Abstract
Reduced, transition metal cations commonly enhance oxidative damage to cells caused by hydroperoxides formed as a result of oxygen metabolism or added externally. As expected, the cations Fe2+ and Cu+ enhanced killing of Streptococcus mutans GS-5 by hydroperoxides. However, unexpectedly, they also induced lethal damage under fully anaerobic conditions in a glove box with no exposure to O2 or hydroperoxides from initial treatment with the cations. Sensitivities to anaerobic killing by Fe2+ varied among the organisms tested. The oral streptococci Streptococcus gordonii ATCC 10558, Streptococcus rattus FA-1, and Streptococcus sanguis NCTC 10904 were approximately as sensitive as S. mutans GS-5. Enterococcus hirae ATCC 9790, Actinomyces viscosus OMZ105E, and Actinomyces naeslundii WVU45 had intermediate sensitivity, while Lactobacillus casei ATCC 4646 and Escherichia coli B were insensitive. Killing of S. mutans GS-5 in response to millimolar levels of added Fe2+ occurred over a wide range of temperatures and pH. The organism was able to take up ferrous iron, but ferric reductase activity could not be detected. Chelators, uric acid, and thiocyanate were not effective inhibitors of the lethal damage. Sulfhydryl compounds, ferricyanide, and ferrocyanide were protective if added prior to Fe2+ exposure. Fe2+, but not Fe3+, acted to reduce the acid tolerance of glycolysis by intact cells of S. mutans. The reduction in acid tolerance appeared to be related directly to Fe2+ inhibition of F-ATPase, which could be assayed with permeabilized cells, isolated membranes, or F1 enzyme separated from membranes. Cu+ and Cu2+ also inhibited F-ATPase and sensitized glycolysis by intact cells to acid. All of these damaging actions occurred anaerobically and thus did not appear to involve reactive oxygen species.
Oxidative damage involving reduced, transition metal cations is well-known in a variety of biological systems. The generally accepted mechanism for damage involves so-called Fenton reactions, in which the reduced cations react with an oxidant to produce hydroxyl radical, OH·, the agent considered generally to cause the major damage to nucleic acids, proteins, or lipids. However, other views of the specifics of damage are developing, including ones in which oxidants such as superoxide radical act to leach iron from storage proteins. The leached iron then catalyzes damage to DNA or other biopolymers (11). If the oxidant produced by cells or added from the outside is H2O2, then the products of the Fenton reaction involving Fe2+ are Fe3+, hydroxyl anion, and hydroxyl radical. If the oxidant is an organic peroxide, then the products include Fe3+ and an organic radical. If the oxidant is HOCl, then the products are Fe3+, hydroxyl radical, and Cl− (5).
Multiple experimental systems to produce oxidative damage have been developed. The simplest systems include a reduced, transition metal cation, say, Fe2+ or Cu+, and oxygen. More-elaborate systems may include NAD(P)H oxidase-NAD(P)H-O2-Fe3+, xanthine-xanthine oxidase-O2-Fe3+, or other combinations. In many systems, metabolizing organisms may act as catalysts for their own destruction by reducing O2 to produce intermediates involved in Fenton reactions, such as H2O2, superoxide radical (O2·−), or hydroperoxyl radical (HO2·). Many organisms also produce ferric reductases (4, 23), so Fe3+ added to the systems is reduced by the organisms to Fe2+, which is then a source of reducing equivalents for Fenton reactions.
Although all the systems described above are very much dependent on O2 or hydroperoxide, there are a few reports in the literature of damage by transition metal cations apparently in the absence of O2 or hydroperoxide. For example, Sagripanti et al. (20) found that Fe3+ or Cu2+ alone at concentrations of about 16 to 18 mM could cause inactivation of a variety of viruses, although their actions were greatly enhanced by addition of H2O2. Jain et al. (10) found that O2 was not required for degradation of isolated DNA by glutathione plus Cu2+, possibly because of formation of glutathione radicals, which then damaged the DNA.
In this paper, we present a primary description of damage, including lethal damage, to oral streptococci induced by Fe2+ and Cu+ involving mechanisms which appear to be independent of oxygen because the damage can occur even under anaerobic conditions.
MATERIALS AND METHODS
Bacteria.
Streptococcus mutans GS-5, Streptococcus gordonii ATCC 10558, Streptococcus rattus FA-1, and Streptococcus sanguis NCTC10904 are maintained routinely in our laboratory with weekly subculture on tryptic soy agar (Difco Laboratories, Detroit, Mich.) plates and long-term storage at −70°C in 50% glycerol solution. The organisms were routinely grown in static culture at 37°C in tryptone-yeast extract medium (1). Other organisms used that are maintained routinely in the laboratory include Actinomyces viscosus OMZ105E, Enterococcus hirae ATCC 9790, Escherichia coli B, and Lactobacillus casei ATCC 4646. Actinomyces naeslundii WVU45 was obtained from E. Morou-Bermudez of the Department of Dental Research, University of Rochester. It was maintained by anaerobic growth on tryptic soy agar plates and was grown for experiments in static culture in tryptone-yeast extract broth.
Assays of lethality.
