Abstract
A fungus, Fusarium lateritium, with dimethylsulfoniopropionate (DMSP) lyase activity was isolated from both seawater and a salt marsh due to its ability to grow on DMSP (with the evolution of dimethyl sulfide) as the sole source of carbon. This is the first reported case of DMSP lyase activity in a fungus. Several other common fungal genera tested did not have DMSP lyase activity. DMSP was taken up more rapidly by F. lateritium than it was utilized, leading to its intracellular accumulation. Inhibitor studies with nystatin and cyanide indicated that DMSP uptake was an energy-dependent process. The lyase was inducible by its substrate, DMSP (Km, 1.2 mM), and by the substrate analogs choline and glycine betaine. During induction, DMSP lyase activity increased with time and then dropped rapidly. This loss of activity could be prevented by spiking the culture with fresh DMSP or choline. The Vmax for DMSP lyase was 34.7 mU · mg of protein−1. The inhibitory effects of nystatin, and p-chloromercuriphenylsulfonate on DMSP lyase activity suggested that the enzyme is cytosolic. Because plants like Spartina (a marsh grass) and marine algae contain high concentrations of DMSP, we speculate that DMSP-utilizing fungi may be involved in their decay.
The role of marine fungi in biodegradation is well documented; however the details involving specific degradative processes have not been closely examined (15, 19, 24). Much of the research on marine fungi has focused on taxonomy, or determining which fungal species is associated with a particular host and whether the relationship is symbiotic, parasitic, or saprophytic (19). Furthermore, considerable work has been done on the mineralization (lignocellulolysis) of marine plant litter by fungi (14, 27, 28) and its impact on the carbon and nitrogen cycles (12). Fungi, possibly including marine fungi, are believed to play a significant role in the global sulfur cycle through mineralization and immobilization processes; however, the specific enzymatic reactions involved in these processes are not well characterized (23, 31). One aspect of the global sulfur cycle to which marine ecosystems are major contributors is the production of dimethyl sulfide (DMS) (2, 18). DMS arises primarily from the enzymatic cleavage of dimethylsulfoniopropionate (DMSP), which is produced as an osmoprotectant by salt marsh cordgrasses (6) and by certain species of macroalgae (3, 4, 16) and phytoplankton (1, 13, 17, 30). The physiology and biochemistry of the DMSP-degrading enzyme, DMSP lyase, has been studied in bacteria (9, 10, 21, 38), macroalgae (3, 4, 11, 16, 28a, 33), and phytoplankton (17, 32). However, no data on fungal DMSP lyase have been reported, although there was a brief mention of DMS emissions from added DMSP by a penicillium (4a), and the potential contribution by fungi in cleaving DMSP was suggested by Kiene (18). This paper reports the discovery and in vivo characterization of DMSP lyase in isolates of the fungus Fusarium lateritium. The potentially significant role of this species, and others that may contain this enzyme, in marine DMS emissions is discussed.
MATERIALS AND METHODS
Isolation of Fusarium spp.
Plates of a minimal medium, which contained 1 mM DMSP as the sole source of carbon and energy, were inoculated with either seawater or liquid from the surface of a decaying jellyfish found on the beach near North Myrtle Beach, S.C. Fungal hyphae, which appeared 5 days after inoculation, were subcultured on this medium. Pure cultures were obtained by restreaking onto separate plates containing 25 μg of chloramphenicol · ml−1. Cultivation of fungal biomass for the experiments described below was carried out in Vogel’s medium (37) modified by the addition of a trace elements solution (8). This medium was further supplemented with 1.5% NaCl, 2 mg of biotin · liter−1 25 mg of chloramphenicol · liter−1, and 2% sucrose.
DMSP lyase induction.
After 5 days of growth on sucrose medium, Fusarium cultures were filtered onto a 0.45-μm-pore-size membrane filter (Gelman), washed with deionized water, and resuspended in 50 mM sodium phosphate buffer (unless otherwise indicated). Cell suspensions were incubated for 30 min prior to experimentation so that ethanol, a sucrose fermentation product (which has a peak on our gas chromatograph interfering with that of DMS), could be metabolized or released to the air. DMSP lyase was induced by adding 2 mM DMSP to this buffered cell suspension, which was then incubated for 3 h at room temperature on a rotary shaker at 100 rpm. The same method was used to test acrylate and DMSP analogs as potential inducers of DMSP lyase. The lyase-induced cells were collected by filtration, rinsed, and resuspended in fresh buffer for use in the various experiments described below.
