Abstract
We assayed the tolerance to solvents of three toluene-degrading Pseudomonas putida strains and Pseudomonas mendocina KR1 in liquid and soil systems. P. putida DOT-T1 tolerated concentrations of heptane, propylbenzene, octanol, and toluene of at least 10% (vol/vol), while P. putida F1 and EEZ15 grew well in the presence of 1% (vol/vol) propylbenzene or 10% (vol/vol) heptane, but not in the presence of similar concentrations of octanol or toluene. P. mendocina KR1 grew only in the presence of heptane. All three P. putida strains were able to become established in a fluvisol soil from the Granada, Spain, area, whereas P. mendocina KR1 did not survive in this soil. The tolerance to organic solvents of all three P. putida strains was therefore assayed in soil. The addition to soil of 10% (vol/wt) heptane or 10% (vol/wt) propylbenzene did not affect the survival of the three P. putida strains. However, the addition of 10% (vol/wt) toluene led to an immediate decrease of several log units in the number of CFU per gram of soil for all of the strains, although P. putida F1 and DOT-T1 subsequently recovered. This recovery was influenced by the humidity of the soil and the incubation temperature. P. putida DOT-T1 recovered from the shock faster than P. putida F1; this allowed the former strain to become established at higher densities in polluted sites into which both strains had been introduced.
Toluene is widely used as an organic solvent, and its worldwide production is estimated to be more than 80,000 metric tons per year (1). Toluene and other solvents, such as xylenes, benzene, and ethylbenzene, are ubiquitous pollutants (2, 8), and several environmental protection agencies have declared the removal of these pollutants to be a high priority. In many environments indigenous microorganisms are able to remove aromatic hydrocarbons. This is to be expected, as many catabolic pathways for the metabolism of these compounds have been described, particularly in strains belonging to the genus Pseudomonas. Gibson et al. (7) reported that Pseudomonas putida F1 used a toluene dioxygenase pathway that yielded the corresponding cis-glycol, which was subsequently converted into 3-methylcatechol. Worsey and Williams (29) found that the TOL plasmid of P. putida mt-2 metabolized toluene via oxidation to benzoate. More recently, three cresol-yielding pathways have been described for the metabolism of this compound. Depending on the position at which the hydroxyl group is incorporated, the pathways are known as the o-, m-, and p-cresol pathways for toluene metabolism (14, 17, 24, 28, 30).
Duetz et al. (5) showed with chemostat experiments that in competition assays performed with pairs of Pseudomonas strains that used different toluene degradation pathways, the strain that became dominant was the strain with the higher affinity for toluene. In these experiments Pseudomonas mendocina KR1, which metabolized toluene via the p-cresol pathway, was the winning strain in competition with P. putida mt-2 (bearing the TOL plasmid), P. putida F1, or Pseudomonas cepacia G4, a strain which metabolized toluene via o-cresol. However, in heterogeneous habitats, such as sewage treatment plants, underground waters, and soils, other factors in addition to affinity for a compound are important. These factors include the ability to use multiple substrates simultaneously, the ability to adhere to surfaces and colonize the corresponding niche, and the ability to respond to physicochemical alterations (6, 12, 16, 21). In addition, aromatic hydrocarbons are extremely toxic for microorganisms, because they dissolve in the cell membrane (25). Therefore, a critical issue in the degradation of aromatic hydrocarbons in polluted sites is tolerance to these compounds.
Recently, a number of Pseudomonas strains have been shown to be tolerant to organic solvents such as toluene, xylenes, styrene, and others, when the solvents are supplied at supersaturating concentrations in liquid medium (3, 10, 15, 18, 27). Solvent tolerance in bacteria belonging to the genus Pseudomonas involves (i) an increase in cell membrane rigidity as a result of increases in both the level of the trans isomers of unsaturated fatty acids and the level of cardiolipin, a component of phospholipid head groups (18, 26), and (ii) removal of solvents from membranes via efflux pumps (11, 17).
