Abstract
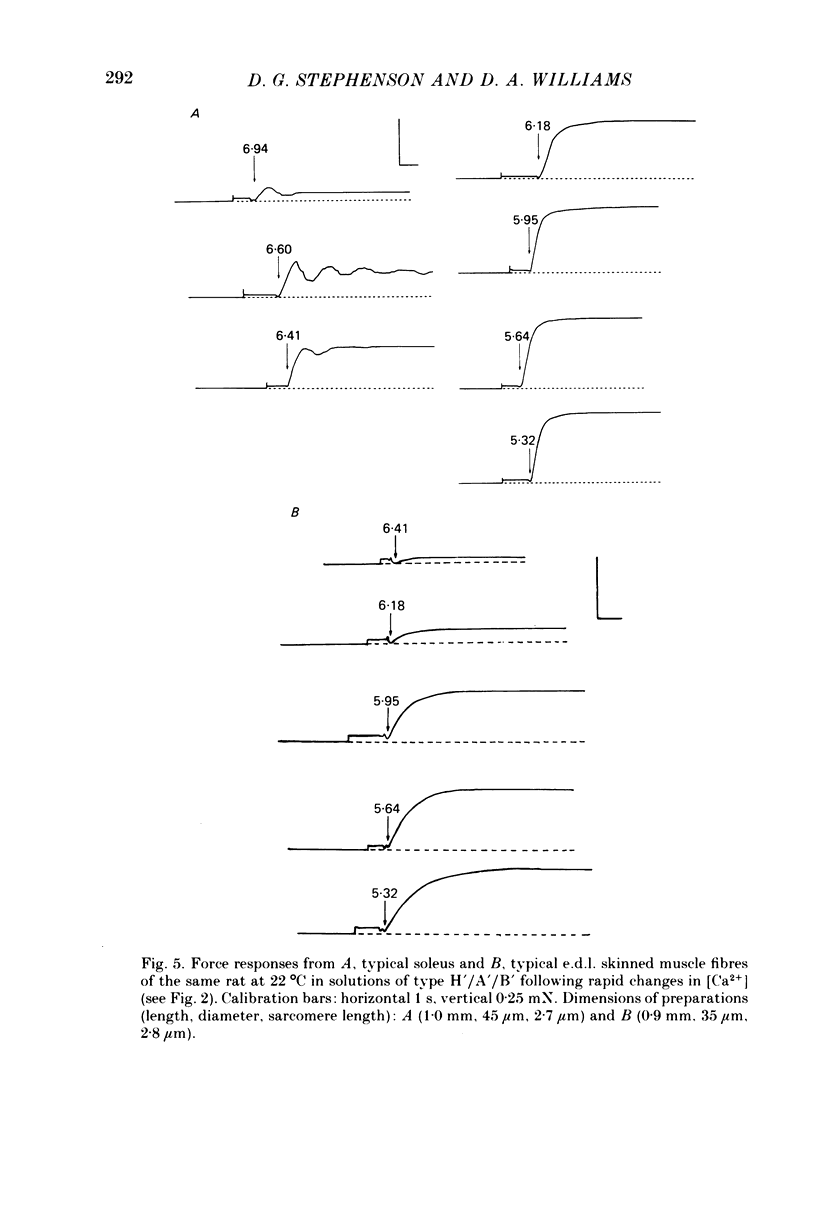
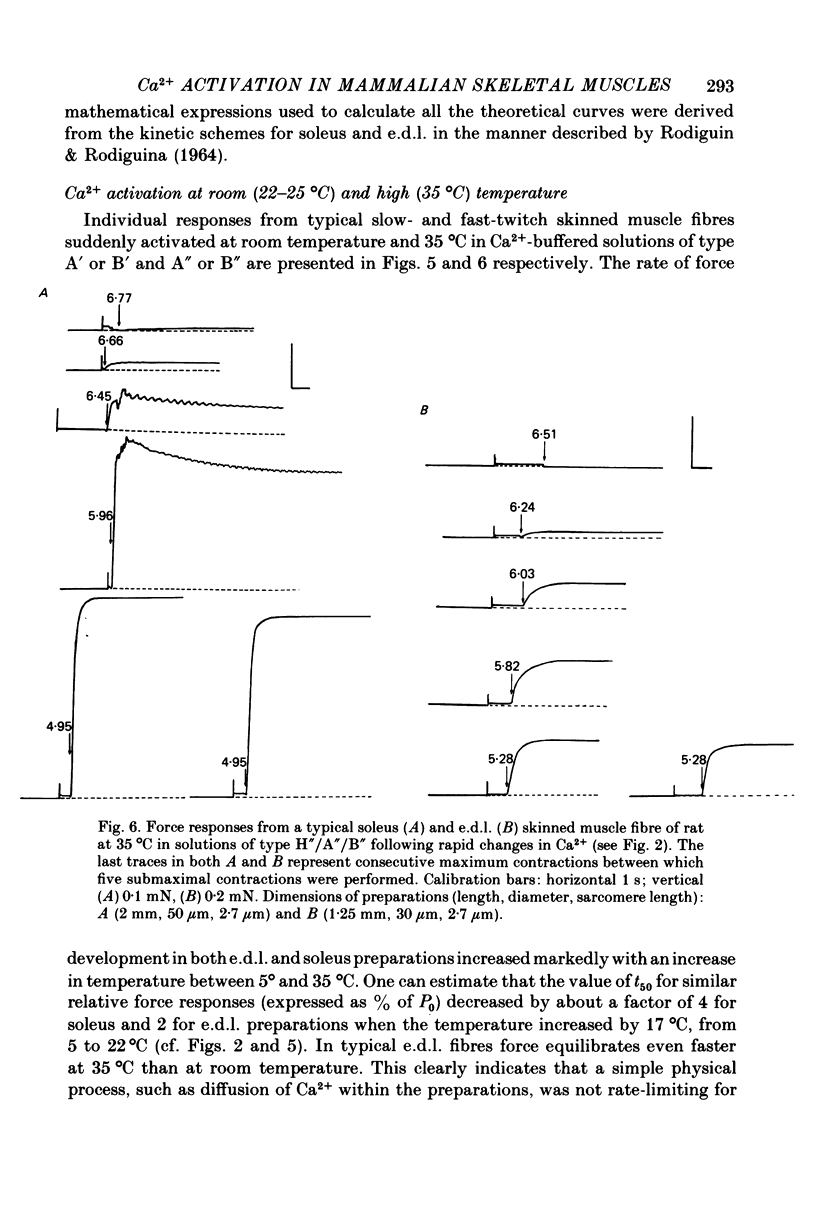
1. Force responses from mechanically skinned fibres of rat skeletal muscles (extensor digitorum longus and soleus) were measured at different temperatures in the range 3-35 degrees C following sudden changes in Ca2+ concentration in the preparations. 2. At all temperatures there were characteristic differences between the slow- and fast-twitch muscle fibres with respect to the relative steady-state force-[Ca2+] relation: such as a lower [Ca2+] threshold for activation and a less steep force-pCa curve in slow-twitch muscle fibres. 3. At 3-5 degrees C the force changes in both types of muscle fibres lagged considerably behind the estimated changes in [Ca2+] within the preparations and this enabled us to perform a comparative analysis of the Ca2+ kinetics in the process of force development in both muscle fibre types. This analysis suggest that two and six Ca2+ ions are involved in the regulatory unit for contraction of slow- and fast-twitch muscle fibres respectively. 4. The rate of relaxation following a sudden decrease in [Ca2+] was much lower in the slow-twitch than in the fast-twitch muscle at 5 degrees C, suggesting that properties of the contractile apparatus could play an essential role in determining the rate of relaxation in vivo. 5. There was substantial variation in Ca2+ sensitivity between muscle fibres of the same type from different animals at each temperature. However the steepness of the force-[Ca2+] relation was essentially the same for all fibres of the same type. 6. A change in temperature from 5 to 25 degrees C had a statistically significant effect on the sensitivity of the fast-twitch muscle fibres, rendering them less sensitive to Ca2+ by a factor of 2. However a further increase in temperature from 25 to 35 degrees C did not have any statistically significant effect on the force-[Ca2+] relation in fast-twitch muscle fibres. 