DMSP lyase activity.
DMSP lyase activity in fungal suspensions was determined by measuring the amount of DMS emitted from 1 mM DMSP. DMS was analyzed on a GC-8A gas chromatograph (Shimadzu, Kyoto, Japan) equipped with a flame ionization detector and a Poropak R column. The column temperature was maintained at 170°C, and the carrier gas flow rate was 74 ml · min−1. The amount of DMS in the liquid phase was calculated from the measured amount of gas in the headspace by using Henry’s law constants (7). Estimations of total protein were made by boiling hyphae in 2 M NaOH for 5 min, centrifuging for 3 min, and adding Bradford reagent (Bio-Rad) to an aliquot of the supernatant. Bovine serum albumin was the standard.
Effect of pH and temperature on DMSP lyase activity.
The optimum temperature for DMSP lyase activity was determined by incubating induced cells at the temperatures indicated for 15 min before adding the substrate and during the assay. The optimum pH was determined by resuspending rinsed, induced cells in buffers ranging from pH 4.0 to 10.0. After 15 min, 1 mM DMSP substrate was added and the enzyme activity was measured.
Effect of inhibitors on DMSP lyase induction and activity.
Fungal cells were incubated with the inhibitor nystatin or p-chloromercuriphenylsulfonate (p-CMBS) at the concentrations indicated. To determine their effect on DMSP lyase induction or turnover (activity), the inhibitors were incubated with the cells 15 min before addition of the inducer or substrate. The data shown are representative of at least two experiments.
DMSP uptake.
The rate of DMSP uptake by Fusarium was measured by adding DMSP (250 μM) to fungal hyphae in 50 ml of phosphate buffer. Aliquots of the fungus were removed and filtered every 5 min after the addition of DMSP. The intracellular DMSP concentration was determined by the addition of 1 ml of 4 M NaOH to the filtered hyphae to cleave DMSP to DMS and acrylate. The effect of inhibitors (cyanide, nystatin, and p-CMBS) on the rate of DMSP uptake is presented as percent inhibition and was calculated as follows: [(1 − rate of inhibitor-treated sample)/rate of sample with DMSP only] × 100.
Chemicals.
DMSP was synthesized from DMS and acrylate by the method of Chambers et al. (5). DMSP was standardized against the pure commercial reagent obtained from Research Plus, Inc., Bayonne, N.J. Acrylate was obtained from Aldrich, Milwaukee, Wis. Nystatin was obtained from Sigma Chemical Co., St. Louis, Mo.
RESULTS AND DISCUSSION
Characterization of the fungus.
Two fungal isolates growing on minimal medium containing DMSP as the sole source of carbon were purified and tested for the production of DMS. DMS emissions were inhibited by nystatin but not chloramphenicol, indicating that a fungus, not a bacterial contaminant, was responsible for DMSP lyase activity. Comparative analysis of macroconidia (25) indicated that both isolates were of the genus Fusarium (Fig. 1). Comparison of fungal growth on various media and further analysis of the reproductive structures revealed that both isolates were the same genus and species, F. lateritium (12a).
FIG. 1.
Scanning electron micrograph of F. lateritium macroconidia. Fungal cultures were fixed in 2% glutaraldehyde and postfixed in 1% OsO4. Magnification, ×1,200.
Substrate utilization.
