Abstract
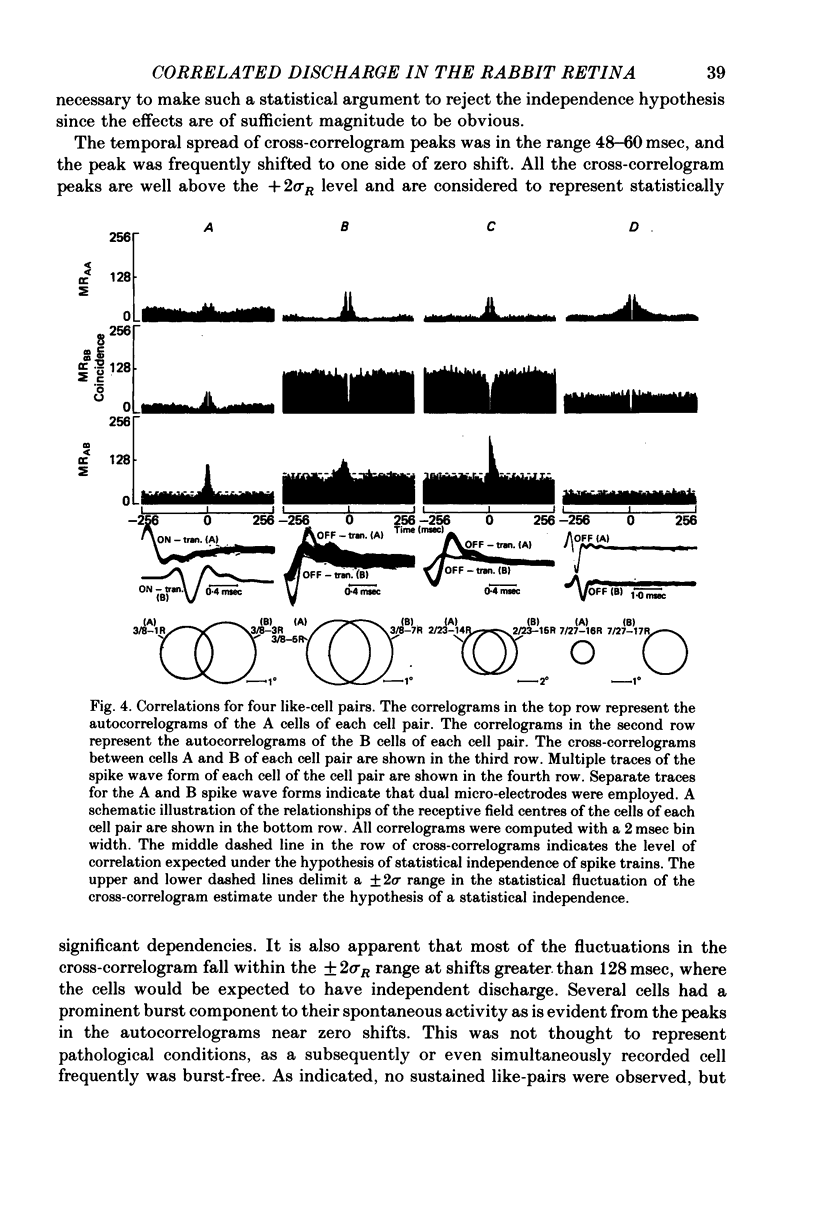
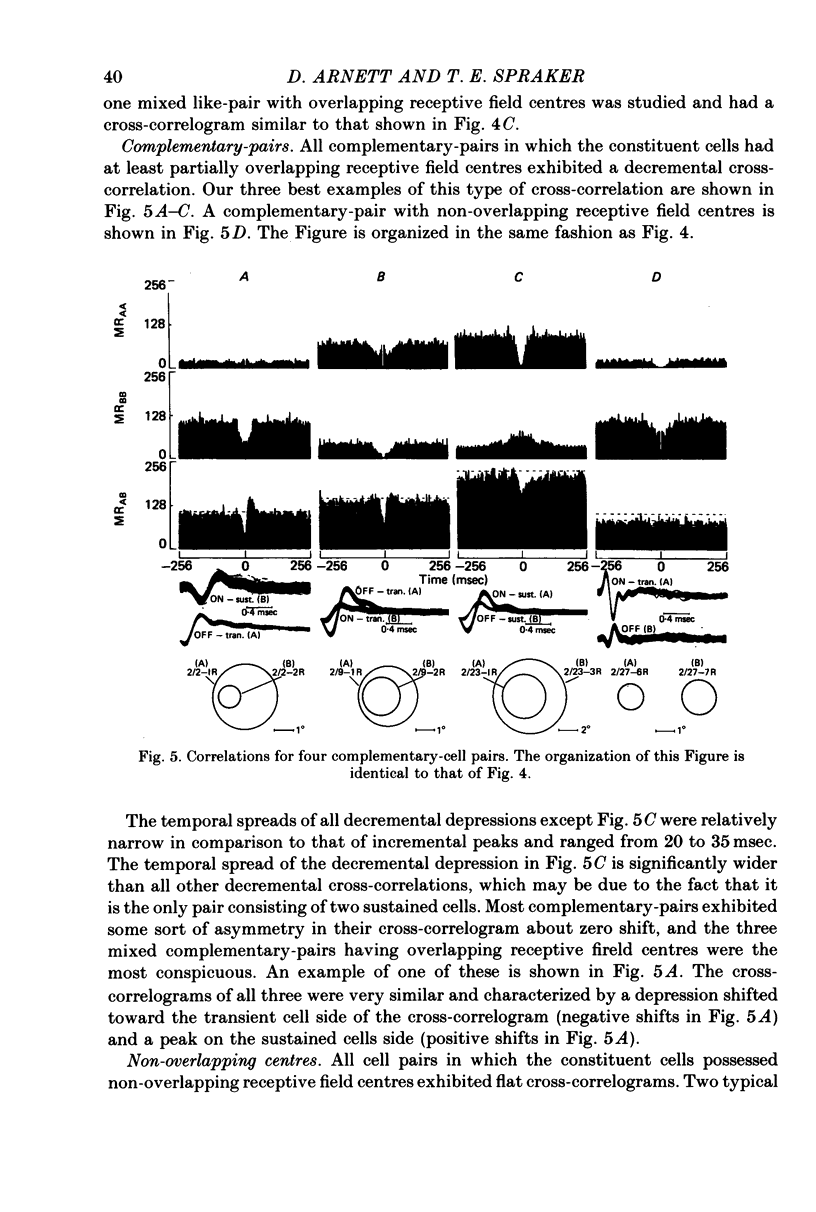
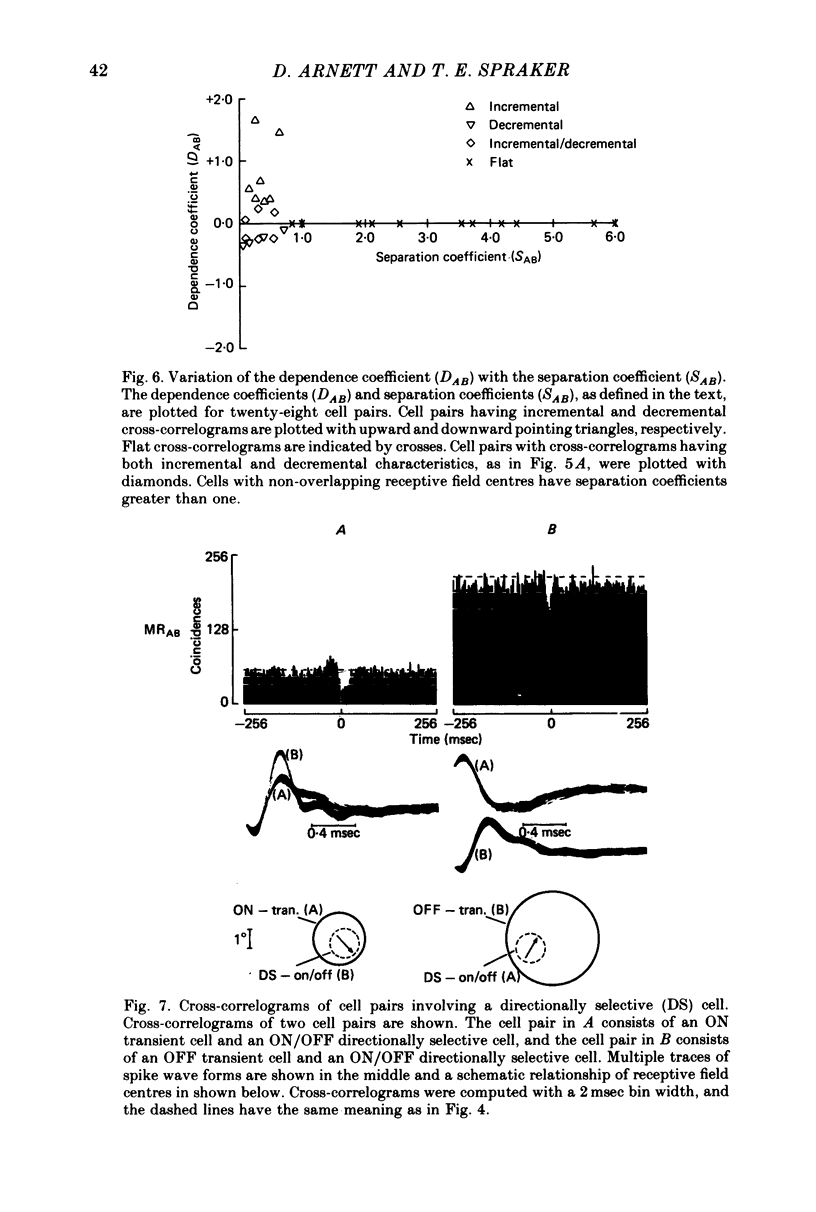
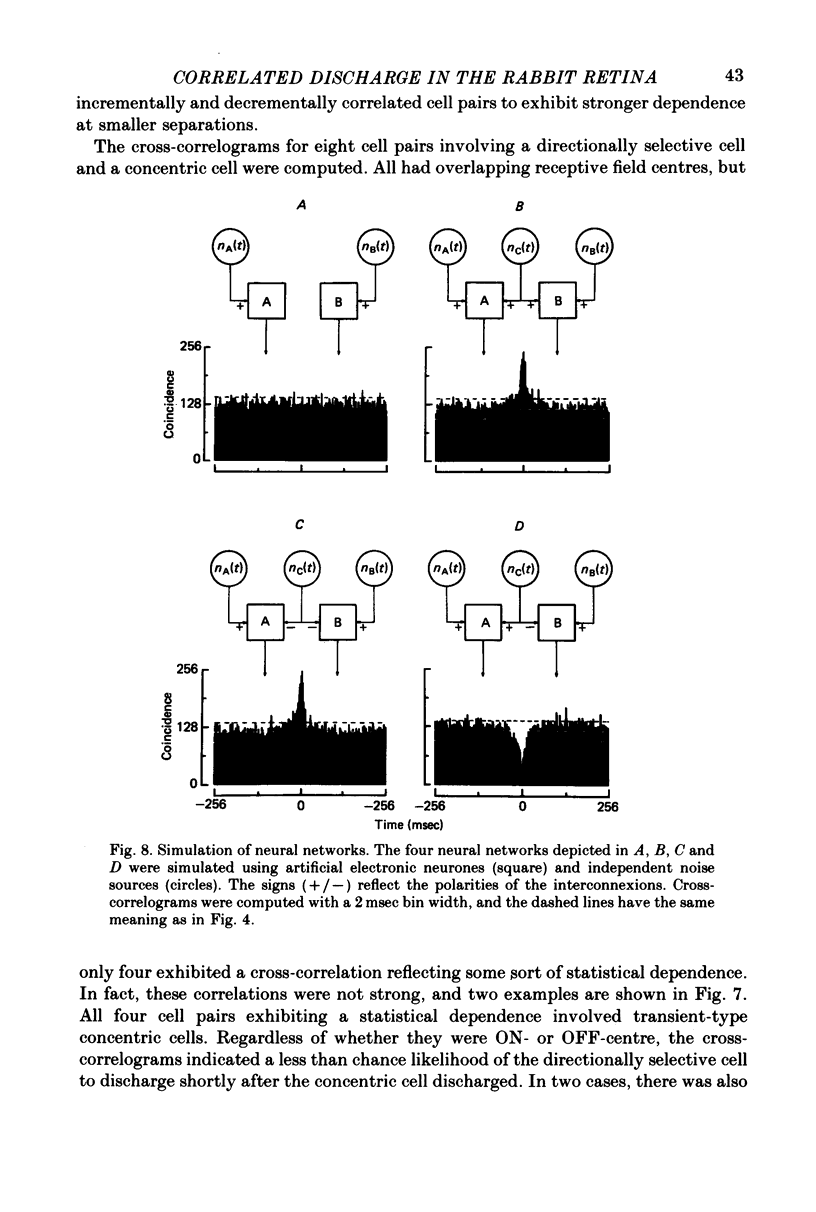
1. Simultaneous recordings were made from pairs of rabbit retinal ganglion cells. Physiological tests were used to classify the receptive field properties of each cell and the receptive field locations were mapped. 2. The statistical dependence between simultaneously recorded retinal ganglion cells was assessed by cross-correlating the maintained discharge of the simultaneously recorded cells. Cross-correlations from cell pairs in which the constituent cells had non-overlapping receptive field centres were statistically flat, reflecting no statistical dependence. 3. Most cell pairs consisting of transient and sustained concentric cells and having overlapping receptive field centres exhibited a correlated maintained discharge indicative of statistical dependence. The strength of the statistical dependence varied approximately inversely with the degree of overlap between the two cells comprising the cell pair. 4. Cell pairs consisting of two ON-centre cells or two OFF-centre cells and having overlapping receptive field centres possessed incremental cross-correlations which were characterized by a peak centred near zero. Cell pairs consisting of an ON-centre cell with an OFF-centre and having overlapping receptive field centres possessed decremental cross-correlations which were characterized by a valley centred near zero. 5. The results are consistent with the hypothesis that a noise source provides shared input to two or more retinal ganglion cells. Bipolar and photoreceptors are the most likely sources of noise responsible for the statistical dependency between retinal ganglion cells.