Abstract
A polyester polyurethane (PUR)-degrading enzyme, PUR esterase, derived from Comamonas acidovorans TB-35, a bacterium that utilizes polyester PUR as the sole carbon source, was purified until it showed a single band in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). This enzyme was bound to the cell surface and was extracted by addition of 0.2% N,N-bis(3-d-gluconamidopropyl)deoxycholamide (deoxy-BIGCHAP). The results of gel filtration and SDS-PAGE showed that the PUR esterase was a monomer with a molecular mass of about 62,000 Da. This enzyme, which is a kind of esterase, degraded solid polyester PUR, with diethylene glycol and adipic acid released as the degradation products. The optimum pH for this enzyme was 6.5, and the optimum temperature was 45°C. PUR degradation by the PUR esterase was strongly inhibited by the addition of 0.04% deoxy-BIGCHAP. On the other hand, deoxy-BIGCHAP did not inhibit the activity when p-nitrophenyl acetate, a water-soluble compound, was used as a substrate. These observations indicated that this enzyme degrades PUR in a two-step reaction: hydrophobic adsorption to the PUR surface and hydrolysis of the ester bond of PUR.
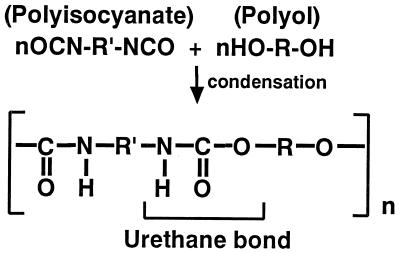
Polyurethane (PUR), which is a kind of plastic, is widely used as base material in various industries. PUR is synthesized by condensation of polyol and polyisocyanate (Fig. 1). There are two types of PUR, named according to the polyol used in the synthesis: PUR which uses polyester as polyol in synthesis is called polyester PUR, and the one which uses polyether is called polyether PUR. PUR is a material which is resistant to microbial attack, but generally, the ester-type PUR is more easily degraded than the ether-type PUR (15). Several kinds of fungi and bacteria that can degrade ester-type PUR have been reported (2, 3, 11, 22). In all cases, the degradation was considered to be initiated by hydrolysis of the ester bond by some hydrolytic enzyme(s), such as esterase.
FIG. 1.
Structure of PUR.
In solid-polyester biodegradation, degradation of poly(3-hydroxyalkanoate) (PHA) by PHA depolymerase is well-known (7, 8, 13, 16, 18, 21, 24, 25). This enzyme, which is one of the esterases, has a hydrophobic surface-binding domain and a catalytic domain and accomplishes the degradation of PHA film via two steps (9, 10, 17). The first step is adsorption of the enzyme on the surface of the film via the surface-binding domain, and the second step is hydrolysis of the ester bond. Unfortunately, the mechanisms of enzymatic degradation of polyester-based plastics, such as polyester PUR, are still unclear.
Pathirana and Seal (23) reported the activities of several enzymes, namely, esterase, protease, and urease, in culture broth during ester-type PUR degradation by fungi. Crabbe et al. (2) also obtained fractions containing esterase activity from the broth of a fungus culture which could degrade colloidal PUR. In the case of bacteria, Kay et al. (12) detected extracellular esterase activity in the medium during PUR degradation by a Corynebacterium sp. However, the relationship between the esterase activity and PUR degradation was unclear. There have been no reports about purification and detailed characterization of degrading enzymes from solid-PUR-degrading microorganisms.
Previously (19), a bacterium, strain TB-35, which could utilize solid polyester PUR as the sole carbon and nitrogen source, was isolated and identified as Comamonas acidovorans. We also found that an esterase plays a major role in PUR degradation by this strain (20). This strain constitutively secreted two kinds of extracellular esterase: one is secreted into the culture broth, and the other is bound to the cell surface. Only the cell-bound esterase could degrade the PUR. In this study, we purified the cell-bound esterase, which is a PUR degradation enzyme, and examined some of its characteristics.
MATERIALS AND METHODS
Materials.
The polyester PUR used in this study was synthesized by reacting poly(diethylene glycol adipate) with 2,4-tolylene diisocyanate under anhydrous conditions as described previously (19). The poly(diethylene glycol adipate) was supplied by Suzuki Motor Co. (Hamamatsu, Japan). Various detergents used in enzyme extraction were purchased from Dojindo Co., Tokyo, Japan. Poly(3-hydroxybutyric acid) and poly(3-hydroxybutyric acid-co-3-hydroxyvaleric acid) were bought from Aldrich Chemical Co. Poly(lactic acid)s were purchased from Wako Pure Chemical Co., Tokyo, Japan.
