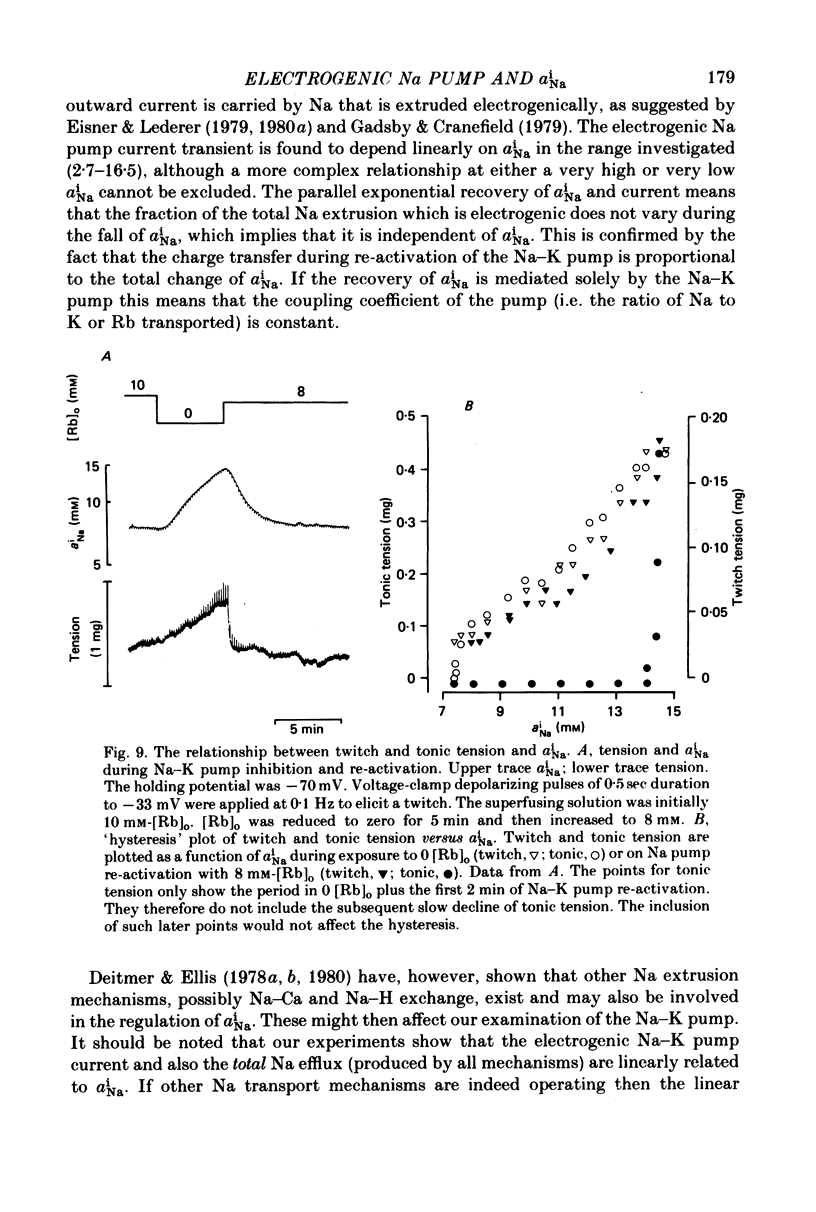
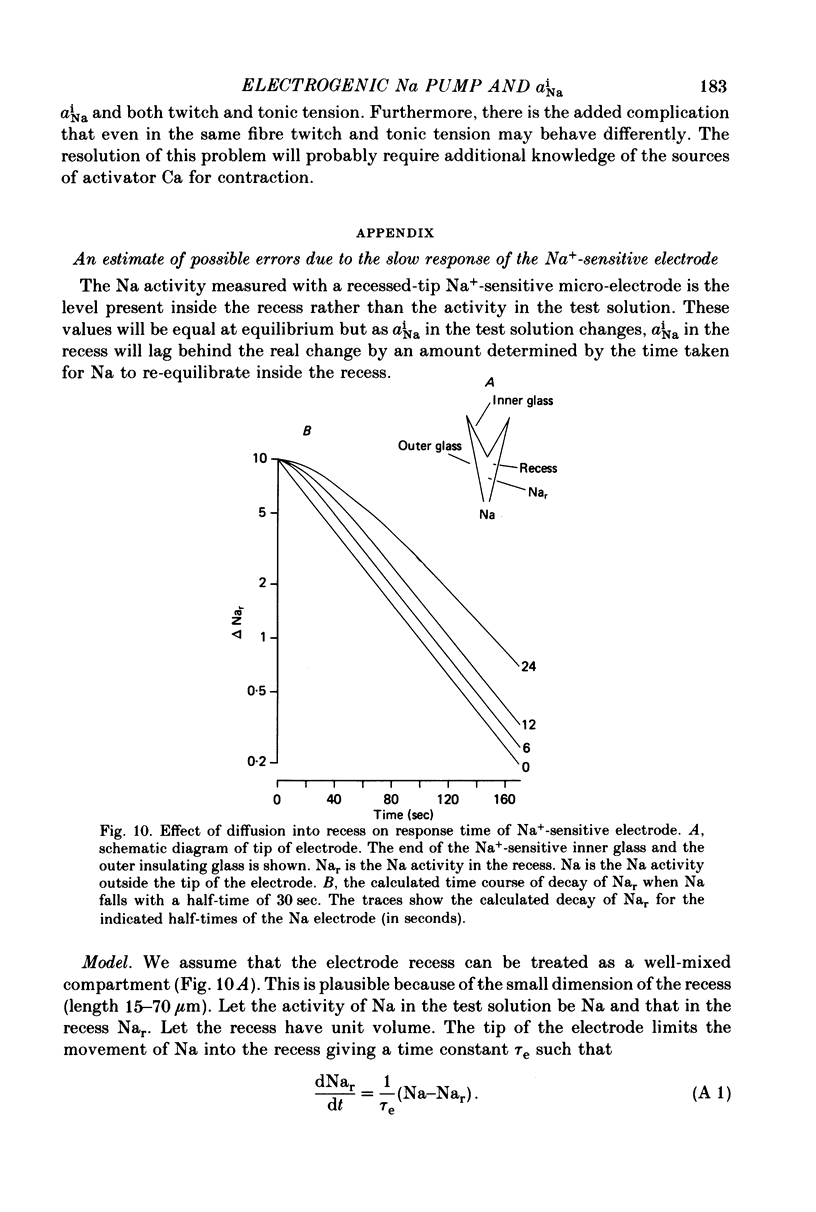
Abstract
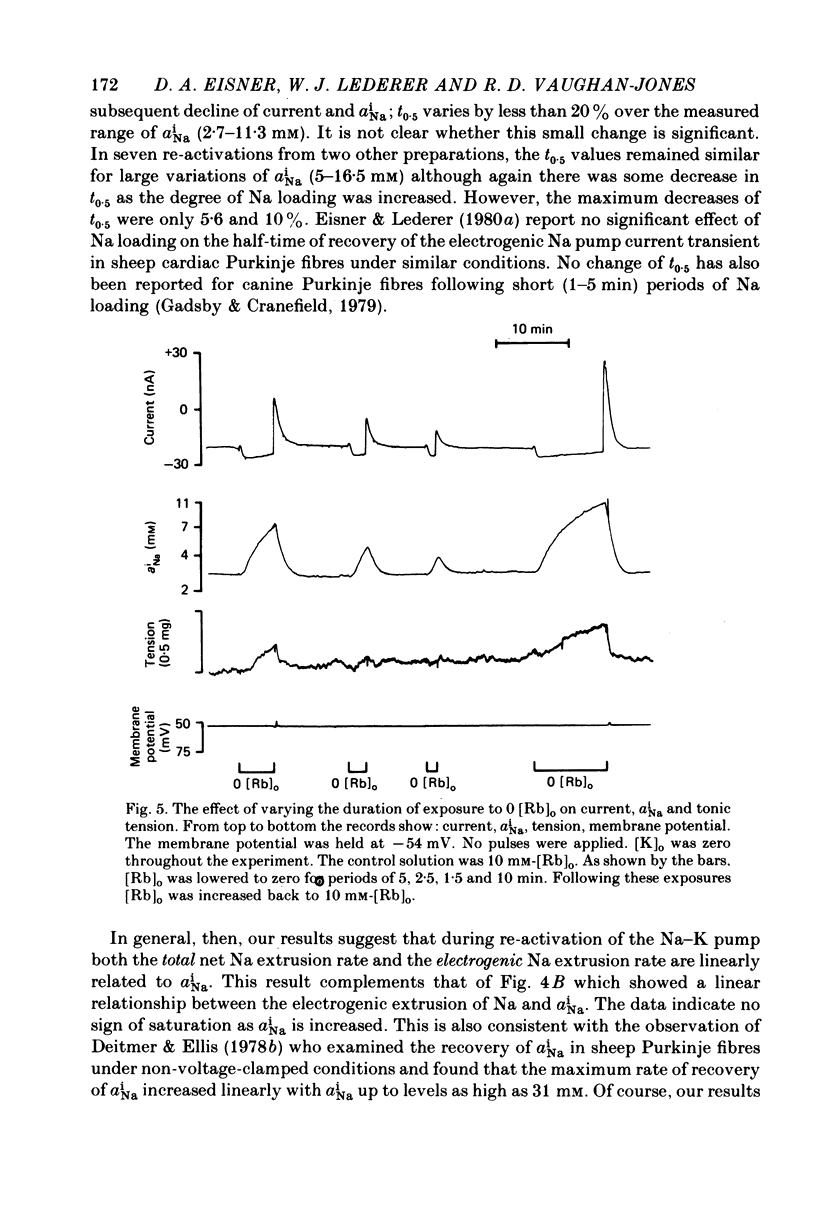
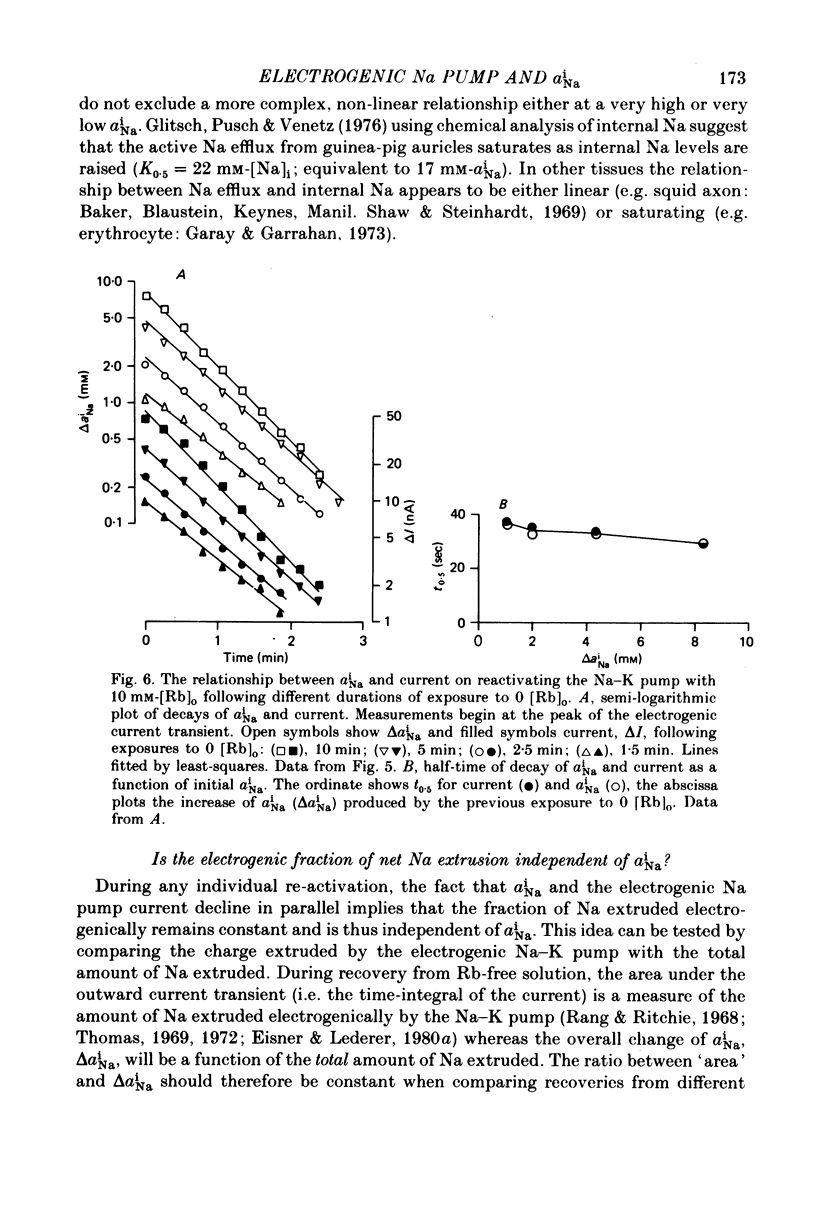
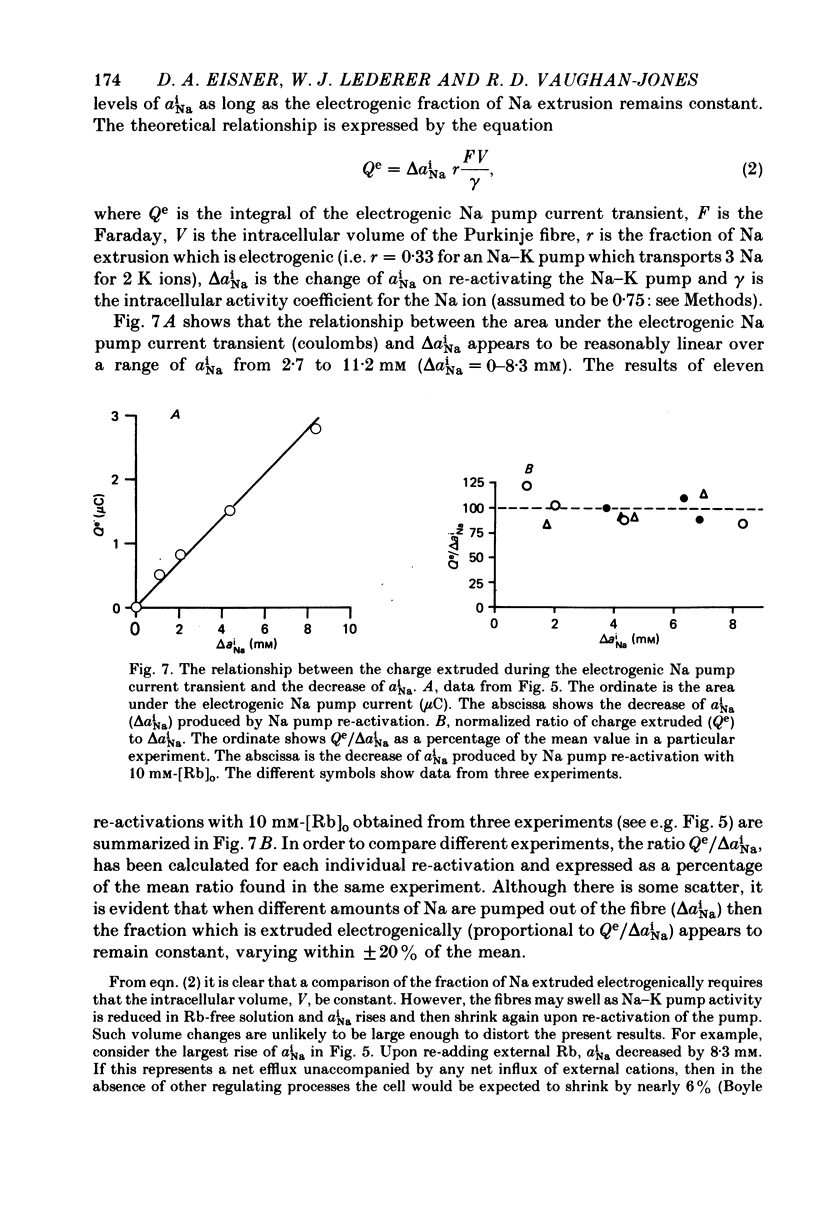
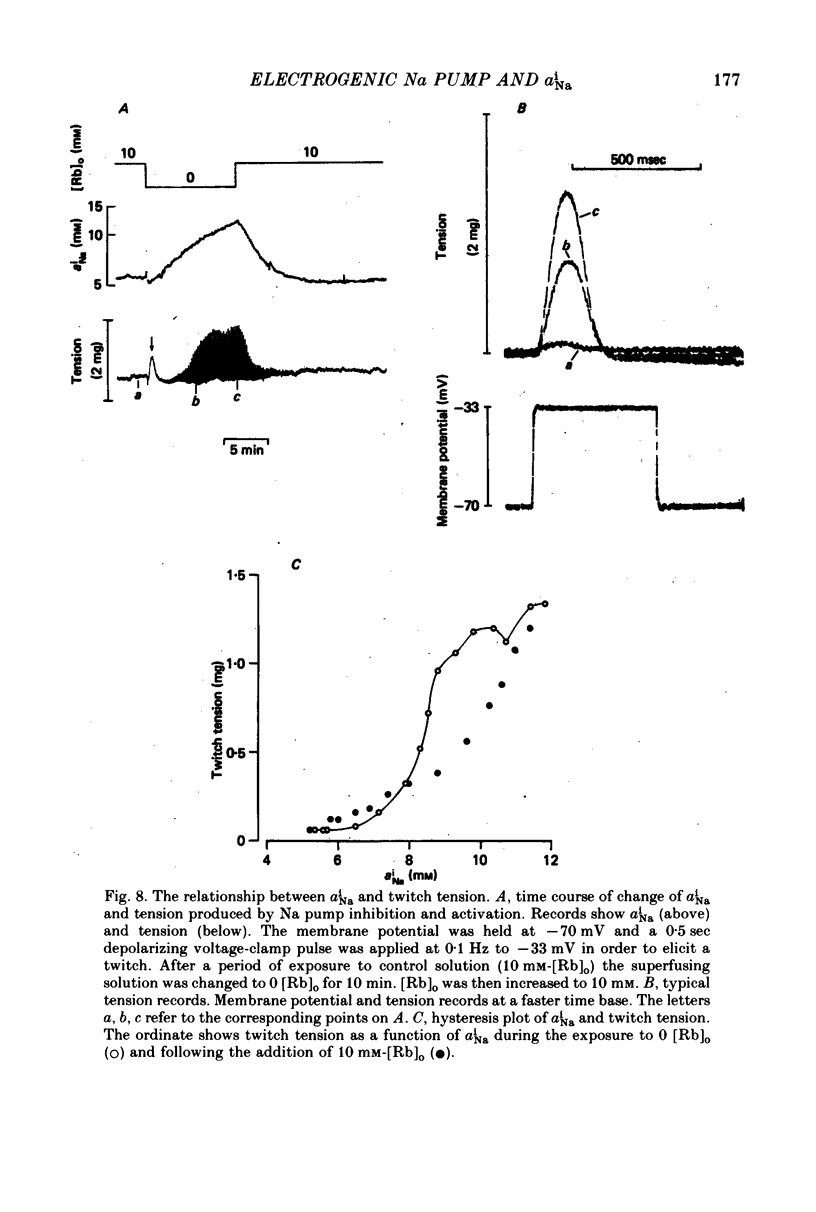
1. Intracellular Na activity (aiNa) was measured in sheep cardiac Purkinje fibres using a recessed-tip Na+-sensitive micro-electrode. The membrane potentials was controlled with a two-micro-electrode voltage clamp. Tension was measured simultaneously. 2. Removing external K produced a rise of aiNa and both twitch and tonic tension. On adding 4-10 mM-[Rb]0 to reactivate the Na-K pump aiNa and tension declined. An electrogenic Na pump current transient accompanied the fall of aiNa. 3. The half-time of decay of the electrogenic Na pump current transient was similar to that of aiNa, (mean tNa0.5/tI0.5 = 0.97 +/- 0.03 (S.E.M.; n = 28)). Following the re-activation of the Na-K pump, the electrogenic Na pump current transient was linearly related to aiNa. 4. The duration of exposure to K-free, Rb-free solutions was varied to change the level of aiNa. On subsequently re-activating the Na-K pump with 10 mM-[Rb]0, the ratio of the charge extruded to the total change of aiNa was constant. It is concluded that the fraction of Na extruded electrogenically is unaffected by changes of aiNa. About 26% of the total Na extrusion appeared as charge transfer. 5. The relationship between tonic tension and aiNa was usually different during Na-K pump inhibition in a K-free, Rb-free solution compared with the relationship during Na-K pump re-activation. In general, a given aiNa was associated with a greater level of tonic tension during Na-K pump inhibition compared with that during pump re-activation. A similar hysteresis was often seen between twitch tension and aiNa.
