Abstract
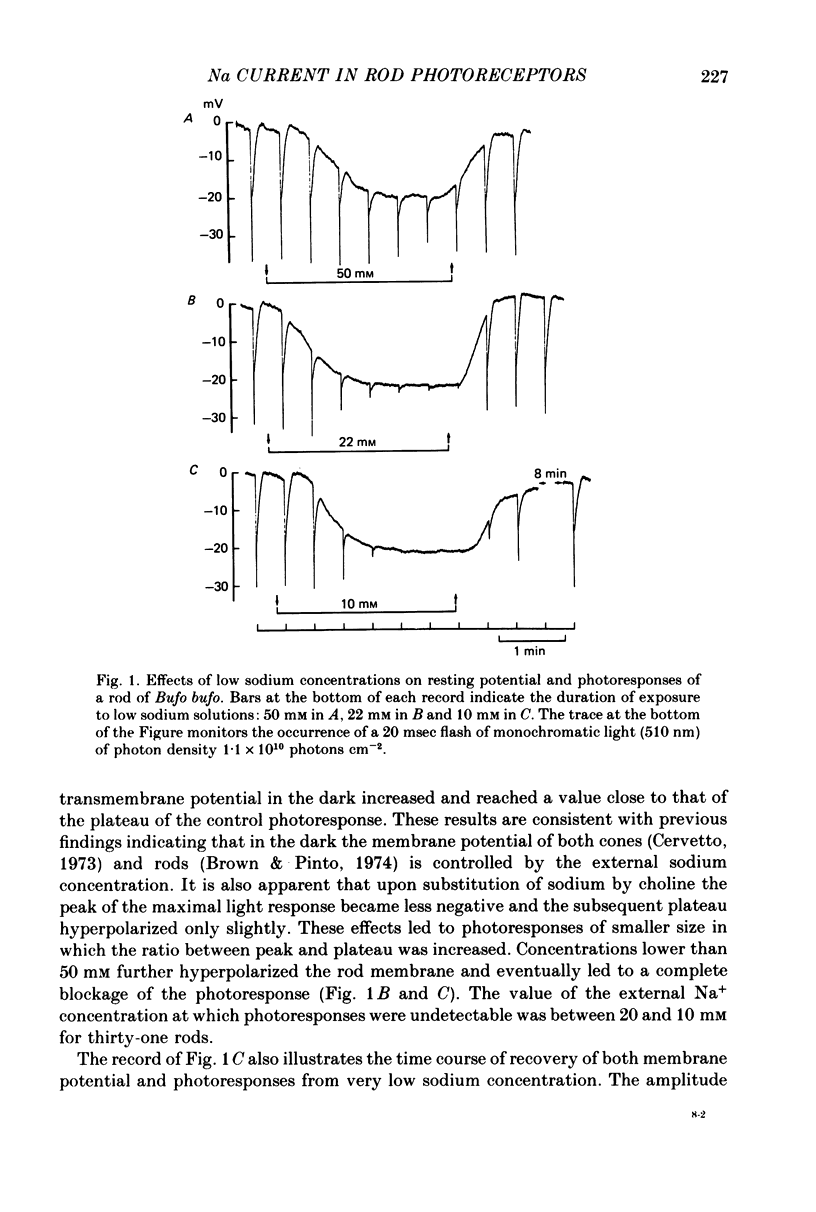
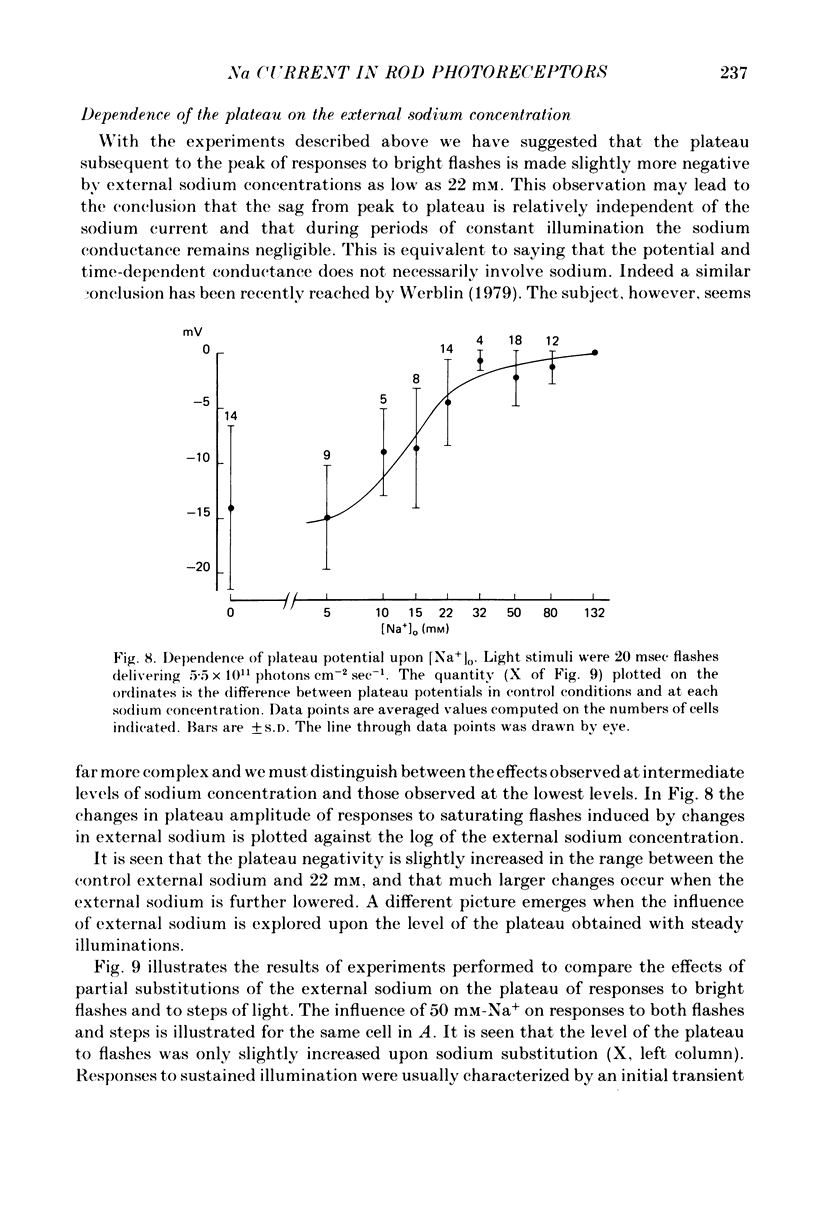
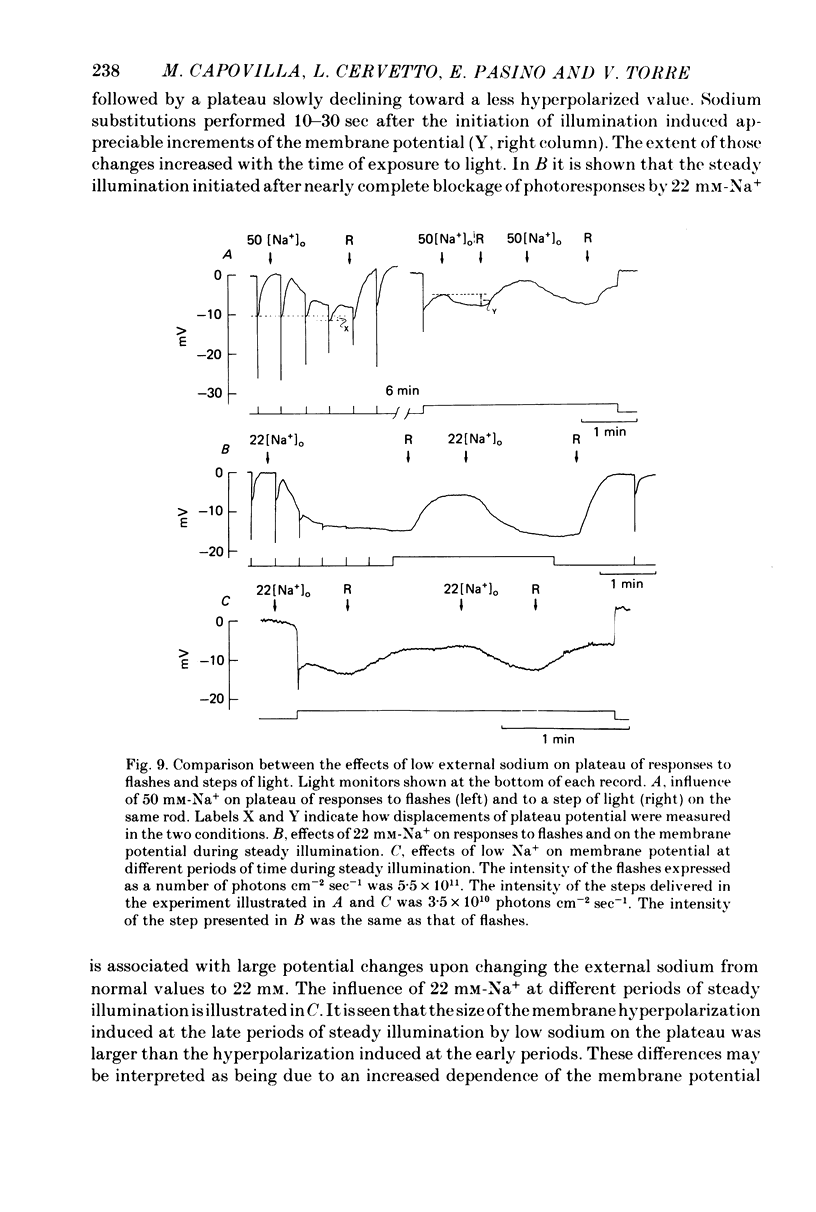
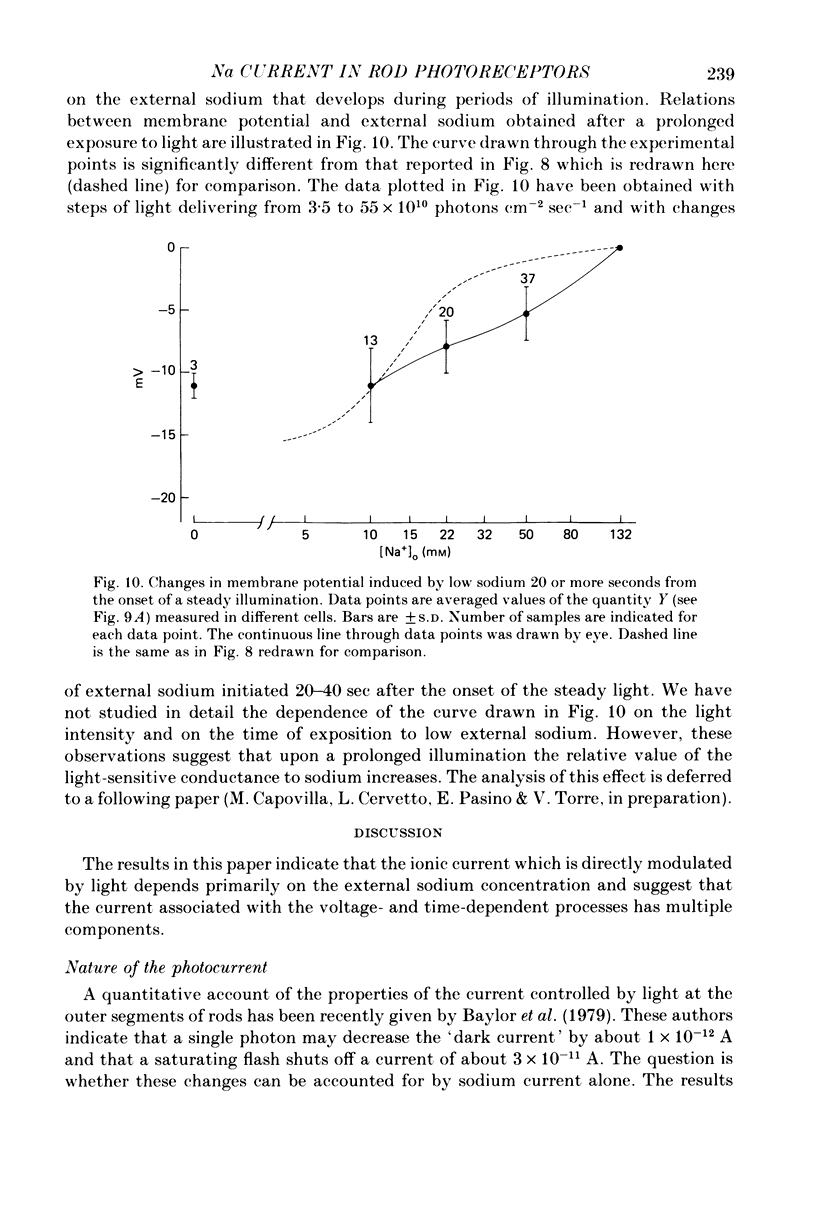
1. Intracellular responses were recorded from single rods in the retina of the toads Bufo bufo and Bufo marinus during exposure to solutions in which sodium was replaced by equimolar amounts of choline. 2. Upon moderate reduction (80 and 50 mM) of the external sodium the size of responses to bright flashes decreased as a consequence of both an increase in the resting potential and a decrease of the membrane potential at the peak, while the level of the plateau remained fairly constant. 3. Upon reduction of the external sodium to 22 mM or less, rods hyperpolarized to about the plateau level and failed to respond to illumination. Under these circumstances, membrane depolarization induced by an increased external potassium did not restore the cell responsiveness. Addition of 2-5 mM caesium hyperpolarized the membrane and partially restored the photoresponse. 4. Complete replacements of external sodium with potassium depolarized the rod by 40 +/- 10 mV, and no voltage responses to light could be detected. 5. In the presence of caesium, a nearly complete blockage of the photoresponses was obtained when the external sodium was 5 mM or less. Further reductions of the external sodium did not invert the photoresponses. Application of caesium when the external sodium was nominally zero induced a transient hyperpolarization followed by a slow decay. 6. During exposure to steady illumination, the dependence of the plateau level on the external sodium slowly increased. 7. These results indicate that the ionic current which is directly modulated by the light depends primarily on the external sodium. They suggest also that the current associated with the voltage- and time-dependent process responsible for the sag from peak to plateau of the response to a bright flash of light may have multiple components.