Bacterial strains and cultivation.
C. acidovorans TB-35, which had been isolated as a degrader of solid polyester PUR, was used throughout this study. The composition of the basal medium for polyester PUR degradation was described previously (19). One loopful of cells was used to inoculate three 500-ml Erlenmeyer flasks, each containing 100 ml of basal medium and 1.0 g of PUR cubes (5 by 5 by 5 mm) as the sole carbon source. These flasks were shaken on a rotary shaker for 6 days at 30°C. Then the culture broths were transferred to a 5-liter jar fermentor (model MD-50; B. E. Marubishi Co., Tokyo, Japan) containing 3 liters of the basal medium and 30 g of PUR cubes. Cultivation was performed under the following conditions: aeration, 1.5 liters/min; temperature, 30°C; and agitation, 500 rpm. Cells were harvested after 7 days’ cultivation and were stored at −80°C until use.
Extraction of cell-bound PUR esterase.
Cells (1.5 g [wet weight]) were suspended in 15 ml of 20 mM potassium phosphate buffer (pH 7.0). The suspension was distributed into small test tubes at 1 ml each, and then 1 ml each of detergent solutions (0.2% final concentration) was added. The tubes were shaken vigorously for 2 h, after which they were centrifuged and the supernatants were collected. An enzyme activity assay was performed with p-nitrophenyl acetate as the substrate. The total cell-bound esterase activity was measured by adding the untreated cell suspension to the cuvette.
Purification of PUR degradation enzyme.
All the purification procedures were performed at room temperature since the enzyme activity was stable at room temperature for at least 24 h. A 500-ml volume of 20 mM potassium phosphate buffer (pH 7.0) which contained 0.2% N,N-bis(3-d-gluconamidopropyl)deoxycholamide (deoxy-BIGCHAP) was added to the frozen bacterial cells (55 g [wet weight]), and the cell-bound PUR esterase was extracted by vigorous stirring for 30 min. The treated cell suspension was centrifuged, and the supernatant was used as crude extract. Solid (NH4)2SO4 was added to the crude extract with gentle stirring until the solution reached 45% saturation. The solution was stirred for a further 30 min. The solution was centrifuged at 7,000 × g for 10 min, and the precipitate was washed once with 100 ml of 20 mM potassium phosphate buffer (pH 7.0) which was saturated with (NH4)2SO4 to 45%. After centrifugation, the washed precipitate was dissolved in 400 ml of 20 mM potassium phosphate buffer (pH 7.0). A 100-ml volume of Phenyl-TOYOPEARL 650M was added to the (NH4)2SO4 fractionated enzyme solution, and then the mixture was gently stirred to allow absorption of the PUR esterase. The Phenyl-TOYOPEARL 650M to which the PUR esterase was absorbed was washed with 20 mM potassium phosphate buffer (pH 7.0) four times (300 ml each). After that, the PUR esterase was eluted with 300 ml of the same buffer containing 0.25% deoxy-BIGCHAP by stirring for 45 min. Phenyl-TOYOPEARL 650M was removed by filtration, and solid (NH4)2SO4 was added to the enzyme solution until it reached 50% saturation. The solution was centrifuged at 7,000 × g, and the precipitate was resuspended in 6 ml of 20 mM potassium phosphate buffer (pH 7.0) containing 0.2% deoxy-BIGCHAP and then was desalted by Sephadex G-25 (Pharmacia, Uppsala, Sweden) column chromatography. A Q-Sepharose FF (Pharmacia) column (1.0 by 10 cm) was equilibrated with 20 mM potassium phosphate buffer (pH 7.0) containing 0.2% deoxy-BIGCHAP. The desalted solution prepared in the previous step was then loaded onto the column at a flow rate of 3 ml/min. Fractions which were not bound to the column were collected and concentrated, and deoxy-BIGCHAP was removed by ammonium sulfate precipitation (50% saturation). The purified enzyme was stored at −80°C until use.
Molecular weight determination.
The native size of the purified cell-bound PUR esterase was determined by gel filtration on a Superose 12 HR 10/30 (Pharmacia) column equilibrated with 20 mM potassium phosphate buffer (pH 7.0) containing 0.2 M NaCl and 0.2% deoxy-BIGCHAP at a flow rate of 0.6 ml/min. The subunit size of cell-bound PUR esterase was determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) using standard proteins (Amersham, Buckinghamshire, United Kingdom) as a reference. SDS-PAGE was performed with a 12.5% polyacrylamide gel as described by Laemmli (14), and the proteins were silver stained.