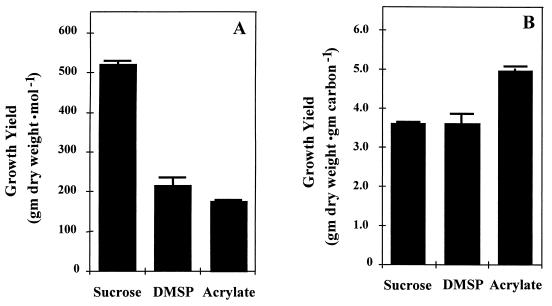
Yields of F. lateritium grown in minimal medium with sucrose, DMSP, or acrylate as the sole source of carbon were compared (Fig. 2). After 8 days of growth, the fungal biomass was filtered, dried, and weighed. Figure 2A compares the fungal biomass produced per mole of added substrate, and Fig. 2B compares the fungal biomass produced per gram of carbon. While the biomass yield per mole on sucrose was ca. 2.5-fold higher than on DMSP or acrylate, on a gram-of-carbon basis, yields on acrylate were significantly higher (ca. 30%) than were yields on DMSP or sucrose, which were approximately equal. Since methyl carbon is ca. two-fifths of the total carbon, it is probable that the lower growth yield (per gram of carbon) on DMSP was because the DMS released could not be recaptured and utilized by this fungus.
FIG. 2.
Growth of F. lateritium on various carbon sources. (A) Fungal dry weight produced per mole of substrate; (B) fungal dry weight per gram of carbon. Substrate concentrations were as follows: sucrose, 2.5 mM; DMSP and acrylate, 5 mM each. The results show the mean and standard deviation of three replicas.
Fungal DMS production.
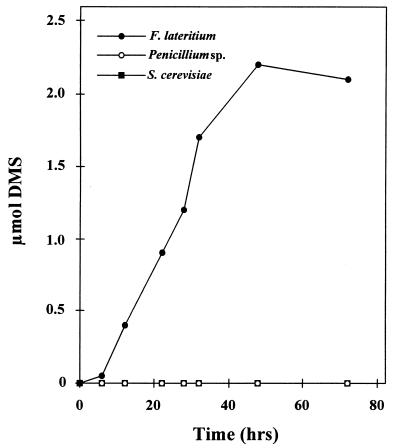
To determine if DMS emissions from added DMSP occur in fungal metabolism generally, both Penicillium spp. and Saccharomyces cerevisiae were tested for DMSP lyase activity. Only F. lateritium had this activity (Fig. 3). Several other unidentified fungal species were also tested, but none evolved DMS from DMSP.
FIG. 3.
DMS emissions by various fungal genera. DMSP (1 mM) was added to fungal cell suspensions containing approximately 40 mg of biomass; the appearance of DMS in the gas phase was monitored by gas chromatography.
Induction of DMSP lyase.
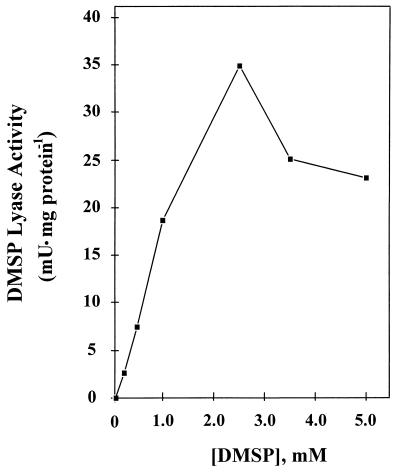
As seen in Fig. 3, DMS was not immediately evolved from F. lateritium upon addition of DMSP, suggesting that DMSP lyase is an inducible enzyme. The optimum DMSP concentration for lyase induction was 2.5 mM, although concentrations as low as 0.25 mM would induce the enzyme (Fig. 4). In F. lateritium, this enzyme was induced not only by its substrate DMSP (Km, 1.2 mM) but also by the substrate analogs choline, glycine betaine, and dimethyl glycine (Table 1). The selenium analog of DMSP, dimethylselenoniopropionate, did not induce DMSP lyase. Furthermore, neither proline, an osmoprotectant for many organisms (22), nor acrylate, the product of the lyase reaction, was effective as an inducer of DMSP lyase.
FIG. 4.
Effect of DMSP concentration on DMSP lyase induction. The kinetics of induction were monitored by assaying washed cells for DMSP lyase activity after 3 h of induction.
TABLE 1.