Full text
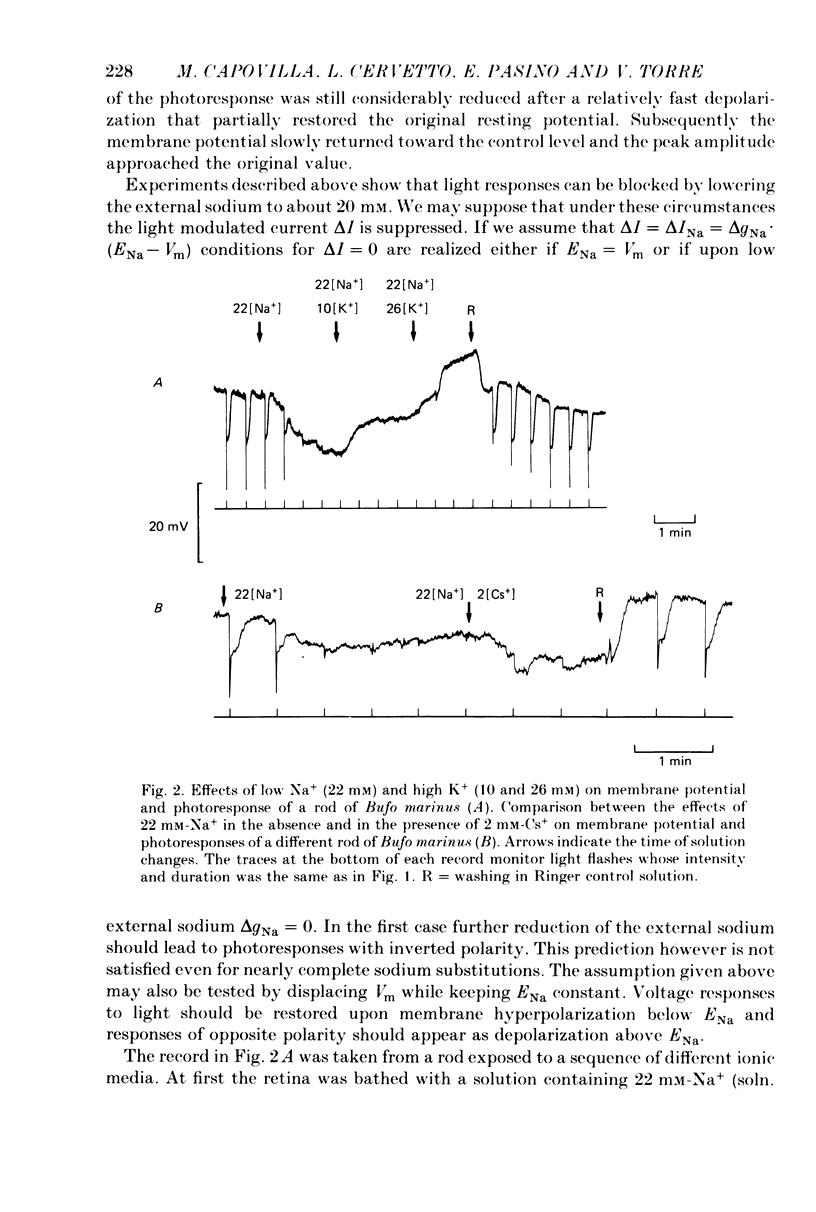
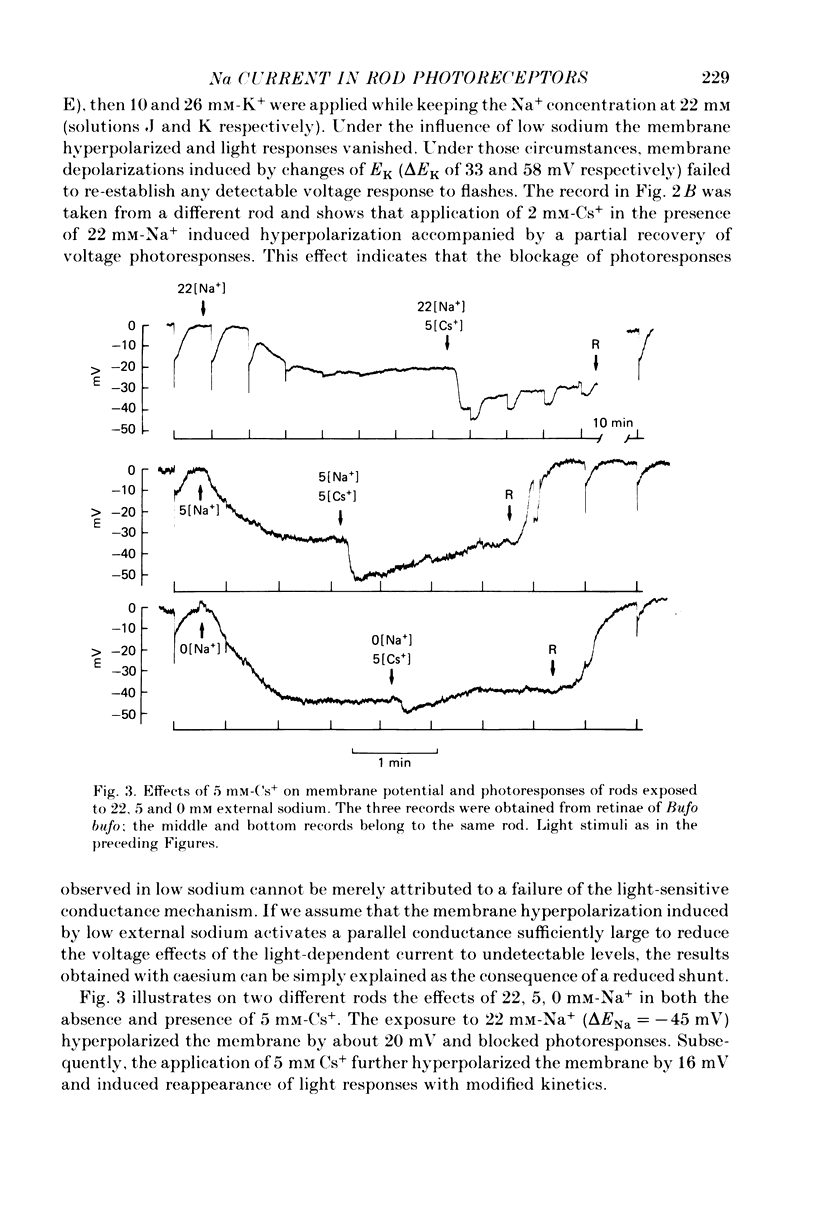
PDF




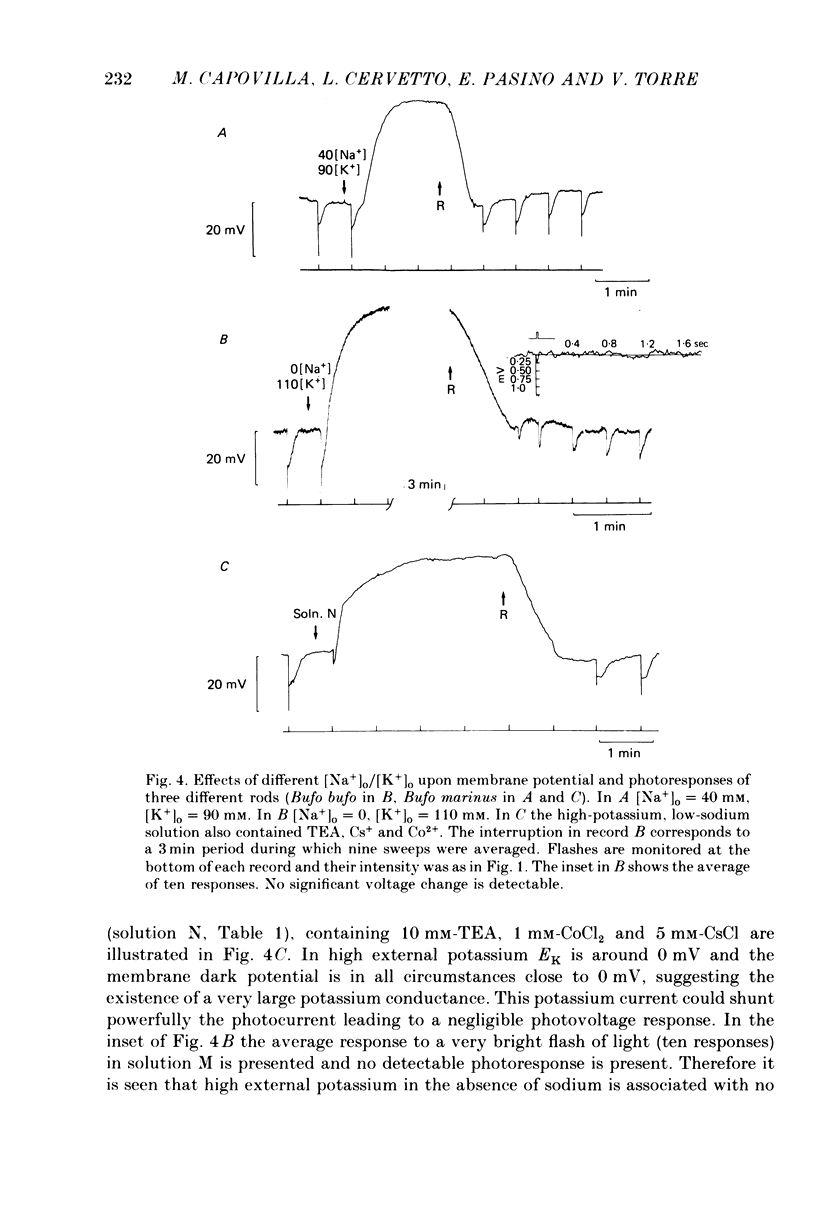
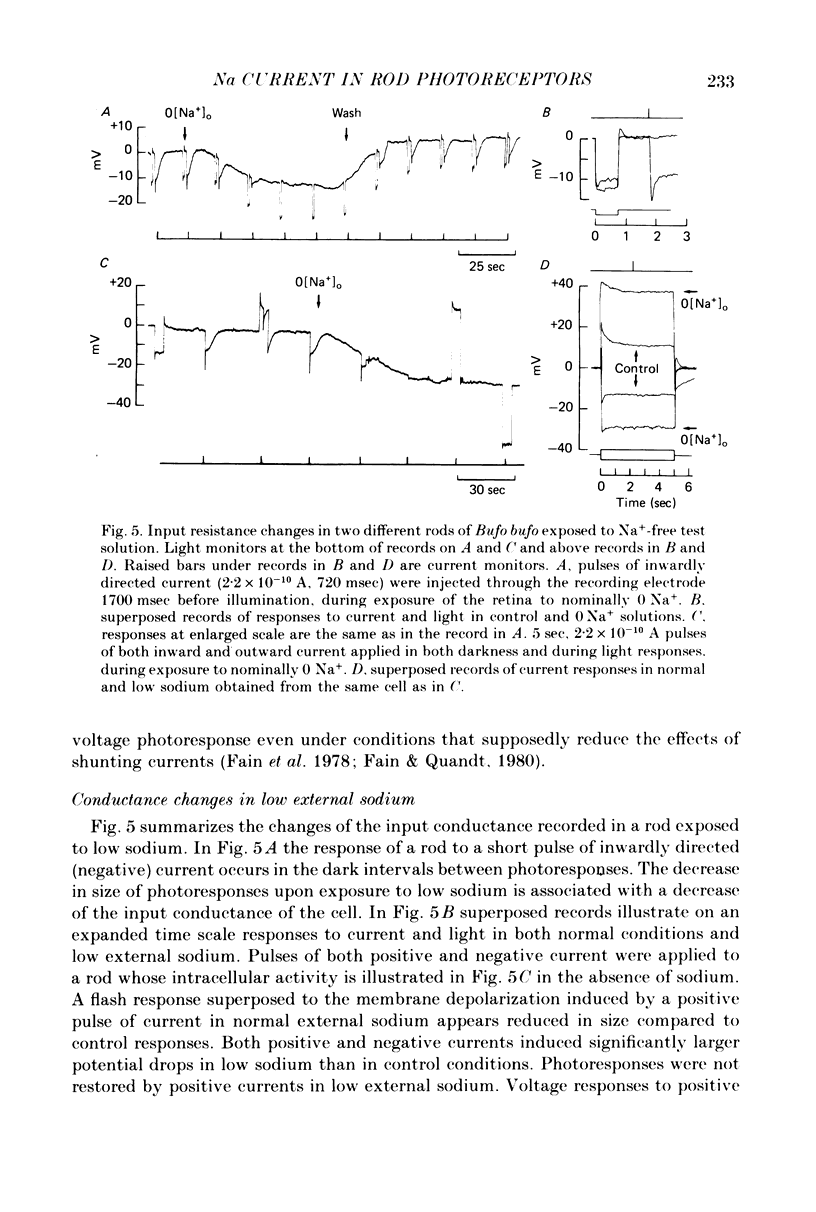
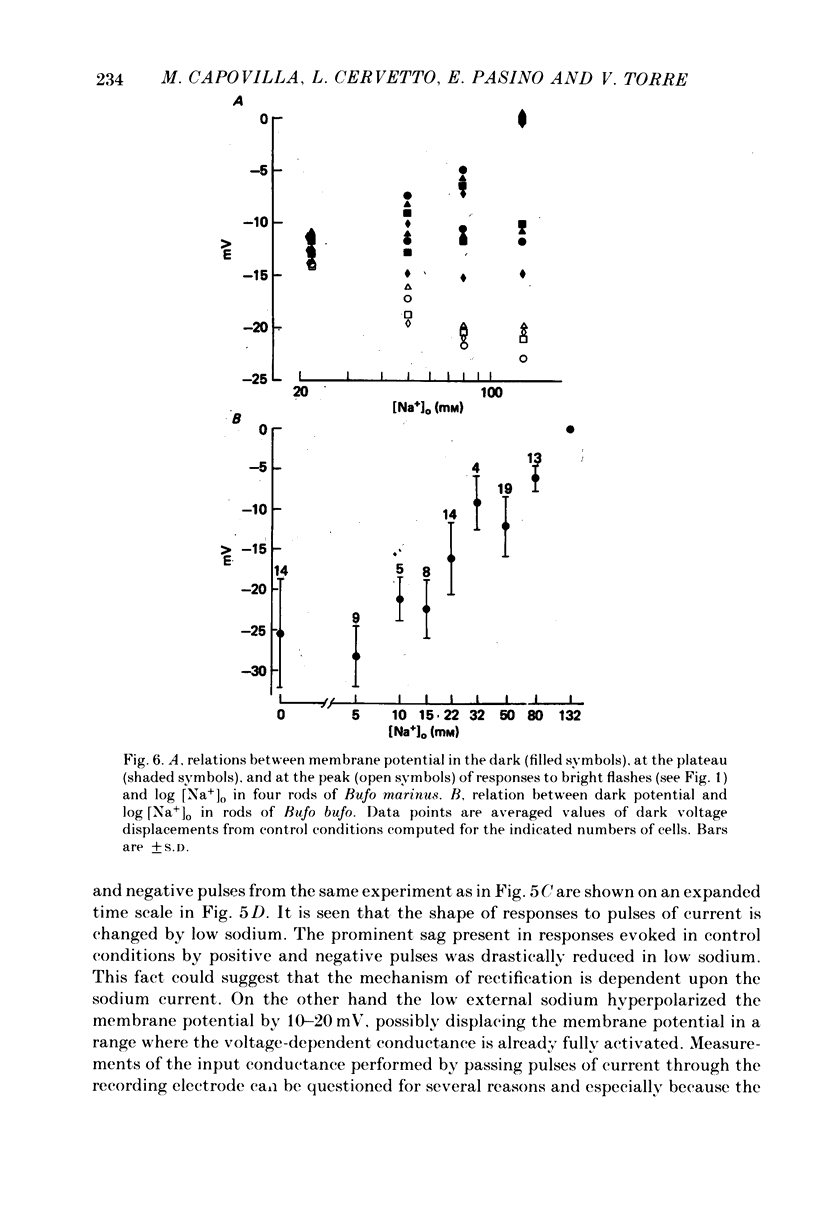

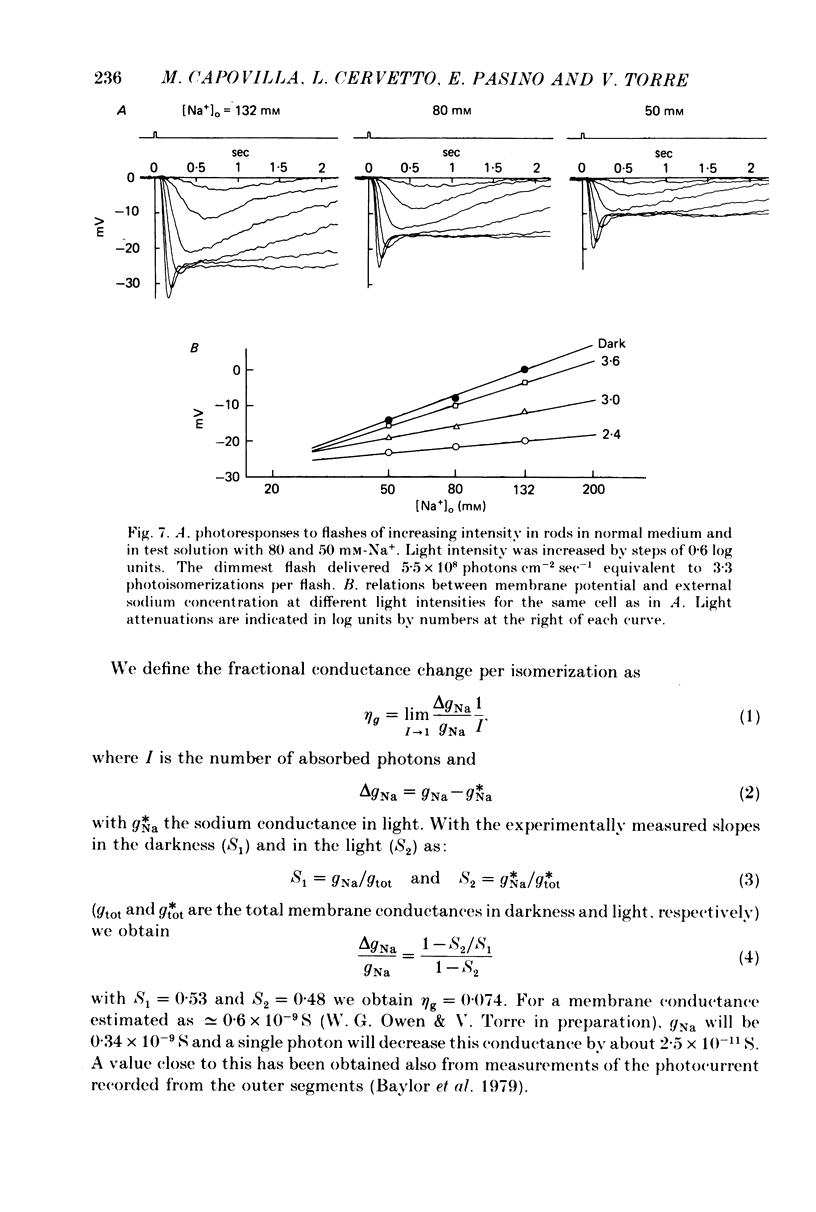



Selected References
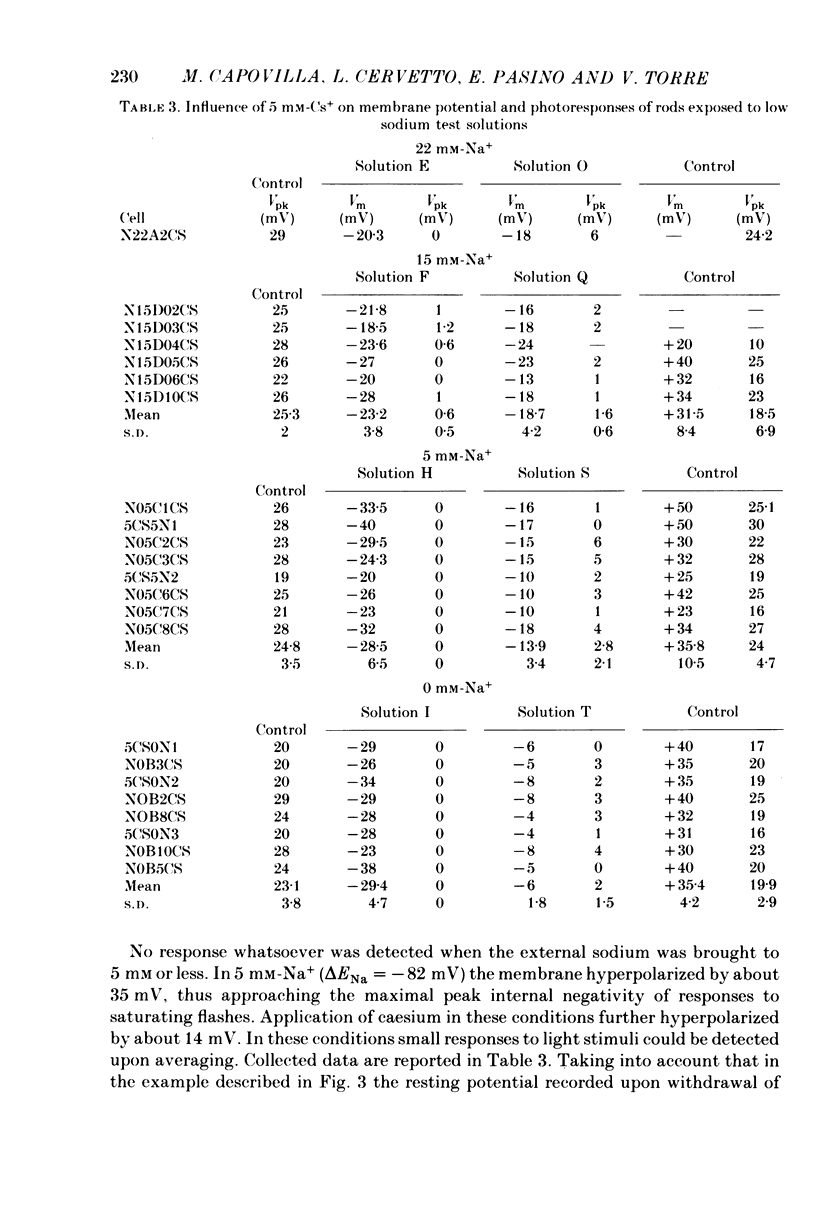
These references are in PubMed. This may not be the complete list of references from this article.
- Bader C. R., Macleish P. R., Schwartz E. A. A voltage-clamp study of the light response in solitary rods of the tiger salamander. J Physiol. 1979 Nov;296:1–26. doi: 10.1113/jphysiol.1979.sp012988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Lamb T. D., Yau K. W. The membrane current of single rod outer segments. J Physiol. 1979 Mar;288:589–611. [PMC free article] [PubMed] [Google Scholar]