Electron microscopy.
The morphological changes of PUR during the enzyme reaction were observed by scanning electron microscopy. Samples were gold coated by evaporation and observed with a JSM-T330 (JEOL DATUM Ltd., Tokyo, Japan) microscope at 5 kV.
Enzyme activity assays.
The PUR degradation activity of the cell-bound PUR esterase was determined as follows. Samples (0.5 ml each) of 100 mM potassium phosphate buffer (pH 7.0) which contained PUR esterase (0.05 U) were transferred to small test tubes. The reaction was started by adding a PUR cube (2 by 2 by 1 mm) to each of the tubes. After 24-h incubation at 30°C, the PUR cubes were removed, and then the amount of free diethylene glycol which was produced upon hydrolysis of the ester bond of the PUR was quantified by gas chromatography (GC) using a model GC-8A gas chromatograph (Shimadzu Co., Tokyo, Japan). A glass column (2.6 mm by 1 m) was packed with Unisole 30T on an 80/100-mesh Uniport HP (GL Sciences Co., Tokyo, Japan) and used for GC analysis. The injection, oven, and detector temperatures were 250, 190, and 250°C, respectively. A flame ionization detector was used. PUR-degrading activity was also determined by measuring the weight loss of the PUR cube.
Esterase activity of the water-soluble substrate (p-nitrophenyl acetate) was determined by the method of Kay et al. (12). One unit was defined as the amount of enzyme required to liberate 1 μmol of p-nitrophenol per min.
Substrate specificity assay.
Degradation activities for various substrates were assayed by the decrease in turbidity of their homogeneous emulsions. Emulsions were prepared with detergent (Plysurf A; Daiichi Kogyo Seiyaku Co., Tokyo, Japan) by using a sonic oscillator. The assay mixture (1.0 ml) contained 0.5 mg of substrate, 0.1 M potassium phosphate buffer, and 0.1 U of PUR esterase. The mixtures were incubated at 30°C for 60 min. The decrease of turbidity was measured at 600 nm. The initial turbidities of the substrates were taken as 100%.
RESULTS
Purification of cell-bound PUR esterase.
In our previous study (20), the cell-bound esterase could be extracted with 2% sodium cholate, but enzyme purification was very difficult with such a high detergent concentration. Table 1 shows the effects of various detergents on enzyme extraction from the cell surface. The highest extraction activity was found with 0.2% deoxy-BIGCHAP. The critical micelle concentration of deoxy-BIGCHAP is about 0.12%, and the micelle was easily broken by dilution. In addition, deoxy-BIGCHAP simplifies ion-exchange chromatography, since it has no ionic charge. deoxy-BIGCHAP was used for further study.
TABLE 1.
Effects of detergents on extraction of cell-bound PUR esterase
| Detergent | PUR esterase activity (U/ml) |
|---|---|
| None | 0.0 |
| CHAPSa | 3.5 |
| CHAPSOb | 3.5 |
| BIGCHAP | 2.1 |
| deoxy-BIGCHAP | 6.6 |
| n-Octyl-β-d-glucoside | 1.2 |
| n-Heptyl-β-d-thioglucoside | 0.9 |
| n-Octyl-β-d-thioglucoside | 0.9 |
| n-Dodecyl-β-d-maltoside | 5.8 |
| MEGA-8c | 0.9 |
| MEGA-9d | 0.9 |
| MEGA-10e | 2.6 |
| Sucrose monocaprate | 0.9 |
| Sucrose monolaurate | 2.6 |
| Sodium cholate | 1.6 |
| Digitonin | 3.3 |
| Total cell-bound esterase activity | 5.7 |
CHAPS, 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate.
CHAPSO, 3-[(3-cholamidopropyl)dimethylammonio]-2-hydroxypropanesulfonic acid.
MEGA-8, n-octanoyl-N-methylglucamide.
MEGA-9, n-nonanoyl-N-methylglucamide.
MEGA-10, n-decanoyl-N-methylglucamide.
