Abstract
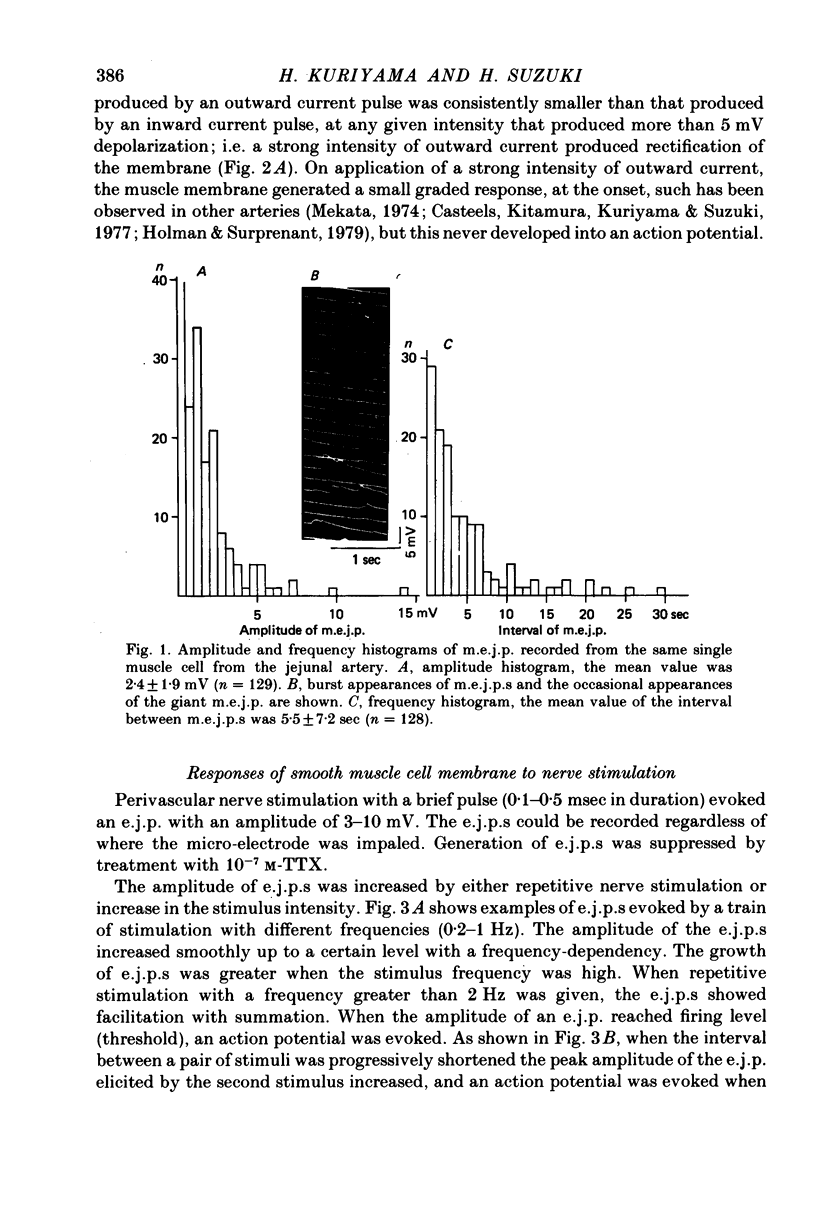
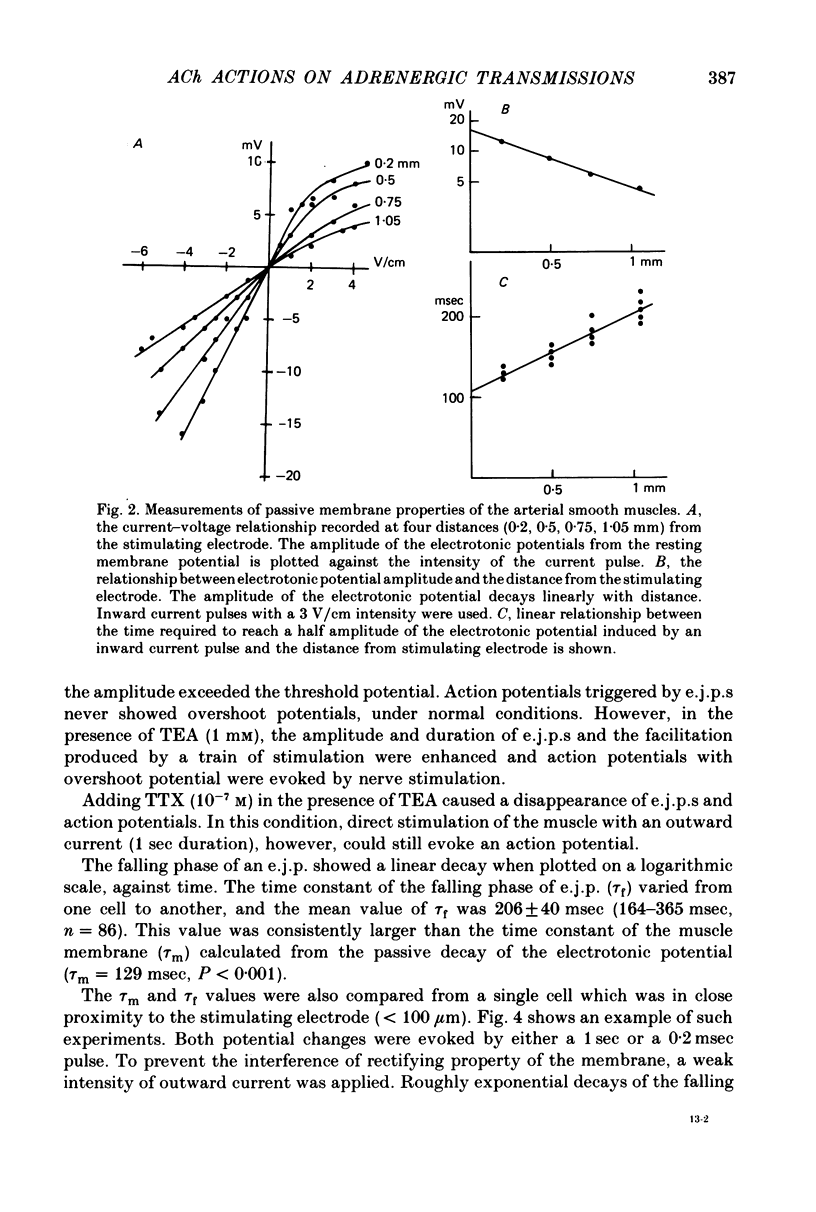
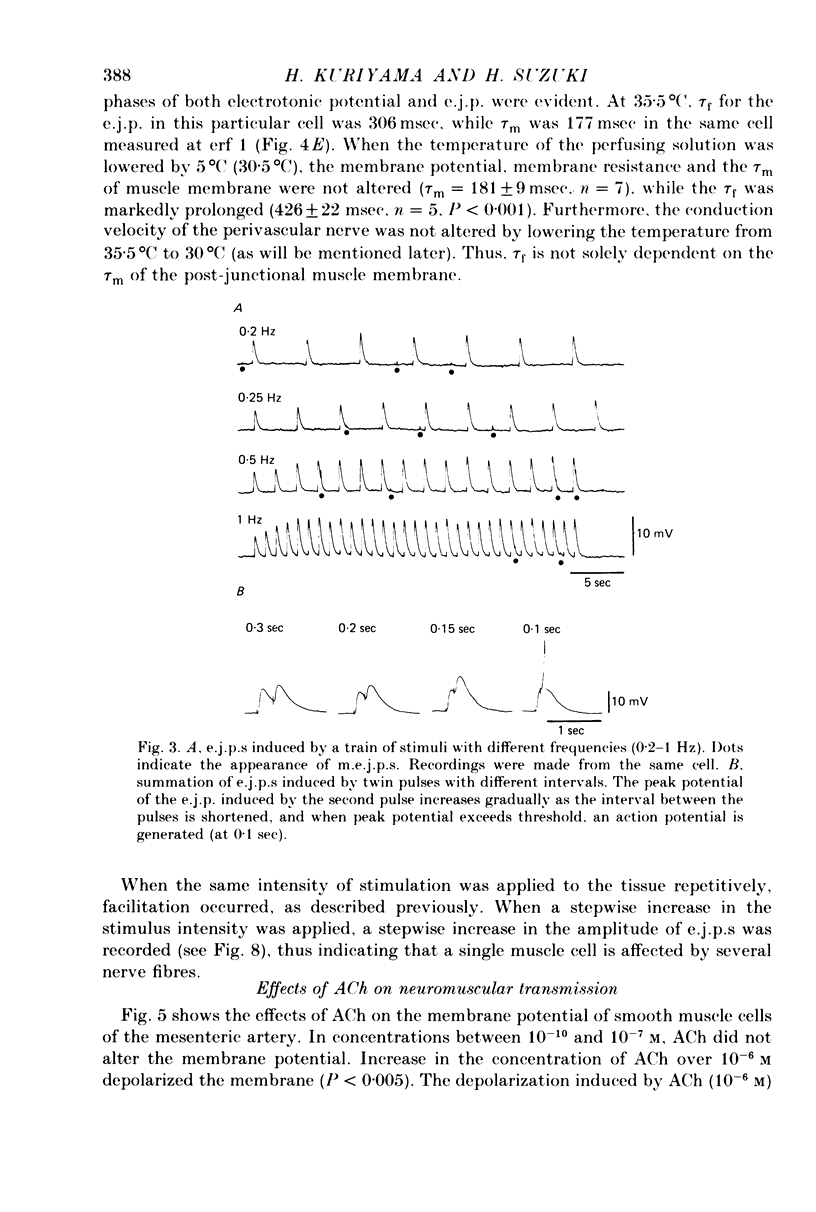
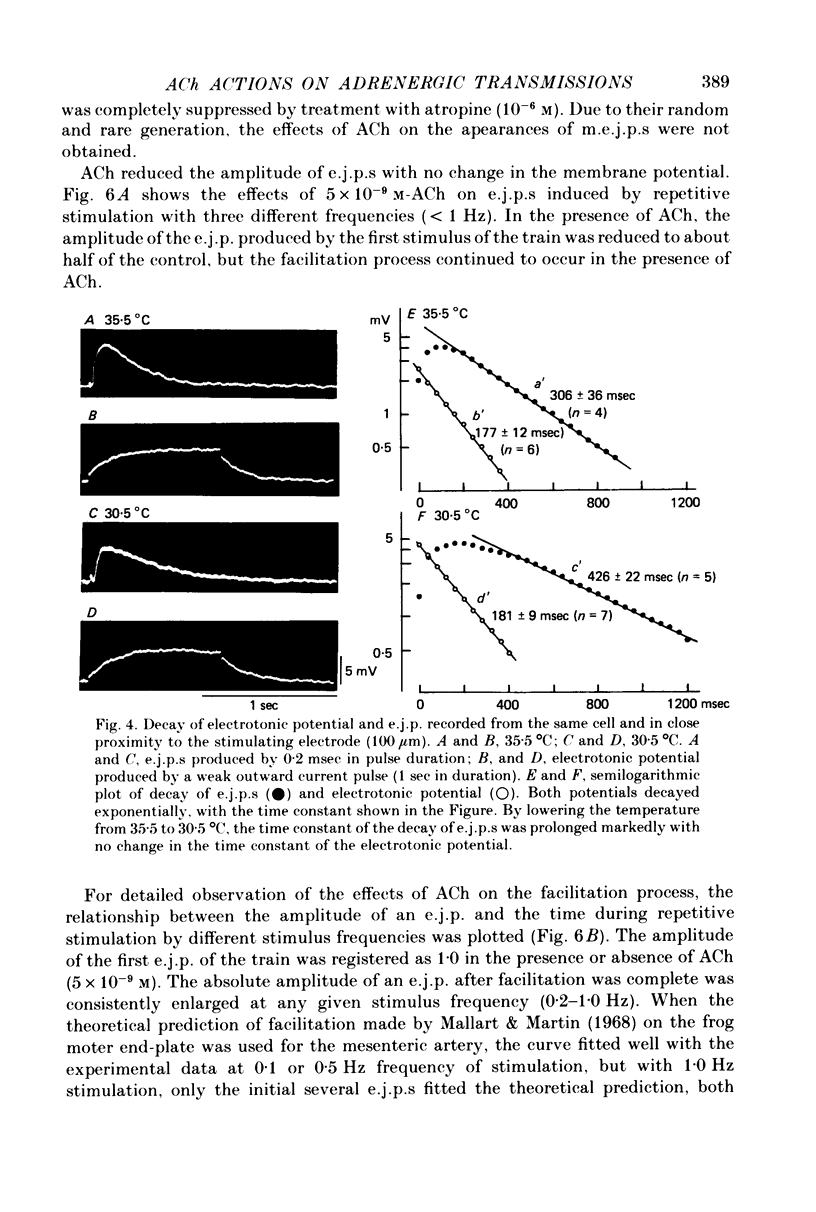
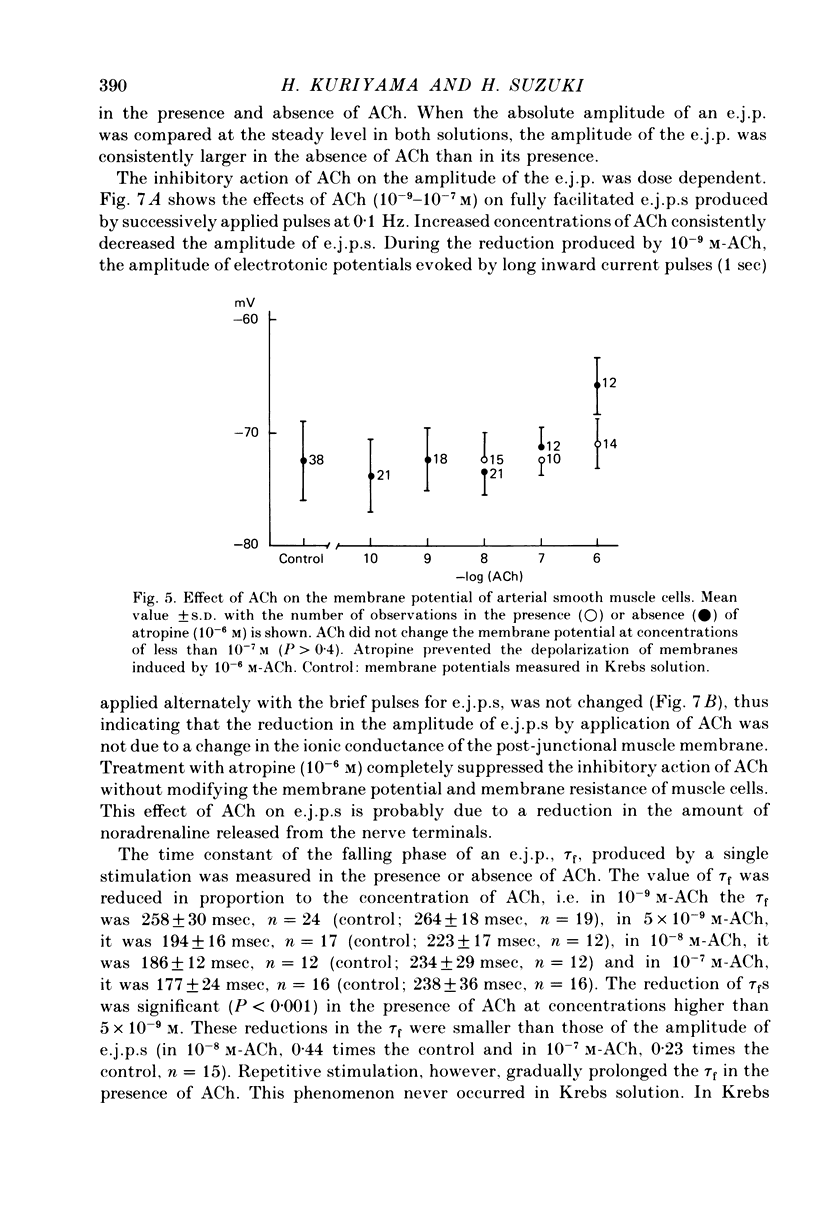
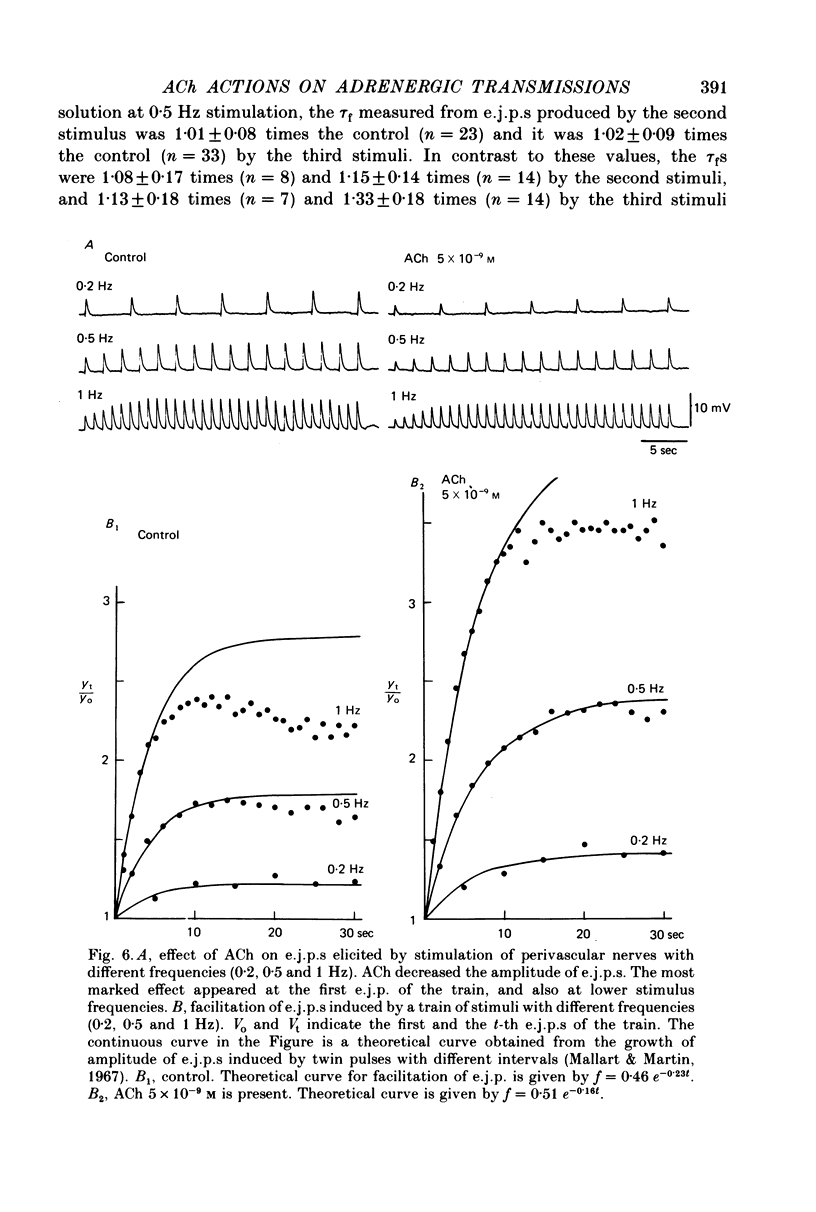
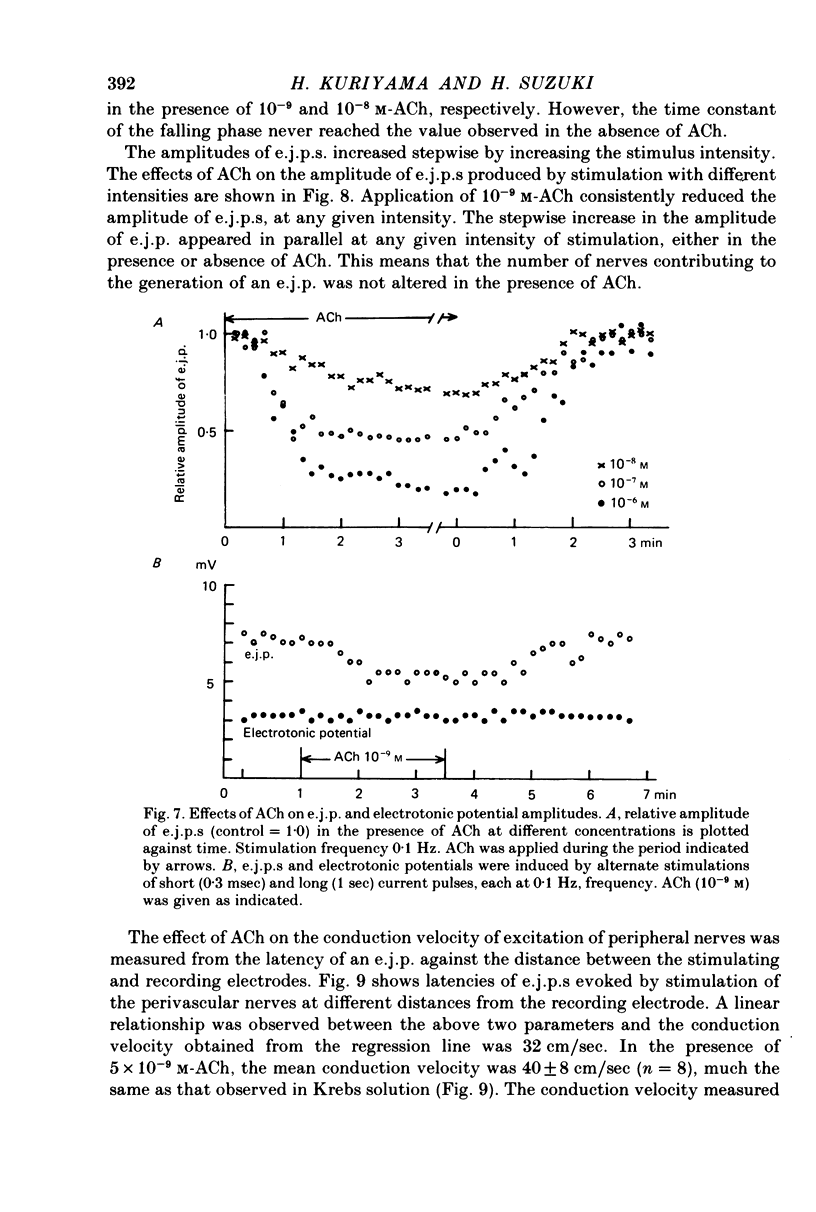
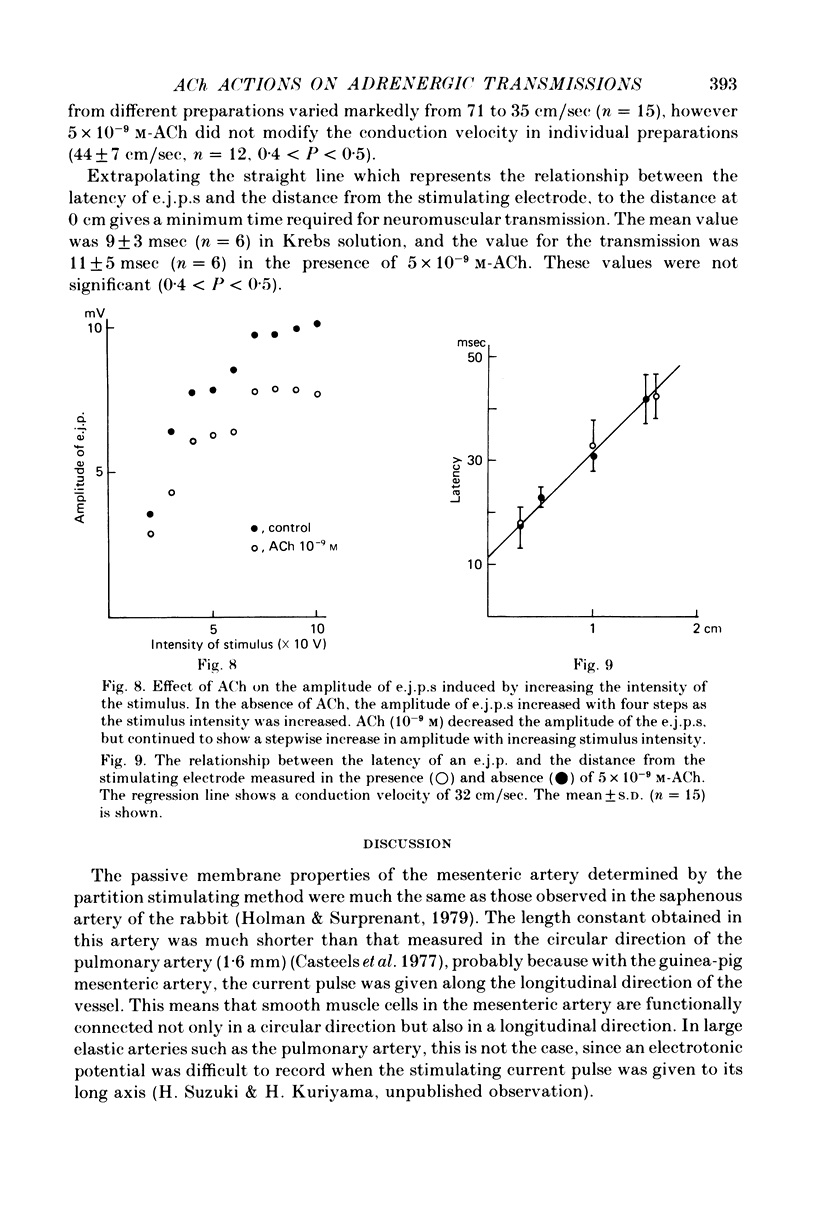
1. Neuromuscular transmission in the guinea-pig mesenteric artery and the effect of acetylcholine (ACh) on transmission were investigated by the micro-electrode method. 2. The membrane potential, length and time constant of the smooth muscle of the mesenteric artery were found to be -69.6 +/- 1.9 mV, 0.81 +/- 0.15 mm and 129 +/- 42 msec, respectively. 3. Perivascular nerve stimulation produced an excitatory junction potential (e.j.p.) and repetitive stimulation produced facilitation. When the amplitude of an e.j.p. reached threshold, a spike was evoked. 4. Very rarely, miniature e.j.p.s. were recorded. The amplitude histogram showed a skew distribution. Increases in the stimulus intensity enlarged the amplitude of e.j.p.s. as a stepwise manner. These results indicate multiple innervation to the muscle cells. 5. The time constant of the falling phase of an e.j.p. was consistently larger than that of the muscle membrane. 