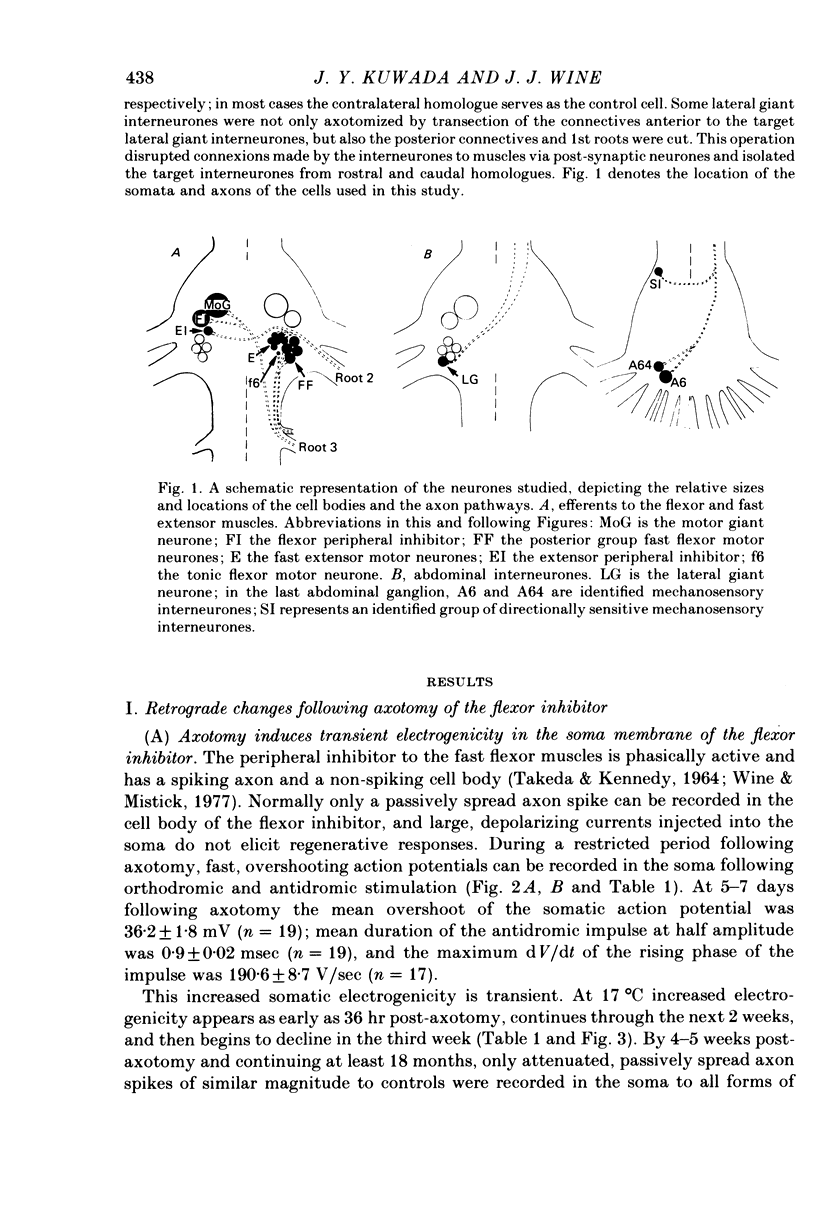
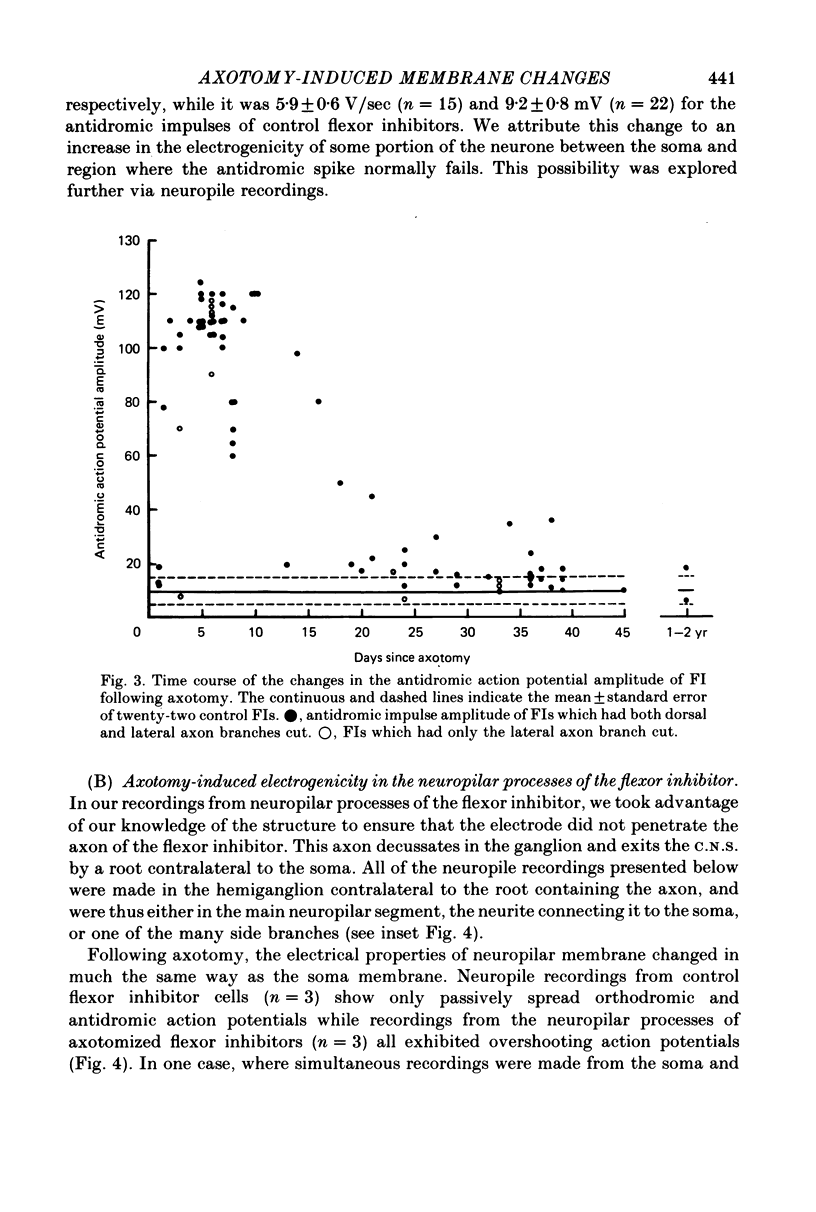
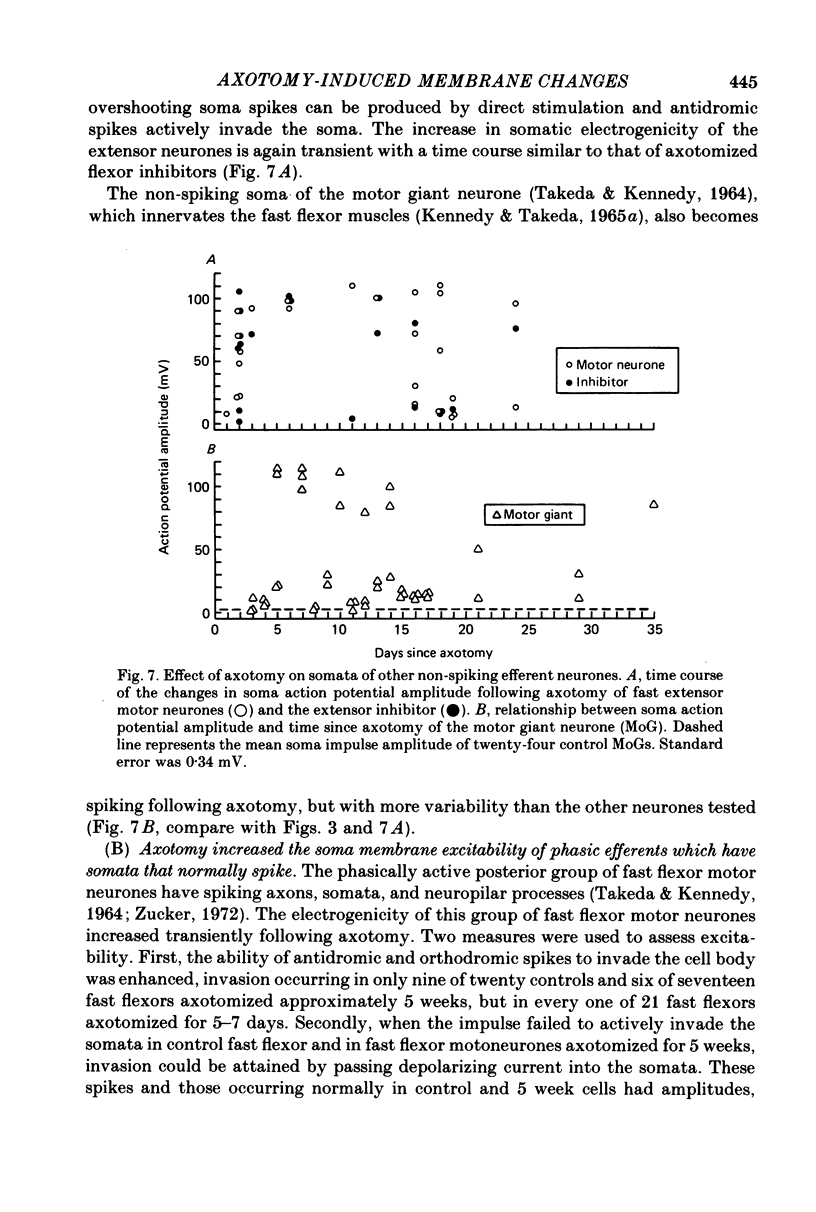
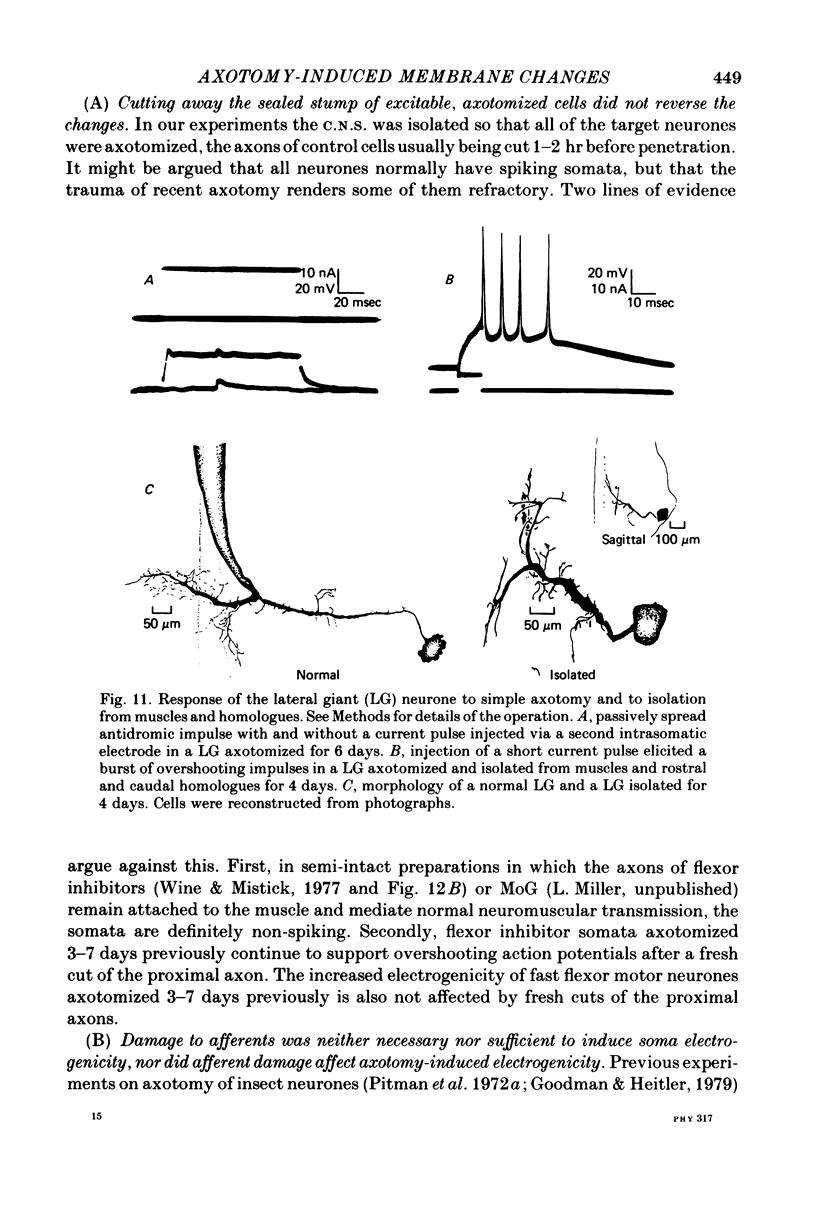
Abstract
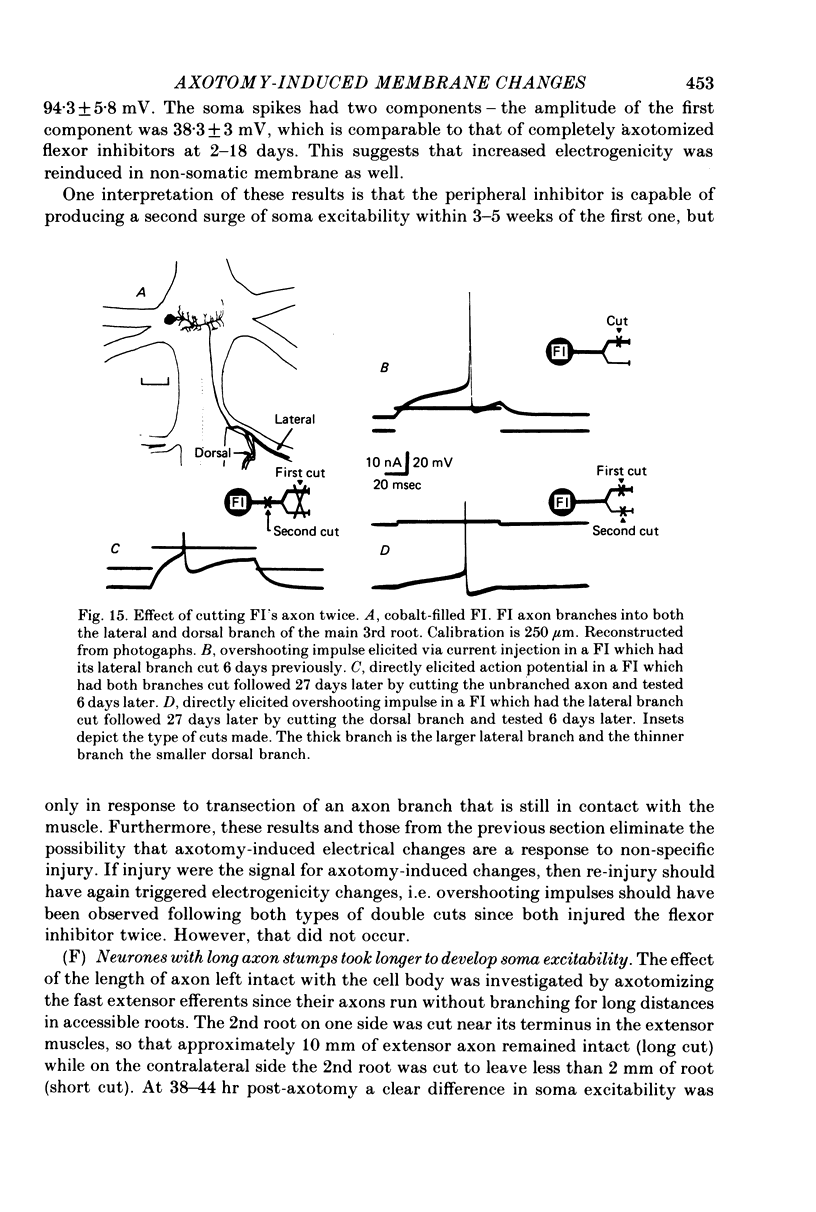
1. In crayfish, the normally passive, non-spiking somata of certain unipolar, efferent neurones became spiking within 36 hr of axotomy. 2. The changes persisted for approximately 2 weeks and then waned. The decline in excitability occurred independently of regeneration, and excitability was not restored by recutting the axon stump. 3. The neuropilar processes also became capable of supporting spikes, but synaptic transmission onto the cells and the spike threshold for orthodromic activation were unchanged, as was the gross structure of the neurone. 4. In somata which normally spike, electrogenicity was nevertheless increased, as evidenced by soma spikes that were larger, faster rising, and easier to evoke. 5. We tested for post-axotomy excitability changes in a variety of identified neurones. Every type (n = 5) of phasically active efferent we tested responded as above, as did all three phasic interneurones. One class of spontaneously active interneurones and one spontaneously active efferent did not respond to axotomy. 6. Extensive damage to afferents did not initiate changes in efferents of the same ganglion, nor did it interfere with changes induced by axotomy of the efferents. 7. Transection of the larger of the two main branches of the phasic flexor inhibitor induced soma excitability, but cutting the smaller branch did not. However, after the excitability caused by cutting the larger branch waned, transection of the smaller branch then induced excitability. 8. Neurones with longer axon stumps took longer to develop soma excitability.