We report here the solvent tolerance of different toluene-degrading Pseudomonas strains and the survival of these organisms in soil after solvent shock. Our results show that the viability of P. putida DOT-T1 and F1 was not lost upon heptane shock and propylbenzene shock; however, the viability of these strains was severely affected by toluene shock, although both strains recovered and colonized polluted niches. This recovery was influenced by the soil humidity and the incubation temperature.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The strains used in this study are shown in Table 1. Strains were grown on Luria-Bertani (LB) medium or M9 minimal medium supplemented with toluene in the gas phase or 0.5% (wt/vol) glucose in the liquid phase as the C source. Spontaneous rifampin-resistant mutants of all of the Pseudomonas strains were isolated. The rifampin resistance marker was introduced into the strains because no rifampin-resistant bacteria were recovered from the fluvisol soil used in this study (see below). When required, antibiotics were added to the culture medium as follows: ampicillin, 50 μg/ml; chloramphenicol, 30 μg/ml; kanamycin, 25 μg/ml; rifampin, 20 μg/ml; and streptomycin, 50 μg/ml.
TABLE 1.
Catabolic pathways and tolerance to organic solvents of the strains used in this study
| Strain | Catabolic pathway | Marker(s)a | Reference(s) |
|---|---|---|---|
| P. putida DOT-T1 | Toluene dioxygenase | Rifr, Cmr | 13, 17 |
| P. putida DOT-T1-Km | Toluene dioxygenase | Cmr, Kmr | 13 |
| Mini-Tn5-Km derivative of DOT-T1 | |||
| P. mendocina KR1 | p-Cresol | Rifr | 28 |
| P. putida F1 | Toluene dioxygenase | Rifr, Cmr | 6 |
| P. putida F1-Te | Toluene dioxygenase | Ter, mini-Tn5-Te derivative of F1 | This study |
| P. putida EEZ15(pWWO-EB62) | Benzyl alcohol | Apr, Kmr | 19 |
Apr, Cmr, Kmr, Rifr, and Ter, resistance to ampicillin, chloramphenicol, rifampin, kanamycin, and telurate, respectively.
Mutagenesis of Pseudomonas strains by mini-Tn5 transposons.
Triparental matings involving P. putida F1 as the recipient, Escherichia coli CC118λpir harboring the suicide vector pJMSB4 as the mini-Tn5 transposon donor strain (23), and E. coli HB101(pRK600) as the helper strain were performed as described by de Lorenzo and Timmis (4). Transconjugants of P. putida F1 were selected on minimal medium plates containing toluene in the gas phase as the sole C source and supplemented with telurate (20 μg/ml), the selection marker in the pJMSB4 mini-transposon. P. putida DOT-T1 was labelled with mini-Tn5Km basically as described above except that the donor strain was E. coli CC118λpir(pUT-Km) (4) and the selection medium contained kanamycin instead of telurate.
Soil microcosm assays.
For soil assays we used a fluvisol soil (6.4% [wt/wt] organic matter, 3.5% [wt/wt] CaCO3). The soil was sieved through a 4-mm mesh, and the humidity was usually adjusted to 30% of the field water-carrying capacity (16). Pots containing 90 g of soil were incubated at room temperature (17 to 20°C) or at the temperature indicated below. Bacteria were uniformly distributed in the soil. The number of CFU per gram of soil was determined as described by Ramos et al. (16) in minimal medium containing toluene as the sole C source and the appropriate antibiotics.
RESULTS
Solvent tolerance of several toluene-degrading strains in liquid medium.
