). In 1974, a scientific status summary by the Institute of Food Technologists was communicated with the public to address concerns relating to the presence of phthalates in food. Consumers were assured that phthalates were not readily extracted from plastics by substances they came into contact with, and that no evidence linked phthalate exposure from food, beverages, or household items to toxicity in humans (Institute of Food Technologists’ Expert Panel on Safety and Nutrition and The Committee On Public Information, 1974). However, between the 1980s to early 2000s, the perspective surrounding the safety of phthalates shifted when they were listed as a possible carcinogen (International Agency for Research on Cancer, 2000). Around this time, data was produced suggesting the pathological effects of phthalates, such as subtle alterations in hepatic function, respiratory symptoms and disease, and testicular cellular damage (Strauss, 2004; Seth, 1982; Hoppin et al., 2004; Gangolli, 1982).
Due to the short half-life of phthalates of approximately 12 h, their bio-metabolism in the human body is relatively rapid. After uptake by the cell, phthalates are hydrolyzed and then conjugated to form the hydrophilic glucuronide conjugate, which is catalyzed by uridine 5’-diphosphoglucuronyl transferase (Wang and Qian, 2021; Frederiksen et al., 2007). Biomonitoring studies detect the presence of phthalates in 75–90 % of the general population, with evidence suggesting exposure may result in dysfunction of developmental, metabolic, endocrinologic, and immunologic processes (Ramadan et al., 2020; Mondal et al., 2022). A study with a cohort of 102 women detected the presence of the following compounds in all participants: DEHP, BBP, DBP, di-ethyl phthalate, bisphenol A, and bisphenol S. Results from this study also revealed a positive association between phthalate exposure and breast cancer incidence, showing significantly higher serum phthalate concentrations in women with breast cancer compared to those without (Segovia-Mendoza et al., 2022). In addition, the utilization of phthalates in personal care items and cosmetic products such as lacquers, perfumes, and lotions suggest that women are more susceptible to phthalate exposure than men, due to the marketing of these products often targeting women (Mughees et al., 2022). By crossing the blood-placental barrier, phthalates also pose as a risk to fetuses in utero by entering the circulation of the fetus. Studies have indicated prenatal phthalate exposure is correlated with negative fetal health outcomes, such as small gestational age, premature birth, and abnormal fetal growth (Jin et al., 2023).
3. Phthalates and the induction of cancer
Phthalates’ widespread use as plasticizers accounts for their presence in a diverse range of consumer products, including building materials, household furnishings, children’s toys, and automobiles. DEHP is one of the most common phthalate plasticizers, which can also be found in medical products. At its peak, global production of DEHP reached up to 3–4 million tons annually before starting to decline in recent years.
Women between the ages of 18 and 34 are likely to be heavy users of personal care and hair products, which increases their risk of exposure to endocrine disruptors. A study by Casiano et al. revealed six hair products commonly used by Black or African American women in the Greater New York Area all affected hormonal activity (Santaliz Casiano et al., 2022). As an endocrine-responsive organ, the breast is particularly vulnerable to disruption by products containing EDCs, such as phthalates (Darbre, 2021).
The hallmarks of cancer are a conceptual framework developed by Hanahan and Weinberg that provides a comprehensive perspective on the biochemical processes driving cancer development (Hanahan and Weinberg, 2000). EDCs have the capability to enable all ten of these cancer hallmarks in the context of breast cancer, including replicative immortality, sustained proliferation, and evasion of growth suppressors (Darbre, 2021). Yet, there are inconsistencies in evidence from epidemiological studies on the effect of phthalates on breast cancer incidence. The role of phthalates as potential endocrine disruptors is evident in their ability to induce estrogen receptor signaling in the context of breast cancer, but they also have demonstrated antiestrogenic effects (Ahern et al., 2019). A study examining the implications of urinary concentrations of nine phthalate metabolites found that exposure to diethyl phthalate, the parent compound of monoethyl phthalate (MEP), may be associated with increased risk of breast cancer. In contrast, evidence from this study also revealed an inverse association between exposure to the parent compounds of monobenzyl phthalate (MBzP) and mono(3-carboxypropyl) phthalate (MCPP) and breast cancer risk, suggesting that higher exposure was linked to a decrease likelihood of developing breast cancer (López-Carrillo et al., 2010).
Although results from epidemiological studies remain controversial, experimental evidence suggests that phthalates may induce breast carcinogenesis through activation of cell proliferation pathways, particularly the PI3K/AKT/mTOR pathway (Fig. 3). This highly regulated signaling mechanism is involved in cell growth, survival, and cell cycle progression, interacting with a variety of indispensable pathways that may become oncogenic if dysregulated (Glaviano et al., 2023; Yu et al., 2022). A study aiming to characterize the effect of low-dose exposures of BBP, DBP, and DEHP on MCF-10A breast tumorigenesis revealed that exposure of phthalates at both 10 nM and 100 nM resulted in significantly higher cell viability, lower apoptosis, and increased cell numbers in the S and G2/M phases compared to the control group. Results from this study further suggested that phthalates can induce cell cycle progression via upregulation of the PI3K/AKT/mTOR pathway (Chen et al., 2023). A gene-profiling analysis of fine needle aspirates from the breasts of healthy volunteers before and after a 28-day discontinuation of paraben- and phthalate-containing personal care products revealed a reversal of cancer-related phenotypes, including the PI3K-AKT/mTOR pathway, as well as autophagy and apoptotic signaling. These findings correlated with a significant reduction in urinary parabens and phthalate metabolites after the 28-day reduction period (Dairkee et al., 2023).
Fig. 3.