Freshly harvested cells were suspended routinely in 1% (wt/vol) Difco peptone solution to yield suspensions with approximately 109 CFU/ml. A sample was taken for a zero time count, and then the various chemicals to be tested were added to the suspension. Generally, chloride or sulfate salts were used, but the anionic counterion did not significantly affect the results. At intervals, samples were taken and immediately diluted in 1% Difco peptone broth. Samples (0.1 ml) from 1:10 dilution series in peptone broth were spread over the surfaces of tryptic soy agar plates, and the plates were incubated appropriately to allow colony formation and subsequent counting. For a few experiments, cells were suspended in a solution containing 50 mM KCl and 1 mM MgCl2. The extent of killing of the cells was reduced by about 50% compared with that of cells in peptone; nevertheless, killing of more than 99% of the population could be achieved after exposure to 10 mM Fe2+.
For experiments in which all manipulations were carried out anaerobically, a Coy (Grass Lake, Mich.) glove box chamber was used. It was gassed with 5% CO2, 10% H2, and 85% N2. In a series of experiments, it was found that the lethal effect of Fe2+ for S. mutans GS-5 was the same when the experiment was carried out totally anaerobically or when static suspensions were exposed to the cation with subsequent growth of plated samples aerobically. However, if the suspensions were stirred aerobically after exposure to Fe2+, the cells were much more resistant to the lethal action of Fe2+. In fact, in the extreme, cells suspended in 1% Difco peptone which were treated with 10 mM Fe2+ and vigorously aerated on a shaker at 37°C showed essentially no killing, while cells in the same experiment incubated statically or anaerobically showed about a 4-log reduction in viable count.
Fe3+ reductase activity.
Ferric reductase activities were assayed with whole cells, permeabilized cells (1), isolated membranes (22), and cytoplasmic extracts of lysed spheroplasts according to the procedures described by Mazoy and Lemos (15).
Glycolysis and pH drop assays.
Cells for pH drop assays were harvested by centrifugation, washed once with water, and resuspended in dense suspension (2 mg [dry weight] of cells per ml) in a solution containing 50 mM KCl and 1 mM MgCl2. The suspension was titrated with KOH to about 7.2. Then glucose was added to yield an initial concentration of 55.6 mM. The resulting fall in pH was monitored with a glass electrode. Glucose utilization was assessed by use of glucose oxidase kits from Sigma Chemical Co. (St. Louis, Mo.). Assays of the phosphotransferase system were carried out as described previously (2).
ATPase assays.
Permeabilization of cells, membrane isolation, and extraction of F1-ATPase were carried out as described previously (22), as were assessments of ATPase activity in terms of phosphate release from ATP.
Permeability measurements.
The space, or dense-suspension, technique was used to assess the permeability of S. mutans cells to Fe2+ (13). Centrifuged cell pellets were mixed with equal volumes of Fe2+ solution, incubated at 25°C for 45 min, and recentrifuged. Uptake values and R values were calculated from knowledge of the original and final Fe2+ concentrations, the relative volumes of cells and suspending medium, and the volume of interstitial fluid. The interstitial space in cell pellets was assessed by use of high-molecular-weight dextrans (Dextran T-2000; Pharmacia Corp., Piscataway, N.J.). Iron was assayed by the method of Horak et al. (9), which involves formation of ferrozine-ferrous complexes that absorb light of 562-nm wavelength.
RESULTS
Killing of S. mutans GS-5 by hydroperoxides and iron or copper cations.
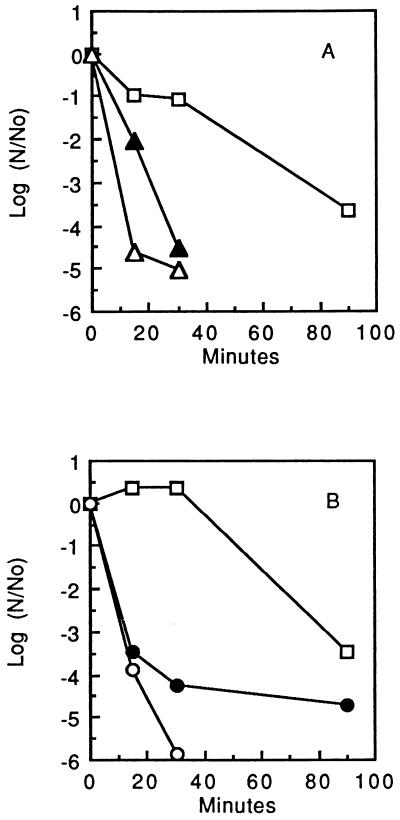
The data presented in Fig. 1 show that S. mutans GS-5 cells in dense suspension were killed by 0.1% hydrogen peroxide (32.6 mM) with a time for killing of 90% of the initial population (D value) of about 25 min and that killing could be markedly enhanced by addition of transition metal ions. The reduced cations Fe2+ and Cu+ were more effective, at a 10 mM level, than the oxidized cations Fe3+ and Cu2+. In fact, with Fe2+ added (Fig. 1A), the initial D value was reduced to less than 3 min, and with Cu+ added (Fig. 1B), the value was about 3.8 min. This type of enhancement of hydroperoxide killing is found commonly and is thought to be due to the reduced, transition metal cations acting to catalyze formation of hydroxyl radicals from hydroperoxides via Fenton reactions.