- Brown J. E., Pinto L. H. Ionic mechanism for the photoreceptor potential of the retina of Bufo marinus. J Physiol. 1974 Feb;236(3):575–591. doi: 10.1113/jphysiol.1974.sp010453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capovilla M., Cervetto L., Torre V. Effects of changing external potassium and chloride concentrations on the photoresponses of Bufo bufo rods. J Physiol. 1980 Oct;307:529–551. doi: 10.1113/jphysiol.1980.sp013452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavaggioni A., Sorbi R. T., Turini S. Efflux of potassium from isolated rod outer segments: a photic effect. J Physiol. 1973 Aug;232(3):609–620. doi: 10.1113/jphysiol.1973.sp010288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervetto L. Influence of sodium, potassium and chloride ions on the intracellular responses of turtle photoreceptors. Nature. 1973 Feb 9;241(5389):401–403. doi: 10.1038/241401a0. [DOI] [PubMed] [Google Scholar]
- Cervetto L., Pasino E., Torre V. Electrical responses of rods in the retina of Bufo marinus. J Physiol. 1977 May;267(1):17–51. doi: 10.1113/jphysiol.1977.sp011799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detwiler P. B., Hodgkin A. L., McNaughton P. A. Temporal and spatial characteristics of the voltage response of rods in the retina of the snapping turtle. J Physiol. 1980 Mar;300:213–250. doi: 10.1113/jphysiol.1980.sp013159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fain G. L., Quandt