Abstract
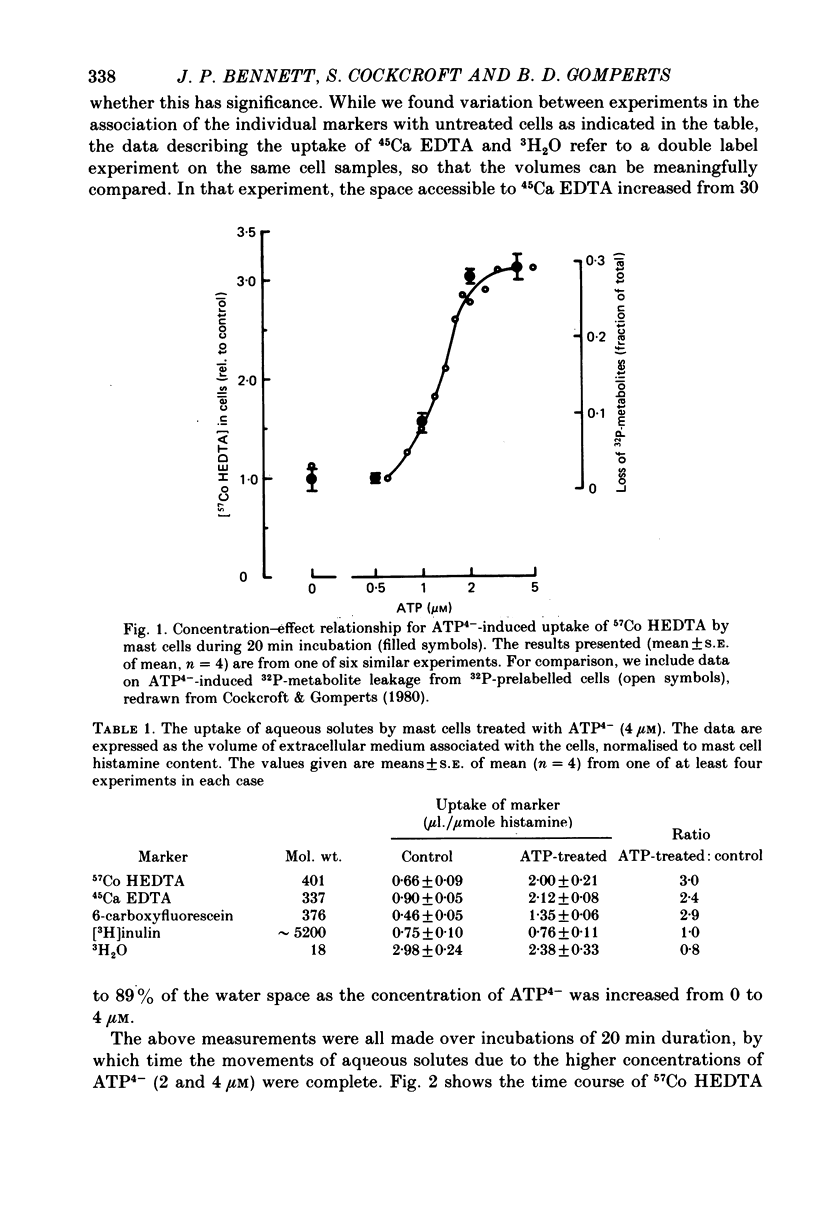
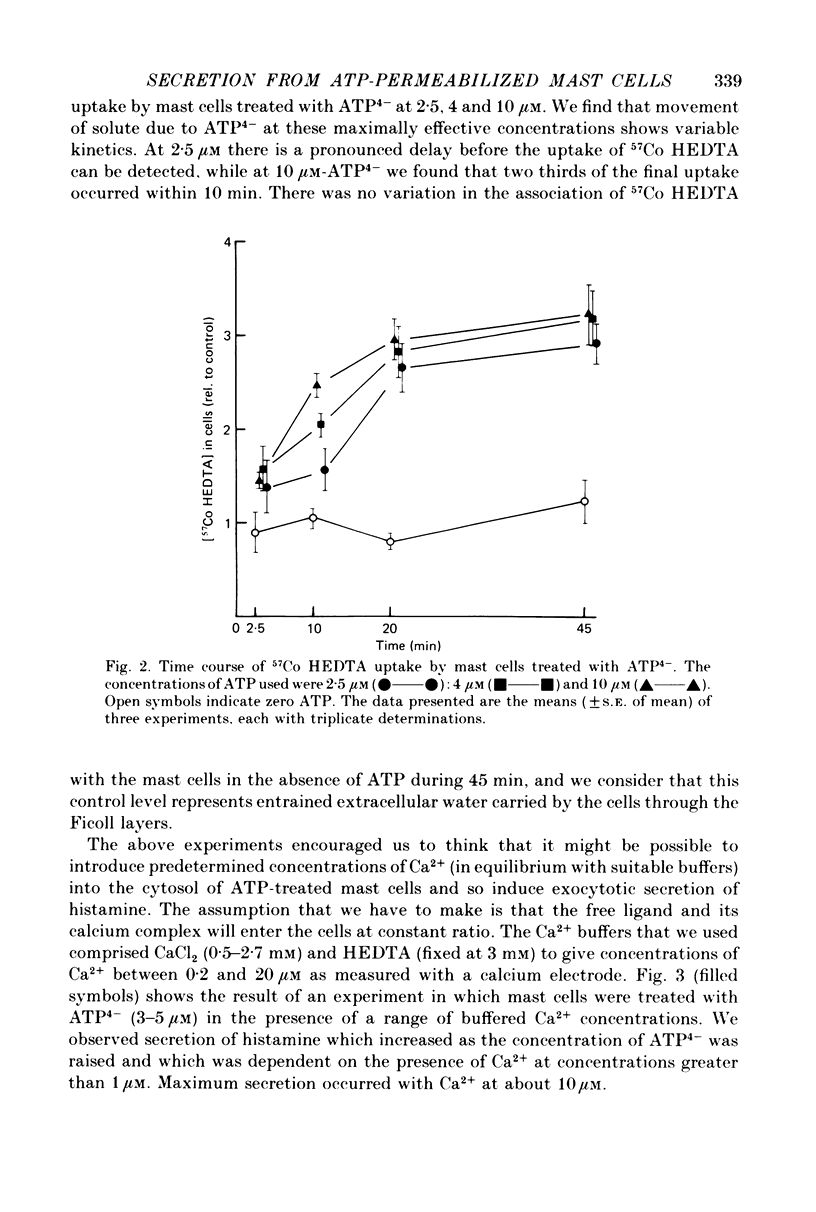
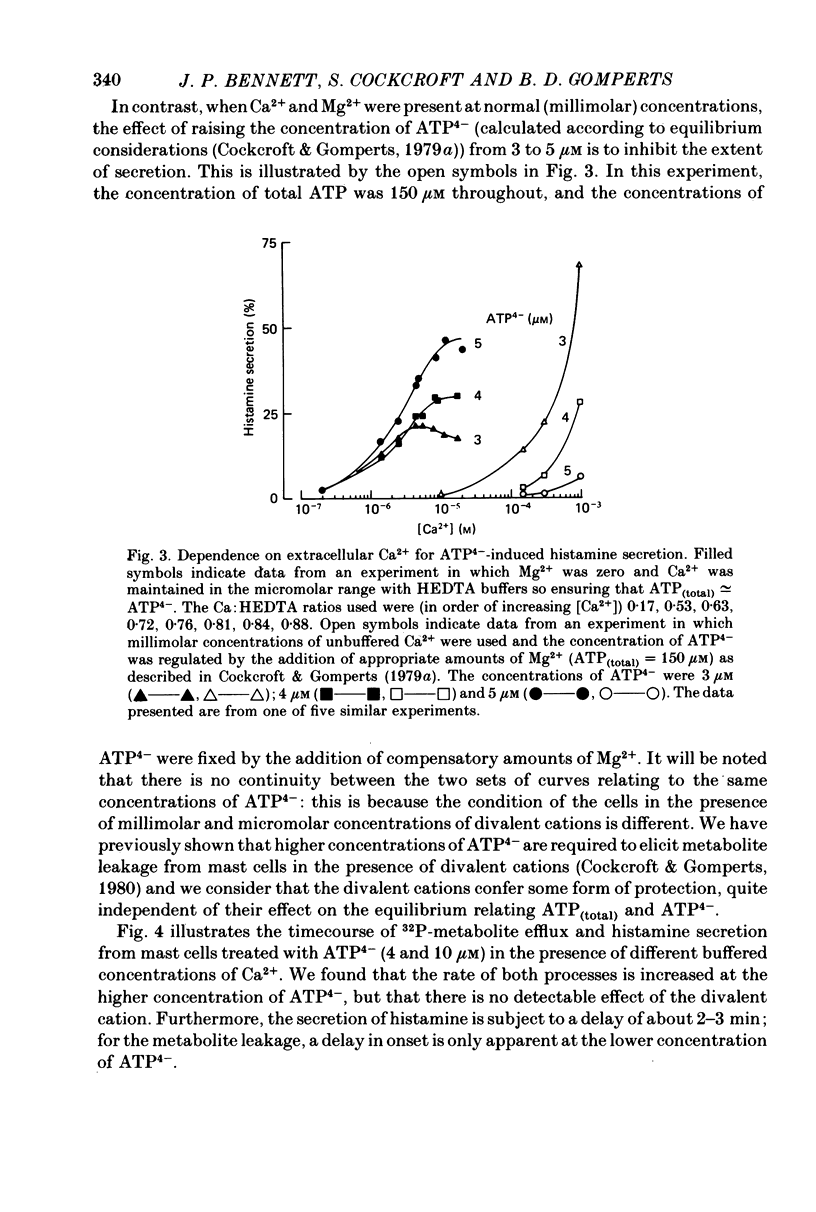
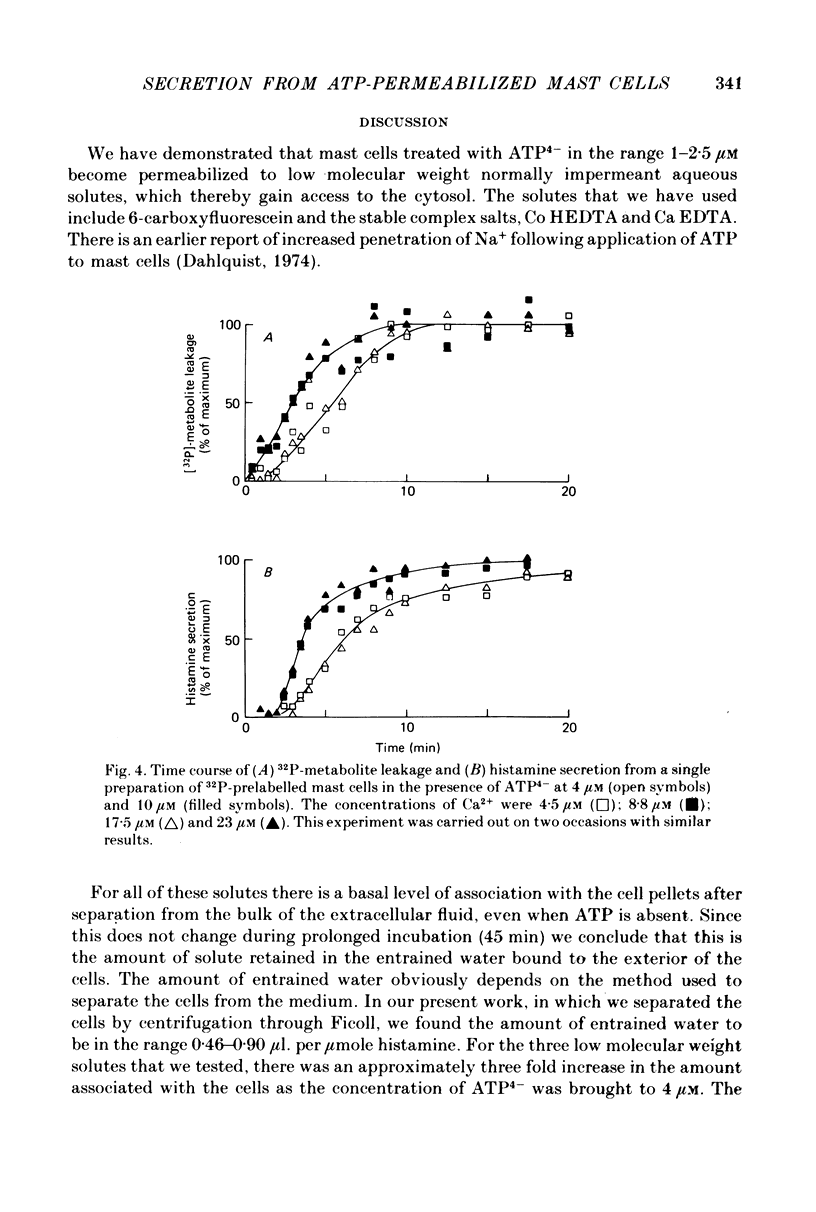
1. Rat mast cells with ATP (0.5-4 micro M) in the absence of divalent cations (so that almost all the ATP is present as ATP4-) became permeable to normally impermeant aqueous solutes, added extracellularly. These include the stable complexes Co HEDTA and Ca EDTA, and 6-carboxyfluorescein but not inulin. At 4 micro M-ATP4- the space accessible to Ca EDTA is 89% of that occupied by 3H2O. 2. The kinetics of solute entry are regulated by the concentration of ATP4-. 3. Ca2+, buffered in the range 1-10 micro M with HEDTA, causes histamine secretion from mast cells that have been rendered permeable with ATP4-. The extent of secretion increases as the concentration of ATP4- is raised from 3 to 5 micro M. With extracellular Ca2+ present as physiological (millimolar) concentrations, the effect of increasing ATP4- through this range is to inhibit secretion. 4. The rates of histamine secretion and of 32P-metabolite leakage from 32P-prelabelled cells in the presence of micromolar concentrations of Ca2+, were compared. The kinetics of both processes are regulated by the concentration of ATP4-, but not Ca2+.