Effect of various DMSP analogues and osmoprotectants on DMSP lyase induction in F. lateritium
| Inducera | DMSP lyase activityb (mU · mg of protein−1) |
|---|---|
| DMSP | 23 ± 3.7 |
| DMSeP | 0 |
| Choline | 6.9 ± 1.3 |
| Glycine betaine | 5.9 ± 0.1 |
| Dimethylglycine | 0.8 ± 0.6 |
| Acrylate | 0 |
| Proline | 0 |
Putative inducers were added at 2 mM. After 3 h, cells were washed free of inducer, 1 mM DMSP substrate was added, and the enzyme activity was monitored.
One unit of activity is defined as the amount of enzyme required to cleave 1 μmol of added DMSP to DMS in 1 min. Data represent the mean and standard deviation of three replicate determinations.
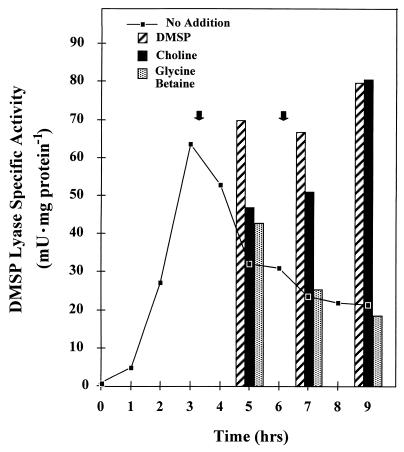
On examining the activity of DMSP lyase during induction in this fungus, we observed that enzyme specific activity, as measured by DMS emissions from added DMSP, increased with time up to about 3 h and then began to drop rapidly as the inducer was depleted from the cell suspension (Fig. 5, no addition). Since this drop in activity occurred even though fungal aliquots had been filtered and fresh DMSP had been added, the reason for the loss was unrelated to substrate availability. Furthermore, since the addition of the reaction end product acrylate or DMS or lowering the pH had no effect on enzyme induction or activity (data not shown), this loss of activity was apparently not due to feedback inhibition. However, spiking the cell suspension with fresh DMSP or choline (1 mM), but not glycine betaine (1 mM), at 3 and 6 h resulted in the fungal lyase activity remaining constant (Fig. 5, bar graphs). These molecules either stabilized the lyase or induced the synthesis of new enzyme. Since glycine betaine induced the lyase but was not effective in maintaining lyase activity after spiking, it appears that the molecules which caused the lyase activity to remain high did so by stabilizing the enzyme rather than inducing new enzyme. It is not uncommon for enzymes to be stabilized by their substrates occupying the active site (35), and these results may be examples of such a phenomenon.
FIG. 5.
Effect of DMSP and various DMSP analogs on DMSP lyase stability. DMSP (2 mM) was added to four identical fungal cell suspensions at time zero. At the times indicated, an aliquot of cells was removed, filtered, washed, and resuspended with 1 mM DMSP. The cultures were amended after 3 and 6 h, as indicated by the arrows, with 1 mM choline, glycine betaine, or DMSP. The line indicates the course of an unamended culture. Bars show the level of activity after the additions listed.
DMSP storage.
During the induction process, the fungus took up more DMSP than could be immediately used. After 3 h of induction, 1 ml of filtered, washed hyphae could contain between 0.35 and 1.0 μmol of DMSP per mg of cell protein. This stored DMSP could be released by the addition of nystatin to the cell suspension (data not shown).
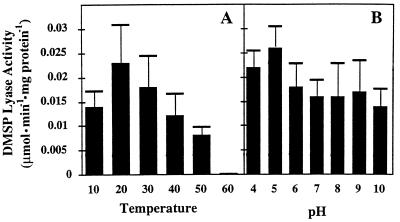
Optimum conditions for DMSP lyase activity.
Although both F. lateritium isolates came from the marine environment, salinity variations in the growth or resuspension media had no effect on DMSP lyase activity (data not shown). The temperature for optimal DMS emissions was between 20 and 30°C (Fig. 6A). This compares with 37°C for the DMSP lyase of aerobic marine bacteria (10). The lyase activity was essentially independent of pH over the range measured (pH 4 to 10) (Fig. 6B), which may be considered unusual. However, since the activity was measured in vivo, it may have been unaffected by the external pH. DMSP lyases in bacteria were strongly dependent on [H+], with maxima at approximately pH 8 (10). The kinetics of DMSP utilization by DMSP lyase could not be determined in these fungal isolates because they concentrated DMSP during the induction period, and therefore the lyase did not respond to added DMSP (data not shown).