7. The effect of temperature on the Ca2+ sensitivity of slow-twitch muscle fibres was not statistically significant, mainly because of the large variation in sensitivity amongst these preparations at room temperature. 8. Two types of oscillatory processes not associated with intracellular membranes were observed in the force response of all slow-twitch muscle fibres when submaximally activated (less than 60% maximum force) at 25 and 35 degrees C, but never at 3-5 degrees C. The frequency of oscillations increased with temperature. 9. Maximum Ca2+-activated force in both muscle fibre types was greatly dependent upon temperature over the range 0-25 degrees C, but increased only slightly above 25 degrees C. 10. Experiments on the rigor state suggest that the number of possible actomyosin interacting sites diminishes considerably as temperature is decreased below 25 degrees C.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashley C. C., Moisescu D. G. Effect of changing the composition of the bathing solutions upon the isometric tension-pCa relationship in bundles of crustacean myofibrils. J Physiol. 1977 Sep;270(3):627–652. doi: 10.1113/jphysiol.1977.sp011972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashley C. C., Moisescu D. G. Model for the action of calcium in muscle. Nat New Biol. 1972 Jun 14;237(76):208–211. doi: 10.1038/newbio237208a0. [DOI] [PubMed] [Google Scholar]
- BENDALL J. R. A study of the kinetics of the fibrillar adenosine triphosphatase of rabbit skeletal muscle. Biochem J. 1961 Dec;81:520–535. doi: 10.1042/bj0810520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CULLINGHAM P. J., LIND A. R., MORTON R. J. The maximal isometric tetanic tensions developed by mammalian muscle, in situ, at different temperatures. Q J Exp Physiol Cogn Med Sci. 1960 Apr;45:142–156. doi: 10.1113/expphysiol.1960.sp001452. [DOI] [PubMed] [Google Scholar]