Although many aromatic hydrocarbon-degrading strains have been isolated (8), they are usually solvent sensitive, and degradation of aromatic hydrocarbons occurs only when these compounds are supplied at low concentrations (8, 25). However, a number of toluene-resistant Pseudomonas strains, some of which were unable to use this aromatic compound as a sole C source, were recently isolated (3, 10, 17, 27). The results described above and other results from the four laboratories that isolated these strains suggested that metabolism of monocyclic aromatic hydrocarbons and tolerance to organic solvents are unrelated characteristics (3, 10, 16, 18, 25, 27). To further explore this possibility, we tested the solvent tolerance of four gram-negative toluene degraders, P. putida DOT-T1, EEZ15(pWW0-EB62), and F1 and P. mendocina KR1, which metabolized toluene by using the catabolic pathways shown in Table 1. The degrees of tolerance to organic solvents with logPo/w values between 4.5 and 2 (heptane [logPo/w = 4.5], propylbenzene [logPo/w = 3.5], toluene [logPo/w = 2.5], and benzene [logPo/w = 2.0] [logPo/w values are from references 10 and 25) and to different amounts of solvents (0 to 10%, vol/vol) were tested in LB liquid medium cultures. In these assays the cultures were incubated under aerobic conditions at 30°C with moderate shaking (100 strokes per min). All four strains grew in LB medium supplemented with 0, 0.1, 1, and 10% (vol/vol) heptane (data not shown), and none of the strains grew in the presence of benzene (logPo/w = 2.0) supplied at concentrations of 0.2 to 10% (vol/vol) (data not shown). The growth of the four strains when propylbenzene was added to the liquid phase was also assayed. P. putida DOT-T1 tolerated concentrations of this aromatic compound greater than 10% (vol/vol), whereas P. putida F1 and EEZ15-Km tolerated 1% (vol/vol) propylbenzene and P. mendocina KR1 tolerated only 0.1% (vol/vol) propylbenzene (data not shown). P. putida DOT-T1 was also shown to be tolerant to toluene concentrations greater than 10% (vol/vol), in agreement with previous findings (16). P. putida F1 and EEZ15-Km(pWW0) and P. mendocina KR1 tolerated up to 0.1% (vol/vol) toluene in the culture medium. These results corroborate the finding that solvent tolerance is a strain characteristic rather than a property associated with the metabolism of a compound by a microorganism.
Survival of toluene degraders in soils after organic solvent shock.
To study the response of the Pseudomonas strains described above to solvent shocks in a different context, we determined survival in a fluvisol soil from the Granada, Spain, area before and after solvent shocks. This fluvisol soil is relatively rich in organic matter, and previous studies have shown that certain Pseudomonas strains are able to become established in it at a level of 105 to 106 CFU per g of soil (16, 22).
The four strains described above were introduced into unsterile soil at a density of approximately 106 CFU per g of soil, and the number of cells of each strain was followed with time in each of the microcosms. In short-term assays (30 days) the number of CFU of each P. putida strain introduced per g of soil remained relatively constant with time. In contrast, P. mendocina KR1 was no able to become established in this soil, and this strain could not be recovered after 30 days (data not shown). For this reason in subsequent studies we used only the three P. putida strains.
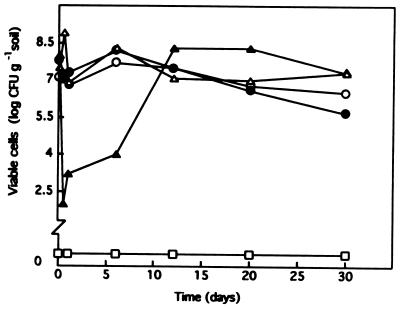
To soil microcosms into which the P. putida strains had been introduced at a density of about 106 CFU/g we added 0.1 to 10% (vol/wt) heptane or 0.1 to 10% (vol/wt) propylbenzene. After heptane or propylbenzene was added, the three strains survived well. The number of CFU per gram of soil remained relatively constant (range, 107 to 106 CFU/g of soil) throughout the assay (Fig. 1).
FIG. 1.
Survival in soil of P. putida F1 after solvent shock. About 107 CFU of P. putida F1 per g of soil was introduced into independent pots to which 10% (vol/wt) heptane (•), 10% (vol/wt) propylbenzene (▵), 1% (vol/wt) toluene (▴), 1% (vol/wt) benzene (□), or nothing (○) was added. At different times the number of CFU per g of soil was determined.