Full text
PDF





















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BODENSTEIN D. Studies on nerve regeneration in Periplaneta americana. J Exp Zool. 1957 Oct;136(1):89–115. doi: 10.1002/jez.1401360107. [DOI] [PubMed] [Google Scholar]
- Bacon J. P., Altman J. S. A silver intensification method for cobalt-filled neurones in wholemount preparations. Brain Res. 1977 Dec 16;138(2):359–363. doi: 10.1016/0006-8993(77)90753-3. [DOI] [PubMed] [Google Scholar]
- Blinzinger K., Kreutzberg G. Displacement of synaptic terminals from regenerating motoneurons by microglial cells. Z Zellforsch Mikrosk Anat. 1968;85(2):145–157. doi: 10.1007/BF00325030. [DOI] [PubMed] [Google Scholar]
- Borgens R. B., Jaffe L. F., Cohen M. J. Large and persistent electrical currents enter the transected lamprey spinal cord. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1209–1213. doi: 10.1073/pnas.77.2.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner H. R., Johnson E. W. Physiological and morphological effects of post-ganglionic axotomy on presynaptic nerve terminals. J Physiol. 1976 Aug;260(1):143–158. doi: 10.1113/jphysiol.1976.sp011508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner H. R., Martin A. R. Reduction in acetylcholine sensitivity of axotomized ciliary ganglion cells. J Physiol. 1976 Aug;260(1):159–175. doi: 10.1113/jphysiol.1976.sp011509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CERF J. A., CHACKO L. W. Retrograde reaction in motoneuron dendrites following ventral root section in the frog. J Comp Neurol. 1958 Apr;109(2):205–219. doi: 10.1002/cne.901090205. [DOI] [PubMed] [Google Scholar]
- COHEN M. J., JACKLET J. W. NEURONS OF INSECTS: RNA CHANGES DURING INJURY AND REGENERATION. Science. 1965 May 28;148(3674):1237–1239. doi: 10.1126/science.148.3674.1237. [DOI] [PubMed] [Google Scholar]
- Cragg B. G. What is the signal for chromatolysis? Brain Res. 1970 Sep 29;23(1):1–21. doi: 10.1016/0006-8993(70)90345-8. [DOI] [PubMed] [Google Scholar]
- ECCLES J. C., LIBET B., YOUNG R. R. The behaviour of chromatolysed motoneurones studied by intracellular recording. J Physiol. 1958 Aug 29;143(1):11–40. doi: 10.1113/jphysiol.1958.sp006041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman C. S., Heitler W. J. Electrical properties of insect neurones with spiking and non-spiking somata: normal, axotomized, and colchicine-treated neurones. J Exp Biol. 1979 Dec;83:95–121. doi: 10.1242/jeb.83.1.95. [DOI] [PubMed] [Google Scholar]
- Horridge G. A., Burrows M. Synapses upon motoneurons of locusts during retrograde degeneration. Philos Trans R Soc Lond B Biol Sci. 1974 Sep 12;269(896):95–108. doi: 10.1098/rstb.1974.0042. [DOI] [PubMed] [Google Scholar]
- Hoy R. R., Bittner G. D., Kennedy D. Regeneration in crustacean motoneurons: evidence for axonal fusion. Science. 1967 Apr 14;156(3772):251–252. doi: 10.1126/science.156.3772.251. [DOI] [PubMed] [Google Scholar]
- Kennedy D., Evoy W. H., Fields H. L. The unit basis of some crustacean reflexes. Symp Soc Exp Biol. 1966;20:75–109. [PubMed] [Google Scholar]
- Kristensson K., Olsson Y. Retrograde transport of horseradish peroxidase in transected axons. 1. Time relationships between transport and induction of chromatolysis. Brain Res. 1974 Oct 11;79(1):101–109. doi: 10.1016/0006-8993(74)90569-1. [DOI] [PubMed] [Google Scholar]