6. ACh, in concentrations of less than 10(-8) M, suppressed the e.j.p. amplitude without change in the membrane potential and resistance of the muscle membrane, but accelerated facilitation. These ACh actions were suppressed by pre-treatment with atropine. 7. ACh suppressed neither the conduction velocity of excitation of adrenergic nerves nor the number of nerves contributing to the generation of an e.j.p. 8. These results suggest that pre- and post-junctional muscarinic receptors possess different sensitivities to ACh, and a low concentration of ACh (less than 10(-8)M solely suppressed the release of noradrenaline by activating the pre-junctional muscarinic receptors.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe Y., Tomita T. Cable properties of smooth muscle. J Physiol. 1968 May;196(1):87–100. doi: 10.1113/jphysiol.1968.sp008496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altura B. M., Malaviya D., Reich C. F., Orkin L. R. Effects of vasoactive agents on isolated human umbilical arteries and veins. Am J Physiol. 1972 Feb;222(2):345–355. doi: 10.1152/ajplegacy.1972.222.2.345. [DOI] [PubMed] [Google Scholar]
- BULBRING E. Membrane potentials of smooth muscle fibres of the taenia coli of the guinea-pig. J Physiol. 1954 Aug 27;125(2):302–315. doi: 10.1113/jphysiol.1954.sp005159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell C. Control of uterine blood flow in pregnancy. Med Biol. 1974 Aug;52(4):219–228. [PubMed] [Google Scholar]
- Bell C. Mechanism of enhancement by angiotensin II of sympathetic adrenergic transmission in the guinea pig. Circ Res. 1972 Sep;31(3):348–355. doi: 10.1161/01.res.31.3.348. [DOI] [PubMed] [Google Scholar]
- Bell C. Transmission from vasoconstrictor and vasodilator nerves to single smooth muscle cells of the guinea-pig uterine artery. J Physiol. 1969 Dec;205(3):695–708. doi: 10.1113/jphysiol.1969.sp008991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteels R., Kitamura K., Kuriyama H., Suzuki H. The membrane properties of the smooth muscle cells of the rabbit main pulmonary artery. J Physiol. 1977 Sep;271(1):41–61. doi: 10.1113/jphysiol.1977.sp011989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehinger B., Gennser G., Owman C., Persson H., Sjöberg N. O. Histochemical and pharmacological studies on amine mechanisms in the umbilical cord, umbilical vein and ductus venosus of the human fetus. Acta Physiol Scand. 1968 Jan-Feb;72(1):15–24. doi: 10.1111/j.1748-1716.1968.tb03821.x. [DOI] [PubMed] [Google Scholar]