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnett D. W. Correlation analysis of units recorded in the cat dorsal lateral geniculate nucleus. Exp Brain Res. 1975 Dec 22;24(2):111–130. doi: 10.1007/BF00234058. [DOI] [PubMed] [Google Scholar]
- Arnett D. Optical pattern generator for visual research. Med Biol Eng. 1976 Sep;14(5):532–537. doi: 10.1007/BF02478052. [DOI] [PubMed] [Google Scholar]
- BROWN K. T., WIESEL T. N. Intraretinal recording with micropipette electrodes in the intact cat eye. J Physiol. 1959 Dec;149:537–562. doi: 10.1113/jphysiol.1959.sp006360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Fettiplace R. Transmission from photoreceptors to ganglion cells in turtle retina. J Physiol. 1977 Oct;271(2):391–424. doi: 10.1113/jphysiol.1977.sp012006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell J. H., Daw N. W. New properties of rabbit retinal ganglion cells. J Physiol. 1978 Mar;276:257–276. doi: 10.1113/jphysiol.1978.sp012232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland B. G., Levick W. R. Brisk and sluggish concentrically organized ganglion cells in the cat's retina. J Physiol. 1974 Jul;240(2):421–456. doi: 10.1113/jphysiol.1974.sp010617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creutzfeldt O., Innocenti G. M., Brooks D. Vertical organization in the visual cortex (area 17) in the cat. Exp Brain Res. 1974;21(3):315–336. doi: 10.1007/BF00235750. [DOI] [PubMed] [Google Scholar]