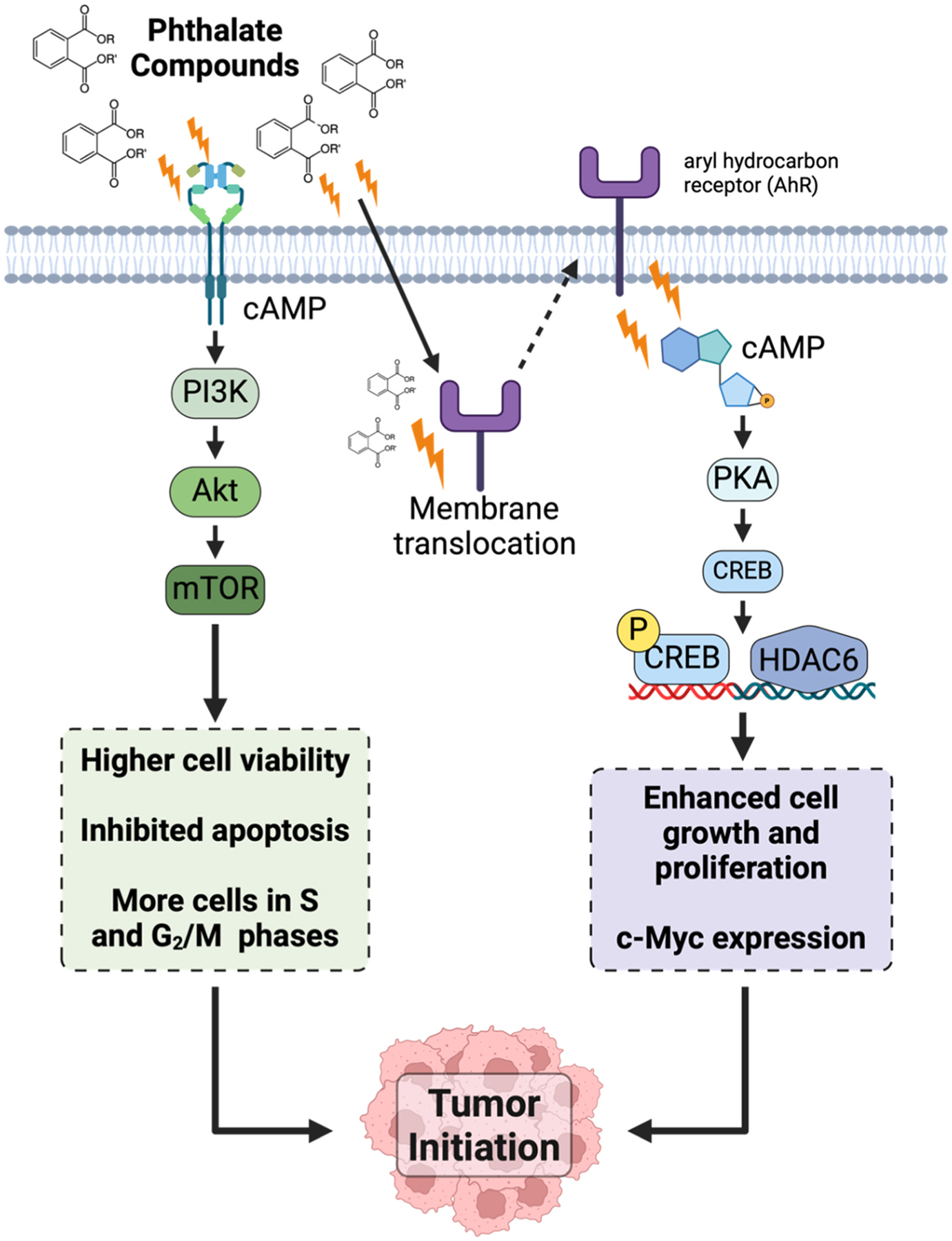
Phthalates can induce signaling pathways implicated in tumor initiation. The PI3K/Akt/mTOR and cAMP/PKA/CREB signaling pathways can create a highly favorable microenvironment and biological state for tumor formation, such as expression of oncogenes and apoptosis evasion.
Higher concentrations of phthalates also have marked effects on cell signaling pathways. ER-negative breast cancer cells, MDA-MB-231, exposed to a 1 mM combination of BBP and DBP revealed increased carcinogenicity due to phthalate exposure. More specifically, phthalate stimulation of the cell surface aryl hydrocarbon receptor (AhR) initiated a nongenomic cascade that led to cAMP/PKA activation, CREB1 activation, and transactivation of the HDAC6 promoter (Hsieh et al., 2012a). The cAMP/PKA/CREB signaling pathway plays several pivotal roles in tumor cells, particularly due to its involvement in cell signaling and its impact on tumor growth (Ahmed et al., 2022). Indeed, overexpression of the R subunit of PKA is associated with cell proliferation in normal breast and malignant transformation of breast epithelial cells (Zhang et al., 2020; Paul et al., 2020). Additionally, data suggested that HDAC6 activation is mandatory for phthalate-induced cell growth, motility, and invasiveness in MDA-MB-231 breast cancer cells by demonstrating the lack of these observed effects in HDAC6-depleted cells. Moreover, results indicated that the nuclear translocation of the B-catenin/LEF1/TCF4 transactivation complex, facilitated by HDAC6, promotes c-Myc expression. In breast cancer, Myc is overexpressed in about 30–50 % of high-grade tumors (Stine et al., 2015; Dang, 2012; Fallah et al., 2017). Consistent with the findings in vitro, elevated expression of HDAC6 and c-Myc was also exhibited in the tumor tissues of nude mice that were exposed to phthalates following the implantation of MDA-MB-231 cells. Mice exposed to phthalates also had significantly increased tumor growth compared to mice treated with the control vehicle (Hsieh et al., 2012a).