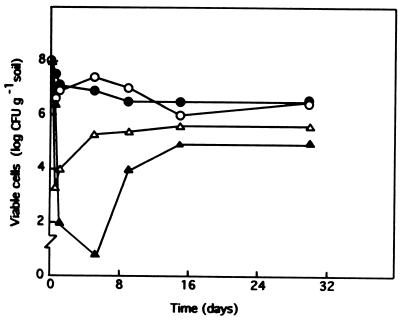
To soil microcosms into which we introduced the different P. putida strains toluene was also added to an initial concentration of 0, 0.1, 1, or 10% (vol/wt). Then the number of cells of each strain was followed. The addition of 0.1% (vol/wt) toluene had no significant effect on the survival of any of the strains (Fig. 2). However, the strains were extremely sensitive to toluene shock at a toluene concentration of 1% (vol/wt) or higher. Immediately after the addition of 1% (vol/wt) toluene the number of P. putida EEZ15(pWW0-EB62) cells decreased by more than 6 orders of magnitude, and after 24 h no bacteria were recovered from the soils (data not shown). For P. putida F1 and DOT-T1 the initial decrease was also large, and the number of organisms decreased to 102 to 104 CFU/g of soil. Then the number of organisms increased to about 105 to 106 CFU/g of soil (Fig. 1 and 2). When 10% (vol/wt) toluene was added, the number of CFU per gram of soil for all three introduced strains was below our detection limit. Since our detection limit was about 100 CFU/g of soil, we attempted to determine whether viable cells remained in the preparations. One gram of soil was suspended in 10 ml of LB medium containing the antibiotics to which each strain was resistant, and after growth for 20 h at 30°C serial dilutions of the cultures were spread onto minimal medium containing toluene as a C source and the appropriate antibiotics. Recovery of cells was taken as evidence that viable cells were present, whereas the absence of recovery was considered to indicate complete disappearance of cells. P. putida EEZ15(pWW0-EB62) disappeared completely from soils after a 10% (vol/wt) toluene shock, whereas a few P. putida DOT-T1 and F1 cells remained viable, but the cell density was very low. The soils were incubated further, and the numbers of CFU of P. putida DOT-T1 and F1 per g of soil were followed with time. Both strains were able to multiply in the soils, and they reached densities of about 105 to 106 CFU per g of soil 30 days after the addition of toluene (Fig. 2).
FIG. 2.
Survival in soil of P. putida DOT-T1 after toluene shock. About 108 CFU of P. putida DOT-T1 per g of soil was introduced into independent pots, which were supplemented or not supplemented with toluene. Symbols: ○, no addition; •, 0.1% (vol/wt) toluene; ▵, 1% (vol/wt) toluene; ▴, 10% (vol/wt) toluene. At different times the number of CFU per g soil was determined.
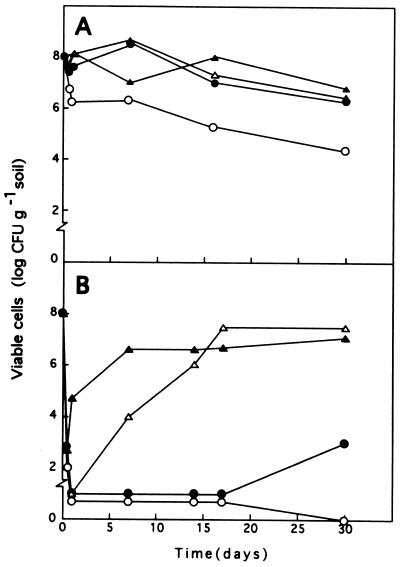
We previously found that the survival of certain P. putida strains in soils is influenced by the humidity of the soil and the incubation temperature (16). We assayed how these two factors influence the multiplication in soils of P. putida DOT-T1 and F1 cells after toluene shock. As a control, cells were incubated under the same conditions, but no toluene was added. In the absence of toluene, survival of these two strains was high when the water content of the soil was 15 to 45% of the field carrying capacity, but survival decreased (by about 2 log units) when the water content was lower (Fig. 3A). After the addition of toluene, regardless of the soil water content, the number of viable cells decreased by 5 to 6 log units. No P. putida DOT-T1 or F1 cells were recovered from soil with a humidity below 15% of the field carrying capacity, whereas cells were recovered from soils with humidities in the range from 15 to 45% of the field carrying capacity (Fig. 3B). The lag time required for recovery was influenced by the soil humidity; the higher the humidity, the faster the recovery (Fig. 3B). At a humidity of 45%, cells were present at a relatively high density 24 h after the shock, whereas at a humidity of 30%, cells were found 1 week after the shock and at a humidity of 15% cells were found at a density greater than 102 CFU per g of soil 15 days after the toluene shock. When the soil humidity was about 5% of the field carrying capacity, we never found more than 102 CFU per g of soil, and eventually cells could not be recovered (Fig. 3B).
FIG. 3.
Survival of P. putida DOT-T1 in a fluvisol soil maintained at different humidities in the absence of toluene (A) or after a 1% (vol/wt) toluene shock (B). The humidity of the soil was adjusted to 5% (○), 15% (•), 30% (▵), or 45% (▴) of the field carrying capacity before bacteria were introduced. Then 108 CFU per g of soil was added, and 1% (vol/wt) toluene was added to one set of pots (B). At different times the number of CFU per g of soil was determined.