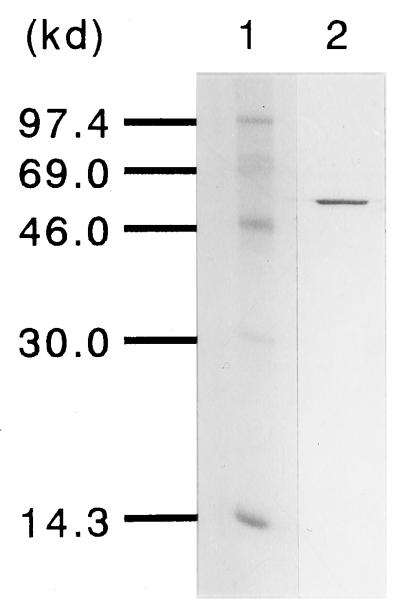
The purification of cell-bound PUR esterase is shown in Table 2. All purification steps except the final step were done in batches. The crude enzyme extracted with 0.2% deoxy-BIGCHAP was fractionated with 45% ammonium sulfate precipitation. Under this condition, almost all the cell-bound PUR esterase was adsorbed to Phenyl-TOYOPEARL 650M and was eluted with 20 mM potassium phosphate buffer (pH 7.0) containing 0.25% deoxy-BIGCHAP. Most contaminants were removed during this step. The rest of the impurities that remained in the previous step were absorbed in a Q-Sepharose FF column, and the cell-bound PUR esterase was allowed to pass through the column. The cell-bound PUR esterase was purified by about eightfold, giving a 25% yield. The purified cell-bound PUR esterase showed a single band in SDS-PAGE (Fig. 2).
TABLE 2.
Purification of PUR esterasea
| Step | Vol (ml) | Total protein (mg) | Total activity (U) | Sp act (U/mg) | Fold | Recovery (%) |
|---|---|---|---|---|---|---|
| Crude enzyme | 500 | 1,500 | 3,300 | 2.2 | 1 | 100 |
| (NH4)2SO4 precipitation | 400 | 600 | 2,460 | 4.4 | 2.0 | 80 |
| Phenyl-TOYOPEARL 650M | 300 | 135 | 1,560 | 11.6 | 5.3 | 47 |
| Q-Sepharose FF | 156 | 46 | 840 | 18.3 | 8.3 | 25 |
Enzyme activity was measured by using p-nitrophenyl acetate as a substrate.
FIG. 2.
SDS-PAGE of the purified cell-bound esterase. Lane 1, molecular mass markers; lane 2, purified PUR esterase.
Time course of PUR degradation by the PUR esterase.
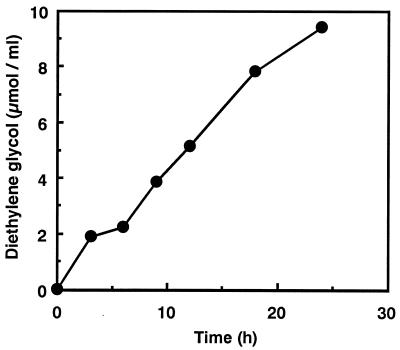
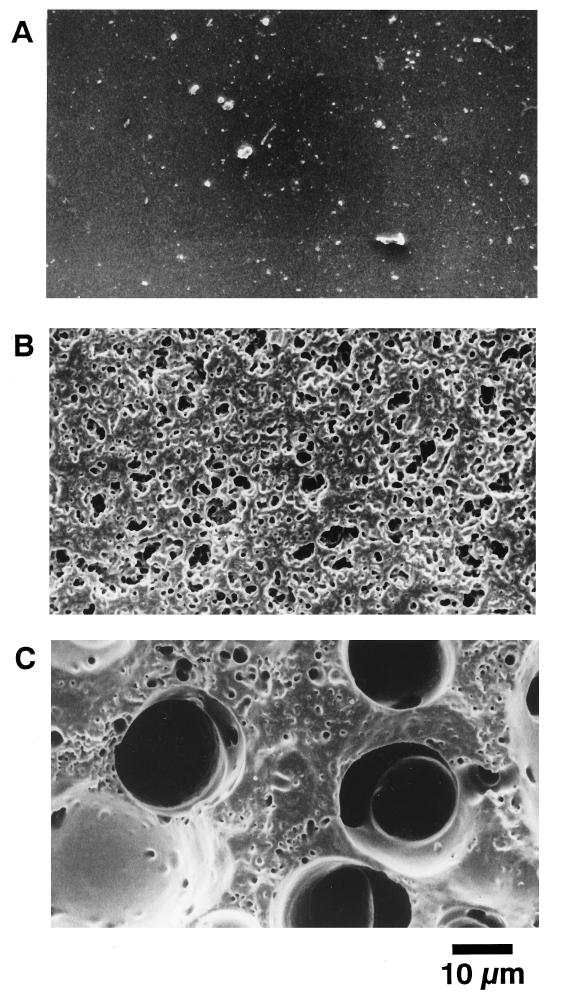
Figure 3 shows the time course of PUR degradation by the PUR esterase. The PUR (2 by 2 by 1 mm) was degraded by the purified PUR esterase, producing diethylene glycol, a degradation product which was detected by GC analysis. Adipic acid, another PUR degradation product, was also detected, by high-performance liquid chromatography analysis. These products were identified by GC-mass spectrometry by a method described previously (20). The mass spectra of these products were identical to those of authentic compounds. The amount of diethylene glycol gradually increased as the weight of PUR decreased. After 24-h incubation, 1.2 mg of PUR was degraded and 0.5 mg of diethylene glycol was produced. The amount of diethylene glycol was almost equal to the theoretical amounts derived from degraded PUR. Figure 4 shows the morphological changes of PUR observed by scanning electron microscopy. After 24-h incubation, the PUR developed a large number of pits at the surface.