F. N., Bastian B. L., Gerschenfeld H. M. Contribution of a caesium-sensitive conductance increase to the rod photoresponse. Nature. 1978 Mar 30;272(5652):466–469. doi: 10.1038/272467a0. [DOI] [PubMed] [Google Scholar]
- Fain G. L., Quandt F. N. The effects of tetraethylammonium and cobalt ions on responses to extrinsic current in toad rods. J Physiol. 1980 Jun;303:515–533. doi: 10.1113/jphysiol.1980.sp013301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagins W. A., Penn R. D., Yoshikami S. Dark current and photocurrent in retinal rods. Biophys J. 1970 May;10(5):380–412. doi: 10.1016/S0006-3495(70)86308-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B., Schwarz W. Potassium channels as multi-ion single-file pores. J Gen Physiol. 1978 Oct;72(4):409–442. doi: 10.1085/jgp.72.4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korenbrot J. I., Cone R. A. Dark ionic flux and the effects of light in isolated rod outer segments. J Gen Physiol. 1972 Jul;60(1):20–45. doi: 10.1085/jgp.60.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillman A. J., Ito H., Tomita T. Studies on the mass receptor potential of the isolated frog retina. I. General properties of the response. Vision Res. 1969 Dec;9(12):1435–1442. doi: 10.1016/0042-6989(69)90059-5. [DOI] [PubMed] [Google Scholar]
- Werblin F. S. Time- and voltage-dependent ionic components of the rod response. J Physiol. 1979 Sep;294:613–626. doi: 10.1113/jphysiol.1979.sp012949. [DOI] [PMC free article] [PubMed] [Google Scholar]