At a humidity of 30% of the field carrying capacity, temperature also affected recovery. Recovery was best at 25 to 30°C, and higher or lower incubation temperatures decreased the recovery rate. At 4 and 37°C no cells were recovered (Table 2).
TABLE 2.
Effect of incubation temperature on recovery of P. putida DOT-T1 and F1 after toluene shock in soila
| Incubation temp (°C) | Density (CFU/g of soil)
|
|
|---|---|---|
| P. putida DOT-T1 | P. putida F1 | |
| 4 | <102 | <102 |
| 18 | 104 | 103 |
| 25 | 4 × 105 | 2 × 103 |
| 30 | 2 × 105 | 5 × 103 |
| 37 | <102 | <102 |
P. putida DOT-T1 and F1 were introduced at a density of 106 CFU per g of fluvisol soil which contained water at a level of 30% of the field carrying capacity. Then 10% (vol/wt) toluene was added, and samples were incubated for 8 days at different temperatures. Serial dilutions were then plated onto selective culture medium, and the numbers of CFU per g of soil were determined.
In these studies the loss of viability by P. putida DOT-T1 and F1 in soils upon toluene shock may have been influenced not only by the addition of the solvent, but also by the fact that cells might not have had enough time to become established in the soil. We therefore tested whether introduction of P. putida DOT-T1 or F1 into soils before the toluene shock had a beneficial effect on tolerance. Incubation of P. putida DOT-T1 for 2 or 14 days before the toluene shock had no beneficial effect on tolerance to this compound; immediately after the addition of the aromatic hydrocarbon the number of CFU per g of soil decreased by between 4 and 5 orders of magnitude (data not shown). Similar results were obtained when the assays were done with P. putida F1 (data not shown).
Given that the levels of toluene tolerance of P. putida F1 and EEZ15(pWW0-EB62) in soil were greater than the levels of tolerance in liquid medium, we also tested whether P. putida DOT-T1 and F1 could tolerate benzene shocks. After the addition of 1% (vol/wt) benzene to the soil, the number of CFU decreased to below our detection limits, and cells could not be recovered even after enrichment as described above (data not shown).
Establishment in soil of different strains able to degrade toluene after toluene shock.
To determine how the two toluene-tolerant strains behaved when they were introduced simultaneously into soil in the absence and in the presence of toluene, the strains were labelled with different resistance markers so that they could be easily distinguished from each other and from indigenous soil microbiota. We generated kanamycin-resistant and telurate-resistant derivatives of P. putida DOT-T1 and F1, respectively. When these labelled strains were introduced independently into soils, they behaved like the unmarked wild-type strains described above (data not shown). The two labelled strains were introduced into soil microcosms at a ratio of 1:1 and at a cell density of either 108 or 106 CFU per g of soil with and without 10% (vol/wt) toluene.
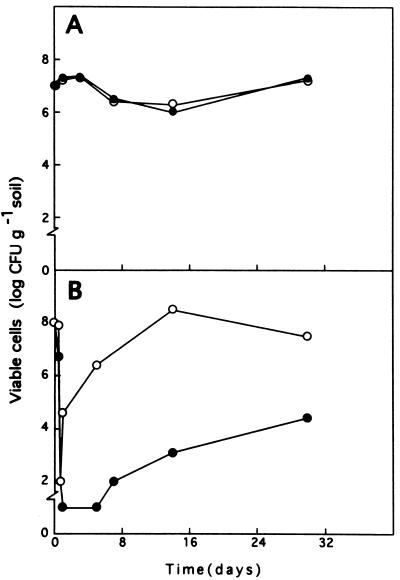
In nonpolluted soils with a humidity of 30% of the field carrying capacity, no apparent competition between the strains was observed (Fig. 4A). Regardless of the initial cell load, both strains tended to become established at a level of approximately 106 to 107 CFU per g of soil (Fig. 4A).
FIG. 4.