FIG. 6.
Effects of pH and temperature on DMSP lyase activity in F. lateritium. Fungal cultures were induced with 1 mM DMSP; the temperature and pH were adjusted for each aliquot of fungal biomass. DMSP lyase rates were averaged from three separate experiments and are shown with their standard deviations.
DMSP uptake.
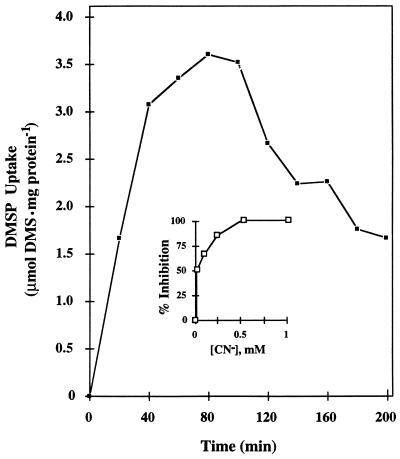
The kinetics of DMSP uptake by cells uninduced for DMSP lyase (i.e., having no internal DMSP) are shown in Fig. 7. The intracellular pool of DMSP peaked at ca. 80 min and then declined as the lyase that was induced during this period began to turn over and deplete the pool. A steady-state level of DMSP (1.5 to 2 μmol · mg of protein−1) was reached at about 3 h.
FIG. 7.
DMSP uptake by F. lateritium as measured by increases in intracellular DMSP concentrations with time. The inset shows the effect of cyanide on DMSP uptake. The 0% inhibition of DMSP uptake represents 1.62 μmol of DMS · mg of protein−1 after 40 min of uptake.
Effect of inhibitors on DMSP metabolism.
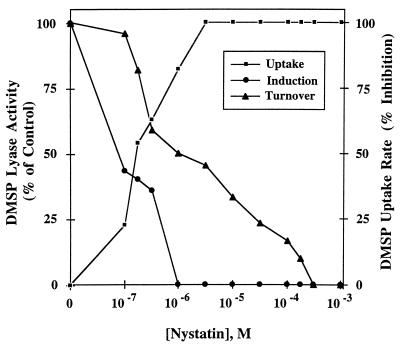
The energy requirement for the DMSP uptake process was seen by its strong inhibition by the electron transport inhibitor cyanide (Fig. 7, inset). To determine if DMS emission (DMSP lyase activity) and induction of DMSP lyase were energy-requiring processes and to confirm that DMSP uptake is energy dependent, the effects of nystatin on DMSP metabolism were examined. Nystatin, which attacks fungal membrane sterols, strongly inhibited DMSP uptake (Ki, ca. 2 × 10−7 M) and DMSP lyase induction (Ki, ca. 8 × 10−8 M) when added to cell suspensions (Fig. 8). While it might be expected that DMSP uptake and turnover would be identical in their sensitivity to nystatin, lyase activity (turnover) was less sensitive to this inhibitor (Ki, ca. 10−6 M). This difference in sensitivities could be explained by the fungus storing DMSP during the induction period. The stored DMSP would be available to the enzyme (for turnover) and therefore would be insensitive to low levels of added nystatin.
FIG. 8.
Effect of nystatin concentrations on DMSP uptake and induction and turnover of DMSP lyase. The 100% DMSP lyase activities for induction and turnover were 0.06 and 0.03 μmol of DMS · min−1 · mg of protein−1, respectively. DMSP uptake in the absence of inhibitor (0% was 1.6 μmol of DMS · mg of protein−1; all uptake measurements were done at 40 min.