Full text
PDF


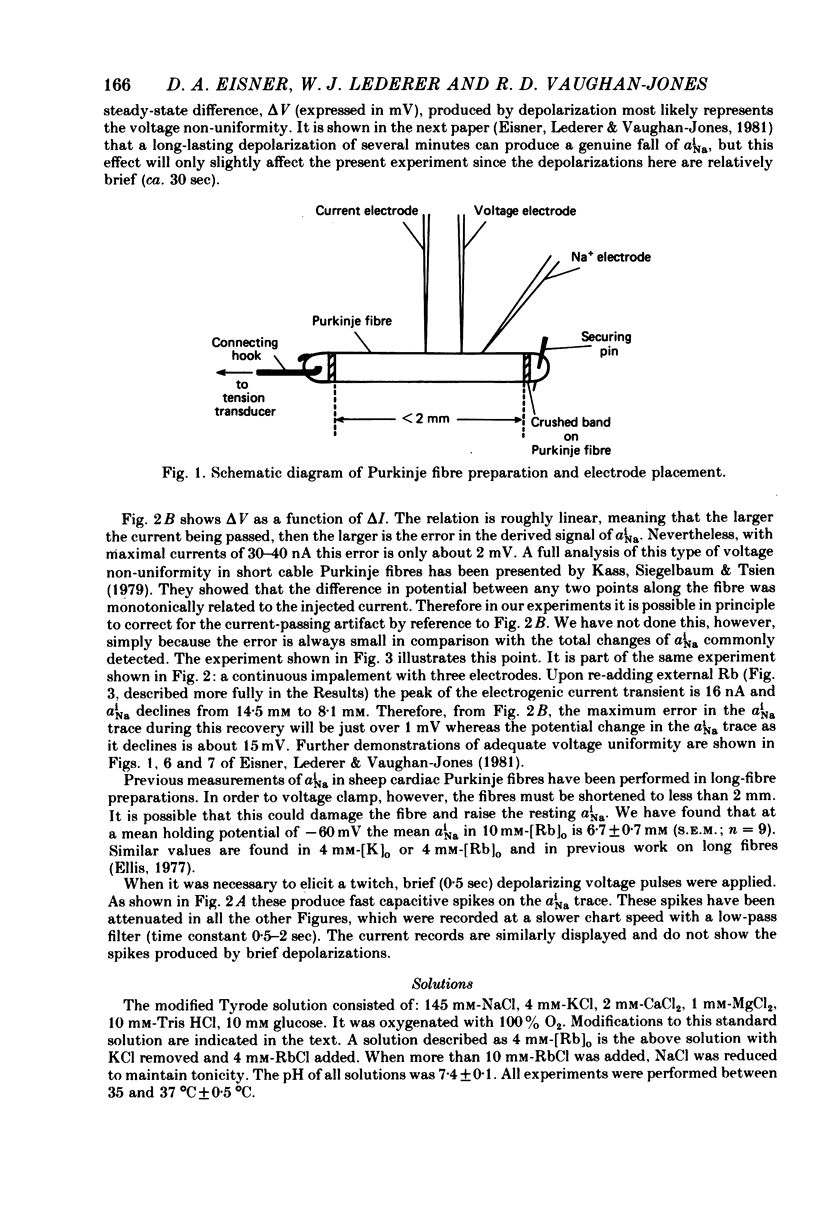
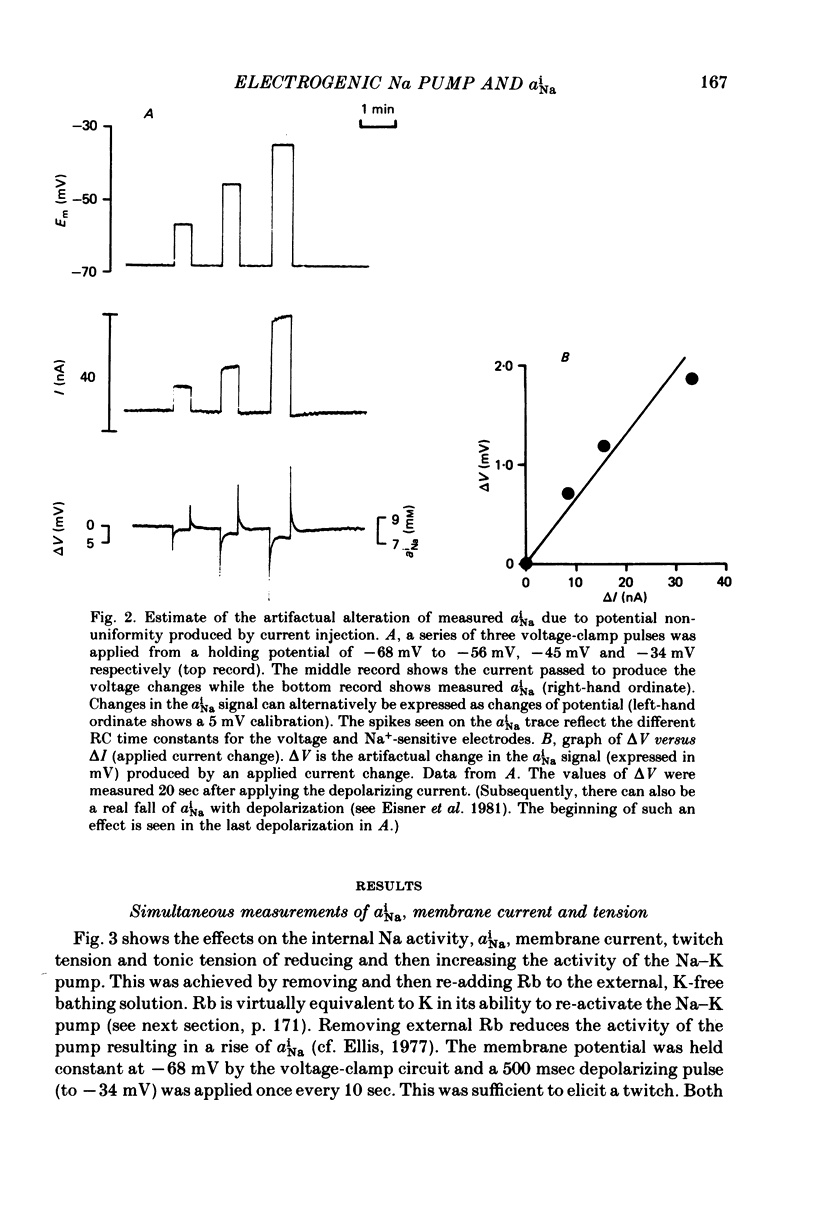
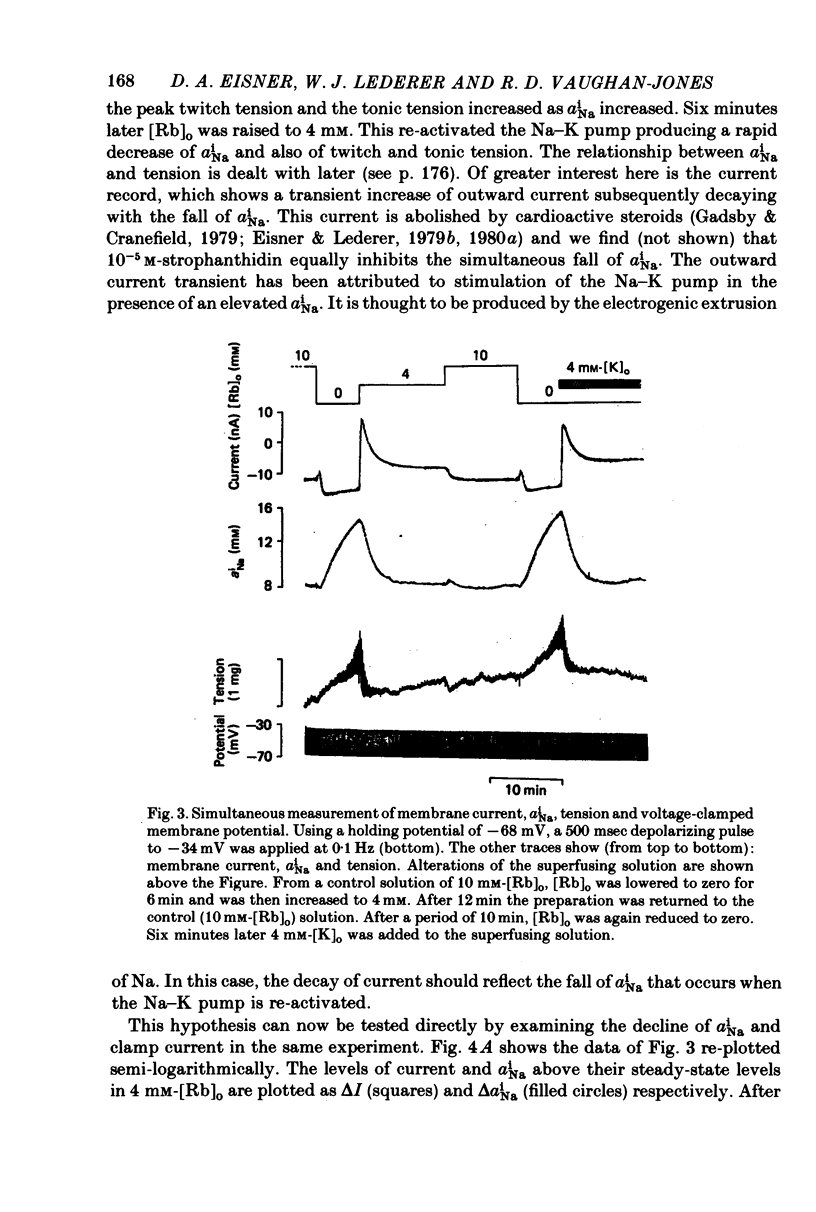
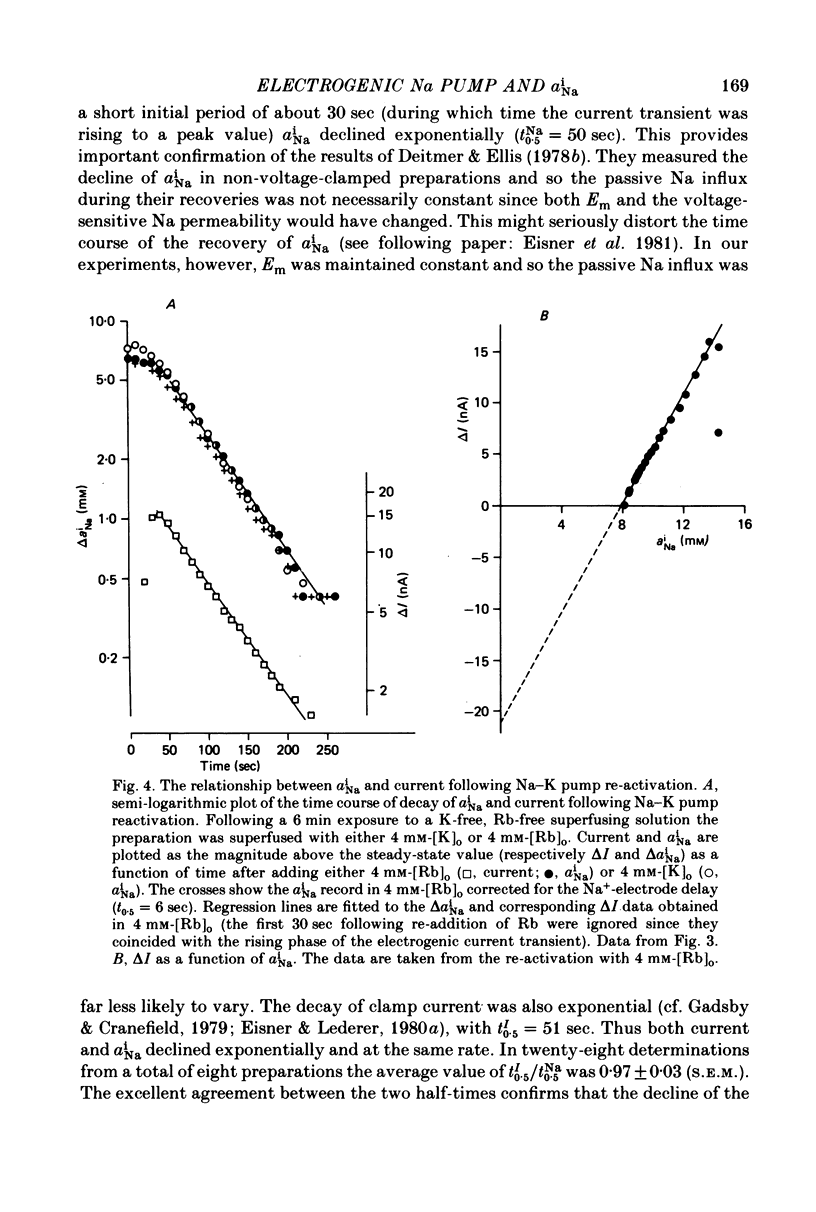












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian R. H., Slayman C. L. Membrane potential and conductance during transport of sodium, potassium and rubidium in frog muscle. J Physiol. 1966 Jun;184(4):970–1014. doi: 10.1113/jphysiol.1966.sp007961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Blaustein M. P., Hodgkin A. L., Steinhardt R. A. The influence of calcium on sodium efflux in squid axons. J Physiol. 1969 Feb;200(2):431–458. doi: 10.1113/jphysiol.1969.sp008702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Blaustein M. P., Keynes R. D., Manil J., Shaw T. I., Steinhardt R. A. The ouabain-sensitive fluxes of sodium and potassium in squid giant axons. J Physiol. 1969 Feb;200(2):459–496. doi: 10.1113/jphysiol.1969.sp008703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle P. J., Conway E. J. Potassium accumulation in muscle and associated changes. J Physiol. 1941 Aug 11;100(1):1–63. doi: 10.1113/jphysiol.1941.sp003922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DECK K. A., KERN R., TRAUTWEIN W. VOLTAGE CLAMP TECHNIQUE IN MAMMALIAN CARDIAC FIBRES. Pflugers Arch Gesamte Physiol Menschen Tiere. 1964 Jun 9;280:50–62. doi: 10.1007/BF00412615. [DOI] [PubMed] [Google Scholar]
- DELEZE J. Possible reasons for drop of resting potential of mammalian heart preparations during hypothermia. Circ Res. 1960 May;8:553–557. doi: 10.1161/01.res.8.3.553. [DOI] [PubMed] [Google Scholar]
- Deitmer J. W., Ellis D. Changes in the intracellular sodium activity of sheep heart Purkinje fibres produced by calcium and other divalent cations. J Physiol. 1978 Apr;277:437–453. doi: 10.1113/jphysiol.1978.sp012283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deitmer J. W., Ellis D. Interactions between the regulation of the intracellular pH and sodium activity of sheep cardiac Purkinje fibres. J Physiol. 1980 Jul;304:471–488. doi: 10.1113/jphysiol.1980.sp013337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deitmer J. W., Ellis D. The intracellular sodium activity of cardiac Purkinje fibres during inhibition and re-activation of the Na-K pump. J Physiol. 1978 Nov;284:241–259. doi: 10.1113/jphysiol.1978.sp012539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehara T. Effects of adrenaline, ouabain, and some ionic conditions on twitch contraction of bullfrog ventricle. Jpn J Physiol. 1974 Jun;24(3):317–328. doi: 10.2170/jjphysiol.24.317. [DOI] [PubMed] [Google Scholar]