- Kuno M., Llinás R. Alterations of synaptic action in chromatolysed motoneurones of the cat. J Physiol. 1970 Nov;210(4):823–838. doi: 10.1113/jphysiol.1970.sp009244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuno M., Miyata Y., Muñoz-Martinez E. J. Differential reaction of fast and slow alpha-motoneurones to axotomy. J Physiol. 1974 Aug;240(3):725–739. doi: 10.1113/jphysiol.1974.sp010631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuno M., Miyata Y., Muñoz-Martinez E. J. Properties of fast and slow alpha motoneurones following motor reinnervation. J Physiol. 1974 Oct;242(1):273–288. doi: 10.1113/jphysiol.1974.sp010706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwada J. Y. Ionic and metabolic dependence of axotomy-induced somatic membrane changes in crayfish. J Physiol. 1981 Aug;317:463–473. doi: 10.1113/jphysiol.1981.sp013836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman A. R. The axon reaction: a review of the principal features of perikaryal responses to axon injury. Int Rev Neurobiol. 1971;14:49–124. doi: 10.1016/s0074-7742(08)60183-x. [DOI] [PubMed] [Google Scholar]
- Matthews M. R., Nelson V. H. Detachment of structurally intact nerve endings from chromatolytic neurones of rat superior cervical ganglion during the depression of synaptic transmission induced by post-ganglionic axotomy. J Physiol. 1975 Feb;245(1):91–135. doi: 10.1113/jphysiol.1975.sp010837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McINTYRE A. K., BRADLEY K., BROCK L. G. Responses of motoneurons undergoing chromatolysis. J Gen Physiol. 1959 May 20;42(5):931–958. doi: 10.1085/jgp.42.5.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer M. R., Bittner G. D. Histological studies of trophic dependencies in crayfish giant axons. Brain Res. 1978 Mar 24;143(2):195–211. doi: 10.1016/0006-8993(78)90564-4. [DOI] [PubMed] [Google Scholar]
- Mittenthal J. E., Wine J. J. Segmental homology and variation in flexor motoneurons of the crayfish abdomen. J Comp Neurol. 1978 Jan 15;177(2):311–334. doi: 10.1002/cne.901770209. [DOI] [PubMed] [Google Scholar]
- Njå A., Purves D. The effects of nerve growth factor and its antiserum on synapses in the superior cervical ganglion of the guinea-pig. J Physiol. 1978 Apr;277:53–75. [PMC free article] [PubMed] [Google Scholar]
- Nordlander R. H., Singer M. Degeneration and regeneration of severed crayfish sensory fibers: an ultrastructural study. J Comp Neurol. 1973 Nov 15;152(2):175–191. doi: 10.1002/cne.901520205. [DOI] [PubMed] [Google Scholar]
- Payton B. W., Bennett M. V., Pappas G. D. Permeability and structure of junctional membranes at an electrotonic synapse. Science. 1969 Dec 26;166(3913):1641–1643. doi: 10.1126/science.166.3913.1641. [DOI] [PubMed] [Google Scholar]
- Pilar G., Landmesser L. Axotomy mimicked by localized colchicine application. Science. 1972 Sep 22;177(4054):1116–1118. doi: 10.1126/science.177.4054.1116. [DOI] [PubMed] [Google Scholar]
- Pitman R. M., Tweedle C. D., Cohen M. J. Branching of central neurons: intracellular cobalt injection for light and electron microscopy. Science. 1972 Apr 28;176(4033):412–414. doi: 10.1126/science.176.4033.412. [DOI] [PubMed] [Google Scholar]
- Pitman R. M., Tweedle C. D., Cohen M. J. Electrical responses of insect central neurons: augmentation by nerve section or colchicine. Science. 1972 Nov 3;178(4060):507–509. doi: 10.1126/science.178.4060.507. [DOI] [PubMed] [Google Scholar]
- Purves D. Functional and structural changes in mammalian sympathetic neurones following interruption of their axons. J Physiol. 1975 Nov;252(2):429–463. doi: 10.1113/jphysiol.1975.sp011151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purves D., Njå A. Effect of nerve growth factor on synaptic depression after axotomy. Nature. 1976 Apr 8;260(5551):535–536. doi: 10.1038/260535a0. [DOI] [PubMed] [Google Scholar]