FIG. 3.
Time course of PUR degradation by purified PUR esterase. The reaction conditions are described in Materials and Methods.
FIG. 4.
Scanning electron micrograph of PUR. (A) Undegraded control; (B and C) PUR after degradation by 0.02 and 0.2 U of PUR esterase, respectively, for 24 h.
Molecular mass determination.
The molecular mass of the PUR esterase was 62,000 Da, as determined by SDS-PAGE. The molecular mass of the native PUR esterase was also estimated to be about 62,000 Da by gel filtration. These observations indicate that the native PUR esterase exists as a monomer. Determination of the molecular mass of the purified PUR esterase by gel filtration without the addition of 0.2% deoxy-BIGCHAP to the buffer was unsuccessful. This result was due to binding of the PUR esterase to Superose because of its high hydrophobicity.
Optimal reaction conditions.
The optimum pH for PUR esterase was determined by activity assays using PUR at 30°C. The PUR degradation activity was detected at the pH range from 4.0 to 8.0, and the highest activity was observed at pH 6.5. The optimum temperature for PUR esterase was 45°C. The thermostability was also determined: PUR esterase was stable within 30 min of incubation at 55°C but almost inactivated (85%) at 60°C.
Substrate specificity.
The purified PUR esterase degraded poly(diethylene glycol adipate), which consists of soft segments of the PUR, producing diethylene glycol and adipic acid as degradation products. The PUR esterase also degrades low-molecular-weight (5,000) poly(l-lactic acid). In contrast, this enzyme did not degrade other polyesters such as poly(3-hydroxybutyric acid), poly(3-hydroxybutyric acid-co-4-hydroxybutyric acid), and high-molecular-weight (20,000) poly(l-lactic acid). In the case of triglyceride, the PUR esterase could degrade tributyrin but not triolein and olive oil, which are typical substrates for lipase (Table 3).
TABLE 3.
Substrate specificity of PUR esterase
| Substrate | Activity (% degradation) |
|---|---|
| Poly(diethylene glycol adipate) | 36.4 |
| Tributyrin | 100.0 |
| Triolein | 0.0 |
| Poly(3-hydroxybutyrate) | 0.0 |
| Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) | 0.0 |
| Poly(l-lactic acid) | |
| MW, 5,000a | 7.7 |
| MW, 20,000 | 0.0 |
MW, molecular weight.
Effect of detergent on enzyme activity.
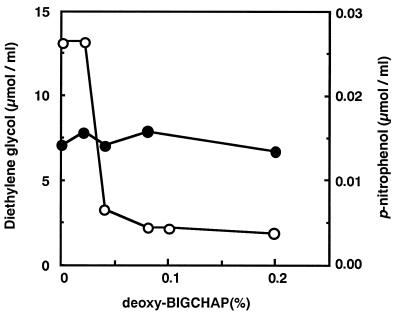
Various concentrations of deoxy-BIGCHAP were added to the reaction mixture, and their effects on enzyme activity were examined. PUR (water-insoluble, solid substrate) and p-nitrophenyl acetate (water-soluble substrate) were used as substrates. As shown in Fig. 5, when the deoxy-BIGCHAP concentration in the reaction mixture exceeded 0.04%, PUR degradation activity decreased drastically. In contrast, when p-nitrophenyl acetate was used as the substrate, the effects of deoxy-BIGCHAP were not observed.