- Eisner D. A., Lederer W. J. Characterization of the electrogenic sodium pump in cardiac Purkinje fibres. J Physiol. 1980 Jun;303:441–474. doi: 10.1113/jphysiol.1980.sp013298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner D. A., Lederer W. J. Inotropic and arrhythmogenic effects of potassium-depleted solutions on mammalian cardiac muscle. J Physiol. 1979 Sep;294:255–277. doi: 10.1113/jphysiol.1979.sp012929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner D. A., Lederer W. J. The relationship between sodium pump activity and twitch tension in cardiac Purkinje fibres. J Physiol. 1980 Jun;303:475–494. doi: 10.1113/jphysiol.1980.sp013299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner D. A., Lederer W. J. The role of the sodium pump in the effects of potassium-depleted solutions on mammalian cardiac muscle. J Physiol. 1979 Sep;294:279–301. doi: 10.1113/jphysiol.1979.sp012930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner D. A., Lederer W. J., Vaughan-Jones R. D. The effects of rubidium ions and membrane potentials on the intracellular sodium activity of sheep Purkinje fibres. J Physiol. 1981 Aug;317:189–205. doi: 10.1113/jphysiol.1981.sp013820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis D. The effects of external cations and ouabain on the intracellular sodium activity of sheep heart Purkinje fibres. J Physiol. 1977 Dec;273(1):211–240. doi: 10.1113/jphysiol.1977.sp012090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Effects of pH on the myofilaments and the sarcoplasmic reticulum of skinned cells from cardiace and skeletal muscles. J Physiol. 1978 Mar;276:233–255. doi: 10.1113/jphysiol.1978.sp012231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadsby D. C. Activation of electrogenic Na+/K+ exchange by extracellular K+ in canine cardiac Purkinje fibers. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4035–4039. doi: 10.1073/pnas.77.7.4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadsby D. C., Cranefield P. F. Direct measurement of changes in sodium pump current in canine cardiac Purkinje fibers. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1783–1787. doi: 10.1073/pnas.76.4.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garay R. P., Garrahan P. J. The interaction of sodium and potassium with the sodium pump in red cells. J Physiol. 1973 Jun;231(2):297–325. doi: 10.1113/jphysiol.1973.sp010234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrahan P. J., Glynn I. M. The stoicheiometry of the sodium pump. J Physiol. 