4. Phthalates and the progression of cancer
The pathological impact of phthalates does not cease at the initiation of the tumor via increased cell viability, proliferation, and evasion of apoptotic signaling. The presence of these compounds magnifies the ability of cancer-initiating cells to exploit otherwise normal physiological functions, such as hormone secretion.
In hormone-dependent, ER-positive breast tumor cells, estrogens play a crucial role in the modulation of cell growth and progression. Once estrogen (E2) binds to ERα, the receptor undergoes conformational activation, dimerizes, and translocates into the nucleus, where it interacts with transcriptional coactivators. The ligand-activated ERα then binds to estrogen-responsive elements, regulating the transcription of hundreds of target genes involved in cell growth, apoptosis, and differentiation. Once activated, ERα promotes the expression of potentially oncogenic factors, including MYC, BCL2, CXCL12, and cyclin D1 (Clusan et al., 2023). Increasing evidence from epidemiological, animal, and in vitro studies suggests an association between endogenous estrogens and breast carcinogenesis (Samavat and Kurzer, 2015). Xenoestrogenic effects of phthalates can impact the endocrine system by modulating ER signaling, through agonist and antagonist activity (Dairkee et al., 2023; Gachowska et al., 2024; Mukherjee Das et al., 2022). A study investigating the interactions between phthalates and 17β-estradiol found estrogenic activity of BBP, DEHP, and DBP, even at very low concentrations (Fig. 4). Results also demonstrated the induction of an additive proliferative effect through the PI3K/AKT signaling pathway and impeded apoptotic activation of MCF-7 cells in the presence of E2 (Chen et al., 2016). Another study using MCF-7 breast cancer cells revealed BBP exposure led to demethylation of estrogen receptor (ESR1) promoter-associated CpG islands, suggesting that modified ESR1 mRNA expression by BBP could be a result of irregular DNA methylation (Singh and Li, 2012).
Fig. 4.

Phthalates contribute to the progression of cancer through mimicking estrogens, inducing chemoresistance, promoting metabolic dysfunction, and enhancing stemness. Observations referenced in this figure were obtained from in vitro and in vivo studies.
Apart from endocrine disruption, the carcinogenicity of phthalates is also linked to their ability to confer drug resistance. An investigation using MCF-7 and MDA-MB-231 breast cancer cells demonstrated the antagonistic effect of DEHP on the chemotherapeutic drugs paclitaxel and doxorubicin. These results were observed when exposing the cells to 1 mM DEHP in combination with the anticancer drugs across a range of doses. Cell growth analyses exhibited the inhibition of the effects of paclitaxel and doxorubicin, resulting in increased tumor cell survival. Similar results were observed in vivo after 1 × 104 MDA-MB-231-GFP cells were implanted into the yolk sac of zebrafish embryos at 48 h post-fertilization. Whole-body fluorescence imaging provided evidence that DEHP suppressed the inhibitory effect of paclitaxel and doxorubicin on tumor cell growth (Hsieh et al., 2022). Additionally, the study revealed that DEHP increases doxorubicin metabolism and clearance in cell culture medium.
DEHP is capable of directly targeting AhR, thus impacting down-stream signaling in human breast cancer cell lines, including cytochrome P450 enzyme (CYP) activation. Data suggests that genetic variations in CYP2D6, CYP2C8, and CYP3A4 can promote drug metabolism and decrease the efficacy of anticancer therapeutics (Jin et al., 2005; Marcath et al., 2019; Alzahrani and Rajendran, 2020). RT-PCR analysis revealed that CYP2D6, CYP2C8, and CYP3A4 were induced by DEHP alone, AhR plasmid alone, and DEHP + AhR plasmid combined; however, AhR siRNA decreased the expression of the CYP1A2, CYP2CB, CYP3A4, and CYP2D6 in MCF-7 and MDA-MB-231 cells (Hsieh et al., 2022). Another study also demonstrated the inhibitory effect of phthalates on tamoxifen-induced apoptosis of MCF-7 breast cancer cells, seemingly due to an increase in intercellular Bcl-2 to Bax ratio (Kim et al., 2004).
There is a correlation between body mass index (BMI) and urinary phthalate metabolite levels, strongly suggesting that phthalates can impact metabolic function (Yaghjyan et al., 2015; Waits et al., 2020; Zhao et al., 2025). Metabolic dysfunction is linked to an increased risk of breast cancer and can profoundly influence tumor progression and treatment response (Karra et al., 2022; Dong et al., 2021). Malignant cells often undergo metabolic reprogramming, known as “the Warburg effect,” that allows the tumor cell to sustain extremely rapid growth which involves a metabolic shift from fully oxidizing glucose toward aerobic glycolysis, where glucose is primarily converted into lactate (Kocianova et al., 2022).
Breast cancer stem cells (BCSCs) play a crucial role in tumor progression. BCSCs are stem cells that can self-renew, self-repair, and differentiate multi-directionally. These phenotypic characteristics allow these cells to maintain and expand tumor populations and resist conventional therapies, thus promoting tumor recurrence, metastasis and therapeutic failure (Zhang et al., 2025a, 2025b). Analysis of MCF-7 and MDA-MB-231 breast cancer stem cells after exposure to BBP, DBP, and DEHP revealed enhanced tumorsphere formation ability and higher levels of cancer stem cell markers (CD133, CD44, ALDH1A1, OCT-4, Nanog). The promotion of this stemness is proposed to be mediated by the Sonic hedgehog (Shh) signaling pathway and ΔNp63α upregulation (Cao et al., 2023). The Sonic hedgehog pathway is a highly conserved process, modulating the differentiation of stem cells and sustaining their ability to self-renew. ΔNp63α is a common isoform of Tp63, a crucial member of the p53 family and a tumor-suppressing gene that regulates the growth of mammary gland epithelial cells by maintaining genomic stability (Park et al., 2016). Results showed that the increased expression of cancer stem cell markers and Shh pathway molecules was attenuated in the presence of ΔNp63α siRNA, further supporting the role of Shh in cancer progression.