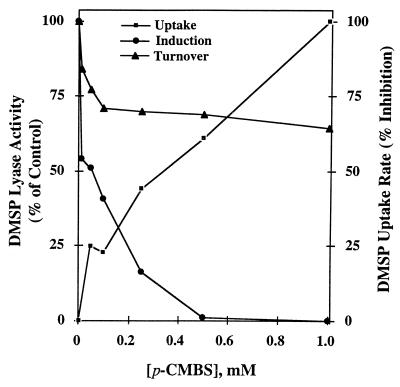
Addition to fungal suspensions of the thiol-binding reagent p-CMBS, which cannot cross membrane barriers (36), helped determine which of the proteins (or enzymes) involved in DMSP metabolism are exposed to the cell surface. Figure 9 shows that DMSP uptake was inhibited 50% by ca. 0.3 mM p-CMBS. This relatively low sensitivity suggests that the DMSP uptake system, presumably located in the cell membrane, is only partially exposed to the thiol-binding reagent. Since induction of DMSP lyase also requires DMSP uptake, the much stronger inhibition of this process by p-CMBS (Ki = 0.05 mM) suggests that this thiol reagent may also be interfering with other cellular processes. Finally, DMSP lyase activity was inhibited ca. 30% by 0.1 mM p-CMBS, and higher concentrations had no further effect. This observation may be explained by the fact, already mentioned, that F. lateritium stores DMSP during the induction period and therefore the rate of enzyme turnover is affected by the inhibition of DMSP uptake by p-CMBS. These results suggest that DMSP lyase in F. lateritium is not exposed to the surface and could possibly be cytosolic. Furthermore, the fact that DMSP lyase activity in this fungus requires the active uptake of substrate supports the cytosolic location of this enzyme.
FIG. 9.
Effect of p-CMBS concentrations on DMSP uptake and induction and turnover of DMSP lyase. The 100% DMSP lyase activities for induction and turnover were 0.037 and 0.031 μmol of DMS · min−1 · mg of protein−1, respectively. DMSP uptake was 1.2 μmol of DMS · mg of protein−1 in the absence of inhibitor (0% inhibition); all uptake measurements were made at 40 min.
In summary, we have definitively established that fungi are capable of causing DMS emission from DMSP by the action of an inducible DMSP lyase. This activity in F. lateritium requires the uptake of DMSP by an energy-dependent process. Potential sources of DMSP for F. lateritium are phytoplankton (17) and macroalgae (16) and salt marsh cordgrasses (20, 29). The salt marsh cordgrass Spartina alterniflora contains between 80 and 280 μmol · g (dry weight) of DMSP−1 (6, 20, 29). Studies have shown an increased rate of DMS emission during senescence and decay of this grass (6, 34). Furthermore, it has been shown that fungi are the major mediators of this decay (26). Although the significance of fungus-related DMS emissions in the ocean and salt marsh is not known, the high concentrations of DMSP in marsh grasses and marine algae lead to the notion that DMSP-utilizing fungi may be found in these cordgrasses and could be involved in their senescence and decay.
ACKNOWLEDGMENTS
We thank R. Hanlin and his technician, C. Rodriguez, University of Georgia, Athens, Ga., for identifying the fungal isolates and Steven Y. Newell for a critical reading of the manuscript.