- Ellison G. D., Zanchetti A. Diffuse and specific activation of sympathetic cholinergic fibers of the cat. Am J Physiol. 1973 Jul;225(1):142–149. doi: 10.1152/ajplegacy.1973.225.1.142. [DOI] [PubMed] [Google Scholar]
- Feigl E. O. Parasympathetic control of coronary blood flow in dogs. Circ Res. 1969 Nov;25(5):509–519. doi: 10.1161/01.res.25.5.509. [DOI] [PubMed] [Google Scholar]
- Hirst G. D., Neild T. O. An analysis of excitatory junctional potentials recorded from arterioles. J Physiol. 1978 Jul;280:87–104. doi: 10.1113/jphysiol.1978.sp012374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst G. D. Neuromuscular transmission in arterioles of guinea-pig submucosa. J Physiol. 1977 Dec;273(1):263–275. doi: 10.1113/jphysiol.1977.sp012093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holman M. E., Surprenant A. M. Some properties of the excitatory junction potentials recorded from saphenous arteries of rabbits. J Physiol. 1979 Feb;287:337–351. doi: 10.1113/jphysiol.1979.sp012663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes J., Roth R. H. Evidence that angiotensin enhances transmitter release during sympathetic nerve stimulation. Br J Pharmacol. 1971 Feb;41(2):239–255. doi: 10.1111/j.1476-5381.1971.tb08025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y., Kitamura K., Kuriyama H. Effects of acetylcholine and catecholamines on the smooth muscle cell of the porcine coronary artery. J Physiol. 1979 Sep;294:595–611. doi: 10.1113/jphysiol.1979.sp012948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y., Kitamura K., Kuriyama H. Nitroglycerine and catecholamine actions on smooth muscle cells of the canine coronary artery. J Physiol. 1980 Dec;309:171–183. doi: 10.1113/jphysiol.1980.sp013502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y., Tajima K. An electrophysiological analysis of the actions of prostaglandin on neuromuscular transmission in the guinea-pig vas deferens. J Physiol. 1979 Dec;297(0):521–537. doi: 10.1113/jphysiol.1979.sp013054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura K., Kuriyama H. Effects of acetylcholine on the smooth muscle cell of isolated main coronary artery of the guinea-pig. J Physiol. 1979 Aug;293:119–133. doi: 10.1113/jphysiol.1979.sp012881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurihara S., Kuriyama H., Magaribuchi T. Effects of rapid cooling on the electrical properties of the smooth muscle of the guinea-pig urinary bladder. J Physiol. 