5. Phthalates and the metastasis of cancer
As a breast tumor progresses through the stages of disease – intensified by the effects of phthalate exposure on hormone activity, drug resistance, metabolic dysfunction, and cancer cell stemness – the tumor and its surrounding microenvironment become more aggressive, ultimately leading to possibly fatal outcomes.
Metastasis, the deadliest manifestation of cancer, is a systemic process characterized as a sequence of biological events in which primary tumor cells acquire invasive properties, breach mucosal barriers, and spread through the bloodstream, lymphatic system, or directly infiltrate tissues. These disseminated cells then seed and colonize distant organs where they resume proliferation to form secondary tumor sites (Gerstberger et al., 2023). Breast cancer can metastasize to several organs, including the liver, skin, bone, lungs, and brain (Di Micco et al., 2019; Wrobel and Toborek, 2016). Although evidence suggests that fewer than 0.1 % of tumor cells successfully metastasize, most cancer-related deaths result from metastatic disease rather than primary tumors (Gerstberger et al., 2023; Fares et al., 2020). The dissemination of cancer cells occurs early in the metastatic cascade (Lambert et al., 2017), driven by epithelial-mesenchymal transition (EMT), a transformative process in which epithelial cells acquire mesenchymal traits (Bakir et al., 2020), thus enhancing their invasive and metastatic potential (Fig. 5). Initiation of this program facilitates the loss of cell-cell junctions, degradation of local basement membranes via elevated expression of various matrix-degrading enzymes, loss of apical-basal polarity, increased resistance to apoptosis, and increased ability in extracellular matrix production (Debnath et al., 2022; Zhang et al., 2017). EMT plasticity creates an epithelial-mesenchymal spectrum, allowing cells to exist in transitional states where they exhibit both epithelial and mesenchymal characteristics. This intermediate stage makes the cells more effective in circulation, colonization, and overall metastasis development (Fares et al., 2020).
Fig. 5.

Phthalates activate metastasis-promoting programs, such as epithelial-to-mesenchymal transition (EMT) and angiogenesis. These modifications allow tumor cells to become more motile and invasive, making them more efficient at establishing secondary tumor sites once they can colonize and become vascularized via angiogenesis.
In a study designed to investigate the EMT-inducing effects of BBP and DBP, R2d cells, a stem-cell derived human breast epithelial cell line, were treated with phthalates for 24, 48, or 72 h. After exposure to BBP or DBP (1 μmol/L) for 24 h, the R2d cells exhibited enhanced expression of mesenchymal markers vimentin and CD90 and decreased expression of epithelial markers cytokeratin 7 and E-cadherin. After 72 h of exposure, the cells also began to resemble mesenchymal-like morphological traits, such as spindle-like shape and scattered colony growth (Hsieh et al., 2012b).
BBP exposure is implicated in angiogenesis, a crucial component of the metastatic pathway that facilitates the recruitment of new blood vessels (Zetter, 1998). Newly formed vessels serve as the primary pathway for tumor cells to escape the primary tumor and enter the bloodstream through intravasation. Vascular density of tumors may serve as a prognostic marker for metastatic potential, as highly vascularized primary tumors exhibit a higher chance of metastasis than those that are poorly vascularized. The angiogenic process, which involves matrix degradation, endothelial cell proliferation, migration, sprouting, and mural cell recruitment, is dynamically balanced, dependent on the presence of pro-angiogenic or anti-angiogenic factors. A hypoxic tumor microenvironment can trigger the release of growth factors that promote the sprouting and migration of endothelial cells, resulting in the release of proteases that degrade the basal lamina of blood vessels (Yehya et al., 2018; Wicks and Semenza, 2022).
A cohort of mice intraperitoneally exposed to 500 mg/kg BBP every 2 days for 1 month demonstrated higher rates of hepatocellular carcinoma metastasis in the lungs, kidneys, and spleen than those in a control group (Tsai et al., 2014). An in vivo Matrigel plug angiogenesis assay model was utilized to determine the angiogenic effects of BBP exposure in mice. Huh7 cells mixed with Matrigel were injected into the flanks of nude mice and measured for hemoglobin levels after 3 weeks. The Matrigel plug treated with BBP had significantly higher levels of hemoglobin compared to the control group, indicative of increased angiogenesis (Tsai et al., 2014). Further analysis revealed BBP promoted angiogenesis via nongenomic AhR mechanisms. An increase in levels of vascular endothelial growth factor (VEGF), a promoter of angiogenesis (Zhang et al., 2012), was also observed, initiated by ERK1/2 phosphorylation. In fact, a dose-dependent relationship between phthalates and VEGF secretion has been demonstrated in breast cancer cell lines MCF-7 and MELN, which can result in increased angiogenesis as well as enhanced capillary permeability (Buteau-Lozano et al., 2008; Geindreau et al., 2021).