REFERENCES
- 1.Ackman R G, Tocher C S, McLaachlan J. Occurrence of dimethyl-β-propiothetin in marine phytoplankton. J Fish Res Board Can. 1966;23:357–364. [Google Scholar]
- 2.Andreae M O, Raemdonck H. Dimethyl sulfide in the surface ocean and the marine atmosphere: a global view. Science. 1983;221:744–747. doi: 10.1126/science.221.4612.744. [DOI] [PubMed] [Google Scholar]
- 3.Cantoni G L, Anderson D G. Enzymatic cleavage of dimethylpropiothetin by Polysiphonia lanosa. J Biol Chem. 1956;222:171–177. [PubMed] [Google Scholar]
- 4.Challenger F, Simpson M I. Studies on biological methylation. XII. A precursor of the dimethyl sulfide evolved by Polysiphonia fastigata, dimethyl-2-carboxyethyl-sulfonium hydroxide and its salts. J Chem Soc. 1948;3:1591–1597. doi: 10.1039/jr9480001591. [DOI] [PubMed] [Google Scholar]
- 4a.Challenger F, Liu Y C. The elimination of methylthiol and dimethylsulphide from methylthiol and dimethylsulphonium compounds by moulds. Rec Trav Chim Pays-Bas. 1950;69:334–342. [Google Scholar]
- 5.Chambers S T, Kunin C M, Miller D, Hamada A. Dimethylthetin can substitute for glycine betaine as an osmoprotectant molecule for Escherichia coli. J Bacteriol. 1987;169:4845–4847. doi: 10.1128/jb.169.10.4845-4847.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dacey J W H, King G M, Wakeham S G. Factors controlling emission of dimethylsulfide from salt marshes. Nature (London) 1987;330:643–645. [Google Scholar]
- 7.Dacey J W H, Wakeham S G, Howes B L. Henry’s law constants for dimethylsulfide in fresh water and seawater. Geophys Res Lett. 1984;11:991–994. [Google Scholar]
- 8.Davis R H, de Serres F J. Genetic and microbiological research techniques for Neurospora crassa. Methods Enzymol. 1970;17A:79–143. [Google Scholar]
- 9.de Souza M P, Yoch D C. Purification and characterization of dimethylsulfoniopropionate lyase from an Alcaligenes-like dimethyl sulfide-producing marine isolate. Appl Environ Microbiol. 1995;61:21–26. doi: 10.1128/aem.61.1.21-26.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Souza M P, Yoch D C. Comparative physiology of dimethyl sulfide production by dimethylsulfoniopropionate lyase in Pseudomonas doudoroffii and Alcaligenes sp. strain M3A. Appl Environ Microbiol. 1995;61:3986–3991. doi: 10.1128/aem.61.11.3986-3991.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Souza M P, Chen Y P, Yoch D C. Dimethylsulfoniopropionate lyase from the marine macroalga Ulva curvata: purification and characterization of the enzyme. Planta. 1996;199:433–438. [Google Scholar]
- 12.Fell J W, Newell S Y. Role of fungi in carbon flow and nitrogen immobilization in coastal marine plant litter systems. Mycol Ser. 1981;2:665–678. [Google Scholar]
- 12a.Hanlin, R. (University of Georgia, Athens). Personal communication.
- 13.Ishida Y. 30 years of research on dimethylsulfoniopropionate. In: Kiene R P, Visscher P T, Keller M D, Kirst G O, editors. Environmental and biological chemistry of DMSP and related sulfonium compounds. New York, N.Y: Plenum Press; 1996. pp. 1–12. [Google Scholar]
- 14.Jennings D H. Some aspects of the physiology and biochemistry of marine fungi. Biol Rev. 1983;58:423–459. [Google Scholar]
- 15.Johnson T W, Sparrow F K., Jr . Fungi in oceans and estuaries. J. New York, N.Y: Cramer; 1961. [Google Scholar]
- 16.Karsten U, Wiencke C, Kirst G O. The β-dimethylsulfoniopropionate (DMSP) content of macroalgae from Antarctica and southern Chile. Bot Mar. 1990;33:143–146. [Google Scholar]
- 17.Keller M D, Bellows W K, Guillard R R L. Dimethyl sulfide production in marine phytoplankton. ACS Symp Ser. 1989;393:167–182. [Google Scholar]
- 18.Kiene R P. Dimethyl sulfide production from dimethylsulfoniopropionate in coastal seawater samples and bacterial cultures. Appl Environ Microbiol. 1990;56:3292–3297. doi: 10.1128/aem.56.11.3292-3297.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kohlmeyer J, Kohlmeyer E. Marine mycology, the higher fungi. New York, N.Y: Academic Press, Inc.; 1979. [Google Scholar]