- Close R. I. Dynamic properties of mammalian skeletal muscles. Physiol Rev. 1972 Jan;52(1):129–197. doi: 10.1152/physrev.1972.52.1.129. [DOI] [PubMed] [Google Scholar]
- Close R., Hoh J. F. Influence of temperature on isometric contractions of rat skeletal muscles. Nature. 1968 Mar 23;217(5134):1179–1180. doi: 10.1038/2171179a0. [DOI] [PubMed] [Google Scholar]
- Donaldson S. K., Hermansen L., Bolles L. Differential, direct effects of H+ on Ca2+ -activated force of skinned fibers from the soleus, cardiac and adductor magnus muscles of rabbits. Pflugers Arch. 1978 Aug 25;376(1):55–65. doi: 10.1007/BF00585248. [DOI] [PubMed] [Google Scholar]
- Ebashi S., Endo M. Calcium ion and muscle contraction. Prog Biophys Mol Biol. 1968;18:123–183. doi: 10.1016/0079-6107(68)90023-0. [DOI] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Effects of magnesium on contractile activation of skinned cardiac cells. J Physiol. 1975 Aug;249(3):497–517. doi: 10.1113/jphysiol.1975.sp011027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Myofilament-generated tension oscillations during partial calcium activation and activation dependence of the sarcomere length-tension relation of skinned cardiac cells. J Gen Physiol. 1978 Nov;72(5):667–699. doi: 10.1085/jgp.72.5.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederiksen D. W. Myosin phosphorylation. Nature. 1980 Sep 18;287(5779):191–192. doi: 10.1038/287191a0. [DOI] [PubMed] [Google Scholar]
- Griffiths P. J., Kuhn H. J., Güth K., Rüegg J. C. Rate of isometric tension development in relation to calcium binding of skinned muscle fibres. Pflugers Arch. 1979 Nov;382(2):165–170. doi: 10.1007/BF00584218. [DOI] [PubMed] [Google Scholar]
- Hess A. Vertebrate slow muscle fibers. Physiol Rev. 1970 Jan;50(1):40–62. doi: 10.1152/physrev.1970.50.1.40. [DOI] [PubMed] [Google Scholar]