6. Conclusion and limitations
Phthalates are pervasive environmental toxicants that influence the initiation, progression and metastasis of breast cancer. Phthalates can increase activation of cell growth and proliferation programs such as the PI3K/AKT/mTOR and cAMP/PKA/CREB pathways, whose aberrant upregulation can create an ideal environment for cancer initiation. These compounds have demonstrated their ability to increase cell viability, decrease apoptotic signaling, and promote the expression of oncogenes such as C-Myc (Chen et al., 2023, 2016). As xenoestrogens, phthalates can interact with estrogen receptors in ER-positive breast cancers, subsequently yielding additive proliferative effects and dysregulated ER signaling (Ahern et al., 2019; Singh and Li, 2012). Data also suggests phthalates can contribute to the progression of cancer by enhancing chemoresistance, as shown by increased metabolism of chemotherapeutics in vitro and the upregulation of cytochrome P450 enzymes, which can decrease the effectiveness of anticancer treatments (Hsieh et al., 2022). The ability of phthalates to negatively impact metabolic function and promote breast cancer stemness also contributes to the carcinogenicity of these environmental pollutants (Yaghjyan et al., 2015; Karra et al., 2022; Cao et al., 2023). Metastasis, the most lethal component of cancer, is promoted by phthalates due to their angiogenic and EMT-inducing effects, increasing tumor vasculature and enhancing the ability of breast tumor cells to invade and colonize distant organs to form secondary tumor sites (Hsieh et al., 2012b; Tsai et al., 2014; Buteau-Lozano et al., 2008).
Despite growing evidence, studies on phthalate carcinogenicity face notable limitations. Namely, a frequent use of nonphysiological phthalate concentrations in experimental studies limits the inferences that can be made from these studies about health outcomes. Epidemiological data remain inconclusive, partly due to reliance on urinary metabolite measurements, which reflect only short-term exposure. Future longitudinal studies should acquire biological samples from large-scale populations in all stages of life to strengthen conclusions regarding causality, since humans are exposed to phthalates beginning in utero and throughout their lifetime. Additionally, phthalates are rarely encountered in isolation, and STRING network analysis suggest complex interactions among targets modulated by different phthalate mixtures (Chuang et al., 2024). Co-exposure to other endocrine-disrupting chemicals such as BPA and parabens may further affect the conclusions drawn. Thus, future studies need to thoroughly investigate the cumulative effect of combined toxicants and EDCs to draw biologically relevant conclusions that reflect accurate health outcomes. It is also imperative to expand the models that are used to investigate the effect of phthalates on human health to enhance scientific rigor. MDA-MB-231 and MCF-7 are the breast cancer cell lines frequently used in phthalate toxicology studies as well as animal models, subsequently disregarding the heterogeneity of breast tumors. However, advancements in in vitro systems may allow for more physiologically relevant insights through the utilization of three-dimensional breast tissue models such as spheroids, organoids, and microfluidic breast tumor-on-a-chip applications. In addition to enhancement of models, the evaluation of effect biomarkers can more precisely characterize the biological impact of phthalates and bridge the results from traditional assays to epidemiological data. The integration of probing for biomarkers related to hormonal activity, oxidative stress and epigenetic modifications in future investigations can offer valuable insight into the mechanistic effects of phthalate exposure, thus enhancing translational relevance of traditional in vivo and in vitro toxicological assays.
Beyond identifying risk and causality, recent studies have begun exploring strategies to mitigate the harmful effects of phthalate exposure. Melatonin, green tea polyphenols and their derived antioxidants, as well as Korean red ginseng were proposed to attenuate adverse phthalate-induced effects, via reduced oxidative stress, decreased lesion growth, and improved inflammatory signaling (Chuang et al., 2024; Liu et al., 2025; Mondal and Bandyopadhyay, 2024; Song et al., 2022). Comprehensive education on phthalates falls short when only health risks are investigated, thus it is critical to prioritize explorative investigations such as those that reveal preventative methods to better inform consumer choices that can have health impacts. Understanding phthalate-driven carcinogenicity, and how to mitigate it, is essential to inform public health policy, clinical guidance, and individual risk reduction methods.
Acknowledgments
The figures were drawn by BioRender (app.biorender.com).
Funding
This work was supported in part by a grant from the Howard Hughes Medical Institute GT17100 to the University of Miami through the Gilliam Fellows Program. In addition, support for this work was provided by the University of Miami Team Science Award, U-LINK Resilience Challenge grant, and funding from the National Institutes of Health ES036983.
Abbreviation:
- ER
estrogen receptor
- PR
progesterone receptor
- HER2/ERBB2
human epidermal growth factor receptor 2
- TNBC
triple negative breast cancer
- SEER
surveillance, epidemiology, and end results
- EDCs
endocrine disrupting chemicals
- PFAS
polyfluoroalkyl substances
- BPA
bisphenol A
- PCBs
polychlorinated biphenyls
- DEHP
di2-ethylhexyl phthalate
- ATSDR
agency for toxic substances and disease registry
- MEP
monoethyl phthalate
- MBzP
monobenzyl phthalate
- MCPP
mono3-carboxypropyl phthalate
- BBP
butyl benzyl phthalate
- DBP
di-butyl-phthalate
- AhR
aryl hydrocarbon receptor
- E2
estrogen
- ESR1
estrogen receptor 1
- CYP
cytochrome P450 enzyme
- ROS
reactive oxygen species
- BMI
body mass index
- Shh
sonic hedgehog
- EMT
epithelial-mesenchymal transition
- VEGF
vascular endothelial growth factor
Footnotes
CRediT authorship contribution statement
Sarah Adolphe: Writing – review & editing, Writing – original draft, Investigation. Hannah Shapiro: Writing – review & editing, Writing – original draft, Investigation. Makenna Parsell: Writing – review & editing, Writing – original draft, Investigation. Destiny Tiburcio: Writing – review & editing, Writing – original draft, Methodology, Investigation, Conceptualization. Michal Toborek: Writing – review & editing, Resources, Funding acquisition, Conceptualization. Sophia George: Writing – review & editing, Writing – original draft, Methodology. Oandy Naranjo: Writing – review & editing, Writing – original draft, Visualization, Investigation.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data Availability
Data will be made available on request.