FIG. 1.
Transition metal cation enhancement of hydroperoxide killing of S. mutans GS-5. (A) Effects of 10 mM Fe2+ (▵) or Fe3+ (▴) on killing by 0.1% H2O2 (□). (B) Effects of 10 mM Cu+ (○) or Cu2+ (•) on killing by 0.1% H2O2 (□).
Similar enhancement by Fe2+ and Cu+ of killing of S. mutans GS-5 cells by the organic hydroperoxide tertiary butyl hydroperoxide at a concentration of 7.2 mM was found (data not shown).
In subsequent experiments, we found that Fe2+ alone, but not Fe3+, in the absence of any hydroperoxide was lethal for S. mutans GS-5 cells with approximately the same kinetics and to the same extent as shown in Fig. 1A for Fe2+ combined with 0.1% H2O2. These results were somewhat unexpected but still were interpretable in terms of knowledge that the organism has active oxygen metabolism, mainly involving NADH oxidases, one of which produces H2O2 (8). Thus, the organism in suspensions in contact with air could have produced sufficient H2O2 through endogenous metabolism of O2 diffusing into the suspension to allow for Fe2+-H2O2 killing. However, a completely unexpected finding (Fig. 2A) was that the cells were killed when the entire experiment was carried out in an anaerobic glove box at 37°C. At no time from first exposure to Fe2+ to growth of colonies were the cells exposed to air or oxygen. The extent of killing varied somewhat from experiment to experiment. In 59 experiments carried out over a 9-month period, the average final log (N/N0) was −4.6 with a standard deviation of ±0.99, where N/N0 is the ratio of the final plate count to the initial plate count. The important point is that nearly all of the cells in populations exposed to Fe2+ or Cu+ were killed, so that generally fewer than 1 cell in 1,000 survived. Under anaerobic conditions, Fe3+ was essentially ineffective for killing. Addition of a reducing agent, such as phenazine methosulfate, was not found to be effective for enhancing killing, although Evans et al. (7) had found that phenazine methosulfate could enhance iron uptake by S. mutans (sobrinus) OMZ176, which is thought to involve only the reduced cation.
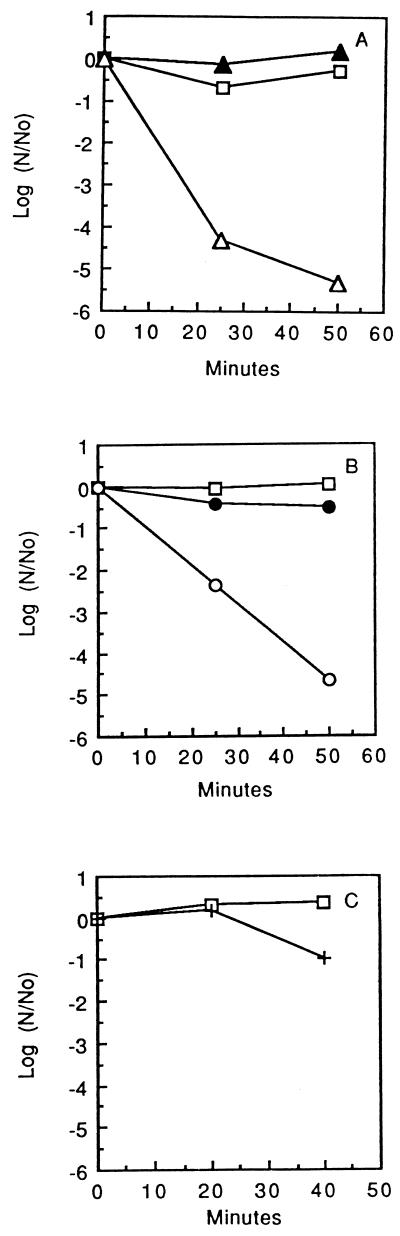
FIG. 2.
Anaerobic killing of S. mutans GS-5 by iron, copper, and cobalt cations. Data are shown for control cells (□) (A to C) and cells exposed to 5 mM Fe2+ (▵) or Fe3+ (▴) (A), 5 mM Cu+ (○) or Cu2+ (•) (B), and 5 mM Co2+ (+) (C).
Cu+, but not Cu2+, proved to be nearly as effective as Fe2+ for anaerobic killing of S. mutans GS-5 (Fig. 2B), but reduced cobalt cations, at a level of 5 mM, were much less effective, although some killing was evident (Fig. 2C). However, in most experiments, killing was delayed and then resulted in at most 90% reduction in viable counts of populations. Mg2+ or Ca2+ at a concentration of 20 mM had no damaging effect on the bacteria. The rates of killing by Fe2+ or Cu+ alone under anaerobic conditions were approximately the same as those for killing by combinations of the ions with 0.1% H2O2 or 7.2 mM tertiary butyl hydroperoxide. In addition, the extent of killing by the transition metal ions alone was generally approximately the same as that with combinations of 0.1% H2O2 and Fe2+ or Cu+, as indicated by the data of Fig. 1 and 2.