- Hill D. K. Resting tension and the form of the twitch of rat skeletal muscle at low temperature. J Physiol. 1972 Feb;221(1):161–171. doi: 10.1113/jphysiol.1972.sp009746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson A., Hinkes M. J., Taylor S. R. Contracture and twitch potentiation of fast and slow muscles of the rat at 20 and 37 C. Am J Physiol. 1970 Jan;218(1):33–41. doi: 10.1152/ajplegacy.1970.218.1.33. [DOI] [PubMed] [Google Scholar]
- Johnson J. D., Charlton S. C., Potter J. D. A fluorescence stopped flow analysis of Ca2+ exchange with troponin C. J Biol Chem. 1979 May 10;254(9):3497–3502. [PubMed] [Google Scholar]
- Julian F. J. The effect of calcium on the force-velocity relation of briefly glycerinated frog muscle fibres. J Physiol. 1971 Oct;218(1):117–145. doi: 10.1113/jphysiol.1971.sp009607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerrick W. G., Secrist D., Coby R., Lucas S. Development of difference between red and white muscles in sensitivity to Ca2+ in the rabbit from embryo to adult. Nature. 1976 Apr 1;260(5550):440–441. doi: 10.1038/260440a0. [DOI] [PubMed] [Google Scholar]
- Miller D. J., Moisescu D. G. The effects of very low external calcium and sodium concentrations on cardiac contractile strength and calcium-sodium antagonism. J Physiol. 1976 Jul;259(2):283–308. doi: 10.1113/jphysiol.1976.sp011466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moisescu D. G., Ashley C. C., Campbell A. K. Comparative aspects of the calcium-sensitive photoproteins aequorin and obelin. Biochim Biophys Acta. 1975 Jul 8;396(1):133–140. doi: 10.1016/0005-2728(75)90196-6. [DOI] [PubMed] [Google Scholar]
- Moisescu D. G. Kinetics of reaction in calcium-activated skinned muscle fibres. Nature. 1976 Aug 12;262(5569):610–613. doi: 10.1038/262610a0. [DOI] [PubMed] [Google Scholar]