- 20.Larher F, Hamelin J, Stewart G R. L’acidic dimethylsufonium-3-propanoique de Spartina anglica. Phytochemistry. 1977;16:2019–2020. [Google Scholar]
- 21.Ledyard K M, Dacey J W H. Dimethylsulfide production from dimethylsulfoniopropionate by a marine bacterium. Mar Ecol Prog Ser. 1994;110:95–103. [Google Scholar]
- 22.Le Rudulier D, Strom A R, Dandekar A M, Smith L T, Valentine R C. Molecular biology of osmoregulation. Science. 1984;224:1064–1068. doi: 10.1126/science.224.4653.1064. [DOI] [PubMed] [Google Scholar]
- 23.Marzluf G A. Regulation of sulfur and nitrogen metabolism in filamentous fungi. Annu Rev Microbiol. 1993;47:31–55. doi: 10.1146/annurev.mi.47.100193.000335. [DOI] [PubMed] [Google Scholar]
- 24.Moss S T, editor. The biology of marine fungi. London, United Kingdom: Cambridge University Press; 1986. [Google Scholar]
- 25.Nelson P E, Toussoun T A, Marass W F O. Fusarium species, an illustrated manual for identification. University Park: Pennsylvania State University Press; 1983. pp. 124–127. [Google Scholar]
- 26.Newell S Y. Decomposition of shoots of a salt-marsh cordgrass. Adv Microb Ecol. 1993;13:301–326. [Google Scholar]
- 27.Newell S Y. Established and potential impacts of eukaryotic mycelial decomposers in marine/terrestrial ecotones. J Exp Mar Biol Ecol. 1996;200:187–206. [Google Scholar]
- 28.Newell S Y, Porter D, Lingle W L. Lignocellulosis by Ascomycetes (fungi) of a saltmarsh grass (smooth cordgrass) Microbiol Res Tech. 1996;33:32–46. doi: 10.1002/(SICI)1097-0029(199601)33:1<32::AID-JEMT5>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 28a.Nishiguchi M K, Goff L J. Isolation, purification, and characterization of DMSP lyase (dimethylpropiothetin dethiomethylase (4.4.1.3)) from the red alga Polysiphonia paniculata. J Phycol. 1995;31:567–574. [Google Scholar]
- 29.Otte M L, Morris J T. Dimethylsulfoniopropionate (DMSP) in Spartina alterniflora Loisel. Aquat Bot. 1994;48:239–259. [Google Scholar]
- 30.Reed R H. Measurement and osmotic significance of β-dimethylsulfoniopropionate in marine microalgae. Mar Biol Lett. 1983;4:173–178. [Google Scholar]
- 31.Slaughter J C. Sulfur compounds in fungi. In: Boddy L, Marchant R, Read D J, editors. Nitrogen, phosphorus and sulphur utilization by fungi. Cambridge, United Kingdom: Cambridge University Press; 1989. pp. 91–105. [Google Scholar]
- 32.Stefels J, van Boekel W H M. Production of DMS from dissolved DMSP in axenic cultures of the marine phytoplankton species Phaeocystis sp. Mar Ecol Prog Ser. 1993;97:11–18. [Google Scholar]
- 33.Steinke M, Daniel C, Kirst G O. DMSP lyase in marine macro- and microalgae. In: Kiene R P, Visscher P T, Keller M D, Kirst G O, editors. Biological and environmental chemistry of DMSP and related sulfonium compounds. New York, N.Y: Plenum Press; 1996. pp. 317–324. [Google Scholar]
- 34.Steudler P A, Peterson B J. Contribution of gaseous sulphur from salt marshes to the global sulfur cycle. Nature (London) 1984;311:455–457. [Google Scholar]
- 35.Stryer L. Catalytic strategies. In: Stryer L, editor. Biochemistry. 4th ed. New York, N.Y: W. H. Freeman & Co.; 1995. pp. 206–236. [Google Scholar]
- 36.van Iwaarden P R, Driessen A J M, Konings W N. What we can learn from the effects of thiol reagents on transport proteins. Biochim Biophys Acta. 1992;1113:161–170. doi: 10.1016/0304-4157(92)90037-b. [DOI] [PubMed] [Google Scholar]
- 37.Vogel H, Bonner D M. Acetylornithase of E. coli: partial purification and properties. J Biol Chem. 1956;218:97–106. [PubMed] [Google Scholar]
- 38.Yoch D C, Ansede J H, Rabinowitz K S. Evidence for intracellular and extracellular dimethylsulfoniopropionate (DMSP) lyases and DMSP uptake sites in two species of marine bacteria. Appl Environ Microbiol. 1997;63:3182–3188. doi: 10.1128/aem.63.8.3182-3188.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]