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker P. F., Knight D. E. Calcium-dependent exocytosis in bovine adrenal medullary cells with leaky plasma membranes. Nature. 1978 Dec 7;276(5688):620–622. doi: 10.1038/276620a0. [DOI] [PubMed] [Google Scholar]
- Bennett J. P., Cockcroft S., Gomperts B. D. Ionomycin stimulates mast cell histamine secretion by forming a lipid-soluble calcium complex. Nature. 1979 Dec 20;282(5741):851–853. doi: 10.1038/282851a0. [DOI] [PubMed] [Google Scholar]
- Bennett J. P., Cockcroft S., Gomperts B. D. Use of cytochalasin B to distinguish between early and late events in neutrophil activation. Biochim Biophys Acta. 1980 Oct 2;601(3):584–591. doi: 10.1016/0005-2736(80)90560-x. [DOI] [PubMed] [Google Scholar]
- Cockcroft S., Gomperts B. D. ATP induces nucleotide permeability in rat mast cells. Nature. 1979 Jun 7;279(5713):541–542. doi: 10.1038/279541a0. [DOI] [PubMed] [Google Scholar]
- Cockcroft S., Gomperts B. D. Activation and inhibition of calcium-dependent histamine secretion by ATP ions applied to rat mast cells. J Physiol. 1979 Nov;296:229–243. doi: 10.1113/jphysiol.1979.sp013002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockcroft S., Gomperts B. D. The ATP4- receptor of rat mast cells. Biochem J. 1980 Jun 15;188(3):789–798. doi: 10.1042/bj1880789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford L. E., Podolsky R. J. Calcium uptake and force development by skinned muscle fibres in EGTA buffered solutions. J Physiol. 1972 May;223(1):1–19. doi: 10.1113/jphysiol.1972.sp009830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foreman J. C., Mongar J. L. The role of the alkaline earth ions in anaphylactic histamine secretion. J Physiol. 1972 Aug;224(3):753–769. doi: 10.1113/jphysiol.1972.sp009921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinl P., Kuhn H. J., Rüegg J. C. Tension responses to quick length changes of glycerinated skeletal muscle fibres from the frog and tortoise. J Physiol. 1974 Mar;237(2):243–258. doi: 10.1113/jphysiol.1974.sp010480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helander H. F., Bloom G. D. Quantitative analysis of mast cell structure. J Microsc. 1974 Apr;100(3):315–321. doi: 10.1111/j.1365-2818.1974.tb03943.x. [DOI] [PubMed] [Google Scholar]