- Dowling J. E., Boycott B. B. Neural connections of the retina: fine structure of the inner plexiform layer. Cold Spring Harb Symp Quant Biol. 1965;30:393–402. doi: 10.1101/sqb.1965.030.01.039. [DOI] [PubMed] [Google Scholar]
- Dubin M. W., Cleland B. G. Organization of visual inputs to interneurons of lateral geniculate nucleus of the cat. J Neurophysiol. 1977 Mar;40(2):410–427. doi: 10.1152/jn.1977.40.2.410. [DOI] [PubMed] [Google Scholar]
- Dubin M. W. The inner plexiform layer of the vertebrate retina: a quantitative and comparative electron microscopic analysis. J Comp Neurol. 1970 Dec;140(4):479–505. doi: 10.1002/cne.901400406. [DOI] [PubMed] [Google Scholar]
- Enroth-Cugell C., Robson J. G. The contrast sensitivity of retinal ganglion cells of the cat. J Physiol. 1966 Dec;187(3):517–552. doi: 10.1113/jphysiol.1966.sp008107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French A. S., Stein R. B. A flexible neural analog using integrated circuits. IEEE Trans Biomed Eng. 1970 Jul;17(3):248–253. doi: 10.1109/tbme.1970.4502739. [DOI] [PubMed] [Google Scholar]
- Gestri G., Maffei L., Petracchi D. Spatial and temporal organization in retinal units. Kybernetik. 1966 Nov;3(4):196–202. doi: 10.1007/BF00290257. [DOI] [PubMed] [Google Scholar]
- Hughes A. Topographical relationships between the anatomy and physiology of the rabbit visual system. Doc Ophthalmol. 1971 Sep 12;30:33–159. doi: 10.1007/BF00142518. [DOI] [PubMed] [Google Scholar]