Dose-response data for killing of S. mutans GS-5 by Fe2+ are presented in Fig. 3. Extensive killing occurred in response to addition of Fe2+ to a final millimolar concentration. The D value for cells exposed to 2.0 mM Fe2+ was about 15 min, and this value fell to less than 5 min for cells exposed to 10 mM Fe2+. The results of repeated experiments to determine the optimal concentration of Fe2+ or Cu+ for killing of S. mutans GS-5 showed that 5 to 10 mM levels were close to saturating for the lethal effect, and higher concentrations were not more effective, possibly because of precipitation. In addition, when cells were first treated with 10 mM Fe2+ and incubated for up to 45 min at 25°C before addition of a second dose of Fe2+ to raise the total concentration to 20 mM, there was no additional killing detected, at least not within the errors of plate counting. Again, it appeared that 5 to 10 mM levels of Fe2+ or Cu+ were saturating in terms of the killing response under the experimental conditions.
FIG. 3.
Anaerobic killing of cells of S. mutans GS-5 mediated by Fe2+ at 0 (□), 0.25 (▴), 0.50 (▪), 1.0 (▵), and 2.0 (○) mM (A) or 0 (□), 2.5 (×), 5.0 (+), and 10.0 (•) mM (B).
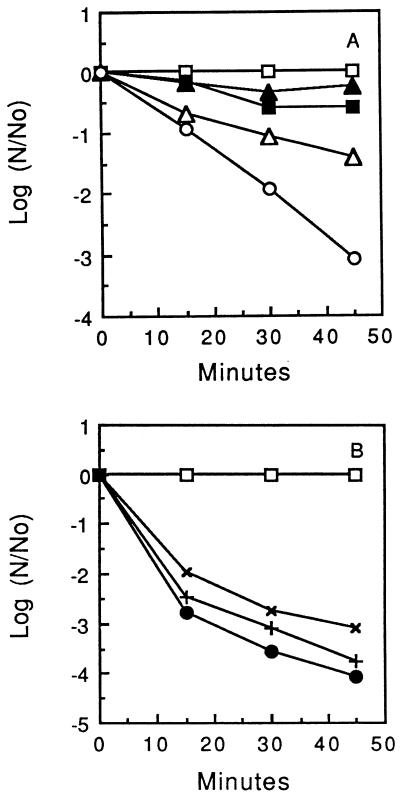
Fe2+ killing of other bacteria.
Anaerobic killing by Fe2+ could be demonstrated for the other oral streptococci tested (S. gordonii ATCC 10558, S. rattus FA-1, and S. sanguis NCTC 10904) (Fig. 4A to C) but not for L. casei ATCC 4646 or E. coli B (data not shown because control and treated suspensions all maintained essentially the same counts). Thus, anaerobic killing by Fe2+ did not seem to be universal among bacteria but was common for oral streptococci. E. hirae ATCC 9790 was intermediate in its sensitivity to Fe2+, and exposure to 10 mM Fe2+ resulted in only about 90% killing of the organism over a period of an hour. A. viscosus OMZ105E was somewhat more sensitive (Fig. 4D), but again, the maximum killing was generally only about 99% of the population. Catalase-negative A. naeslundii WVU45 was only slightly more sensitive than catalase-positive A. viscosus (Fig. 4D).
FIG. 4.
Iron-induced killing of various plaque bacteria. (A to C) S. gordonii, S. rattus FA-1, and S. sanguis NCTC10904, respectively (□, control cells; ▵ and ▴, cells exposed to 10 mM Fe2+ or Fe3+, respectively). (D) A. viscosus OMZ105E (□, control cells; ▵ and +, cells exposed to 10 or 20 mM Fe2+, respectively) and A. naeslundii WVU45 (▪, cells exposed to 10 mM Fe2+).
Factors affecting ferrous-ion killing of S. mutans GS-5.
As shown by the data presented in Table 1, optimal pH values for killing of S. mutans GS-5 by 10 mM Fe2+ were between 7 and 8. However, the range of pH over which killing occurred was broad, from about 4 to 9.
TABLE 1.
pH profile for anaerobic killing of S. mutans GS-5 by 10 mM Fe2+
| pH | −log10 (N/N0)a
|
||
|---|---|---|---|
| 15 min | 30 min | 45 min | |
| 9 | 2.43 ± 0.84 (3) | 2.71 ± 1.0 (3) | 2.66 ± 0.77 (3) |
| 8 | 3.56 ± 0.031 (2) | 4.32 ± 0.76 (2) | 4.13 ± 0.35 (2) |
| 7 | 3.02 ± 0.29 (2) | 3.61 ± 0.78 (2) | 4.33 ± 0.89 (2) |
| 6 | 2.26 ± 0.51 (2) | 3.00 ± 0.11 (2) | 3.45 ± 0.04 (2) |
| 5 | 2.10 ± 0.25 (3) | 2.75 ± 0.17 (3) | 2.85 ± 0.16 (3) |
| 4 | 1.39 ± 0.60 (3) | 1.53 ± 0.39 (4) | 1.69 ± 0.19 (4) |
Minus log10 of the ratio of the plate count after the indicated exposure times to the initial plate count. Values are averages ± standard deviations, with the number of trials given in parentheses.