FIG. 5.
Effect of deoxy-BIGCHAP on the activity of the PUR esterase. The substrates used were PUR (○) and p-nitrophenyl acetate (•). The PUR degradation activity was estimated as the amount of diethylene glycol derived from PUR, and the p-nitrophenyl acetate degradation activity was estimated as the amount of p-nitrophenol derived from p-nitrophenyl acetate. The reaction conditions for each substrate are described in Materials and Methods.
Effect of enzyme concentration on PUR degrading activity.
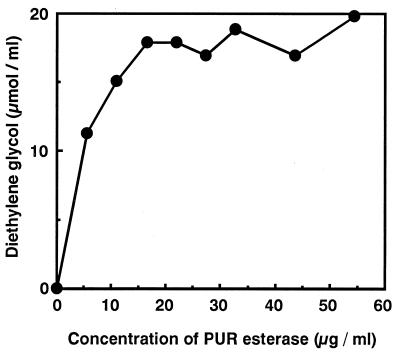
For PHA depolymerase, which is known as a solid-substrate degradation enzyme, a decrease in enzyme activity was reported when the enzyme concentration was increased with the surface area of solid substrate kept constant (17). The effects of the concentration of PUR esterase were examined. Various concentrations of PUR esterase and solid PURs with constant surface area were mixed, and the PUR degradation activities were measured. As shown in Fig. 6, the PUR degradation activities increased in proportion with the increase in enzyme concentration up to 15 μg/ml, above which the activities remained constant. Reduction of PUR-degrading activity was not observed.
FIG. 6.
Effect of PUR esterase concentration on PUR degradation.
DISCUSSION
The cell-bound PUR esterase of strain TB-35 was purified easily, because all of the purification steps except for the final step consist of batch procedures. This enzyme was able to degrade polyester PUR, yielding adipic acid and diethylene glycol as degradation products. The amounts of these degradation products showed good agreement with the theoretical amounts calculated from the degraded PUR. These observations strongly indicate that the ester bonds of the PUR were hydrolyzed by the PUR esterase and that adipic acid and diethylene glycol were released as degradation products. On the other hand, it is unclear whether the urethane bond is hydrolyzed by the PUR esterase, because degradation products derived from polyisocyanate segments of the PUR were not detected.
Among the esterolytic enzymes which can degrade solid polyesters, lipase and PHA depolymerase have been well studied. In contrast, the purified PUR esterase used in this study did not exhibit either lipase activity or PHA depolymerase activity (Table 3). This observation suggests that this enzyme is a novel plastic-degrading esterase with properties different from those of lipase or PHA depolymerase. The substrate specificity of the PUR esterase was thought to be narrow, since with the exception of low-molecular-weight poly(l-lactic acid), it had no degradation activities for other polyesters.
Unlike water-soluble substrates, solid substrates are thought to have extremely low efficacy of contact with enzyme molecules. Enzymes which degrade solid substrates are considered to have some functions which enable them to adsorb to the surface of solid substrates (4, 5, 26). For example, in PHA depolymerase, the existence of a hydrophobic PHA-binding domain has already been determined by analyses of its amino acid sequence (1, 6). The purified PUR esterase had a high hydrophobicity, and this hydrophobicity was thought to be important for PUR degradation. That is, we considered the PUR esterase to have a hydrophobic PUR surface-binding domain and a catalytic domain and the degradation of the PUR to be done by two steps. The first step is hydrophobic adsorption of the enzyme to the surface of the PUR via the surface-binding domain, and the second step is hydrolysis of the ester bond. This speculation is strongly supported by the fact that the PUR degradation activity of this enzyme was extremely inhibited by the addition of detergent, such as deoxy-BIGCHAP (Fig. 5). This detergent did not inhibit the active site of the PUR esterase, because when p-nitrophenyl acetate, a water-soluble compound, was used as a substrate, deoxy-BIGCHAP did not inhibit its esterase activity.