1974 Apr;238(2):413–426. doi: 10.1113/jphysiol.1974.sp010533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama H., Suzuki H. The effects of acetylcholine on the membrane and contractile properties of smooth muscle cells of the rabbit superior mesenteric artery. Br J Pharmacol. 1978 Dec;64(4):493–501. doi: 10.1111/j.1476-5381.1978.tb17310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer S. Z. Sixth gaddum memorial lecture, National Institute for Medical Research, Mill Hill, January 1977. Presynaptic receptors and their role in the regulation of transmitter release. Br J Pharmacol. 1977 Aug;60(4):481–497. doi: 10.1111/j.1476-5381.1977.tb07526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallart A., Martin A. R. An analysis of facilitation of transmitter release at the neuromuscular junction of the frog. J Physiol. 1967 Dec;193(3):679–694. doi: 10.1113/jphysiol.1967.sp008388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath M. A. 5-hydroxytryptamine and neurotransmitter release in canine blood vessels. Inhibition by low and augmentation by high concentrations. Circ Res. 1977 Oct;41(4):428–435. doi: 10.1161/01.res.41.4.428. [DOI] [PubMed] [Google Scholar]
- McGrath M. A., Shepherd J. T. Inhibition of adrenergic neurotransmission in canine vascular smooth muscle by histamine: mediation by H2-receptors. Circ Res. 1976 Oct;39(4):566–573. doi: 10.1161/01.res.39.4.566. [DOI] [PubMed] [Google Scholar]
- Mekata F. Current spread in the smooth muscle of the rabbit aorta. J Physiol. 1974 Oct;242(1):143–155. doi: 10.1113/jphysiol.1974.sp010698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton J. M., Detar R. Potassium and isolated coronary vascular smooth muscle. Am J Physiol. 1972 Feb;222(2):474–479. doi: 10.1152/ajplegacy.1972.222.2.474. [DOI] [PubMed] [Google Scholar]
- Speden R. N. Adrenergic transmission in small arteries. Nature. 1967 Oct 21;216(5112):289–290. doi: 10.1038/216289a0. [DOI] [PubMed] [Google Scholar]
- Starke K. Alpha sympathomimetic inhibition of adrenergic and cholinergic transmission in the rabbit heart. Naunyn Schmiedebergs Arch Pharmacol. 1972;274(1):18–45. doi: 10.1007/BF00501004. [DOI] [PubMed] [Google Scholar]
- Steinsland O. S., Furchgott R. F., Kirpekar S. M. Inhibition of adrenergic neurotransmission by parasympathomimetics in the rabbit ear artery. J Pharmacol Exp Ther. 1973 Feb;184(2):346–356. [PubMed] [Google Scholar]