References
- Ahern TP, et al. , 2019. Phthalate exposure and breast cancer incidence: a danish nationwide cohort study. J. Clin. Oncol 37 (21), 1800–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed MB, et al. , 2022. cAMP signaling in cancer: a PKA-CREB and EPAC-Centric approach. Cells 11 (13). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzahrani AM, Rajendran P, 2020. The multifarious link between cytochrome P450s and cancer. Oxid. Med Cell Longev 2020, 3028387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Cancer Society, Cancer Facts & Figures 2025. 2025. p. 3.
- Bakir B, et al. , 2020. EMT, MET, plasticity, and tumor metastasis. Trends Cell Biol 30 (10), 764–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buteau-Lozano H, et al. , 2008. Xenoestrogens modulate vascular endothelial growth factor secretion in breast cancer cells through an estrogen receptor-dependent mechanism. J. Endocrinol 196 (2), 399–412. [DOI] [PubMed] [Google Scholar]
- Cao WS, et al. , 2023. Low-dose phthalates promote breast cancer stem cell properties via the oncogene ΔNp63α and the sonic hedgehog pathway. Ecotoxicol. Environ. Saf 252, 114605. [DOI] [PubMed] [Google Scholar]
- Chen FP, Chien MH, Lee CH, 2023. The no-observed-adverse-effect level of phthalates promotes proliferation and cell cycle progression in normal human breast cells. Taiwan J. Obstet. Gynecol 62 (6), 874–883. [DOI] [PubMed] [Google Scholar]
- Chen F-P, Chien M-H, Chern IY-Y, 2016. Impact of low concentrations of phthalates on the effects of 17β-estradiol in MCF-7 breast cancer cells. Taiwan. J. Obstet. Gynecol 55 (6), 826–834. [DOI] [PubMed] [Google Scholar]
- Chuang Y-T, et al. , 2024. Natural products modulate phthalate-associated miRNAs and targets. Ecotoxicol. Environ. Saf 284, 117015. [DOI] [PubMed] [Google Scholar]
- Clusan L, et al. , 2023. A basic review on estrogen receptor signaling pathways in breast cancer. Int J. Mol. Sci 24 (7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dairkee SH, et al. , 2023. Reduction of daily-use parabens and phthalates reverses accumulation of cancer-associated phenotypes within disease-free breast tissue of study subjects. Chemosphere 322, 138014. [DOI] [PubMed] [Google Scholar]
- Dang CV, 2012. MYC on the path to cancer. Cell 149 (1), 22–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darbre PD, 2021. Chapter thirteen - endocrine disrupting chemicals and breast cancer cells. In: Vandenberg LN, Turgeon JL (Eds.), Advances in Pharmacology. Academic Press, pp. 485–520. [DOI] [PubMed] [Google Scholar]
- Debnath P, et al. , 2022. Epithelial-mesenchymal transition and its transcription factors. Biosci. Rep 42 (1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Pup L, et al. , 2016. Carcinogenetic mechanisms of endocrine disruptors in female cancers (Review). Oncol. Rep 36 (2), 603–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSantis CE, et al. , 2019. Breast cancer statistics, 2019. CA Cancer J. Clin 69 (6), 438–451. [DOI] [PubMed] [Google Scholar]
- Di Micco R, et al. , 2019. Rare sites of breast cancer metastasis: a review. Transl. Cancer Res 8 (5), S518–S552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong S, et al. , 2021. Metabolic syndrome and breast cancer: prevalence, treatment response, and prognosis. Front Oncol 11, 629666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallah Y, et al. , 2017. MYC-Driven pathways in breast cancer subtypes. Biomolecules 7 (3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fares J, et al. , 2020. Molecular principles of metastasis: a hallmark of cancer revisited. Signal Transduct. Target. Ther 5 (1), 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederiksen H, Skakkebaek NE, Andersson AM, 2007. Metabolism of phthalates in humans. Mol. Nutr. Food Res 51 (7), 899–911. [DOI] [PubMed] [Google Scholar]
- Gachowska M, et al. , 2024. The influence of environmental exposure to xenoestrogens on the risk of cancer development. Int. J. Mol. Sci 25 (22). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangolli SD, 1982. Testicular effects of phthalate esters. Environ. Health Perspect 45, 77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geindreau M, Ghiringhelli F, Bruchard M, 2021. Vascular endothelial growth factor, a key modulator of the Anti-Tumor immune response. Int. J. Mol. Sci 22 (9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstberger S, Jiang Q, Ganesh K, 2023. Metastasis. Cell 186 (8), 1564–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliani A, et al. , 2020. Critical review on the presence of phthalates in food and evidence of their biological impact. Int. J. Environ. Res. Public Health 17 (16). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaviano A, et al. , 2023. PI3K/AKT/mTOR signaling transduction pathway and targeted therapies in cancer. Mol. Cancer 22 (1), 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González MC, 2022. Prenatal exposure to persistent organic pollutants as a risk factor of offspring metabolic syndrome development during childhood. Rev. Environ. Health 37 (1), 61–70. [DOI] [PubMed] [Google Scholar]
- Guo JY, et al. , 2020. The undervalued effects of polychlorinated biphenyl exposure on breast cancer. Clin. Breast Cancer 20 (1), 12–18. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA, 2000. The hallmarks of cancer. Cell 100 (1), 57–70. [DOI] [PubMed] [Google Scholar]
- Heudorf U, Mersch-Sundermann V, Angerer J, 2007. Phthalates: toxicology and exposure. Int. J. Hyg. Environ. Health 210 (5), 623–634. [DOI] [PubMed] [Google Scholar]
- Holahan MR, Smith CA, 2015. Phthalates and neurotoxic effects on hippocampal network plasticity. Neurotoxicology 48, 21–34. [DOI] [PubMed] [Google Scholar]
- Hoppin JA, Ulmer R, London SJ, 2004. Phthalate exposure and pulmonary function. Environ. Health Perspect 112 (5), 571–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlader N, et al. , 2018. Differences in breast cancer survival by molecular subtypes in the United States. Cancer Epidemiol. Biomark. Prev 27 (6), 619–626. [DOI] [PubMed] [Google Scholar]
- Hsieh TH, et al. , 2012a. Phthalates induce proliferation and invasiveness of estrogen receptor-negative breast cancer through the AhR/HDAC6/c-Myc signaling pathway. Faseb J 26 (2), 778–787. [DOI] [PubMed] [Google Scholar]
- Hsieh T-H, et al. , 2012b. Phthalates stimulate the epithelial to mesenchymal TransitionThrough an HDAC6-Dependent mechanism in human BreastEpithelial stem cells. Toxicol. Sci 128 (2), 365–376. [DOI] [PubMed] [Google Scholar]
- Hsieh T-H, et al. , 2022. DEHP mediates drug resistance by directly targeting AhR in human breast cancer. Biomed. Pharmacother 145, 112400. [DOI] [PubMed] [Google Scholar]
- Institute of Food Technologists’ Expert Panel on Safety and Nutrition & The Committee On Public Information, Phthalates in food, Nutr. Rev 1974. p. 126–128.