- Jakiela H. G., Enroth-Cugell C. Adaptation and dynamics in X-cells and Y-cells of the cat retina. Exp Brain Res. 1976 Feb 26;24(4):335–342. doi: 10.1007/BF00235001. [DOI] [PubMed] [Google Scholar]
- Johnson D. H., Kiang N. Y. Analysis of discharges recorded simultaneously from pairs of auditory nerve fibers. Biophys J. 1976 Jul;16(7):719–734. doi: 10.1016/S0006-3495(76)85724-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight B. W. Dynamics of encoding in a population of neurons. J Gen Physiol. 1972 Jun;59(6):734–766. doi: 10.1085/jgp.59.6.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb T. D., Simon E. J. The relation between intercellular coupling and electrical noise in turtle photoreceptors. J Physiol. 1976 Dec;263(2):257–286. doi: 10.1113/jphysiol.1976.sp011631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B. B., Cleland B. G., Creutzfeldt O. D. The retinal input to cells in area 17 of the cat's cortex. Exp Brain Res. 1977 Dec 19;30(4):527–538. doi: 10.1007/BF00237642. [DOI] [PubMed] [Google Scholar]
- Levick W. R., Cleland B. G., Dubin M. W. Lateral geniculate neurons of cat: retinal inputs and physiology. Invest Ophthalmol. 1972 May;11(5):302–311. [PubMed] [Google Scholar]
- Naka K. Functional organization of catfish retina. J Neurophysiol. 1977 Jan;40(1):26–43. doi: 10.1152/jn.1977.40.1.26. [DOI] [PubMed] [Google Scholar]
- Perkel D. H., Gerstein G. L., Moore G. P. Neuronal spike trains and stochastic point processes. II. Simultaneous spike trains. Biophys J. 1967 Jul;7(4):419–440. doi: 10.1016/S0006-3495(67)86597-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodieck R. W. Maintained activity of cat retinal ganglion cells. J Neurophysiol. 1967 Sep;30(5):1043–1071. doi: 10.1152/jn.1967.30.5.1043. [DOI] [PubMed] [Google Scholar]