The data presented in Table 2 show that killing occurred over a broad range of temperatures, from 4 to 48°C. Killing was detected even when the exposure to 10 mM Fe2+ was at 4°C. Thus, it appeared that metabolism by the cells during initial exposure to Fe2+ was not required for killing. However, since this assay of damage involves growth of the cells at 37°C to form colonies, Fe2+ may be bound at a low temperature and then cause damage only when the cells are warmed. The temperature range for killing is broader than the growth range for the organism. The minimal and maximal growth temperatures are about 30 and 48°C (12).
TABLE 2.
Temperature effects on anaerobic killing of S. mutans GS-5 by 10 mM Fe2+
| Temp (°C) | −log10 (N/N0)a
|
||
|---|---|---|---|
| 15 min | 30 min | 45 min | |
| 4 | 2.14 ± 0.08 (5) | 2.37 ± 0.29 (5) | 2.48 ± 0.44 (5) |
| 15 | 2.49 ± 0.58 (4) | 2.91 ± 0.71 (4) | 3.05 ± 0.68 (4) |
| 20 | 2.58 ± 0.60 (4) | 3.31 ± 0.43 (4) | 3.41 ± 0.74 (4) |
| 37 | 3.68 ± 0.25 (4) | 3.84 ± 0.36 (4) | 3.98 ± 0.95 (4) |
| 48 | 3.23 ± 0.22 (2) | 3.60 ± 0.18 (2) | 3.63 ± 0.31 (2) |
Minus log10 of the ratio of the plate count after the indicated exposures to the initial plate count. Values are averages ± standard deviations, with the number of trials given in parentheses.
Attempts to reverse killing by treating cells initially exposed to Fe2+ at 4°C (or at 37°C) with chelators were not very successful, even with use of chelators such as 10 to 80 mM deferoxamine, dipyridine, or EDTA. Also, extensive washing of the cells with water or peptone solution did not restore viability. Conversely, the use of various chelators also did not enhance killing, probably because the Fe2+ form of iron was sufficiently water soluble under the experimental conditions, while the less soluble Fe3+ was not effective even in a chelate complex for this organism which appears not to have highly active ferric reductases. Sulfhydryl compounds such as dithiothreitol, cysteine, and methionine were only partially protective. For example, in typical experiments, at a concentration as high as 100 mM, methionine increased the final average (n = 2) log (N/N0) from approximately −5.1 to −4.2 (increase in percent survivors from 0.0008 to 0.0063%). Dithiothreitol was more protective, and the average percent survivors was increased by 10 mM dithiothreitol to approximately 1%. Histidine or tryptophan had almost no protective effect. Ferricyanide and ferrocyanide were highly protective against killing and at concentrations as low as 1 mM could totally protect cells of S. mutans GS-5 against killing by 10 mM Fe2+. However, ferricyanide and ferrocyanide were not effective in reversing the effects of prior exposure to Fe2+. The major antioxidant in saliva is uric acid (17), but even at 10 mM, it did not protect against Fe2+ killing. Moreover, thiocyanate, which is also in saliva, had no protective effect, even at a concentration of 10 mM.
Aeration of cells of S. mutans GS-5 in flasks on a shaker incubator during exposure to Fe2+ protected them from killing, presumably because of oxidation of Fe2+ to Fe3+ (6). Cells grown in shaker culture, rather than static culture, were not found to have enhanced resistance. Moreover, growth of the bacteria in cultures to which H2O2 was added repeatedly during the growth phase to yield a concentration of 0.005 or 0.010% did not increase resistance to anaerobic Fe2+. Cells from the mid-exponential phase of growth in static or shaken cultures appeared to be slightly more sensitive to anaerobic Fe2+ killing than cells from late-exponential-phase or early-stationary-phase cultures, but the difference was marginal.
Permeability of cells of S. mutans GS-5 to Fe2+.
As shown by the data presented in Table 3, cells of S. mutans GS-5 took up Fe2+ present in suspensions at initial concentrations in the lethal range. The pattern of uptake suggests that binding is an important component of uptake at lower Fe2+ levels (R values of >1). However, at higher concentrations (10 mM in this case), it seems that Fe2+ uptake involves mainly nonconcentrative movement into the cell water (R = 0.86). Overall, the data suggest that cells of S. mutans GS-5 are permeable to Fe2+.
TABLE 3.
Uptake of Fe2+ by S. mutans GS-5 cells in dense suspensions
| Initial Fe2+ concn (mM) | Uptake (μmol/g [dry wt] of cells)a | R valueb |
|---|---|---|
| 1.0 | 2.4 ± 0.2 (4) | 2.64 |
| 2.5 | 5.1 ± 1.3 (2) | 2.39 |
| 5.0 | 9.6 ± 1.0 (3) | 1.42 |
| 10.0 | 16.5 ± 2.3 (3) | 0.86 |
Values are averages ± standard deviations, with the number of trials given in parentheses.
R is the corrected space value. R values greater than 1.0 indicate concentrative uptake, while values less than 1 indicate that only part of the cell volume is penetrated by the solute.
Ferric reductase activity.
Attempts to detect ferric reductase activity in S. mutans GS-5 with intact cells, permeabilized cells, membranes, or cytoplasmic extracts indicated that activity was minimal, basically at the limit of detection. The major reductant tested was NADH in the presence of Mg2+ and flavin adenine dinucleotide, but other reductants, such as NADPH, also were ineffective.
Inhibition of glycolysis by Fe2+ and Cu+.