- International Agency for Research on Cancer, 2000. di(2-ethylhexyl) phthalate. IARC Monogr. Eval. Carcinog. Risks Hum 77, 41–148. [PMC free article] [PubMed] [Google Scholar]
- Jaeger RJ, Rubin RJ, 1970. Plasticizers from plastic devices extraction, metabolism, and accumulation by biological systems. Science 170 (3956), 460–462. [DOI] [PubMed] [Google Scholar]
- Jaeger RJ, Rubin RJ, 1972. Migration of a phthalate ester plasticizer from polyvinyl chloride blood bags into stored human blood and its localization in human tissues. N. Engl. J. Med 287 (22), 1114–1118. [DOI] [PubMed] [Google Scholar]
- Jin S, et al. , 2023. Associations between prenatal exposure to phthalates and birth weight: a meta-analysis study. Ecotoxicol. Environ. Saf 262, 115207. [DOI] [PubMed] [Google Scholar]
- Jin Y, et al. , 2005. CYP2D6 genotype, antidepressant use, and tamoxifen metabolism during adjuvant breast cancer treatment. J. Natl. Cancer Inst 97 (1), 30–39. [DOI] [PubMed] [Google Scholar]
- Karra P, et al. , 2022. Metabolic dysfunction and obesity-related cancer: beyond obesity and metabolic syndrome. Obesity (Silver Spring) 30 (7), 1323–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kentsis A, 2020. Why do young people get cancer? Pedia Blood Cancer 67 (7), e28335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim IY, Han SY, Moon A, 2004. Phthalates inhibit tamoxifen-induced apoptosis in MCF-7 human breast cancer cells. J. Toxicol. Environ. Health A 67 (23–24), 2025–2035. [DOI] [PubMed] [Google Scholar]
- Kocianova E, Piatrikova V, Golias T, 2022. Revisiting the warburg effect with focus on lactate. Cancers (Basel) 14 (24). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari M, Pulimi M, 2023. Phthalate esters: occurrence, toxicity, bioremediation, and advanced oxidation processes. Water Sci. Technol 87 (9), 2090–2115. [DOI] [PubMed] [Google Scholar]
- Lambert AW, Pattabiraman DR, Weinberg RA, 2017. Emerging biological principles of metastasis. Cell 168 (4), 670–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei S, et al. , 2021. Global patterns of breast cancer incidence and mortality: a population-based cancer registry data analysis from 2000 to 2020. Cancer Commun. (Lond.) 41 (11), 1183–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JC, et al. , 2025. Proteomic analysis reveals the alleviation of follicular development defects in offspring mice under DEHP exposure by melatonin. BMC Biol 23 (1), 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Carrillo L, et al. , 2010. Exposure to phthalates and breast cancer risk in Northern Mexico. Environ. Health Perspect 118 (4), 539–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcath LA, et al. , 2019. Patients carrying CYP2C8*3 have shorter systemic paclitaxel exposure. Pharmacogenomics 20 (2), 95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald ES, et al. , 2016. Clinical diagnosis and management of breast cancer. J. Nucl. Med 57 (Suppl. 1), 9s–16s. [DOI] [PubMed] [Google Scholar]
- Mondal S, Bandyopadhyay A, 2024. Antioxidants in mitigating phthalate-induced Male reproductive toxicity: a comprehensive review. Chemosphere 364, 143297. [DOI] [PubMed] [Google Scholar]
- Mondal T, et al. , 2022. Phthalates - a family of plasticizers, their health risks, phytotoxic effects, and microbial bioaugmentation approaches. Environ. Res 214 (Pt 3), 114059. [DOI] [PubMed] [Google Scholar]
- Mughees M, Chugh H, Wajid S, 2022. Mechanism of phthalate esters in the progression and development of breast cancer. Drug Chem. Toxicol 45 (3), 1021–1025. [DOI] [PubMed] [Google Scholar]
- Mukherjee Das A, et al. , 2022. Urinary concentration of endocrine-disrupting phthalates and breast cancer risk in Indian women: a case-control study with a focus on mutations in phthalate-responsive genes. Cancer Epidemiol 79, 102188. [DOI] [PubMed] [Google Scholar]
- National Institute of Environmental Health Sciences. Endocrine disruptors. [cited 2025 Jan 23]; Available from: 〈https://www.niehs.nih.gov/health/topics/agents/endocrine〉.