- Moisescu D. G., Thieleczek R. Calcium and strontium concentration changes within skinned muscle preparations following a change in the external bathing solution. J Physiol. 1978 Feb;275:241–262. doi: 10.1113/jphysiol.1978.sp012188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons R. M., Szent-Györgyi A. G. Control of tension development in scallop muscle fibres with foreign regulatory light chains. Nature. 1980 Aug 7;286(5773):626–628. doi: 10.1038/286626a0. [DOI] [PubMed] [Google Scholar]
- Solaro R. J., Wise R. M., Shiner J. S., Briggs F. N. Calcium requirements for cardiac myofibrillar activation. Circ Res. 1974 Apr;34(4):525–530. doi: 10.1161/01.res.34.4.525. [DOI] [PubMed] [Google Scholar]
- Stephenson D. G., Forrest Q. G. Different isometric force - [Ca2+] relationships in slow and fast twitch skinned muscle fibres of the rat. Biochim Biophys Acta. 1980 Feb 8;589(2):358–362. doi: 10.1016/0005-2728(80)90052-3. [DOI] [PubMed] [Google Scholar]
- Stephenson D. G., Williams D. A. Activation of skinned arthropod muscle fibres by Ca2+ and Sr2+. J Muscle Res Cell Motil. 1980 Mar;1(1):73–87. doi: 10.1007/BF00711926. [DOI] [PubMed] [Google Scholar]
- TRUONG X. T., WALL B. J., WALKER S. M. EFFECTS OF TEMPERATURE ON ISOMETRIC CONTRACTION OF RAT MUSCLE. Am J Physiol. 1964 Aug;207:393–396. doi: 10.1152/ajplegacy.1964.207.2.393. [DOI] [PubMed] [Google Scholar]
- Takagi A., Endo M. Guinea pig soleus and extensor digitorum longus: a study on single-skimmed fibers. Exp Neurol. 1977 Apr;55(1):95–101. doi: 10.1016/0014-4886(77)90161-3. [DOI] [PubMed] [Google Scholar]
- Thames M. D., Teichholz L. E., Podolsky R. J. Ionic strength and the contraction kinetics of skinned muscle fibers. J Gen Physiol. 1974 Apr;63(4):509–530. doi: 10.1085/jgp.63.4.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
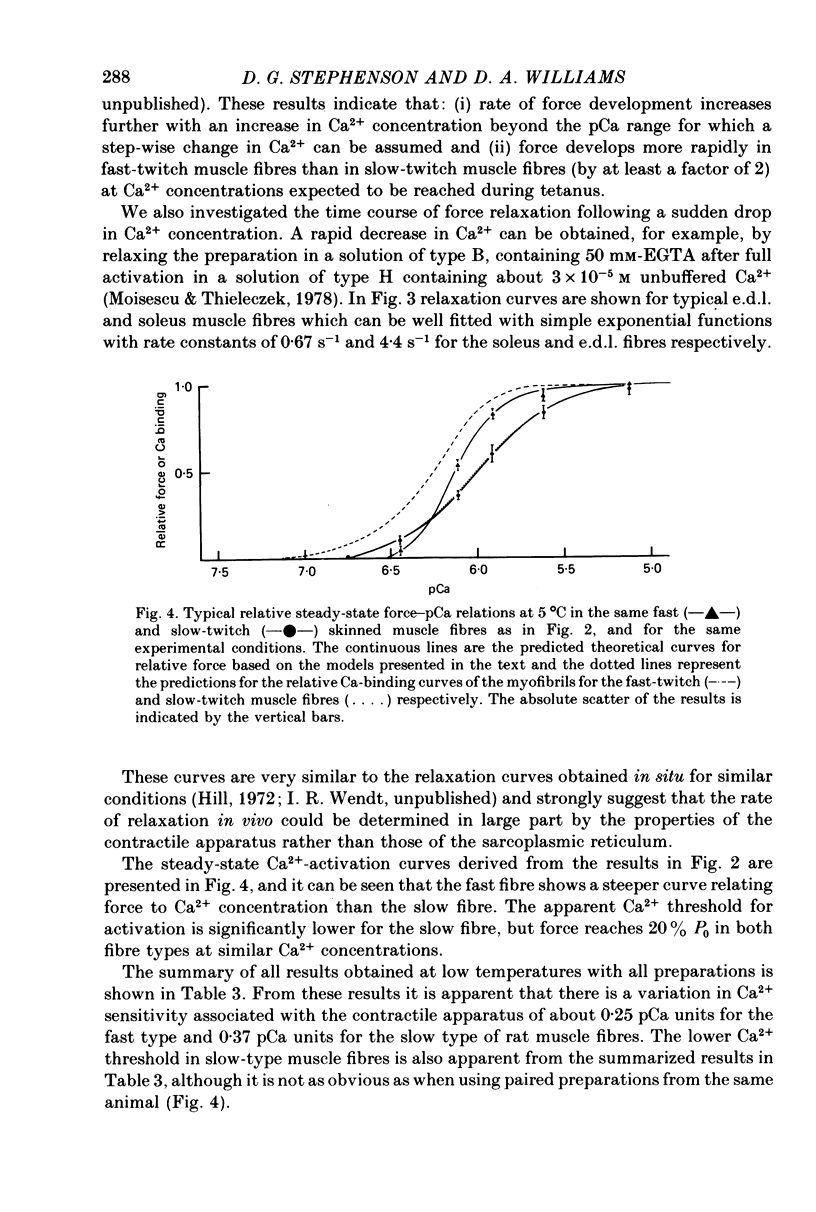
- Weber A., Murray J. M. Molecular control mechanisms in muscle contraction. Physiol Rev. 1973 Jul;53(3):612–673. doi: 10.1152/physrev.1973.53.3.612. [DOI] [PubMed] [Google Scholar]