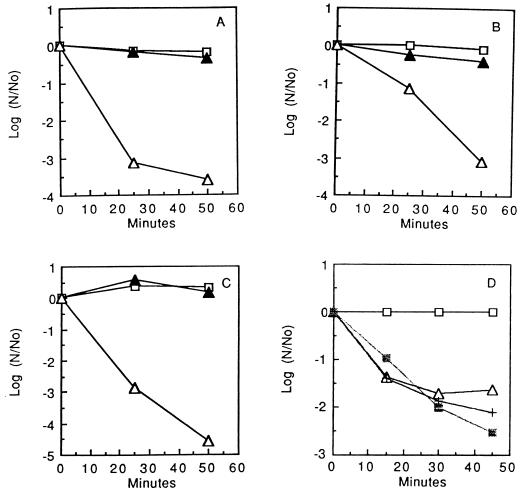
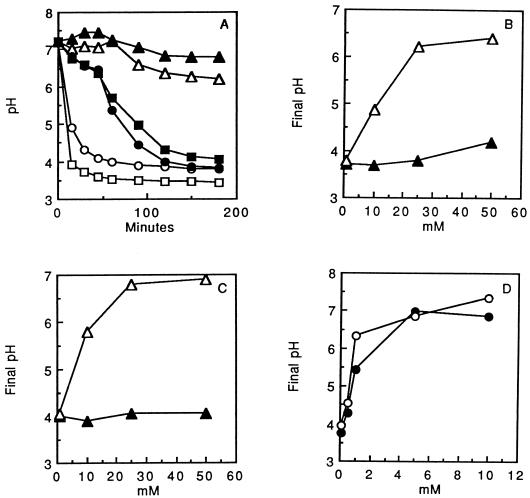
Fe2+, but not Fe3+, was found to reduce the acid tolerance of S. mutans GS-5 cells in standard pH drop assays (Fig. 5A to C). In the suspensions used for these experiments, the extent of pH drop reflects the acid tolerance of the organism. Glycolytic acid tolerance in oral streptococci depends on the capacities of F-ATPases to move protons out of the cytoplasm, and the final pH values in glycolyzing suspensions with excess glucose indicate minimal environmental values at which the F-ATPases can operate to maintain a sufficient ΔpH across the cell membrane to allow glycolysis (22). Acidification only reversibly inhibited glycolysis in control suspensions (3). Examples of pH drop curves are presented in Fig. 5A. The plots in Fig. 5B and C show final pH values as a function of the concentrations of Fe2+ or Fe3+ added to the suspensions. The data indicate almost no effect of Fe3+ on acid tolerance but major reductions in acid tolerance due to Fe2+. Fe2+ was somewhat more potent when the experiments were carried out with suspensions in the anaerobic glove box (Fig. 5C) rather than in air (Fig. 5B). Moreover, cells treated with Fe2+ were irreversibly impaired, and extensive washing did not restore glycolytic capacity, even though cells from control suspensions readily produced acid from glucose after being washed and resuspended in KCl-MgCl2 solution.
FIG. 5.
Reduced pH drop in suspensions of S. mutans GS-5 cells in response to added Fe2+ under aerobic or anaerobic conditions. Data are shown for control cells incubated aerobically (□) or anaerobically (•), cells exposed to 10 mM Fe3+ incubated aerobically (○) or anaerobically (▪), and cells exposed to 10 mM Fe2+ incubated aerobically (▵) or anaerobically (▴) (A); for cells exposed aerobically to Fe3+ (▴) or Fe2+ (▵) (B); for cells exposed anaerobically to Fe3+ (▴) or Fe2+ (▵) (C); and for cells exposed anaerobically to Cu2+ (•) or Cu+ (○) (D).
Copper acted also to decrease acid tolerance (Fig. 5D), but even for anaerobic suspensions, Cu2+ was nearly as potent as Cu+. Again, the inhibition was not reversible.
Results of experiments to assess inhibition of glycolysis by Fe2+ at pH 6.5 in terms of changes in the rate of disappearance of glucose from suspensions assayed by means of the glucose oxidase reaction indicated an average control value for glucose utilization of 0.82 mmol/h/g (dry weight) of cells, compared with values of 0.89 and 0.42 for cells exposed to 20 mM Fe3+ or Fe2+, respectively.
The results of assays of the phosphotransferase system with permeabilized cells (2) indicated essentially no inhibition over a range of Fe2+ concentrations from 0 to 10 mM.
Effects on F-ATPase.
Fe2+ was found to be an effective inhibitor of the F-ATPase of S. mutans GS-5 in permeabilized cells. For example, activities (means ± standard deviations) were reduced from 59.4 ± 8.20 (n = 4) μmol of Pi released/min/g (dry weight) of cells to values of 28.0 ± 6.58 (n = 4), 19.95 ± 6.27 (n = 4), and 5.53 ± 1.04 (n = 3) in the presence of 2.5, 5.0, or 10.0 mM added Fe2+, respectively. Fe3+ served mainly to slightly stimulate ATPase activity (data not shown). Fe2+ inhibition of F-ATPase activity appeared to be irreversible in that extensive washing of permeabilized cells with solutions containing 10 mM Mg2+ or treatment with chelators did not restore activity. Fe2+ also inhibited F-ATPase activities of isolated membranes and F1 complexes dissociated from the membrane. For example, the activity of isolated membranes (mean ± standard deviation) was reduced from 0.469 ± 0.032 (n = 3) μmol of Pi released/min/mg of membrane protein to 0.031 ± 0.008 (n = 3) in the presence of 5 mM added Fe2+. The activity of F1 complexes (mean ± standard deviation) was reduced from 2.43 ± 0.22 (n = 4) μmol of Pi released/min/mg of protein to 0.24 ± 0.07 (n = 2) in the presence of 5 mM added Fe2+.