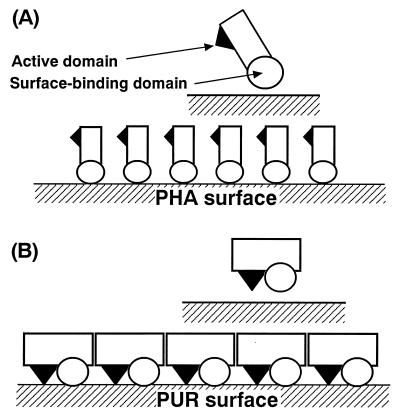
Recently, Mukai et al. (17) reported that the degradation activity of the PHA depolymerase was inhibited by excess amounts of the enzyme. They explain this finding as follows. When the enzyme concentration was increased, the surface of the PHA film became saturated by the surface-binding domain of the enzyme. However, the catalytic domain could not gain access to the film surface (Fig. 7A). This phenomenon is thought to be attributed to the three-dimensional structure of the enzyme, which exhibits a large distance between the surface-binding domain and the catalytic domain of the enzyme polypeptide chain.
FIG. 7.
Kinetic models of the surface binding and hydrolysis of PHA by PHA depolymerase (A) (17) and of PUR by the PUR esterase (B).
On the other hand, in the case of PUR esterase, the degradation activity did not decrease but rather remained constant when an excess amount of the enzyme was present (Fig. 6). From this observation, it was thought that the surface binding site and catalytic site of the PUR esterase exist in three-dimensionally close positions, unlike those of PHA depolymerase. In this model, the catalytic domain can gain access to the PUR surface even if the PUR surface has been saturated by the enzyme molecules. However, since the number of adsorbable enzyme molecules per surface area of the PUR is fixed (Fig. 7B), the PUR degradation activity remained constant. Furthermore, in contrast to PHA depolymerase, which is strictly extracellular, the PUR esterase is bound to the cell surface; therefore, the PUR esterase may have a cell surface-binding domain in addition to catalytic and PUR surface-binding domains. The molecular cloning of the genes for the PUR esterase is under way in our laboratory.
ACKNOWLEDGMENTS
We thank Noriko Kimpara, Suzuki Motor Co., for performing the electron microscopy. We also thank Takashi Nishida, in our laboratory, for his technical assistance.
This work was partly supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science and Culture, Japan.
REFERENCES
- 1.Behrends A, Klingbeil B, Jendrossek D. Poly(3-hydroxybutyrate) depolymerases bind to their substrate by a C-terminal located substrate binding site. FEMS Microbiol Lett. 1996;143:191–194. doi: 10.1111/j.1574-6968.1996.tb08479.x. [DOI] [PubMed] [Google Scholar]
- 2.Crabbe J R, Campbell J R, Thompson L, Walz S L, Schultz W W. Biodegradation of a colloidal ester-based polyurethane by soil fungi. Int Biodeterior Biodegrad. 1994;33:103–113. [Google Scholar]
- 3.Darby R T, Kaplan A M. Fungal susceptibility of polyurethanes. Appl Microbiol. 1968;16:900–905. doi: 10.1128/am.16.6.900-905.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fukui T, Narikawa T, Miwa K, Shirakura Y, Saito T, Tomita K. Effect of limited tryptic modification of a bacterial poly(3-hydroxybutyrate) depolymerase on its catalytic activity. Biochim Biophys Acta. 1988;952:164–171. doi: 10.1016/0167-4838(88)90112-4. [DOI] [PubMed] [Google Scholar]
- 5.Hansen C K. Fibronectin type III-like sequences and a new domain type in prokaryotic depolymerases with insoluble substrates. FEBS Lett. 1992;305:91–96. doi: 10.1016/0014-5793(92)80871-d. [DOI] [PubMed] [Google Scholar]
- 6.Jendrossek D, Frisse A, Behrends A, Andermann M, Kratzin H, Stanislawski T, Schlegel H G. Biochemical and molecular characterization of the Pseudomonas lemoignei polyhydroxyalkanoate depolymerase system. J Bacteriol. 1995;177:596–607. doi: 10.1128/jb.177.3.596-607.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kanesawa Y, Tanahashi N, Doi Y. Enzymatic degradation of microbial poly(3-hydroxyalkanoates) Polym Degrad Stabil. 1994;45:179–185. [Google Scholar]
- 8.Kasuya K, Doi Y. Enzymatic degradation of poly[(R)-3-hydroxybutyrate] by Comamonas testosteroni ATSU of soil bacterium. Polym Degrad Stabil. 1994;45:379–386. [Google Scholar]