- Selverston A. I., Remler M. P. Neural geometry and activation of crayfish fast flexor motoneurons. J Neurophysiol. 1972 Nov;35(6):797–814. doi: 10.1152/jn.1972.35.6.797. [DOI] [PubMed] [Google Scholar]
- Somers M. E., Nunnemacher R. F. Microanatomy of the ganglionic roots of the abdominal cord of the crayfish, Orconectes virilis (Hagen). J Comp Neurol. 1970 Feb;138(2):209–218. doi: 10.1002/cne.901380207. [DOI] [PubMed] [Google Scholar]
- Spitzer N. C., Lamborghini J. E. The development of the action potential mechanism of amphibian neurons isolated in culture. Proc Natl Acad Sci U S A. 1976 May;73(5):1641–1645. doi: 10.1073/pnas.73.5.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart W. W. Functional connections between cells as revealed by dye-coupling with a highly fluorescent naphthalimide tracer. Cell. 1978 Jul;14(3):741–759. doi: 10.1016/0092-8674(78)90256-8. [DOI] [PubMed] [Google Scholar]
- Tweedle C. D., Pitman R. M., Cohen M. J. Dendritic stability of insect central neurons subjected to axotomy and de-afferentation. Brain Res. 1973 Oct 12;60(2):471–476. doi: 10.1016/0006-8993(73)90805-6. [DOI] [PubMed] [Google Scholar]
- WIERSMA C. A., HUGHES G. M. On the functional anatomy of neuronal units in the abdominal cord of the crayfish, Procambarus clarkii (Girard). J Comp Neurol. 1961 Apr;116:209–228. doi: 10.1002/cne.901160209. [DOI] [PubMed] [Google Scholar]
- Watson W. E. An autoradiographic study of the incorporation of nucleic-acid precursors by neurones and glia during nerve regeneration. J Physiol. 1965 Oct;180(4):741–753. doi: 10.1113/jphysiol.1965.sp007728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson W. E. Observations on the nucleolar and total cell body nucleic acid of injured nerve cells. J Physiol. 1968 Jun;196(3):655–676. doi: 10.1113/jphysiol.1968.sp008528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson W. E. The response of motor neurones to intramuscular injection of botulinum toxin. J Physiol. 1969 Jun;202(3):611–630. doi: 10.1113/jphysiol.1969.sp008830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiese K., Calabrese R. L., Kennedy D. Integration of directional mechanosensory input by crayfish interneurons. J Neurophysiol. 1976 Jul;39(4):834–843. doi: 10.1152/jn.1976.39.4.834. [DOI] [PubMed] [Google Scholar]
- Willard A. L. Electrical excitability of outgrowing neurites of embryonic neurones in cultures of dissociated neural plate of Xenopus laevis. J Physiol. 1980 Apr;301:115–128. doi: 10.1113/jphysiol.1980.sp013193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wine J. J. Crayfish neurons with electrogenic cell bodies: correlations with function and dendritic properties. Brain Res. 1975 Feb 21;85(1):92–98. doi: 10.1016/0006-8993(75)91011-2. [DOI] [PubMed] [Google Scholar]
- Wine J. J., Mistick D. C. Temporal organization of crayfish escape behavior: delayed recruitment of peripheral inhibition. J Neurophysiol. 1977 Jul;40(4):904–925. doi: 10.1152/jn.1977.40.4.904. [DOI] [PubMed] [Google Scholar]
- Wine J. J. Neuronal organization of crayfish escape behavior: inhibition of giant motoneuron via a disynaptic pathway from other motoneurons. J Neurophysiol. 1977 Sep;40(5):1078–1097. doi: 10.1152/jn.1977.40.5.1078. [DOI] [PubMed] [Google Scholar]
- Zucker R. S. Crayfish escape behavior and central synapses. 3. Electrical junctions and dendrite spikes in fast flexor motoneurons. J Neurophysiol. 1972 Sep;35(5):638–651. doi: 10.1152/jn.1972.35.5.638. [DOI] [PubMed] [Google Scholar]