Cu+ also was inhibitory for the F-ATPase, with a 50% inhibitory concentration about equal to that of Fe2+. However, Cu2+ had approximately the same inhibitory potency as Cu+. Thus, the effect on F-ATPases appeared to be related to reduction of acid tolerance of glycolysis by intact cells but not to lethality.
DISCUSSION
We present here a description of what appears to be a phenomenon not previously described, anaerobic killing of bacterial cells induced by the reduced, transition metal cations Fe2+ and Cu+. There is at least some precedent in the literature in the report of Sagripanti et al. (20) that viruses can be inactivated by iron or copper cations in the absence of hydroperoxides. The phenomenon we describe has unique characteristics, especially the effectiveness of only the reduced cations for damage. The detailed nature of the damage caused by Fe2+ and Cu+ remains to be determined. Presumably, damage involves formation of organic radicals, such as glutathione radicals, with subsequent electron transfer to or from various biopolymers. In our initial search for enzyme targets, we found also that Fe2+ and Cu+ could irreversibly inhibit the F-ATPase of S. mutans GS-5 and, as a consequence, reduce the capacity of the organism to carry out glycolysis in acid environments. Previously, Senior et al. (21) had found that Fe2+ was bound by beef heart F1 enzyme with high avidity, and there was some 40% loss of activity when 4 mol of Fe was bound per mol of F1 at about 20 μM Fe. Inhibition of F-ATPase could be partly responsible for lethal damage. However, it seems that other enzymes or structures must be the prime targets for lethality, since Cu+ and Cu2+ both inhibited F-ATPase, while only Cu+ was effective for anaerobic killing.
The type of killing described here seems to be limited in terms of types of susceptible bacteria. The oral streptococci were generally susceptible, but lactic acid bacteria are not all susceptible, as shown by the high level of resistance of L. casei and the moderate susceptibility of E. hirae. Both catalase-positive and catalase-negative oral actinomycetes were susceptible but not as sensitive as the oral streptococci. E. coli was insensitive. The role of iron in the physiology of oral streptococci has remained somewhat unclear. The organisms do produce a superoxide dismutase that is active with iron as metal. However, the enzyme is active also with Mn, and at least for S. sobrinus OMZ176, iron is not required for anaerobic growth (14). It can substitute partly for the Mn required for aerobic growth. Evans et al. (7) concluded that only the ferrous form is transported across the cell membrane by S. sobrinus OMZ176, and the organism did not appear to produce iron siderophores. Iron was taken up mainly under anaerobic conditions. Overall, it appears that oral streptococci seem not well adapted to manage iron. However, they are not unique in this regard, and other lactic acid bacteria do not produce hemes and do not have major needs for iron.
It appears from our work and that of others that iron may be useful as a plaque control agent. In fact, there is already evidence in the literature that iron and copper are anticaries agents. Previously, Oppermann and Rölla (18) found that FeCl3 can reduce acid production by plaque in vivo. Our findings indicate that Fe3+ is actually slightly stimulatory for glycolysis at near-neutral pH. However, it is probable that polymicrobic plaque in vivo may bring about reduction of Fe3+ to Fe2+, which may then be inhibitory. Iron in the diet has been correlated with a low incidence of caries, and recently, Rosalen et al. (19) and Miguel et al. (16) have shown that iron cocrystalized with sugar can reduce caries in rats. Copper had a similar effect, and the effects of Cu or Fe were found to be separate from any anticaries effects of fluoride. The work described here provides a basis for consideration of antimicrobial actions in attempts to interpret the known anticaries effects of Fe and Cu.
ACKNOWLEDGMENTS
This study was supported by grants R01DE06127 and P01DE11549 from the U.S. National Institute of Dental Research.
We thank Anne Clancy for help with the experiments involving hydroperoxide killing.