1967 Sep;192(1):217–235. doi: 10.1113/jphysiol.1967.sp008297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glitsch H. G., Pusch H. Correlation between changes in membrane potential and intracellular sodium activity during K activated response in sheep Purkinje fibres. Pflugers Arch. 1980 Mar;384(2):189–191. doi: 10.1007/BF00584438. [DOI] [PubMed] [Google Scholar]
- Glitsch H. G., Pusch H., Venetz K. Effects of Na and K ions on the active Na transport in guinea-pig auricles. Pflugers Arch. 1976 Sep 3;365(1):29–36. doi: 10.1007/BF00583625. [DOI] [PubMed] [Google Scholar]
- Glitsch H. G., Reuter H., Scholz H. The effect of the internal sodium concentration on calcium fluxes in isolated guinea-pig auricles. J Physiol. 1970 Jul;209(1):25–43. doi: 10.1113/jphysiol.1970.sp009153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauswirth O., Noble D., Tsien R. W. The mechanism of oscillatory activity at low membrane potentials in cardiac Purkinje fibres. J Physiol. 1969 Jan;200(1):255–265. doi: 10.1113/jphysiol.1969.sp008691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass R. S., Siegelbaum S. A., Tsien R. W. Three-micro-electrode voltage clamp experiments in calf cardiac Purkinje fibres: is slow inward current adequately measured? J Physiol. 1979 May;290(2):201–225. doi: 10.1113/jphysiol.1979.sp012768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunze D. L. Rate-dependent changes in extracellular potassium in the rabbit atrium. Circ Res. 1977 Jul;41(1):122–127. doi: 10.1161/01.res.41.1.122. [DOI] [PubMed] [Google Scholar]
- Langer G. A. The role of sodium ion in the regulation of myocardial contractility. J Mol Cell Cardiol. 1970 Sep;1(3):203–207. doi: 10.1016/0022-2828(70)90001-5. [DOI] [PubMed] [Google Scholar]
- Lederer W. J., Spindler A. J., Eisner D. A. Thick slurry bevelling: a new technique for bevelling extremely fine microelectrodes and micropipettes. Pflugers Arch. 1979 Sep;381(3):287–288. doi: 10.1007/BF00583261. [DOI] [PubMed] [Google Scholar]
- Lee C. O., Kang D. H., Sokol J. H., Lee K. S. Relation between intracellular Na ion activity and tension of sheep cardiac Purkinje fibers exposed to dihydro-ouabain. Biophys J. 1980 Feb;29(2):315–330. doi: 10.1016/S0006-3495(80)85135-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobley B. A., Page E. The surface area of sheep cardiac Purkinje fibres. J Physiol. 