- Schellart N. A., Spekreijse H. Origin of the stochastic nature of ganglion cell activity in isolated goldfish retina. Vision Res. 1973 Feb;13(2):337–345. doi: 10.1016/0042-6989(73)90111-9. [DOI] [PubMed] [Google Scholar]
- Schwartz E. A. Voltage noise observed in rods of the turtle retina. J Physiol. 1977 Nov;272(2):217–246. doi: 10.1113/jphysiol.1977.sp012042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer W., Creutzfeldt O. D. Reciprocal lateral inhibition of on- and off-center neurones in the lateral geniculate body of the cat. Exp Brain Res. 1970;10(3):311–330. doi: 10.1007/BF00235054. [DOI] [PubMed] [Google Scholar]
- Steinberg R. H., Walker M. L., Johnson W. M. A new microelectrode positioner for intraretinal recording from the intact mammalian eye. Vision Res. 1968 Dec;8(12):1521–1523. doi: 10.1016/0042-6989(68)90126-0. [DOI] [PubMed] [Google Scholar]
- Stevens J. K., Gerstein G. L. Interactions between cat lateral geniculate neurons. J Neurophysiol. 1976 Mar;39(2):239–256. doi: 10.1152/jn.1976.39.2.239. [DOI] [PubMed] [Google Scholar]
- Stevens J. K., Gerstein G. L. Spatiotemporal organization of cat lateral geniculate receptive fields. J Neurophysiol. 1976 Mar;39(2):213–238. doi: 10.1152/jn.1976.39.2.213. [DOI] [PubMed] [Google Scholar]
- Stone J., Fukuda Y. Properties of cat retinal ganglion cells: a comparison of W-cells with X- and Y-cells. J Neurophysiol. 1974 Jul;37(4):722–748. doi: 10.1152/jn.1974.37.4.722. [DOI] [PubMed] [Google Scholar]