REFERENCES
- 1.Belli W A, Marquis R E. Adaptation of Streptococcus mutans and Enterococcus hirae to acid stress in continuous culture. Appl Environ Microbiol. 1991;57:1134–1138. doi: 10.1128/aem.57.4.1134-1138.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belli W A, Marquis R E. Catabolite modification of acid tolerance of Streptococcus mutans GS-5. Oral Microbiol Immunol. 1994;9:29–34. doi: 10.1111/j.1399-302x.1994.tb00211.x. [DOI] [PubMed] [Google Scholar]
- 3.Belli W A, Buckley D H, Marquis R E. Weak acid effects and fluoride inhibition of glycolysis by Streptococcus mutans GS-5. Can J Microbiol. 1995;41:785–791. doi: 10.1139/m95-108. [DOI] [PubMed] [Google Scholar]
- 4.Deneer H G, Healey V, Boychuk I. Reduction of exogenous ferric iron by a surface-associated ferric reductase of Listeria spp. Microbiology. 1995;141:1985–1991. doi: 10.1099/13500872-141-8-1985. [DOI] [PubMed] [Google Scholar]
- 5.Dukan S, Touati D. Hypochlorous acid stress in Escherichia coli: resistance, DNA damage, and comparison with hydrogen peroxide stress. J Bacteriol. 1996;178:6145–6150. doi: 10.1128/jb.178.21.6145-6150.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ehrlich H L. Geomicrobiology. 3rd ed. New York, N.Y: Marcel Dekker, Inc.; 1995. [Google Scholar]
- 7.Evans S L, Arceneaux J E L, Byers B R, Martin M E, Aranha H. Ferrous iron transport in Streptococcus mutans. J Bacteriol. 1986;168:1096–1099. doi: 10.1128/jb.168.3.1096-1099.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Higuchi M, Shimada M, Yamamoto Y, Hayashi T, Koga T, Kamio Y. Identification of two distinct NADH oxidases corresponding to H2O2-forming oxidase and H2O-forming oxidase induced in Streptococcus mutans. J Gen Microbiol. 1993;139:2343–2351. doi: 10.1099/00221287-139-10-2343. [DOI] [PubMed] [Google Scholar]
- 9.Horak E, Hohnadel D C, Sunderman F W., Jr Modified method for analysis of serum iron. Ann Clin Lab Sci. 1975;5:303–307. [PubMed] [Google Scholar]
- 10.Jain A, Alvi N K, Parish J H, Hadi S M. Oxygen is not required for degradation of DNA by glutathione and Cu(II) Mutat Res. 1996;357:83–88. doi: 10.1016/0027-5107(96)00085-1. [DOI] [PubMed] [Google Scholar]
- 11.Keyer K, Imlay J A. Superoxide accelerates DNA damage by elevating free-iron levels. Proc Natl Acad Sci USA. 1996;93:13635–13640. doi: 10.1073/pnas.93.24.13635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma Y, Marquis R E. Thermophysiology of Streptococcus mutans and related lactic-acid bacteria. Antonie Leeuwenhoek. 1997;72:91–100. doi: 10.1023/a:1000290426248. [DOI] [PubMed] [Google Scholar]
- 13.Marquis R E. Permeability and transport. In: Gerhardt P, Murray R G E, Wood W A, Krieg N R, editors. Methods for general and molecular bacteriology. Washington, D.C: American Society for Microbiology; 1994. pp. 587–599. [Google Scholar]
- 14.Martin M E, Strachan R C, Aranha H, Evans S L, Salin M L, Welch B, Arceneaux J E L, Byers B R. Oxygen toxicity in Streptococcus mutans: manganese, iron, and superoxide dismutase. J Bacteriol. 1984;159:745–749. doi: 10.1128/jb.159.2.745-749.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mazoy R, Lemos M L. Ferric-reductase activities in whole cells and cell fractions of Vibrio (Listonella) anguillarum. Microbiology. 1996;142:3187–3193. [Google Scholar]
- 16.Miguel J C, Bowen W H, Pearson S K. Effects of iron salts in sucrose on dental caries and plaque in rats. Arch Oral Biol. 1997;42:377–383. doi: 10.1016/s0003-9969(97)00018-6. [DOI] [PubMed] [Google Scholar]
- 17.Moore S, Calder K A C, Miller N J, Rice-Evans C A. Antioxidant activity of saliva and periodontal disease. Free Rad Res. 1994;21:417–425. doi: 10.3109/10715769409056594. [DOI] [PubMed] [Google Scholar]
- 18.Oppermann R V, Rölla G. Effect of some polyvalent cations on the acidogenicity of dental plaque in vivo. Caries Res. 1980;14:422–427. doi: 10.1159/000260485. [DOI] [PubMed] [Google Scholar]
- 19.Rosalen P L, Pearson S K, Bowen W H. Effects of copper, iron and fluoride co-crystalized with sugar on caries development and acid formation in desalivated rats. Arch Oral Biol. 1996;41:1003–1010. doi: 10.1016/s0003-9969(96)00082-9. [DOI] [PubMed] [Google Scholar]
- 20.Sagripanti J-L, Routson L B, Lytle C D. Virus inactivation by copper or iron ions alone and in the presence of peroxide. Appl Environ Microbiol. 1993;59:4374–4376. doi: 10.1128/aem.59.12.4374-4376.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Senior A E, Richardson L V, Baker K, Wise J G. Tight divalent cation-binding sites of soluble adenosine triphosphatase (F1) from beef heart mitochondria and Escherichia coli. J Biol Chem. 1980;255:7211–7217. [PubMed] [Google Scholar]
- 22.Sturr M G, Marquis R E. Comparative acid tolerances and inhibitor sensitivities of isolated F-ATPases of oral lactic acid bacteria. Appl Environ Microbiol. 1992;58:2287–2291. doi: 10.1128/aem.58.7.2287-2291.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wakatsuki T. Metal oxidoreduction by microbial cells. J Ind Microbiol. 1995;14:169–177. doi: 10.1007/BF01569900. [DOI] [PubMed] [Google Scholar]