Survival of P. putida DOT-T1 and F1 in a fluvisol soil without toluene (A) and after a 10% (vol/wt) toluene shock (B). A telurate-resistant derivative of P. putida F1 (•) and a kanamycin-resistant derivative of P. putida DOT-T1 (○) were introduced into the same soil at a density of about 108 CFU per g of soil. The number of CFU per g of soil was determined at different times.
The addition of 10% (vol/wt) toluene to soils inoculated with 108 CFU of the two strains per g of soil led to an immediate decrease of 5 to 6 log units in the number of CFU per g of soil. Twenty-four hours later we detected between 102 and 103 CFU of each strain per g of soil (Fig. 4B). P. putida DOT-T1 recovered faster than strain F1; P. putida DOT-T1 reached a density of about 105 CFU per g of soil 3 days after the shock, whereas the density of P. putida F1 remained about 102 CFU per g of soil and reached 105 CFU per g of soil after 30 days. After 30 days the density of P. putida DOT-T1 was about 107 CFU per g of soil (Fig. 4B).
DISCUSSION
Toluene and related aromatic hydrocarbons are highly toxic for living organisms because the preferential partitioning of these compounds in cell membranes disrupts the membrane structure, which leads to cell death (25). Toluene-degrading microbes are not immune to this general toxic effect and are sensitive to toluene shocks. This is particularly relevant for P. putida EEZ15 (a P. putida mt-2 derivative), P. putida F1, and P. mendocina KR1, which in liquid culture medium were not able to grow when toluene was present at a concentration of 0.2% (vol/vol). In contrast to these sensitive strains is P. putida DOT-T1, one of the two strains described so far that are able to tolerate high concentrations of toluene in liquid medium (10, 17).
Our results showed that the three P. putida strains studied were able to become established in nonpolluted soil at levels of approximately 105 to 106 CFU per g of soil, whereas P. mendocina KR1 was not able to become established in this soil. Also, P. mendocina KR1 was the winning strain in competition assays in C-limited chemostats (5). The inability of P. mendocina KR1 to become established in soil makes this strain the least competitive. This finding suggests that the results of competition assays performed in the laboratory cannot always be extrapolated to a complex environment, such as soil.
Compared with liquid cultures, P. putida EEZ15(pWW0-EB62) and F1 tolerated greater amounts of organic solvents in soil. Both of these strains tolerated shocks consisting of 10% (vol/wt) propylbenzene in soil, whereas this concentration prevented cell growth in liquid medium. Furthermore, P. putida F1 tolerated shocks consisting of 1 and 10% (vol/wt) toluene, in contrast to the results observed in liquid cultures. This might reflect the limited access of the solvent to the cells in soil either because of sequestration of part of the toluene by soil particles or because of the absence of homogeneous mixing of toluene in soil, in contrast to the situation in agitated liquid culture medium.
P. putida DOT-T1 has been shown previously to tolerate high concentrations of toluene in liquid medium (17), and in agreement with this observation is our finding that this strain tolerates shocks of propylbenzene and toluene in liquid medium and in soil. However, the number of CFU of P. putida F1 and DOT-T1 per g of soil after shocks consisting of 1% (vol/wt) (or more) toluene deserves attention. We found that after the addition of toluene, the number of CFU of both P. putida F1 and DOT-T1 per g of soil decreased by about 5 log units and then increased. This suggested that most cells were not able to tolerate the initial solvent shock and that those cells able to respond multiplied and colonized the niche. The recovery of the more tolerant strain, P. putida DOT-T1, was faster than the recovery of P. putida F1. In assays performed with both strains in the same soil, P. putida DOT-T1 colonized the site more rapidly than P. putida F1 (Fig. 4B).
The rate of recovery of both strains after toluene shock was influenced by the soil humidity and the incubation temperature. Both strains recovered best at a humidity between 30 and 45% of the soil carrying capacity; lower water levels resulted in slower recovery or no recovery. Humidities below 5% of the field carrying capacity in the soil reduced the survival of P. putida strains, whereas the survival was optimal at a humidity of 30% of the field carrying capacity (16). The faster recovery at the higher humidity was unexpected, as the presence of water probably facilitated the movement of unbound toluene. The faster recovery probably reflected the fact that under these conditions the metabolic activity of the cells was higher, which helped to remove toluene from the cell membrane, to increase cell membrane rigidity, or to metabolize toluene (9, 11, 18, 27). The activity of the Pseudomonas strains was optimal in the temperature range from 25 to 30°C, which should favor metabolic activities and therefore solvent exclusion. In general, our results show that Pseudomonas strains are more resistant to solvents in soils than in liquid culture medium; this may explain why these microbes are able to deal with these pollutants in biofilms and soils (12, 20). However, the level of tolerance in soil is related to the level of tolerance in liquid; the higher the tolerance in liquid, the faster the recovery of the strain in soil after toluene shock. Therefore, in sites heavily polluted by aromatic hydrocarbons, solvent-tolerant strains would be expected to become established first, to colonize the site, and to become predominant in the removal of these compounds.