- 9.Kasuya K, Inoue Y, Doi Y. Adsorption kinetics of bacterial PHB depolymerase on the surface of polyhydroxyalkanoate films. Int J Biol Macromol. 1996;19:35–40. doi: 10.1016/0141-8130(96)01097-5. [DOI] [PubMed] [Google Scholar]
- 10.Kasuya K, Inoue Y, Yamada K, Doi Y. Kinetics of surface hydrolysis of poly[(R)-3-hydroxybutyrate] film by PHB depolymerase from Alcaligenes faecalis T1. Polym Degrad Stabil. 1995;48:167–174. [Google Scholar]
- 11.Kay M J, Morton L H G, Prince E L. Bacterial degradation of polyester polyurethane. Int Biodeterior. 1991;27:205–222. [Google Scholar]
- 12.Kay M J, McCabe R W, Morton L H G. Chemical and physical changes occurring in polyester polyurethane during biodegradation. Int Biodeterior Biodegrad. 1993;31:209–225. [Google Scholar]
- 13.Kita K, Ishimaru K, Teraoka M, Yanase H, Kato N. Properties of poly(3-hydroxybutyrate) depolymerase from a marine bacterium, Alcaligenes faecalis AE122. Appl Environ Microbiol. 1995;61:1727–1730. doi: 10.1128/aem.61.5.1727-1730.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 15.Morton L H G, Surman S B. Biofilms in biodeterioration—a review. Int Biodeterior Biodegrad. 1994;32:203–221. [Google Scholar]
- 16.Mukai K, Yamada K, Doi Y. Extracellular poly(hydroxyalkanoate) depolymerases and their inhibitor from Pseudomonas lemoignei. Int J Biol Macromol. 1992;14:235–239. doi: 10.1016/s0141-8130(05)80034-0. [DOI] [PubMed] [Google Scholar]
- 17.Mukai K, Yamada K, Doi Y. Kinetics and mechanism of heterogeneous hydrolysis of poly[(R)-3-hydroxybutyrate] film by PHA depolymerases. Int J Biol Macromol. 1993;15:361–366. doi: 10.1016/0141-8130(93)90054-p. [DOI] [PubMed] [Google Scholar]
- 18.Müller B, Jendrossek D. Purification and properties of poly(3-hydroxyvaleric acid) depolymerase from Pseudomonas lemoignei. Appl Microbiol Biotechnol. 1993;38:487–492. [Google Scholar]
- 19.Nakajima-Kambe T, Onuma F, Kimpara N, Nakahara T. Isolation and characterization of a bacterium which utilizes polyester polyurethane as a sole carbon and nitrogen source. FEMS Microbiol Lett. 1995;129:39–42. doi: 10.1016/0378-1097(95)00131-N. [DOI] [PubMed] [Google Scholar]
- 20.Nakajima-Kambe T, Onuma F, Akutsu Y, Nakahara T. Determination of the polyester polyurethane breakdown products and distribution of the polyurethane degrading enzyme of Comamonas acidovorans strain TB-35. J Ferment Bioeng. 1997;83:454–458. [Google Scholar]
- 21.Nojima S, Mineki S, Iida M. Purification and characterization of extracellular poly(3-hydroxybutyrate) depolymerases produced by Agrobacterium sp. K-03. J Ferment Bioeng. 1996;81:72–75. [Google Scholar]
- 22.Pathirana R A, Seal K J. Studies on polyurethane deteriorating fungi. Part 1. Isolation and characterization of the test fungi employed. Int Biodeterior. 1984;20:163–168. [Google Scholar]
- 23.Pathirana R A, Seal K J. Studies on polyurethane deteriorating fungi. Part 2. An examination of their enzyme activities. Int Biodeterior. 1984;20:229–235. [Google Scholar]
- 24.Schirmer A, Jendrossek D, Schlegel H G. Degradation of poly(3-hydroxyoctanoic acid) [P(3HO)] by bacteria: purification and properties of a P(3HO) depolymerase from Pseudomonas fluorescens GK13. Appl Environ Microbiol. 1993;59:1220–1227. doi: 10.1128/aem.59.4.1220-1227.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tanio T, Fukui T, Shirakura Y, Saito T, Tomita K, Kaiho T, Masamune S. An extracellular poly(3-hydroxybutyrate) depolymerase from Alcaligenes faecalis. Eur J Biochem. 1982;124:71–77. doi: 10.1111/j.1432-1033.1982.tb05907.x. [DOI] [PubMed] [Google Scholar]
- 26.van Tilbeurgh H, Tomme P, Claeyssens M, Bhikhabhai R, Pettersson G. Limited proteolysis of the cellobiohydrolase I from Trichoderma reesei. FEBS Lett. 1986;204:223–227. [Google Scholar]