- Suzuki H., Kuriyama H. Observation of quantal release of noradrenaline from vascular smooth muscles in potassium-free solution. Jpn J Physiol. 1980;30(4):665–670. doi: 10.2170/jjphysiol.30.665. [DOI] [PubMed] [Google Scholar]
- Vanhoutte P. M. Inhibition by acetylcholine of adrenergic neurotransmission in vascular smooth muscle. Circ Res. 1974 Mar;34(3):317–326. doi: 10.1161/01.res.34.3.317. [DOI] [PubMed] [Google Scholar]
- Vanhoutte P. M., Lorenz R. R. Effect of temperature on reactivity of saphenous, mesenteric, and femoral veins of the dog. Am J Physiol. 1970 Jun;218(6):1746–1750. doi: 10.1152/ajplegacy.1970.218.6.1746. [DOI] [PubMed] [Google Scholar]
- Vanhoutte P. M., Lorenz R. R., Tyce G. M. Inhibition of norepinephrine- 3 H release from sympathetic nerve endings in veins by acetylcholine. J Pharmacol Exp Ther. 1973 May;185(2):386–394. [PubMed] [Google Scholar]
- Zimmerman B. G., Whitmore L. Effect of angiotensin and phenoxybenzamine on release of norepinephrine in vessels during sympathetic nerve stimulation. Int J Neuropharmacol. 1967 Jan;6(1):27–38. doi: 10.1016/0028-3908(67)90039-1. [DOI] [PubMed] [Google Scholar]