ACKNOWLEDGMENTS
This work was supported by grant BIO 97-0641 from the CICYT and by grant BIO4-CT97-2270 from the European Community.
REFERENCES
- 1.Anonymous. Toluene. Environmental health criteria. Vol. 52. Geneva, Switzerland: World Health Organization; 1985. [Google Scholar]
- 2.Atlas R M. Bioremediation: The Tokyo ’94 Workshop. Paris, France: OECD Documents; 1995. Efficacy of bioremediation: chemical and risk-based determinations. [Google Scholar]
- 3.Cruden D L, Wolfram J H, Rogers R D, Gibson D T. Physiological properties of a Pseudomonas strain which grows with p-xylene in a two-phase (organic-aqueous) medium. Appl Environ Microbiol. 1992;58:2723–2729. doi: 10.1128/aem.58.9.2723-2729.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Lorenzo V, Timmis K N. Analysis and construction of stable phenotypes in Gram-negative bacteria with Tn5- and Tn10-derived minitransposons. Methods Enzymol. 1994;235:386–405. doi: 10.1016/0076-6879(94)35157-0. [DOI] [PubMed] [Google Scholar]
- 5.Duetz W A, Marqués S, De Jong C, Ramos J L, Van Andel J G. Inducibility of the TOL catabolic pathway in Pseudomonas putida(pWW0) growing on succinate in continuous culture: evidence of carbon catabolite repression control. J Bacteriol. 1994;176:2354–2361. doi: 10.1128/jb.176.8.2354-2361.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Egli T, Lendenmann U, Snozzi M. Kinetics of microbial growth with mixtures of carbon sources. Antonie Leeuwenhoek. 1993;63:289–298. doi: 10.1007/BF00871224. [DOI] [PubMed] [Google Scholar]
- 7.Gibson D T, Hensley M, Yoshioka H, Mabry T J. Formation of (+)-cis-2,3-dihydroxy-1-methylcyclohexa-4,6-diene from toluene by Pseudomonas putida. Biochemistry. 1970;9:1626–1630. doi: 10.1021/bi00809a023. [DOI] [PubMed] [Google Scholar]
- 8.Gibson D T, Subramanian V. Microbial degradation of aromatic hydrocarbons. In: Gibson D T, editor. Microbial degradation of organic compounds. New York, N.Y: Marcel Dekker, Inc.; 1984. pp. 181–252. [Google Scholar]
- 9.Heipieper H J, Weber F J, Sikkema J, Keweloh H, de Bont J A M. Mechanisms behind resistance of whole cells to toxic organic solvents. Trends Biotechnol. 1994;12:409–415. [Google Scholar]
- 10.Inoue A, Horikoshi K. A Pseudomonas thrives in high concentrations of toluene. Nature. 1989;338:264–266. [Google Scholar]
- 11.Isken S, de Bont J A M. Active efflux of toluene in a solvent-resistant bacterium. J Bacteriol. 1996;178:6056–6058. doi: 10.1128/jb.178.20.6056-6058.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Møller S, Pedersen A R, Poulsen L K, Arvin E, Molin S. Activity and three-dimensional distribution of toluene-degrading Pseudomonas putida in a multispecies biofilm assessed by quantitative in situ hybridization and scanning confocal laser microscopy. Appl Environ Microbiol. 1996;62:4632–4640. doi: 10.1128/aem.62.12.4632-4640.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mosqueda, G., and J. L. Ramos. 1997. Unpublished data.