- Park JH, et al. , 2016. p53 as guardian of the mitochondrial genome. FEBS Lett 590 (7), 924–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul MR, et al. , 2020. Genomic landscape of metastatic breast cancer identifies preferentially dysregulated pathways and targets. J. Clin. Invest 130 (8), 4252–4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pironti C, et al. , 2021. Endocrine-Disrupting compounds: an overview on their occurrence in the aquatic environment and human exposure. Water 13 (10), 1347. [Google Scholar]
- Ramadan M, Cooper B, Posnack NG, 2020. Bisphenols and phthalates: plastic chemical exposures can contribute to adverse cardiovascular health outcomes. Birth Defects Res 112 (17), 1362–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samavat H, Kurzer MS, 2015. Estrogen metabolism and breast cancer. Cancer Lett 356 (2 Pt A), 231–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santaliz Casiano A, et al. , 2022. Endocrine-Disrupting chemicals and breast cancer: disparities in exposure and importance of research inclusivity. Endocrinology 163 (5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segovia-Mendoza M, et al. , 2022. Association of serum levels of plasticizers compounds, phthalates and bisphenols, in patients and survivors of breast cancer: a real connection? Int J. Environ. Res. Public Health 19 (13). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth PK, 1982. Hepatic effects of phthalate esters. Environ. Health Perspect 45, 27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha R, et al. , 2024. Global burden of breast cancer and attributable risk factors in 204 countries and territories, from 1990 to 2021: results from the global burden of disease study 2021. Biomark. Res 12 (1), 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shien T, Iwata H, 2020. Adjuvant and neoadjuvant therapy for breast cancer. Jpn. J. Clin. Oncol 50 (3), 225–229. [DOI] [PubMed] [Google Scholar]
- Siegel RL, Giaquinto AN, Jemal A, 2024. Cancer statistics, 2024. CA Cancer J. Clin 74 (1), 12–49. [DOI] [PubMed] [Google Scholar]
- Singh S, Li SS, 2012. Epigenetic effects of environmental chemicals bisphenol a and phthalates. Int. J. Mol. Sci 13 (8), 10143–10153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H, et al. , 2022. Korean red ginseng attenuates Di-(2-ethylhexyl) phthalate-induced inflammatory response in endometrial cancer cells and an endometriosis mouse model. J. Ginseng Res 46 (4), 592–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stine ZE, et al. , 2015. MYC, metabolism, and cancer. Cancer Discov 5 (10), 1024–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss AA, 2004. Di(2-ethylhexyl)phthalate (DEHP). J. Pedia Pharm. Ther 9 (2), 89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai CF, et al. , 2014. Benzyl butyl phthalate induces migration, invasion, and angiogenesis of Huh7 hepatocellular carcinoma cells through nongenomic AhR/G-protein signaling. BMC Cancer 14, 556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Visser KE, Joyce JA, 2023. The evolving tumor microenvironment: from cancer initiation to metastatic outgrowth. Cancer Cell 41 (3), 374–403. [DOI] [PubMed] [Google Scholar]
- Waits A, et al. , 2020. Urinary phthalate metabolites are associated with biomarkers of DNA damage and lipid peroxidation in pregnant women – tainan birth cohort study (TBCS). Environ. Res 188, 109863. [DOI] [PubMed] [Google Scholar]
- Wang Y, Qian H, 2021. Phthalates and their impacts on human health. Healthcare (Basel) 9 (5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicks EE, Semenza GL, 2022. Hypoxia-inducible factors: cancer progression and clinical translation. J. Clin. Invest 132 (11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrobel JK, Toborek M, 2016. Blood-brain barrier remodeling during brain metastasis formation. Mol. Med 22, 32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaghjyan L, et al. , 2015. Associations of urinary phthalates with body mass index, waist circumference and serum lipids among females: national health and nutrition examination survey 1999–2004. Int. J. Obes. (Lond.) 39 (6), 994–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehya AHS, et al. , 2018. Angiogenesis: managing the culprits behind tumorigenesis and metastasis. Medicina (Kaunas) 54 (1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Wei J, Liu P, 2022. Attacking the PI3K/Akt/mTOR signaling pathway for targeted therapeutic treatment in human cancer. Semin. Cancer Biol 85, 69–94. [DOI] [PubMed] [Google Scholar]
- Zetter BR, 1998. Angiogenesis and tumor metastasis. Annu Rev. Med 49, 407–424. [DOI] [PubMed] [Google Scholar]
- Zhang C, et al. , 2025b. The role of the mTOR pathway in breast cancer stem cells (BCSCs): mechanisms and therapeutic potentials. Stem Cell Res. Ther 16 (1), 156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Lowery FJ, Yu D, 2017. Intracarotid cancer cell injection to produce mouse models of brain metastasis. J. Vis. Exp (120). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, et al. , 2020. Complex roles of cAMP-PKA-CREB signaling in cancer. Exp. Hematol. Oncol 9 (1), 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, et al. , 2012. VEGF is essential for the growth and migration of human hepatocellular carcinoma cells. Mol. Biol. Rep 39 (5), 5085–5093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y-J, et al. , 2021. Phthalate metabolites: characterization, toxicities, global distribution, and exposure assessment. Environ. Pollut 291, 118106. [DOI] [PubMed] [Google Scholar]
- Zhang Z, et al. , 2025a. Cancer stem cell specificity as new targets in breast tumor treatment. Oncol. Res 33 (4), 811–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M, Ye M, Zhao Y, 2025. Causal link between dietary antioxidant vitamins intake, oxidative stress injury biomarkers and colorectal cancer: a mendelian randomization study. Medicine (Baltimore) 104 (7), e41531. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.