- Johansen T. Utilization of adenosine triphosphate in rat mast cells during histamine release induced by the ionophore A23187. Br J Pharmacol. 1979 Jan;65(1):103–109. doi: 10.1111/j.1476-5381.1979.tb17338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makan N. R., Heppel L. A. Control of glycolysis and the pentose phosphate shunt in transformed 3T3 cultures rendered permeable by ATP. J Cell Physiol. 1978 Jul;96(1):87–94. doi: 10.1002/jcp.1040960111. [DOI] [PubMed] [Google Scholar]
- Meech R. W. The sensitivity of Helix aspersa neurones to injected calcium ions. J Physiol. 1974 Mar;237(2):259–277. doi: 10.1113/jphysiol.1974.sp010481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pant H. C., Terakawa S., Yoshioka T., Tasaki I., Gainer H. Evidence for the utilization of extracellular [gamma-32P]ATP for the phosphorylation of intracellular proteins in the squid giant axon. Biochim Biophys Acta. 1979 Jan 4;582(1):107–114. doi: 10.1016/0304-4165(79)90293-9. [DOI] [PubMed] [Google Scholar]
- Pollard H. B., Zinder O., Hoffman P. G., Nikodejevic O. Regulation of the transmembrane potential of isolated chromaffin granules by ATP, ATP analogs, and external pH. J Biol Chem. 1976 Aug 10;251(15):4544–4550. [PubMed] [Google Scholar]
- Rorive G., Kleinzeller A. The effect of ATP and Ca 2+ on the cell volume in isolated kidney tubules. Biochim Biophys Acta. 1972 Jul 3;274(1):226–239. doi: 10.1016/0005-2736(72)90296-9. [DOI] [PubMed] [Google Scholar]
- Rozengurt E., Heppel L. A., Friedberg I. Effect of exogenous ATP on the permeability properties of transformed cultures of mouse cell lines. J Biol Chem. 1977 Jul 10;252(13):4584–4590. [PubMed] [Google Scholar]
- Schanne F. A., Kane A. B., Young E. E., Farber J. L. Calcium dependence of toxic cell death: a final common pathway. Science. 1979 Nov 9;206(4419):700–702. doi: 10.1126/science.386513. [DOI] [PubMed] [Google Scholar]
- Trams E. G. Evidence for ATP action on the cell surface. Nature. 1974 Dec 6;252(5483):480–482. doi: 10.1038/252480a0. [DOI] [PubMed] [Google Scholar]
- Westcott K. R., Engelhard V. H., Storm D. R. Production of cyclic AMP from extracellular ATP by intact LM cells. Biochim Biophys Acta. 1979 Feb 19;583(1):47–54. doi: 10.1016/0304-4165(79)90308-8. [DOI] [PubMed] [Google Scholar]