- 14.Olsen R H, Kukor J J, Kaphammer B. A novel toluene-3-monooxygenase pathway cloned from Pseudomonas pickettii PKO1. J Bacteriol. 1994;176:3749–3756. doi: 10.1128/jb.176.12.3749-3756.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pinkart H C, Wolfram J W, Rogers R, White D C. Cell envelope changes in solvent-tolerant and solvent-sensitive Pseudomonas putida strains following exposure to o-xylene. Appl Environ Microbiol. 1996;62:1129–1132. doi: 10.1128/aem.62.3.1129-1132.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramos J L, Duque E, Ramos-González M I. Survival in soils of an herbicide-resistant Pseudomonas putida strain bearing a recombinant TOL plasmid. Appl Environ Microbiol. 1991;57:260–266. doi: 10.1128/aem.57.1.260-266.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramos J L, Duque E, Huertas M J, Haïdour A. Isolation and expansion of the catabolic potential of a Pseudomonas putida strain able to grow in the presence of high concentrations of aromatic hydrocarbons. J Bacteriol. 1995;177:3911–3916. doi: 10.1128/jb.177.14.3911-3916.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramos J L, Duque E, Ródiguez-Herva J J, Godoy P, Fernández-Barrero A. Mechanisms for solvent tolerance in bacteria. J Biol Chem. 1997;272:3887–3890. doi: 10.1074/jbc.272.7.3887. [DOI] [PubMed] [Google Scholar]
- 19.Ramos-González M I, Duque E, Ramos J L. Conjugational transfer of recombinant DNA in cultures and in soils: host range of Pseudomonas putida TOL plasmid. Appl Environ Microbiol. 1991;57:3020–3027. doi: 10.1128/aem.57.10.3020-3027.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reihard M, Shang S, Kitanides P K, Orwin E, Hopkins G D, Lebron A C. In situ BTEX biotransformation under enhanced nitrate- and sulfate-reducing conditions. Environ Sci Technol. 1997;31:28–36. [Google Scholar]
- 21.Rodríguez-Herva, J. J., D. Reniero, M.-A. Ramos-Díaz, L. Molina, C. Ramos, E. Galli, and J. L. Ramos. Unpublished results.
- 22.Ronchel M C, Ramos C, Jensen L B, Molin S, Ramos J L. Construction and behavior of biologically contained bacteria for environmental applications in bioremediation. Appl Environ Microbiol. 1995;61:2990–2994. doi: 10.1128/aem.61.8.2990-2994.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sánchez-Romero, J. M. 1997. Personal communication.
- 24.Shields M S, Montgomery S O, Chapman P J, Cuskey S M, Pritchard P H. Novel pathway of toluene catabolism in the trichloroethylene-degrading bacterium G4. Appl Environ Microbiol. 1989;55:1624–1629. doi: 10.1128/aem.55.6.1624-1629.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sikkema J, de Bont J A M, Poolman B. Mechanisms of membrane toxicity of hydrocarbons. Microbiol Rev. 1995;59:201–222. doi: 10.1128/mr.59.2.201-222.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weber F J. Toluene: biological waste-gas treatment, toxicity and microbial adaptation. Ph.D. thesis. Wageningen, The Netherlands: University of Wageningen; 1995. [Google Scholar]
- 27.Weber F J, Isken S, de Bont J A M. Cis/trans isomerization of fatty acids as a defence mechanism of Pseudomonas putida strains to toxic concentrations of toluene. Microbiology. 1994;140:2013–2017. doi: 10.1099/13500872-140-8-2013. [DOI] [PubMed] [Google Scholar]
- 28.Whited G M, Gibson D T. Separation and partial characterization of the enzymes of the toluene-4-monooxygenase catabolic pathway in Pseudomonas mendocina KR1. J Bacteriol. 1991;173:3017–3020. doi: 10.1128/jb.173.9.3017-3020.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Worsey M J, Williams P A. Metabolism of toluene and xylenes by Pseudomonas putida mt-2: evidence for a new function of the TOL plasmid. J Bacteriol. 1975;124:7–13. doi: 10.1128/jb.124.1.7-13.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yen K-M, Karl M R, Blatt L M, Simon M J, Winter R B, Fausset P R, Lu H S, Harcourt A A, Chen K K. Cloning and characterization of a Pseudomonas mendocina KR1 gene cluster encoding toluene-4-monooxygenase. J Bacteriol. 1991;173:5315–5327. doi: 10.1128/jb.173.17.5315-5327.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]