1972 Feb;220(3):547–563. doi: 10.1113/jphysiol.1972.sp009722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins L. J., Brinley F. J., Jr Potassium fluxes in dialyzed squid axons. J Gen Physiol. 1969 Jun;53(6):704–740. doi: 10.1085/jgp.53.6.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAGE E., STORN S. R. CAT HEART MUSCLE IN VITRO. 8. ACTIVE TRANSPORT OF SODIUM IN PAPILLARY MUSCLES. J Gen Physiol. 1965 May;48:957–972. doi: 10.1085/jgp.48.5.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POST R. L., JOLLY P. C. The linkage of sodium, potassium, and ammonium active transport across the human erythrocyte membrane. Biochim Biophys Acta. 1957 Jul;25(1):118–128. doi: 10.1016/0006-3002(57)90426-2. [DOI] [PubMed] [Google Scholar]
- Rang H. P., Ritchie J. M. On the electrogenic sodium pump in mammalian non-myelinated nerve fibres and its activation by various external cations. J Physiol. 1968 May;196(1):183–221. doi: 10.1113/jphysiol.1968.sp008502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter H., Seitz N. The dependence of calcium efflux from cardiac muscle on temperature and external ion composition. J Physiol. 1968 Mar;195(2):451–470. doi: 10.1113/jphysiol.1968.sp008467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjodin R. A., Beaugé L. A. Strophanthidin-sensitive components of potassium and sodium movements in skeletal muscle as influenced by the internal sodium concentration. J Gen Physiol. 1968 Sep;52(3):389–407. doi: 10.1085/jgp.52.3.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjodin R. A., Ortiz O. Resolution of the potassium ion pump in muscle fibers using barium ions. J Gen Physiol. 1975 Sep;66(3):269–286. doi: 10.1085/jgp.66.3.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R. C. Electrogenic sodium pump in nerve and muscle cells. Physiol Rev. 1972 Jul;52(3):563–594. doi: 10.1152/physrev.1972.52.3.563. [DOI] [PubMed] [Google Scholar]
- Thomas R. C. Membrane current and intracellular sodium changes in a snail neurone during extrusion of injected sodium. J Physiol. 1969 Apr;201(2):495–514. doi: 10.1113/jphysiol.1969.sp008769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan-Jones R. D. The effect of lowering external sodium on the intracellular sodium activity of crab muscle fibres. J Physiol. 1977 Jan;264(1):239–265. doi: 10.1113/jphysiol.1977.sp011666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Weer P., Geduldig D. Contribution of sodium pump to resting potential of squid giant axon. Am J Physiol. 1978 Jul;235(1):C55–C62. doi: 10.1152/ajpcell.1978.235.1.C55. [DOI] [PubMed] [Google Scholar]


